Single missense mutations in a conserved motif of PP2C.D proteins abolish SAUR binding, leading to constitutive phosphatase activity that restricts cell expansion.
Abstract
The phytohormone auxin promotes the growth of plant shoots by stimulating cell expansion via plasma membrane (PM) H+-ATPase activation, which facilitates cell wall loosening and solute uptake. Mechanistic insight was recently obtained by demonstrating that auxin-induced SMALL AUXIN UP RNA (SAUR) proteins inhibit D-CLADE TYPE 2C PROTEIN PHOSPHATASE (PP2C.D) activity, thereby trapping PM H+-ATPases in the phosphorylated, activated state, but how SAURs bind PP2C.D proteins and inhibit their activity is unknown. Here, we identified a highly conserved motif near the C-terminal region of the PP2C.D catalytic domain that is required for SAUR binding in Arabidopsis (Arabidopsis thaliana). Missense mutations in this motif abolished SAUR binding but had no apparent effect on catalytic activity. Consequently, mutant PP2C.D proteins that could not bind SAURs exhibited constitutive activity, as they were immune to SAUR inhibition. In planta expression of SAUR-immune pp2c.d2 or pp2c.d5 derivatives conferred severe cell expansion defects and corresponding constitutively low levels of PM H+-ATPase phosphorylation. These growth defects were not alleviated by either auxin treatment or 35S:StrepII-SAUR19 coexpression. In contrast, a PM H+-ATPase gain-of-function mutation that results in a constitutively active H+ pump partially suppressed SAUR-immune pp2c.d5 phenotypes, demonstrating that impaired PM H+-ATPase function is largely responsible for the reduced growth of the SAUR-immune pp2c.d5 mutant. Together, these findings provide crucial genetic support for SAUR-PP2C.D regulation of cell expansion via modulation of PM H+-ATPase activity. Furthermore, SAUR-immune pp2c.d derivatives provide new genetic tools for elucidating SAUR and PP2C.D functions and manipulating plant organ growth.
The phytohormone auxin coordinates plant growth and development by regulating the fundamental processes of cell division, expansion, and differentiation (Strader and Zhao, 2016; Zhao, 2018). Plants perceive the auxin signal through a coreceptor complex comprising TRANSPORT INHIBITOR RESPONSE1/AUXIN SIGNALING F-BOX PROTEINS (TIR1/AFBs) and AUXIN/INDOLE-3-ACETIC ACID (AUX/IAA) transcriptional repressors in the nucleus, which upon auxin binding leads to AUX/IAA ubiquitylation by the SCFTIR1/AFB complex and subsequent degradation by the 26S proteasome (Gray et al., 2001; Dharmasiri et al., 2005; Kepinski and Leyser, 2005). Aux/IAA degradation results in the derepression of AUXIN RESPONSE FACTOR transcription factors, leading to changes in auxin-responsive gene expression that subsequently direct auxin-induced physiological and developmental responses (Lavy and Estelle, 2016). Early auxin-responsive genes are induced within minutes, including AUX/IAAs, GRETCHEN HAGEN3s (GH3s), and SMALL AUXIN UP RNAs (SAURs; Hagen and Guilfoyle, 2002). While induction of AUX/IAA and GH3 expression attenuates auxin signaling through feedback regulation (Lavy and Estelle, 2016), up-regulation of SAUR expression has been proposed to promote cell expansion leading to organ growth (Spartz et al., 2012, 2014).
In Arabidopsis (Arabidopsis thaliana), the plant-specific SAUR gene family consists of 81 members (including two pseudogenes), which share high sequence similarity. Most, but not all, SAUR genes are auxin inducible (Ren and Gray, 2015). SAUR expression is also controlled through mRNA instability and posttranslational control through the ubiquitin-26S proteasome pathway (Gil and Green, 1996; Knauss et al., 2003; Chae et al., 2012; Spartz et al., 2012; Vi et al., 2013), although the regulation of these aspects of SAUR expression is poorly understood. In many cases, the rapid degradation of SAUR proteins can be overcome with the addition of GFP or epitope tags, which partially stabilize the proteins (Chae et al., 2012; Spartz et al., 2012). This strategy has enabled gain-of-function genetic approaches to elucidate SAUR function. Arabidopsis plants expressing stabilized SAUR19 or SAUR63 fusion proteins display increased cell expansion phenotypes, suggesting that these SAURs promote cell expansion. For example, 35S:GFP-SAUR19 plants display increased hypocotyl length and leaf size, while PSAUR63:SAUR63:GFP plants exhibit long hypocotyl, petal, and stamen filament phenotypes (Franklin et al., 2011; Chae et al., 2012; Spartz et al., 2012).
Recent findings suggest that SAURs activate plasma membrane (PM) H+-ATPases to promote cell expansion via an acid growth mechanism. Based on physiological studies correlating auxin-induced expansion with apoplastic acidification, the acid growth theory was first proposed in 1970 (Rayle and Cleland, 1970, 1980, 1992; Hager, 2003). H+ pump activation reduces apoplastic pH, which activates expansins and cell wall modification enzymes to enable cell expansion (McQueen-Mason et al., 1992; Cosgrove, 2016). Additionally, increased PM H+-ATPase activity hyperpolarizes the PM, leading to solute and water uptake and increased turgor that drives cell expansion. Takahashi et al. (2012) provided mechanistic insight into this process by demonstrating auxin-induced phosphorylation of the penultimate Thr within the autoinhibitory domain of PM H+-ATPases. Phosphorylation of this residue (corresponding to Thr-947 of ARABIDOPSIS H+ ATPASE [AHA2]) promotes 14-3-3 protein binding and H+ pump activation (Fuglsang et al., 1999; Kinoshita and Shimazaki, 1999; Jelich-Ottmann et al., 2001). In addition to increased cell expansion phenotypes, 35S:GFP-SAUR19 seedlings also exhibit increased PM H+-ATPase activity and Thr-947 phosphorylation (Spartz et al., 2014). SAUR proteins promote PM H+-ATPase activation by inhibiting D-CLADE TYPE 2C PROTEIN PHOSPHATASES (PP2C.Ds), which can directly dephosphorylate Thr-947 of PM H+-ATPases (Spartz et al., 2014, 2017). This suggests a model whereby auxin induction of SAUR expression leads to a reduction in PP2C.D activity, thus leading to increased PM H+-ATPase phosphorylation, which drives cell expansion. Consistent with this model, plants constitutively overexpressing GFP-SAUR19 exhibit reduced apoplastic pH and auxin-independent elongation growth (Spartz et al., 2014, 2017; Fendrych et al., 2016; Barbez et al., 2017).
The Arabidopsis genome encodes nine PP2C.D members, which belong to the Mg2+/Mn2+-dependent PP2C family of protein phosphatases (Fuchs et al., 2013). Individual members display unique subcellular localizations, however, suggesting functional specificity (Tovar-Mendez et al., 2014; Ren et al., 2018). Notably, like SAUR19 and PM H+-ATPases, PP2C.D2, PP2C.D5, and PP2C.D6 localize exclusively to the PM. We recently demonstrated that the pp2c.d2/5/6 triple mutant phenocopies 35S:GFP-SAUR19 gain-of-function plants in a variety of growth assays and in biochemical assays examining PM H+-ATPase Thr-947 phosphorylation status (Ren et al., 2018). Together with our biochemical findings that SAURs inhibit PP2C.D enzymatic activity, these findings provide strong evidence supporting the hypothesis that SAUR and PP2C.D proteins function antagonistically to regulate PM H+-ATPase activity and expansion growth. Missing from this model, however, is strong genetic support derived from saur loss-of-function mutants. Recently, saur16/50 double mutants were shown to exhibit modest organ-specific cell expansion defects (Sun et al., 2016). However, these mutant phenotypes were dramatically weaker than those conferred by PP2C.D overexpression (Spartz et al., 2014; Ren et al., 2018). While this difference could be due to ectopic PP2C.D expression, given the large number of SAUR genes in plant genomes, it seems likely that extensive functional redundancy exists within the large SAUR gene family. Given the plethora of SAUR genes in Arabidopsis and other plants, we investigated the possibility that the SAUR binding and phosphatase activities of PP2C.D proteins were genetically separable. If so, pp2c.d mutants that cannot bind SAURs yet retain phosphatase activity may be able to serve as genetic proxies for saur loss-of-function plants. Here, we identify a highly conserved, unique motif near the C terminus of the catalytic domain of PP2C.D proteins that is essential for SAUR binding. We find that single missense mutations within this motif abolish SAUR binding and inhibition, resulting in phosphatases with constitutive enzymatic activity. Our findings demonstrate that SAUR-immune pp2c.d2 and pp2c.d5 derivatives constitutively dephosphorylate and inhibit PM H+-ATPases, thus restricting plant cell elongation and organ growth.
