Abstract
Polysaccharides from Ganoderma lucidum have been demonstrated to possess diverse biological activities. Despite lots of studies on the biological activities of Ganoderma lucidum polysaccharide (GLP), little is known regarding the medicinal potential of low–molecular weight enzymatically hydrolyzed Ganoderma lucidum polysaccharide (EGLP). EGLP was prepared by enzymatic degradation and its potential effects in U14 cervical tumor–bearing mice were evaluated. Both GLP and EGLP delayed tumor growth of the tumor xenograft. The EGLP was superior to native polysaccharide. Moreover, EGLP treatment could effectively protect the immune organs of U14 cervical carcinoma–bearing mice. In addition, the EGLP treatment ameliorated oxidative stress as compared with cyclophosphamide (CTX). Compared with the MC group, the expression of Bcl-2 and COX-2 was obviously decreased by EGLP treatment, whereas the expression of Bax and cleaved caspase-3 was obviously increased. These results indicated that EGLP showed stronger antitumor activity with lower toxic effects and had the potential to be a novel antitumor agent.
Keywords: apoptosis, cervical carcinoma, enzymatically hydrolyzed polysaccharide, G. lucidum
Introduction
Cervical cancer is a common malignant tumor in female genital organs, which has more than 500,000 new cases diagnosed each year globally, seriously influencing women’s health and life.1 Radiotherapy, surgery, and chemotherapy are the most common methods in the treatment of cervical cancer. However, surgical treatment is only applicable for these patients in the early stage. Chemotherapy and radiotherapy often cause many adverse effects, for instance, immunotoxicity and organ toxicity.2 Cyclophosphamide (CTX), a traditional DNA alkylating agent, is commonly used in cervical cancer chemotherapy. Its metabolite can eliminate cancer cells and result in immunosuppressive effects.2 Thus, novel therapeutic strategies are urgently required to alleviate those adverse effects and possess antitumor activity associated with this malignancy.
During the past few years, lots of progresses have been achieved in our comprehension of the carcinogenic process at the molecular level. This has promoted the development of a promising way to carcinoma prevention, named “chemoprevention,” which aims to suppress the development of precancerous cells through the use of natural nutrients and pharmacological agents.3 Hence, the utilization of natural products and dietary components as potential carcinoma chemopreventive agents in the form of nutraceuticals has become a significant issue in this cancer-related study. Ganoderma lucidum is an edible mushroom and has long been used as a traditional Chinese medicine with medicinal properties. Polysaccharides are the main components of G. lucidum and possess a wide range of pharmacological activities, such as antimicrobial, antitumor, and antioxidant effects.4 The biological activities of polysaccharides are closely related to their molecular structures, including molecular weight, sulfate content, conformation, and type of glycosidic linkage.4 Increasing evidence indicates that low–molecular weight polysaccharides exhibit anticancer activities toward different types of tumors, such as colon cancer, ovarian cancer, and prostate cancer.5 Low–molecular weight polysaccharides can be prepared by enzymolysis and acidolysis; however, in acidolysis it is difficult to produce the proper size of polysaccharides and the sulfate groups can be removed. The major advantages of enzymolysis are high selectivity, mild conditions, and substrate specificity, which can prepare oligosaccharides with well-defined structures.6 Therefore, we prepared low–molecular weight enzymatically hydrolyzed Ganoderma lucidum polysaccharides (EGLPs) by cellulase enzymolysis and hypothesized that EGLP may suppress cervical cancer development. Therefore, this research aimed to investigate the antitumor activity and regulatory mechanism of EGLP on a U14 cervical carcinoma–bearing mice model and evaluate the safety of EGLP in mice.
Materials and methods
Preparation of GLP and EGLP
The fruiting body of G. lucidum was purchased from Beijing Tong Ren Tang Group Co., Ltd (Beijing, China) and was identified as artificial cultivar. The extraction conditions of Ganoderma lucidum polysaccharide (GLP) are as follows: extraction temperature of 90°C, water/solid ratio of 10:1, and extraction time of 4 h. The extract was centrifuged to obtain the supernatant and then concentrated under vacuum; the condensate was collected and precipitated with 80% ethanol at 2°C for 12 h. After centrifugation at 5000 r/min for 15 min at 4°C, the ethanol precipitate was deproteinated by Sevag method. Then the crude polysaccharide was purified using the dialysis membrane (cutoff = 10 kDa) against pure water for 72 h. Finally, the high–molecular weight crude polysaccharide was lyophilized (GLP). The cellulase (Sigma-Aldrich, St Louis, MO, USA) was selected to catalyze GLP hydrolysis under the condition of 50°C, pH 4.5, and incubation for 2 h, thereafter the enzyme reaction was terminated by heating at 100°C for 5 min, and the precipitate was eliminated by centrifugation. Finally, the supernatant was obtained and purified with the dialysis membrane (cutoff = 10 kDa) against pure water for 72 h and thereafter lyophilized (EGLP).
