Abstract
Process conditions that are applied to make structured soy-protein-based food commonly include high temperatures. Those conditions can induce protein oxidation, leading to a decrease in their susceptibility to proteolysis by digestive enzymes. We aimed to investigate the effects of thermomechanical processing on oxidation and in vitro gastric digestion of commercial soy protein ingredients. Samples were sheared at 100 to 140 °C and characterized for acid uptake, carbonyl content, electrophoresis, and surface hydrophobicity. The enzymatic hydrolysis was determined in simulated gastric conditions. Protein ingredients were already oxidized and showed higher surface hydrophobicity and hydrolysis rate compared with those of the processed matrices. However, no clear correlation between the level of carbonyls and the hydrolysis rate was found. Therefore, we conclude that gastric digestion is mostly driven by the matrix structure and composition and the available contact area between the substrate and proteolytic enzymes.
Keywords: protein oxidation, gastric digestion, soy proteins, meat analogues, processing
Introduction
The interest in using plant proteins as an alternative to animal proteins in foods has raised over the past years for environmental, health, and animal welfare reasons.1 Currently, many plant-protein-based products have been developed to substitute meat and are available on the market. Those products are known as meat analogues. The most common ingredient used in that respect is soy proteins, which are often combined with polysaccharides.2,3 Some techniques, such as extrusion and shear cell technology, are based on applying deformation at high temperatures, allowing for making fibrous plant-based products that aim at mimicking whole muscle meat products. However, temperature affects the physiochemical status of the proteins, which can result in protein denaturation, protein oxidation, loss of essential amino acids, change in surface-exposed hydrophobicity, and aggregation. Protein oxidation in foods can be induced directly by free radicals and indirectly by lipid and Maillard reaction products, such as α-dicarbonyls.4 It can notably lead to amino acid side chain or protein backbone modifications, such as the formation of carbonyls, loss of thiol groups and tryptophan, and cross-links between amino acids residues.5,6 These modifications can result in fragmentation of the protein backbone, protein aggregation, and polymerization. Consistently, protein carbonyls are commonly analyzed as markers of the oxidative damage to food proteins.6
Protein oxidation is temperature- and pro-oxidant concentration-dependent and can alter protein digestion in different ways. For instance, at low pro-oxidant concentration or temperatures below 100 °C, minor modifications and partial unfolding of proteins can enhance digestion by exposure of susceptible sites of the proteins to digestive enzymes. These modifications have been described to increase the protein digestibility value of extrudates compared to nonextruded products.7 However, high pro-oxidant concentrations or heating proteins above 100 °C can induce extensive protein oxidation. This, in turn, decreases protein susceptibility to digestive enzymes, because of amino acid side-chain modifications or protein aggregation.8,9 Though it is known that in vivo oxidation is related to aging and diseases,10 exposure to dietary oxidized proteins may also have adverse impacts on human health. Recent studies have shown that that dietary oxidized proteins may promote some organ dysfunctions using in vitro and animal models.11 However, oxidized protein levels used in those studies have not been reported in food so far.
Besides the chemical status of the proteins, gastric protein digestion can be affected by food structure, matrix composition (e.g., other ingredients), and pH.12 Protein digestion is promoted by pepsin activity (optimal activity between pH 1.5 and 2.5) and mechanical forces, which help to grind and disintegrate the food into smaller particles.13,14 The rate of disintegration indicates how fast food is broken down into small particles. The breakdown is a result of surface erosion and texture softening.15 The effect of food structure on pepsin hydrolysis has been shown for protein gels already, in which hydrolysis was limited to a thin layer at the surface of the gel. Therefore, the main constraints for pepsin hydrolysis were proven to be the surface area and surface erosion rate of the gels.16,17
In this study, we investigate the effect of thermomechanical processing on the oxidation of commercial soy proteins ingredients, which may contain preformed protein-bound carbonyls.18 The subsequent impact of processing on the in vitro gastric digestion of soy-protein-based matrices was also assessed.
Materials and Methods
Materials
Soy protein isolate (SPI, 83.4% protein, SUPRO 500E IP) and soy protein concentrate (SPC, 59.4% protein, ALPHA 8 ZP) (N x 5.7) were obtained from Solae (St Louis, MO, U.S.A.). Pectin from citrus peel (P9135), pepsin from porcine gastric mucosa (400–800 units/mg, P1725), NaCl (ReagentPlus, ≥ 99%), sodium tetraborate decahydrate (Borax, ≥ 99.5%), DL-dithiothreitol (DTT) (≥98%), o-phtaldialdehyde (OPA) (≥97%), trifluoracetic acid (TFA), acetonitrile (ACN), 8-anilino-1-napthalenesulfonic acid ammonium salt (ANSA, ≥ 97%), β-mercaptoethanol, sodium phosphate monobasic dihydrate (≥99%), sodium phosphate dibasic (≥99%), diaminoethane tetraacetic acid (EDTA), tris(hydroxymethyl) aminomethane (Tris), KCl, 2,4-dinitrophenylhydrazine (DNPH), trichloroacetic acid (TCA), sodium dodecyl sulfate (SDS), and guanidine hydrochloride were obtained from Sigma-Aldrich (Darmstadt, Germany). HCl 37% was purchased from VWR Chemicals (Fontenay-sous-Bois, France), and solvents such as ethanol (ACS 99%) and ethyl acetate (ACS 99%) were purchased from Emsure (Merck Millipore, Darmstadt, Germany). A bicinchoninic acid (BCA) protein assay kit was obtained from Thermo Scientific (Pierce, Rockford, U.S.A.). Mini-Protean TGX gels, Biosafe Coomassie G-250 stain, 2× Laemmli sample native buffer: 10× Tris/glycine/SDS buffer (25 mM Tris, 192 mM glycine and 0.1 w/v% SDS, 1× solution, pH 8.3), and Precision plus protein dual color standard were purchase from Bio-Rad Laboratories (Munchen, Germany). l-Serine was purchase from Alfa Aesar (99%, Thermo Fisher Scientific, Kandel, Germany). Ultrapure water obtained from Millipore Milli-Q system was used for all experiments, unless otherwise mentioned.
Preparation of Unheated Protein Suspensions and Processed Samples
Soy ingredients (SPC and SPI) were used to prepare the unheated 6 wt % protein suspensions (based on protein content in dry basis) in 100 mM Tris/5 mM EDTA buffer pH 7.5. Four independent suspensions were prepared and allowed to rotate at 40 rpm, at 4 °C overnight. The suspensions were analyzed on the following day.
Processed protein-based matrices were prepared using a high-temperature shear cell (HTSC). Samples based on SPC were made with 45 wt % SPC, 1 wt % NaCl, and 54 wt % demineralized water as described by Grabowska et al.2 Samples with SPI were prepared with 44 wt % SPI or 41.8 wt % SPI with 2.2 wt % pectin, 1 wt % NaCl, and 55 wt % demineralized water.3 Samples were sheared in the HTSC using different temperatures (100, 120, and 140 °C) at 30 rpm for 15 min and cooled down to 25 °C in 5 min. After preparation, samples were stored at −18 °C prior to further analysis. Processed samples were prepared in duplicate per condition.
Determination of Protein-Bound Carbonyl Content
The separation of protein fractions from processed protein-based matrices to measure the protein-bound carbonyl content and the 2,4-dinitrophenylhydrazine (DNPH) method were done according to Soglia et al.,19 with minor modifications as described previously.18
Processed protein-based matrices were cut into cylinders of 8 × 8 mm size, with sampling at various locations in the matrix, then 9 g of sample was homogenized with 100 mM Tris/5 mM EDTA buffer pH 7.5 (1:3 w/v) using a rotor-stator homogenizer (IKA T18 UltraTurrax, Thermo Fisher Scientific, Staufen, Germany) at 13 600 rpm for 1 min in an ice bath. Then, the samples were centrifuged at 18 000g at 2 °C for 20 min. The supernatant was collected as the soluble fraction. Since previous research18 showed low protein solubility in Tris/EDTA/NaCl buffer, we suspended the remaining pellet in 0.15 M KCl solution (1:3, w/v) and homogenized the mixture using the same rotor-stator homogenizer at 13 600 rpm for 30 s in an ice bath. This final suspension was called the pellet fraction. Afterward, both fractions were filtered with a 0.2 μm syringe filter, and the soluble protein concentration was determined by the BCA method.18 The same procedure was done for the unheated protein suspensions.
