Abstract
The expression of indoleamine 2,3 dioxygenase (IDO) by tumors can contribute to immunotolerance, and IDO induced by inflammation can also increase risk for the development of behavioral alterations. Thus, this study was initiated to determine whether IDO inhibition, intended to facilitate tumor clearance in response to treatment, attenuates behavioral alterations associated with tumor growth and treatment. We used a murine model of human papilloma virus–related head and neck cancer. We confirmed that tumor cells express IDO and expression was increased by radiotherapy. Interestingly, inhibition of IDO activation by the competitive inhibitor 1-methyl tryptophan mildly exacerbated treatment-associated burrowing deficits (burrowing is a sensitive index of sickness in tumor-bearing mice). Genetic deletion of IDO worsened tumor outcomes and had no effect on the behavioral response as by decreased burrowing or reduced voluntary wheel running. In contrast, oral administration of a specific inhibitor of IDO1 provided no apparent benefit on the tumor response to cancer therapy, yet decreased voluntary wheel-running activity independent of treatment. These results indicate that, independent of its potential effect on tumor clearance, inhibition of IDO does not improve cancer-related symptoms.
Keywords: Cancer; Indoleamine 2,3-dioxygenase; Behavior; Fatigue; Mouse
Introduction
Indoleamine 2,3 dioxygenase (IDO) is an inducible enzyme that metabolizes tryptophan along the kynurenine pathway. This enzyme plays an important role in the development of an immunosuppressive milieu for the tumor.1 Many tumor types constitutively express IDO and this expression is enhanced by local inflammation present in the tumor microenvironment. Interferon gamma (IFN-γ) is the main inducer of IDO activity. In addition to its expression in tumor cells, IDO is expressed by tumor-associated cells including dendritic cells and macrophages. IDO activation has counter-regulatory (controlling inflammation) and tolerogenic (creating acquired antigen-specific tolerance in T cells) properties.1 Because of the potent immunosuppressive effects of IDO and its expression by various types of cancer,2-6 much effort has been invested in the development of IDO inhibitors with the objective of breaking immunotolerance of the tumor and improving the efficacy of cancer therapy.7,8 This task has turned out to be more complicated than initially thought as IDO exists in 2 molecular forms, IDO1 and its paralog IDO2, which are not entirely redundant.9-11 In addition, another enzyme known as tryptophan 2,3 dioxygenase (TDO) has the same activity as IDO1 although it is not inducible by inflammation.12,13 Although initially promising, clinical outcomes obtained with IDO1 inhibitors have been disappointing.14-16
Cancer and its therapy are associated with nonspecific symptoms such as malaise or sickness, pain, fatigue, and cognitive dysfunction. The development of these symptoms and their persistence in cancer survivors after completion of therapy is usually attributed to the propagation of inflammation from the tumor to the brain.17 Inflammatory mediators can act directly on the brain or induce secondary processes including the formation of neurotoxic kynurenine metabolites as a result of activation of the kynurenine pathway.18,19 This last mechanism mediates the switch from sickness to symptoms of depression that can develop in inflamed individuals.
Therefore, we set out to determine whether IDO1 activation contributes to the development of cancer-related symptoms. We selected a validated murine syngeneic model of human papilloma virus (HPV)-induced head and neck cancer. In this model, mice injected with murine oropharyngeal epithelial cells stably expressing E6 and E7 oncogenes of HPV16 and H-ras develop behavioral signs of sickness.20 The HPV-positive tumor cells can be injected orthotopically under the tongue or heterotopically (eg, the hind leg or flank). Faithful to the human disease, these tumors respond to a combined treatment of cisplatin and radiotherapy (chemoradiation). As tumor growth is not associated with cachexia or sarcopenia, this tumor model allows us to study the pathophysiology of cancer-related symptoms and their relation to inflammation.21,22 The inducible expression of IDO1 that develops downstream of IFN-γ has been proposed to mediate at least part of the immunosuppressive properties of HPV.23 Although little is known about its possible role in HPV-related head and neck cancer, IDO1 expression has been identified as a key contributor to the development and progression of HPV-related cervical cancer.24-27 Using biochemical and behavioral approaches, we show that HPV-positive murine epithelial tumor cells express IDO1 and that radiotherapy increased IDO1-expression. While chronic administration of the nonspecific competitive inhibitor of IDO (1-methyl tryptophan) tended to improve the response to chemoradiation, this treatment increased the behavioral alterations induced by chemoradiation. Furthermore, chronic treatment with a selective IDO1 inhibitor, which did not potentiate the tumor response to chemoradiation, also enhanced behavioral side effects. These findings suggest that targeting IDO1 during the treatment of HPV-related cancer is unlikely to alleviate the neurotoxicities associated with cancer therapy.
Materials and Methods
Animals
Experiments were conducted using adult male C57BL/6J mice or Ido1−/− mice originally purchased from Jackson Laboratory. The mice were maintained in temperature and humidity–controlled environments. Food and water was available ad libitum. All procedures were conducted in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals and approved by the Institution Animal Care and Use Committees of The University of Texas MD Anderson Cancer Center and Sanford Research.
Tumor model and chemoradiation treatment
A heterotopic syngeneic murine model of HPV-related head and neck cancer was used within these studies.20,28 The tumor cells were derived from mouse oropharyngeal epithelial cells stably expressing HPV16 E6/E7 and H-ras (mEER). We used either the mEER or the luciferase-tagged mEER (mEERL) cells in the present experiments. As previously described, mice were injected with a 1 × 105 or 1 × 106 tumor cells into the right hind leg or flank.22,29 In some experiments, mice were exposed to a regimen of concurrent cisplatin and radiotherapy (CRT; 3 weekly rounds of 5.28 mg/kg cisplatin + 8 Gy radiation).