Cell line and animals
Uterine cervical cancer (U14) cell line was purchased from the Institute of Material Medical (Chinese Academy of Medical Sciences, Beijing, China). Female Kunming mice (6–8 weeks old and 18–22 g weight) were purchased from the Experimental Animal Center of the Chinese Academy of Medical Sciences. The mice were fed with basic diet and water in plastic cages and housed under 50%–60% relative humidity, a laboratory temperature of 22°C–24°C, and illumination between 06:00 am and 06:00 pm. Animal experimentation was performed according to the National Institute of Health Guide for the Use and Care of Laboratory Animals and approved by the local animal ethics committee.
Animal model and treatment
Animals were fasted for 12 h before the establishment of the cervical carcinoma model, and then U14 carcinoma cells at logarithmic phase were propagated in the abdominal cavity of the mice for a week. The ascites were collected from the mice and diluted with sterile normal saline to 2 × 107 cells/mL. Then, 0.3 mL of U14 cell suspension was subcutaneously injected into the right axilla to establish the U14 carcinoma model.7 After 24 h of inoculation, all mice were divided into four groups (eight mice per group) as follows: model control group treated with sterile physiological saline alone (MC); CTX group treated with CTX at a dose of 25 mg/kg b.w. (CTX); and the mice of the GLP and EGLP groups orally administered with GLP and EGLP at a dose of 80 mg/kg b.w., respectively. All animals were administrated one time daily for 14 days. After 24 h of the last administration, all the animals were sacrificed by cervical dislocation. Then, the samples of the thymus, spleen, and tumor tissues were immediately collected and weighed. The tumor inhibition ratio was calculated according to the following formula: ((mean tumor weight of the model control group – the mean tumor weight of the treated group)/the mean tumor weight of the model control group) × 100%.
Measurement of the spleen and thymus indexes
The spleen and thymus of the U14 tumor–bearing mice were weighed to measure the spleen and thymus indexes, which were calculated using the following formula: (weight of the spleen or thymus/body weight) × 100.
Observation of tumor volume
The width and length (mm) of tumor were measured with calipers on the 3rd, 6th, and 12th days, and the tumor volume was calculated by the following formula: tumor volume (mm3) = length × width2 × 0.5, where width is the perpendicular short diameter and length is the longest diameter.
High-performance gel permeation chromatography refractive index measurement
The polysaccharide fractions were measured by high-performance gel permeation chromatography (HPGPC) (Waters 2695; Waters Corporation, Milford Massachusetts, USA) combined with a refractive index detector. High-performance gel permeation chromatography refractive index (HPGPC-RI) measurement was performed with a TSKgel G4000PWxl column (7.8 mm × 300 mm) at 30°C. All samples were dissolved in 0.1 M sodium chloride, which used as the mobile phase. The flow was set at 0.7 mL/min and the injection volume was 100 μL.
Determination of the malondialdehyde contents and enzyme activities in U14 tumor–bearing mice
Serum samples were obtained by retro-orbital venous puncture and immediately measured for superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and malondialdehyde (MDA) contents using a commercial assay kit with a spectrophotometer (Cary 60; Agilent, Santa Clara, CA, USA) according to manufacturer’s instructions (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Analysis of the liver and renal function
Serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), serum creatinine (CRE), and blood urea nitrogen (BUN) were measured by a commercial assay kit according to manufacturer’s instructions (Nanjing Jiancheng Bioengineering Institute).