Aliquots from the protein fractions (1–6 mg of soluble protein) were taken to measure the carbonyl content by the DNPH method. After hydrazone derivatization, samples were incubated at 37 °C overnight in 6 M guanidine hydrochloride prepared in 20 mM sodium phosphate buffer pH 6.5. Then, the absorbance was measured at 370 nm using a UV–visible spectrophotometer (DR-3900, HACH Lange, Germany) using 6 M guanidine hydrochloride as a blank. A control was prepared for all samples following the same procedure, except no DNPH was added. The soluble protein concentration in 6 M guanidine hydrochloride was determined by the BCA method. The carbonyl content was calculated with the following equation:
| 1 |
where ABSsample is the absorbance of the sample; ABScontrol is the absorbance of the control; and ε is the molar extinction coefficient of carbonyl, set as 22 000 M–1 cm–1. The carbonyl content was measured in independent samples, each one measured in triplicate (per sample and per blank).
It should be noted that all measurements were done on the soluble protein fraction, which is about 0.8 to 6% of the total protein in the processed protein-based matrices. The measurement on the soluble protein fraction is a fair representation of the levels of protein-bound carbonyls, and it has been commonly used to determine protein oxidation in a broad range of protein-based samples.18−29
Molecular Weight Distribution Profile: Electrophoresis
The protein fractions were characterized by their molecular weight profile by 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions. The reducing sample buffer was prepared with 950 μL of native buffer and 50 μL of 2-mercaptoethanol. Only the soluble fraction was diluted to a final concentration of 3 mg/mL. Then samples were mixed with the reducing buffer in a ratio 1:1, heated at 95 °C for 5 min in an Eppendorf thermomixer, and cooled at room temperature for 30 min. Afterward the samples were centrifuged using 10 000g for 5 min. Then, 15 μL of samples and molecular weight standards were placed in the gels, and 10× Tris/Glycine/SDS running buffer was used. The electrophoresis was carried out at 200 V for approximately 30 min. Subsequently, the gel was washed three times with ultrapure water and then stained with Biosafe Coomassie Stain overnight. The next day the gel was washed with ultrapure water for 30 min before gel images were taken using a GS-900 Calibrated Densitometry System (Bio-Rad Laboratories, Inc., U.S.A.). The gel images were analyzed using the Image Lab (version 2.0.1, Bio-Rad Laboratories, Munchen, Germany). Two independent samples were analyzed per SDS-PAGE, done in duplicate.
Protein Surface-Exposed Hydrophobicity
The surface-exposed hydrophobicity was determined in ground processed protein-based matrices according to Berton-Carabin et al.27 The samples were ground with four cycles of 10 s each using a kitchen mixer (Multiquick 5, Braun, Kronberg, Germany).30 The average size of ground samples was determined with measuring the smallest (0.64 ± 0.25 mm) and largest (1.46 ± 0.37 mm) dimension of the particles from pictures obtained with an automated digital 3D microscope Smartzoon 5 (Carl Zeiss, Breda, The Netherlands). Then ground samples were homogenized in 10 mM sodium phosphate buffer pH 7.0 at 13 600 rpm for 1 min in ice bath using an Ultra Turrax. As a next step, the samples were centrifuged at 18 000g and 2 °C for 20 min, and the soluble fractions were collected. Suspensions of unheated soy protein-ingredients were prepared with 6 wt% net protein (dry basis) in the 10 mM sodium phosphate buffer, as described in the section Preparation of unheated protein suspensions and processed samples. The soluble protein concentration was measured with BCA method and samples were diluted to obtain a final concentration of 1 mg/mL soluble protein. The anionic fluorescence ANSA probe (2.4 mM) was prepared in 10 mM sodium phosphate buffer at pH 7.0 and stirred overnight at 4 °C. The fluorescence emission spectra were measured between 400 and 650 nm with steps of 0.5 nm using a RF-6000 spectrofluorometer (Shimadzu Corporation, Kyoto, Japan). The excitation wavelength was set at 385 nm, and the emission was measured at 480 nm, with a scan rate of 60 nm/min and spectral bandwidth of 5.0 nm. For this measurement, quartz cuvettes with dimensions of 10 × 10 mm were used (Hellma Analytics, Müllheim, Germany). Then 1 mL of sample was mixed with 10 μL of ANSA for 1 min, and the spectra were recorded until the signal reached ANSA saturation. The two blanks were pure buffer with added ANSA with the same concentration as reached in the sample and a sample without ANSA. The results were expressed as the maximum fluorescence intensity at 480 nm, which represents the number of exposed hydrophobic sites of the protein. The experiment was done with two independent samples.
Acid Titration
A titration with HCl was done using an automatic titrator (877 Titrino plus, Metrohm, Herisau, Switzerland) to estimate the acid uptake by protein ingredients and processed protein-based matrices according to Luo et al.,999 with a few modifications. The titration was performed by adding ground processed protein-based matrices containing 1 g of net protein (dry basis) to 25 mL of ultrapure water with NaCl (0.8775 w/v%) in a jacketed vessel connected to a thermostat bath set at 37°C and stirred at 100 rpm. Before titration, the samples were soaked in the solution for 30 min. The titration was done stepwise from their original pH, which was around 7, to pH 2 by adding 0.05 mL of 0.5 M HCl, in a minimum interval of 1 s and a maximum waiting time of 25 s. The experiment was performed in two independent samples, measured twice for each sample.
Preparation of Samples for In Vitro Gastric Digestion
The suspensions of unheated protein ingredients (SPC, SPI, or SPI with pectin (1:19)) were prepared with 5 w/v% protein in ultrapure water according to Luo et al.17 After stirring at room temperature for 30 min, the samples were used for in vitro gastric digestion experiments. Samples were prepared in duplicate. The processed protein-based matrices were cut into cylinders of 3 × 3 mm size31 or ground as previously described in the section Protein surface exposed-hydrophobicity..
In Vitro Gastric Digestion Setup
A static soaking in vitro setup was used to simulate the gastric digestion process. Simulated gastric fluid (SGF) was prepared with pepsin (1 g/L) and NaCl (8.775 g/L) in ultrapure water according to Luo et al.17 with few modifications. The pH of the SGF was adjusted to 2 with 2 M HCl. Then processed protein-based matrices or the suspensions of unheated protein ingredients containing 0.1 g of protein (dry basis) were added to 50 mL of the SGF preheated at 37 °C in a jacketed vessel while stirring at 100 rpm. The vessels were sealed with parafilm to avoid evaporation. One milliliter of sample was taken after 5, 20, 30, 60, 120, and 180 min for further analysis. To inactivate the pepsin activity, the samples were heated at 90 °C32 and mixed at 1400 rpm for 5 min in a preheated Eppendorf thermomixer (Eppendorf AG, Germany). After the inactivation step, the samples were cooled down. The hydrolysis experiments were carried out with two independent samples, and each sample was tested in duplicate, resulting in a total of four digestion experiments per processing condition tested.
Determination of the Degree of Hydrolysis
The degree of hydrolysis (DH) was measured by the ο-phthalaldehyde (OPA) method according to Luo et al.17 The OPA reagent was prepared by first dissolving 3.81 w/v% Borax and 0.1 w/v% SDS in ultrapure water, and subsequently, 0.08 w/v% OPA predissolved in 2 mL of ethanol was added to the Borax-SDS solution. Lastly, 0.088 w/v% DTT was added, and the solution was filled up to 150 mL with ultrapure water. The solution was filtered through a 0.45 μm syringe filter and protected from light exposure.
A calibration curve with L-serine was prepared in a concentration range of 50–400 mg/L. Digestion samples were first centrifuged at 14 000g for 1 min. Then 200 μL of sample, blank, or calibration sample was mixed with 1.5 mL of OPA reagent for 3 min, and the absorbance was measured at 340 nm with a UV–vis spectrophotometer (DU720, Beckman Coulter, Inc., Indianapolis, IN, U.S.A.). Each sample was measured in triplicate. Free amino groups values from digestion samples were corrected by subtracting the contribution of free amino groups from the SGF before digestion without sample addition.