Pharmacologic inhibition of IDO
Indoleamine 2,3 dioxygenase inhibition was achieved by chronic administration of either a nonspecific competitive inhibitor of the enzyme, 1-methyl tryptophan (Sigma-Aldrich, Saint-Louis, Missouri, USA; catalog numbers 447439 and 860646), or a selective IDO1 inhibitor30 (hereafter referred to as IDOInh; kindly provided by Boehringer Ingelheim, Ingelheim, Germany). The levogyre and racemic forms of 1-methyl tryptophan (L-1MT and DL-1MT, respectively) were used in 2 different experiments. Both forms inhibit IDO1 but they are also active against IDO231,32 and TDO, although this last effect is exerted in a noncompetitive manner.33 1L-MT and 1-DL-MT were given in the drinking water starting on day 2 and ending on day 30 after implantation of HPV-positive tumor cells. 1L-MT was administered at a dose of 5 mg/mL, whereas 1-DL-MT was administered at a dose of 2 mg/kg. Due to poor solubility, 1-MT was first dissolved in NaOH and then diluted to concentration and titrated to a pH of 10 using HCl; the solution was freshly made every other day. The pharmacokinetic properties of IDOInh and its ability to selectively block IDO1 have been previously described.30,34 IDOInh was administered by oral gavage at the daily dose of 200 mg/kg, starting on day 10 and ending on day 28 after implantation of HPV-positive tumor cells.
Behavioral data collection
During our early investigation, we assessed sickness behavior using the burrowing task as previously described.21 Burrowing is a naturally rewarding behavior that can be assessed by exposing mice to an elevated cylinder containing food pellets placed within the home cage. Independently from their level of appetite, mice spontaneously remove food pellets from the burrow. The efficacy of burrowing was measured by the amount of food pellets removed from the container. After a couple exposure sessions prior to tumor injection, mice reached a stable performance level and were tested weekly for 30 minutes throughout the duration of the experiment.
We shifted from burrowing to voluntary wheel running during the later phase of this investigation as running wheel activity is more sensitive to the sickness and fatigue associated with tumor growth and cancer therapy.29,35 Mice were individually housed with a home cage running wheel. Mice started wheel running 10 days prior to tumor implantation to give them time to stabilize their performance and were maintained with wheels throughout the study. As mice run almost exclusively during the dark phase of the experiment, the data were quantified as the number of wheel rotations per night.
Blood collection and analysis
Metabolites of the kynurenine pathway were evaluated in plasma of mice at baseline (day 0), prior to the start of chemoradiation (day 7), and postchemoradiation (day 21). Blood was collected by submandibular bleed using lithium heparin tubes. It was spun, the plasma was collected, and samples were stored at −80°C. Samples were analyzed by high-performance liquid chromatography and mass spectrometry (HPLC-MS) at Lundbeck Research USA (Paramus, NJ, USA) as previously described.19 Briefly, plasma was diluted 5-fold with 0.2% acetic acid and filtered with a 3 kDa filter. Standard curves for each metabolite were prepared from pure components purchased from Sigma. Samples were analyzed using the Waters Acquity HPLC System along with the Waters Quattro Premier XE triple quadrupole mass spectrometer.
Tissue collection and analyses
Liver and/or tumor tissue was also collected for analysis by reverse transcription-polymerase chain reaction (RT-PCR) or protein expression. Mice were euthanized by CO2 exposure and transcardially perfused with phosphate-buffered saline. Tissue was collected and flash frozen in liquid nitrogen.
Reverse transcription-polymerase chain reaction
RNA was extracted using E.Z.N.A. RNA Isolation kits (Omega Bio-Tek, Norcross, GA, USA). RNA was reverse transcribed into cDNA (complementary DNA) and RT-PCR was performed with TaqMan gene expression assays for Ido1 (Mm.PT.42.8645095), Il6 (Mm.PT.58.13354106), Il1b (Mm.PT.58.41616450), Tnf (Mm.PT.58.12575861), and Gapdh (Mm.PT.39a.1) from Integrated DNA Technologies (Coralville, IA, USA) and Itgam (Mm01271259_g1) from Applied Biosystems (Foster City, CA, USA).
Western blot of IDO1 expression for cell lines
Cells were grown to 90% confluence and harvested in lysis buffer (50 mM Tris HCl pH 7.5, 150 mM NaCl, 5 mM ethylenediaminetetraacetic acid [EDTA], 2 mN Na3VO4, 100 mM NaF, 10 mM NaPPi, 10% glycerol, 1% Triton X-100, 17.4 µg/mL paramethylsulfonylfluoride, 1× HALT with EDTA (Pierce). Cellular lysates were centrifuged at 10 000 r/min for 15 minutes at 4°C. Tx100 soluble cell lysates (40 µg/lane) were boiled, separated by SDS-PAGE (sodium dodecyl sulfate-polyacrylamide gel electrophoresis), transferred to polyvinylidene difluoride membrane and analyzed by Western blot with the following antibodies: IDO, mIDO-048 (Santa Cruz Biotechnology, Dallas, TX, USA; sc-53978) 1:500 and GAPDH (Ambion AM4300) 1:1000. Standard horseradish peroxidase secondary antibodies (1:10 000) and ECL reagent (Thermo Scientific, Waltham, Massachussets, USA) were used for visualization with a CCD camera imaging system (UVP).
