SPX4 localizes to both the cytosol and nucleus, acting as a dose-dependent modulator of PHR1-dependent and PHR1-independent phosphate-starvation responses in shoots.
Abstract
Phosphorus (P) is an essential macronutrient for all living organisms and limits plant growth. Four proteins comprising a single SYG1/Pho81/XPR1 (SPX) domain, SPX1 to SPX4, are putative phosphate-dependent inhibitors of Arabidopsis (Arabidopsis thaliana) PHOSPHATE STARVATION RESPONSE1 (PHR1), the master transcriptional activator of phosphate starvation responses. This work demonstrated that SPX4 functions as a negative regulator not only of PHR1-dependent but also of PHR1-independent responses in P-replete plants. Transcriptomes of P-limited spx4 revealed that, unlike SPX1 and SPX2, SPX4 modulates the shoot phosphate starvation response but not short-term recovery after phosphate resupply. In roots, transcriptional regulation of P status is SPX4 independent. Genes misregulated in spx4 shoots intersect with both PHR1-dependent and PHOSPHATE2-dependent signaling networks associated with plant development, senescence, and ion/metabolite transport. Gene regulatory network analyses suggested that SPX4 interacts with transcription factors other than PHR1, such as SUPPRESSOR OF OVEREXPRESSION OF CONSTANS1 and ARABIDOPSIS NAC DOMAIN CONTAINING PROTEIN55, known regulators of shoot development. Transient expression studies in protoplasts indicated that PHR1 retention in the cytosol by SPX4 occurs in a dose- and P-status-dependent manner. Using a luciferase reporter in vivo, SPX4 expression kinetics and stability revealed that SPX4 is a short-lived protein with P-status-dependent turnover. SPX4 protein levels were quickly restored by phosphate resupply to P-limited plants. Unlike its monocot ortholog, AtSPX4 was not stabilized by the phosphate analog phosphite, implying that intracellular P status is sensed by its SPX domain via phosphate-rich metabolite signals.
Phosphorus (P) is an essential macronutrient for all living organisms. Apart from nitrogen, it is the most limiting factor for plant growth and development in both natural and agricultural ecosystems. Phosphate (Pi), the inorganic form of P, that is taken up and metabolized by plants, is a key substrate for most biochemical reactions in the cell. Even though P can be abundant in soils, it is mostly unavailable to plants due to the conversion of Pi into organic compounds by microorganisms (Richardson and Simpson, 2011) and fixation to soil particles (Hinsinger, 2001). In order to maintain agricultural productivity and provide food security for a growing global population, our already heavy reliance on Pi fertilizers is bound to increase. Yet, this practice is neither economically nor environmentally sustainable, as it leads to depletion of natural resources and eutrophication of water bodies. Hence, achieving sustainable P use in crop plants has become a major goal in plant breeding (Veneklaas et al., 2012; Heuer et al., 2017).
To cope with Pi scarcity, plants have evolved a range of adaptations, collectively known as the Pi-starvation response (PSR; Raghothama, 1999). PSR typically aims at increasing Pi uptake from soil particles (e.g. through modifications in root system architecture alongside enhanced exudation of carboxylates and phosphatases) or maximizing Pi utilization, through increased Pi translocation from shoot to root, release from vacuolar stores, and remobilization within tissues (Raghothama, 1999). Two common root adaptations to increase Pi uptake in higher plants (i.e. cluster-root formation and mycorrhizal associations; Péret et al., 2014) are absent in the Arabidopsis (Arabidopsis thaliana) model, yet this model has allowed the identification of key regulators of most acclimation responses (Rubio et al., 2001; Aung et al., 2006; Bari et al., 2006; Chen et al., 2007; Devaiah et al., 2007, 2009; Camacho-Cristóbal et al., 2008; Duan et al., 2008; Bustos et al., 2010; Lundmark et al., 2011; Arpat et al., 2012; Puga et al., 2014; Chen and Schmidt, 2015; Baek et al., 2017). The discoveries that there are also negative regulators of PSR in P-sufficient plants (Mukatira et al., 2001) and that many PSR components are shut down within hours of Pi resupply (Burleigh and Harrison, 1999; Müller et al., 2004; Woo et al., 2012; Secco et al., 2013) pose a challenge to improving Pi uptake in agricultural systems: due to negative feedback loops acting on root Pi uptake, crops such as wheat (Triticum aestivum), oat (Avena sativa), or maize (Zea mays) currently use only between 10% and 30% of the Pi fertilizer applied (Bezzola et al., 1994; Glendinning and Fertilizer Industry Federation of Australia, 2000). It is hence of utmost importance to gain a better understanding of the role of these negative regulators in modulating P efficiency in plants.
Transcriptional reprogramming in response to Pi starvation in Arabidopsis is largely regulated by the GARP family of R2R3 MYB DOMAIN PROTEIN (MYB) transcription factors, namely PHOSPHATE STARVATION RESPONSE1 (PHR1), PHR1-LIKE1 (PHL1), and PHL2 (Rubio et al., 2001; Sun et al., 2016). Regulatory regions of almost all PSR genes are greatly enriched in the P1BS cis-acting motif, and the absence of PHR1 and PHL1 leads to misregulation of about 75% and 65% of Pi-starvation-induced and -repressed genes, respectively (Bustos et al., 2010). Other MYB factors such as MYB62 (Devaiah et al., 2009), GARP COILED-COIL7 (GCC7; Lundmark et al., 2011), HRS1 HOMOLOG2, and three R3-type MYBs (Chen and Schmidt, 2015) have been implicated in a number of PHR1-dependent and -independent Pi starvation responses, indicating both functional redundancy and/or discrete regulatory networks that regulate PSR in different plant organs (Wu et al., 2003; Devaiah et al., 2009) or biochemical pathways (Acevedo-Hernández et al., 2012).
Information on the molecular basis of P sensing and early signaling upstream of PHR1 is scarce (Abel et al., 2002). While PHR1 transcripts are regulated by light and ethylene signals (Liu et al., 2017), they are not directly affected by changes in Pi availability (Rubio et al., 2001). Instead, PHR1 activity seems to rely on additional layers of posttranscriptional regulation, including SUMOylation by SAP AND MIZ1 (Miura et al., 2005) and interaction with SYG1/PHO81/XPR1 (SPX) domain-containing proteins (Lv et al., 2014; Puga et al., 2014; Wang et al., 2014; Qi et al., 2017).
The latter form a group of four proteins in Arabidopsis, SPX1 to SPX4, made up of a single SPX domain (Duan et al., 2008). Transcripts of SPX1, SPX2, and SPX3 accumulate in roots and shoots of P-limited plants in a PHR1-dependent manner (Duan et al., 2008; Bustos et al., 2010). SPX4 transcripts, on the other hand, show only a weak suppression by Pi withdrawal or knockout of PHR1 (Duan et al., 2008). Nucleus-localized SPX1 binds to and inhibits PHR1 activity in the presence of Pi (Puga et al., 2014). Similar findings have been reported for the interaction of rice (Oryza sativa) orthologs, OsSPX1 and OsSPX2, with the PHR1 ortholog OsPHR2 (Wang et al., 2014). Arabidopsis SPX1 binds to monomeric PHR1 in the presence of either 5 mm Pi or 50 μm inositol hexakisphosphate in vitro but not to PHR1 dimers bound to twin P1BS-binding sites (Qi et al., 2017). Eukaryotic SPX domains were found to directly bind to inositol polyphosphate signaling molecules (IPs) in response to shifts in cellular Pi levels (Wild et al., 2016), positioning SPX proteins as prime candidates for P(i) sensors in plants. In both Arabidopsis and rice, SPX1 and SPX2 display a significant degree of redundancy, with only double knockout mutants showing substantial accumulation of Pi in shoots and changes in the expression of PHR1-dependent PSR genes (Puga et al., 2014; Wang et al., 2014). Shoot accumulation of Pi in OsPHR2 overexpression lines in rice could be reversed by simultaneous overexpression of OsSPX1; this was attributed to differential expression of the Pi transporter gene OsPT2 in the root of these lines (Liu et al., 2010).
PHOSPATE2 (PHO2)/UBIQUITIN-CONJUGATING ENZYME24, a ubiquitin E2 conjugase, is another well-characterized repressor of select PSR gene function in roots. PHO2 regulates root Pi uptake and its translocation to shoots via targeting of Pi transporter proteins of the PHOSPHATE TRANSPORTER1 (PHT1) family (Huang et al., 2013; Park et al., 2014) as well as of the Pi exporter PHO1 (Liu et al., 2012) for proteasomal degradation. The complex down-regulation of PHO2 transcripts in roots by select shoot-derived microRNA399 species and their proposed sequestration by antagonistic noncoding small RNAs INDUCED BY PI STARVATION1 (IPS1) and IPS2/AT4 are known to occur downstream of PHR1 (Fujii et al., 2005; Aung et al., 2006; Bari et al., 2006; Franco-Zorrilla et al., 2007; Pant et al., 2008). In rice, both Ospho2 knockout and OsmiR399k overexpression lines showed enhanced transcript accumulation of OsSPX1 in roots but not shoots (Liu et al., 2010). OsPHO2 expression in turn was also higher in roots, not shoots, of OsSPX1 overexpression lines. Liu et al. (2010) concluded at the time that a root-specific negative feedback loop existed between OsSPX1 and OsPHO2. The role of PHO2 in shoots, however, remains largely unknown, with evidence suggesting a possible role in flowering time regulation in both Arabidopsis and rice (Kant et al., 2011; Kim et al., 2011; Li et al., 2017).
The function of a third negative regulator of PSR gene expression, SPX4, has recently been studied in rice. Lv et al. (2014) found that it, too, interacts with OsPHR2 in a P-status-dependent manner. However, unlike OsSPX1, OsSPX4-GFP fusion protein was detected in both cytosol and nucleus of transgenic root cells as well as in rice protoplasts. OsSPX4 was also shown to inhibit the translocation of OsPHR2 from the cytosol into the nucleus by bimolecular fluorescence complementation assays (Lv et al., 2014). The authors were able to show that there is no functional redundancy in rice, given that the spx4 T-DNA insertion line showed Pi accumulation and derepression of the PSR gene IPS1 in P-replete shoots (Lv et al., 2014). The fact that this negative regulator also undergoes rapid turnover in extracts of P-deficient plants and is stabilized in the presence of either Pi or its analog phosphite (Phi; Lv et al., 2014) would furthermore suggest its immediate role in the sensing of cellular P status. In addition, organ-specific differences between root and shoot regulatory networks have been shown for OsSPX6, which is only degraded in P-limited leaves but accumulates in roots (Zhong et al., 2018).
Given the importance of overcoming negative regulators of Pi uptake for increasing P use in plants, and the distinct differences in PSR regulatory networks in monocots and dicots, we focused on dissecting SPX4-dependent gene regulatory networks in Arabidopsis. Use of a luciferase reporter, protoplast transfection with fluorescent fusion proteins, mutants of spx4, transcriptome analyses under Pi-replete, -limited, and -resupply conditions, and comparison with PHR1 and PHO2 master regulators revealed that SPX4 acts as a modulator of transcription factor activities in P-replete and P-limited shoots. Potential downstream targets of SPX4, other than PHR1, and the role of PHO2 are discussed.
RESULTS
SPX4 Is a Short-Lived Protein with P-Dependent Turnover
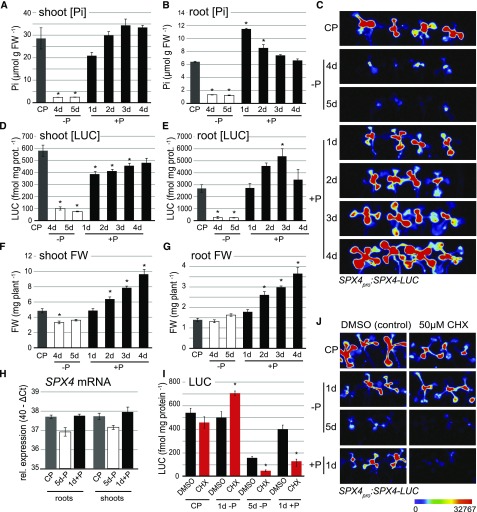
Among the four Arabidopsis genes encoding single SPX domain proteins, named SPX1 to SPX4, SPX4 is the only one not induced by Pi starvation. In fact, SPX4 transcripts were mildly repressed by Pi deprivation in roots and shoots, going back to control levels within 24 h of Pi resupply (Duan et al., 2008; Fig. 1H). A similar expression pattern was described for the rice ortholog OsSPX4 (Lv et al., 2014). In vitro, OsSPX4 protein levels fluctuate with Pi availability, with 26S proteasome-dependent degradation dominating under P deficiency (Lv et al., 2014). To determine the stability of AtSPX4 in vivo, a SPX4pro:SPX4-LUC reporter line was created in which the coding region of SPX4 is translationally fused to firefly luciferase (LUC) under the control of its native promoter, allowing us to monitor SPX4 protein kinetics under varying Pi supplies in seedlings. Pi resupply to P-starved seedlings led to distinct Pi and SPX4-LUC accumulation kinetics in each organ: in shoots, Pi pools were restored within 2 d of Pi resupply (Fig. 1A), marked by a concomitant but slower recovery of SPX4-LUC protein and activity (Fig. 1, C and D). In roots, SPX4-LUC levels were restored to those in control plants within 24 h of Pi resupply but increased further until day 3 of Pi resupply (Fig. 1E). This overshooting of SPX4-LUC levels in roots is counterintuitive, given that Pi accumulation in this organ peaks after 1 d of resupply (Fig. 1B). SPX4-LUC levels in roots thus appear to be coupled to the stabilization of intracellular Pi pools in shoots rather than local Pi concentration.
Figure 1.
SPX4 protein stability is affected by P-dependent changes in protein turnover. A, B, and D to G, Organ Pi concentration (A and B), quantification of LUC activity (D and E), and organ biomass (F and G) in seedlings grown under varying Pi supplies. CP = control (P-replete), −P = P-limited, and +P = Pi-resupplied plants. Values are means ± se of at least three independent biological replicates, each comprising pools of four to six organs. Asterisks indicate significant differences from P-replete plants (P < 0.05, ANOVA/Tukey). FW, Fresh weight. H, Relative SPX4 transcript abundance in Col-0 roots and shoots grown under varying Pi supplies. Relative expression is shown as 40−ΔCt, compared with ACT7, UBC9, and UBC21 reference genes (n = 3 independent biological replicates). No significant differences were found across treatments (P < 0.05, ANOVA/Tukey). I, Quantification of LUC activity in SPX4pro:SPX4-LUC shoots treated for 24 h with DMSO (control; black bars) or 50 µM CHX (red bars) under varying Pi supplies. Values are means ± se of at least three independent biological replicates. Asterisks indicate significant differences from DMSO treatment for each condition (P < 0.05, ANOVA/Tukey). C and J, LUC imaging of Arabidopsis SPX4pro:SPX4-LUC shoots over a time course of Pi limitation and resupply (C) and upon CHX treatment (J). Luminescence is shown as units of pixel intensity.
