CDK8 positively regulates SAR by interacting with NPR1, transcription factors, and connecting RNA polymerase II to regulate NPR1 and PR1 expression.
Abstract
NPR1 (NONEXPRESSER OF PR GENES1) functions as a master regulator of the plant hormone salicylic acid (SA) signaling and plays an essential role in plant immunity. In the nucleus, NPR1 interacts with transcription factors to induce the expression of PR (PATHOGENESIS-RELATED) genes and thereby promote defense responses. However, the underlying molecular mechanism of PR gene activation is poorly understood. Furthermore, despite the importance of NPR1 in plant immunity, the regulation of NPR1 expression has not been extensively studied. Here, we show that SA promotes the interaction of NPR1 with both CDK8 (CYCLIN-DEPENDENT KINASE8) and WRKY18 (WRKY DNA-BINDING PROTEIN18) in Arabidopsis (Arabidopsis thaliana). NPR1 recruits CDK8 and WRKY18 to the NPR1 promoter, facilitating its own expression. Intriguingly, CDK8 and its associated Mediator subunits positively regulate NPR1 and PR1 expression and play a pivotal role in local and systemic immunity. Moreover, CDK8 interacts with WRKY6, WRKY18, and TGA transcription factors and brings RNA polymerase II to NPR1 and PR1 promoters and coding regions to facilitate their expression. Our studies reveal a mechanism in which NPR1 recruits CDK8, WRKY18, and TGA transcription factors along with RNA polymerase II in the presence of SA and thereby facilitates its own and target gene expression for the establishment of plant immunity.
In nature, plants constantly face all kinds of abiotic and biotic stresses, including pathogens such as fungi, bacteria, oomycetes, nematodes, and viruses. Plants employ a battery of immune mechanisms that overcome infections caused by these pathogens (Jones and Dangl, 2006). The first layer of defense is called pathogen-associated molecular patterns (PAMP)-triggered immunity (PTI), in which plants utilize pattern recognition receptors to perceive conserved molecules in pathogens called PAMPs and induce defense responses against pathogen infection. However, many plant pathogens deliver PTI-suppressing effectors into plant cells, inducing effector-triggered susceptibility to cause diseases (Block et al., 2008). Facing these challenges, plants developed a second layer of defense, which involves Resistance proteins that recognize pathogen-specific effectors to mount effector-triggered immunity. Effector-triggered immunity is associated with a rapid and localized cell death called the hypersensitive response (Wu et al., 2014). Besides the induction of defense at the site of infection, a systemic defense response is often triggered in the distal parts of the infected plants, which protects uninfected tissues against subsequent infections by a wide range of pathogens (Mishina and Zeier, 2007; Fu and Dong, 2013). This long-lasting and broad-spectrum induced disease resistance is referred to as systemic acquired resistance (SAR; Pieterse et al., 2009). SAR is associated with increased levels of the plant hormone salicylic acid (SA) in plants (Shah, 2009; Vlot et al., 2009). Upon pathogen infection, an elevated level of SA activates the expression of PR (PATHOGENESIS-RELATED) genes, some of which encode proteins with antimicrobial activities (van Loon et al., 2006). Through genetic screens of Arabidopsis (Arabidopsis thaliana) mutants in which the expression of PR genes is abolished after treatment with SA or its active analogs, a locus called NPR1 (NONEXPRESSOR OF PR1) was identified (Cao et al., 1994; Ryals et al., 1997; Shah et al., 1997). The npr1 mutants, which are unable to activate the expression of PR genes, are more susceptible to virulent pathogen infection and are completely compromised in SAR (Cao et al., 1997). Later, NPR1 was identified as a receptor for SA by different groups (Wu et al., 2012; Ding et al., 2018). Even though it is known that NPR1 interacts with TGA and TCP transcription factors (TFs) in the nucleus to activate the expression of PR genes, including PR1, PR2, and PR5 (Després et al., 2000, 2003; Zhou et al., 2000; Kesarwani et al., 2007; Boyle et al., 2009; Lindermayr et al., 2010; Li et al., 2018), further studies are required to elucidate how NPR1-TF protein complexes promote the expression of PR genes.
Whereas posttranslational modifications of NPR1 protein including phosphorylation, S-nitrosylation, and sumoylation have been extensively studied (Tada et al., 2008; Xie et al., 2010; Lee et al., 2015; Saleh et al., 2015), transcriptional regulation of NPR1 is poorly understood. Yu et al. (2001) reported that the W-box sequence in the promoter of NPR1 gene is specifically recognized by the SA-induced WRKY18 protein in Arabidopsis. Mutations in the W-box sequence prevent WRKY18 from binding to the NPR1 promoter. A wild-type copy of the NPR1 gene with mutated W-box is unable to complement npr1 mutants and is unable to induce SA-dependent defense gene expression and disease resistance. This suggests that WRKY binding to the W-box sequence in the NPR1 promoter is essential for NPR1 gene expression. Interestingly, a number of WRKY genes were found to be induced by SA, which further suggests that these WRKYs could be involved in the regulation of SA-dependent responses (Wang et al., 2006). Indeed, it was found that WRKY6 protein can bind directly to the NPR1 promoter containing the W-box motif in a chromatin immunoprecipitation (ChIP) assay (Chai et al., 2014). The NPR1 mRNA level is reduced in wrky6 mutants and increased in WRKY6-overexpressing transgenic Arabidopsis in response to SA treatment (Chai et al., 2014).
In this study, we show that NPR1 protein positively regulates its own expression and binds to its own NPR1 promoter. Furthermore, we show that SA substantially promotes the interactions between NPR1 and CDK8 (CYCLIN-DEPENDENT KINASE8) and between NPR1 and WRKY18. In cdk8 and CDK8-associated Mediator mutants, SAR is compromised and the expression of NPR1 and NPR1-dependent defense genes including PR1 is significantly reduced compared with wild-type plants. CDK8 interacts with WRKY6 and WRKY18, which are associated with the NPR1 promoter, to positively regulate the expression of NPR1. In addition, CDK8 interacts with both TGA5 and TGA7 and is associated with the PR1 promoter to regulate PR1 gene expression. Moreover, we found that CDK8 is involved in the recruitment of RNA polymerase II to the NPR1 and PR1 promoters and coding regions to promote the expression of these two genes. Taken together, our study uncovers a unique mechanism in which NPR1 recruits CDK8 and thereby promotes its own and target gene expression for the establishment of plant immunity.
RESULTS
NPR1 Protein Facilitates NPR1 Gene Expression
The npr1-1 and npr1-2 mutants were identified by screening for ethyl methanesulfonate (EMS)-induced mutants in which SA and its active analogs could not induce PR gene expression (Cao et al., 1997). The npr1-1 mutant carries a C1000-to-T mutation, changing the conserved His residue at position 334 in the third ankyrin-repeat sequence to a Tyr (Cao et al., 1997), whereas the npr1-2 mutant has a G449-to-A mutation that changes Cys-150 to Tyr (Cao et al., 1997; Zhou et al., 2000; Fan and Dong, 2002). We are fascinated by the fact that a single nucleotide mutation, which results in a single amino acid change, completely abolishes the function of NPR1 in plant defense. The first question we asked was whether any npr1-1 or npr1-2 transcript or npr1-1 or npr1-2 protein was present in these mutants (Cao et al., 1997; Spoel et al., 2009).