Western blotting
U14 cells were lysed in lysis buffer (Pierce, Rockford, IL, USA). Total protein of the lysates was measured using the Bicinchoninic Acid Protein Assay Kit (Nanjing Jiancheng Bioengineering Institute). The proteins were separated by 10% sodium dodecyl sulfate polyacrylamide gels and then transferred to a polyvinylidene difluoride (PVDF) membrane (Millipore, Boston, MA, USA). After blocking with 5% nonfat dried milk for 1 h at room temperature, the membranes were incubated with the following primary antibodies overnight at 4°C: anti-Bcl-2 (1:1000; Invitrogen), anti-COX-2 (1:1000; Invitrogen), anti-cleaved caspase-3 (1:1000; Invitrogen), anti-Bax (1:1000; Invitrogen), and GAPDH (1:1000; Invitrogen, Shanghai, China). The membranes were washed with Tris-buffered saline with Tween 20 (TBST) and subsequently incubated with horseradish peroxidase–conjugated secondary antibody for 45 min at room temperature. Visualization was performed with chemiluminescence detection reagent (ECL; Millipore). The relative protein levels were analyzed using Gel-Pro 4.0 software (Media Cybernetics, Inc., Rockville, MD, USA) and normalized to GAPDH.
Analysis of cell apoptosis by flow cytometry
The U14 cells were seeded in six-well palates at 37°C in a humidified 5% CO2 atmosphere and grown for 3 days. U14 cells were cultured in complete culture medium including 10 µM GLP or EGLP for 48 h. Then, the U14 cells were obtained by centrifugation at 2000 r/min and 4°C for 5 min and resuspended in Annexin V-FITC binding buffer. U14 cells were incubated with 5 µL of propidium iodide and 5 µL of Annexin V-FITC in the dark for 15 min according to the manufacturer’s protocol. The apoptosis rates were measured with a FACSCalibur flow cytometer.
Statistical analysis
All experimental results were expressed as means ± standard deviation (SD). Statistical comparison between groups under different conditions was utilized by one-way analysis of variance and Student’s t-test. Data were analyzed by SPSS 13.0 software (SPSS, Inc., Chicago, IL, USA). P-values < 0.05 were considered as statistically significant.
Results
High-performance size exclusion chromatography refractive index chromatograms for GLP and EGLP
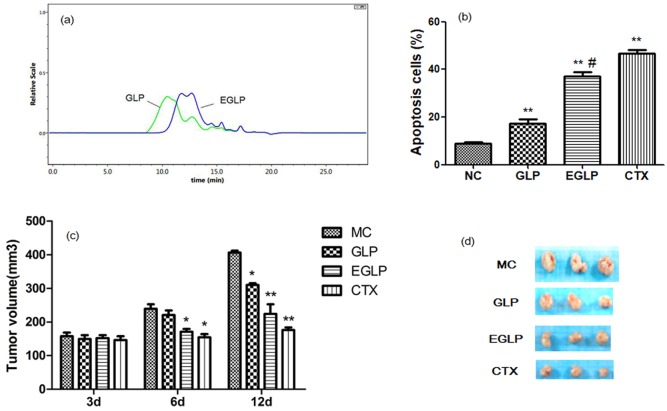
As displayed in Figure 1(a), after enzymatic hydrolysis by cellulase, the peak retention time of GLP was shorter than that of EGLP. These results implied that the GLP was enzymatically degraded into low–molecular weight polysaccharide.
Figure 1.
(a) HPSEC-RI chromatograms for low–molecular weight enzymatically hydrolyzed G. lucidum (EGLP) and G. lucidum polysaccharide (GLP) dissolved in 0.1 M sodium chloride solution. (b) GLP- and EGLP-induced apoptosis in U14 cells. The data are reported as mean ± SD (three independent experiments). **P < 0.01 (vs the control group) and #P < 0.01 (vs the GLP group). (c) Effects of EGLP and GLP on the tumor volume curve in U14-bearing mice. The data are reported as mean ± SD (n = 8/group). **P < 0.01 (vs the MC group) and *P < 0.05 (vs the MC group). (d) Images of the tumor tissue of U14-bearing mice.
Effects of EGLP and GLP on tumor growth
The mice were transplanted with U14 carcinoma cells and the antitumor effects of EGLP and GLP were investigated (Table 1 and Figure 1(d)). GLP treatment inhibited tumor growth (26.34% tumor inhibition ratio). Compared to the GLP group, EGLP treatment suppressed tumor growth (45.31% tumor inhibition ratio). Histogram depicting transplanted tumor growth of different groups is displayed in Figure 1(c). After 12 days of drug administration, the mean tumor volumes showed obvious differences among the treatment groups and the MC group (P < 0.05). These results indicated that the effect of EGLP treatment is preferable to GLP treatment in inhibiting U14 tumor growth, implying that the antitumor effect of EGLP in tumor suppression may be potentially improved by enzymolysis.