The DH was determined as the percentage of peptide bonds cleavage regarding a total number of peptide bonds, following the equations:
| 2 |
| 3 |
where h is the number of peptide bonds cleavage in 1 kg protein; htot is the total number of peptide bonds in 1 kg protein set as 7.8 meq/g for soy proteins; serine-NH2 represents free amino groups as serine amino equivalents obtained from the calibration curve; and α is 0.970, and β is 0.342 for soy proteins.33
Size-Exclusion Chromatography
The peptide size distribution profile was measured with high-performance size-exclusion chromatography (HPSEC) using TSKgel G3000SWxl column (7.8 mm × 300 mm) and TSKgel G2000SWxl (7.8 mm × 300 mm) (Tosoh Bioscience LLC, King of Prussia, PA, U.S.A.) in an Ultimate 3000 UHPLC system (ThermoFisher Scientific Inc., U.S.A.). Digested samples were first filtered using a 0.2 μm Spartan 13/0.2 R 6 Whatman filter (VWR), and then 10 μL of sample was injected for each measurement. The mobile phase was made with 30% acetonitrile, 0.1% trifluoroacetic acid, and 70% ultrapure water. Signals were measured with a UV detector set at 214 nm, at 30 °C and with a flow rate of 1.5 mL/min. The calibration was performed with standard solutions of α-lactalbumin, aprotinin, insulin, bacitracin, phenylalanine, g-globulin, and ovalbumin. Then the calibration curve was made by plotting the retention time of each standard solution as a function of the protein’s molecular weight. Data analysis was performed in Dionex Chromeleon 7.2 Chromatography Data System software (ThermoFisher Scientific Inc., U.S.A.). A chromatogram from the system resulted in peaks showing the amount of molecules with the sizes >50 kDa, 50–10 kDa, 10–4 kDa, 4–2 kDa, and <2 kDa. The experiments were done in duplicate per digestion sample, resulting in four experiments per sample.
Statistical Analysis
Statistical analysis was done using the Statistical Package for the Social Sciences (SPSS software v. 23, IBM Inc.). The Mann–Whitney test was used to compare the results pertaining to the degree of hydrolysis. One way-ANOVA with post hoc analysis using Tukey’s multiple comparison test was used to compare the means pertaining to carbonylation level, soluble protein concentration, maximum fluorescence intensity, and peptide size distribution profile regarding processing conditions and ingredients. An independent t test was performed to compare the maximum fluorescence intensity of samples processed with the same conditions, but starting from different ingredients. The significance level was set at p < 0.05.
Results and Discussion
Physicochemical Characterization
Protein-Bound Carbonyl Content
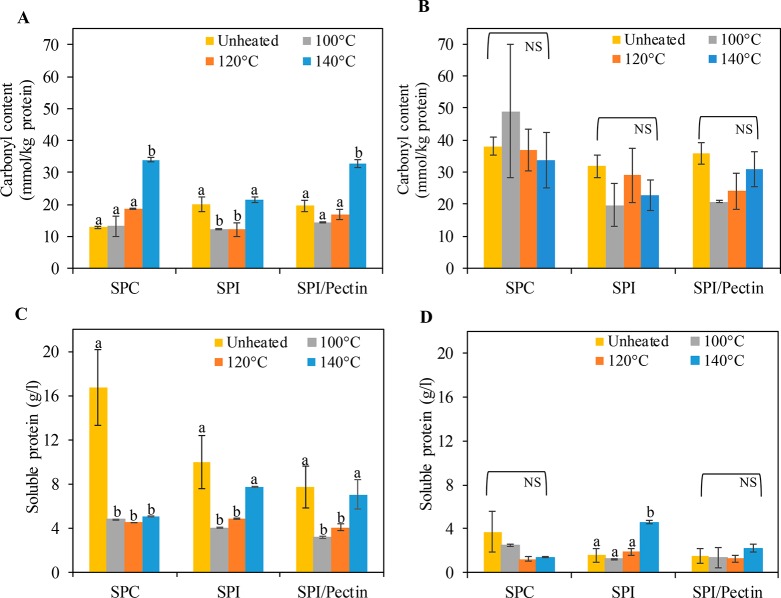
The effect of process conditions on protein oxidation was quantified through measuring the protein-bound carbonyl content. The soluble protein fraction of all unheated protein ingredients had a carbonyl content between 12.9 and 20.2 mmol carbonyl/kg soluble protein (Figure 1A), which did not increase further with processing at 100 and 120 °C, independently of the matrix. However, processing at 140 °C increased the carbonyl content in SPC- and SPI/pectin-based matrices significantly (p < 0.05) when compared to samples that were unheated or processed at lower temperatures (Figure 1A). Figure 1B shows the carbonyl content of the pellet fractions. The carbonyl content of the unheated protein ingredients was above 30 mmol carbonyl/kg soluble protein, which was higher than in the soluble fraction. However, there was no significant increase in carbonyl content in this fraction with processing, whatever the applied temperature. In addition, the carbonyl content in the pellet fractions was similar for all tested ingredients (SPC, SPI, and SPI/pectin).
Figure 1.
Carbonyl content (mmol per kg soluble protein) (A, B) and soluble protein concentration (g/L) in Tris/EDTA (C) or KCl (D) buffers of unheated protein ingredients suspensions and processed protein-based matrices (100, 120, and 140 °C): soluble fraction (A and C) and pellet fraction (B and D). Different letters indicate a significant difference between unheated protein ingredients and processed protein-based matrices made with the same ingredient. Significance level at p < 0.05. NS: no significant difference.
Heat- and shear-based process significantly decreased the soluble protein concentration in the processed SPC-based matrices compared with the starting ingredient, especially in the soluble fraction (Figure 1C). Processing SPI and SPI/pectin at 140 °C caused a small increase in the soluble concentration compared with lower temperatures, but it was not significant compared to unheated protein ingredients. For all samples, the soluble protein concentration in the pellet fraction was lower than that in the soluble fraction. Processing SPI at higher temperatures caused an increase in the soluble concentration in the pellet fraction compared with unheated protein ingredients. Overall, we see that a heat- and shear-based process can promote soy protein oxidation, which is in line with previous research.18
Although the effect of handling, processing, and storage on protein oxidation is largely unexplored for plant-protein-based ingredients and foods, such data are readily available for muscle protein-based foods. For example, in processed meat, Soladoye et al.34 reported carbonyl content of 80 mmol carbonyl/kg soluble protein in raw bacon, which further increased with cooking. These results suggested that the quality of the raw material, the use of additional ingredients in formulated products, and the processing conditions largely determine protein oxidation and can lead to high levels of oxidation. Regarding the ingredients of processed food, the presence of reducing sugars and their oxidation products, such as α-dicarbonyl compounds, can induce oxidative deamination of basic amino acids resulting in protein carbonylation via a Maillard-mediated mechanism.35 For instance, Luna and Estévez4 found that the SPI glycation reaction formed more carbonyls than the metal-catalyzed hydroxyl-radical generating system, while a combination of both oxidation systems further increased carbonylation. Nevertheless, such reactions are probably highly dependent on the ingredient’s composition, notably regarding reducing sugars, which may largely vary between different ingredients and supplier.
Furthermore, we found that the proteins in the starting ingredients used (i.e., unheated SPC or SPI) were already oxidized to a certain extent. We made similar observations in a previous study using commercial soy ingredients.18 Likewise, Chen et al.36 reported a carbonyl content of 15.1 mmol carbonyl/kg soluble proteins in commercial SPI, which increased to 22.4 mmol carbonyl/kg soluble proteins upon dry heating at 100 °C for 8 h. The fractionation process and/or storage conditions applied to protein ingredients could have caused protein oxidation. In industrial processes, spray drying is often used as the final drying step to obtain the SPC and SPI, and preheating treatments may be applied prior to drying, which can promote protein oxidation.18 Conversely, the carbonyl content of lab-made SPI is often lower, with a typical value range of 1.5 to 6.5 mmol carbonyl/kg soluble proteins reported in different studies.37−222 This may be because those SPI samples were prepared with freeze-drying as the final drying step. Preheating SPI suspensions from 5 to 30 min at 90 °C followed by spray drying resulted in increased protein carbonyls and decreased protein solubility during 8 weeks of storage.333 The fractionation process prior to drying also seems of importance. For example, Wu et al.41 prepared a low-oxidized SPI (1.7 mmol carbonyl/kg soluble proteins) by using soybeans with low moisture content and isolating proteins by fractionation process under low oxygen conditions. This suggests that a large variability in that respect may exist from one plant protein material to another and that considering the oxidative status of protein ingredients is relevant to control oxidation during processes.