RESULTS
PP2C.D1 Deletion Analysis
To begin to investigate the SAUR-binding determinants of PP2C.D phosphatases, we conducted a deletion analysis of PP2C.D1 and assessed binding activity in yeast two-hybrid assays. We chose PP2C.D1 as it exhibits the most robust interaction with SAUR19 in this system. PP2C.D phosphatases are composed primarily of the core PP2C catalytic domain (amino acids 41–342 of PP2C.D1) with short N- and C-terminal extensions. PP2C.D1 containing a deletion of the C-terminal 26 amino acids (Δ345-370) retained the ability to interact with SAUR19. Longer C-terminal deletions (Δ332-370 and Δ292-370), however, abolished SAUR binding (Supplemental Fig. S1A). Expression of these truncated proteins was confirmed by immunoblot analysis (Supplemental Fig. S1B). Likewise, a short N-terminal deletion (Δ1-36) retained SAUR-binding activity, but the longer Δ1-116 mutant derivative did not. In this case however, we were unable to detect expression of the Δ1-116 mutant derivative, so it remains unclear whether this region is involved in SAUR binding. We also tested a deletion within the catalytic domain (Δ227-255) that had no effect on SAUR-binding activity (Supplemental Fig. S1, A and C).
Identification of PP2C.D Missense Mutations That Prevent SAUR Binding
Our deletion studies indicated that the C-terminal region of PP2C.D1 was required for SAUR binding. However, since we could not conclusively eliminate other regions, we generated a library of pp2c.d1 missense mutants using error-prone PCR and tested interactions with SAUR19 in a reverse yeast two-hybrid screen. Upon screening ∼1,500 library clones, 56 candidates were identified that abolished interaction with SAUR19 and that also passed retesting following plasmid rescue and retransformation and immunoblot analysis to verify expression. Sequence analysis revealed that 19 of the 56 candidates contained single missense mutations within the PP2C.D1 coding sequence (Fig. 1A). The remaining candidates contained two or more mutations or rearrangements and were not analyzed further.
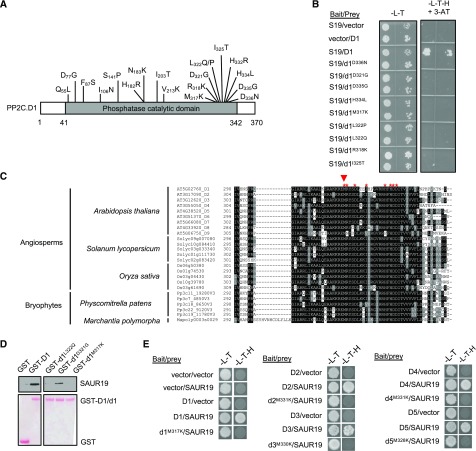
Figure 1.
Screening of SAUR interaction mutants of PP2C.D1. A, Diagram of the PP2C.D1 protein with point mutations that abolish SAUR19 binding indicated. B, Reverse yeast two-hybrid assay of wild-type PP2C.D1 or mutant pp2c.d1 derivatives with SAUR19. Growth on SC-Leu,Trp,His (-L-T-H) + 3-amino-1′, -2′, -4′ triazole (3-AT) medium is indicative of PP2C.D1-SAUR19 interaction. C, Multiple sequence alignment of partial PP2C.D amino acid sequences from a variety of plants. Not all tomato (Solanum lycopersicum) and rice (Oryza sativa) members are included. PP2C.D orthologs from other species were retrieved from https://phytozome.jgi.doe.gov/pz/portal.html. Red asterisks indicate the positions of PP2C.D1 residues identified in the reverse two-hybrid screen. The red arrowhead indicates the conserved Met residue, which was mutated to Lys in other PP2C.D family members. D, In vitro pull-down assay of SAUR19 by wild-type PP2C.D1 or mutated pp2c.d1 derivatives. Immunoblots are shown in the top row, whereas Ponceau S-stained membranes are shown in the bottom row. All samples were run on the same gel and blotted together. Extraneous lanes were digitally spliced out of the blot as indicated. E, Yeast two-hybrid assay of wild-type PP2C.D or mutant pp2c.d isoforms with SAUR19. In B and E, images are composites where individual images were cropped and digitally extracted for comparison.
Strikingly, over half of the missense mutations identified localized to the very C-terminal end of the catalytic domain (amino acids 317–336; Fig. 1, A and B). Multiple sequence alignment of the Arabidopsis PP2C.D proteins revealed that this region was nearly perfectly conserved among all nine Arabidopsis PP2C.D members, as well as PP2C.D orthologs from a variety of angiosperms as well as bryophytes (Fig. 1C). Furthermore, this sequence is unique to D-clade phosphatases and represents an insertion that is missing in all other PP2C families, with the exception of a loosely related sequence in C-clade PP2Cs (Supplemental Fig. S2). To further test the importance of this motif in SAUR binding, select pp2c.d1 missense mutants were tested in in vitro pull-down assays with SAUR19. Both the pp2c.d1M317K and pp2c.d1L322Q mutations completely abolished SAUR19 binding, whereas the pp2c.d1D321G mutation modestly reduced binding in this assay (Fig. 1D).
Given the high degree of sequence conservation of this motif among PP2C.D family members, we examined the possibility that mutations analogous to those identified in PP2C.D1 could also disrupt SAUR binding when introduced into other PP2C.D family members. Since the pp2c.d1M317K mutation completely abolished SAUR binding in both two-hybrid and pull-down assays, we chose this residue to test this possibility and generated pp2c.d2M331K, pp2c.d3M330K, pp2c.d4M331K, and pp2c.d5M328K mutant derivatives. Indeed, yeast two-hybrid assays revealed that these analogous mutations conferred the same effect on these PP2C.D family members by preventing interaction with SAUR19 (Fig. 1E).
The above findings indicate that a highly conserved motif near the C terminus of the PP2C.D catalytic domain is essential for SAUR-binding activity. To investigate whether this domain is sufficient to mediate SAUR interaction, we replaced the C-terminal amino acids (407–434) of the A-clade PP2C, ABA INSENSITIVE1 (ABI1), with the C terminus (amino acids 313–370) of PP2C.D1. In yeast two-hybrid assays, however, SAUR19 did not interact with this ABI1-PP2C.D1 fusion protein (Supplemental Fig. S3A). Expression of the hybrid protein was verified by western blotting (Supplemental Fig. S3B). Together, our findings suggest that the C terminus of the PP2C.D catalytic domain is essential, but not sufficient, for SAUR binding.
SAUR-Immune PP2C.Ds Exhibit Constitutive Phosphatase Activity
We next asked if mutations in the C-terminal motif affect PP2C.D phosphatase activity. Wild-type PP2C.D1 and the M317K, R318K, D321G, and L322Q mutant derivatives were purified from Escherichia coli as GST fusion proteins and tested in in vitro phosphatase assays with the chromogenic substrate, p-nitrophenylphosphate (pNPP). All mutant derivatives exhibited phosphatase activity comparable to wild-type PP2C.D1, indicating that the mutations do not have major effects on catalytic activity (Fig. 2A).
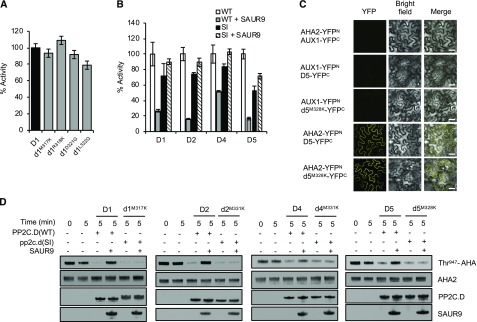
Figure 2.
Analogous mutations in other PP2C.D members also confer SAUR immunity. A, In vitro phosphatase assay of pp2c.d1 missense mutant proteins. Values represent means (n = 3) ± sd. B, Phosphatase activities of wild-type PP2C.D (WT) or pp2c.d SAUR-immune (SI) M→K missense mutant proteins in the absence or presence of SAUR9. Values represent means (n = 3) ± sd. C, BiFC assay demonstrating protein-protein interaction of wild-type PP2C.D5 or pp2c.d5M328K with AHA2 after transient expression in N. benthamiana leaves. Images are composites where individual images were cropped and digitally extracted for comparison. Bars = 50 µm. D, In vitro phosphatase assay showing Thr-947 dephosphorylation of yeast-expressed AHA2 by wild-type PP2C.Ds or SAUR-immune pp2c.d isoforms. Where indicated, 6xHis-SAUR9 was added to a threefold molar excess relative to PP2C.D. The slight mobility difference between wild-type PP2C.D1 and pp2c.d1M317K is due to a second 6xHis tag in the mutant construct.