Results
IDO1 is expressed by head and neck tumor cell lines and in mEERL tumors
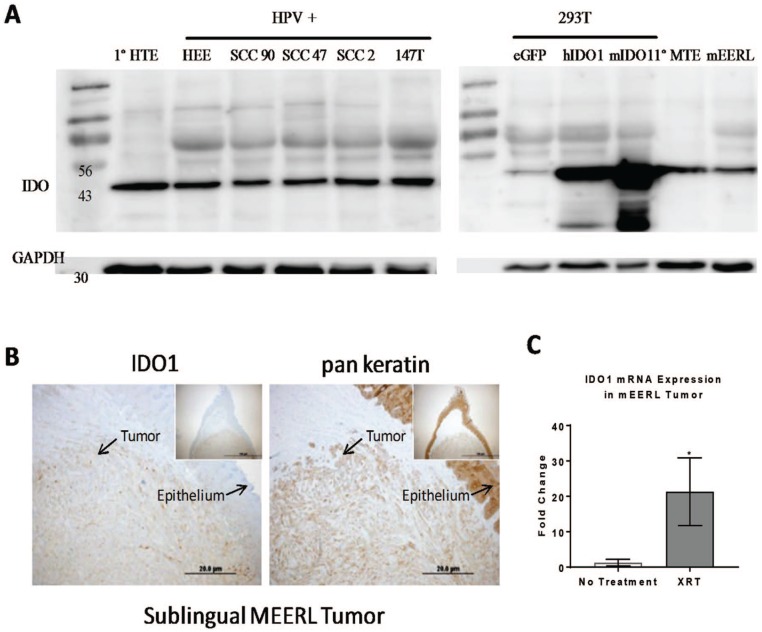
IDO1 protein expression was observed in HPV+ human tumor cell lines (SCC90, SCC47, SCC2, ant 147T) as well as in the mEERL tumor cell line (Figure 1A and B). Human embryonic kidney cells (293T) transfected or not with IDO1 were used as positive and negative controls. IDO1 expression level appears to be intrinsic to epithelial cells, as expression was also noted in primary epithelial cells from both mice and humans (human tonsillar epithelium and murine tonsillar epithelium). Furthermore, the levels of IDO1 appear to be enhanced by cancer therapy, as indicated by an increased expression of IDO1 messenger RNA (mRNA) in response to radiation in tumor tissue collected from mice 24 hours after 1 × 10 Gy leg irradiation, t(11) = 2.27, P < .05 (Figure 1C).
Figure 1.
IDO1 is expressed in head and neck tumor cell lines. (A) Western blot analysis for IDO and GAPDH for human HPV+ cell lines as well as in a mouse HPV+ cell line (MEERL). The 293T cells spiked with human or mouse IDO1 served as a positive control. (B) A representative image of an MEERL tumor showing the co-localization of the IDO1 with the tumor. (C) RT-PCR analysis of IDO1 mRNA expression from hind limb MEERL tumors that were treated or not with a single dose of 10 Gy radiation 24 hours prior to tissue collection, n = 6 to 7 mice/group. Mean ± SEM, *P < .05. HEE indicates human tonsil epithelial cells transfected with E6 E7; HPV, human papilloma virus; HTE, human tonsil epithelial cells; IDO, indoleamine 2,3-dioxygenase; MEERL, mouse tonsil epithelial cells with E6, E7, h-RAS, and luciferase; mRNA, messenger RNA; MTE, mouse tonsil epithelial cells; RT-PCR, reverse transcription-polymerase chain reaction; SCC, squamous cell carcinoma.
Administration of 1-MT tended to enhance the tumor response to chemoradiation but did not protect from tumor CRT-induced burrowing deficits
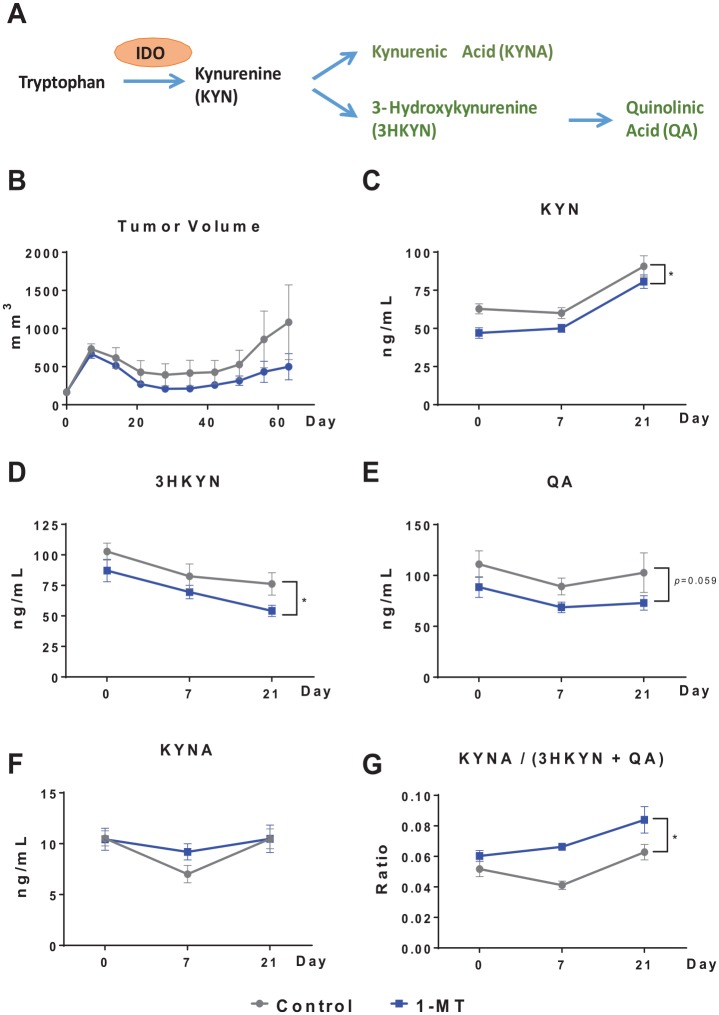
We wanted to first verify that chronic 1-MT treatment would produce the anticipated effect on metabolites of the kynurenine pathway (Figure 2A) in plasma. Tumor-bearing mice were treated with a regimen of CRT with or without the 1-MT in the drinking water. There was a trend toward reduced tumor burden with the addition of 1L-MT to the CRT regimen (Figure 2B) and a nonsignificant increase in survival (data not shown, which we did not pursue as this was not the main objective of this study). As expected, 1L-MT reduced the levels of kynurenine (F1,18 = 7.21, P < .05; Figure 2C) and 3-hydroxykynurenine (F1,18 = 6.81, P < .05; Figure 2D) with a trend for quinolinic acid (F1,18 = 4.05, P < .10; Figure 2E). Treatment with 1-MT did not significantly affect the levels of kynurenic acid (Figure 2F), but increased the ratio of neuroprotective (kynurenic acid) to neurotoxic (3-hydroxykynurenine + quinolinic acid) kynurenine metabolites, F1,18 = 23.13, P < .001 (Figure 2G). The CRT by itself significantly increased plasma kynurenine levels following CRT (day 21) and this increase was independent of 1-MT treatment, F2,36 = 49.71, P < .001; Figure 2C.