Moreover, information regarding the consequences of dietary oxidized proteins on human health is still limited. The effect of postprandial protein oxidation causing cellular and tissue damage has been related to the progression of diseases such as inflammatory bowel disease, diabetes, and fibrosis.10 Studies with in vitro and animal models have shown that dietary oxidized proteins may promote some organ dysfunctions.11 For instance, animal studies showed that the intake of oxidized tyrosine (2 to 8 g/kg diet for 24 weeks) resulted in oxidative stress and dysfunction of kidney and pancreas cells, combined with inflammation.42,43 However, the doses used in these studies are much higher than values typically reported in food proteins, and thus more studies are needed to elucidate dose-related effects to establish whether usual oxidation levels occurring in foods could give rise to similar consequences.
Molecular Weight Distribution Profile: SDS-PAGE
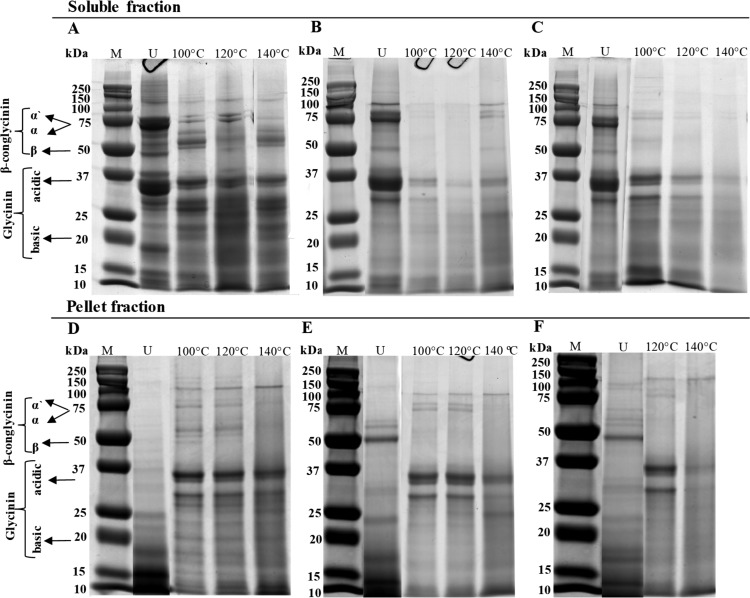
The molecular weight distribution of proteins in the soluble fractions (Figure 2A–C) and pellet fractions (Figure. 2D–F) was determined with SDS-PAGE under reducing conditions. For all samples, we see differences in the protein molecular weight distribution between the unheated ingredients and the processed matrices, for both fractions (Figure 2). In the soluble fraction of unheated SPC (Figure 2A), β-conglycinin and glycinin subunits, the major constituents of soy proteins, are present, but the intensity of the bands corresponding to the α and α′ subunits of β-conglycinin decreased after processing at 140 °C. The soluble fraction of unheated SPI and SPI/pectin ingredients had a similar molecular weight distribution. The same was observed when comparing SPI and SPI/pectin-based matrices processed at temperatures below 140 °C (Figure 2B,C). These findings were expected since both matrices were prepared with the same source of protein ingredient. However, the bands around 75 kDa in SPI/pectin-based matrices processed at 140 °C were not visible anymore.
Figure 2.
SDS-PAGE under reducing conditions of the soluble fraction and pellet fraction of unheated protein ingredients and processed protein-based matrices at different temperatures. A and D: SPC samples; B and E: SPI samples; C and F: SPI/pectin samples; U: unheated ingredients; M: molecular weight standard; α′/α/β: peptides of β-conglycinin.
The molecular weight distribution profiles of the pellet fractions of unheated samples were different from those of the soluble fractions (Figure 2D–F), for a given sample. In the pellet fractions, bands around 75 kDa were not visible, and an accumulation of low molecular weight components appeared. For unheated SPI and SPI/pectin samples (Figure 2E,F), the band around 50 kDa was still visible as seen in the soluble fraction (Figure 2B,C). The pellet fraction of all processed samples at 140 °C did not show bands around 75 kDa, which represents the molecular weight of α- and α′ subunits of β-conglycinin. Overall, we observed a systematic difference between unheated and processed samples with a decrease in the intesity of some particular bands after processing, regardlles of the protein fraction. In addition, bands at molecular weights above 37 kDa for the pellet fractions appeared as faded, or were not even visible sometimes, compared with the soluble fraction.
Protein Surface-Exposed Hydrophobicity
Figure 3 shows the maximum fluorescence intensity (Fmax) of unheated protein ingredients and processed protein-based matrices made with different ingredients, which represents the number of surface-exposed hydrophobic sites in the protein. In the case of unheated protein ingredients, we found that SPC had more exposed hydrophobic sites than SPI and SPI/pectin. All processed protein-based matrices showed a strong and significant decrease in the number of surface-exposed hydrophobic sites compared with unheated protein ingredients (p < 0.05). SPC-based matrices processed at 100 °C had a 90% decrease on Fmax compared with the unheated SPC ingredient. For all three starting ingredients, the temperatures applied had only a minor additional effect on protein hydrophobicity. The protein surface hydrophobicity in the processed SPI/pectin-based matrices decreased further upon processing at a higher temperature. It seems therefore that the presence of pectin decreases protein hydrophobicity. A possible explanation could be that pectin would interact with proteins via hydrophobic interactions involving the methoxyl groups of pectin.44 It is, however, difficult to generalize this effect, as opposite trends were also reported in the literature.45 Presumably, the type of pectin (and notably its degree of methoxylation), the type of protein, and the probe used to assess surface-exposed hydrophobicity are of importance in that respect. Previous studies showed that protein oxidation is also a relevant parameter that affects surface hydrophobicity. We observed a substantial formation of protein-bound carbonyls in processed SPC- and SPI/pectin-based matrices made at 140 °C (Figure 1A), which could reduce the number of exposed hydrophobic sites. Likewise, studies have shown that at low levels of carbonyl content, the surface hydrophobicity was higher compared with high levels of carbonyl content.27,37,46−48
Figure 3.
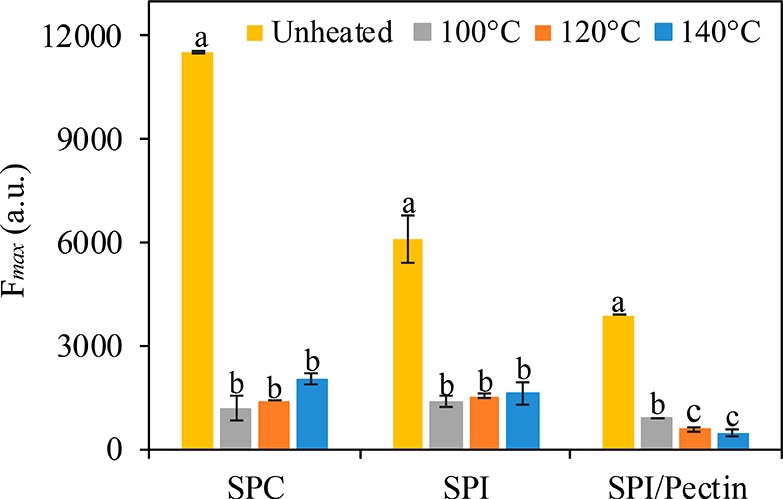
Maximum fluorescence intensity (Fmax) based on ANSA probe of unheated protein ingredients and processed protein-based matrices made with different ingredients and process conditions. Excitation wavelength set at 385 nm and emission at 480 nm. Results are expressed as mean and error bars as standard deviation. Different letters indicate a significant difference among unheated protein ingredients and processed protein-based matrices, made with the same ingredient. Significance level at p < 0.05.