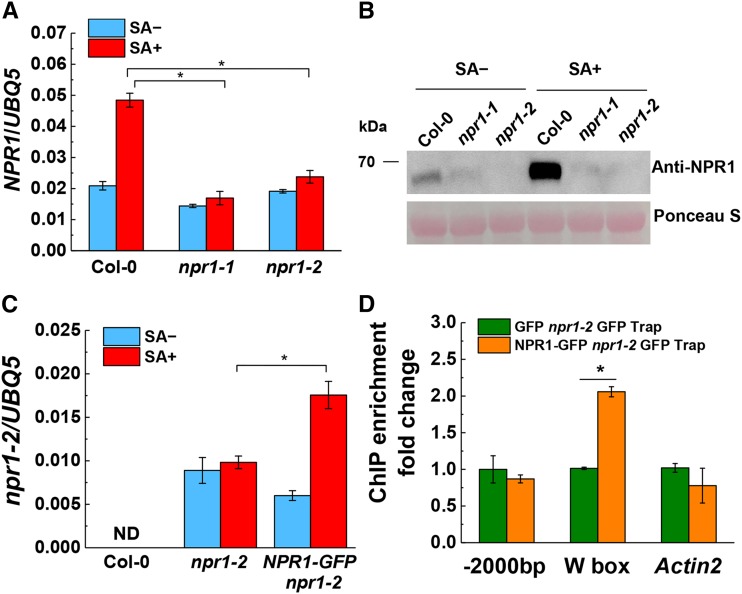
To address this question, we first checked NPR1 gene expression in Columbia-0 (Col-0) wild-type and npr1-1 and npr1-2 mutant plants. Interestingly, we found that both mutants accumulated significantly lower amounts of NPR1 transcript compared with Col-0 after SA treatment (Fig. 1A). To determine npr1 protein levels in npr1-1 and npr1-2 mutants, we first checked if an anti-NPR1 antibody can detect the npr1-1 and npr1-2 protein expressed in yeast (Saccharomyces cerevisiae) cells. As shown in Supplemental Figure S1, distinct bands were observed using lysate from yeast cells expressing npr1-1 or npr1-2, but not using the lysate of control yeast cells with an empty vector, demonstrating that this anti-NPR1 antibody detects npr1-1 and npr1-2 proteins efficiently (Supplemental Fig. S1). To test whether npr1-1 and npr1-2 mutants are indeed null mutants, we used this anti-NPR1 antibody to detect npr1-1 and npr1-2 protein levels in npr1-1 and npr1-2 mutants. As shown in Figure 1B, after SA treatment, a strong NPR1 band was detected in wild-type Col-0 plants; however, in npr1-1 and npr1-2 mutants, no npr1 protein was detected. It has been shown that NPR1-green fluorescent protein (GFP) expressed under the control of the 35S promoter complements npr1-2 mutants (Spoel et al., 2009). When we checked the NPR1-GFP protein level in 35S:NPR1-GFP transgenic plants in the npr1-2 background using this anti-NPR1 antibody, we also detected a strong npr1-2 band that was absent in the npr1-2 mutant, indicating that a functional NPR1 protein is required for the SA induction of NPR1 protein (Supplemental Fig. S2). To exclude the possibility that the npr1-2 band was due to degradation of NPR1-GFP protein, we ran a separate gel using the same samples and probed the blot with an anti-GFP antibody. As shown in Supplemental Figure S2, we observed a strong NPR1-GFP band but no GFP band in the 35S:NPR1-GFP transgenic plants in the npr1-2 background, suggesting that the npr1-2 band in 35S:NPR1-GFP npr1-2 plants was not due to degradation of NPR1-GFP protein.
Figure 1.
NPR1 protein facilitates its own expression and binds to its own promoter. A, NPR1 gene expression level in Col-0 wild type, npr1-1, and npr1-2. The plants were sprayed with 0.5 mm SA or water as a control for 4 h. Leaves were collected for gene expression analysis. B, NPR1 protein level in Col-0 wild type, npr1-1, and npr1-2. Plants were treated as in A. Western blots were probed with an anti-NPR1 antibody. C, npr1-2 gene expression level in Col-0 wild type, npr1-2, and NPR1-GFP npr1-2. Seedlings were treated with 0.5 mm SA or water as a control for 4 h. Samples were assayed for gene expression analysis with primers specifically recognizing npr1-2 but not NPR1. The expression levels of NPR1 and npr1-2 were normalized to UBQ5 expression. ND, Not detected. D, NPR1 associates with its own promoter. Seedlings of transgenic GFP npr1-2 and NPR1-GFP npr1-2 were treated with 0.5 mm SA for 4 h. ChIP assay was performed using GFP-Trap (ChromoTek) or control beads with the following primers: -2000bp, amplifies upstream of the NPR1 promoter at the −2,000-bp region; W box, amplifies the sequence containing the W-box at the NPR1 promoter; Actin2 was used as a negative control. All experiments were repeated at least two times with similar results. Data represent means of three independent samples with se. Asterisks indicate significant differences (Student’s t test, *, P < 0.05). Ponceau S, Ponceau staining.
These data prompt us to speculate that a functional NPR1 protein promotes NPR1 gene expression. To further demonstrate that NPR1 promotes its own expression, we designed a pair of specific primers that amplify the npr1-2 coding region sequence from 429 to 541 with an additional T448-to-G mutation in the forward primer. In this case, there is only a nucleotide change in the forward primer compared with the npr1-2 complementary DNA (cDNA) sequence, but two nucleotides are altered compared with wild-type NPR1 cDNA, so this pair of primers can amplify npr1-2 but not wild-type NPR1 cDNA. As shown in Figure 1C, the npr1-2 transcript was detected in npr1-2 mutants but not in Col-0 wild-type plants. Intriguingly, we observed a strong induction of npr1-2 mRNA in 35S:NPR1-GFP npr1-2 plants compared with npr1-2 mutants after they were treated with SA, indicating that NPR1-GFP promotes npr1-2 expression.
NPR1 Binds to Its Own Promoter
As a transcriptional coactivator, NPR1 binds to the promoters of the PR gene to facilitate their expression (Saleh et al., 2015). Based on these observations, we hypothesized that NPR1 protein positively regulates its own expression by binding to its own promoter. To demonstrate that NPR1 indeed binds to its own promoter, a ChIP assay was performed using 35S:GFP transgenic plants and 35S:NPR1-GFP transgenic plants in the npr1-2 background treated with 0.5 mm SA for 4 h. Interestingly, we found that the NPR1-GFP association with the W-box of the NPR1 promoter was enriched twofold compared with the GFP control (Fig. 1D). By contrast, no difference was found when the Actin2 gene was used as a negative control. Taken together, these data indicate that NPR1 protein positively regulates NPR1 gene expression and is associated with its own promoter.