Since our previous work demonstrated strong SAUR9 inhibition of several Arabidopsis PP2C.D phosphatases (Spartz et al., 2014), we used SAUR9 to examine the ability of SAURs to inhibit the pp2c.d1M317K mutant as well as the analogous mutant derivatives of PP2C.D2, PP2C.D4, and PP2C.D5. The addition of recombinant SAUR9 protein to pNPP phosphatase assays strongly inhibited wild-type PP2C.D1, PP2C.D2, and PP2C.D5 and, to a somewhat lesser extent, PP2C.D4 (Fig. 2B). In contrast, phosphatase activities of the M→K pp2c.d mutant derivatives were not inhibited whatsoever by SAUR9 addition. Together with our protein interaction data, these findings demonstrate that SAUR binding activity is required for SAUR inhibition of PP2C.D activity. pp2c.d mutations that disrupt SAUR binding result in constitutively active phosphatases that are immune to SAUR inhibition.
Our previous findings demonstrated that several PP2C.Ds can interact with AHA2 and dephosphorylate the C-terminal Thr-947 residue (Spartz et al., 2014; Ren et al., 2018). To investigate whether SAUR-immune PP2C.D proteins could still bind AHA2, we performed bimolecular fluorescence complementation (BiFC) assays where AHA2 was coexpressed in Nicotiana benthamiana leaves with either wild-type PP2C.D1, PP2C.D2, PP2C.D5, and PP2C.D6 or the M→K mutant pp2c.d derivatives. Strong PM-localized yellow fluorescent protein (YFP) signal was observed in all cases (Fig. 2C; Supplemental Fig. S4), demonstrating that the M→K mutants retain the ability to interact with PM H+-ATPases.
To determine if SAUR-immune PP2C.Ds could still dephosphorylate the Thr-947 residue of AHA2, we conducted in vitro AHA2 dephosphorylation assays as previously described (Spartz et al., 2014). This assay used yeast-expressed AHA2 that is phosphorylated on Thr-947. Both wild-type and SAUR-immune PP2C.Ds could dephosphorylate AHA2, as shown by GST-14-3-3 far-western gel-blotting assays, which are widely employed for assessing Thr-947 phosphorylation status (Fuglsang et al., 1999; Kinoshita and Shimazaki, 1999; Hayashi et al., 2010). Consistent with our pNPP assay findings, SAUR9 strongly inhibited wild-type PP2C.D proteins from dephosphorylating AHA2 but had no effect on the SAUR-immune PP2C.D mutant derivatives (Fig. 2D). These findings clearly demonstrate that SAUR-immune PP2C.Ds are specifically defective in SAUR binding and retain both phosphatase activity and the ability to interact with PM H+-ATPases.
SAUR-Immune pp2c.d2 and pp2c.d5 Repress Cell Expansion
Our prior genetic analysis of the PP2C.D family revealed that PP2C.D2, PP2C.D5, and PP2C.D6 are the primary family members involved in controlling AHA phosphorylation status in planta to regulate cell elongation (Ren et al., 2018). To examine the physiological effects of constitutively active SAUR-immune PP2C.D enzymes, we generated native promoter:pp2c.d2M331K-GFP and native promoter:pp2c.d5M328K-GFP constructs and introduced them into the respective pp2c.d2 or pp2c.d5 mutant backgrounds. Wild-type native promoter:PP2C.D2-GFP and native promoter:PP2C.D5-GFP lines were included as controls. Both the wild-type and SAUR-immune pp2c.d2/d5-GFP fusions decorated the PM, indicating that the M→K mutations did not compromise localization of the SAUR-immune PP2C.Ds in either cotyledon pavement cells or root tips (Supplemental Fig. S5, A and B). Notably, however, the SAUR-immune pp2c.d2- and pp2c.d5-expressing plants exhibited pavement cells significantly smaller than wild-type PP2C.D2-GFP and PP2C.D5-GFP controls (Supplemental Fig. S5, C and D).
In our previous analysis of PPP2C.D:PP2C.D2/5/6-HA/GFP transgenic lines, we found that plant growth is highly sensitive to PP2C.D dosage (Ren et al., 2018). In that prior study, the majority of transgenic lines expressed low levels of PP2C.D-HA/GFP and complemented (or slightly overcomplemented) pp2c.d mutant phenotypes. Due to position effects, however, a small subset of transgenic lines expressed high levels of PP2C.D-HA/GFP, resulting in reductions in cell expansion and organ growth due to overexpression. Therefore, to assess the functional consequences of SAUR-immune pp2c.d mutants and avoid the complication of overexpression, we intentionally selected pp2c.d2M331K-GFP and pp2c.d5M328K-GFP lines that expressed lower levels of the fusion protein than the corresponding PP2C.D2-GFP and PP2C.D5-GFP wild-type controls (Fig. 3B, bottom; Supplemental Fig. S6B, bottom).
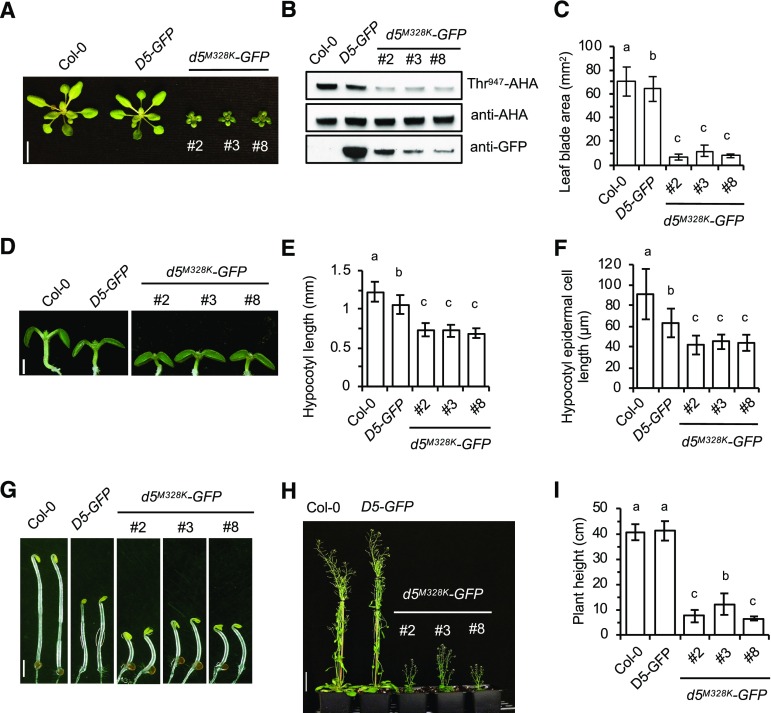
Figure 3.
Expression of pp2c.d5M328K in Arabidopsis confers dramatic cell expansion defects. A, Twenty-four-day-old plants. Three independent pp2c.d5M328K-GFP lines are shown. Bar = 1 cm. B, Examination of AHA Thr-947 phosphorylation in 7-d-old light-grown seedlings by GST-14-3-3 far-western assay. PP2C.D5-GFP and pp2c.d5M328K-GFP protein expression levels are shown in the bottom image. C, Leaf blade area of 24-d-old plants. Values represent means (n = 30, three leaf blades per plant) ± sd. D, Seven-day-old light-grown seedlings. Bar = 1 mm. E, Hypocotyl length of 7-d-old light-grown seedlings. Error bars indicate sd (n = 20). F, Mean hypocotyl epidermal cell length of 7-d-old light-grown seedlings. The apical-most five cells from 10 seedlings were measured (n = 50). Error bars indicate sd. G, Five-day-old etiolated seedlings. Bar = 2 mm. H, Seven-week-old plants. Bar = 5 cm. I, Plant height of 7-week-old plants. Error bars indicate sd (n = 10). Different letters above bars indicate significant differences (P < 0.05) when analyzed by one-way ANOVA with Tukey’s honestly significant difference (HSD) test. In D and G, images are composites where individual images were cropped and digitally extracted for comparison.
Plants expressing the M→K SAUR-immune pp2c.d2/d5-GFP derivatives exhibited severe dwarf phenotypes with dramatic reductions in leaf size and petiole length (Fig. 3, A and C; Supplemental Fig. S6, A and C). In contrast, PP2C.D2-GFP and PP2C.D5-GFP plants largely resembled wild-type controls, although minor reductions in organ size were observed. Overexpression of PP2C.D1 or PP2C.D5 was previously reported to confer severe dwarf phenotypes (Spartz et al., 2014; Ren et al., 2018). However, the dwarfism resulting from expression of the SAUR-immune pp2c.d2/d5-GFP constructs is clearly not the result of overexpression, as mutant protein levels were lower than those of the corresponding wild-type PP2C.D2/D5-GFP proteins (Fig. 3B; Supplemental Fig. S6B). Rather, we hypothesize that the severe phenotypes resulting from pp2c.d2M331K and pp2c.d5M328K expression are the consequence of the constitutively active phosphatase activity of these mutant derivatives due to the loss of SAUR binding.