In this study, heat- and shear-based process decreased surface-exposed hydrophobicity in protein-based matrices compared with protein ingredients, which is a result of protein rearrangements induced by processing and protein oxidation. The reduced hydrophobicity of the proteins can decrease the proteolytic susceptibility of the proteins.9,49
Acid Uptake
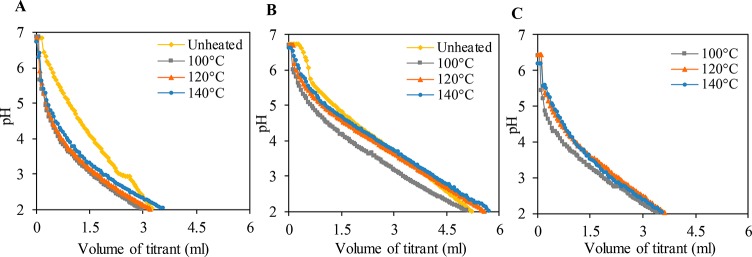
We investigated the volume of acid (HCl) uptake by unheated protein ingredients and ground processed protein-based matrices made with different ingredients that are necessary to reach pH 2 (Figure 4). The volume of HCl solution needed to reach pH 2.0 was higher for SPC-based suspensions than for SPI- and SPI/pectin-based ones. The patterns for unheated protein ingredients and processed protein matrices were similar for SPI and SPI/pectin, implying that the presence of pectin did not affect acid uptake and hence the protein buffering capacity. Processing can modulate acid uptake, but the temperature treatment did not have a large effect. A higher acid uptake is a measure for a strong buffer capacity, which can affect the gastric digestion rate since the activity of pepsin is highly pH-dependent.50 Therefore, differences in gastric digestion are expected because of different ingredients and heating. However, the exact process conditions are likely to have a limited effect in that respect.
Figure 4.
pH as a function of the volume of titrant 0.5 M HCl of suspensions of unheated protein ingredients or of ground processed protein-based matrices made with (A) SPI, (B) SPC, and (C) SPI/pectin at different temperatures. The protein content (dry basis) in all samples is similar.
In Vitro Gastric Digestion
Degree of Hydrolysis
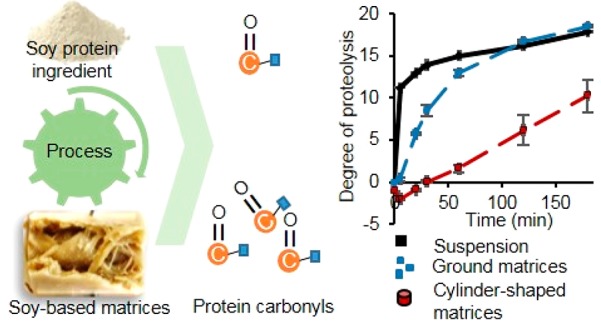
First, we investigated the effect of process conditions, the effect of grinding the processed protein-based matrices, and the effect of the starting protein ingredient on the degree of hydrolysis using in vitro gastric conditions. Second, we aimed to correlate the obtained results with protein oxidation.
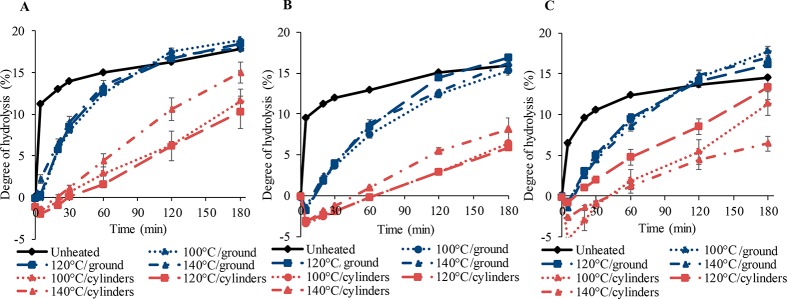
Figure 5 shows that all unheated protein ingredients had a rapid increase in DH within the first 5 min of simulated gastric digestion. Then proteolysis slowed down, before reaching a plateau. This fast increase within the first minutes of digestion is in line with previous studies with protein suspensions.17,51,52 Processed protein-based matrices (cylinder-shaped) showed a slower increase in DH and lower final value than unheated protein ingredients after 180 min. After 5 min of gastric digestion, samples were hardly digested, indicating a long sample disintegration time. The combination of pepsin diffusion, hydrolysis rate, microstructure, and mechanical strength was previously used to explain differences in disintegration and subsequent digestion of whey protein isolate gels.16 The pepsin diffusion was limited to a depth of 2 mm within the gels, explaining why hydrolysis only occurred in the thin layer at the surface of the gels. Thus, the digestion rate was constrained by the surface area of the gel and the surface erosion.
Figure 5.
Degree of hydrolysis (%) of unheated protein ingredients and processed protein-based matrices made with SPC (A), SPI (B), and SPI/pectin (C) prepared at different temperatures. The processed matrices cut as small cylinders are represented by red lines, and the processed ground samples are represented as blue lines. Error bars represent the standard deviation (two independent samples, each digested in duplicate).
After cutting the processed protein-based matrices into cylindrical shapes, the SPI-based one (Figure 5B) had slower disintegration than SPC- and SPI/pectin-based ones, which could be associated with a higher sample hardness. Previous research revealed that SPI-based matrices processed at 140 °C had higher tensile stress compared to SPC- and SPI/pectin-based ones.2,3,53 The lower tensile stress in processed SPI/pectin-based matrices is associated with the presence of pectin, which forms a weaker and elongated separated phase and induces air pockets within the matrix, resulting in fiber formation at 140 °C.2,3,53 The results apply with the general observation that soft protein gels can be subjected to faster disintegration in digestive conditions compared with hard gels.52
Besides the sample texture, the sample size could influence the rate of hydrolysis. To test this hypothesis, we ground the processed protein-based matrices. Unfortunately, the broad particle size distribution obtained and the roughness of the samples prevented calculation of the exact surface area. Ground processed protein-based matrices had significantly (p < 0.05) higher DH than cylinder-shaped ones but a slower rate of hydrolysis than unheated protein ingredients. Most likely, the reduced sample size facilitated disintegration and the diffusion of pepsin and HCl into the samples, increasing the DH. Interestingly, ground SPI/pectin-based matrices had equal or even higher DH than unheated protein ingredients after 180 min of digestion. The exact reason for this effect is not clear yet.
Although the applied processing temperature seemed to influence the DH of the processed protein matrices, no generic trend across the different materials could be observed. After grinding the products, the differences due to processing became small. Regarding the effect of the starting protein ingredient, ground SPC-based matrices had faster increase in the DH compared with SPI and SPI/pectin-based matrices. Since the buffering capacity of SPC was the largest among the tested ingredients, this could have led to a lower digestibility. However, this was clearly not the case. Nevertheless, increased surface-exposed hydrophobicity in SPC might have increased the exposure of hydrophobic sites in which pepsin has more affinity. The excess of gastric juice is most probably able to compensate for the effect and to keep the conditions favorable for pepsin to be active. The fractionation process applied to obtain concentrated and isolated proteins could be another factor that can affect the status of the proteins and as a consequence the digestion rate. Opazo-Navarrete et al.54 reported a lower degree of protein hydrolysis in isolated quinoa protein obtained by wet fractionation than in the protein-enriched quinoa fraction obtained by dry fractionation. They suggested that the changes in pH and the thermal treatment during the drying step involved in the wet fractionation process affect the protein conformation and supramolecular structures, which would impact protein digestibility.
When carbonyl levels of all samples were related to DH, we concluded that oxidation had no obvious relationship with the DH (see Figure 1 in Supporting Information). Likewise, Chen et al.55 found no significant difference in the DH after a 1 h pepsin digestion of SPI samples treated with increasing concentration of pro-oxidants. We also did not observe a relationship between the DH and surface-exposed hydrophobicity or carbonyl content and hydrophobicity (see Figures 2 and 3 in Supporting Information). Santé-Lhoutellier et al.22 did not find a correlation between carbonyl content and pepsin activity in myofibrillar proteins from pork, which was attributed to the level of protein oxidation and the oxidative susceptibility of amino acids that pepsin has a preference for cleaving (e.g., aromatic amino acids). Therefore, the physicochemical modifications induced by the shear- and heat-based process of soy protein ingredients contributed to slowing down the degree of hydrolysis but did not impair the DH after 120 min of digestion.
