Abstract
This study investigated the properties of extracts of Camellia oleifera Abel seed dregs, which were extracted using polyol compounds. Solvents employed to extract the Camellia seed dregs included water, methanol, ethanol, and polyol compounds. The examined properties included ultraviolet (UV) absorbance, total polyphenol content, total flavonoid content, and 2,2-diphenyl-1-picrylhydrazyl (DPPH) scavenging efficiency as an antioxidant ability. The results revealed that the glycerol, glycerol plus ethanol, and propylene glycol plus ethanol solvents yielded extracts with greater DPPH scavenging efficiency and total polyphenol and flavonoid content than the water, methanol, and ethanol solvents did. In addition, the polyol plus ethanol solvents yielded extracts with greater DPPH scavenging efficiency, total polyphenol and flavonoid content, and antioxidant ability than the single polyol solvents did. Furthermore, both the UV and infrared absorption spectra and color reaction test of triterpenoid glycosides revealed that polyol or polyol–ethanol solvents effectively extracted quintessential ingredients such as flavonoids and triterpenoid glycosides and other polyphenols from the Camellia seed dregs. In addition, because polyols are moisture-retaining and nontoxic solvents, the extracts can be directly added to cosmetics after simple filtration. Without the need for solvent separation, C. oleifera Abel dregs extracts exhibit excellent potential for application in products such as body washes, shampoos, hair conditioners, skin care products, cosmetics, and sunscreen lotions.
Keywords: Analytical chemistry, Materials chemistry, Natural product chemistry, Physical chemistry, Free radical scavenger, Cosmetic, Antioxidation, Extraction, Polyol, Camellia oleifera Abel dreg
1. Introduction
This study investigated the cosmetic applications of natural ingredients extracted from Camellia oleifera Abel dregs. C. oleifera Abel dregs are the remnants of C. oleifera Abel seeds after the oil has been extracted; these remnants contain approximately 8%–14% saponins. C. oleifera Abel dregs exhibit excellent foaming properties and can be used for as a hemolytic, anti-inflammatory, or antimicrobial agent. C. oleifera Abel dregs contain flavonoid and triterpenoid glycosides; the flavonoids and other phenolic compounds in these dregs exhibit free radical scavenging, anti-inflammatory, and anticancer properties [1, 2]. The extracts of C. oleifera Abel dregs can inhibit the formation of conjugated dienes and lipid peroxides, and they exhibit excellent ability in 2,2-diphenyl-1-picrylhydrazyl (DPPH) scavenging and restoration, rendering them suitable for cosmetic applications [3, 4, 5, 6, 7].
Supercritical fluid extraction (SFE) with carbon dioxide is a method for extracting pure, high-quality C. oleifera Abel seed oils with a high extraction rate. However, because of the high equipment cost of SFE, mechanical expeller pressing is the common method for extracting C. oleifera Abel seed oils [8, 9]. Because a substantial amount of dregs are trapped after pressing, hexane solvents are also used for extraction. However, the toxicity of the remaining solvents hinders further applications.
C. oleifera is rich in flavonoid glycosides, polyphenols and triterpenoid glycosides, while flavonoids and polyphenols have scavenging free radicals, anti-inflammatory and anti-cancer functions. C. oleifera extract can delay the production of conjugated diene and lipid peroxide, and has good DPPH scavenging ability and reducing power, which is very suitable for cosmetics.
However, the current extraction of bitter tea oilseed oil is mainly based on mechanical pressing method. The crushing method of bitter tea oil has a high residual oil rate. After the bitter tea oil seed extract oil, the bitter tea oil is mostly used for dish washing. It is a pity that soaps and other oil-free ingredients are not fully utilized for soapy and other applications that are less economical. Therefore, it is necessary to seek an appropriate method to extract the essential ingredients and reuse them.
Most applications of C. oleifera Abel dregs are in products with low economic value, such as dishwashing detergents and soaps, but the quintessence of C. oleifera Abel dregs has not been fully utilized. Studies have investigated the extraction of the quintessence of C. oleifera Abel dregs using solvents such as water vapor, methanol, ethanol, propanol, and other low-carbon primary alcohols [10, 11]. However, these solvents, except for water vapor, require separation and purification before use, resulting in complex processing and increased production costs. Methanol provides the optimal extractability among the aforementioned solvents, but it requires complete separation and purification because of its high toxicity.