Table 1.
Comparison of body weight, spleen indexes, thymus indexes, tumor weights, and tumor inhibition after medication.
| Groups | MC | CTX | GLP | EGLP |
|---|---|---|---|---|
| Body weight (g) | 24.54 ± 0.88 | 14.40 ± 1.72** | 25.72 ± 0.92 | 26.12 ± 1.19 |
| Spleen index (mg/g) | 3.90 ± 0.34 | 2.69 ± 0.36* | 4.49 ± 0.31* | 6.48 ± 0.37** |
| Thymus index (mg/g) | 2.24 ± 0.33 | 1.07 ± 0.28* | 3.34 ± 0.46* | 4.10 ± 0.60** |
| Tumor weight (g) | 3.22 ± 0.26 | 1.29 ± 0.17** | 2.37 ± 0.26* | 1.76 ± 0.19** |
| Inhibition rate (%) | – | 59.98 | 26.34 | 45.31 |
MC: model control group; CTX: cyclophosphamide; GLP: Ganoderma lucidum polysaccharide; EGLP: enzymatically hydrolyzed Ganoderma lucidum polysaccharide; SD: standard deviation.
The data were expressed as the mean ± SD (n = 8/group).
P < 0.05 (vs the model group);**P < 0.01 (vs the model group).
Effects of EGLP and GLP on the activities of antioxidant enzymes
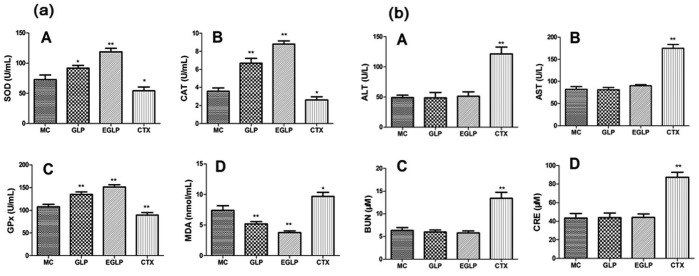
As displayed in Figure 2(a), after being treated with EGLP and GLP, the activities of antioxidant enzymes were significantly increased and the content of MDA was significantly decreased as compared with the MC group (P < 0.05). After the CTX treatment, the activities of antioxidant enzymes were lower than those in the MC group (P < 0.01). These data indicated that the activities of antioxidant enzymes could be reduced by the CTX treatment. However, the EGLP treatment can increase the activities of antioxidant enzymes compared to the CTX treatment in tumor-bearing mice.
Figure 2.
(a) Effects of EGLP and GLP on the serum levels of antioxidant enzymes and lipid peroxide in U14-bearing mice. (b) Effects of EGLP and GLP on the serum levels of (A) ALT, (B) AST, (C) BUN, and (D) CRE in U14-bearing mice. The data are reported as mean ± SD (n = 8/group).
**P < 0.01 (vs the MC group); *P < 0.05 (vs the MC group).
Effects of EGLP and GLP on the thymus and spleen indexes
To evaluate potential immune organ toxicity of EGLP and GLP, the spleen and thymus indexes of treated mice were investigated. As shown in Table 1, compared with the model group, the spleen and thymus indexes were significantly decreased in the CTX group (P < 0.05). However, after the administration of GLP or EGLP, the spleen and thymus indexes were significantly increased as compared with the MC group (P < 0.05 and P < 0.01, respectively). These results indicated that CTX exerted strong killing effects in U14 cervical cells with serious adverse effects, while EGLP showed not only greater antitumor effect than GLP, but also lower immune organ toxicity than CTX.
Toxicological effects of EGLP and GLP in U14-bearing mice
Perfect therapeutics possesses not only the advantage of inhibiting the growth of tumor cells but also the minimum toxicity to their tissue. Hence, the serum levels of hepatic function and renal function indicators, such as ALT, AST, BUN, and CRE, were determined to evaluate the toxicological effects of EGLP or GLP in U14-bearing mice (Figure 2(b)). The CTX treatment increased the serum levels of ALT, AST, BUN, and CRE as compared with the MC group (P < 0.01). More importantly, the EGLP or GLP treatment had no apparent effect on hepatic and renal function indicators as compared with the MC group (P > 0.05). These results also provide scientific evidence for the application of EGLP in cervical carcinoma chemoprevention.