SPX4 protein turnover changed in a P-dependent manner when SPX4pro:SPX4-LUC seedlings were treated (via their roots) with cycloheximide (CHX), an inhibitor of de novo protein synthesis. LUC activity in shoots was found to be lower in P-replete and in Pi-resupply conditions compared with the corresponding mock treatments (0.01% dimethyl sulfoxide [DMSO]). This was shown both by LUC imaging (Fig. 1J) and quantification of LUC activity (Fig. 1I). Thus, increasing SPX4-LUC levels in the presence of Pi requires de novo protein synthesis. Simultaneous CHX treatment of seedlings upon transfer from P-replete to P-deficient medium for 24 h resulted in increased LUC activity (Fig. 1, I and J, 1d −P), while 24-h CHX treatment of seedlings already starved of Pi for 4 d resulted in a significant decrease in LUC activity (Fig. 1, I and J, 5d −P). Reduction of LUC protein and activity levels in CHX-treated plants irrespective of Pi supply indicates that faster SPX4 degradation, not reduced de novo protein synthesis, is a major factor affecting SPX4 turnover, ensuring that basal levels of functional SPX4 are still being maintained in P-starved shoots. Protein(s) responsible for triggering SPX4 degradation seem to be synthesized de novo in the early phase of Pi depletion, which would explain the higher SPX4 accumulation in CHX-treated shoots at the 1-d −P time point compared with control plants.
To demonstrate the contribution of ubiquitin-mediated SPX4 degradation, the effect of an inhibitor of the 26S proteasome, MG132, on SPX4-LUC protein levels in P-starved seedlings was assessed. Unlike the stabilizing effect of MG132 treatment on OsSPX4 levels in extracts from P-starved rice seedlings (Lv et al., 2014), no differences in LUC activity were observed in vivo by treating plants with MG132 in combination with CHX (Park et al., 2014; Crozet et al., 2016; Supplemental Fig. S1). E-64d, an inhibitor of endosomal Cys proteases (Yamada et al., 2005), was also tested to no effect (Supplemental Fig. S1). The divergent results between OsSPX4 and AtSPX4 are likely due to our finding that SPX4 degradation requires de novo protein synthesis in Arabidopsis (Fig. 1I, 1d −P), but the main finding of P-dependent SPX4 turnover is consistent in both species.
Phi Supply Does Not Mimic Pi Resupply in Restoring SPX4 Levels
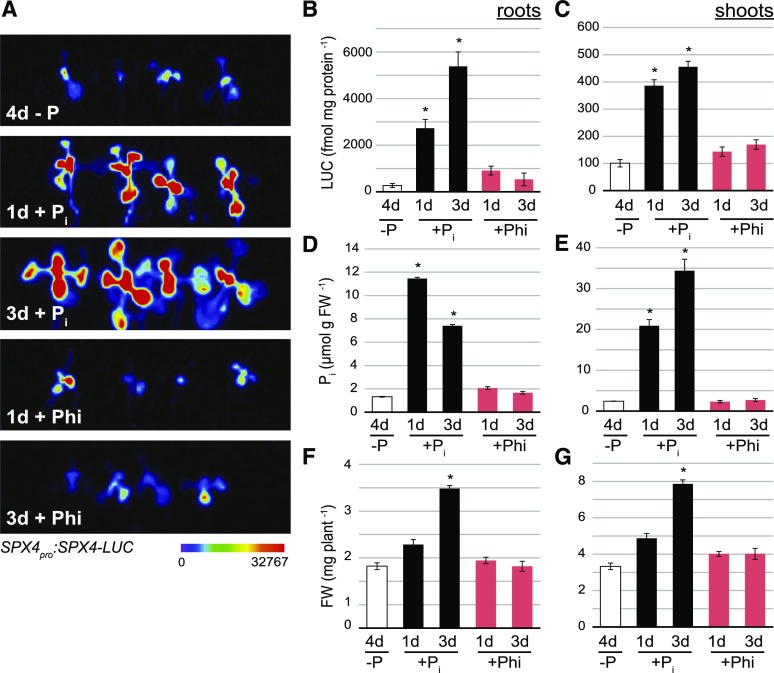
Phi (H2PO3−) is a more reduced form of P and a Pi mimetic that cannot be metabolized by plants (McDonald et al., 2001). It can be used to discriminate between processes that are directly dependent on Pi and those that require an organic P metabolite (Jost et al., 2015). Hence, we tested its ability to substitute Pi in stabilizing SPX4 levels in vivo. We compared the effect of short-term (24 h) or medium-term (72 h) Pi or Phi supply to Pi-starved seedlings grown on vertical plates. Phi treatment did not recover Pi pools (Fig. 2, D and E; Jost et al., 2015), leading to a complete arrest of root and shoot growth (Fig. 2, F and G). In contrast to OsSPX4 (Lv et al., 2014) and AtSPX1 (Puga et al., 2014), AtSPX4 levels in P-limited roots and shoots were not restored by provision of Phi (Fig. 2, B and C). In P-starved shoots, LUC activity increased by fourfold within 24 h of Pi resupply, remaining highly induced at the 3-d time point (Fig. 2C). LUC activity in Phi-treated shoots, on the other hand, was not significantly different (P > 0.05) from that in P-starved shoots. In roots, SPX4 levels steadily increased upon Pi resupply, with 10- and 20-fold increases after 1 and 3 d of treatment, respectively (Fig. 2B). Phi treatment of P-starved roots led to a transient threefold higher LUC activity within 24 h. This weak LUC induction in Phi-treated roots coincided with a slight transient increase in root Pi concentration (Fig. 2D), most likely caused by scavenging of residual Pi present on fresh agar plates. Our results indicate that SPX4 does not recognize the metabolically inert Pi mimetic Phi, further supporting the involvement of organic P molecules, such as IPs, in primary sensing of intracellular P status via direct binding to the SPX domain (Wild et al., 2016).
Figure 2.
SPX4 protein levels in P-limited shoots recover upon Pi but not Phi treatment. A, Luciferase imaging of Arabidopsis SPX4pro:SPX4-LUC shoots comparing the effects of Pi resupply and Phi supply to P-limited seedlings. Luminescence is shown as units of pixel intensity. B to G, Quantification of LUC activity (B and C), tissue Pi concentration (D and E), and biomass (F and G) in roots and shoots of P-limited, Pi-resupplied, and Phi-supplied seedlings. Values are means ± se of at least three independent biological replicates, comprising pools of four to six organs each. Asterisks indicate significant differences from P-limited seedlings grown for 4 d without Pi supply (P < 0.05, ANOVA/Tukey). FW, Fresh weight.
Coexpression with SPX4 Leads to Dose- and Pi Treatment-Dependent Retention of PHR1 in the Cytosol
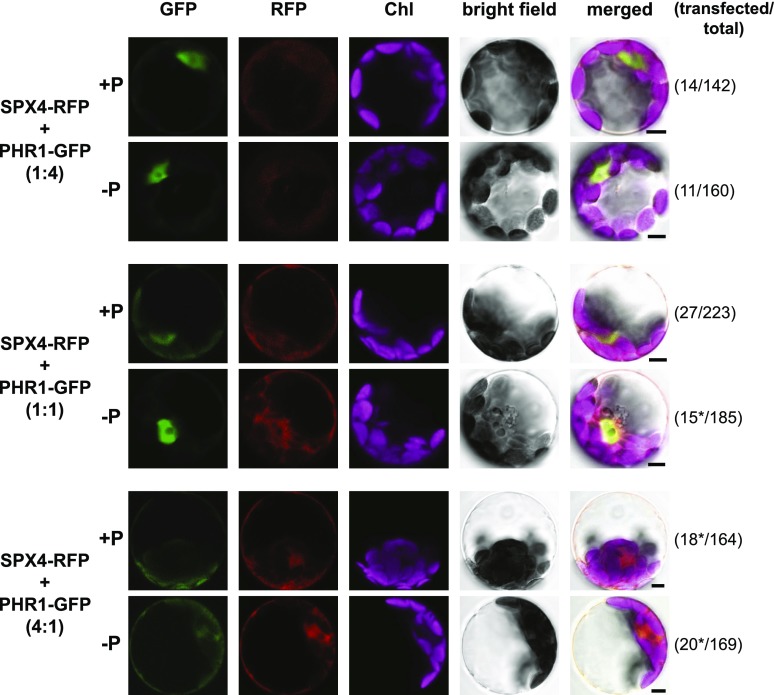
In rice, OsSPX4 and OsPHR2 were shown to interact in vivo via bimolecular fluorescence complementation analysis, resulting in retention of OsPHR2 in the cytosol (Lv et al., 2014). Here, we wanted to address whether the cytosolic accumulation of PHR1 in Arabidopsis can be induced upon AtSPX4 coexpression and whether this depends on SPX4 abundance and/or cellular P status. Upon transfection of Arabidopsis Columbia-0 (Col-0) protoplasts with a construct expressing a C-terminal SPX4-RFP fusion under the control of the 35S promoter, mRFP fluorescence was detected with similar intensity in both cytosol and nucleus, irrespective of Pi treatment (Supplemental Fig. S2). This would suggest that, unlike the SPX4-LUC fusion (Fig. 1), the fluorescent protein tag prevents P-status-dependent SPX4 turnover. This is most likely a reflection of the better suitability of luciferase as a reporter for protein turnover, given its much shorter half-life (4 min to 4 h) compared with, for example, 18 h for GFP in plants (de Ruijter et al., 2003). Consistent with stably transformed 35S:PHR1-GFP lines (Rubio et al., 2001), transfection with a 35Spro:PHR1-GFP construct resulted in strong nuclear fluorescence, irrespective of Pi supply (Supplemental Fig. S3). Coexpression of SPX4-RFP and PHR1-GFP fusions led to a partial retention of eGFP fluorescence in the cytosol, but only in P-replete Col-0 protoplasts (Supplemental Fig. S3). In P-limited protoplasts, the eGFP signal was almost exclusively found in the nucleus. This differential localization was not observed when the 35Spro:SPX4-RFP construct was coexpressed with a 35S:GFP control (Supplemental Fig. S2). In P-replete spx4-1 cells, the relative shift in eGFP fluorescence from nucleus to cytosol upon coexpression of PHR1-GFP and SPX4-RFP appeared weaker than in Col-0 protoplasts (Supplemental Fig. S4). Expression of SPX4-RFP on its own resulted in a much stronger nuclear signal than in the other two genotypes. In P-limited phr1-2 protoplasts, a weak cytosolic PHR1-GFP signal was already detected upon single transfection with the 35Spro:PHR1-GFP construct (Supplemental Fig. S5, top). A stronger nuclear SPX4-RFP signal in P-limited phr1-2 protoplasts was observed upon PHR1-GFP coexpression. These results indicated a fine balance between SPX4 and PHR1 protein abundance and their respective subcellular localization. To investigate this further, we used different ratios of SPX4-RFP and PHR1-GFP constructs for transfection of spx4-1 protoplasts (Fig. 3). With increasing 35Spro:SPX4-RFP construct input, ranging from 0.25-fold to 4-fold excess over 35Spro:PHR1-GFP, the cytosolic eGFP signal became stronger, while the nuclear signal got fainter. At a 1:1 SPX4-to-PHR1 ratio, this response was P-status dependent (see Supplemental Fig. S4). At a 4:1 SPX4-to-PHR1 ratio, PHR1-GFP signal was almost exclusively cytosolic, irrespective of Pi treatment. A similar dose response was observed upon transfection of phr1-2 protoplasts (Supplemental Fig. S6). Together, these data suggest an interaction of AtSPX4 and AtPHR1 in the cytosol, as previously observed for the rice orthologs (Lv et al., 2014). In contrast to rice, sequestration of AtPHR1 by AtSPX4 in the cytosol is weaker, with both proteins still detected in the nucleus. PHR1 accumulation in the cytosol occurs at lower SPX4-to-PHR1 ratios in P-replete protoplasts, while a large excess of SPX4 over PHR1 is required to achieve cytosolic retention of PHR1 in P-limited cells. The fact that these changes in subcellular localization of PHR1 occur despite the lack of turnover of the SPX4-RFP fusion in P-limited protoplasts would suggest P-status-dependent modification of their interaction.
Figure 3.
SPX4 affects the translocation of PHR1 to the nucleus in a dose- and Pi-dependent manner. Transfecting spx4-1 mutant protoplasts with equal amounts of 35Spro:SPX4-RFP and 35Spro:PHR1-GFP constructs leads to partial retention of PHR1-GFP in the cytosol, but only in P-replete conditions (+P). In P-limited (−P) spx4-1 protoplasts, PHR1-GFP fluorescence is almost exclusively found in the nucleus. Increasing the ratio of SPX4-RFP over PHR1-GFP fusion construct in the transfection reaction (from 0.25-fold in the top row of images to 4-fold in the bottom row of images) leads to more prominent eGFP fluorescence in the cytosol. At the same time, RFP fluorescence intensity increases from the top to bottom row of images, but the signal remains evenly distributed between nucleus and cytosol. Almost exclusive nuclear localization of the PHR1-GFP fusion protein in P-limited protoplasts is disrupted only in the presence of a 4-fold excess of SPX4-RFP protein (bottom row of images). On the right, the number of transfected protoplasts over the total number of cells scanned for fluorescence is given in parentheses. The images shown are representative of each transfection reaction. Asterisks indicate that between one (1:1 ratio, −P) and five (4:1 ratio, +P) protoplasts had detectable levels of RFP fluorescence only, indicating reduced cotransfection rates with PHR1-GFP. Chl, Chlorophyll. Bars = 5 µm.
Disruption of SPX4 Function Leads to Pi Overaccumulation in Shoots
In order to assess the physiological effects of disrupting SPX4, we isolated two T-DNA insertion lines, SALK_019826 and SK40726, named spx4-1 and spx4-2, respectively. The molecular characterization of these lines is shown in Supplemental Figure S7. Sequencing of T-DNA left-border amplicons confirmed that the single insertions in spx4-1 and spx4-2 are in the second and third (last) exons of the SPX4 gene, respectively. Both insertions disrupt the third portion of the SPX tripartite domain (PFam:03105; Supplemental Fig. S7A), upstream of the last amino acid residue forming the phosphate-binding cluster and of the Lys-binding cluster, which are active sites involved in binding to IPs (Wild et al., 2016; Azevedo and Saiardi, 2017). SPX4 transcripts downstream of the insertion site were not detected in spx4-1 and were reduced in the weaker spx4-2 allele compared with the wild type (Supplemental Fig. S7C). When mutant alleles were grown side by side with their respective wild-type backgrounds (Col-0 for spx4-1 and Col-4 for spx4-2) in nutrient-rich soil, an average of 22% reduction in rosette biomass was observed (Supplemental Fig. S7D).