The Interaction between NPR1 and WRKY18 Is Enhanced by SA
To investigate how NPR1 is associated with its own promoter, we used yeast two-hybrid (Y2H) assays to test the interaction between NPR1 and WRKY6 and WRKY18, which are TFs that are known to bind the NPR1 promoter (Yu et al., 2001; Chai et al., 2014). The yeast strain AH109 cotransformed with pGBKT7-WRKY18 and pGADT7-NPR1, but not pGADT7-npr1-1 or -npr1-2, grew on the triple synthetic dropout (TD; −Leu, −Trp, and −His) plate, indicating that NPR1 but not npr1-1 or npr1-2 interacts with WRKY18 (Fig. 2A). Interestingly, the growth of yeast AH109 cotransformed with pGADT7-NPR1 and pGBKT7-WRKY18 increased on the TD plate supplemented with 200 μm SA, indicating that SA promotes the interaction between NPR1 and WRKY18. No interaction between WRKY6 and NPR1 was found in Y2H assays (Fig. 2A). To further confirm the interaction between NPR1 and WRKY18, we performed a coimmunoprecipitation (Co-IP) assay in which GFP, WRKY6-GFP, or WRKY18-GFP and human influenza hemagglutinin (HA)-NPR1 were coexpressed in Nicotiana benthamiana leaves. We found that HA-NPR1 coimmunoprecipitated with WRKY18-GFP but not with WRKY6-GFP, indicating that WRKY18 but not WRKY6 interacts with NPR1 in planta (Fig. 2B). To further demonstrate the effect of SA on the interaction between NPR1 and WRKY18, we performed an in vitro pull-down assay. N. benthamiana leaves were used to transiently express GFP or WRKY18-GFP by agroinfiltration. GFP or WRKY18-GFP was then immunoprecipitated from leaf extracts by GFP-Trap. The beads were washed and incubated with 1 μg of 6xHis-Maltose Binding Protein (MBP)-NPR1 with or without 200 μm SA overnight. We found that 6xHis-MBP-NPR1 was pulled down by WRKY18-GFP but not by the GFP control and substantially more 6xHis-MBP-NPR1 protein was pulled down by WRKY18-GFP in the presence of SA than in its absence (Fig. 2C), indicating that SA promotes the interaction between WRKY18 and NPR1.
Figure 2.
The interaction between NPR1 and WRKY18 is substantially enhanced by SA. A, NPR1 but not npr1-1 or npr1-2 interacts with WRKY18 in Y2H assays. Yeast strain AH109 was cotransformed with pGBKT7-WRKY6 or WRKY18 and pGADT7-NPR1, npr1-1, npr1-2, or an empty vector (EV). Cotransformed yeast selected from a double dropout (DD) plate (−Leu, −Trp) was recultured, diluted to OD600 nm = 1, 0.1, or 0.01, and then 10 µL of the diluted liquid cultures was placed onto a DD plate and a TD plate (−Leu, −Trp, −His) with or without 200 μm SA. B, Co-IP between HA-NPR1 and GFP, WRKY6-GFP, or WRKY18-GFP. HA-NPR1 and GFP, WRKY6-GFP, or WRKY18-GFP proteins, expressed in N. benthamiana leaves by agroinfiltration, were immunoprecipitated with GFP-Trap for 2 h. The beads were washed, eluted with 2× loading buffer, and immunoblotted with an anti-GFP or an anti-HA antibody. C, In vitro pull-down between 6xHis-MPB-NPR1 and WRKY18-GFP. GFP or WRKY18-GFP was immunoprecipitated from N. benthamiana leaf extracts with GFP-Trap. The beads were washed and incubated with purified 6xHis-MBP-NPR1 with or without 200 μm SA. After overnight incubation, beads were washed three times with washing buffer, then eluted, and detected with anti-GFP or anti-6xHis antibody. The number beneath each blot indicates the relative strength of the band. The experiments were repeated two times with similar results.
SA Promotes the Interaction between NPR1 and CDK8
NPR1 controls the expression of over 2,000 genes (Wang et al., 2006). Being a transcriptional coactivator, NPR1 may play a role in communicating with RNA polymerase II to promote its own expression; however, we did not observe any direct interaction between NPR1 and RNA polymerase II C-terminal domain (CTD) in our Y2H assays (data not shown). Since CDK8 phosphorylates RNA polymerase II CTD (Wang and Chen, 2004), we hypothesize that CDK8 could be the link between NPR1 and RNA polymerase II. To determine whether CDK8 interacts with NPR1, we performed Y2H assays using NPR1 as the bait and CDK8 as the prey. We found that NPR1 but not npr1-1 or npr1-2 interacts with CDK8 strongly on the TD plate. To determine whether SA can enhance this interaction, we tested the yeast growth on the TD plate supplemented with 1 mm 3-aminotriazole (3-AT) or 1 mm 3-AT and 200 μm SA. Indeed, SA dramatically promoted the yeast growth on the TD plates supplemented with 1 mm 3-AT (Fig. 3A), indicating that SA facilitates the interaction between NPR1 and CDK8 in Y2H assays. To further confirm the interaction between NPR1 and CDK8, a glutathione S-transferase (GST) pull-down assay was performed. We found that 6xHis-MBP-CDK8 was pulled down by GST-NPR1 but not by the GST control (Fig. 3B). In the presence of SA, more CDK8 protein was pulled down by GST-NPR1, indicating that SA promotes the direct interaction between NPR1 and CDK8 in vitro. To determine whether CDK8 and NPR1 expressed in plants interact with each other, a Co-IP assay was performed. GFP or NPR1-GFP and HA-CDK8 were coexpressed in N. benthamiana NahG transgenic plants. NahG encodes SA hydroxylase, which degrades SA into catechol (Delaney et al., 1994). We use NahG transgenic plants to eliminate SA induced by agroinfiltration in N. benthamiana plants. We found that HA-CDK8 was coimmunoprecipitated with NPR1-GFP but not with the GFP control. To test the effect of SA on the interaction between NPR1 and CDK8, we added 2,6-dichloroisonicotinic acid (INA), an active SA analog that cannot be degraded by NahG (Clarke et al., 1998; Fu et al., 2012; Li et al., 2017; Qi et al., 2018), in the NahG plant extract during the immunoprecipitation step. We found that INA strongly increased the amount of HA-CDK8 that was coimmunoprecipitated (Fig. 3C), supporting that INA promotes the interaction between NPR1 and CDK8.
Figure 3.