Although research has been conducted to extract the essential components of bitter tea oil by using low-carbon number alcohols such as water vapor, methanol, ethanol, and propanol as solvents, these solvents are used, except for water vapor, which must be separated and purified. After the solvent can be used, the procedure is complicated and the cost is increased. Among them, methanol should be used as the solvent to extract the best. However, methanol is toxic to the human body and cannot be directly used. It must be completely separated and purified, which will increase the cost.
The goal of this study was to extract the remaining essence of bitter tea oil using a polyol that has always been used as a moisturizer in cosmetics, and to use it directly by simple filtration. In the study, the effects of various polyol extracts were investigated and compared with water, methanol and ethanol to confirm the multiple benefits of using polyol as a solvent for extracting bitter tea. The results revealed that polyols easily extracted the quintessential ingredients of the dregs including flavonoid and triterpenoid glycosides and other phenolic compounds. In addition, because polyols are moisture-retaining and nontoxic solvents, the extracts can be directly added to cosmetics after simple filtration. Without the need for solvent separation, C. oleifera Abel dregs have excellent application potential in cosmetic products.
2. Experimental
The bitter tea oil sputum material of this study was taken from the bitter tea oil simmered by the bitter tea oil farmer after mechanical cold pressing, and then ground into a fine powder by a sickle, and then extracted with various solvents. The solvents used for extracting C. oleifera Abel dregs and their abbreviated names are listed as follows: water (W), methanol (M), ethanol (E), propylene glycol (PG), butylene glycol (BG), glycerol (G), and mixtures of them.
2.1. Extraction of C. oleifera Abel dregs
The solid/liquid ratio currently used in this study is: bitter tea oil powder (g)/solvent (g) = 1/3, the solution after extraction and filtration is a stock solution, and various properties are tested. The aforementioned solid/liquid ratio can be adjusted.
Table 1 shows the content ratio of each solvent used for extracting C. oleifera Abel dregs. The extraction steps are listed as follows (using No. 1 extract in Table 1 as an example):
-
(1)
Grind the mechanically pressed cake of C. oleifera Abel dregs to powder by using a file.
-
(2)
Take 30 g of the aforementioned powder, E-PG-12, a solvent mixture containing 30 g of E and 60 g of PG is added. First soaked in solvent (E-PG-12) for 12 h and then subjected to microwave extraction thrice for 30 s at 5-minute intervals.
-
(3)
The mixture is cooled to room temperature and then filtered. The filtrate is then stored in the freezer for later use.
Table 1.
Experimental ratios for extracting C. oleifera Abel dregs. C. oleifera Abel dregs were filtered with the solvents after saturating them for 12 h (some were microwaved three times before filtering; 30 s each heating with a 5-min interval between heating).
| No. | C. oleifera Abel dregs (g) | Water (W) (g) | Methanol (M) (g) | Ethanol (E) (g) | Propylene glycol (PG) (g) | Butylene glycol (BG) (g) | Glycerol (G) (g) |
|---|---|---|---|---|---|---|---|
| 1 | 30 | 30 | 60 | ||||
| 2 | 30 | 30 | 60 | ||||
| 3 | 30 | 30 | 60 | ||||
| 4 | 30 | 90 | |||||
| 5 | 30 | 90 | |||||
| 6 | 30 | 90 | |||||
| 7 | 30 | 90 | |||||
| 8 | 30 | 90 | |||||
| 9 | 30 | 90 | |||||
| 10 | 30 | 45 | 45 | ||||
| 11 | 30 | 30 | 30 | 30 |
2.2. Determining the content of extracts of C. oleifera Abel dregs
-
(1)
Determine the extractive content by conducting a functional group analysis through Fourier transform infrared (FTIR) spectroscopy.
-
(2)
Determine the light absorbance of the extracts by using ultraviolet–visible (UV–Vis) spectroscopy.
Fourier transform infrared (FTIR) spectra were recorded in the range 4000–650 cm−1 using a Japan Spectroscopic FT/IR-3 spectrophotometer. Each test compound was spread as a thin layer on a KBr tablet and then 32 scans were collected at a resolution of 4 cm−1. A series of test solutions with various solvents were prepared and mixed using a solvent solution (1:100 ratio) before using the Shimadzu UV-240 spectrophotometer to measure the UV absorption of the solution.