Peptide Size Distribution (HPSEC)
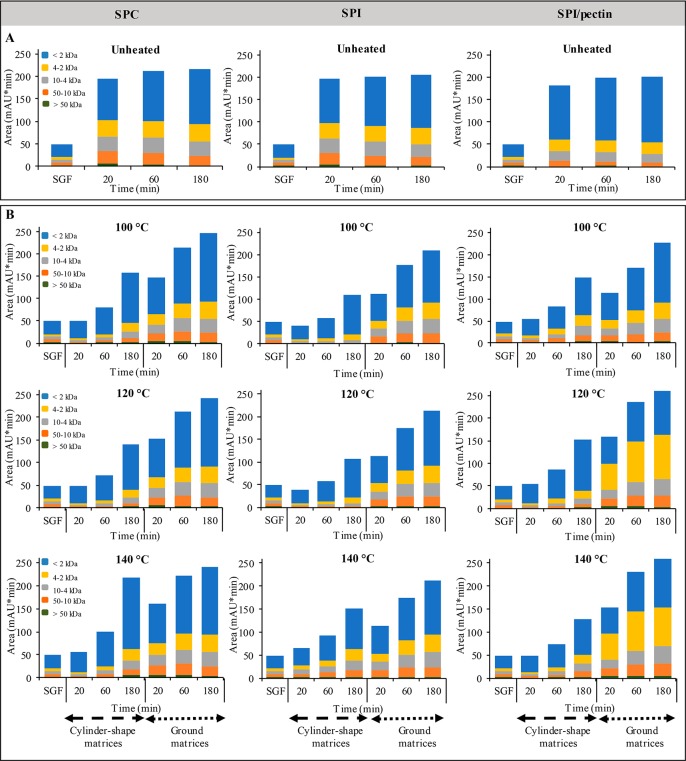
The peptide size distribution of unheated SPC and SPI ingredients showed more peptides larger than 10 kDa compared with the SPI/pectin ingredient (p < 0.05) (Figure 6A). Additionally, the SPI/pectin ingredient had more peptides smaller than 2 kDa (p < 0.05). Overall, the peptide size distribution of the unheated protein ingredients seemed already quite stable after 20 min of incubation in simulated gastric conditions, which is in agreement with the fast digestion observed in Figure 5. This is different for the processed protein-based matrices, either in a cylinder-shape or ground. Figure 6B shows that more small peptides were present in digested ground samples over time compared with the digested cylinder-shaped samples (p < 0.05). These results are in line with the lower digestion rate found for the cylinder-shaped samples in Figure 5. However, after 180 min of digestion, there was no difference for peptides smaller than 4 kDa between digested cylinder-shaped and ground samples made of processed SPC-based matrices at 140 °C (p > 0.05).
Figure 6.
Peak area (mAU*min) of peptides distribution after 20, 60, and 180 min of gastric digestion of unheated SPC, SPI, SPI/pectin ingredients (A) and protein-based matrices processed at different temperatures (cylinder-shape and ground matrices) (B). SGF: simulated gastric fluid. Results are shown as mean values (two independent samples, measured in duplicate). The mean values, standard deviations, and statistics results are shown in Tables 1 and 2 in the Supporting Information.
When comparing the different starting ingredients, SPC-based matrices processed at different temperatures showed a similar peptide distribution, though the amounts formed were distinct (Figure 6B). This suggests that the same reaction products are formed, regardless of the reaction rate of pepsin. The higher digestion rate of cylinder-shaped of those samples processed at 140 °C as shown in Figure 5A is nicely confirmed by the higher amount of peptides present in the gastric juice after 180 min of digestion. Interestingly, the digestion of ground SPI/pectin-based matrices resulted in more peptides between 2 and 4 kDa than SPC and SPI-based matrices (p < 0.05) when processed at 120 and 140 °C. Conversely, SPC-based matrices (cylinder-shape and ground) processed at 120 and 140 °C had more peptides lower than 2 kDa than both SPI and SPI/pectin (p < 0.05). Even though after 180 min of gastric digestion SPC and SPI/pectin-based matrices reach a similar DH (Figure 5A,C), the distribution of their peptides was different, confirming the relatively faster rate of hydrolysis of SPC-based matrices. This can be related to the reduced hydrophobicity of SPI/pectin-based matrices at 120 and 140 °C or to the presence of pectin hindering pepsin activity. The effect of polysaccharides on protein digestion was previously investigated for protein solutions;56,57 for instance, the presence of 1% w/w pectin in whey protein isolate solutions (pH 7.0) heated at 85 °C for 30 min has been shown to form aggregates due to extensive intragastric gelation, which were not digested after 2 h under in vitro conditions.57 Most of the pectin and more than half of the protein remained in the gels. Therefore, the presence of pectin decreased the digestion rate of whey protein.
The commercial soy protein ingredients used in this work contained a substantial amount of preformed protein-bound carbonyls, revealing a certain initial degree of protein oxidation. Application of a thermomechanical process to make structured matrices resulted in additional protein oxidation in SPC- and SPI/pectin-based matrices at 140 °C. The process also led to reduced protein surface-exposed hydrophobicity. Structuring the starting ingredients via this process slowed down proteolysis, even though the actual rate showed no correlation with the level of carbonyls or the applied temperature during processing. The peptide distribution during simulated gastric digestion was not affected by the process conditions, but the presence of pectin resulted in the formation of larger peptides. Therefore, we foresee that developing fractionation and storage methods to yield plant protein ingredients with a low level of protein oxidation is becoming an essential matter in the current transition to more plant protein-based food products.
Glossary
Abbreviations Used
- ABS
absorbance
- ACN
acetonitrile
- ANSA
8-anilino-1-napthalenesulfonic acid ammonium salt
- BCA
bicinchoninic acid
- Borax
sodium tetraborate decahydrate
- DNPH
2,4- dinitrophenylhydrazine
- DTT
DL-dithiothreitol
- ε
molar absorptivity
- EDTA
diaminoethane tetraacetic acid
- Fmax
maximum fluorescence intensity
- HCl
hydrochloric acid
- HTSC
high temperature shear cell
- KCl
potassium chloride
- OPA
o-phtaldialdehyde
- NaCl
sodium chloride
- SDS
sodium dodecyl sulfate
- SPC
soy protein concentrate
- SPI
soy protein isolate
- TCA
trichloroacetic acid
- TFA
trifluoracetic acid
- Tris
tris(hydroxymethyl) aminomethane
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.jafc.9b02423.
Correlation graphs and statistics of peptide size distribution results (HPSEC) (PDF)
(PDF)
The authors thank the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq/Brazil), for supporting P. Duque-Estrada’s Ph.D. scholarship (process number 233663/2014-2).
The authors declare no competing financial interest.