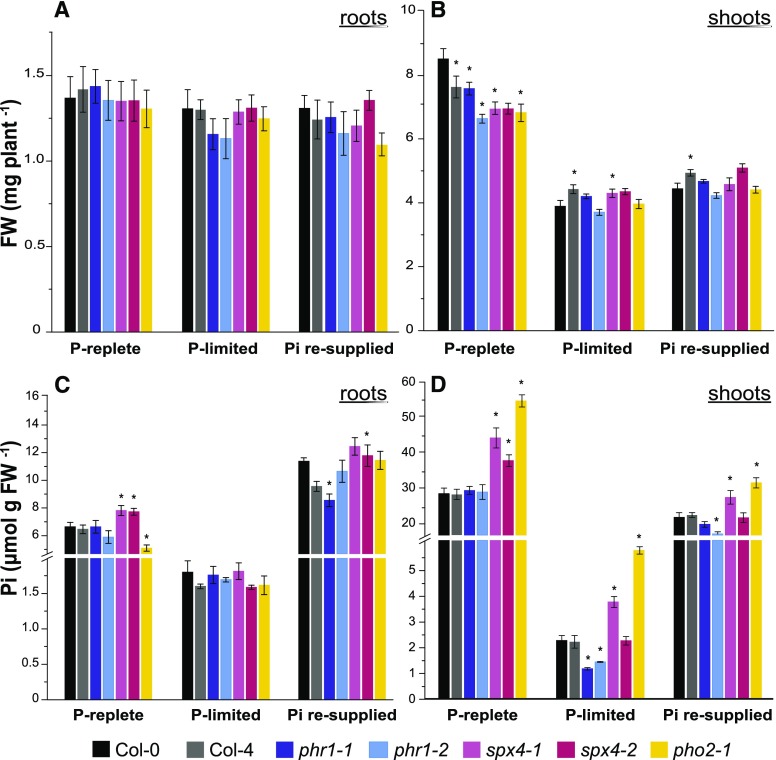
For the physiological characterization of the two SPX4 mutant alleles under varying Pi supplies, seedlings were grown vertically on one-half-strength Murashige Skoog (MS) agar plates (see “Materials and Methods”). In addition to wild-type lines (Col-0 and Col-4), mutants of PHR1, phr1-1 (Rubio et al., 2001) and phr1-2 (SALK_067629), displaying reduced shoot Pi concentration under Pi deficiency (Nilsson et al., 2007; Bustos et al., 2010), and PHO2 (pho2-1/CS8508), known to overaccumulate Pi in shoots (Dong et al., 1998), were added for comparison. The experiment comprised three conditions causing changes in plant Pi status (Fig. 4): under Pi-replete conditions, seedlings were maintained on sufficient Pi throughout the experiment (including two transfers to fresh medium), thus having fully replete root and shoot Pi pools (Fig. 4, C and D) for optimal plant growth (Fig. 4, A and B). Under Pi limitation, 7-d-old seedlings were deprived of Pi for 4 d, followed by an additional 1 d on fresh P-deficient medium prior to harvest. This 5-d treatment was enough to deplete root and shoot Pi pools in the wild type, leading to a 50% reduction in shoot growth (Fig. 4B). For Pi resupply, P-limited seedlings were transferred to 1 mm Pi for 1 d after 4 d of Pi deprivation. At this time point, accelerated Pi uptake and root-to-shoot translocation were observed in the wild type, with roots accumulating almost twofold more Pi than under continuous Pi supply (Fig. 4C), while shoot Pi pools were 70% replete within 24 h (Fig. 4D), with shoots just starting to resume growth (Fig. 4B).
Figure 4.
Disruption of SPX4 function leads to Pi overaccumulation in shoots. Tissue biomass (A and B) and phosphate concentration (C and D) are shown for Arabidopsis roots and shoots grown under varying Pi supplies. Plants defective in two key regulators of P signaling with contrasting Pi accumulation profiles (PHR1 and PHO2) were included for comparison. Values are means ± se of at least three independent biological replicates, comprising pools of four to six shoots and eight to 12 roots each. Asterisks indicate significant differences from the wild type under each condition (P < 0.05, ANOVA/Fisher lsd). FW, Fresh weight.
Compared with the wild type, all mutant lines tested displayed lower shoot biomass under optimal growth conditions (Fig. 4B, P-replete), asserting that the maintenance of Pi homeostasis is linked to plant fitness (Rouached et al., 2011; Linn et al., 2017). Mutants were not impaired in root growth (Fig. 4A).
In our system, phr1 and pho2-1 mutants displayed the expected trends in organ Pi accumulation. Both phr1-1 and phr1-2 showed faster depletion of their shoot Pi pools under Pi-limiting conditions (Fig. 4D). This is in line with the positive role that PHR1 exerts over the regulation of Pi uptake and translocation (Nilsson et al., 2007). On the other hand, pho2-1 displayed the typical shoot Pi overaccumulation phenotype irrespective of Pi supply (Fig. 4D) as well as reduced Pi concentration in P-replete roots (Fig. 4C). This is due to the negative effect of PHO2/UBC24 on xylem Pi loading in roots via suppression of PHO1 (Liu et al., 2012). By contrast, disrupting SPX4 led to higher intracellular Pi accumulation in both P-replete roots and shoots. In shoots, the true knockout allele, spx4-1, accumulated more Pi than Col-0 irrespective of Pi supply and to similar levels as pho2-1 (Fig. 4D). The weaker knockdown allele, spx4-2, showed this effect only in P-replete seedlings. Since the T-DNA insertion in this line is in the C-terminal portion of the functional domain, just 5′ of the coding region for the active Lys-binding cluster but after the phosphate-binding cluster residues (Supplemental Fig. S7A; Wild et al., 2016), a partially functional SPX domain might explain the weaker response. The observed changes in Pi allocation place SPX4 as a central negative regulator of Pi acquisition, alongside PHO2.
SPX4 Is a Negative Regulator of PSR Gene Expression in Shoots, Not Roots
Knocking out the negative regulators SPX1 and SPX2 led to derepression of PHR1 in P-replete as well as Pi-resupplied seedlings (Puga et al., 2014; Fig. 5; Supplemental Table S1). This is in contrast to PSR genes in the wild type that are active upon Pi withdrawal. The shoot Pi overaccumulation phenotype of the spx4 mutants prompted the hypothesis that SPX4 knockout would result in a similar misregulation of PSR genes, both Pi-starvation induced (PSI) and Pi-starvation suppressed (PSS), in P-replete plants. Using an RNA sequencing (RNA-seq) approach, the root and shoot transcriptome profiles from the stronger spx4-1 allele and the two phr1 alleles were compared with those of Col-0 seedlings across all three conditions (P replete, P limited, and Pi resupply; Supplemental Data Sets S1 and S2). The two phr1 alleles showed the expected misregulated PSR gene expression in P-limited roots and shoots and wild-type-like expression profiles in P-replete and Pi-resupplied seedlings (Fig. 5; Supplemental Table S1). In P-replete spx4-1 shoots, 166 genes were differentially expressed compared with the wild type (Fig. 5; Supplemental Table S1; Supplemental Data Set S2, B and C). Seventy-two percent of differentially induced genes in P-replete spx4-1 shoots were PSI genes (i.e. responsive to changes in Pi supply in Col-0), but only 16% of these were affected by PHR1 knockout (Supplemental Table S1). The induction of PHO1 found in P-replete spx4-1 shoots (Supplemental Data Set S2B) may account for their higher Pi accumulation (Fig. 4D). It is important to note that there is very little overlap (less than 5%) between SPX1/2- and SPX4-controlled differentially expressed genes (DEGs) in P-replete seedlings, indicating that SPX1/2 control different subsets of PSR genes compared with SPX4. SPX4 knockout had very little effect on transcript profiles in roots (Fig. 5; Supplemental Data Set S1). Among the 50 genes with more than 2-fold lower expression (FDR < 0.05) in P-replete spx4-1 versus Col-0 shoots, only 6% were PSS and only 22% were PHR1-dependent genes (Supplemental Table S1). SPX4 is one of these genes, confirming gene knockout in spx4-1. In contrast to whole seedlings of the spx1 spx2 double mutant (Puga et al., 2014), Pi resupply to P-limited spx4-1 seedlings did not lead to significant deregulation of PSR genes (Fig. 5; Supplemental Table S1). It is possible that the missing effect of SPX4 knockout on PHR1-dependent genes could be masked by genetic redundancy with SPX1 and SPX2.
Figure 5.
Knockout of SPX4 leads to ectopic PSR gene expression in P-replete shoots but not in roots. Knockout of SPX genes should lead to the release of PHR1-activated gene expression under P-replete and Pi-resupply conditions, as shown here for DEGs in whole seedlings of the spx1 spx2 double mutant (asterisk; Puga et al., 2014). PHR1 knockout primarily affects PSR gene expression in P-limited organs, shown here as the common response to changes in Pi supply of both phr1-1 and phr1-2 alleles (this work; greater than 2-fold change in mutant versus the wild type; false discovery rate [FDR] < 0.05). The spx4-1 mutant shows the expected response in P-replete shoots, but not under Pi resupply. spx4-1 mutant roots show a similar transcript profile to the wild type. See Supplemental Data Sets S1 and S2 for details.
In summary, these expression profiles suggest that SPX4 is a negative regulator of a subset of PSR genes in shoots that are not all controlled by PHR1 but possibly by another, yet-to-be-identified transcription factor. Candidates could potentially be found in differentially expressed transcription factors highlighted in Supplemental Data Set S2, B and C.
SPX4 Knockout Disrupts PSR Gene Expression in P-Limited Shoots
Given the relatively small number of DEGs in P-replete spx4-1 shoots compared with the wild type, the magnitude of the transcriptome response to changes in Pi supply was analyzed in each genotype using two comparisons (Fig. 6; Supplemental Data Sets S3 and S4). In the first comparison, differences between P-limited and P-replete organs were assessed in each genotype (Fig. 6A). The second comparison identified expression changes in P-limited versus Pi-resupplied organs (Fig. 6B). In P-limited shoots of Col-0, 1,148 genes (71%) were induced (PSI genes) and 469 genes (29%) were suppressed (PSS genes) compared with P-replete conditions. Relative to P-limited Col-0 shoots, Pi resupply to this organ led to suppression of 857 genes (56%) and induction of 663 genes (44%), respectively. Within 24 h of Pi resupply, the expression of 49% of PSI and 10% of PSS genes was back to control levels (Supplemental Data Set S4B). Roots were less responsive to Pi withdrawal, with 459 genes induced (59%) and 313 suppressed (41%), and to Pi resupply, with 264 genes suppressed (68%) and 125 induced (32%; Supplemental Data Set S3B). Within 1 d of Pi resupply to roots, the expression of 49% of PSI and 27% of PSS genes was back to control levels. Overall, similar trends were observed in Col-4 (Fig. 6).
Figure 6.
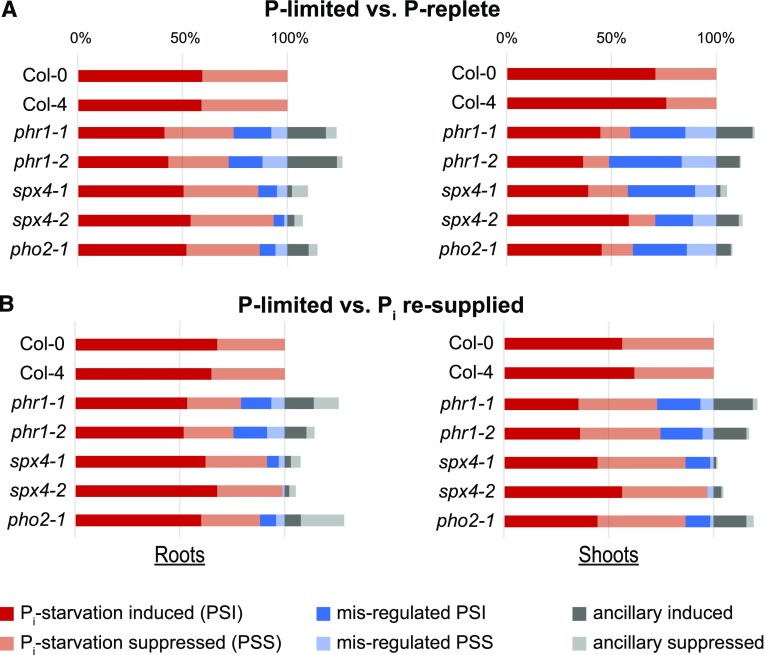
Knockout of SPX4 specifically modulates the shoot Pi starvation response. A, Proportion of genes with altered expression in roots or shoots with changes in Pi supply across genotypes. The two phr1 alleles show 27% and 46% perturbation of PSR gene expression in roots and shoots, respectively. The two spx4 alleles and pho2-1 show a similar disturbance of PSR gene expression in P-limited shoots but not in roots. B, Proportion of genes with an altered response to Pi resupply in P-limited roots and shoots across genotypes. phr1 mutants are impaired in their response to Pi resupply, with 23% and 26% of PSR genes misregulated in roots and shoots, respectively. The spx4 and pho2-1 mutants have an almost wild-type-like response to Pi resupply. Shown are PSR DEGs with a more than twofold altered expression ratio in each treatment comparison (red bars; FDR < 0.05). Misregulated genes (blue bars) represent PSR genes with altered (greater than twofold expression difference) response in each mutant compared with the wild type. Ancillary genes (gray bars) show a greater than fourfold change in expression ratio in individual mutants (FDR < 0.05) but not in the wild type. See Supplemental Data Sets S3 and S4 for details.
Compared with the wild type, all mutants failed to fully respond to the changes in Pi availability (Supplemental Data Sets S3, C–E, and S4, C–E). As expected, lines disrupted in PHR1 were the most affected in all conditions. phr1-1 was reported to affect about 47% and 69% of P-responsive genes by at least twofold in P-starved roots and shoots, respectively (Bustos et al., 2010). Using the same selection criteria, about 27% of root and 46% of shoot PSR genes showed a reduced response in both phr1 mutants following 5 d of Pi withdrawal (Fig. 6A, blue bars). The effect of PHR1 disruption was also evident on Pi resupply, with 23% of root PSR genes and 26% of shoot PSR genes not responding to the same extent as in Col-0 (Fig. 6B, blue bars). SPX4 or PHO2 knockout also had a strong effect on the PSR response in shoots, with 42% and 40% of PSR genes misregulated in spx4-1 and pho2-1, respectively. The weaker spx4-2 allele still affected 29% of shoot PSR genes (Fig. 6). In contrast to phr1 mutants, the response to Pi resupply in shoots (or roots) was not significantly altered, with less than 13% of PSR genes misregulated in spx4-1 and pho2-1 compared with Col-0, respectively (Fig. 6B). Knockout of these two genes also had very little effect on the Pi-responsive transcriptome in roots, with less than 14% of PSR genes misregulated in the stronger spx4-1 allele and pho2-1 compared with Col-0 (Fig. 6A; Supplemental Data Set S3). Together, the expression profiles shown in Figures 5 and 6 suggest that SPX4 acts as a negative regulator of a subset of shoot PSR genes in P-replete shoots, while also being required for the expression of more than 40% of PSR genes in P-limited shoots.