2.3. Determining the ability of the extracts on scavenging DPPH free radicals
-
(1)
The extraction method of C. oleifera Abel dregs is listed on the embodiment of this study. The solvents were methanol (M), ethanol (E), propylene glycol (P), ethanol plus glycerol (E + G; E:G = 1:2), and glycerol (G).
-
(2)
Weigh 0.06 g of the extracts. Add 10 mL of methanol solvent as a diluent. Add 1 mL of the diluent to a formulated solution with 0.02 mM DDPH and 1 mL of methanol solution, and then stir the mixture. React for 10, 20, 30, 60, 90, and 120 min. Measure the light absorbance at 517 nm. The extract of 0.06 g refers to the aforementioned bitter tea oil powder (g)/solvent (g) = 1/3, and the extracted stock solution after extraction and filtration is not dried and directly tested.
-
(3)
Add 1 mL of methanol to 1 mL of the sample (diluent) as a sample blank.
-
(4)
Add 1 mL of methanol to the 1 mL methanol solution containing 0.02 mM DPPH as the control group.
where
A is the absorbance of the sample at 517 nm wavelength.
B is the absorbance of the control group at 517 nm wavelength.
C is the absorbance of the sample blank at 517 nm wavelength.
2.4. Color reaction test of triterpenoid glycosides
Using the Liberman–Burchard reaction [12]:
-
(1)
Weigh 0.5 g of each extract and heat until the solvents evaporate. Dissolve with glacial acetic acid.
-
(2)
Add 1 mL of acetic anhydride and 0.2 mL of concentrated sulfuric acid.
-
(3)
If the color turns pink, red or purple this indicates the presence of triterpenoid glycoside. For this experiment, methanol, ethanol, propylene glycol, ethanol plus glycerol, and glycerol served as the solvent extracts.
2.5. Determining the total polyphenol contents
We adopted the methodology of Christel et al. [13] to determine the total polyphenol content (TPC) of the extracts.
-
(1)
Mix the extracted samples with methanol to an appropriate concentration, and add 0.1 mL of the sample solution to a test tube. Dilute 0.5 mL of the Folin–Ciocalteu reagent (FCR) 10x with pure water. Set for 5 min after mixing. Add 0.4 mL of 0.7M Na2CO3 and set for 45 min after mixing. Finally, measure the light absorbance of the mixtures at 675 nm.
-
(2)
Use gallic acid as the standard calibration curve and obtain the TPC of the samples through interpolation.
-
(3)
Blank: 0.1 mL of methanol + 0.5 mL of FCR + 0.4 mL of Na2CO3
-
(4)
Sample: 0.1 mL of sample + 0.5 mL of FCR + 0.4 mL of Na2CO3
-
(5)
Calibration curve: 0.5 mL of gallic acid + 0.5 mL of FCR + 0.4 mL of Na2CO3
2.6. Determining the total flavonoid contents
We adopted the methodology of Christel et al. [13] to determine the total flavonoid content (TFC) of the extracts.
-
(1)
Mix the extracted samples with methanol to an appropriate concentration, and add 1 mL of methanol sample solution to 1 mL of 2% methanol AlCl3•6H2O solution. Set for 10 min at room temperature and measure the light absorbance of the mixtures at 430 nm.
-
(2)
Use quercetin (Sigma–Aldrich) as the standard calibration curve and obtain the TFC of the samples through interpolation.
3. Results and discussion
3.1. Appearance of the extracts
Fig. 1 shows the appearance of the extract after immersion in solvent and the filtrate after microwave extraction. The extract dissolved by glycerol (G) exhibited the darkest color, with thick and pasty solid matter; the extracted supernatant also showed the deepest color. The supernatant extracted from the propylene glycol (PG) and butylene glycol (BG) solvents exhibited a light yellow color; that dissolved by methanol (M) and ethanol (E) had the lightest colors among all the extraction liquids in Fig. 1.
Fig. 1.
Filtrates of C. oleifera Abel dregs extract by microwave-heated.