To extend our in vitro findings that SAUR-immune phosphatases exhibit constitutive activity, we examined AHA Thr-947 phosphorylation status in pp2c.d2M331K-GFP and pp2c.d5M328K-GFP seedlings. Despite the fact that the SAUR-immune derivatives were expressed at lower levels than their wild-type counterparts, a dramatic reduction in Thr-947 phosphorylation was observed in the mutants compared with the PP2C.D2-GFP and PP2C.D5-GFP control lines (Fig. 3B; Supplemental Fig. S6B). This finding strongly suggests that, as per our in vitro findings, the SAUR-immunity mutations result in constitutively active phosphatases and supports our prior genetic and biochemical findings that SAURs negatively regulate PP2C.D activity to activate PM H+-ATPases (Spartz et al., 2014; Ren et al., 2018).
In young seedlings, hypocotyl lengths of the SAUR-immune pp2c.d2 and pp2c.d5 mutants were significantly reduced in both light- and dark-grown seedlings (Fig. 3, D, E, and G; Supplemental Fig. S6, D, E, G, and H). The reduction of hypocotyl length was the result of decreased cell elongation, as hypocotyl epidermal cells in the SAUR-immune mutants were approximately 30% to 40% shorter than in wild-type Columbia-0 (Col-0; Fig. 3F; Supplemental Fig. S6F). In addition, the inflorescences of both SAUR-immune pp2c.d2 and pp2c.d5 mutants were approximately 70% shorter than those of control plants, giving them very short stature (Fig. 3, H and I; Supplemental Fig. S6, I and J). This dwarf phenotype contrasts with the increased organ growth phenotypes resulting from overexpression of stabilized SAUR19 (Spartz et al., 2012). The SAUR-immune pp2c.d2/d5 plants also produced small flowers with short carpels and stamens (Supplemental Fig. S7, A and B). Stamen filaments of pp2c.d5M328K flowers were shorter than those of pp2c.d2M331K, consistent with the higher expression of PP2C.D5 in stamen filaments as previously reported (Ren et al., 2018). The short filaments resulted in a mechanical defect where self-fertilization could not occur, and hence led to undeveloped siliques bearing no seeds in the case of pp2c.d5M328K or reduced seed yields in pp2c.d2M331K plants (Supplemental Fig. S7, C and D). We observed rare cases of pp2c.d5M328K flowers producing short siliques with a few seeds, but manual pollination was generally needed to obtain seeds. Similar findings were reported for PP2C.D5 overexpression lines (Ren et al., 2018).
SAUR-Immune pp2c.d2 and pp2c.d5 Block Auxin-Mediated Hypocotyl Elongation
Together, the above findings suggest that SAUR-immune pp2c.d2 and pp2c.d5 mutants exhibit constitutive phosphatase activity via loss of SAUR inhibition, resulting in continual PM H+-ATPase dephosphorylation that prevents proper cell expansion for plant growth. Therefore, if auxin normally promotes cell expansion by inducing SAUR expression to inhibit PP2C.D activity and activate H+ pumps, the SAUR-immune pp2c.d2/d5 mutants should not exhibit auxin-mediated organ growth. To test this possibility, we examined hypocotyl lengths of SAUR-immune pp2c.d2/d5-GFP seedlings and the respective PP2C.D2-GFP and PP2C.D5-GFP controls following treatment with picloram. This synthetic auxin promotes robust hypocotyl elongation and has been shown to rapidly induce the expression of many SAURs in hypocotyls (Chapman et al., 2012). Treatment with 2 µm picloram increased wild-type Col-0 hypocotyl length by approximately 1.7-fold (Fig. 4, A–D). Likewise, the PP2C.D2-GFP and PP2C.D5-GFP lines exhibited significantly longer hypocotyls on picloram compared with control medium. The slightly less robust increase in hypocotyl lengths of these transgenics compared with wild-type controls is presumably the result of weak overexpression (Ren et al., 2018). Importantly, however, unlike Col-0 and the wild-type PP2C.D2/D5-GFP seedlings, lines expressing the SAUR-immune pp2c.d2/d5-GFP constructs displayed no significant increase in hypocotyl length following picloram treatment (Fig. 4, A–D). Cell length measurements confirmed that picloram promoted an increase in hypocotyl cell length of the control genotypes but not the SAUR-immune lines (Fig. 4E). Similar results were obtained using 602 proauxin (Savaldi-Goldstein et al., 2008), with 602 treatment having no significant effect on SAUR-immune pp2c.d2 hypocotyl length (Fig. 4F). SAUR-immune pp2c.d5 seedlings exhibited a slight response to 602, but this was dramatically reduced compared with the wild-type controls. In contrast to the auxin-resistant hypocotyl phenotype conferred by the expression of SAUR-immune pp2c.d2/d5-GFP constructs, these lines exhibited wild-type-like sensitivity to auxin in root growth inhibition assays (Supplemental Fig. S8), demonstrating that SAUR-immune pp2c.d seedlings can still respond to auxin.
Figure 4.
SAUR immunity confers resistance to auxin and SAUR19 overexpression. A, Three-day-old light-grown Col-0, pp2c.d5, PP2C.D5-GFP, and pp2c.d5M328K-GFP seedlings were transferred to Arabidopsis with Suc (ATS) medium ± 2 µm picloram and grown an additional 4 d. Bar = 1 mm. B, Mean hypocotyl length of seedlings grown as described in A. Error bars indicate sd (n = 20). C and D, pp2c.d2M331K-GFP and control seedlings grown as described in A. Error bars indicate sd (n = 20). E, Mean hypocotyl epidermal cell length of seedlings in A and C. Error bars indicate sd (n = 50). Asterisks indicate statistically significant differences (P < 0.001) when analyzed by two-tailed Student’s t test. n.s., Not significant (P > 0.05). F, Mean hypocotyl length of seedlings treated with or without 2 µm 602 proauxin according to the procedure in A. Error bars indicate sd (n = 17). G to L, The effects of 35S:StrepII-SAUR19 (S19-OX) expression on the growth of control and pp2c.d5M328K-GFP plants. G, Seven-day-old light-grown seedlings grown on unsupplemented ATS medium. Bar = 1 mm. H, Hypocotyl length of 7-d-old light-grown seedlings. Error bars indicate sd (n = 20). I, Five-day-old etiolated seedlings. Bar = 2 mm. J, Hypocotyl length of 5-d-old etiolated seedlings. Error bars indicate sd (n = 20). K, Twenty-five-day-old plants. Bar = 1 cm. L, Leaf blade area of 25-d-old plants. Values represent means (n = 30, three leaf blades per plant) ± sd. Different letters above bars indicate significant differences (P < 0.05) when analyzed by one-way ANOVA with Tukey’s HSD test. In A, C, G, and I, images are composites where individual images were cropped and digitally extracted for comparison.
The above findings suggest that auxin-induced expression of SAURs (or any other auxin-regulated genes) cannot overcome the inhibitory effects of the SAUR-immune PP2C.D derivatives on cell expansion. To examine this possibility more directly, we crossed the pp2c.d2M331K-GFP and pp2c.d5M328K-GFP lines with plants expressing a 35S:StrepII-SAUR19 overexpression construct (Spartz et al., 2012). We previously demonstrated that overexpression of SAUR19 could suppress the growth defects conferred by overexpression of wild-type PP2C.D5 (Ren et al., 2018). While SAUR19 overexpression promoted increases in the hypocotyl lengths and leaf sizes of Col-0, PP2C.D2-GFP, and PP2C.D5-GFP control plants, no effect whatsoever was apparent when SAUR19 was overexpressed in the pp2c.d2M331K-GFP and pp2c.d5M328K-GFP plants (Fig. 4, G–L; Supplemental Fig. S9). These findings further support our conclusion that these mutations confer SAUR immunity and constitutive phosphatase activity regardless of the SAUR protein dosage.
SAUR-Immune PP2C.D Phenotypes Are Partially Suppressed by Constitutive PM H+-ATPase Activity
There are 11 genes in Arabidopsis encoding PM H+-ATPases. AHA1 and AHA2 are the major contributors, together constituting 70% to 80% of the total H+-ATPase activity in most plant organs (Haruta et al., 2010). The open stomata2 (ost2-2) mutant contains two point mutations (L169F and G867S) in AHA1 that result in constitutive H+ pump activity (Merlot et al., 2007). Although not yet confirmed, this has been proposed to be the consequence of the mutations abolishing intramolecular interactions between the autoinhibitory C-terminal domain and cytosolic regions elsewhere in the protein, thus trapping the H+ pump in the activated state. The resulting gain-of-function ost2-2 phenotypes partially phenocopy those resulting from SAUR19 overexpression or pp2c.d2/5/6 mutation (Spartz et al., 2014; Ren et al., 2018). Furthermore, ost2-2 mutant phenotypes are enhanced when the mutation is combined with the aha2-5 null mutation (Fendrych et al., 2016).