SA promotes the interaction between NPR1 and CDK8. A, NPR1 but not npr1-1 or npr1-2 interacts with CDK8 in Y2H assays. Y2H assays were carried out using CDK8 as bait (in pGBKT7 vector with a DNA-binding domain) and NPR1, npr1-1, npr1-2, or an EV as prey (in pGADT7 vector with an activation domain). Cotransformed yeast selected from a DD plate were recultured, diluted to OD600 nm = 1, 0.1, or 0.01, and then 10 µL of the diluted liquid cultures was placed onto a DD plate and a TD plate with or without 1 mm 3-AT or 200 μm SA. B, In vitro pull-down assay between GST-NPR1 and 6xHis-MBP-CDK8. Purified 6xHis-MBP-CDK8 was incubated with magnetic beads conjugated with GST or GST-NPR1 with or without 200 μm SA. After overnight incubation, beads were washed four times with washing buffer, then eluted, and detected with anti-GST or anti-6xHis antibody. C, Co-IP between HA-CDK8 and NPR1-GFP proteins in N. benthamiana NahG transgenic plants. Proteins, extracted from N. benthamiana leaves transiently expressing NPR1-GFP and HA-CDK8 or GFP and HA-CDK8 proteins by agroinfiltration, were immunoprecipitated with GFP-Trap with or without 200 μm INA overnight. The beads were washed, eluted with 2× loading buffer, and immunoblotted with an anti-GFP or an anti-HA antibody. The number beneath each blot indicates the relative strength of the band. The experiments were repeated two times with similar results.
CDK8 Plays an Important Role in Plant Immunity
To determine whether CDK8 plays a role in plant immunity, we first checked the expression of CDK8 in Arabidopsis Col-0 plants after they were infected by Pseudomonas syringae pv maculicola ES4326 (Psm 4326) carrying the avirulent gene avrRpt2, which is a trigger for SAR. We found that Psm avrRpt2 infection induced CDK8 gene expression slightly at 4 h and significantly (P < 0.05) by 3.3- and 3.4-fold at 8 and 12 h post inoculation, respectively (Fig. 4A). We also observed an even higher level of CDK8 gene expression at 24 h after inoculation (Fig. 4A). These data suggest that CDK8 plays a role in plant immunity. To determine whether CDK8 indeed plays a role in SAR, we performed a SAR assay in Col-0 wild-type, npr1-2, cdk8-4, and cdk8-1 mutant plants. We found that both cdk8-4 and cdk8-1 mutants showed increased susceptibility compared with that of Col-0 in the absence of SAR induction (Fig. 4B). In terms of SAR, unlike in Col-0 plants, the growth of the virulent Psm 4326 on systemic leaves of npr1-2, cdk8-4, and cdk8-1 mutants was not significantly reduced, even after preinoculation with the avirulent Psm avrRpt2, indicating that cdk8-4 and cdk8-1 mutants were SAR defective (Fig. 4B).
Figure 4.
SAR and defense gene expression are compromised in the cdk8 mutants. A, CDK8 gene expression level during the time-course infection of the avirulent pathogen. Col-0 plants were infiltrated with Psm avrRpt2 (OD600 nm = 0.005) or MgCl2 buffer as a control. Leaf samples were collected at the indicated times for gene expression analysis. hpi, Hours post inoculation. B, SAR phenotypes in Col-0 wild-type, npr1-2, cdk8-4, and cdk8-1 mutant plants. Two lower leaves were infiltrated with Psm avrRpt2 (OD600 nm = 0.02; SAR+) or MgCl2 buffer (SAR−) as a control. After 2 d, two upper healthy leaves were challenged with virulent Psm (OD600 nm = 0.0005). The leaf discs from the second inoculation were collected at 3 d post inoculation for counting bacterial colonies. CFU, Colony-forming unit. C, NPR1 gene expression levels in the local leaves of Col-0 wild-type, npr1-2, and cdk8-4 mutant plants infected with Psm avrRpt2 (OD600 nm = 0.02) or MgCl2 buffer as a control. Leaves were collected 4 h after infection for gene expression analysis. D, PR1 gene expression level in the local leaves of Col-0 wild-type, npr1-2, and cdk8-4 mutant plants. Leaves were infected with Psm avrRpt2 (OD600 nm = 0.001) or MgCl2 buffer as a control. After 24 h, the infected leaves were collected for gene expression analysis. E, PR1 gene expression levels in the systemic leaves of Col-0 wild-type, npr1-2, and cdk8-4 mutant plants. Two lower leaves were infected with Psm avrRpt2 (OD600 nm = 0.02) or MgCl2 buffer as a control. After 2 d, the upper uninfected leaves were collected for gene expression analysis. F, NPR1 gene expression level in Col-0 wild type, npr1-2, and cdk8-4 mutants. Col-0 and mutant plants were irrigated with 0.5 mm SA or water as a control, and then leaf samples were collected after 4 h for NPR1 gene expression analysis. G, NPR1 protein accumulation in Col-0 wild type, npr1-2, and cdk8-4 mutants during SA treatment. H to J, Col-0 wild type, npr1-2, and cdk8-4 mutants were treated with SA for 24 h, and leaf samples were collected for PR1 (H), PR2 (I), and WRKY38 (J) gene expression analysis. The expression levels of CDK8, NPR1, PR1, PR2, and WRKY38 were normalized to UBQ5 expression. Data represent means of three independent samples with se. Asterisks indicate significant differences (Student’s t test, *, P < 0.05 and **, P < 0.01). The experiments were repeated three times with similar results.
To investigate how CDK8 contributes to the establishment of SAR, we analyzed NPR1 gene expression in the local leaves of Col-0, npr1-2, and cdk8-4 mutants 4 h after infection with Psm avrRpt2, because NPR1 is a master regulator of local and systemic plant immunity (Cao et al., 1997). We found that the NPR1 gene was dramatically induced by Psm avrRpt2 in Col-0. Interestingly, the expression of the NPR1 gene was significantly (P < 0.05) reduced in npr1-2 and cdk8-4 mutants compared with Col-0 (Fig. 4C). We next analyzed the expression of PR1, a widely used marker gene of SAR, in the local leaves of Col-0, npr1-2, and cdk8-4 mutants after 24 h of infection with Psm avrRpt2. We found that PR1 gene expression was dramatically reduced in the cdk8-4 mutant compared with Col-0 (Fig. 4D). Similarly, we also observed a reduction of PR1 gene expression in the local leaves of the cdk8-4 mutant compared with Col-0 when challenged with virulent Psm (Supplemental Fig. S3). In addition, we found that PR1 gene expression was drastically reduced in npr1-2 and cdk8-4 mutant systemic leaves when compared with Col-0 after 2 d of infection with Psm avrRpt2 (Fig. 4E). Taken together, these results indicate that CDK8 plays an important role in plant immunity.