3.2. FTIR spectroscopy of the extracts
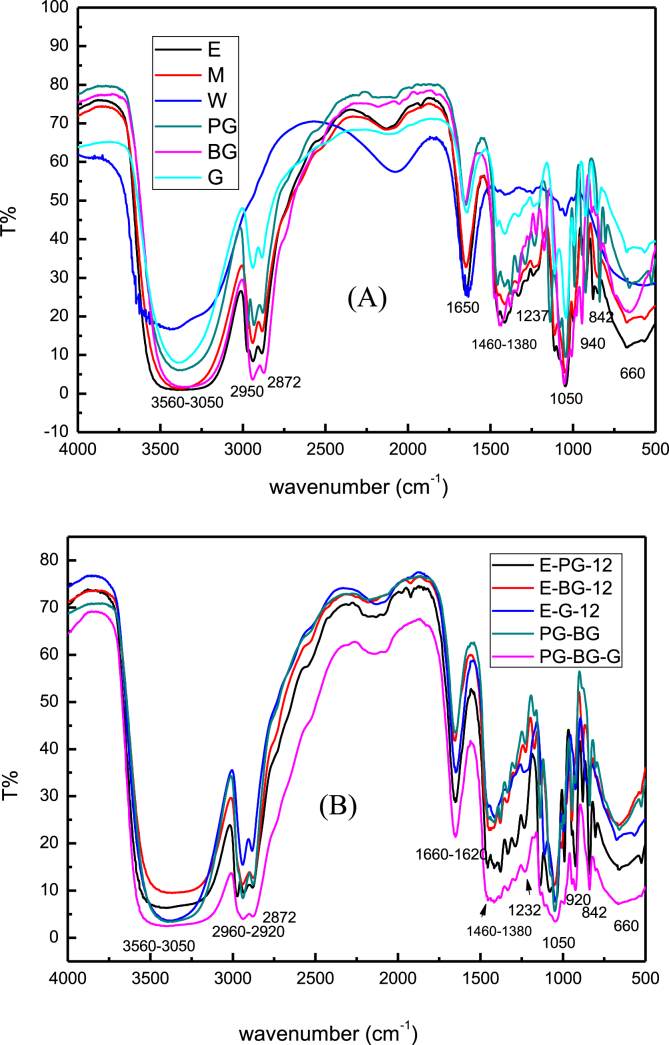
Fig. 2 illustrates the FTIR spectrums of the extracts of C. oleifera Abel dregs. Fig. 2A shows the extracts dissolved by mixed solvents under room temperature, whereas Fig. 2B shows the extracts dissolved by single solvents under room temperature. The triterpenoid glycosides absorbed hydroxyl group at 3350 cm−1,C–H bonds off C=C double bonds at 3050–3080 cm−1, terminal double bonds at 1630–1660 and 840–850 cm−1, C=C double bonds at 900–940cm−1, C–O bonds at 1045 and 1020 cm−1, (C–H) bonds at 3050 and 2920–2960 cm−1, (–CH2) at 1450 cm−1, and (–CH3) at 1380cm−1. These results revealed that the triterpenoid glycosides were unsaturated hydrocarbons. The polyphenols absorbed hydroxyl group at 3500–3000cm−1, benzene rings at 1610, 1550, 1460, and 800–900 cm−1, and C–O bonds at 1050and 1237cm−1. The flavonoid glycosides absorbed benzene rings at 3050 cm−1, C=O bonds at 1620–1680 cm−1, and C=C double bonds at 900–940cm−1 [14, 15, 16]. These results revealed that polyol solvents in this invention effectively extracted the quintessential ingredients of C. oleifera Abel dregs for applications. These extracts exhibited free radical scavenging, anti-inflammatory, and anticancer properties, indicating their high suitability for application in skin and hair care products.
Fig. 2.
FTIR spectrums of C. oleifera Abel dregs extracts. (A) Extract by single solvents at room temperature, and (B) extract by mixed solvents at room temperature.