Effects of EGLP and GLP on the protein expression of Bcl-2, COX-2, Bax, and cleaved caspase-3 in U14 cervical carcinoma–bearing mice
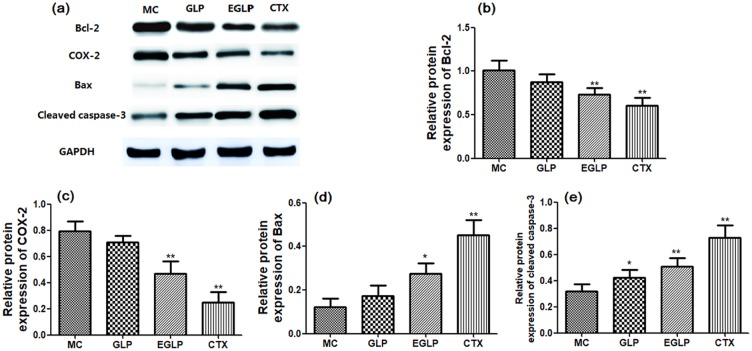
The protein expression of Bcl-2, COX-2, Bax, and cleaved caspase-3 was measured to investigate the regulatory mechanism of EGLP and CTX. After treatment with EGLP or CTX, Bcl-2 and COX-2 expression in cervical carcinoma–bearing mice was significantly lower than that in the MC group, and the expression of Bax and cleaved caspase-3 was significantly increased (Figure 3).
Figure 3.
Effects of EGLP and GLP on the protein expression of Bcl-2 (b), COX-2 (c), Bax (d), and cleaved caspase-3 (e) in U14-bearing mice. The data are reported as mean ± SD (n = 8/group).
**P < 0.01 (vs the MC group); *P < 0.05 (vs the MC group).
EGLP and GLP induce apoptosis in U14 cells
We investigated the anticancer activity of EGLP and GLP at 48 h in U14 cells. As shown in Figure 1(b), flow cytometry analysis indicated that EGLP and GLP both obviously induced U14 apoptosis as compared with the control group (P < 0.01). In addition, compared to the GLP group, EGLP treatment obviously accelerated U14 apoptosis (P < 0.01). Those results indicated that the effect of EGLP is preferable to GLP treatment in accelerating U14 cells’ apoptosis.
Discussion
Cancer is considered as one of the most challenging problematic human diseases. Despite the fact that some chemotherapeutic agents could effectively inhibit the growth of tumor, their clinical application is limited due to the severe adverse effects.2 Therefore, it is very significant to develop high-efficiency anticancer drugs with low toxicity. G. lucidum is a functional food and it has received considerable attention because GLP exhibits antitumor activity.4 However, detailed work on antitumor and organ protection of its enzymatically hydrolyzed polysaccharide has not been fully exploited. In this research, we compared the antitumor effects of GLP and EGLP in tumor-bearing mice. Our results showed that EGLP exhibits better antitumor effect than the original polysaccharide, implying the potential of EGLP in improving quality of life of the organism as well as inhibiting tumor growth, which was in accordance withthe previous research indicating that the enzymatic extraction of crude polysaccharide from Trichosanthes fructus exerts higher antitumor activity than the native polysaccharide.8
The immune system plays a vital role in cancer prevention. As an important immune organ, the thymus is the central hematopoietic site for generating T cells, which are primary participants of the adaptive immune system in hosts. The spleen as the largest secondary immune organ in the organism plays a vital role in keeping immune homeostasis.9 Growing evidence shows that increased oxidative stress has long been acknowledged to play a vital role in cancers and cancer chemotherapy–induced adverse effects.9 In this study, our findings revealed that EGLP could protect the immune organs, improve activities of antioxidant enzyme, and inhibit the growth of tumors, while CTX exhibited serious adverse effects on immune organs, which showed that EGLP exhibited strong antitumor activity in vivo with little toxicity.
Apoptosis is a very widespread phenomenon in cytotoxicity induced by antitumor treatment and it was an important cellular homeostatic process for the host to balance cell death and cell division.10 Bcl-2 families, including Bcl-2 and Bax, are commonly considered as major mediators of cell apoptosis.11 Moreover, cleaved caspase-3 is also regarded as an important mediator of mitochondrial apoptosis and the activation of cleaved caspase-3 can arouse cell apoptosis in cervical carcinoma cells.12 An accumulation of evidence indicates that the overexpression of COX-2 in cervical carcinoma tissues is involved in the pathogenesis of cervical cancer.13 This is consistent with a previous study, in which the polysaccharide obtained from G. lucidum regulated Bcl-2, Bax, and cleaved caspase-3 protein expression and exerted antitumor activity against HL-60 acute leukemia cells.14 Therefore, we speculated that EGLP may inhibit tumor development via an increase in the expression of Bax and cleaved caspase-3, and a decrease in the expression of Bcl-2 and COX-2.