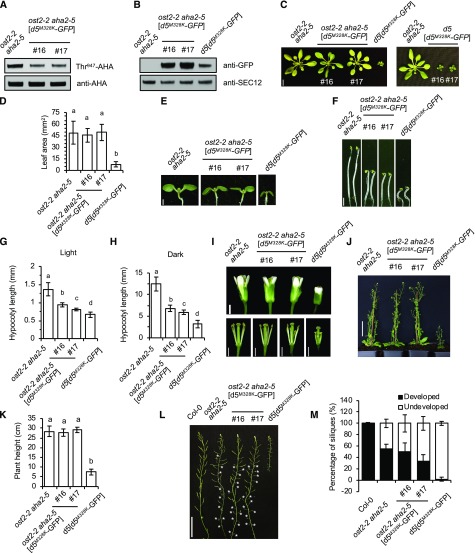
To investigate whether constitutive PM H+-ATPase activity could suppress the dwarf phenotype conferred by SAUR-immune phosphatases, we introduced the PPP2C.D5:pp2c.d5M328K-GFP construct into the ost2-2 aha2-5 genetic background. Analysis of two independent lines expressing the SAUR-immune pp2c.d5 construct revealed reduced AHA Thr-947 phosphorylation status compared with ost2-2 aha2-5 controls (Fig. 5, A and B). Despite this reduction in AHA phosphorylation, pp2c.d5M328K-GFP expression in the ost2-2 aha2-5 plants did not result in severe dwarfism (Fig. 5, C and D). However, when these lines were put through a backcross to remove the ost2-2 and aha2-5 mutations, the pp2c.d5M328K-GFP transgene once again conferred a dramatic dwarf phenotype (Fig. 5C, right), indicating that the SAUR-immune pp2c.d5 growth defects are PM H+-ATPase dependent. Likewise, the reduction in hypocotyl lengths resulting from pp2c.d5M328K-GFP expression was partially suppressed in the ost2-2 aha2-5 background (Fig. 5, E–H). That said, ost2-2 aha2-5 [pp2c.d5M328K-GFP] hypocotyls were still significantly shorter than those of the ost2-2 aha2-5 parental line. This modest reduction could be due to decreased phosphorylation status of the remaining AHA proteins, or this finding may indicate that PP2C.D5 regulates additional proteins that contribute to elongation growth. Lastly, several adult phenotypes of pp2c.d5M328K expression were also largely suppressed in the ost2-2 aha2-5 background, including the severe reductions in stature, stamen filament length, and fertility (Fig. 5, I–M). These findings suggest that the constitutively active ost2-2 mutant protein functions independently of Thr-947 phosphorylation status and hence is less responsive to constitutively active SAUR-immune pp2c.d5. Importantly, these findings support our contention that reduced PM H+-ATPase activity is a major contributor to the cell expansion phenotypes conferred by SAUR-immune PP2C.D phosphatases.
Figure 5.
pp2c.d5M328K is partially suppressed in ost2-2 aha2-5. A, Examination of AHA Thr-947 phosphorylation in 7-d-old light-grown seedlings by GST-14-3-3 far-western gel assay. B, Immunoblot examining pp2c.d5M328K-GFP protein expression level in 7-d-old light-grown seedlings. SEC12 is shown as a loading control. C, Twenty-four-day-old plants. The left image shows a comparison between ost2-2 aha2-5 with or without the pp2c.d5M328K-GFP transgene and pp2c.d5M328K-GFP in the pp2c.d5 mutant background as a reference. The right image shows two independent lines of ost2-2 aha2-5[pp2c.d5M328K-GFP] after backcrossing the transgene into the pp2c.d5 AHA1 AHA2 background. Bar = 1 cm. D, Leaf blade area of 25-d-old plants. Values represent means (n = 30, three leaf blades per plant) ± sd. E, Seven-day-old light-grown seedlings. Bar = 1 mm. F, Five-day-old etiolated seedlings. Bar = 2 mm. G, Mean hypocotyl length of 7-d-old light-grown seedlings. Error bars indicate sd (n = 20). H, Mean hypocotyl length of 5-d-old etiolated seedlings. Error bars indicate sd (n = 20). I, Flowers. The bottom images show flowers with sepals and petals removed to expose carpels and stamens. Bars = 1 mm. J, Seven-week-old plants. Bar = 5 cm. K, Plant height of 7-week-old plants. Error bars indicate sd (n = 10). L, Inflorescence stems showing siliques. White asterisks indicate undeveloped siliques in ost2-2 aha2-5 double mutant and ost2-2 aha2-5 [pp2c.d5M328K-GFP] independent lines. Bar = 5 cm. M, Percentage of developed and undeveloped siliques. Error bars indicate sd (n = 200, 20 siliques per plant). Different letters above bars indicate significant difference (P < 0.05) when analyzed by one-way ANOVA with Tukey’s HSD test. In C, E, F, and I, images are composites where individual images were cropped and digitally extracted for comparison.
DISCUSSION
Cell elongation is a crucial process that affects organ growth and development to give shape and form to the plant. Our previous studies demonstrated that auxin-induced cell elongation in hypocotyls is mediated by SAUR and PP2C.D proteins, which function in an antagonistic manner to control phosphorylation and activity of PM H+-ATPases and perhaps other protein substrates (Spartz et al., 2014, 2017; Ren et al., 2018). Among the nine D-clade PP2C phosphatases, PP2C.D2, PP2C.D5, and PP2C.D6 act in a largely redundant manner to inhibit cell expansion in hypocotyls and several other organs, while the remaining family members do not appear to contribute to these growth processes (Ren et al., 2018). While analysis of pp2c.d loss-of-function mutants by Ren et al. (2018) clearly implicated PP2C.D2, PP2C.D5, and PP2C.D6 as negative regulators of cell expansion, due to the plethora of SAUR genes present in plant genomes, the evidence that auxin-induced SAUR proteins antagonize these phosphatases has been limited to gain-of-function SAUR overexpression and in vitro biochemical approaches. In this study, we provide an approach to potentially circumvent the complications of SAUR genetic redundancy by identifying mutant PP2C.D derivatives that retain phosphatase activity but are incapable of interacting with SAUR proteins, thus rendering the phosphatases immune to SAUR inhibition.
Our random mutagenesis of PP2C.D1 identified a motif near the C terminus of the catalytic domain that is essential for SAUR binding. This motif is extremely highly conserved in all D-clade family members from Arabidopsis as well other species representing both vascular and nonvascular plants. Compared with other PP2Cs, this motif is present as a unique insertion, with the lone exception of the C-clade phosphatases, which contain a related but loosely conserved sequence. The seven member C-clade PP2Cs include POLTERGEIST and the related POLTERGEIST-LIKE (PLL) phosphatases (Yu et al., 2003), which regulate stem cell initiation and maintenance through CLAVATA3/WUSCHEL-RELATED HOMEOBOX signaling pathways (Gagne and Clark, 2010). Since SAUR19 overexpression confers a variety of cell expansion phenotypes but no obvious meristematic defects, it seems unlikely that SAURs regulate C-clade phosphatase activity. Furthermore, we examined potential interactions between SAUR19 and PLL4 in both yeast two-hybrid and BiFC interaction assays but obtained negative results using both approaches (Supplemental Fig. S10).
While mutations in the C-terminal SAUR-interaction motif abolish SAUR binding, these mutations do not appear to affect catalytic activity of PP2C.D enzymes. Consequently, PP2C.D proteins containing mutations in this motif are immune to SAUR inhibition and display constitutive phosphatase activity both in vitro and in planta. For example, absolutely no SAUR inhibition was detected in our phosphatase assays of mutant derivatives of pp2c.d1, pp2c.d2, pp2c.d4, or pp2c.d5 in biochemical assays employing either the chemical substrate, pNPP, or a native substrate, AHA2. Furthermore, when expressed in Arabidopsis from their respective native promoters, the pp2c.d2 or pp2c.d5 SAUR-immune derivatives conferred constitutive low Thr-947 phosphorylation status of PM H+-ATPases, severe dwarfism, and several other cell expansion phenotypes. While overexpression of wild-type PP2C.D5 confers very similar phenotypes, co-overexpression of SAUR19 suppresses all of the growth defects resulting from PP2C.D5 overexpression (Ren et al., 2018). In marked contrast, however, the growth defects of the SAUR-immune pp2c.d2M331K and pp2c.d5M328K mutants were not restored whatsoever by overexpression of StrepII-SAUR19. Also consistent with this premise, auxin treatment did not promote hypocotyl elongation in these SAUR-immune pp2c.d mutants. Together, these findings provide a strong complement to our previous analysis of pp2c.d2 and pp2c.d5 loss-of-function mutants (Ren et al., 2018), clearly demonstrating that these phosphatases play vital roles in regulating PM H+-ATPase activity and cell expansion.