Supplementary Material
References
- Day L. Proteins from Land Plants - Potential Resources for Human Nutrition and Food Security. Trends Food Sci. Technol. 2013, 32 (1), 25–42. 10.1016/j.tifs.2013.05.005. [DOI] [Google Scholar]
- Grabowska K. J.; Zhu S.; Dekkers B. L.; de Ruijter N. C. A.; Gieteling J.; van der Goot A. J. Shear-Induced Structuring as a Tool to Make Anisotropic Materials Using Soy Protein Concentrate. J. Food Eng. 2016, 188, 1–10. 10.1016/j.jfoodeng.2016.05.01. [DOI] [Google Scholar]
- Dekkers B. L.; Nikiforidis C. V.; van der Goot A. J. Shear-Induced Fibrous Structure Formation from a Pectin/SPI Blend. Innovative Food Sci. Emerging Technol. 2016, 36, 193–200. 10.1016/j.ifset.2016.07.003. [DOI] [Google Scholar]
- Luna C.; Estévez M. Oxidative Damage to Food and Human Serum Proteins: Radical-Mediated Oxidation vs. Glyco-Oxidation. Food Chem. 2018, 267, 111–118. 10.1016/j.foodchem.2017.06.154. [DOI] [PubMed] [Google Scholar]
- Hu L.; Ren S.; Shen Q.; Chen J.; Ye X.; Ling J. Proteomic Study of the Effect of Different Cooking Methods on Protein Oxidation in Fish Fillets. RSC Adv. 2017, 7 (44), 27496–27505. 10.1039/C7RA03408C. [DOI] [Google Scholar]
- Estévez M. Protein Carbonyls in Meat Systems: A Review. Meat Sci. 2011, 89 (3), 259–279. 10.1016/j.meatsci.2011.04.025. [DOI] [PubMed] [Google Scholar]
- Singh S.; Gamlath S.; Wakeling L. Nutritional Aspects of Food Extrusion: A Review. Int. J. Food Sci. Technol. 2007, 42 (8), 916–929. 10.1111/j.1365-2621.2006.01309.x. [DOI] [Google Scholar]
- Santé-Lhoutellier V.; Aubry L.; Gatellier P. Effect of Oxidation on In Vitro Digestibility of Skeletal Muscle Myofibrillar Proteins. J. Agric. Food Chem. 2007, 55 (13), 5343–5348. 10.1021/jf070252k. [DOI] [PubMed] [Google Scholar]
- Santé-Lhoutellier V.; Astruc T.; Marinova P.; Greve E.; Gatellier P. Effect of Meat Cooking on Physicochemical State and in Vitro Digestibility of Myofibrillar Proteins. J. Agric. Food Chem. 2008, 56 (4), 1488–1494. 10.1021/jf072999g. [DOI] [PubMed] [Google Scholar]
- Estévez M.; Luna C. Dietary Protein Oxidation: A Silent Threat to Human Health?. Crit. Rev. Food Sci. Nutr. 2017, 57 (17), 3781–3793. 10.1080/10408398.2016.1165182. [DOI] [PubMed] [Google Scholar]
- Estévez M.; Xiong Y. Intake of Oxidized Proteins and Amino Acids and Causative Oxidative Stress and Disease: Recent Scientific Evidences and Hypotheses. J. Food Sci. 2019, 84 (3), 387–396. 10.1111/1750-3841.14460. [DOI] [PubMed] [Google Scholar]
- Laguna L.; Picouet P.; Guàrdia M. D.; Renard C. M. G. C.; Sarkar A. In Vitro Gastrointestinal Digestion of Pea Protein Isolate as a Function of pH, Food Matrices, Autoclaving, High-Pressure and Re-Heat Treatments. LWT - Food Sci. Technol. 2017, 84, 511–519. 10.1016/j.lwt.2017.06.021. [DOI] [Google Scholar]
- Piper D. W.; Fenton B. H. pH Stability and Activity Curves of Pepsin with Special Reference to Their Clinical Importance. Gut 1965, 6 (5), 506–508. 10.1136/gut.6.5.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh H.; Ye A.; Ferrua M. J. Aspects of Food Structures in the Digestive Tract. Curr. Opin. Food Sci. 2015, 3, 85–93. 10.1016/j.cofs.2015.06.007. [DOI] [Google Scholar]
- Lorieau L.; Halabi A.; Ligneul A.; Hazart E.; Dupont D.; Floury J. Impact of the Dairy Product Structure and Protein Nature on the Proteolysis and Amino Acid Bioaccessiblity during in Vitro Digestion. Food Hydrocolloids 2018, 82, 399–411. 10.1016/j.foodhyd.2018.04.019. [DOI] [Google Scholar]
- Luo Q.; Borst J. W.; Westphal A. H.; Boom R. M.; Janssen A. E. M. Pepsin Diffusivity in Whey Protein Gels and Its Effect on Gastric Digestion. Food Hydrocolloids 2017, 66, 318–325. 10.1016/j.foodhyd.2016.11.046. [DOI] [Google Scholar]
- Luo Q.; Boom R. M.; Janssen A. E. M. Digestion of Protein and Protein Gels in Simulated Gastric Environment. LWT - Food Sci. Technol. 2015, 63 (1), 161–168. 10.1016/j.lwt.2015.03.087. [DOI] [Google Scholar]
- Duque Estrada P.; Berton-Carabin C. C.; Schlangen M.; Haagsma A.; Pierucci A. P. T. R.; van der Goot A. J. Protein Oxidation in Plant Protein-Based Fibrous Products: Effects of Encapsulated Iron and Process Conditions. J. Agric. Food Chem. 2018, 66 (42), 11105–11112. 10.1021/acs.jafc.8b02844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soglia F.; Petracci M.; Ertbjerg P. Novel DNPH-Based Method for Determination of Protein Carbonylation in Muscle and Meat. Food Chem. 2016, 197, 670–675. 10.1016/j.foodchem.2015.11.038. [DOI] [PubMed] [Google Scholar]
- Luo S.; Wehr N. B. Protein Carbonylation: Avoiding Pitfalls in the 2,4-Dinitrophenylhydrazine Assay. Redox Rep. 2009, 14 (4), 159–166. 10.1179/135100009X392601. [DOI] [PubMed] [Google Scholar]
- Santé-Lhoutellier V.; Engel E.; Aubry L.; Gatellier P. Effect of Animal (Lamb) Diet and Meat Storage on Myofibrillar Protein Oxidation and in Vitro Digestibility. Meat Sci. 2008, 79 (4), 777–783. 10.1016/j.meatsci.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Colombo G.; Clerici M.; Garavaglia M. E.; Giustarini D.; Rossi R.; Milzani A.; Dalle-Donne I. A Step-by-Step Protocol for Assaying Protein Carbonylation in Biological Samples. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2016, 1019, 178–190. 10.1016/j.jchromb.2015.11.052. [DOI] [PubMed] [Google Scholar]
- Semedo Tavares W. P. S.; Dong S.; Yang Y.; Zeng M.; Zhao Y. Influence of Cooking Methods on Protein Modification and in Vitro Digestibility of Hairtail (Thichiurus Lepturus) Fillets. Lwt 2018, 96 (June), 476–481. 10.1016/j.lwt.2018.06.006. [DOI] [Google Scholar]
- Berghout J. A. M.; Marmolejo-Garcia C.; Berton-Carabin C. C.; Nikiforidis C. V.; Boom R. M.; van der Goot A. J. Aqueous Fractionation Yields Chemically Stable Lupin Protein Isolates. Food Res. Int. 2015, 72, 82–90. 10.1016/j.foodres.2015.03.039. [DOI] [Google Scholar]
- Berardo A.; Claeys E.; Vossen E.; Leroy F.; De Smet S. Protein Oxidation Affects Proteolysis in a Meat Model System. Meat Sci. 2015, 106, 78–84. 10.1016/j.meatsci.2015.04.002. [DOI] [PubMed] [Google Scholar]
- Berton-Carabin C. C.; Schröder A.; Rovalino-Cordova A.; Schroën K.; Sagis L. Protein and Lipid Oxidation Affect the Viscoelasticity of Whey Protein Layers at the Oil – Water Interface. Eur. J. Lipid Sci. Technol. 2016, 118, 1630–1643. 10.1002/ejlt.201600066. [DOI] [Google Scholar]
- Berton C.; Ropers M. H.; Guibert D.; Solé V.; Genot C. Modifications of Interfacial Proteins in Oil-in-Water Emulsions Prior to and during Lipid Oxidation. J. Agric. Food Chem. 2012, 60 (35), 8659–8671. 10.1021/jf300490w. [DOI] [PubMed] [Google Scholar]
- Freitas D.; Le Feunteun S.; Panouillé M.; Souchon I. The Important Role of Salivary α-Amylase in the Gastric Digestion of Wheat Bread Starch. Food Funct. 2018, 9 (1), 200–208. 10.1039/C7FO01484H. [DOI] [PubMed] [Google Scholar]
- Luo Q.; Zhan W.; Boom R. M.; Janssen A. E. M. Interactions between Acid and Proteins under in Vitro Gastric Condition-A Theoretical and Experimental Quantification. Food Funct. 2018, 9 (10), 5283–5289. 10.1039/C8FO01033A. [DOI] [PubMed] [Google Scholar]