In conclusion, this research indicates that EGLP was capable of suppressing the growth of tumor through the regulation of the apoptotic process. In addition, EGLP showed no toxicity to the liver, the kidney, and body weight simultaneously. This therefore indicates that EGLP can be a potential candidate as an antitumor agent with little side effect.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Hongmei Zhang  https://orcid.org/0000-0002-5378-5011
https://orcid.org/0000-0002-5378-5011
References
- 1. Siegel R, Miller K, Jemal A. (2016) Cancer statistics, 2016. CA: A Cancer Journal for Clinicians 66(1): 7–30. [DOI] [PubMed] [Google Scholar]
- 2. Ehrke MJ. (2003) Immunomodulation in cancer therapeutics. International Immunopharmacology 3(8): 1105–1119. [DOI] [PubMed] [Google Scholar]
- 3. Ouyang MZ, Lin LZ, Lv WJ, et al. (2016) Effects of the polysaccharides extracted from Ganoderma lucidum on chemotherapy-related fatigue in mice. International Journal of Biological Macromolecules 91: 905–910. [DOI] [PubMed] [Google Scholar]
- 4. Cor D, Knez Z, Knez Hrncic M. (2018) Antitumour, antimicrobial, antioxidant and antiacetylcholinesterase effect of Ganoderma lucidum terpenoids and polysaccharides: A review. Molecules 23(3): E649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li Q, Zhou S, Jing J, et al. (2013) Oligosaccharide from apple induces apoptosis and cell cycle arrest in HT29 human colon cancer cells. International Journal of Biological Macromolecules 57: 245–254. [DOI] [PubMed] [Google Scholar]
- 6. McCleary BV. (1986) Enzymatic modification of plant polysaccharides. International Journal of Biological Macromolecules 8: 349–354. [Google Scholar]
- 7. Zhai Q, Li X, Yang Y, et al. (2014) Antitumor activity of a polysaccharide fraction from Laminaria japonica on U14 cervical carcinoma-bearing mice. Tumour Biology 35(1): 117–122. [DOI] [PubMed] [Google Scholar]
- 8. Chen F, Li D, Shen H, et al. (2017) Polysaccharides from Trichosanthes fructus via ultrasound-assisted enzymatic extraction using response surface methodology. BioMed Research International 2017; 2017: 6160785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cui H, Li T, Wang L, et al. (2016) Dioscorea bulbifera polysaccharide and cyclophosphamide combination enhances anti-cervical cancer effect and attenuates immunosuppression and oxidative stress in mice. Scientific Reports 6: 19185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu JR, Dong HW, Chen BQ, et al. (2009) Fresh apples suppress mammary carcinogenesis and proliferative activity and induce apoptosis in mammary tumors of the Sprague−Dawley rat. Journal of Agricultural and Food Chemistry 57(1): 297–304. [DOI] [PubMed] [Google Scholar]
- 11. Risso A, Mercuri F, Quagliaro L, et al. (2001) Intermittent high glucose enhances apoptosis in human umbilical vein endothelial cells in culture. American Journal of Physiology-Endocrinology and Metabolism 281(5): E924–E930. [DOI] [PubMed] [Google Scholar]
- 12. Wang W, Liu X, Guo X, et al. (2018) Mitofusin-2 triggers cervical carcinoma cell HeLa apoptosis via mitochondrial pathway in mouse model. Cellular Physiology and Biochemistry 46(1): 69–81. [DOI] [PubMed] [Google Scholar]
- 13. Jawanjal P, Salhan S, Dhawan I, et al. (2016) Augmented activity of cyclooxygenase-2 in tissue and serum of patients with cervical cancer. Journal of Clinical Laboratory Analysis 30(6): 1198–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yang G, Yang L, Zhuang Y, et al. (2016) Ganoderma lucidum polysaccharide exerts anti-tumor activity via MAPK pathways in HL-60 acute leukemia cells. Journal of Receptor and Signal Transduction Research 36(1): 6–13. [DOI] [PubMed] [Google Scholar]