Figure 2.
Administration of 1-MT tends to improve response to chemoradiation and significantly affects tryptophan metabolism. (A) The kynurenine pathway metabolites assessed in this experiment. (B) Treatment with 1L-MT starting 2 days prior to tumor cell injection tended to improve response to CRT administered weekly starting on day 7 (n = 10 mice/group). (C-G) HPLC-MS of kynurenine pathway metabolites in control and 1-MT–treated mice. Inhibition of IDO by 1-MT produced the expected decrease in kynurenine and its downstream metabolites. *P < .05. CRT indicates concurrent cisplatin and radiotherapy; HPLC-MS, high-performance liquid chromatography and mass spectrometry; IDO, indoleamine 2,3-dioxygenase.
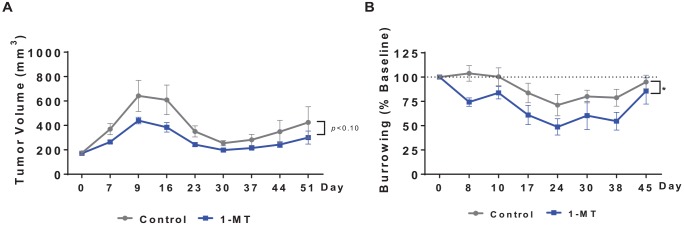
Next, we explored the impact of 1-DL-MT on tumor growth and burrowing behavior in tumor-bearing mice treated concurrently with CRT. As in the previous study, there was a trend toward a reduced tumor burden (P < .10; Figure 3A) and a nonstatistically significant increase in survival rates (data not shown). Tumor-bearing mice subjected to CRT showed a reduction in burrowing behavior (significant effect of time, F7,119 = 8.57, P < .001; Figure 3B). This effect was not improved by 1-MT, rather 1-MT–treated mice performed more poorly (significant main effect of 1-MT treatment, F1,17 = 4.87, P < .05.
Figure 3.
Administration of 1-MT tends to improve response to chemoradiation but does not improve behavioral deficits. (A) A treatment regimen of CRT plus 1-DL-MT showed a trend toward reduced tumor burden and improved survival compared with CRT alone (n = 9-10 mice/group). (B) However, 1-MT treatment exacerbated CRT-induced burrowing deficits. *P < .05. CRT indicates concurrent cisplatin and radiotherapy.
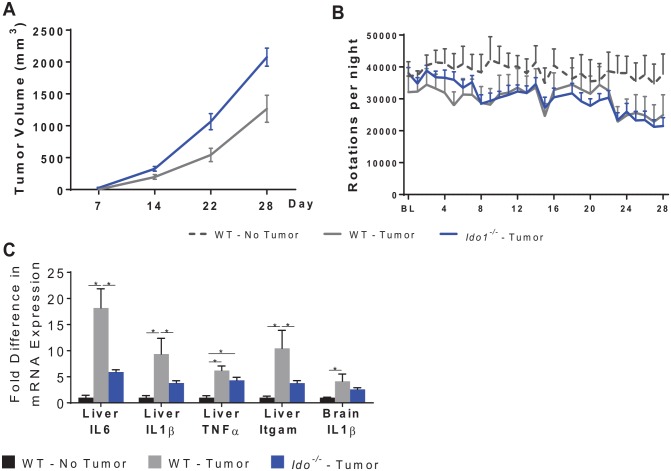
Genetic deletion of IDO1 increased tumor growth and did not improve neurotoxicities associated with chemoradiation
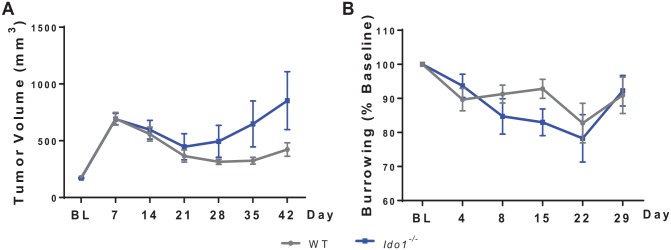
Ido1−/− mice implanted with mEERL tumors displayed poorer response to CRT compared with wild-type (WT) mice as indicated by increased tumor volume (significant time by group interaction, F6,96 = 2.58, P < .05, Figure 4A). Burrowing deficits in response to CRT did not differ significantly according to genotype (Figure 4B).