3.3. UV–Vis absorbance spectrum of the extracts
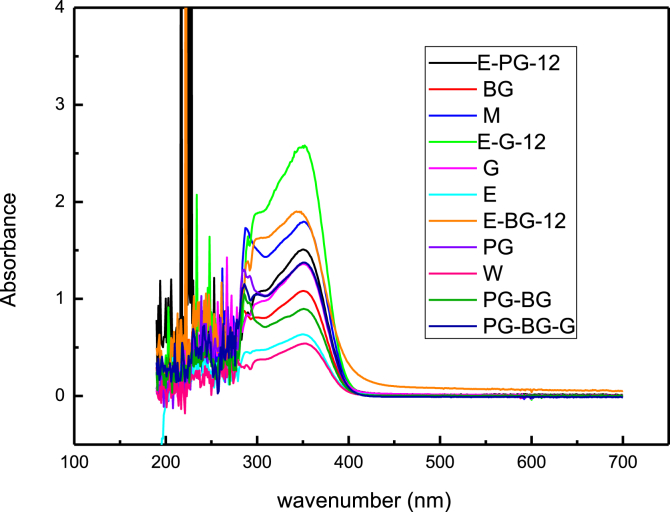
There are two main ways of sunscreen. One is to use the reflection or refraction of powder particles to block the ultraviolet rays from entering the skin and causing damage, such as TiO2 and ZnO inorganic powders. The other is to absorb UVA and UVB by using organic compounds. Ability to absorb the energy of ultraviolet light and release it with lower energy to achieve the purpose of protecting the skin from UV rays. The extract of this study has been proven to have UV absorption capacity, so it can be used to make sunscreen. The amount of inorganic physical sunscreen and organic compound sunscreen can be reduced. Commercially available sunscreen products are usually combined with the aforementioned organic and inorganic sunscreens, and have a synergistic sunscreen effect.
Fig. 3 shows the UV–Vis absorbance spectrum of the C. oleifera Abel dregs extracts that had undergone room temperature extraction and then diluted 100 times. The triterpenoid glycosides absorbed UV at 200–240 nm, the polyphenols absorbed UV at 254, 280, and 329 nm, and the flavonoid glycosides absorbed a substantial amount of UV at 230–400 nm. These results revealed that the polyol solvents in this experiment effectively extracted the quintessential ingredients of C. oleifera Abel dregs. The extracts in this study exhibited strong UVA and UVB absorbance, rendering them ideal for application in sunscreens [17, 18, 19, 20].
Fig. 3.
UV–Vis absorbance spectrum of C. oleifera Abel dregs extracts at room temperature extracted and at 100x dilution.
3.4. DPPH scavenging efficiency of the extracts
The antioxidant activities of phenolics are related to a number of different mechanisms, such as free radical-scavenging, hydrogen-donation, singlet oxygen quenching, metal ion chelation, and acting as a substrate for radicals such as superoxide and hydroxyl. A direct relationship has been found between the content of total phenolics and antioxidant capacity of plants [21]. The DPPH radical inhibition assay is a widely used and comparably facile method to evaluate antioxidant activity. DPPH, a stable free radical, produces a violet solution in ethanol. It is reduced and decolorized in the presence of the antioxidant molecule, and is often used in evaluating the radical scavenging activity of antioxidants such as natural and synthetic pure compounds as well as plant extracts [22].
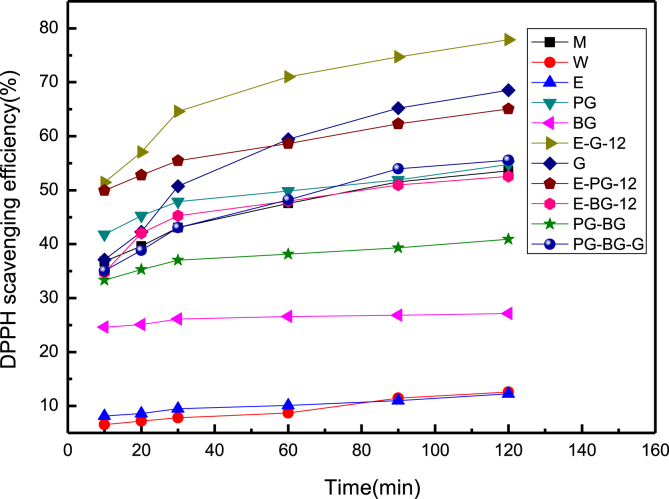
Fig. 4 shows the DPPH scavenging efficiency of the C. oleifera Abel dregs extracts. The results are listed as follows: (1) The extracts of C. oleifera Abel dregs dissolved with all types of polyol solvents exhibited DPPH scavenging efficiency, and greater antioxidant ability than those dissolved with ethanol solvents. (2) The extracts of C. oleifera Abel dregs dissolved by both of ethanol plus glycerol and pure glycerol solvents exhibited DPPH scavenging efficiency, and greater antioxidant ability than those dissolved by methanol solvents. (3) The extract of C. oleifera Abel dregs dissolved by ethanol plus glycerol solvent exhibited the highest DPPH scavenging efficiency and greater antioxidant ability than those dissolved by methanol solvents. Polysaccharides from Codonopsis pilosula were prepared. The polysaccharides with different molecular weights could have reducing power and antioxidant activities, namely removing hydroxyl radicals and DPPH free radicals. Their antioxidant activities increased with the increase of molecular weight, and the concentrations were positively correlated with activities. It was suggested that the antioxidant activity of polysaccharide by sonicate was higher than that by water extraction. The removal ability of polysaccharides to DPPH free radicals, it showed the scavenging effect to DPPH was up to 63.91% [23].