SPX4 Predominantly Acts as a PHO2- and PHR1-Dependent Modulator of PSR Gene Expression in Shoots
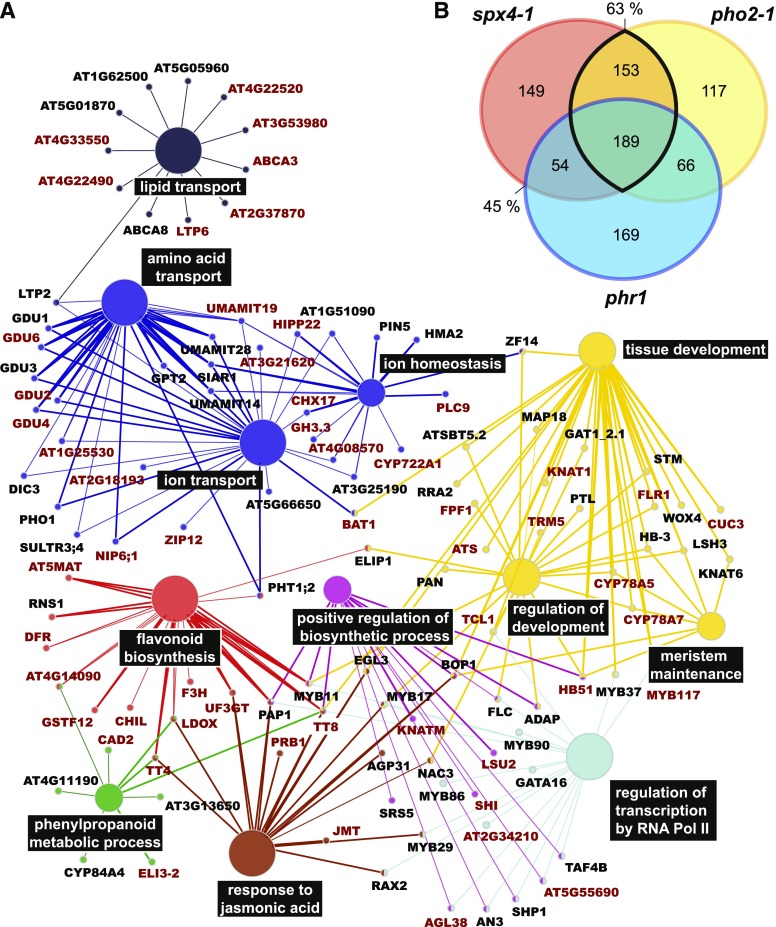
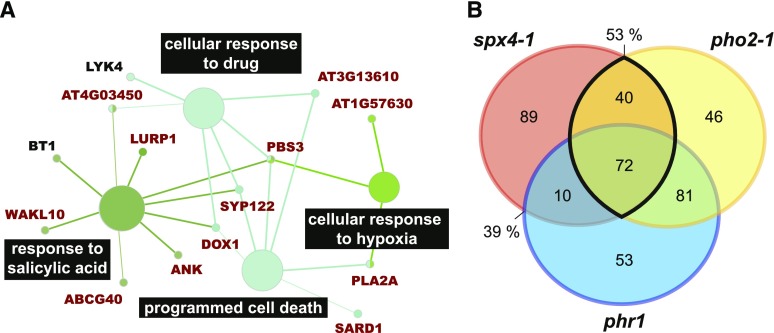
The finding that, in contrast to SPX1/2, SPX4 has a significant impact on the orderly launch of the transcriptomic PSR in P-limited shoots poses the question of whether this response is PHR1 dependent. In addition to the PSR genes with reduced responsiveness in the mutants described above, genes that were responsive in the mutants only (ancillary genes in Fig. 6 [gray bars]; log2 > 2, FDR < 0.05) were also identified, as these show an additional response to Pi withdrawal or resupply not observed in the wild type. Together, these genes were termed genes misregulated in mutants (GMMs). Given similar patterns of perturbation of GMM expression in spx4-1 and pho2-1 shoots, the extent of overlap in GMMs between these two mutants, as well as between spx4-1 and phr1, was investigated further. Of the 545 GMMs induced in P-limited spx4-1 shoots, 63% and 45% were similarly affected in pho2-1 and phr1, respectively, and 35% were misregulated in all three mutants (Fig. 7B; Supplemental Data Set S4). Gene Ontology (GO) term enrichment analysis of the 342 GMMs shared by both spx4-1 and pho2-1 revealed that genes associated with classical acclimation responses to Pi starvation, such as amino acid, ion, and lipid transport as well as flavonoid biosynthesis and jasmonic acid signaling, were overrepresented (Fig. 7A). A large number of transcription factors were among these genes. Many are associated with the regulation of the aforementioned acclimation responses, while others are regulators of organ development and meristem maintenance. About half the genes associated with these processes are PHR1 dependent. PHO2 was identified as an upstream regulator of 53% of the 211 GMMs suppressed by Pi withdrawal in spx4-1 shoots (Fig. 8B; Supplemental Data Set S4D). PHR1 targets 39% of these SPX4-dependent GMMs; 34% of the PSS GMMs are shared between all three mutants. GMMs shared by spx4-1 and pho2-1 mutants show enrichment of GO terms associated with defense-related processes, and most of these genes are dependent on PHR1 (Fig. 8A).
Figure 7.
GMMs induced in P-limited spx4-1 and pho2-1 shoots are associated with classical acclimation responses as well as plant development. A, GO terms enriched in the 342 GMMs induced by Pi withdrawal in both spx4-1 (514 misregulated + 31 ancillary) and pho2-1 (414 misregulated + 111 ancillary; 63% overlap in B) mutants are associated with classical acclimation processes such as ion, amino acid, and lipid transport, flavonoid biosynthesis, and jasmonic acid response. There is a strong association with transcriptional regulation and development. Shown in dark red are genes differentially expressed in both phr1 alleles. B, Overlap of GMMs in P-limited versus P-replete shoots of spx4-1, pho2-1, and phr1. Misregulated and ancillary GMMs for each mutant are listed in Supplemental Data Set S4.
Figure 8.
GMMs suppressed in P-limited spx4-1 and pho2-1 shoots are associated with plant defense. A, GO terms enriched in the 116 GMMs suppressed by Pi withdrawal and shared by spx4-1 (161 misregulated + 50 ancillary) and pho2-1 (227 misregulated + 12 ancillary; 53% overlap in B) are associated with defense-related processes such as programmed cell death and salicylic acid response. Most of these genes are also GMMs in both phr1 alleles (dark red). B, Overlap of GMMs in P-limited versus P-replete shoots of spx4-1, pho2-1, and phr1. Misregulated and ancillary GMMs for each mutant are listed in Supplemental Data Set S4.
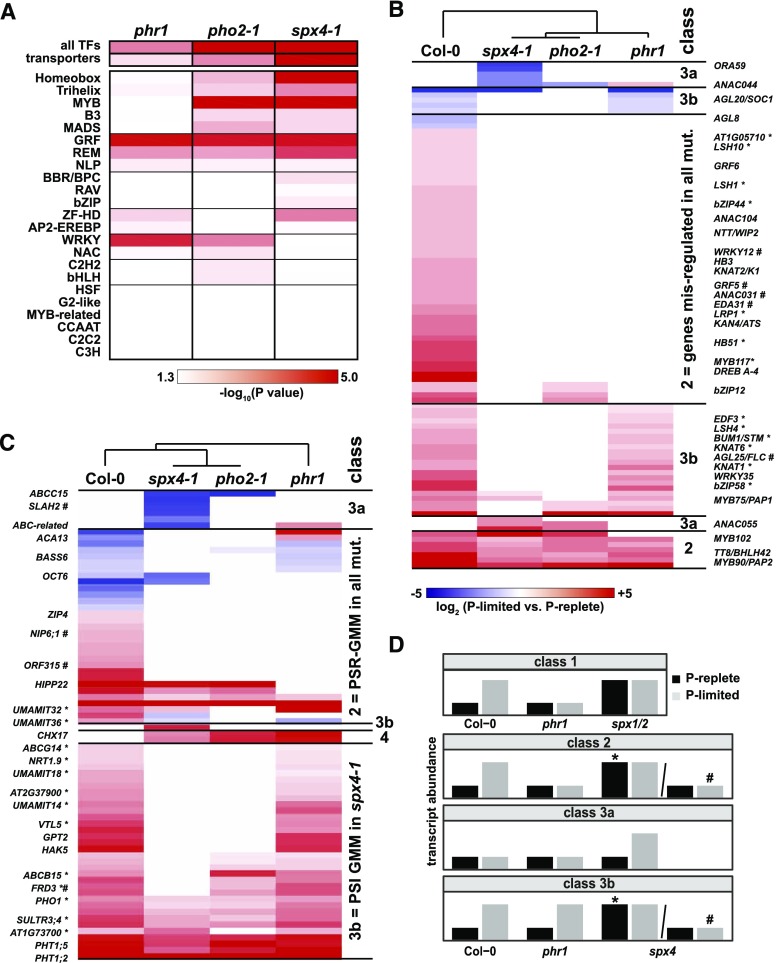
Many of the downstream targets misregulated in P-limited shoots of all three mutants are transcription factors, hinting at a more complex regulatory network. To investigate whether specific transcription factor families were overrepresented among the GMMs, an in-depth enrichment analysis was performed (Fig. 9A). Analysis of individual transcription factor families revealed that plant-specific growth-regulating factors (GRFs) were misregulated in all three genotypes, as were REPRODUCTIVE MERISTEM (part of the B3 superfamily of transcription factors), trihelix (GT-element binding), and NIN-LIKE PROTEIN transcription factors.
Figure 9.
Transcription factors and transporters show significantly altered expression profiles in spx4-1, pho2-1, and phr1 mutant shoots. A, Heat map of transporters and individual transcription factor (TF) families enriched among spx4-1 GMMs (misregulated and ancillary genes in Supplemental Data Set S4). B and C, Hierarchical clusters of expression ratios of individual transcription factor (B) or transporter (C) transcripts across genotypes that fail to be correctly induced (red) or suppressed (blue) in P-limited versus P-replete spx4-1 shoots compared with the wild type (misregulated PSR-GMMs) or that are ectopically expressed in spx4-1 only (ancillary GMMs). Genes differentially expressed in spx4-1 versus wild-type shoots (Supplemental Data Set S2) are marked by asterisks (P replete) or hash marks (P limited). D, Genes were assigned to classes according to their expression profiles depicted in stylized bar charts (shown for PSI genes only). Class 1 = SPX1/2, which act as negative regulators of PHR1. Class 2 = altered expression of PSR genes in spx4-1, (pho2-1), and phr1. SPX4 acts as a negative (*) or positive (#) modulator of PHR1. Class 3a = ectopic expression in spx4-1 but not in Col-0. SPX4 acts as a repressor of target genes in P-limited shoots. Class 3b = genes with altered PSR gene expression in spx4-1 (and pho2-1). SPX4 acts as a PHR1-independent negative (*) or positive (#) modulator of PSR gene expression. Class 4 (not shown) = ectopic expression in all three mutants but not in Col-0.
Hierarchical clustering of the expression ratios (log2 fold difference in transcript abundance in P-limited versus P-replete mutant shoots) of misregulated transcription factors in spx4-1 revealed that the majority of transcription factors were PSI genes that failed to be induced in P-limited mutant shoots (Fig. 9B; Supplemental Data Set S5A), acting downstream of SPX4 (and PHO2; class 3b) or of SPX4 (PHO2) and PHR1 (class 2). Among this group of genes, many were found to be ectopically induced in P-replete spx4-1 shoots (marked by asterisks in Fig. 9B), with ectopic meaning that these genes are PSR genes that would normally respond to low P status (Supplemental Data Set S4B) but are already induced in P-replete spx4-1 shoots (Supplemental Data Set S2B). This particular profile suggests that they are part of a PSR network controlled by SPX4 via negative regulation of a transcription factor, such as PHR1 (class 2) or an unknown factor (class 3b). SPX4 also seems to act as a positive regulator of four transcription factors, AGAMOUS-LIKE25 (AGL25)/FLOWERING LOCUS C (FLC), GRF5, ARABIDOPSIS NAC DOMAIN CONTAINING PROTEIN31 (ANAC031), and EMBRYO SAC DEVELOPMENT ARREST1, in P-limited shoots (marked by hash marks in Fig. 9B; Supplemental Data Set S2C). A smaller group of transcription factors showed an ancillary response to changes in P status in spx4-1 (and the other two mutants; class 3a in Fig. 9B). This class contained a number of candidates for direct downstream targets of SPX4: ANAC044 is an ancillary, up-regulated gene in P-replete spx4-1 and pho2-1 shoots as well as an ancillary PSI gene in P-limited phr1 shoots (Fig. 9B; Supplemental Fig. S8). This makes ANAC044 a target gene with the expected contrasting expression between SPX4 and PHR1 knockout plants, as suggested in the rice model (Lv et al., 2014). ANAC055 is an ancillary PSI gene in P-limited spx4-1 and pho2-1 shoots (Fig. 9B; Supplemental Fig. S8). AGL25/FLC showed the opposite response and failed to be induced in P-limited spx4-1 shoots compared with the wild type (Fig. 9B; Supplemental Data Set S2C; Supplemental Fig. S8). Both ANAC055 and AGL25/FLC expression were wild type like in phr1-2 shoots. SPX4 (and PHO2) thus appear to modulate the expression of both transcription factors in P-limited shoots in a PHR1-independent manner. GMM transporters showed similar expression profiles (Fig. 9C; Supplemental Data Set 5B). This could indicate that transporters are prominent downstream targets of the transcription factors mentioned above. The largest group was again formed by PSI genes misregulated in all three mutants (Fig. 9C, class 2) or in spx4-1 (and pho2-1; class 3b). Class 3b contained many ancillary GMMs that were ectopically expressed in P-replete spx4-1 shoots compared with Col-0 (marked by asterisks in Fig. 9C; Supplemental Data Set S2B). Notably, these comprised not only PHO1 but also iron transporter genes FERRIC REDUCTASE DEFECTIVE3 and VACUOLAR IRON TRANSPORTER-LIKE5, low-affinity nitrate transporter NITRATE TRANSPORTER1.9, and four USUALLY MULTIPLE ACIDS MOVE IN AND OUT TRANSPORTERS (UMAMIT) transporters (Supplemental Fig. S8).
Genes with asterisks or hash marks in Figure 9 hint at separate functions of SPX4 as a negative regulator (asterisks) of PSI genes in P-replete shoots or a positive regulator (hash marks) in P-limited shoots. However, the majority of GMMs appear to be targets of both SPX4 activities, showing a slight derepression in P-replete shoots and a lack of full induction in P-limited shoots. This is possibly the reason why absolute differences from treated Col-0 shoots are small (Fig. 5) while the effect on log2 fold changes in P-limited over P-replete shoots is significant (Fig. 6A).