Figure 4.
Genetic deletion of host IDO1 increases tumor growth without a significant impact on behavior. (A and B) WT and ido1−/− mice were injected with MEERL tumor cells and were treated with CRT. Ido1−/− mice displayed poorer tumor control as indicated by a trend toward larger tumors and no significant change in burrowing behavior. CRT indicates concurrent cisplatin and radiotherapy; IDO, indoleamine 2,3-dioxygenase; MEERL, mouse tonsil epithelial cells with E6, E7, h-RAS, and luciferase; WT, wild type.
We also implanted mEER tumors into the flank of WT and Ido1−/− mice to monitor tumor growth and behavior in the absence of CRT. Consistent with the previous experiment, Ido1−/− mice displayed increased tumor growth (significant group by time interaction, F3,36 = 6.51, P < .01, Figure 5A). Tumor growth was associated with reduced wheel running (group by time interaction, F54,432 = 1.40, P < .05) the extent of which was identical in WT and Ido1−/− mice (Figure 5B). Despite the more rapid tumor growth, Ido1−/− mice displayed an attenuation of tumor-associated liver IL-6 and IL-1β mRNA expression (Figure 6C). As this study was run concurrently with another study, the data from the control group, but not the data in Ido1−/−mice, were previously reported.29
Figure 5.
Genetic deletion of host IDO1 increases tumor growth without a significant impact on behavior. (A and B) WT and ido1−/− mice were injected with MEERL tumor cells and the tumor was allowed to grow, untreated. Ido1−/− mice displayed more rapid tumor growth. While both WT and ido1−/− mice displayed deficits in voluntary home cage wheel running, there were no differences in tumor-induced deficits between the genotypes. (C) Analyses of mRNA expression of inflammatory markers in the liver and brain show an attenuation of tumor-induced liver IL-6, IL-1β, and Itgam/CD11b in ido1−/− mice. n = 5-10 mice/group. *P < .05. IDO indicates indoleamine 2,3-dioxygenase; MEERL, mouse tonsil epithelial cells with E6, E7, h-RAS, and luciferase; WT, wild type.
Figure 6.
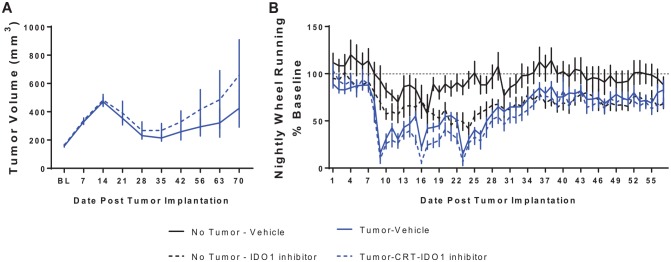
Administration of IDOInh tends to increase tumor recurrence and exacerbates wheel running deficits. (A) In this experiment, control mice and MEERL tumor + CRT mice were treated with IDOInh. Tumor-bearing mice treated with the IDOInh showed a tendency for poorer tumor control. (B) While tumor-bearing mice treated with CRT showed a dramatic reduction in wheel running, a main effect of IDOInh was also present such that it reduced wheel running in tumor-bearing and control mice. N = 6 to 11 mice/group. CRT indicates concurrent cisplatin and radiotherapy; IDO, indoleamine 2,3-dioxygenase; MEERL, mouse tonsil epithelial cells with E6, E7, h-RAS, and luciferase.
Administration of a specific IDO1 inhibitor (IDOInh) did not improve tumor response to chemoradiation and decreased voluntary wheel running
Administration of a specific IDO1 inhibitor, IDOInh, showed no tumor growth protection (Figure 6A). Furthermore, mice treated with the compound showed impaired wheel running independent of the presence of the tumor (main effect of IDOInh, F1,29 = 6.6, P < .05, Figure 6B). This effect developed more rapidly in the tumor-bearing mice than in the control mice, as a significant time by tumor by IDOInh interaction was observed, F42,1218 = 1.6, P < .001.
Discussion
The primary objective of this study was to determine whether inhibition of IDO1 could prevent the severe behavioral toxicities that develop in response to chemoradiation. We confirmed that our HPV-positive model of head and neck cancer expresses IDO1. In addition, we confirmed that inhibition of IDO by 1-MT attenuated activation of the kynurenine pathway and that showed that it tended to enhance the efficacy of chemoradiation to treat the tumor. However, in contrast to our expectation, IDO inhibition did not alleviate the behavioral toxicities of chemoradiation.
There are several possible explanations for this apparently paradoxical result. The most obvious one is based on the counter-regulatory role of IDO1 in inflammation.1 IDO1 is activated by inflammation and its expression helps to contain inflammation. This counter-regulatory activity is beneficial when IDO1 helps to control harmful inflammation, for instance, in the case of autoimmune processes.36 Our data suggest that in the context of cancer, inhibition of IDO1 potentiates the inflammation associated with the tumor or that induced by chemoradiation. This explanation, however, is unlikely to account for our results as in the mEERL tumor model inflammation does not play a significant role in the behavioral alterations associated with tumor growth and behavioral toxicity induced by cancer therapy.21,29 In addition, data collected in Ido1−/− mice implanted with HPV-positive tumor cells show that despite accelerated tumor growth, deletion of IDO1 actually attenuated the late expression of inflammatory cytokines in the liver and brain. Because there was no difference in behavior between Ido1−/− and WT mice, it could be speculated that IDO inhibitors have an intrinsic toxicity independent of their ability to block IDO activation. However, this should result in general signs of toxicity (eg, decreased body weight and reduced food consumption), which were not observed in our experiments.