- Peyron M. A.; Mishellany A.; Woda A. Particle Size Distribution of Food Boluses after Mastication of Six Natural Foods. J. Dent. Res. 2004, 83 (7), 578–582. 10.1177/154405910408300713. [DOI] [PubMed] [Google Scholar]
- Casey E. J.; Laidler K. J. The Kinetics and Mechanism of the Heat Inactivation of Pepsin. J. Am. Chem. Soc. 1951, 73 (4), 1455–1457. 10.1021/ja01148a015. [DOI] [Google Scholar]
- Adler-Nissen J.Proteases. In Nagodawithana T., Reed G., Eds.; Enymes in Food Processing; Academic Press: San Diego, 1993; pp 159–203. [Google Scholar]
- Soladoye O. P.; Shand P.; Dugan M. E. R.; Gariépy C.; Aalhus J. L.; Estévez M.; Juárez M. Influence of Cooking Methods and Storage Time on Lipid and Protein Oxidation and Heterocyclic Aromatic Amines Production in Bacon. Food Res. Int. 2017, 99 (May), 660–669. 10.1016/j.foodres.2017.06.029. [DOI] [PubMed] [Google Scholar]
- Poulsen M. W.; Hedegaard R. V.; Andersen J. M.; de Courten B.; Bügel S.; Nielsen J.; Skibsted L. H.; Dragsted L. O. Advanced Glycation Endproducts in Food and Their Effects on Health. Food Chem. Toxicol. 2013, 60, 10–37. 10.1016/j.fct.2013.06.052. [DOI] [PubMed] [Google Scholar]
- Chen X.; Chen Y. P.; Wu D. W.; Wen C.; Zhou Y. M. Effects of Heat-Oxidized Soy Protein Isolate on Growth Performance and Digestive Function of Broiler Chickens at Early Age. Asian-Australas. J. Anim. Sci. 2015, 28 (4), 544–550. 10.5713/ajas.14.0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W.; Zhang C.; Hua Y. Structural Modification of Soy Protein by the Lipid Peroxidation Product Malondialdehyde. J. Sci. Food Agric. 2009, 89, 1416–1423. 10.1002/jsfa.3606. [DOI] [Google Scholar]
- Chamba M. V. M.; Hua Y.; Katiyo W. Oxidation and Structural Modification of Full-Fat and Defatted Flour Based Soy Protein Isolates Induced by Natural and Synthetic Extraction Chemicals. Food Biophys. 2014, 9 (3), 193–202. 10.1007/s11483-014-9333-8. [DOI] [Google Scholar]
- Chen N.; Zhao Q.; Sun W.; Zhao M. Effects of Malondialdehyde Modification on the in Vitro Digestibility of Soy Protein Isolate. J. Agric. Food Chem. 2013, 61 (49), 12139–12145. 10.1021/jf404099y. [DOI] [PubMed] [Google Scholar]
- Huang Y.; Hua Y.; Qiu A. Soybean Protein Aggregation Induced by Lipoxygenase Catalyzed Linoleic Acid Oxidation. Food Res. Int. 2006, 39 (2), 240–249. 10.1016/j.foodres.2005.07.012. [DOI] [Google Scholar]
- Wu W.; Zhang C.; Kong X.; Hua Y. Oxidative Modification of Soy Protein by Peroxyl Radicals. Food Chem. 2009, 116 (1), 295–301. 10.1016/j.foodchem.2009.02.049. [DOI] [Google Scholar]
- Chen N.; Zhao M.; Sun W.; Ren J.; Cui C. Effect of Oxidation on the Emulsifying Properties of Soy Protein Isolate. Food Res. Int. 2013, 52 (1), 26–32. 10.1016/j.foodres.2013.02.028. [DOI] [Google Scholar]
- Guo F.-X.; Xiong Y. L.; Qin F.; Jian H.-J.; Huang X.-L.; Chen J. Examination of the Causes of Instability of Soy Protein Isolate during Storage through Probing of the Heat-Induced Aggregation. J. Am. Oil Chem. Soc. 2015, 92 (8), 1075–1084. 10.1007/s11746-015-2684-6. [DOI] [Google Scholar]
- Li Z. L.; Shi Y.; Ding Y.; Ran Y.; Le G. Dietary Oxidized Tyrosine (O-Tyr) Stimulates TGF-B1-Induced Extracellular Matrix Production via the JNK/P38 Signaling Pathway in Rat Kidneys. Amino Acids 2017, 49 (2), 241–260. 10.1007/s00726-016-2353-6. [DOI] [PubMed] [Google Scholar]
- Ding Y. Y.; Cheng X. R.; Li Z. Q.; Wu S. J.; Yang Y.; Shi Y. H.; Le G. W. Effect of Dietary Oxidized Tyrosine Products on Insulin Secretion Via the Oxidative Stress-Induced Mitochondria Damage in Mice Pancreas. RSC Adv. 2017, 7 (43), 26809–26826. 10.1039/C7RA02945D. [DOI] [Google Scholar]
- Wang C.; Zhang X.; Wang H.; Wang J.; Guo M. Effects of amidated low methoxyl pectin on physiochemical and structural properties of polymerized whey proteins. CyTA--J. Food 2018, 16 (1), 923–930. 10.1080/19476337.2018.1508074. [DOI] [Google Scholar]
- Benjamin O.; Lassé M.; Silcock P.; Everett D. W. Effect of Pectin Adsorption on the Hydrophobic Binding Sites of β-Lactoglobulin in Solution and in Emulsion Systems. Int. Dairy J. 2012, 26 (1), 36–40. 10.1016/j.idairyj.2011.12.007. [DOI] [Google Scholar]
- Sun W.; Zhou F.; Zhao M.; Yang B.; Cui C. Physicochemical Changes of Myofibrillar Proteins during Processing of Cantonese Sausage in Relation to Their Aggregation Behaviour and in Vitro Digestibility. Food Chem. 2011, 129 (2), 472–478. 10.1016/j.foodchem.2011.04.101. [DOI] [PubMed] [Google Scholar]
- Shao Y.; Lin K.; Kao Y. Modification of Foaming Properties of Commercial Soy Protein Isolates and Concentrates By Heat Treatments. J. Food Qual. 2016, 39, 695–706. 10.1111/jfq.12241. [DOI] [Google Scholar]
- Tan Y.; Wang J.; Chen F.; Niu S.; Yu J. Effect of Protein Oxidation on Kinetics of Droplets Stability Probed by Microrheology in O/W and W/O Emulsions of Whey Protein Concentrate. Food Res. Int. 2016, 85, 259–265. 10.1016/j.foodres.2016.05.005. [DOI] [PubMed] [Google Scholar]
- Filgueras R. S.; Gatellier P.; Ferreira C.; Zambiazi R. C.; Santé-Lhoutellier V. Nutritional Value and Digestion Rate of Rhea Meat Proteins in Association with Storage and Cooking Processes. Meat Sci. 2011, 89 (1), 6–12. 10.1016/j.meatsci.2011.02.028. [DOI] [PubMed] [Google Scholar]
- Kondjoyan A.; Daudin J. D.; Santé-Lhoutellier V. Modelling of Pepsin Digestibility of Myofibrillar Proteins and of Variations Due to Heating. Food Chem. 2015, 172, 265–271. 10.1016/j.foodchem.2014.08.110. [DOI] [PubMed] [Google Scholar]
- Yang Y.; Wang Z.; Wang R.; Sui X.; Qi B.; Han F.; Li Y.; Jiang L. Secondary Structure and Subunit Composition of Soy Protein In Vitro Digested by Pepsin and Its Relation with Digestibility. BioMed Res. Int. 2016, 2016, 1–11. 10.1155/2016/5498639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opazo-Navarrete M.; Altenburg M. D.; Boom R. M.; Janssen A. E. M. The Effect of Gel Microstructure on Simulated Gastric Digestion of Protein Gels. Food Biophys. 2018, 13 (2), 124–138. 10.1007/s11483-018-9518-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekkers B. L.; Hamoen R.; Boom R. M.; van der Goot A. J. Understanding Fiber Formation in a Concentrated Soy Protein Isolate - Pectin Blend. J. Food Eng. 2018, 222, 84–92. 10.1016/j.jfoodeng.2017.11.014. [DOI] [Google Scholar]
- Opazo-Navarrete M.; Tagle Freire D.; Boom R. M.; Janssen A. E. M. The Influence of Starch and Fibre on In Vitro Protein Digestibility of Dry Fractionated Quinoa Seed (Riobamba Variety). Food Biophys. 2019, 14 (1), 49–59. 10.1007/s11483-018-9556-1. [DOI] [Google Scholar]
- Chen N.; Zhao M.; Sun W. Effect of Protein Oxidation on the in Vitro Digestibility of Soy Protein Isolate. Food Chem. 2013, 141 (1), 3224–3229. 10.1016/j.foodchem.2013.05.113. [DOI] [PubMed] [Google Scholar]
- Mouécoucou J.; Villaume C.; Sanchez C.; Méjean L. Effects of Gum Arabic, Low Methoxy Pectin and Xylan on in Vitro Digestibility of Peanut Protein. Food Res. Int. 2004, 37 (8), 777–783. 10.1016/j.foodres.2004.04.002. [DOI] [Google Scholar]
- Zhang S.; Vardhanabhuti B. Intragastric Gelation of Whey Protein-Pectin Alters the Digestibility of Whey Protein during in Vitro Pepsin Digestion. Food Funct. 2014, 5, 102–110. 10.1039/C3FO60331H. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.