Gene Regulatory Network Analysis Reveals Central Upstream Regulators of PSR Affected by SPX4
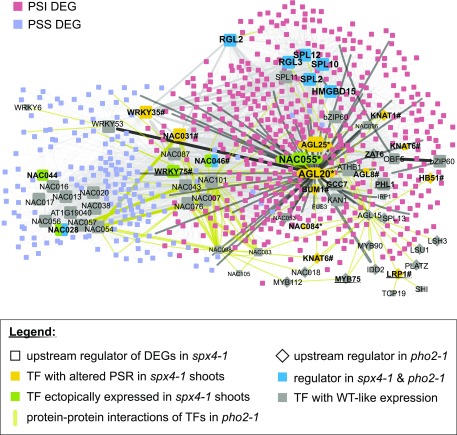
Given the high number and complex Pi-dependent expression profiles of transcription factors misregulated in spx4-1 shoots, we conducted a gene regulatory network (GRN) analysis using the TF2Network tool to identify upstream regulators (Kulkarni et al., 2018). The tool searched the corresponding GMM promoters for enriched transcription factor-binding sites and cross-compared these with coexpression profiles and experimentally confirmed protein-DNA interactions. The network was further extended by adding experimental evidence for protein-protein interactions between these regulators and downstream targets. This resulted in 719 nodes and 3,900 edges that were visualized in Cytoscape (Fig. 10). Data from pho2-1 and phr1 GRN analyses were subsequently used to further annotate and color code this list (Fig. 10; Supplemental Data Set S6).
Figure 10.
Gene regulatory networks of SPX4-dependent transcriptional responses. The TF2Network tool (Kulkarni et al., 2018) identified ANAC055 as a potential upstream regulator of PSR in all three mutants (Supplemental Data Set S6). It is itself a target of HMGBD15, RGL2/3, and SPL2/10/11 (predicted protein-DNA interaction). ANAC055 transcript abundance is high in P-limited spx4-1 and pho2-1 shoots, with no Pi response in the wild type (WT). AGL20/SOC1 binds to the ANAC055 promoter (confirmed protein-DNA interaction), and AGL20 and AGL25/FLC are both connected to ANAC055 through protein-protein interactions. ANAC028, ANAC031, and ANAC046 are candidates for SPX4-, PHO2-, and PHR1-dependent regulators, as they target promoters of PSI and PSS GMMs in all three mutants. Predicted binding of transcription factors (TFs) to target gene promoters is indicated with light gray lines. Confirmed transcription factor-DNA interactions are depicted in dark gray. Protein-protein interactions are shown as black lines. Upstream regulators are highlighted as larger nodes. Protein-protein interactions between transcription factors in the pho2-1 network are shown in gold. Asterisks mark transcription factors coexpressed in spx4-1 and pho2-1. Hash marks highlight transcription factors with an altered PSR in all three mutants. For better visibility, ANAC was shortened to NAC.
Notably, eight of the identified upstream regulators predicted to bind to the promoters of GMMs were transcription factors misregulated in spx4-1 shoots (Supplemental Data Set S6, highlighted in yellow). Five of these, ANAC028, ANAC031, ANAC046, ANAC055, and WRKY75, were also identified as regulators of pho2-1 and phr1 GMMs (Supplemental Data Set S6, column B, highlighted in purple).
PSI and PSS target genes misregulated in spx4-1 shoots formed two subnetworks that were under the immediate control of distinct transcription factor clusters (Fig. 10): PSS targets were predominantly regulated by NAC and WRKY transcription factors. Of those, WRKY35 and a known regulator of PSR gene expression, WRKY75 (Devaiah et al., 2007), were themselves differentially expressed in shoots of spx4-1 and pho2-1 (WRKY35; class 3b in Fig. 9B) or all three mutants (WRKY75; Supplemental Data Set S2C). ANAC044-binding sites were also enriched in promoters of PSS-GMMs. Due to its differential expression profile mentioned above, ANAC044 is a candidate for a direct target of both SPX4 and PHR1 (Fig. 9B; Supplemental Fig. S8). PSI targets misregulated in spx4-1 shoots, on the other hand, were controlled by a more complex regulatory network comprising HMGBD15, SPLs, RGLs, ANAC055, AGL20/SOC1, AGL25/FLC, and PHR1-related MYBs such as GCC7 and PHL1 (Bustos et al., 2010; Lundmark et al., 2011; Fig. 10). AGL20 was identified as an upstream regulator of ANAC055 and AGL25, with AGL20 experimentally confirmed to bind to the ANAC055 promoter (Supplemental Data Set S6). AGL20 transcripts were suppressed in P-limited over P-replete wild-type and phr1 shoots while being expressed in both spx4-1 and pho2-1 shoots, irrespective of P status (Fig. 9B). This would suggest that SPX4 and PHO2 are negative regulators of AGL20 in P-limited shoots. As mentioned above, transcripts of ANAC055 were highly abundant in P-limited spx4-1 and pho2-1 shoots, while AGL25 transcripts showed the opposite response (Fig. 9B; Supplemental Fig. S8). AGL20 would thus activate ANAC055 and repress AGL25 expression. The contrasting expression profiles of the latter would furthermore support the GRN analysis prediction that ANAC055 also binds to the AGL25 promoter, acting as a repressor (Supplemental Data Set S6). The expression of all three transcription factors is PHR1 independent. The ANAC055 transcription factor is predicted to target the promoters of PSI-GMMs in all three mutants and PSS-GMMs in pho2-1 and phr1 (Supplemental Data Set S6). The latter group shares 80% common targets, so ANAC055 and PHR1 may converge on their promoters to suppress them. In the former group, there is very little overlap (18% of ANAC055 targets among spx4-1 and phr1 PSI-GMMs), hinting at competition between the two transcription factors for common targets. PSI and PSS gene targets appear to be further connected through their regulation by another group of NAC transcription factors. Three of those, ANAC028, ANAC031, and ANAC046, were differentially expressed in spx4-1 shoots compared with the wild type (Fig. 9B; Supplemental Data Set S2). ANAC031 is a predicted downstream target of AGL20, which would therefore be predicted to act as a transcriptional repressor of ANAC031. ANAC028 and ANAC046 target promoters of Pi-responsive GMMs in all three mutants (Supplemental Data Set S6), which would make them common downstream targets of SPX4, PHO2, and PHR1.
PHR1 could not be placed within this network, as no DNA or protein interaction data were available in the TF2Network database. One can assume that target genes predicted for PHL1 and GCC7 are partially overlapping with PHR1 targets. If this were the case, these MYB transcription factors would form a smaller subnetwork controlling GMMs induced upon Pi withdrawal in all three mutants (Fig. 10), consistent with our finding that only about one-third of SPX4-dependent genes are controlled by PHR1.
The regulatory network associated with SPX4 further supports that, unlike SPX1/SPX2, a number of transcription factors other than PHR1 may interact either directly or indirectly with SPX4 and that these transcription factors may be associated with the control of shoot development.
DISCUSSION
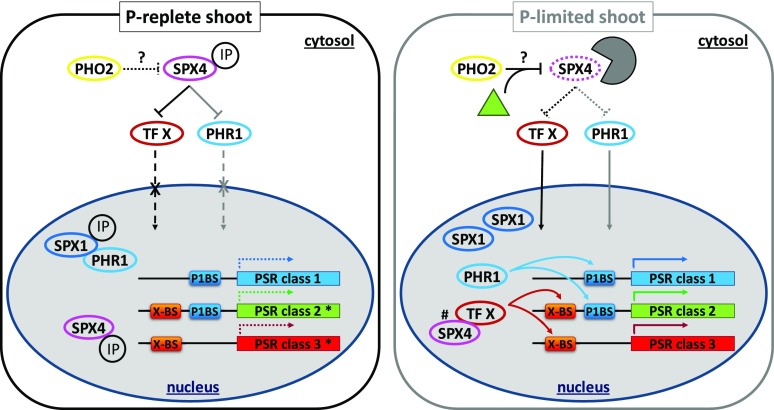
Previous studies have largely focused on unraveling root regulatory PSR networks. This work identified SPX4 as a shoot-specific modulator of PSR gene expression that not only acts as a repressor of PHR1 but potentially targets other transcription factors (Fig. 11). This sets SPX4 apart from SPX1 and SPX2 that are active in both roots and shoots and act as negative regulators of PHR1 (Puga et al., 2014; Wang et al., 2014). Contrasting regulation between root and shoot was proposed for OsSPX6, which shows higher turnover in P-limited leaves while accumulating in roots (Zhong et al., 2018). Differential turnover cannot be the explanation here, given that AtSPX4 is degraded in both P-limited roots and shoots (Fig. 1). The model in Figure 11 suggests at least partial redundancy between SPX1/2 and SPX4 regarding the regulation of PHR1 (class 1 PSR genes in Fig. 11). It is therefore somewhat surprising that SPX4 knockout still resulted in the differential regulation of PSR genes in shoots (Figs. 5 and 6). The fact that less than one-third of these were dependent on PHR1 (class 2 PSR genes in Figs. 9 and 11) implies that SPX4 acts as a modulator of PSR genes by targeting a transcription factor other than PHR1 (class 3 PSR genes in Figs. 9 and 11). Differential expression of genes in P-limited spx4-1 shoots (class 3a and genes marked by hash marks in class 2 and 3b in Fig. 9) suggests that SPX4 acts as a coregulator of PSR gene expression in the nucleus of P-limited leaf cells (Fig. 11, right). This finding is surprising, given that the bulk of SPX4 protein is degraded in P-limited organs (Fig. 1). It is, however, consistent with the continued de novo synthesis of SPX4 observed in P-limited shoots (Fig. 1I) and the observed dual localization of SPX4 in cytosol and nucleus (Fig. 3), potentially allowing for a small pool of nucleus-localized SPX4 to be maintained in P-limiting conditions.
Figure 11.
Model of proposed PHR1-dependent and -independent modulation of subclasses of PSR genes by SPX4. In P-replete shoots (left), SPX4 sequesters transcription factor X (e.g. ANAC055 or SOC1/AGL20; see Fig. 10) and/or PHR1 in the cytosol. SPX1 inhibits PHR1 in the nucleus (Puga et al., 2014), due to conformational changes induced by binding of either Pi or IPs, preventing its association with the P1BS element in class 1 and 2 PSR genes (Qi et al., 2017). Because of the dominant effect of SPX1, knockout of SPX4 will not change the expression profiles of class 1 PSR genes. It will, however, lead to differential expression of class 2 and 3 PSR genes that are under the direct control of transcription factor X (genes marked by asterisks; see Fig. 9). In P-limited shoots (right), SPX4 is degraded by the ubiquitin-proteasome system, possibly involving PHO2 and an unknown, de novo synthesized factor (green triangle; see Fig. 1I). This releases transcription factor X and/or PHR1 to activate PSR gene expression in the nucleus. The interaction of PHR1 with SPX1 is weaker under this condition (Puga et al., 2014). Changes in transcriptome profiles in the spx4-1 mutant (Figs. 7–9) suggest that a nuclear pool of SPX4, protected from proteasomal degradation, acts as a direct or indirect modulator of a small subset of transcription factor targets (genes marked by hash marks; see Fig. 9).
Overlap in GMM profiles of P-limited over P-replete shoots between spx4-1 and pho2-1 mutants was greater than 60%. These two mutants also showed similar physiological responses, such as accumulation of Pi in leaves and reduced biomass production (Fig. 4; Supplemental Fig. S7D; Aung et al., 2006; Bari et al., 2006; Linn et al., 2017). Common effects on PSR gene expression are still unexpected, given that PHO2 function in roots has been primarily associated with controlling trafficking of PHT1;1/4 transporters and PHO1 exporter from the endoplasmic reticulum to the plasma membrane via its ubiquitin E2 conjugase activity (Liu et al., 2012; Huang et al., 2013; Park et al., 2014). In P-limited roots, PHO2 transcript levels are suppressed systemically by miR399 species (Fujii et al., 2005; Aung et al., 2006; Bari et al., 2006). In this work, PHO2 transcripts were 2-fold higher in P-limited over P-replete shoots, indicating a lack of suppression by miR399 (Supplemental Data Set S4B). Reciprocal grafts between pho2-1 and the wild type showed that PHO2 knockout in shoots had no impact on Pi overaccumulation (Bari et al., 2006), suggesting additional PHO2 functions outside the miR399-PHO2-miR827-NITROGEN LIMITATION ADAPTATION (NLA) regulon in roots (Fujii et al., 2005; Aung et al., 2006; Bari et al., 2006; Lin et al., 2013; Park et al., 2014). In both pho2-1 and nla mutants, leaf nitrate and Pi levels had antagonistic effects on flowering time, affecting the expression of the floral repressor AGL25/FLC and positive regulators such as APETALA1, FLOWERING LOCUS T (FT), and LEAFY (Kant et al., 2011). Together with the results presented here, these data outline additional PHO2 functions in controlling shoot meristem activity and flowering time in response to nutrient availability (Mouradov et al., 2002; Vidal et al., 2014; Cho et al., 2017; Landrein et al., 2018). PHO2-dependent SPX4 could be involved in sensing P status and conveying this information to the nucleus (Fig. 11).
SPX domain-containing proteins have long been associated with the sensing of P status in yeast (Lenburg and O’Shea, 1996). Among these, cyclin-dependent kinase inhibitor Pho81 conveys information on P status to the transcriptional machinery via reversible binding of inositol heptakisphosphate (Lee et al., 2007). In plants, no SPX domain-containing kinase inhibitor has been identified (Secco et al., 2012). Instead, a new class of proteins containing only a single SPX domain has emerged that interact with transcriptional regulators such as PHR1. The work on SPX4 presented here (Fig. 3) and work in rice (Lv et al., 2014) demonstrated that SPX4 differs from its two nucleus-localized homologs SPX1 and SPX2 (Duan et al., 2008) in that it localizes to both cytosol and nucleus, irrespective of Pi supply. We show here that retaining PHR1 in the cytosol requires excess SPX4 protein and works more effectively in P-replete cells, most likely due to modification of their interaction by organic P molecules, such as IPs, as well as P status-dependent turnover (Fig. 11). SPX4 transcripts are not responsive to changes in Pi supply, while SPX4 protein is rapidly degraded in P-limited plants (Fig. 1, D and E). In contrast to OsSPX4, AtSPX4 protein turnover was not altered by the Pi analog Phi (Fig. 2). This suggests that SPX4 is stabilized in the presence of an organic P molecule, such as IP (Fig. 11). In this context, it is worth noting that inositol heptakisphosphate levels specifically increase in P-limited Arabidopsis shoots but not roots (Kuo et al., 2018). The discrimination between Pi and organic P in triggering SPX4 degradation hints at its ability to convey information on overall shoot P status. This is also supported by the wild-type-like response of the spx4-1 shoot transcriptome to 24 h of Pi resupply, when organic P pools are unlikely to be fully restored (Figs. 5 and 6). By contrast, the spx1 spx2 double mutant shows ectopic expression of PSR genes in both P-replete and Pi-resupplied seedlings (Fig. 5; Puga et al., 2014), reinforcing their proposed role in directly sensing changes in Pi availability (Puga et al., 2014; Wang et al., 2014; Jost et al., 2015).