The Expression of NPR1 and Its Target Genes Including PR1 Requires NPR1 and CDK8 in SA Signaling
To further validate the function of CDK8 in SA signaling, we irrigated soil-grown Col-0 and npr1-2 and cdk8-4 mutant plants with 0.5 mm SA, then analyzed NPR1 gene expression after 4 h of treatment. We found that SA could induce NPR1 gene expression in the cdk8-4 mutant, but the level was significantly (P < 0.01) lower than that in Col-0 (Fig. 4F). Meanwhile, we also checked NPR1 gene expression in these same genotypes 4 h after they were sprayed with SA. Consistent with the results shown in Figure 4F, we observed significantly reduced NPR1 gene expression in cdk8-4 and npr1-2 mutants compared with Col-0 (Supplemental Fig. S4A). To determine the NPR1 protein level in npr1-2 and cdk8-4 mutants, we performed immunoblot assays using Col-0 wild-type and npr1-2 and cdk8-4 mutant plants subjected to SA treatment. We found that both npr1-2 and cdk8-4 mutants accumulated much less NPR1 protein compared with that in Col-0 (Fig. 4G). In the npr1-2 mutant, SA treatment causes the cotyledons to appear yellow due to overaccumulation of SA (Zhang et al., 2010). We reasoned that the cdk8-4 mutant would also show this phenotype, because NPR1 gene expression is reduced in these mutant plants. To test this, we placed cdk8-4 seeds on Murashige and Skoog plates containing 0.35 mm SA and then recorded the ratio of seedlings showing yellow cotyledons after 7 d. Surprisingly, we found that 95% of cdk8-4 seedlings developed yellow cotyledons on SA plates, which is very close to the 100% yellow cotyledon phenotype observed in npr1-2 mutants (Supplemental Fig. S4B). We next checked the expression of NPR1-dependent genes in these mutants and found that the expression of PR1, PR2, and WRKY38 in the cdk8-4 mutant was dramatically reduced compared with Col-0 (Fig. 4, H–J). Together, these data indicate that CDK8 is required for the full expression of NPR1 and its target genes including PR1 in SA signaling.
CDK8 Regulates NPR1 Protein Accumulation and PR1 Gene Expression in a Kinase-Independent Manner
In mammals, yeast, and Arabidopsis, CDK8 phosphorylates the RNA polymerase II CTD (Liao et al., 1995; Wang and Chen, 2004). We wondered if CDK8 regulates NPR1 and PR1 gene expression in a kinase-dependent manner. To test this, we treated Col-0, cdk8-1, CDK8-MYC cdk8-1, and kinase-dead CDK8 (CDK8KD)-MYC cdk8-1 plants with 0.5 mm SA to detect NPR1 protein level and PR1 gene expression (Zhu et al., 2014). As we expected, cdk8-1, like cdk8-4, accumulated a substantially lower level of NPR1 protein and exhibited reduced PR1 gene expression compared with that in Col-0 (Supplemental Fig. S5A). CDK8-MYC cdk8-1 showed a similar level of NPR1 protein level and PR1 gene expression compared with Col-0 (Supplemental Fig. S5B), indicating that CDK8-MYC is functional and able to complement the cdk8-1 phenotype. Surprisingly, we found that both the NPR1 protein level and PR1 gene expression in CDK8KD-MYC cdk8-1 plants are also higher than in the cdk8-1 mutant (Supplemental Fig. S5B), indicating that CDK8 regulates NPR1 protein accumulation and PR1 gene expression in a kinase-independent manner.
CDK8-Associated Mediator12 and Mediator13 Positively Regulate the Expression of SAR and Defense Genes
Besides phosphorylating CTD of RNA polymerase II, another major function of CDK8 is that it forms the kinase module of the Mediator complex with cyclin C, Mediator12, and Mediator13 (Jeronimo and Robert, 2017). Since we found that CDK8 regulates NPR1 and PR1 gene expression in a kinase-independent manner, we hypothesize that it functions together with its associated subunits in the kinase module of the Mediator complex, so we analyzed the SAR phenotype in Col-0, npr1-2, cycCab (a single T-DNA insertion line in which both CycCa and CycCb genes are down-regulated), med12, and med13 (subunits of the CDK8 kinase module in the Mediator complex) mutants. We found that both med12 and med13 mutants showed significantly (P < 0.05) higher susceptibility compared with Col-0, whereas the cycCab mutant showed modestly increased bacterial growth (Fig. 5A). In terms of SAR, the mediator mutants showed completely compromised SAR. In the cycCab mutant, the Psm growth was significantly reduced by 5-fold after SAR induction, indicating that SAR is not defective in the cycCab mutant (Fig. 5A). This could be because both CycCa and CycCb genes are only partially knocked down in this single T-DNA insertion line, based on a previous report (Zhu et al., 2014).
Figure 5.
CDK8-associated Mediators are involved in SAR and defense gene expression. A, SAR phenotypes in Col-0 wild-type, npr1-2, cycCab, med12, and med13 mutant plants. Two lower leaves were infiltrated with Psm avrRpt2 (OD600 nm = 0.02; SAR+) or MgCl2 buffer (SAR−) as a control. After 2 d, two upper healthy leaves were challenged with virulent Psm (OD600 nm = 0.0005). The leaf discs from the second inoculation were collected at 3 d post infection for counting bacterial colonies. CFU, Colony-forming units. B, PR1 gene expression levels in the systemic leaves of Col-0 wild-type, npr1-2, cycCab, med12, and med13 mutant plants. Two lower leaves were infected with Psm avrRpt2 (OD600 nm = 0.02) or MgCl2 buffer as a control. After 2 d, the upper uninfected leaves were collected for PR1 gene expression analysis. C and D, NPR1 and PR1 gene expression levels in Col-0 wild-type, npr1-2, cycCab, med12, and med13 mutant plants treated with SA. Col-0 and mutant plants were irrigated with 0.5 mm SA, and then leaf samples were collected after 4 h for NPR1 gene expression analysis (C). After 24 h, leaves were collected for PR1 gene expression analysis (D). The expression levels of NPR1 and PR1 were normalized to UBQ5 expression. Data represent means of three independent samples with se. Asterisks indicate significant differences (Student’s t test, *, P < 0.05). Columns with different letters indicate significant differences determined by Duncan’s multiple range test. The experiments were repeated three times with similar results.
We next analyzed the expression of PR1, a widely used marker gene of SAR, in the systemic leaves of Col-0 and npr1-2, med12, and med13 mutants after 48 h infection of Psm avrRpt2 within local leaves. We found that PR1 gene expression was also dramatically reduced in med12 and med13 mutants but only slightly, but not significantly, reduced in the cycCab mutant compared with Col-0 (Fig. 5B). To determine whether CycCa, CycCb, MED12, and MED13 are involved in SA signaling, we analyzed the NPR1 and PR1 gene expression levels in these mutants 4 and 24 h after SA irrigation. Interestingly, we found that the expression levels of both NPR1 and PR1 genes in med12 and med13 mutants were extremely low, similar to in the npr1-2 mutant, indicating that both MED12 and MED13 play important roles in NPR1 and PR1 gene expression (Fig. 5, C and D).
CDK8 Interacts with WRKY6 and WRKY18 and Is Required for RNA Polymerase II to Bind the Promoter and Coding Region of NPR1
Recently, several studies have shown that the Mediator complex plays an important role in plant defense gene expression, but the underlying mechanism has not been fully elucidated (Canet et al., 2012; Zhang et al., 2012, 2013; An and Mou, 2013; Wang et al., 2015, 2016). Mediator regulates gene expression by acting as a bridge between TFs and RNA polymerase II, which catalyzes the transcription of DNA to synthesize precursors of mRNA and most microRNAs (Sims et al., 2004).