Our findings also provide additional support for the long-standing acid growth hypothesis. It has previously been established that auxin promotes PM H+-ATPase phosphorylation and activation (Takahashi et al., 2012) and that this process involves SAUR-PP2C.D2/5/6 regulatory modules (Spartz et al., 2014; Fendrych et al., 2016; Ren et al., 2018). We found that introduction of the dominant ost2-2 mutation encoding a constitutively activated mutant isoform of the AHA1 PM H+-ATPase into the pp2c.d5M328K background significantly suppressed many of the cell expansion defects of this SAUR-immune mutant. This finding strongly suggests that the growth defects conferred by SAUR-immune pp2c.d5 are largely the result of the inability to activate PM H+-ATPases. Interestingly, this suppression occurred despite the fact that Thr-947 phosphorylation was dramatically lower in the pp2c.d5M328K background. We hypothesize that this indicates that the mutant ost2-2 isoform does not require Thr-947 phosphorylation for activation. Rather, as previously suggested, the mutations may disrupt intramolecular contacts with the C-terminal autoinhibitory domain (Merlot et al., 2007), thereby bypassing the normal requirement of phosphorylation for pump activation.
Apart from the PM-localized PP2C.D2, PP2C.D5, and PP2C.D6 family members negatively regulating PM H+-ATPase activity to control cell expansion, the functions of the remaining D-clade phosphatases are less well understood. PP2C.D1 has been implicated in apical hook development (Sentandreu et al., 2011; Spartz et al., 2014) and as a negative regulator of leaf senescence, the latter of which may be mediated through PP2C.D1 control of SENESCENCE-ASSOCIATED RECEPTOR-LIKE KINASE activity (Xiao et al., 2015). PP2C.D3 (also known as PP2C38) was reported to negatively regulate the Arabidopsis cytoplasmic BOTRYTIS-INDUCED KINASE1 (BIK1) to suppress BIK1-mediated plant immunity (Couto et al., 2016). Whether SAURs are involved in regulating PP2C.D1 and PP2C.D3 in these processes remains to be determined. That said, SAUR36 was found to promote leaf senescence (Hou et al., 2013), suggesting that SAUR36 and PP2C.D1 may in fact function antagonistically to regulate senescence. Given the extreme conservation among D-clade members of the C-terminal SAUR interaction motif, and that we have demonstrated that mutations in this motif confer immunity to SAUR inhibition of PP2C.D1, PP2C.D2, PP2C.D4, and PP2C.D5, we hypothesize that analogous mutations in other family members will have similar effects. Unfortunately, we have been unable to test this possibility directly, as we have thus far not been able to develop in vitro phosphatase assays with recombinantly expressed PP2C.D3, PP2C.D6, PP2C.D7, PP2C.D8, or PP2C.D9. If so, however, expression of SAUR-immune derivatives of these additional D-clade members from their respective native promoters may provide unique gain-of-function genetic tools to assign functions to these phosphatases.
Lastly, we suggest that SAUR-immune derivatives of PP2C.D2 and PP2C.D5 may provide novel genetic tools for investigating the roles of PM H+-ATPases throughout plant development. Currently, such genetic studies are hampered by the fact that aha1 and aha2 single mutants only exhibit weak conditional phenotypes and the double mutant is embryo lethal (Haruta et al., 2010, 2015). Since SAUR-immune pp2c.d2 and pp2c.d5 repress H+-ATPase activity via constitutive Thr-947 dephosphorylation, expression of these mutant derivatives from inducible, tissue-specific, and cell type-specific promoters would provide a unique approach for down-regulating PM H+-ATPase activity in a temporal and spatially controlled fashion to probe H+-ATPase functions in specific processes, cells, or organs.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
All Arabidopsis (Arabidopsis thaliana) lines used in this study were in the Col-0 ecotype. Seedlings were grown on ATS medium (Lincoln et al., 1990) at 22°C under long-day conditions (60–80 μE m−2 s−1) unless specified otherwise. Transgenic plants were generated through the floral dip method using Agrobacterium tumefaciens strain GV3101 (helper plasmid pMP90) harboring the binary vector with the desired target genes (Clough and Bent, 1998). The StrepII-SAUR19 and PP2C.D5-HA transgenic plants were described previously (Spartz et al., 2012; Ren et al., 2018).
For auxin treatment, seedlings were grown on ATS plates for 3 d at 22°C before being transferred to plates containing 2 µm picloram (4-amino-3,5,6-trichloro-picolinic acid), 2 µm 602 proauxin, or 1 µm IAA. Seedlings were grown under long-day conditions as described above. The seedlings were grown for 4 d before hypocotyls and roots were imaged and measured at described below.
Gene Cloning
The QuikChange XL site-directed mutagenesis kit (Agilent Technologies) was used to perform site-directed mutagenesis on pENTR/D-TOPO clones harboring the full-length cDNA or genomic DNA of PP2C.D phosphatases using the primer pairs listed in Supplemental Table S1. The PP2C.D genomic DNA clones have been previously described (Ren et al., 2018). The mutated PP2C.D cDNAs were recombined into pET32a-GW or pDEST15-GW for bacterial expression using Gateway LR clonase II enzyme mix (Life Technologies). Mutated PP2C.D genomic DNAs were recombined into pGWB204 for stable expression in Arabidopsis using the floral dip method. For ABI1-D1 fusion construction, overlap-extension PCR was performed. The ABI1-F and SKLA-R primers were used to amplify the N terminus of ABI1 coding sequence, whereas SKLA-F and D1-R primers were used to amplify the C terminus of PP2C.D1 coding sequence as listed in Supplemental Table S1. The resulting PCR products were then mixed and used as template to amplify the ABI1-D1 fusion sequence, which was subsequently cloned into pENTR/D-TOPO entry clone.
Plant Growth Measurement
A SPOT Insight camera mounted on an Olympus SZX12 stereomicroscope was used to photograph seedling hypocotyls for length measurements. For cell length measurements, images of propidium iodide-stained hypocotyl epidermal cells were acquired using a Nikon Ti2 A1si Confocal system (Nikon USA). All image measurements were performed using ImageJ software. The ImageJ plugin PaCeQuant was used to automatically quantify cotyledon pavement cell area and perimeter, together with manual validation to exclude inaccurately segmented pavement cells for each measurement (Möller et al., 2017).
Reverse Two-Hybrid Screen
Yeast parent vectors pBI880 and pBI771 and Saccharomyces cerevisiae strain YPB2 were described by Gray et al. (1999). pBI880 containing full-length SAUR19 and pBI771 containing full-length PP2C.D1 have been previously described (Spartz et al., 2014). To facilitate mutant screening, the PP2C.D1 coding sequence was divided into N-terminal (encoding amino acids 1–107), central (encoding amino acids 105–272), and C-terminal (encoding amino acids 260–370) thirds. Each third was subjected to identical error-prone PCR using pBI771[PP2C.D1] template DNA and employing Sigma RedExtract-n-AMP ready mix supplemented with 0.1 mm MnCl2 and an additional 2 mm dTTP and dCTP. To generate libraries of mutant PP2C.D1 clones, EcoRI and SpeI restriction sites were introduced into the wild-type pBI771[PP2C.D1] vector at nucleotide positions 316 and 780 of the PP2C.D1 cDNA using site-directed mutagenesis. This vector was digested with NotI and EcoRI, EcoRI and SpeI, or SpeI and SalI to generate gapped plasmids corresponding to the above three PP2C.D1 regions. Each gapped vector was then mixed with the corresponding mutagenized PCR products, and the mixture was used to transform YPB2 cells carrying the pBI880[SAUR19] bait vector. Homologous recombination between the ends of the PCR products (corresponding to the amplification primers) and the gapped vector generated libraries of pBI771[PP2C.D1] mutant derivatives. Transformants were selected on SC(-L-T), and then colonies were picked and streaked onto both SC(-L-T) and SC(-L-T-H) + 5 mm 3-AT for screening. Colonies that grew on SC(-L-T) but not SC(-L-T-H) + 3-AT medium were identified as potential SAUR19 noninteractors and characterized further. This included immunoblot screening with purified α-PP2C.D1 antibody to ensure expression and eliminate stop codon mutants, sequencing, retransformation of the plasmid into yeast, and retesting for yeast two-hybrid interaction with SAUR19. Approximately 500 colonies were tested for each of the three PP2C.D1 intervals.
Yeast Two-Hybrid Assays
Additional protein interaction assays of SAUR19 with PP2C.D phosphatases were carried out in both GAL4 (pBI880/pBI771; Kohalmi et al., 1998) and lexA-based (pBTM116/pACT2; Weber et al., 2005) yeast two-hybrid systems. The mutated PP2C.D inserts in pENTR/D-TOPO vectors were cloned into the prey vector pACT2 through Gateway LR recombination as described above. Yeast strain L40ccU3 [MATa, his3-200, trp1-901, leu2-3, 112ade2 LYS:(lexAop)4-HIS3, URA:(lexAop)8-lacZ, GAL4, gal80] was cotransformed with pBTM116-SAUR19 and pACT2-PP2C.D vectors.