OsPHO2 has been shown to interact with GIGANTEA (OsGI) both in vitro and in vivo, in close association with the endoplasmic reticulum (Li et al., 2017). Ospho2 and Osgi knockout mutants showed delayed flowering, correlating with misregulated expression of flowering genes downstream of GI, namely the CONSTANS (CO) rice ortholog HEADING DATE1 and the RAF kinase inhibitor-like protein FT ortholog HEADING DATE3a (Tsuji et al., 2011). Both mutants also showed higher Pi accumulation in shoots (Li et al., 2017). While our regulatory network analysis does not suggest a direct involvement of the GI-CO-FT regulon (Mizoguchi et al., 2005) in GMM expression in both spx4-1 and pho2-1 mutants, we do find an association with transcription factors that regulate shoot and flower development (Fig. 10). Most notably, the FT repressor AGL25/FLC and another common downstream target of both CO and FLC, flower-promoting AGL20/SOC1 (Li et al., 2008; Deng et al., 2011), are among the PSR genes that show contrasting expression in spx4-1 shoots (Fig. 9). Overall, their expression profile would translate into higher AGL20 activity in spx4-1 seedlings. The fact that SPX4 is present in the cytosol may enable its interaction with proteins other than PHR1 (Figs. 9 and 11). SOC1:GFP fusion protein was detected in large speckles in the cytoplasm of leaf protoplasts, suggesting its sequestration by another protein (Lee et al., 2008). From its position in the network (Fig. 10), AGL20/SOC1 is a putative candidate for transcription factor X in Figure 11. AGL20/SOC1 has been shown to integrate multiple flowering signals, derived from temperature, gibberellic, jasmonic, and salicylic acids, photoperiod, and aging (Moon et al., 2003; Lee and Lee, 2010; Yant et al., 2010; Pajoro et al., 2014). AGL20/SOC1 binds to the ANAC055 promoter. ANAC055 is the only differentially expressed transcription factor in P-limited shoots of both spx4-1 and pho2-1 (Fig. 9B; Supplemental Fig. S8) and could hence act downstream of both SPX4 and PHO2. Supporting an integrating function are reports of ANAC055 involvement in jasmonic acid gene regulatory networks (Hickman et al., 2017), Pro-mediated drought tolerance (Fu et al., 2018), as well as abscisic acid-inducible, ethylene-responsive, and developmental leaf senescence (Hickman et al., 2013; Kim et al., 2014; Takasaki et al., 2015). Processes associated with ANAC055 are enriched GO terms in spx4-1 PSI- and PSS-GMMs (Figs. 7 and 8). Many of the downstream transcription factor targets are transporters/transport facilitators shown in Figure 9C, such as UMAMIT amino acid exporters (Müller et al., 2015; Besnard et al., 2016), amino acid exporter-activating GLUTAMINE DUMPER proteins, and SUGAR WILL EVENTUALLY BE EXPORTED TRANSPORTER10 (Chen et al., 2012), which are involved in sink-source regulation and therefore directly impact on plant development and growth.
CONCLUSION
Taken together, our results suggest the involvement of SPX4 in a regulatory network of transcription factors integrating plant nutrient status with developmental processes. SPX4 is a modulator of PHR1-dependent and -independent PSR genes in shoots. Transcription factors that are (direct or indirect) downstream targets of SPX4 other than PHR1 are regulators of plant development, in particular flowering and senescence. Genes responsive to jasmonic and salicylic acids are enriched in the group of downstream targets, alongside many involved in ion, amino acid, and lipid transport. This would suggest that PSR is critical for resource allocation during shoot development and that SPX4 provides crucial feedback on plant P status to these developmental programs.
MATERIALS AND METHODS
Plant Material
The SPX4pro:SPX4-LUC reporter line was produced as follows. An amplicon of 3,129 bp, comprising 1,573 bp of the Arabidopsis (Arabidopsis thaliana) SPX4 (AT5G15330.1) promoter, as defined by the upstream gene encoded on the same strand, followed by 1,556 bp of SPX4 genomic region (comprising 5′ untranslated region, exons, and introns upstream of the stop codon) was cloned into the pCAMBIA1300-LUC vector, where the amplicon was translationally fused to the LUC gene. The construct was then transformed into Arabidopsis Col-0 (N70000) plants via floral dipping. Transgenic lines were selected on hygromycin B (20 μg mL−1) containing one-half-strength MS medium as described by Harrison et al. (2006). A representative single-insert homozygous line with SPX4-specific and stable LUC expression pattern was chosen for downstream analysis. The left-border T-DNA insertion site of the selected line was identified using fusion primers and nested integrated PCR (Wang et al., 2011) followed by sequencing, which confirmed that the insertion was in the intergenic region 323 bp downstream of AT5G67190.1. Stable progeny of this line were used in all downstream experiments.
Mutant lines containing predicted T-DNA insertions in SPX4 (SALK_019826 and SK40726) were ordered from the Nottingham Arabidopsis Stock Centre and were named spx4-1 and spx4-2, respectively. Confirmation of the insertion site and selection of homozygous plants were carried out using gene-specific primers designed with SIGnAL iSect (http://signal.salk.edu/isects.html), in combination with the LBa1.3 T-DNA left-border primer, followed by Sanger sequencing of PCR products. Disruption of SPX4 full-length transcript was confirmed by reverse transcription quantitative PCR (Supplemental Fig. S7C). In all experiments, each mutant line was compared with its parental background, Col-0 (N70000) for spx4-1 and Col-4 (N933) for spx4-2. Mutants disrupted in two known central regulators of Pi signaling, phr1-1 (Rubio et al., 2001) and phr1-2 (SALK_067629C, N686175; Nilsson et al., 2007), and pho2-1 (N8508; Delhaize and Randall, 1995) were also included for comparison.
All primers used to characterize the SPX4pro:SPX4-LUC line and spx4 mutants are listed in Supplemental Table S2.
Construction of Fluorescent Protein Fusions
To construct a vector expressing a translational PHR1-GFP fusion protein under the control of the CaMV 35S promoter, the PHR1 coding sequence without the stop codon was amplified from P-replete Col-0 leaf cDNA, using primers carrying the Gateway attB1 or attB2 recombination sites (Supplemental Table S2). The PCR product was introduced into the Gateway vector pDONR221 using the BP Clonase II Enzyme mix (Thermo Fisher Scientific). The resulting pENTR clone was recombined with the high-copy p2GWF7 destination vector to create a C-terminal fusion of eGFP to PHR1 (35Spro:PHR1-GFP; Karimi et al., 2002) using the LR Clonase II Enzyme mix (Thermo Fisher Scientific).
For expression of the SPX4-RFP fusion protein under the control of the CaMV 35S promoter, the coding sequence of SPX4 without the stop codon was amplified from P-replete Col-0 leaf cDNA, using primers carrying the Gateway attB1 or attB2 recombination sites (Supplemental Table S2). The amplicon was recombined with pDONR221 (Thermo Fisher Scientific). The resulting pENTR clone was introduced into the high-copy p2GWR7 vector (Karimi et al., 2002) using LR Clonase II (Thermo Fisher Scientific), forming the C-terminal fusion of SPX4 to RFP in 35Spro:SPX4-RFP. pENTR vector sequences were verified by DNA sequencing.
Plant Growth Conditions
Genotyping, phenotyping, and propagation of plant material were done in soil (0.5 L of coarse vermiculite, 0.33 L of Perlite, 33 g of Nutricote controlled-release fertilizer, 28 g of ammonium nitrate, 25 g of water-holding granules, 15 g of trace elements, and 7 g of garden lime per kg of potting mix). Seeds were sown in 63-mm pots, stratified at 4°C for 48 h, and grown under short-day conditions (10-h/14-h light/dark cycle, 23°C day/19°C night, 130 µE m−2 s−1 light intensity, and 55% humidity). For induction of flowering, plants were then transferred to long-day conditions (14- to 16-h/10- to 8-h light/dark cycle) after 5 weeks of growth.
Experiments under varying Pi supplies were carried out in vitro using a vertical agar plate system. The standard medium consisted of one-half-strength MS (Murashige and Skoog, 1962) Basal Salt mixture (M524, Phytotech), 0.05% (w/v) MES (Sigma-Aldrich), 0.5% (w/v) Suc (Sigma-Aldrich), and 0.8% (w/v) Difco granulated agar (lot 6173985). The Pi-deficient medium was made with one-half-strength MS Modified Basal Salt mixture (M407, Phytotech) supplemented with 10.3 mm NH4NO3, 9.4 mm KNO3, 0.05% (w/v) MES (Sigma-Aldrich), 0.5% (w/v) Suc (Sigma-Aldrich), 0.8% (w/v) Difco granulated agar, and 625 µm KCl instead of KH2PO4 to maintain the osmolarity of the standard medium. Residual P from the agar added 5.6 µm total P to this medium. Phi treatment was done by adding 625 µm potassium phosphite to the Pi-deficient medium. The 0.1 m Phi stock solution was prepared from a fresh batch of phosphorous acid (99%; Sigma-Aldrich) by adjusting the pH to 5.8 with KOH and was tested for lack of oxidation to Pi via the ammonium molybdate method (Ames, 1966). Arabidopsis seeds were surface sterilized in chlorine gas for 4 h, resuspended in 0.1% (w/v) agarose, stratified at 4°C for 2 d, and then sown in a single line on 10- × 10-cm-square plates sealed with 3M Micropore tape. Plates were vertically placed into racks in the growth chamber with a 12-h light/dark cycle, 120 µE m−2 s−1 light intensity, 23°C (day)/19°C (night), and 60% humidity. Seedlings were initially established for 7 d with standard Pi supply (625 µm), being then transferred to plates with either standard (P-replete) or P-limiting medium. Four days later, seedlings were transferred once more to fresh medium, including the following treatment combinations: from P-replete to P-replete medium, from P-limiting to P-limiting medium, and from P-limiting to P-replete medium (Pi resupply). For the physiological characterization of spx4 mutants and the RNA-seq analyses, shoots and roots were harvested 24 h after the final transfer. For monitoring of SPX4pro:SPX4-LUC expression, seedlings were harvested 24 h after the first transfer (control [P replete] and 1 d −Pi), immediately before and 24 h after the second transfer (4 and 5 d –Pi, respectively), and 1, 2, 3, and 4 d after the second transfer for the time course of Pi resupply (+Pi) or 1 and 3 d after Phi supply (+Phi).
Luciferase Imaging and Quantification
Analysis of SPX4 expression kinetics under varying Pi supplies was performed at selected time points by spraying the SPX4pro:SPX4-LUC reporter line with a 2.5 mm potassium luciferin (GoldBio) solution in 0.01% (v/v) Tween 20 (Sigma-Aldrich). Sprayed seedlings were kept in the dark for 30 min, and luciferase bioluminescence was imaged using the Bio-Rad ChemiDoc. Quantification of luciferase activity was performed on 96-well-plates using the Luciferase Assay System (Promega). Roots and shoots of the SPX4pro:SPX4-LUC reporter line were harvested at the indicated time points, and frozen plant powder was processed according to the manufacturer’s instructions. For absolute LUC protein quantification, a serial dilution of QuantiLum Recombinant Luciferase (Promega) was used. This was then normalized against the total protein concentration of each extract, which was assessed by SDS-PAGE using a BSA standard.
Subcellular Localization of SPX4 and PHR1
Constructs 35Spro:PHR1-GFP and 35Spro:SPX4-RFP, or 35Spro:GFP (pFF19-GFP; Kawashima et al., 2005), as GFP control constructs, were transiently expressed in protoplasts prepared from 8- to 9-week-old soil-grown Arabidopsis Col-0, spx4-1, or phr1-2 plants from a short-day environment (10 h of light/14 h of dark, 23°C day/19°C night, 200 μmol m−2 s−1, and 55% relative humidity). Protoplasts were prepared using the protocol by Yoo et al. (2007) but using the tape-sandwich method (Wu et al., 2009) for the initial release of protoplasts from leaf mesophyll. Ten micrograms of the SPX4-RFP and 12 μg of the PHR1-GFP construct, either individually or in combination, was used in each transfection reaction of 2 × 104 cells in 100 μL of MMG solution (0.4 m mannitol and 15 mm MgCl2 in 4 mm MES, pH 5.7). Transfection was carried out by adding 100 μL of PEG-calcium transfection solution containing 20% (w/v) polyethylene glycol 4000, 0.2 m mannitol, and 100 mm CaCl2. To boost transfection efficiency, 10 μg of carrier plasmid [pGEM-3Zf(+), Promega] was added to single-construct transfection reactions. A 1:4 ratio of SPX4-RFP to PHR1-GFP was achieved by adding 4 μg of the SPX4-RFP and 21 μg of the PHR1-GFP construct. A 4:1 ratio of SPX4-RFP to PHR1-GFP was achieved by adding 16 μg of the SPX4-RFP and 4.5 μg of the PHR1-GFP construct. At the 4:1 ratio, some protoplasts were only positive for RFP, indicating lack of cotransfection and the upper limit of the assay. To modify their P status, transfected protoplasts were transferred to a modified sterile WI solution containing one-half-strength MS salts (Phytotechnology Labs), with either 1 mm KH2PO4 (+P) or 1 mm KCl (−P), 0.5 m mannitol, 20 mm KCl, and 0.5 g L−1 2-morpholinoethanesulfonic acid (pH 5.7). Fluorescent images were captured 16 h after transfection using a Zeiss LSM780 AxioObserver confocal laser scanning microscope and 488- and 594-nm excitation wavelengths from an argon laser for GFP and RFP, respectively. Chlorophyll fluorescence was induced at 633 nm. Fluorescence was detected using emission filters of 493 to 523 nm for eGFP, 599 to 621 nm for RFP, and 667 to 721 nm for chlorophyll. Protoplasts were observed using the 40× water-immersion objective.
Inhibitor Treatments
Analysis of SPX4 stability under varying Pi or Phi supplies was performed as above, except that 24 h prior to luciferin spraying, SPX4pro:SPX4-LUC seedlings were transferred to fresh one-half-strength MS medium containing either 0.01% (v/v) DMSO (control) or 50 μm CHX (Sigma-Aldrich), MG132 (Cayman Chemical), or E-64d (Cayman Chemical) in 0.01% (v/v) DMSO (treatments). The latter two protease inhibitors were also tested in combination with CHX.
Quantification of Intracellular Phosphate Concentration
Twenty volumes of 1% (v/v) acetic acid and two ceramic beads (diameter of 2.8 mm) were added to frozen plant powder (10–50 mg), which was homogenized for 90 s at 30 Hz in a tissue disruptor (Qiagen TissueLyser II). After incubation for 15 min on ice, the homogenization process was repeated once. Cleared supernatants were used to determine organ Pi concentrations via the reduction of a phospho-molybdate complex by ascorbic acid (Ames, 1966; Jost et al., 2015).
Gene Expression Analyses
Total RNA isolation and cDNA synthesis for quantitative PCR were performed as described in Linn et al. (2017). Quantitative PCR and threshold cycle (Ct) determination were performed using a fluorescence baseline setting of 0.3 (QuantStudio 12K Flex Real-Time PCR System, Applied Biosystems). Data were normalized against ACT7, UBC9, and UBC21 reference genes (Czechowski et al., 2005), ranked as the most stable in the conditions tested using NormFinder (Andersen et al., 2004). PCR efficiencies for each primer pair were determined using the LinReg algorithm (Ruijter et al., 2009). Data were expressed as 40–∆Ct values that correlate with the relative transcript expression of the gene of interest (Bari et al., 2006). The detection limit of the assay was calculated to be a 40–∆Ct value of 23.