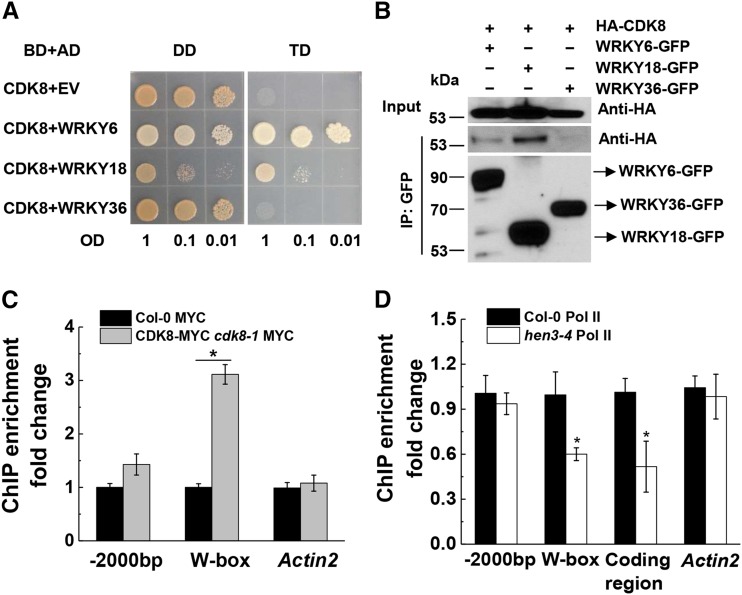
Because Mediator cannot directly interact with DNA (Uthe et al., 2017), it must be associated with TFs to regulate gene expression in response to different stimuli (Yang et al., 2016; Jeronimo and Robert, 2017). It has been shown that SRB10/CDK8 interacts with many TFs in yeast and mammalian cells (Galbraith et al., 2010; Nemet et al., 2014). In addition, CDK8 phosphorylates RNA polymerase II at the CTD domain in Arabidopsis (Wang and Chen, 2004). In previous studies, it was demonstrated that WRKY6 and WRKY18 bind to the NPR1 promoter and that this binding is required for NPR1 gene expression induced by SA (Chai et al., 2014). Furthermore, we found that CDK8 positively regulates NPR1 gene expression in response to SA (Fig. 4F; Supplemental Fig. S4). Therefore, we hypothesize that CDK8 regulates NPR1 gene expression through its association with WRKY6 and/or WRKY18. To test this, we performed Y2H assays using CDK8 as the bait and WRKY6, WRKY18, and WRKY36 as prey. Interestingly, we found that CDK8 interacted with WRKY6 and WRKY18 but not with WRKY36 in Y2H assays (Fig. 6A). To confirm this interaction in planta, we performed a Co-IP assay in N. benthamiana. Agroinfiltration was used to transiently coexpress GFP, WRKY6-GFP, WRKY18-GFP, and WRKY36-GFP proteins with HA-CDK8 in N. benthamiana. We found that HA-CDK8 coimmunoprecipitated with WRKY6-GFP and WRKY18-GFP proteins (Fig. 6B). In contrast, HA-CDK8 did not coimmunoprecipitate with GFP or WRKY36-GFP control protein, confirming that WRKY6 and WRKY18 interact with CDK8 in planta. Taken together, these data indicate that CDK8 interacts with WRKY6 and WRKY18.
Figure 6.
CDK8 connects WRKY6 and WRKY18 with RNA polymerase II to facilitate NPR1 gene expression. A, Y2H assays using CDK8 as bait and WRKY6, WRKY18, WRKY36, or an EV as prey. After cotransformation, yeast was selected on DD medium lacking Leu and Trp. The yeast was then recultured, diluted to OD600 nm = 1, 0.1, or 0.01, and 10 µL of these diluted cultures was placed onto a DD plate and a TD plate. B, Co-IP of HA-CDK8 with WRKY6-GFP, WRKY18-GFP, and WRKY36-GFP in N. benthamiana. WRKY6-GFP, WRKY18-GFP, or WRKY36-GFP was coexpressed with HA-CDK8 proteins in N. benthamiana by agroinfiltration and then immunoprecipitated with magnetic GFP-Trap for 2 h. After washing four times, magnetic beads containing protein complexes associated with GFP or WRKY-GFP were eluted with 2× Laemmli sample buffer and immunoblotted with an anti-GFP antibody. The coimmunoprecipitated proteins were immunoblotted with an anti-HA antibody. Fifty micrograms of the proteins was used as the input and detected with an anti-HA antibody. C, The CDK8 protein is associated with the NPR1 promoter in ChIP assays. Col-0 and CDK8-MYC cdk8-1 transgenic plants were treated with 0.5 mm SA for 4 h. Chromatin was extracted, then immunoprecipitated with an anti-MYC antibody. D, CDK8 is required for RNA polymerase II (Pol II) association with the NPR1 promoter and coding region in ChIP assays. Col-0 and cdk8-4 plants were treated with 0.5 mm SA for 16 h. Chromatin was extracted, then immunoprecipitated with anti-RNA polymerase II antibody or IgG as a negative control. Primers used in the quantitative PCR (qPCR; C and D) amplify the following amplicons: -2000bp, amplifies upstream of the NPR1 promoter at the −2,000-bp region; W-box, amplifies the sequence containing the W-box at the NPR1 promoter; Actin2 was used as a negative control. Data represent means of three independent samples with se. Asterisks indicate significant differences (Student’s t test, *, P < 0.05). The experiments were repeated at least two times with similar results.
Since WRKY6 and WRKY18 can bind to the NPR1 promoter to regulate NPR1 gene expression (Yu et al., 2001; Chai et al., 2014), we speculated that CDK8 regulates NPR1 gene expression by its association with the NPR1 promoter. To test this, we performed ChIP assays using Col-0 and CDK8-MYC cdk8-1 transgenic plants to determine the association of CDK8 with the target gene. Indeed, we found that the CDK8-MYC association with the W-box region was significantly (P < 0.05) enriched compared with that in Col-0 (Fig. 6C). By contrast, no difference was found when Actin2 gene was used as a negative control (Fig. 6C). Collectively, these data indicate that CDK8 interacts with WRKY6 and WRKY18 proteins and regulates NPR1 gene expression through its association with the NPR1 promoter.
To further elucidate how CDK8 regulates NPR1 gene expression, we tested if CDK8 is required for RNA polymerase II binding to the NPR1 promoter using ChIP assays. We found that RNA polymerase II enrichment on the W-box of the NPR1 promoter in the cdk8-4 mutant was significantly (P < 0.05) reduced compared with Col-0 wild-type plants (Fig. 6D), indicating that CDK8 is involved in RNA polymerase II binding to the NPR1 promoter at the W-box motif. Interestingly, we also found that RNA polymerase II enrichment on the NPR1 gene-coding region in the cdk8-4 mutants was significantly (P < 0.05) reduced compared with Col-0 wild-type plants (Fig. 6D), indicating that CDK8 is involved in RNA polymerase II binding to the coding region of NPR1. As a negative control, RNA polymerase II enrichment in the coding region of Actin2 showed no difference between Col-0 and the cdk8-4 mutant (Fig. 6D). Taken together, these results suggest that CDK8 forms a molecular bridge between WRKY6/WRKY18 and RNA polymerase II to facilitate NPR1 gene expression.