In the search for an alternative explanation, it is important to note that IDO blockade using orally administered IDO inhibitors has neglected possible adverse effects due to noncompetitive inhibition of constitutive IDO in the gastrointestinal tract. The most abundant expression of IDO at the mRNA and protein levels is observed in the mouse epididymis, jejunum, and ileum, followed by the colon, prostate, and spleen.37 In the gut, most of the IDO-positive cells are observed in the interstitial space of the mucosa and they resemble macrophages and dendritic cells. There is also abundant IDO-positive staining in Peyer’s patches. These findings indicate that IDO-expressing immune cells probably play an important role in gut physiology and in maintenance of the integrity of the intestinal barrier. While these functions are relatively new areas of investigation, some studies suggest that intestinal IDO may alter the metabolites available to the gut microbiota38-41 and, in this way, affect behavior.41 Although IDO inhibitors are claimed to have an excellent safety profile, a closer examination of adverse effects associated with their administration reveals that patients with cancer treated with the selective IDO1 inhibitor INCB024360 (epacadostat; Incyte) complain of fatigue, nausea, and abdominal pain.42 The still undocumented occurrence of similar toxicities in IDOInh-treated mice would be sufficient to account for the impaired running wheel performance observed with prolonged administration of this compound.
It has been reported that tumoral IDO expression predicts poor outcomes in head and neck squamous cell carcinoma, as well as in other tumors, likely due to the association of IDO with regulatory T cells.2,43-46 In this study, we observed IDO1 expression in human and murine tumor cells. Furthermore, IDO inhibition by 1-MT may improve the tumor response to chemoradiation and decrease the probability of tumor reoccurrence. However, such a favorable effect of IDO inhibition was not observed in IDO1 knockout mice and in mice treated with a selective inhibitor of IDO1. In both cases, there was a tendency for IDO1 inhibition to decrease response of the tumor to chemoradiation. The differences observed between 1-MT treatment and ido1−/− mice could certainly be attributable to the differences in timing of the inhibition. While the knockout mice lacked IDO expression at the time of tumor engraftment, pharmacologic inhibition of IDO with 1-MT did not begin until 10 days after tumor cell injection. However, this does not explain the differences observed between 1-MT and IDOInh-treated mice. The contrasting effects of pharmacologic IDO1 inhibition were apparent despite the fact that our experiments were underpowered to test the efficacy of IDO1 inhibition on the tumor response to chemoradiation (which was not the focus of our study). One tentative explanation for the possible difference between 1-MT and more selective modes of blockade of IDO1 is the possibility that the tumor expresses IDO2 and TDO in addition to IDO1; these 2 enzymes take over when IDO1 is blocked thereby circumventing IDO1 blockade. The ability of HPV16 E7 to induce IDO1 has already been described23 but there has been no systematic study of the possible concomitant expression of IDO2 and TDO in HPV-positive tumors. Another explanation for the adverse effects of selective IDO1 blockade on tumor response to chemoradiation is that IDO1 blockade also inhibits the cytotoxic activity of natural killer cells. This effect has been proposed to be responsible for accelerated tumor growth in another murine model of cancer.47
In conclusion, the present results point to the inability of IDO1 inhibitors to prevent behavioral toxicities induced by chemoradiation even when IDO inhibition has the potential to favorably enhance cancer outcomes. The observation of behavioral impairment associated with administration of a specific IDO1 inhibitor points to the necessity of investing in further studies to clarify the source of this potential adverse effect and minimize it.
Acknowledgments
The authors thank Boehringer Ingelheim (Ingelheim, Germany) for providing the IDOInh.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Cancer Institute of the National Institutes of Health (R01 CA193522, R.D.). Additional support came from the University of Texas MD Anderson Cancer Center and the National Institutes of Health MD Anderson Cancer Center Support Grant (P30 CA016672). The content is solely the responsibility of the authors and does not necessarily represent the official view of the funding sources.
Declaration of Conflicting Interests:The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: R.D. is a consultant/advisory board member for Danone Nutricia Research. No potential conflicts of interest were disclosed by the other authors.
Author Contributions: EGV study conception and design, acquisition and interpretation of data, analysis and interpretation of data, drafting of manuscript, critical revision; DWV acquisition and interpretation of data; DB Acquisition of data; AL Acquisition of data, critical revision; AG acquisition of data, critical revision; PDV acquisition and interpretation of data, critical revision; JHL Critical revision; RD Study conception and design, interpretation of data, drafting of manuscript, critical revision.