Fig. 4.
DPPH scavenging efficiency of C. oleifera Abel dregs extracts at microwave-heating extracted.
3.5. The triterpenoid glycoside color reaction experiment
Table 2 shows the triterpenoid glycosides (saponins) color reaction experiment. The solvents used in this experiment consisted of water (W), ethanol (E), propylene glycol (PG), ethanol plus glycerol (E + G; E:G = 1:2), and glycerol (G). The pink, red, and purple colors indicated triterpenoid glycosides, whereas blue and cyan colors indicated steroidal saponins. The experimental results revealed that the triterpenoid glycosides were present in the extracts of C. oleifera Abel dregs dissolved by polyols, ethanol, and water.
Table 2.
Results of the triterpenoid glycoside (saponin) color reaction experiment.
| Solvent | Pink | Red | Purple | Blue | Cyan |
|---|---|---|---|---|---|
| W | + | - | - | - | |
| M | + | - | - | - | |
| E | + | - | - | - | |
| PG | + | - | - | - | |
| BG | + | - | - | - | |
| G | + | - | - | - | |
| E-PG-12 | + | - | - | - | |
| E-BG-12 | + | - | - | - |
3.6. The total polyphenol and flavonoid contents of the extracts
Previous studies reported that phenols are one of the major plant compounds with antioxidant activity. The antioxidant activity of phenolics and polyphenolics compounds is reported to be mainly due to their redox properties, which can play an important role in adsorbing and neutralizing free radicals, quenching singlet and triplet oxygen or decomposing peroxides.
Table 3 shows the total polyphenol and flavonoid contents of the extracts of C. oleifera Abel dregs. The solvents are arranged in descending order by extraction efficiency, as follows: glycerol plus ethanol, glycerol, propylene glycol plus ethanol, butylene glycol plus ethanol, methanol, propylene glycol, butylene glycol, ethanol, and water. Therefore, polyol–ethanol mixture solvents exhibited the optimal extraction efficiency (greater than single methanol or ethanol solvents) because the relatively small ethanol molecules could swell the C. oleifera Abel dregs by infiltrating the tissue then polyol can easily extracting the triterpenoid glycosides, polyphenols, and flavonoids.
Table 3.
TPC and TFC content of extracts by extract at room temperatures or microwave-heating.
| Solvent | TPC (mg/g) (extract at room temperature) | TPC (mg/g) (extract at microwave) | TFC (mg/g) (extract at room temperature) | TFC (mg/g) (extract at microwave) |
|---|---|---|---|---|
| W | 42.18 ± 0.16 | 65.33 ± 0.05 | 2.36 ± 0.08 | 7.52 ± 0.12 |
| M | 99.19 ± 0.05 | 122.24 ± 0.13 | 11.88 ± 0.02 | 17.14 ± 0.02 |
| E | 52.21 ± 0.04 | 75.36 ± 0.11 | 3.05 ± 0.15 | 8.23 ± 0.06 |
| PG | 95.36 ± 0.13 | 118.41 ± 0.17 | 13.86 ± 0.16 | 19.12 ± 0.01 |
| BG | 78.17 ± 0.19 | 101.22 ± 0.09 | 11.65 ± 0.07 | 16.75 ± 0.06 |
| G | 126.08 ± 0.05 | 149.23 ± 0.12 | 18.17 ± 0.05 | 23.33 ± 0.12 |
| E + PG | 120.57 ± 0.06 | 143.62 ± 0.01 | 16.27 ± 0.08 | 21.45 ± 0.16 |
| E + BG | 118.08 ± 0.11 | 141.23 ± 0.03 | 15.72 ± 0.12 | 20.86 ± 0.10 |
| E + G | 133.11 ± 0.07 | 156.16 ± 0.18 | 21.51 ± 0.05 | 26.65 ± 0.05 |
| PG + BG | 83.06 ± 0.06 | 106.23 ± 0.05 | 12.41 ± 0.06 | 17.56 ± 0.19 |
| PG + BG + G | 114.23 ± 0.03 | 137.21 ± 0.04 | 14.98 ± 0.11 | 20.11 ± 0.04 |
The experimental results in the total polyphenol and flavonoid contents are match the results shown in Fig. 3. The triterpenoid glycosides absorbed a substantial amount of UV at 200–240 nm, as did the polyphenols at 254, 280, and 329 nm, and the flavonoid glycosides at 230–400 nm [24]. These results indicate that polyol solvents are effective for extracting the quintessential ingredients of C. oleifera Abel dregs.