CDK8 Interacts with TGAs and Is Associated with the PR1 Promoter
It is well known that PR1 gene expression is regulated by TGA TFs and the transcriptional coactivator NPR1 in Arabidopsis (Després et al., 2000; Zhou et al., 2000; Kim and Delaney, 2002; Boyle et al., 2009); however, it is not clear how precisely NPR1 coordinates with other transcription machinery to regulate PR1 gene expression. We postulate that NPR1 regulates PR1 gene expression through its interaction with CDK8 and TGA TFs. Although we have already found that NPR1 gene expression is down-regulated in the cdk8-4 mutant, it is still essential to understand how CDK8 coordinates with TFs and other coactivators to regulate PR1 gene expression.
We screened seven TGA TFs to identify which TGA TFs interact with CDK8 in Y2H assays using CDK8 as the bait and TGAs as the prey (Després et al., 2000; Zhou et al., 2000; Kim and Delaney, 2002; Boyle et al., 2009). Our data show that, among seven TGAs, only TGA5 and TGA7 were found to interact with CDK8 specifically in Y2H assays (Fig. 7A), whereas TGA1 showed very weak interaction with CDK8. To confirm the interactions between CDK8 and TGA5/TGA7, we performed a Co-IP assay using N. benthamiana leaves coexpressing GFP, TGA5-GFP, or TGA7-GFP with HA-CDK8. We found that HA-CDK8 coimmunoprecipitated with TGA5-GFP and TGA7-GFP but not with the GFP control (Fig. 7B), confirming that TGA5 and TGA7 interact with CDK8 in planta.
Figure 7.
CDK8 interacts with TGAs and is associated with the PR1 promoter. A, CDK8 interacts with TGA5 and TGA7, but not TGA1, TGA2, TGA3, TGA4, or TGA6, in Y2H assays. Yeast strains Y187 and AH109 were transformed with pGBKT7-CDK8 and pGADT7-TGA1-7 or an EV, respectively. Selected yeast diploids from a DD plate were recultured, diluted to OD600 nm = 1, 0.1, or 0.01, and placed onto a DD plate and a TD plate. B, Co-IP assay between HA-CDK8 and TGA5-GFP or TGA7-GFP in N. benthamiana. Proteins extracted from N. benthamiana leaves transiently expressing GFP, TGA5-GFP, or TGA7-GFP and HA-CDK8 by agroinfiltration were immunoprecipitated with an anti-GFP magnetic trap and immunoblotted with an anti-GFP or an anti-HA antibody. C, CDK8 is associated with the PR1 promoter in ChIP assays. Col-0 and CDK8-MYC cdk8-1 transgenic plants were treated with 0.5 mm SA for 4 h. Chromatin was extracted, then immunoprecipitated with an anti-MYC antibody. D, CDK8 is required for the association of RNA polymerase II (Pol II) with the PR1 gene promoter and coding region. After Col-0 and cdk8-4 mutant plants were treated with 0.5 mm SA for 16 h, chromatin was extracted, then immunoprecipitated with an anti-RNA polymerase II antibody. Primers used in the qPCR (C and D) amplify the following amplicons: -2000bp, amplifies upstream of the PR1 promoter at the −2,000-bp region; as-1, amplifies the sequence containing the as-1 region at the PR1 promoter; Actin2 was used as a negative control. The results are representative data from three independent experiments. Data represent means of three independent samples with se. Asterisks indicate significant differences (Student’s t test, *, P < 0.05). The experiments were repeated at least two times with similar results.
It has been shown that NPR1 monomers interact with TGAs, which target the activation sequence-1 (as-1) element of the PR1 promoter (Zhou et al., 2000). We hypothesized that CDK8 is associated with the PR1 promoter to regulate its expression. Interestingly, we found that CDK8-MYC was significantly enriched (P < 0.05) on the as-1 sequence compared with Col-0 (Fig. 7C), indicating that CDK8 is associated with the PR1 regulatory region. As negative controls, CDK8 did not show specific association with Actin2 or −2,000 bp upstream of the PR1 promoter (Fig. 7C). Together, these data indicate that CDK8 forms a complex with NPR1 and TGAs at the promoter region of PR1 to regulate its expression.
To further elucidate how CDK8 regulates PR1 gene expression, we tested if CDK8 is required for RNA polymerase II binding to the PR1 promoter with ChIP assays. Interestingly, we also found RNA polymerase II enrichment on the as-1 sequence, and the coding region of PR1 was also significantly (P < 0.05) reduced in the cdk8-4 mutants compared with Col-0 wild-type plants (Fig. 7D), indicating that CDK8 is required for the enrichment of RNA polymerase II on the PR1 promoter and coding region. Taken together, these data demonstrate that CDK8 forms a protein complex with NPR1, TGA5, and TGA7 and acts as a molecular bridge between RNA polymerase II and the PR1 promoter and the coding region and thereby facilitates PR1 gene expression.
DISCUSSION
As the master regulator of SA-mediated plant immunity, NPR1 controls the expression of over 2,000 genes (Wang et al., 2006; Fu and Dong, 2013). To exert its function, NPR1 is reduced from an oligomeric state to a monomeric state and then translocated to the nucleus after the accumulation of SA induced by pathogen infection (Mou et al., 2003). Most studies on NPR1 have been focused on posttranslational modifications (Withers and Dong, 2016). NPR1 paralogs NPR3 and NPR4 both function as SA receptors and adaptors for Cullin3 E3 ligase that mediate the degradation of NPR1 and thereby maintain an optimal level of NPR1 protein during plant defense (Fu et al., 2012). Intriguingly, we showed that NPR1 transcripts in the npr1-1 and npr1-2 mutants were dramatically lower compared with wild-type plants (Fig. 1A). Consistent with these observations, Zhang et al. (2012) also showed that Pst DC3000-induced NPR1 transcript accumulation in npr1-3 mutants was also significantly lower than that in Col-0. NPR1 transcript accumulation in the npr1 mutants was not induced in response to INA (Kinkema et al., 2000). Our data indicate that a functional NPR1 protein is required for the full expression of NPR1 (Fig. 8). In addition to npr1-1 and npr1-2 mutants, EMS-induced mutagenesis, which frequently causes a G-to-A mutation, has been widely used for genetic screens to identify loss-of-function mutants. Intriguingly, a single-nucleotide or a single-amino acid change causes the loss of function of many important genes, including FLAGELLIN SENSING2, ENHANCED DISEASE SUSCEPTIBILITY1, RESISTANCE TO PSEUDOMONAS SYRINGAE2, and AVRPPHB SUSCEPTIBLE3, etc. (Falk et al., 1999; Gómez-Gómez and Boller, 2000; Axtell et al., 2001; Nobuta et al., 2007). It is worthwhile to revisit these EMS single-nucleotide mutants to find out whether they are also protein null mutants. For NPR1, dozens of mutants have been identified in Arabidopsis as loss-of-function mutants (Cao et al., 1997; Canet et al., 2010). In addition to NPR1, ABA INSENSITIVE5 and Snail Family Transcriptional Repressor1 have also been shown to positively regulate their own expression by binding to their own promoters (Peiró et al., 2006; Xu et al., 2014). Therefore, our study on NPR1 could provide insights into the autoregulation of ABI5 and Sanil1.