For RNA-seq analysis, libraries were prepared from total RNA using a TruSeq stranded mRNA library prep kit according to the manufacturer’s instructions (Illumina) using samples from three independent experiments for each genotype and treatment. Sequencing runs were performed on a HiSeq1500 platform (Illumina) generating 61-bp single-end reads. Quantification of gene expression was performed using the Kallisto (Bray et al., 2016) and Sleuth (Pimentel et al., 2017) pipelines, using the Araport11 transcript annotation (Cheng et al., 2017). Genes with a fold change of log2 > 1 and FDR < 0.05 were considered as differentially expressed. Comparisons were made between mutant and wild-type plants (Supplemental Data Sets S1 and S2) as well as between P-limited and P-replete (or Pi-resupplied) organs (Supplemental Data Sets S3 and S4). Venn diagrams were drawn using http://bioinformatics.psb.ugent.be/webtools/Venn/. Hierarchical clustering was performed using Partek Genomics Suite v. 6.6 (Partek). GO enrichment was performed using the ClueGO plugin (Bindea et al., 2009) for Cytoscape software (Shannon et al., 2003).
Regulatory Network Analyses
For the identification of regulatory networks and upstream regulators of GMMs in mutant shoots, the TF2Network tool was used (Kulkarni et al., 2018). It identifies transcription factor-binding sites in GMMs and experimentally confirmed protein-DNA and protein-protein interactions between transcription factors and targets. The network was visualized with Cytoscape version 3.5.1 (Shannon et al., 2003); transcription factors were clustered according to the overlap of target genes they regulate, and nodes were positioned using the Organic layout. PSI and PSS GMM promoters were first searched for enriched transcription factor-binding sites, including 2,058 Arabidopsis position weight matrices for 921 transcription factors. All predicted transcription factors (q < 0.05) were additionally analyzed for experimentally confirmed protein-DNA interactions with the predicted target genes. Upstream transcription factors with experimental protein-DNA evidence for regulating the predicted transcription factors were added to the network. In addition, all predicted transcription factors and target genes were searched for known protein-protein interactions.
Statistical Analysis
Significant differences between treatments and genotypes were determined using ANOVA with P < 0.05, followed by Fisher’s lsd or Tukey’s posthoc test to separate means (OriginPro2016 Statistics). At least three independent biological replicates were included in each analysis.
Accession Numbers
Sequence data for the genes characterized in this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following accession numbers: AT5G15330 (SPX4), AT4G28610 (PHR1), and AT2G33770 (PHO2). RNA-seq data were deposited to the National Center for Biotechnology Information Sequence Read Archive database under project identifier PRJNA470732.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. SPX4 protein degradation in P-limited shoots requires de novo synthesis of a ubiquitin-proteasome system component.
Supplemental Figure S2. Neither presence of the SPX4-RFP fusion protein nor P status interferes with subcellular localization of untagged GFP protein.
Supplemental Figure S3. Retention of PHR1-GFP in the cytosol observed upon cotransfection with SPX4-RFP into P-replete Col-0 protoplasts.
Supplemental Figure S4. Retention of PHR1-GFP in the cytosol upon cotransfection with SPX4-RFP is weaker in P-replete spx4-1 protoplasts.
Supplemental Figure S5. Knockout of PHR1 has no effect on the partial retention of PHR1 by SPX4 in P-replete protoplasts.
Supplemental Figure S6. In phr1-2 protoplasts, cotransfection of different ratios of fluorescent protein constructs yields a stronger cytosolic PHR1-GFP signal with higher SPX4-RFP input.
Supplemental Figure S7. SPX4 gene model and characterization of two spx4 T-DNA insertion lines.
Supplemental Figure S8. Expression profiles of transcription factors and transporters show differential expression in P-replete or P-limited spx4-1 shoots.
Supplemental Table S1. Comparison of P-responsive gene expression in spx1 spx2 double and spx4-1 single mutants with that in Col-0 and the phr1-1 mutant.
Supplemental Table S2. List of oligonucleotides used in this study.
Supplemental Data Set S1. Comparison of root RNA-seq data across spx4, phr1, and pho2-1 mutants and treatments against the wild type.
Supplemental Data Set S2. Comparison of shoot RNA-seq data across spx4, phr1, and pho2-1 mutants and treatments against the wild type.
Supplemental Data Set S3. Treatment comparison of root RNA-seq data across spx4, phr1, and pho2-1 mutants.
Supplemental Data Set S4. Treatment comparison of shoot RNA-seq data across spx4, phr1, and pho2-1 mutants.
Supplemental Data Set S5. Hierarchical cluster analysis of differentially expressed transcription factors and transporters in P-limited versus P-replete spx4-1 shoots and their expression profile in Col-0 and pho2-1 and phr1-2 mutants.
Supplemental Data Set S6. Gene regulatory network analysis: input and output data.
Acknowledgments
We thank the Salk Institute Genomic Analysis Laboratory for providing the sequence-indexed Arabidopsis T-DNA insertion mutants. Funding for the SIGnAL indexed insertion mutant collection was provided by the National Science Foundation. We thank Asha Haslem and the La Trobe University Genomics Platform for next-generation sequencing support. We are also grateful for the confocal microscopy support provided by Dr. Peter Lock and the La Trobe University Bioimaging Platform.
Footnotes
This work was supported by the Department of Industry, Innovation, Science, Research, and Tertiary Education, Australian Government/Australian Research Council Centre of Excellence for Plant Energy Biology (CE140100008).
Articles can be viewed without a subscription.
References
- Abel S, Ticconi CA, Delatorre CA (2002) Phosphate sensing in higher plants. Physiol Plant 115: 1–8 [DOI] [PubMed] [Google Scholar]
- Acevedo-Hernández G, Oropeza-Aburto A, Herrera-Estrella L (2012) A specific variant of the PHR1 binding site is highly enriched in the Arabidopsis phosphate-responsive phospholipase DZ2 coexpression network. Plant Signal Behav 7: 914–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames BN. (1966) Assay of inorganic phosphate, total phosphate and phosphatases. In Neufeld E, Ginsburg V, eds, Methods in Enzymology, Vol 8 Academic Press, New York, pp 115–118 [Google Scholar]
- Andersen CL, Jensen JL, Ørntoft TF (2004) Normalization of real-time quantitative reverse transcription-PCR data: A model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res 64: 5245–5250 [DOI] [PubMed] [Google Scholar]
- Arpat AB, Magliano P, Wege S, Rouached H, Stefanovic A, Poirier Y (2012) Functional expression of PHO1 to the Golgi and trans-Golgi network and its role in export of inorganic phosphate. Plant J 71: 479–491 [DOI] [PubMed] [Google Scholar]
- Aung K, Lin SI, Wu CC, Huang YT, Su CL, Chiou TJ (2006) pho2, a phosphate overaccumulator, is caused by a nonsense mutation in a microRNA399 target gene. Plant Physiol 141: 1000–1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azevedo C, Saiardi A (2017) Eukaryotic phosphate homeostasis: The inositol pyrophosphate perspective. Trends Biochem Sci 42: 219–231 [DOI] [PubMed] [Google Scholar]
- Baek D, Chun HJ, Yun DJ, Kim MC (2017) Cross-talk between phosphate starvation and other environmental stress signaling pathways in plants. Mol Cells 40: 697–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari R, Datt Pant B, Stitt M, Scheible WR (2006) PHO2, microRNA399, and PHR1 define a phosphate-signaling pathway in plants. Plant Physiol 141: 988–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besnard J, Pratelli R, Zhao C, Sonawala U, Collakova E, Pilot G, Okumoto S (2016) UMAMIT14 is an amino acid exporter involved in phloem unloading in Arabidopsis roots. J Exp Bot 67: 6385–6397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezzola LC, Lopez SC, Barbaro NO (1994) Effectiveness of different phosphatic fertilizers measured using labeled superphosphate and phosphorus taken up by plants. Fertil Res 39: 31–37 [Google Scholar]
- Bindea G, Mlecnik B, Hackl H, Charoentong P, Tosolini M, Kirilovsky A, Fridman WH, Pagès F, Trajanoski Z, Galon J (2009) ClueGO: A Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 25: 1091–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray NL, Pimentel H, Melsted P, Pachter L (2016) Near-optimal probabilistic RNA-seq quantification. Nat Biotechnol 34: 525–527 [DOI] [PubMed] [Google Scholar]
- Burleigh SH, Harrison MJ (1999) The down-regulation of Mt4-like genes by phosphate fertilization occurs systemically and involves phosphate translocation to the shoots. Plant Physiol 119: 241–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustos R, Castrillo G, Linhares F, Puga MI, Rubio V, Pérez-Pérez J, Solano R, Leyva A, Paz-Ares J (2010) A central regulatory system largely controls transcriptional activation and repression responses to phosphate starvation in Arabidopsis. PLoS Genet 6: e1001102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho-Cristóbal JJ, Rexach J, Conéjéro G, Al-Ghazi Y, Nacry P, Doumas P (2008) PRD, an Arabidopsis AINTEGUMENTA-like gene, is involved in root architectural changes in response to phosphate starvation. Planta 228: 511–522 [DOI] [PubMed] [Google Scholar]
- Chen CY, Schmidt W (2015) The paralogous R3 MYB proteins CAPRICE, TRIPTYCHON and ENHANCER OF TRY AND CPC1 play pleiotropic and partly non-redundant roles in the phosphate starvation response of Arabidopsis roots. J Exp Bot 66: 4821–4834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LQ, Qu XQ, Hou BH, Sosso D, Osorio S, Fernie AR, Frommer WB (2012) Sucrose efflux mediated by SWEET proteins as a key step for phloem transport. Science 335: 207–211 [DOI] [PubMed] [Google Scholar]
- Chen ZH, Nimmo GA, Jenkins GI, Nimmo HG (2007) BHLH32 modulates several biochemical and morphological processes that respond to Pi starvation in Arabidopsis. Biochem J 405: 191–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CY, Krishnakumar V, Chan AP, Thibaud-Nissen F, Schobel S, Town CD (2017) Araport11: A complete reannotation of the Arabidopsis thaliana reference genome. Plant J 89: 789–804 [DOI] [PubMed] [Google Scholar]
- Cho LH, Yoon J, An G (2017) The control of flowering time by environmental factors. Plant J 90: 708–719 [DOI] [PubMed] [Google Scholar]
- Crozet P, Margalha L, Butowt R, Fernandes N, Elias CA, Orosa B, Tomanov K, Teige M, Bachmair A, Sadanandom A, et al. (2016) SUMOylation represses SnRK1 signaling in Arabidopsis. Plant J 85: 120–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czechowski T, Stitt M, Altmann T, Udvardi MK, Scheible WR (2005) Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiol 139: 5–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhaize E, Randall PJ (1995) Characterization of a phosphate-accumulator mutant of Arabidopsis thaliana. Plant Physiol 107: 207–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Ying H, Helliwell CA, Taylor JM, Peacock WJ, Dennis ES (2011) FLOWERING LOCUS C (FLC) regulates development pathways throughout the life cycle of Arabidopsis. Proc Natl Acad Sci USA 108: 6680–6685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ruijter NCA, Verhees J, Leeuwen W, Krol AR (2003) Evaluation and comparison of the GUS, LUC and GFP reporter system for gene expression studies in plants. Plant Biol 5: 103–115 [Google Scholar]
- Devaiah BN, Karthikeyan AS, Raghothama KG (2007) WRKY75 transcription factor is a modulator of phosphate acquisition and root development in Arabidopsis. Plant Physiol 143: 1789–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaiah BN, Madhuvanthi R, Karthikeyan AS, Raghothama KG (2009) Phosphate starvation responses and gibberellic acid biosynthesis are regulated by the MYB62 transcription factor in Arabidopsis. Mol Plant 2: 43–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong B, Rengel Z, Delhaize E (1998) Uptake and translocation of phosphate by pho2 mutant and wild-type seedlings of Arabidopsis thaliana. Planta 205: 251–256 [DOI] [PubMed] [Google Scholar]
- Duan K, Yi K, Dang L, Huang H, Wu W, Wu P (2008) Characterization of a sub-family of Arabidopsis genes with the SPX domain reveals their diverse functions in plant tolerance to phosphorus starvation. Plant J 54: 965–975 [DOI] [PubMed] [Google Scholar]
- Franco-Zorrilla JM, Valli A, Todesco M, Mateos I, Puga MI, Rubio-Somoza I, Leyva A, Weigel D, García JA, Paz-Ares J (2007) Target mimicry provides a new mechanism for regulation of microRNA activity. Nat Genet 39: 1033–1037 [DOI] [PubMed] [Google Scholar]
- Fu Y, Ma H, Chen S, Gu T, Gong J (2018) Control of proline accumulation under drought via a novel pathway comprising the histone methylase CAU1 and the transcription factor ANAC055. J Exp Bot 69: 579–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii H, Chiou TJ, Lin SI, Aung K, Zhu JK (2005) A miRNA involved in phosphate-starvation response in Arabidopsis. Curr Biol 15: 2038–2043 [DOI] [PubMed] [Google Scholar]
- Glendinning JS; Fertilizer Industry Federation of Australia (2000) Australian Soil Fertility Manual., 2 CSIRO Publishing, Collingwood, Australia, 154 pp [Google Scholar]
- Harrison SJ, Mott EK, Parsley K, Aspinall S, Gray JC, Cottage A (2006) A rapid and robust method of identifying transformed Arabidopsis thaliana seedlings following floral dip transformation. Plant Methods 2: 19–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuer S, Gaxiola R, Schilling R, Herrera-Estrella L, López-Arredondo D, Wissuwa M, Delhaize E, Rouached H (2017) Improving phosphorus use efficiency: A complex trait with emerging opportunities. Plant J 90: 868–885 [DOI] [PubMed] [Google Scholar]
- Hickman R, Hill C, Penfold CA, Breeze E, Bowden L, Moore JD, Zhang P, Jackson A, Cooke E, Bewicke-Copley F, et al. (2013) A local regulatory network around three NAC transcription factors in stress responses and senescence in Arabidopsis leaves. Plant J 75: 26–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman R, Van Verk MC, Van Dijken AJH, Mendes MP, Vroegop-Vos IA, Caarls L, Steenbergen M, Van der Nagel I, Wesselink GJ, Jironkin A, et al. (2017) Architecture and dynamics of the jasmonic acid gene regulatory network. Plant Cell 29: 2086–2105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinsinger P. (2001) Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: A review. Plant Soil 237: 173–195 [Google Scholar]