Protein Expression and Phosphatase Assays
GST- or 6xHis-tagged SAUR or PP2C.D proteins were purified from Escherichia coli cultures as previously described (Spartz et al., 2014). For phosphatase assays, 0.15 µm PP2C.D protein was preincubated with 1.2 µm SAUR proteins or an equivalent amount of elution buffer for 10 min at room temperature. Protein mixtures were then added into assay buffer (75 mm Tris, pH 7.6, 10 mm MnCl2, 100 mm NaCl, 0.5 mm EDTA, pH 8, and 5 mm pNPP), and A405 was recorded using a Powerwave 340 plate reader (Biotek Instruments) every 1 min for 20 min.
For in vitro AHA2 dephosphorylation assays, yeast PMs were purified from RS-72 cells expressing AHA2 protein as previously described (Panaretou and Piper, 2006; Spartz et al., 2014). The isolated membranes were resuspended in buffer with 5 mm potassium phosphate, pH 7.8, 3 mm KCl, 0.1 mm EDTA, pH 8, 330 mm Suc, 1 mm DTT, and 1 mm phenylmethylsulfonyl fluoride. The 6xHis-SAUR9 (or buffer control) was preincubated with 6xHis-PP2C.D on ice for 10 min prior to addition to the assay reaction. The protein mixture was then added into reaction buffer containing 2 µg of yeast PM proteins and 1.8 mm MnCl2 with final volume of 15 µL. The reaction mixture was incubated at 25°C for 5 min before stopping with SDS-PAGE sample buffer. GST-14-3-3 far-western gel blotting was performed to assess AHA2 Thr-947 phosphorylation status as previously described (Hayashi et al., 2010; Spartz et al., 2014).
In Vitro Pull-Down Assays
GST-PP2C.D1 and 6xHis-SAUR19 constructs and purification methods were described previously (Spartz et al., 2014). Mutant GST-PP2C.D1 constructs were made by QuikChange site-directed mutagenesis with the primers listed in Supplemental Table S1. Following expression and purification, approximately 1 µg each of GST-PP2C.D1 and SAUR19 was incubated in 250 µL of buffer C (Gray et al., 1999) for 1 h at 4°C, and beads were washed two times in buffer C with a final wash in buffer C + 0.1% (v/v) Nonidet P-40. Immunoblots of pulled down SAUR19 protein were performed with affinity-purified α-SAUR19 (Spartz et al., 2012).
BiFC Assay
pENTR/D-TOPO vectors containing full-length cDNA sequences without stop codons of wild-type or mutated PP2C.D were recombined into the pSPYCE destination vector for BiFC expression constructs (Walter et al., 2004). The expression constructs with PP2C.D1, PP2C.D2, PP2C.D5, PP2C.D6, AHA2, and AUX1 inserts were reported previously (Spartz et al., 2014; Ren et al., 2018). The BiFC assays were performed transiently in approximately 5-week-old Nicotiana benthamiana leaves as previously described (Schütze et al., 2009). YFP fluorescence signals were observed 3 d postinfiltration. The images were acquired using a Nikon Ti2 A1si Confocal system (Nikon USA).
Statistical Analyses
JMP Pro 13.1 software suite (SAS Institute) was used to perform ANOVA for all statistical analyses. Results were grouped by letters, with different letters indicating significant differences (P < 0.05) based on Tukey’s HSD test.
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under the following accession numbers: SAUR19 (At5g18010), SAUR9 (At4g36110), PP2C.D1 (At5g02760), PP2C.D2 (At3g17090), PP2C.D3 (At3g12620), PP2C.D4 (At3g55050), PP2C.D5 (At4g38520), PP2C.D6 (At3g51370), PP2C.D7 (At5g66080), PP2C.D8 (At4g33920), PP2C.D9 (At5g06750), AHA2 (At4g30190), and AUX1 (At2g38120).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. PP2C.D1 deletion analysis.
Supplemental Figure S2. Multiple sequence alignment of Arabidopsis PP2C phosphatases.
Supplemental Figure S3. Yeast two-hybrid assay testing protein-protein interactions between an ABI1-D1 fusion protein and SAUR19.
Supplemental Figure S4. BiFC assay of PP2C.D and SAUR-immune derivatives with AHA2.
Supplemental Figure S5. Subcellular localization of pp2c.d2M331K-GFP and pp2c.d5M328K-GFP.
Supplemental Figure S6. Expression of pp2c.d2M331K in Arabidopsis confers dramatic cell expansion defects.
Supplemental Figure S7. pp2c.d2M331K and pp2c.d5M328K plants have smaller floral organs.
Supplemental Figure S8. Auxin inhibition of root growth.
Supplemental Figure S9. pp2c.d2M331K is insensitive to SAUR19 overexpression.
Supplemental Figure S10. Yeast two-hybrid and BiFC assays testing protein-protein interaction between PLL4 and SAUR19.
Supplemental Table S1. List of oligonucleotides.
Acknowledgments
We thank the College of Biological Sciences Imaging Center for assistance with confocal microscopy and Dr. Anton Sanderfoot (University of Wisconsin-La Crosse) for providing SEC12 antisera.
Footnotes
This work was supported by the National Science Foundation (MCB-1613809 to W.M.G.) and the National Institutes of Health (GM067203 to W.M.G.).
Articles can be viewed without a subscription.
References
- Barbez E, Dünser K, Gaidora A, Lendl T, Busch W (2017) Auxin steers root cell expansion via apoplastic pH regulation in Arabidopsis thaliana. Proc Natl Acad Sci USA 114: E4884–E4893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae K, Isaacs CG, Reeves PH, Maloney GS, Muday GK, Nagpal P, Reed JW (2012) Arabidopsis SMALL AUXIN UP RNA63 promotes hypocotyl and stamen filament elongation. Plant J 71: 684–697 [DOI] [PubMed] [Google Scholar]
- Chapman EJ, Greenham K, Castillejo C, Sartor R, Bialy A, Sun TP, Estelle M (2012) Hypocotyl transcriptome reveals auxin regulation of growth-promoting genes through GA-dependent and -independent pathways. PLoS ONE 7: e36210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Cosgrove DJ. (2016) Catalysts of plant cell wall loosening. F1000Res 5: Faculty Rev-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couto D, Niebergall R, Liang X, Bücherl CA, Sklenar J, Macho AP, Ntoukakis V, Derbyshire P, Altenbach D, Maclean D, et al. (2016) The Arabidopsis protein phosphatase PP2C38 negatively regulates the central immune kinase BIK1. PLoS Pathog 12: e1005811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmasiri N, Dharmasiri S, Estelle M (2005) The F-box protein TIR1 is an auxin receptor. Nature 435: 441–445 [DOI] [PubMed] [Google Scholar]
- Fendrych M, Leung J, Friml J (2016) TIR1/AFB-Aux/IAA auxin perception mediates rapid cell wall acidification and growth of Arabidopsis hypocotyls. eLife 5: e19048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KA, Lee SH, Patel D, Kumar SV, Spartz AK, Gu C, Ye S, Yu P, Breen G, Cohen JD, et al. (2011) Phytochrome-interacting factor 4 (PIF4) regulates auxin biosynthesis at high temperature. Proc Natl Acad Sci USA 108: 20231–20235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs S, Grill E, Meskiene I, Schweighofer A (2013) Type 2C protein phosphatases in plants. FEBS J 280: 681–693 [DOI] [PubMed] [Google Scholar]
- Fuglsang AT, Visconti S, Drumm K, Jahn T, Stensballe A, Mattei B, Jensen ON, Aducci P, Palmgren MG (1999) Binding of 14-3-3 protein to the plasma membrane H+-ATPase AHA2 involves the three C-terminal residues Tyr946-Thr-Val and requires phosphorylation of Thr947. J Biol Chem 274: 36774–36780 [DOI] [PubMed] [Google Scholar]
- Gagne JM, Clark SE (2010) The Arabidopsis stem cell factor POLTERGEIST is membrane localized and phospholipid stimulated. Plant Cell 22: 729–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil P, Green PJ (1996) Multiple regions of the Arabidopsis SAUR-AC1 gene control transcript abundance: The 3′ untranslated region functions as an mRNA instability determinant. EMBO J 15: 1678–1686 [PMC free article] [PubMed] [Google Scholar]
- Gray WM, del Pozo JC, Walker L, Hobbie L, Risseeuw E, Banks T, Crosby WL, Yang M, Ma H, Estelle M (1999) Identification of an SCF ubiquitin-ligase complex required for auxin response in Arabidopsis thaliana. Genes Dev 13: 1678–1691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray WM, Kepinski S, Rouse D, Leyser O, Estelle M (2001) Auxin regulates SCFTIR1-dependent degradation of AUX/IAA proteins. Nature 414: 271–276 [DOI] [PubMed] [Google Scholar]
- Hagen G, Guilfoyle T (2002) Auxin-responsive gene expression: Genes, promoters and regulatory factors. Plant Mol Biol 49: 373–385 [PubMed] [Google Scholar]
- Hager A. (2003) Role of the plasma membrane H+-ATPase in auxin-induced elongation growth: Historical and new aspects. J Plant Res 116: 483–505 [DOI] [PubMed] [Google Scholar]