4. Conclusion
The polyol solvents effectively extracted the flavonoids and triterpenoid glycosides and polyphenols from C. oleifera Abel dregs for extensive applications. The polyol–ethanol solvents yielded extracts that exhibit DPPH scavenging efficiency, and these extracts exhibited greater antioxidant ability than those extracted by methanol or ethanol solvents. The glycerol–ethanol solvent yielded extracts with the highest DPPH scavenging efficiency and greater antioxidant than the single glycerol or ethanol solvent did, only using ethanol solvent revealed a very poor antioxidant activity. The extracts dissolved with polyol solvent exhibited high UV absorbance. The triterpenoid glycosides absorbed a substantial amount of UV at 200–240 nm, as did the polyphenols at 254, 280, and 329 nm, and the flavonoid glycosides at 230–400 nm. These ingredients are suitable for application in sunscreen products to cover the UVA and UVB. The extracts dissolved with polyol solvents can enhance the antioxidant, sunscreen, and moisture-retaining abilities of care products such as shampoos, hair conditioners, and cosmetics. Moreover, polyol solvents extraction could be a reliable step for obtaining C. oleifera Abel dregs extracts with acceptable yields of important phytochemical compounds although some further optimization of extraction procedures is still required. Further studies on these extracts with respect to antioxidant properties in vivo are needed.
Declarations
Author contribution statement
Chun-En Tsai: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data.
Li-Huei Lin: Analyzed and interpreted the data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Li C.C., Lin L.H., Lee H.T., Tsai J.R. Avobenzone encapsulated in modified dextrin for improved UV protection and reduced skin penetration. Chem. Pap. 2016;70:840–847. [Google Scholar]
- 2.Bondet V., Brand-Williams W., Berset C. Kinetics and mechanisms of antioxidant activity using the DPPH free radical method. J. Food Sci. Technol. 1997;30:609–615. [Google Scholar]
- 3.Yin H.W., Liv S.Y., Chen Z.F. Identification, extraction and utilization of Camellia oil, tea seed oil and Camellia. J. Special News For. Res. 2010;17:4–10. [Google Scholar]
- 4.Yen G.C., Chen H.Y. Antioxidant activity of various tea extracts in relation to their anti- mutagenicity. J. Agric. Food Chem. 1995;43:27–32. [Google Scholar]
- 5.Lee C.P., Yen G.C. Antioxidant activity and bioactive compounds of tea seed (Camellia oleifera Abel.) oil. J. Agric. Food Chem. 2006;54:779–784. doi: 10.1021/jf052325a. [DOI] [PubMed] [Google Scholar]
- 6.Zhu X.Y., Lin H.M., Chen X., Xie J., Wang P. Mechanochemical-assisted extraction and antioxidant activities of kaempferol glycosides from Camellia oleifera Abel. meal. J. Agric. Food Chem. 2011;59:3986–3993. doi: 10.1021/jf1042689. [DOI] [PubMed] [Google Scholar]
- 7.Jia Z., Tang M., Wu J. The determination of flavonoid content in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64:555–559. [Google Scholar]
- 8.Cheng Y.T., Wu S.L., Ho C.Y., Huang S.M., Cheng C.L., Yen G.C. Beneficial effects of Camellia oil (Camellia oleifera Abel.) on ketoprofen-induced gastrointestinal mucosal damage through upregulation of HO-1 and VEGF. J. Agric. Food Chem. 2014;62:642–650. doi: 10.1021/jf404614k. [DOI] [PubMed] [Google Scholar]