ORCID iD: Elisabeth G Vichaya  https://orcid.org/0000-0001-9421-7419
https://orcid.org/0000-0001-9421-7419
References
- 1. Munn DH, Mellor AL. IDO in the tumor microenvironment: inflammation, counter-regulation, and tolerance. Trends Immunol. 2016;37:193-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brandacher G, Perathoner A, Ladurner R, et al. Prognostic value of indoleamine 2,3-dioxygenase expression in colorectal cancer: effect on tumor-infiltrating T cells. Clin Cancer Res. 2006;12:1144-1151. [DOI] [PubMed] [Google Scholar]
- 3. Jia Y, Wang H, Wang Y, et al. Low expression of Bin1, along with high expression of IDO in tumor tissue and draining lymph nodes, are predictors of poor prognosis for esophageal squamous cell cancer patients. Int J Cancer. 2015;137:1095-1106. [DOI] [PubMed] [Google Scholar]
- 4. Karanikas V, Zamanakou M, Kerenidi T, et al. Indoleamine 2,3-dioxygenase (IDO) expression in lung cancer. Cancer Biol Ther. 2007;6:1258-1262. [DOI] [PubMed] [Google Scholar]
- 5. Wainwright DA, Balyasnikova IV, Chang AL, et al. IDO expression in brain tumors increases the recruitment of regulatory T cells and negatively impacts survival. Clin Cancer Res. 2012;18:6110-6121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Witkiewicz A, Williams TK, Cozzitorto J, et al. Expression of indoleamine 2,3-dioxygenase in metastatic pancreatic ductal adenocarcinoma recruits regulatory T cells to avoid immune detection. J Am Coll Surg. 2008;206:849-854; discussion 854-856. [DOI] [PubMed] [Google Scholar]
- 7. Prendergast GC, Malachowski WJ, Mondal A, Scherle P, Muller AJ. Indoleamine 2,3-dioxygenase and its therapeutic inhibition in cancer. Int Rev Cell Mol Biol. 2018;336:175-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yentz S, Smith D. Indoleamine 2,3-dioxygenase (IDO) inhibition as a strategy to augment cancer immunotherapy. BioDrugs. 2018;32:311-317. [DOI] [PubMed] [Google Scholar]
- 9. Lob S, Konigsrainer A, Zieker D, et al. IDO1 and IDO2 are expressed in human tumors: levo- but not dextro-1-methyl tryptophan inhibits tryptophan catabolism. Cancer Immunol Immunother. 2009;58:153-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Meininger D, Zalameda L, Liu Y, et al. Purification and kinetic characterization of human indoleamine 2,3-dioxygenases 1 and 2 (IDO1 and IDO2) and discovery of selective IDO1 inhibitors. Biochim Biophys Acta. 2011;1814:1947-1954. [DOI] [PubMed] [Google Scholar]
- 11. Metz R, Smith C, DuHadaway JB, et al. IDO2 is critical for IDO1-mediated T-cell regulation and exerts a non-redundant function in inflammation. Int Immuno. 2014;26:357-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhai L, Spranger S, Binder DC, et al. Molecular pathways: targeting IDO1 and other tryptophan dioxygenases for cancer immunotherapy. Clin Cancer Res. 2015;21:5427-5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zunszain PA, Anacker C, Cattaneo A, et al. Interleukin-1beta: a new regulator of the kynurenine pathway affecting human hippocampal neurogenesis. Neuropsychopharmacology. 2012;37:939-949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Companies scaling back IDO1 inhibitor trials. Cancer Discov. 2018;8:OF5. [DOI] [PubMed] [Google Scholar]
- 15. Muller AJ, Manfredi MG, Zakharia Y, Prendergast GC. Inhibiting IDO pathways to treat cancer: lessons from the ECHO-301 trial and beyond. Semin Immunopathol. 2019;41:41-48. [DOI] [PubMed] [Google Scholar]
- 16. Long GV, Dummer R, Hamid O, et al. Epacadostat (E) plus pembrolizumab (P) versus pembrolizumab alone in patients (pts) with unresectable or metastatic melanoma: results of the phase 3 ECHO-301/KEYNOTE-252 study. J Clin Oncol. 2018;36:108-108. [Google Scholar]
- 17. Dantzer R, Meagher MW, Cleeland CS. Translational approaches to treatment-induced symptoms in cancer patients. Nat Rev Clin Oncol. 2012;9:414-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Walker AK, Budac DP, Bisulco S, et al. NMDA receptor blockade by ketamine abrogates lipopolysaccharide-induced depressive-like behavior in C57BL/6J mice. Neuropsychopharmacology. 2013;38:1609-1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Spanos WC, Nowicki P, Lee DW, et al. Immune response during therapy with cisplatin or radiation for human papillomavirus-related head and neck cancer. Arch Otolaryngol Head Neck Surg. 2009;135:1137-1146. [DOI] [PubMed] [Google Scholar]
- 21. Vichaya EG, Molkentine JM, Vermeer DW, et al. Sickness behavior induced by cisplatin chemotherapy and radiotherapy in a murine head and neck cancer model is associated with altered mitochondrial gene expression. Behav Brain Res. 2016;297:241-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vichaya EG, Vermeer DW, Christian DL, et al. Neuroimmune mechanisms of behavioral alterations in a syngeneic murine model of human papilloma virus-related head and neck cancer. Psychoneuroendocrinology. 2017;79:59-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mittal D, Kassianos AJ, Tran LS, et al. Indoleamine 2,3-dioxygenase activity contributes to local immune suppression in the skin expressing human papillomavirus oncoprotein e7. J Invest Dermatol. 2013;133:2686-2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ferns DM, Kema IP, Buist MR, Nijman HW, Kenter GG, Jordanova ES. Indoleamine-2,3-dioxygenase (IDO) metabolic activity is detrimental for cervical cancer patient survival. Oncoimmunology. 2015;4:e981457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hascitha J, Priya R, Jayavelu S, et al. Analysis of kynurenine/tryptophan ratio and expression of IDO1 and 2 mRNA in tumour tissue of cervical cancer patients. Clin Biochem. 2016;49:919-924. [DOI] [PubMed] [Google Scholar]