- Haruta M, Burch HL, Nelson RB, Barrett-Wilt G, Kline KG, Mohsin SB, Young JC, Otegui MS, Sussman MR (2010) Molecular characterization of mutant Arabidopsis plants with reduced plasma membrane proton pump activity. J Biol Chem 285: 17918–17929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haruta M, Gray WM, Sussman MR (2015) Regulation of the plasma membrane proton pump (H+-ATPase) by phosphorylation. Curr Opin Plant Biol 28: 68–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y, Nakamura S, Takemiya A, Takahashi Y, Shimazaki K, Kinoshita T (2010) Biochemical characterization of in vitro phosphorylation and dephosphorylation of the plasma membrane H+-ATPase. Plant Cell Physiol 51: 1186–1196 [DOI] [PubMed] [Google Scholar]
- Hou K, Wu W, Gan SS (2013) SAUR36, a small auxin up RNA gene, is involved in the promotion of leaf senescence in Arabidopsis. Plant Physiol 161: 1002–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelich-Ottmann C, Weiler EW, Oecking C (2001) Binding of regulatory 14-3-3 proteins to the C terminus of the plant plasma membrane H+-ATPase involves part of its autoinhibitory region. J Biol Chem 276: 39852–39857 [DOI] [PubMed] [Google Scholar]
- Kepinski S, Leyser O (2005) The Arabidopsis F-box protein TIR1 is an auxin receptor. Nature 435: 446–451 [DOI] [PubMed] [Google Scholar]
- Kinoshita T, Shimazaki Ki (1999) Blue light activates the plasma membrane H+-ATPase by phosphorylation of the C-terminus in stomatal guard cells. EMBO J 18: 5548–5558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauss S, Rohrmeier T, Lehle L (2003) The auxin-induced maize gene ZmSAUR2 encodes a short-lived nuclear protein expressed in elongating tissues. J Biol Chem 278: 23936–23943 [DOI] [PubMed] [Google Scholar]
- Kohalmi SE, Reader LJV, Samach A, Nowak J, Haughn GW, Crosby WL (1998) Identification and characterization of protein interactions using the yeast 2-hybrid system. In Gelvin SB, Schilperoort RA, eds, Plant Molecular Biology Manual. Springer, Dordrecht, The Netherlands, pp 95–124 [Google Scholar]
- Lavy M, Estelle M (2016) Mechanisms of auxin signaling. Development 143: 3226–3229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln C, Britton JH, Estelle M (1990) Growth and development of the axr1 mutants of Arabidopsis. Plant Cell 2: 1071–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueen-Mason S, Durachko DM, Cosgrove DJ (1992) Two endogenous proteins that induce cell wall extension in plants. Plant Cell 4: 1425–1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlot S, Leonhardt N, Fenzi F, Valon C, Costa M, Piette L, Vavasseur A, Genty B, Boivin K, Müller A, et al. (2007) Constitutive activation of a plasma membrane H+-ATPase prevents abscisic acid-mediated stomatal closure. EMBO J 26: 3216–3226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möller B, Poeschl Y, Plötner R, Bürstenbinder K (2017) PaCeQuant: A tool for high-throughput quantification of pavement cell shape characteristics. Plant Physiol 175: 998–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panaretou B, Piper P (2006) Isolation of yeast plasma membranes. Methods Mol Biol 313: 27–32 [DOI] [PubMed] [Google Scholar]
- Rayle DL, Cleland R (1970) Enhancement of wall loosening and elongation by acid solutions. Plant Physiol 46: 250–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayle DL, Cleland RE (1980) Evidence that auxin-induced growth of soybean hypocotyls involves proton excretion. Plant Physiol 66: 433–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayle DL, Cleland RE (1992) The acid growth theory of auxin-induced cell elongation is alive and well. Plant Physiol 99: 1271–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren H, Gray WM (2015) SAUR proteins as effectors of hormonal and environmental signals in plant growth. Mol Plant 8: 1153–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren H, Park MY, Spartz AK, Wong JH, Gray WM (2018) A subset of plasma membrane-localized PP2C.D phosphatases negatively regulate SAUR-mediated cell expansion in Arabidopsis. PLoS Genet 14: e1007455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savaldi-Goldstein S, Baiga TJ, Pojer F, Dabi T, Butterfield C, Parry G, Santner A, Dharmasiri N, Tao Y, Estelle M, et al. (2008) New auxin analogs with growth-promoting effects in intact plants reveal a chemical strategy to improve hormone delivery. Proc Natl Acad Sci USA 105: 15190–15195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schütze K, Harter K, Chaban C (2009) Bimolecular fluorescence complementation (BiFC) to study protein-protein interactions in living plant cells. Methods Mol Biol 479: 189–202 [DOI] [PubMed] [Google Scholar]
- Sentandreu M, Martín G, González-Schain N, Leivar P, Soy J, Tepperman JM, Quail PH, Monte E (2011) Functional profiling identifies genes involved in organ-specific branches of the PIF3 regulatory network in Arabidopsis. Plant Cell 23: 3974–3991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spartz AK, Lee SH, Wenger JP, Gonzalez N, Itoh H, Inzé D, Peer WA, Murphy AS, Overvoorde PJ, Gray WM (2012) The SAUR19 subfamily of SMALL AUXIN UP RNA genes promote cell expansion. Plant J 70: 978–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spartz AK, Ren H, Park MY, Grandt KN, Lee SH, Murphy AS, Sussman MR, Overvoorde PJ, Gray WM (2014) SAUR inhibition of PP2C-D phosphatases activates plasma membrane H+-ATPases to promote cell expansion in Arabidopsis. Plant Cell 26: 2129–2142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spartz AK, Lor VS, Ren H, Olszewski NE, Miller ND, Wu G, Spalding EP, Gray WM (2017) Constitutive expression of Arabidopsis SMALL AUXIN UP RNA19 (SAUR19) in tomato confers auxin-independent hypocotyl elongation. Plant Physiol 173: 1453–1462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strader LC, Zhao Y (2016) Auxin perception and downstream events. Curr Opin Plant Biol 33: 8–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun N, Wang J, Gao Z, Dong J, He H, Terzaghi W, Wei N, Deng XW, Chen H (2016) Arabidopsis SAURs are critical for differential light regulation of the development of various organs. Proc Natl Acad Sci USA 113: 6071–6076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Hayashi K, Kinoshita T (2012) Auxin activates the plasma membrane H+-ATPase by phosphorylation during hypocotyl elongation in Arabidopsis. Plant Physiol 159: 632–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar-Mendez A, Miernyk JA, Hoyos E, Randall DD (2014) A functional genomic analysis of Arabidopsis thaliana PP2C clade D. Protoplasma 251: 265–271 [DOI] [PubMed] [Google Scholar]
- Vi SL, Trost G, Lange P, Czesnick H, Rao N, Lieber D, Laux T, Gray WM, Manley JL, Groth D, et al. (2013) Target specificity among canonical nuclear poly(A) polymerases in plants modulates organ growth and pathogen response. Proc Natl Acad Sci USA 110: 13994–13999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter M, Chaban C, Schütze K, Batistic O, Weckermann K, Näke C, Blazevic D, Grefen C, Schumacher K, Oecking C, et al. (2004) Visualization of protein interactions in living plant cells using bimolecular fluorescence complementation. Plant J 40: 428–438 [DOI] [PubMed] [Google Scholar]
- Weber H, Bernhardt A, Dieterle M, Hano P, Mutlu A, Estelle M, Genschik P, Hellmann H (2005) Arabidopsis AtCUL3a and AtCUL3b form complexes with members of the BTB/POZ-MATH protein family. Plant Physiol 137: 83–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao D, Cui Y, Xu F, Xu X, Gao G, Wang Y, Guo Z, Wang D, Wang NN (2015) SENESCENCE-SUPPRESSED PROTEIN PHOSPHATASE directly interacts with the cytoplasmic domain of SENESCENCE-ASSOCIATED RECEPTOR-LIKE KINASE and negatively regulates leaf senescence in Arabidopsis. Plant Physiol 169: 1275–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu LP, Miller AK, Clark SE (2003) POLTERGEIST encodes a protein phosphatase 2C that regulates CLAVATA pathways controlling stem cell identity at Arabidopsis shoot and flower meristems. Curr Biol 13: 179–188 [DOI] [PubMed] [Google Scholar]
- Zhao Y. (2018) Essential roles of local auxin biosynthesis in plant development and in adaptation to environmental changes. Annu Rev Plant Biol 69: 417–435 [DOI] [PubMed] [Google Scholar]