- 9.Ma C.J., Huang Q., Wu D.H., Zhu D.J., Huang C.S., Xiang Y.P. Process Technology: Food Science. Vol. 29. Institute of Food Science, Jishou University; 2008. Study on extraction technologies of tea seed oil by microwave and supercritical CO2; pp. 281–285. [Google Scholar]
- 10.Sun B.X., Qiul H., Deng J., Hu H.C. 2011. Ceramide-containing tea Seed Extract and Preparation Method Thereof. Chinese Patent, CN102058727A. [Google Scholar]
- 11.Tan J.K., Li G.Q., Luo P., Lu H.N., Ban C.J., Su G.F. 2013. Method for Comprehensively Extracting Saponin, Polysaccharides and Polyphenol from Camellia Oleifera Abel Defatted Cakes. Chinese Patent, CN102993329A. [Google Scholar]
- 12.Ye M.H. 2010. Isolation and Identification Potentilla Chinensis Active Fractions in Anti-oxidation and Inhibition of Lipoxygenase. Master's Degree Paper of Yilan University. [Google Scholar]
- 13.Christel Q.D., Bernard G., Jacques V., Thierry D., Claude B., Michel L., Micheline C., Jean- Claude C., François B., Francis T. Phenolic compounds and antioxidant activities of buckwheat (Fagopyrum esculentum Moench) hulls and flour. J. Ethnopharmacol. 2000;72:35–42. doi: 10.1016/s0378-8741(00)00196-3. [DOI] [PubMed] [Google Scholar]
- 14.Xie M.X. Study of the composition of the Maoshan grape plant for the ophthalmology as a folk health medicinal. J. Health Manag. 2013;11:148–155. Yuanpei University. [Google Scholar]
- 15.Michelle R.L., Anna B., Barry A.P., Mary H., David H., Ann M.H. Identification of green tea (Camellia sinensis L.) and tea oil (Camellia oleifera Abel.) by molecular, biological, and anatomical methods. Authentication Food Wine. 2007:290–304. Chapter 19. [Google Scholar]
- 16.Tsai C., Lin L.H., Wu C.S., Kwan C.C. Surface properties of lithospermum-containing multiple phase emulsion systems. J. Appl. Polym. Sci. 2010;117:1041–1046. [Google Scholar]
- 17.Lin L.H., Lai Y.C., Chen K.M., Chang H.M. Oxyethylene chain length affects the physicochemical properties of sugar-based anionic surfactants with phosphates groups. Colloid. Surf. Physicochem. Eng. Asp. 2015;485:118–124. [Google Scholar]
- 18.Lin L.H., Lai Y.C., Chen K.M., Li C.S. Preparation and surface activities of modified soy protein–dextrin surfactants. J. Surfactants Deterg. 2016;19:19–28. [Google Scholar]
- 19.Lin L.H., Chou Y.S. Surface activity and emulsification properties of hydrophobically modified dextrins. Colloids Surf., A. 2010;364:55–60. [Google Scholar]
- 20.Tsai C., Lin L.H., Kwan C.C. Surface properties and morphologies of pheohydrane/liquid crystal moisturizer product. Int. J. Cosmet. Sci. 2010;32:258–265. doi: 10.1111/j.1468-2494.2010.00537.x. [DOI] [PubMed] [Google Scholar]
- 21.Barreira J.C.M., Ferreira I.C.F.R., Oliveira M.B.P.P., Pereira J.A. Food Chem. 2008;107:1106–1113. [Google Scholar]
- 22.Suh H.J., Kim S.R., Lee K.S., Park S., Kang S.C. J. Photochem. Photobiol. B Biol. 2010;99:67–73. doi: 10.1016/j.jphotobiol.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 23.Liu Y., Sun Y., Huang G. Preparation and antioxidant activities of important traditional plant polysaccharides. Int. J. Biol. Macromol. 2018;111:780–786. doi: 10.1016/j.ijbiomac.2018.01.086. [DOI] [PubMed] [Google Scholar]
- 24.Xu S.Z., Wang S.Z., Guo Y.H., Weng Z.Z., Jiang H.Y. Antioxidant activity and phenolic compounds content in Brassica. J. Far East Univ. 2014;31:159–168. [Google Scholar]