- 26. Nakamura T, Shima T, Saeki A, et al. Expression of indoleamine 2, 3-dioxygenase and the recruitment of Foxp3-expressing regulatory T cells in the development and progression of uterine cervical cancer. Cancer Sci. 2007;98:874-881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Inaba T, Ino K, Kajiyama H, et al. Indoleamine 2,3-dioxygenase expression predicts impaired survival of invasive cervical cancer patients treated with radical hysterectomy. Gynecol Oncol. 2010;117:423-428. [DOI] [PubMed] [Google Scholar]
- 28. Spanos WC, Hoover A, Harris GF, et al. The PDZ binding motif of human papillomavirus type 16 E6 induces PTPN13 loss, which allows anchorage-independent growth and synergizes with ras for invasive growth. J Virol. 2008;82:2493-2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Grossberg AJ, Vichaya EG, Christian DL, et al. Tumor-associated fatigue in cancer patients develops independently of IL1 signaling. Cancer Res. 2018;78:695-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cathomas F, Fuertig R, Sigrist H, et al. CD40-TNF activation in mice induces extended sickness behavior syndrome co-incident with but not dependent on activation of the kynurenine pathway. Brain Behav Immun. 2015; 50:125-140. [DOI] [PubMed] [Google Scholar]
- 31. Yuasa HJ, Ball HJ, Austin CJ, Hunt NH. 1-L-methyltryptophan is a more effective inhibitor of vertebrate IDO2 enzymes than 1-D-methyltryptophan. Comp Biochem Physiol B Biochem Mol Biol. 2010;157:10-15. [DOI] [PubMed] [Google Scholar]
- 32. Austin CJ, Mailu BM, Maghzal GJ, et al. Biochemical characteristics and inhibitor selectivity of mouse indoleamine 2,3-dioxygenase-2. Amino Acids. 2010;39:565-578. [DOI] [PubMed] [Google Scholar]
- 33. Uchida K, Usami M, Bandow H, Harada I. Characteristics of substrates and inhibitors in binding to rat liver L-tryptophan 2,3-dioxygenase: a Fourier transform infrared and kinetic study. Biochim Biophys Acta. 1992;1121:153-159. [DOI] [PubMed] [Google Scholar]
- 34. Fuertig R, Azzinnari D, Bergamini G, et al. Mouse chronic social stress increases blood and brain kynurenine pathway activity and fear behaviour: both effects are reversed by inhibition of indoleamine 2,3-dioxygenase. Brain Behav Immun. 2016;54:59-72. [DOI] [PubMed] [Google Scholar]
- 35. Renner M, Feng R, Springer D, et al. A murine model of peripheral irradiation-induced fatigue. Behav Brain Res. 2016;307:218-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kwidzinski E, Bunse J, Kovac AD, et al. IDO (indolamine 2,3-dioxygenase) expression and function in the CNS. Adv Exp Med Biol. 2003;527:113-118. [DOI] [PubMed] [Google Scholar]
- 37. Dai X, Zhu BT. Indoleamine 2,3-dioxygenase tissue distribution and cellular localization in mice: implications for its biological functions. J Histochem Cytochem. 2010;58:17-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Laurans L, Venteclef N, Haddad Y, et al. Genetic deficiency of indoleamine 2,3-dioxygenase promotes gut microbiota-mediated metabolic health. Nat Med. 2018;24:1113-1120. [DOI] [PubMed] [Google Scholar]
- 39. Van der Leek AP, Yanishevsky Y, Kozyrskyj AL. The kynurenine pathway as a novel link between allergy and the gut microbiome. Front Immunol. 2017;8:1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vujkovic-Cvijin I, Swainson LA, Chu SN, et al. Gut-resident lactobacillus abundance associates with IDO1 inhibition and Th17 dynamics in SIV-infected macaques. Cell Rep. 2015;13:1589-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Marin IA, Goertz JE, Ren T, et al. Microbiota alteration is associated with the development of stress-induced despair behavior. Sci Rep. 2017;7:43859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Beatty L, O’Dwyer P, Clark J, Shi J, Newton R, Schaub R. Phase I study of the safety, pharmacokinetics (PK), and pharmacodynamics (PD) of the oral inhibitor of indoleamine 2,3-dioxygenase (IDO1) INCB024360 in patients (pts) with advanced malignancies. J Clin Oncol. 2013;31:3025. [Google Scholar]
- 43. Ye J, Liu H, Hu Y, Li P, Zhang G, Li Y. Tumoral indoleamine 2,3-dioxygenase expression predicts poor outcome in laryngeal squamous cell carcinoma. Virchows Arch. 2013;462:73-81. [DOI] [PubMed] [Google Scholar]
- 44. Riesenberg R, Weiler C, Spring O, et al. Expression of indoleamine 2,3-dioxygenase in tumor endothelial cells correlates with long-term survival of patients with renal cell carcinoma. Clin Cancer Res. 2007;13:6993-7002. [DOI] [PubMed] [Google Scholar]
- 45. Pan K, Wang H, Chen MS, et al. Expression and prognosis role of indoleamine 2,3-dioxygenase in hepatocellular carcinoma. J Cancer Res Clin Oncol. 2008;134:1247-1253. [DOI] [PubMed] [Google Scholar]
- 46. Okamoto A, Nikaido T, Ochiai K, et al. Indoleamine 2,3-dioxygenase serves as a marker of poor prognosis in gene expression profiles of serous ovarian cancer cells. Clin Cancer Res. 2005;11:6030-6039. [DOI] [PubMed] [Google Scholar]
- 47. Kai S, Goto S, Tahara K, Sasaki A, Kawano K, Kitano S. Inhibition of indoleamine 2,3-dioxygenase suppresses NK cell activity and accelerates tumor growth. J Exp Ther Oncol. 2003;3:336-345. [DOI] [PubMed] [Google Scholar]