- Huang TK, Han CL, Lin SI, Chen YJ, Tsai YC, Chen YR, Chen JW, Lin WY, Chen PM, Liu TY, et al. (2013) Identification of downstream components of ubiquitin-conjugating enzyme PHOSPHATE2 by quantitative membrane proteomics in Arabidopsis roots. Plant Cell 25: 4044–4060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost R, Pharmawati M, Lapis-Gaza HR, Rossig C, Berkowitz O, Lambers H, Finnegan PM (2015) Differentiating phosphate-dependent and phosphate-independent systemic phosphate-starvation response networks in Arabidopsis thaliana through the application of phosphite. J Exp Bot 66: 2501–2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kant S, Peng M, Rothstein SJ (2011) Genetic regulation by NLA and microRNA827 for maintaining nitrate-dependent phosphate homeostasis in Arabidopsis. PLoS Genet 7: e1002021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi M, Inzé D, Depicker A (2002) GATEWAY vectors for Agrobacterium-mediated plant transformation. Trends Plant Sci 7: 193–195 [DOI] [PubMed] [Google Scholar]
- Kawashima CG, Berkowitz O, Hell R, Noji M, Saito K (2005) Characterization and expression analysis of a serine acetyltransferase gene family involved in a key step of the sulfur assimilation pathway in Arabidopsis. Plant Physiol 137: 220–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Hong SH, Kim YW, Lee IH, Jun JH, Phee BK, Rupak T, Jeong H, Lee Y, Hong BS, et al. (2014) Gene regulatory cascade of senescence-associated NAC transcription factors activated by ETHYLENE-INSENSITIVE2-mediated leaf senescence signalling in Arabidopsis. J Exp Bot 65: 4023–4036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W, Ahn HJ, Chiou TJ, Ahn JH (2011) The role of the miR399-PHO2 module in the regulation of flowering time in response to different ambient temperatures in Arabidopsis thaliana. Mol Cells 32: 83–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni SR, Vaneechoutte D, Van de Velde J, Vandepoele K (2018) TF2Network: Predicting transcription factor regulators and gene regulatory networks in Arabidopsis using publicly available binding site information. Nucleic Acids Res 46: e31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo HF, Hsu YY, Lin WC, Chen KY, Munnik T, Brearley CA, Chiou TJ (2018) Arabidopsis inositol phosphate kinases IPK1 and ITPK1 constitute a metabolic pathway in maintaining phosphate homeostasis. Plant J 95: 613–630 [DOI] [PubMed] [Google Scholar]
- Landrein B, Formosa-Jordan P, Malivert A, Schuster C, Melnyk CW, Yang W, Turnbull C, Meyerowitz EM, Locke JCW, Jönsson H (2018) Nitrate modulates stem cell dynamics in Arabidopsis shoot meristems through cytokinins. Proc Natl Acad Sci USA 115: 1382–1387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Lee I (2010) Regulation and function of SOC1, a flowering pathway integrator. J Exp Bot 61: 2247–2254 [DOI] [PubMed] [Google Scholar]
- Lee J, Oh M, Park H, Lee I (2008) SOC1 translocated to the nucleus by interaction with AGL24 directly regulates leafy. Plant J 55: 832–843 [DOI] [PubMed] [Google Scholar]
- Lee YS, Mulugu S, York JD, O’Shea EK (2007) Regulation of a cyclin-CDK-CDK inhibitor complex by inositol pyrophosphates. Science 316: 109–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenburg ME, O’Shea EK (1996) Signaling phosphate starvation. Trends Biochem Sci 21: 383–387 [PubMed] [Google Scholar]
- Li D, Liu C, Shen L, Wu Y, Chen H, Robertson M, Helliwell CA, Ito T, Meyerowitz E, Yu H (2008) A repressor complex governs the integration of flowering signals in Arabidopsis. Dev Cell 15: 110–120 [DOI] [PubMed] [Google Scholar]
- Li S, Ying Y, Secco D, Wang C, Narsai R, Whelan J, Shou H (2017) Molecular interaction between PHO2 and GIGANTEA reveals a new crosstalk between flowering time and phosphate homeostasis in Oryza sativa. Plant Cell Environ 40: 1487–1499 [DOI] [PubMed] [Google Scholar]
- Lin WY, Huang TK, Chiou TJ (2013) Nitrogen limitation adaptation, a target of microRNA827, mediates degradation of plasma membrane-localized phosphate transporters to maintain phosphate homeostasis in Arabidopsis. Plant Cell 25: 4061–4074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linn J, Ren M, Berkowitz O, Ding W, van der Merwe MJ, Whelan J, Jost R (2017) Root cell-specific regulators of phosphate-dependent growth. Plant Physiol 174: 1969–1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Wang Z, Ren H, Shen C, Li Y, Ling HQ, Wu C, Lian X, Wu P (2010) OsSPX1 suppresses the function of OsPHR2 in the regulation of expression of OsPT2 and phosphate homeostasis in shoots of rice. Plant J 62: 508–517 [DOI] [PubMed] [Google Scholar]
- Liu TY, Huang TK, Tseng CY, Lai YS, Lin SI, Lin WY, Chen JW, Chiou TJ (2012) PHO2-dependent degradation of PHO1 modulates phosphate homeostasis in Arabidopsis. Plant Cell 24: 2168–2183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Xie Y, Wang H, Ma X, Yao W, Wang H (2017) Light and ethylene coordinately regulate the phosphate starvation response through transcriptional regulation of PHOSPHATE STARVATION RESPONSE1. Plant Cell 29: 2269–2284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundmark M, Nilsson L, Korner CJ, Nielsen TH (2011) Overexpression of the MYB-related transcription factor GCC7 in Arabidopsis thaliana leads to increased levels of Pi and changed P-dependent gene regulation. Funct Plant Biol 38: 151–162 [DOI] [PubMed] [Google Scholar]
- Lv Q, Zhong Y, Wang Y, Wang Z, Zhang L, Shi J, Wu Z, Liu Y, Mao C, Yi K, et al. (2014) SPX4 negatively regulates phosphate signaling and homeostasis through its interaction with PHR2 in rice. Plant Cell 26: 1586–1597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AE, Grant BR, Plaxton WC (2001) Phosphite (phosphorous acid): Its relevance in the environment and agriculture and influence on plant phosphate starvation response. J Plant Nutr 24: 1505–1519 [Google Scholar]
- Miura K, Rus A, Sharkhuu A, Yokoi S, Karthikeyan AS, Raghothama KG, Baek D, Koo YD, Jin JB, Bressan RA, et al. (2005) The Arabidopsis SUMO E3 ligase SIZ1 controls phosphate deficiency responses. Proc Natl Acad Sci USA 102: 7760–7765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi T, Wright L, Fujiwara S, Cremer F, Lee K, Onouchi H, Mouradov A, Fowler S, Kamada H, Putterill J, et al. (2005) Distinct roles of GIGANTEA in promoting flowering and regulating circadian rhythms in Arabidopsis. Plant Cell 17: 2255–2270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon J, Suh SS, Lee H, Choi KR, Hong CB, Paek NC, Kim SG, Lee I (2003) The SOC1 MADS-box gene integrates vernalization and gibberellin signals for flowering in Arabidopsis. Plant J 35: 613–623 [DOI] [PubMed] [Google Scholar]
- Mouradov A, Cremer F, Coupland G (2002) Control of flowering time: Interacting pathways as a basis for diversity. Plant Cell (Suppl) 14: S111–S130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukatira UT, Liu C, Varadarajan DK, Raghothama KG (2001) Negative regulation of phosphate starvation-induced genes. Plant Physiol 127: 1854–1862 [PMC free article] [PubMed] [Google Scholar]
- Müller B, Fastner A, Karmann J, Mansch V, Hoffmann T, Schwab W, Suter-Grotemeyer M, Rentsch D, Truernit E, Ladwig F, et al. (2015) Amino acid export in developing Arabidopsis seeds depends on UmamiT facilitators. Curr Biol 25: 3126–3131 [DOI] [PubMed] [Google Scholar]
- Müller R, Nilsson L, Krintel C, Nielsen TH (2004) Gene expression during recovery from phosphate starvation in roots and shoots of Arabidopsis thaliana. Physiol Plant 122: 233–243 [Google Scholar]
- Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15: 473–497 [Google Scholar]
- Nilsson L, Müller R, Nielsen TH (2007) Increased expression of the MYB-related transcription factor, PHR1, leads to enhanced phosphate uptake in Arabidopsis thaliana. Plant Cell Environ 30: 1499–1512 [DOI] [PubMed] [Google Scholar]
- Pajoro A, Biewers S, Dougali E, Leal Valentim F, Mendes MA, Porri A, Coupland G, Van de Peer Y, van Dijk ADJ, Colombo L, et al. (2014) The (r)evolution of gene regulatory networks controlling Arabidopsis plant reproduction: A two-decade history. J Exp Bot 65: 4731–4745 [DOI] [PubMed] [Google Scholar]
- Pant BD, Buhtz A, Kehr J, Scheible WR (2008) MicroRNA399 is a long-distance signal for the regulation of plant phosphate homeostasis. Plant J 53: 731–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park BS, Seo JS, Chua NH (2014) NITROGEN LIMITATION ADAPTATION recruits PHOSPHATE2 to target the phosphate transporter PT2 for degradation during the regulation of Arabidopsis phosphate homeostasis. Plant Cell 26: 454–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Péret B, Desnos T, Jost R, Kanno S, Berkowitz O, Nussaume L (2014) Root architecture responses: In search of phosphate. Plant Physiol 166: 1713–1723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimentel H, Bray NL, Puente S, Melsted P, Pachter L (2017) Differential analysis of RNA-seq incorporating quantification uncertainty. Nat Methods 14: 687–690 [DOI] [PubMed] [Google Scholar]
- Puga MI, Mateos I, Charukesi R, Wang Z, Franco-Zorrilla JM, de Lorenzo L, Irigoyen ML, Masiero S, Bustos R, Rodríguez J, et al. (2014) SPX1 is a phosphate-dependent inhibitor of Phosphate Starvation Response 1 in Arabidopsis. Proc Natl Acad Sci USA 111: 14947–14952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi W, Manfield IW, Muench SP, Baker A (2017) AtSPX1 affects the AtPHR1-DNA-binding equilibrium by binding monomeric AtPHR1 in solution. Biochem J 474: 3675–3687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghothama KG. (1999) Phosphate acquisition. Annu Rev Plant Physiol Plant Mol Biol 50: 665–693 [DOI] [PubMed] [Google Scholar]
- Richardson AE, Simpson RJ (2011) Soil microorganisms mediating phosphorus availability update on microbial phosphorus. Plant Physiol 156: 989–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouached H, Stefanovic A, Secco D, Bulak Arpat A, Gout E, Bligny R, Poirier Y (2011) Uncoupling phosphate deficiency from its major effects on growth and transcriptome via PHO1 expression in Arabidopsis. Plant J 65: 557–570 [DOI] [PubMed] [Google Scholar]
- Rubio V, Linhares F, Solano R, Martín AC, Iglesias J, Leyva A, Paz-Ares J (2001) A conserved MYB transcription factor involved in phosphate starvation signaling both in vascular plants and in unicellular algae. Genes Dev 15: 2122–2133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruijter JM, Ramakers C, Hoogaars WMH, Karlen Y, Bakker O, van den Hoff MJB, Moorman AFM (2009) Amplification efficiency: Linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Res 37: e45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secco D, Wang C, Arpat BA, Wang Z, Poirier Y, Tyerman SD, Wu P, Shou H, Whelan J (2012) The emerging importance of the SPX domain-containing proteins in phosphate homeostasis. New Phytol 193: 842–851 [DOI] [PubMed] [Google Scholar]
- Secco D, Jabnoune M, Walker H, Shou H, Wu P, Poirier Y, Whelan J (2013) Spatio-temporal transcript profiling of rice roots and shoots in response to phosphate starvation and recovery. Plant Cell 25: 4285–4304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T (2003) Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res 13: 2498–2504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Song L, Zhang Y, Zheng Z, Liu D (2016) Arabidopsis PHL2 and PHR1 act redundantly as the key components of the central regulatory system controlling transcriptional responses to phosphate starvation. Plant Physiol 170: 499–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasaki H, Maruyama K, Takahashi F, Fujita M, Yoshida T, Nakashima K, Myouga F, Toyooka K, Yamaguchi-Shinozaki K, Shinozaki K (2015) SNAC-As, stress-responsive NAC transcription factors, mediate ABA-inducible leaf senescence. Plant J 84: 1114–1123 [DOI] [PubMed] [Google Scholar]
- Tsuji H, Taoka K, Shimamoto K (2011) Regulation of flowering in rice: Two florigen genes, a complex gene network, and natural variation. Curr Opin Plant Biol 14: 45–52 [DOI] [PubMed] [Google Scholar]
- Veneklaas EJ, Lambers H, Bragg J, Finnegan PM, Lovelock CE, Plaxton WC, Price CA, Scheible WR, Shane MW, White PJ, et al. (2012) Opportunities for improving phosphorus-use efficiency in crop plants. New Phytol 195: 306–320 [DOI] [PubMed] [Google Scholar]
- Vidal EA, Moyano TC, Canales J, Gutiérrez RA (2014) Nitrogen control of developmental phase transitions in Arabidopsis thaliana. J Exp Bot 65: 5611–5618 [DOI] [PubMed] [Google Scholar]
- Wang Z, Ye S, Li J, Zheng B, Bao M, Ning G (2011) Fusion primer and nested integrated PCR (FPNI-PCR): A new high-efficiency strategy for rapid chromosome walking or flanking sequence cloning. BMC Biotechnol 11: 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Ruan W, Shi J, Zhang L, Xiang D, Yang C, Li C, Wu Z, Liu Y, Yu Y, et al. (2014) Rice SPX1 and SPX2 inhibit phosphate starvation responses through interacting with PHR2 in a phosphate-dependent manner. Proc Natl Acad Sci USA 111: 14953–14958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild R, Gerasimaite R, Jung JY, Truffault V, Pavlovic I, Schmidt A, Saiardi A, Jessen HJ, Poirier Y, Hothorn M, et al. (2016) Control of eukaryotic phosphate homeostasis by inositol polyphosphate sensor domains. Science 352: 986–990 [DOI] [PubMed] [Google Scholar]
- Woo J, MacPherson CR, Liu J, Wang H, Kiba T, Hannah MA, Wang XJ, Bajic VB, Chua NH (2012) The response and recovery of the Arabidopsis thaliana transcriptome to phosphate starvation. BMC Plant Biol 12: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu FH, Shen SC, Lee LY, Lee SH, Chan MT, Lin CS (2009) Tape-Arabidopsis Sandwich: A simpler Arabidopsis protoplast isolation method. Plant Methods 5: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P, Ma L, Hou X, Wang M, Wu Y, Liu F, Deng XW (2003) Phosphate starvation triggers distinct alterations of genome expression in Arabidopsis roots and leaves. Plant Physiol 132: 1260–1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K, Fuji K, Shimada T, Nishimura M, Hara-Nishimura I (2005) Endosomal proteases facilitate the fusion of endosomes with vacuoles at the final step of the endocytotic pathway. Plant J 41: 888–898 [DOI] [PubMed] [Google Scholar]
- Yant L, Mathieu J, Dinh TT, Ott F, Lanz C, Wollmann H, Chen X, Schmid M (2010) Orchestration of the floral transition and floral development in Arabidopsis by the bifunctional transcription factor APETALA2. Plant Cell 22: 2156–2170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SD, Cho YH, Sheen J (2007) Arabidopsis mesophyll protoplasts: A versatile cell system for transient gene expression analysis. Nat Protoc 2: 1565–1572 [DOI] [PubMed] [Google Scholar]
- Zhong Y, Wang Y, Guo J, Zhu X, Shi J, He Q, Liu Y, Wu Y, Zhang L, Lv Q, et al. (2018) Rice SPX6 negatively regulates the phosphate starvation response through suppression of the transcription factor PHR2. New Phytol 219: 135–148 [DOI] [PubMed] [Google Scholar]