Figure 8.
Schematic model of the roles of NPR1 and CDK8 in the transcriptional regulation of NPR1 and PR1 genes. Left, Activation of NPR1 gene expression by NPR1 and CDK8. Pathogen infection induces the expression of the CDK8 gene. CDK8 interacts with the WRKY6 and WRKY18 TFs, which bind to the NPR1 gene promoter at the W-box motif. SA promotes the interactions between NPR1 and CDK8 and between NPR1 and WRKY18. CDK8 and other proteins in the kinase module of the Mediator complex, including MED12 and MED13, bring RNA polymerase II (Pol II) to the NPR1 gene promoter and coding region to promote its transcription. NPR1 mRNA is then exported to the cytosol for NPR1 protein synthesis. Right, CDK8 facilitates the expression of the PR1 gene. After CDK8 and NPR1 induce the transcription of the NPR1 gene, NPR1 protein is synthesized and reduced from oligomers to monomers upon pathogen infection, then NPR1 monomers enter the nucleus. In the nucleus, NPR1 forms a protein complex with CDK8, TGA5, and TGA7, which bind to the PR1 promoter at the as-1 sequence. RNA polymerase II is then recruited to the promoter and coding region of the PR1 gene by CDK8, MED12, and MED13 to facilitate PR1 gene expression to activate plant defense.
Only two TFs, WRKY18 and WRKY6, have been found to bind to the NPR1 promoter to date (Yu et al., 2001; Chai et al., 2014). We found that both of them interact with CDK8 (Fig. 6, A and B), which brings CDK8 to the W-box region of the NPR1 promoter (Fig. 6C) to form a transcription initiation complex. CDK8 is also required for NPR1 transcription elongation, as it was shown that knockout of CDK8 reduces the binding of RNA polymerase II to the coding region of NPR1 (Fig. 6D). In agreement with our data, a previous report has shown that CDK8 is required for RNA polymerase II binding to the coding region of PDF1.2 to facilitate its expression and confer resistance to that fungal pathogen Alternaria brassicicola (Zhu et al., 2014). Likewise, CDK8 is essential for RNA polymerase II elongation in mammalian cells as well (Galbraith et al., 2013). Together, our data provide evidence that CDK8 functions as a molecular bridge between WRKY6/WRKY18 and the RNA polymerase II transcription machinery (Fig. 8). Thus, our research filled an important knowledge gap between WRKY6/WRKY18 and RNA polymerase II-mediated NPR1 gene transcription. In mammals and yeast, it has been shown that CDK8/SRB10 phosphorylates many TFs, including Ste12, Gcn4, Msn2, GAL4, E2F1, STAT1, and Phd1 (Hirst et al., 1999; Chi et al., 2001; Nelson et al., 2003; Raithatha et al., 2012; Bancerek et al., 2013). Therefore, besides recruiting RNA polymerase II to the gene promoters, CDK8 may also be involved in the phosphorylation of WRKY6 and/or WRKY18 TFs to activate NPR1 gene expression, which warrants further investigation. Surprisingly, we found that NPR1 protein is associated with its own promoter at the W-box motif (Fig. 1D). NPR1 interacts with WRKY18 but not WRKY6 in Y2H assays. The interaction between NPR1 and WRKY18 is greatly enhanced by SA (Fig. 2, A and C). Interestingly, we found that NPR1, but not npr1-1 or npr1-2, interacts with CDK8, which interacts with WRKY6 and WRKY18. Together, these data suggest that NPR1 recruits CDK8, CDK8-associated RNA polymerase II, and WRKY18 to the NPR1 promoter to activate its expression. In agreement with this, we found that npr1-1 and npr1-2 mutants, in which mutated npr1-1 and npr1-2 protein cannot interact with CDK8 to activate the expression of the NPR1 gene, showed a significantly lower NPR1 transcript level than that in Col-0 (Fig. 1A).
In Arabidopsis, NPR1, as a transcriptional coactivator, interacts with TGA and TCP TFs to facilitate PR gene expression (Després et al., 2000; Zhou et al., 2000; Johnson et al., 2003; Li et al., 2018); however, how NPR1 activates PR1 gene expression is still not clear. There is a missing link between NPR1 and the transcription machinery. Our data suggest that CDK8 could be the link, as SA promotes the interaction between NPR1 and CDK8 (Fig. 3). In our screens, we found that CDK8 interacts specifically with TGA5 and TGA7 TFs (Fig. 7, A and B), which function as positive regulators of PR1 gene expression and plant immunity (Kim and Delaney, 2002; Kesarwani et al., 2007; Song et al., 2011). Furthermore, we showed that CDK8 binds to the as-1 sequence in the PR1 promoter (Fig. 7C). Taken together, these data indicate that NPR1 recruits TGAs, CDK8, and RNA polymerase II to the PR1 promoter for transcriptional activation. In addition, our data show that CDK8 is required for RNA polymerase II binding to the as-1 sequence in the PR1 promoter and the PR1 coding region (Fig. 7D). Collectively, our data demonstrate that CDK8 connects the TGA TFs and the coactivator NPR1 with RNA polymerase II to facilitate PR1 gene expression (Fig. 8). Therefore, our data reveal a fundamental mechanism of PR1 gene regulation. A previous study has already shown that SA induces NPR1 sumoylation (Saleh et al., 2015). Sumoylation prevents NPR1 interaction with WRKY70, which functions as a transcription repressor of PR1, and promotes NPR1 interaction with TGA3, to facilitate PR gene expression (Saleh et al., 2015). It would be interesting to test how NPR1 sumoylation affects its interactions with CDK8, WRK18, and TCP8, TCP14, and TCP15 to regulate plant defense (Li et al., 2018).
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Arabidopsis (Arabidopsis thaliana) ecotype Col-0 was used as wild-type plants. The mutants cdk8-4 (GABI_564F11), cycCab (SALK_039400C), med12 (SALK_108241), and med13 (SALK_018056), which have been described in previous studies (Wang and Chen, 2004; Ng et al., 2013; Zhu et al., 2014), were obtained from GABI-KAT or the Arabidopsis Biological Resource Center (Kleinboelting et al., 2012). Seeds of cdk8-1, CDK8-MYC cdk8-1, and CDK8KD-MYC cdk8-1 were previously described in Zhu et al. (2014). The insertions and homozygosity were confirmed by genomic DNA PCR using the primers listed in Supplemental Table S1. Plants were grown in a growth chamber with a cycle of 12 h of light/12 h of dark, 22°C/20°C day/night temperature, and 60% relative humidity.
Plasmid Construction
CDK8, WRKY6, and WRKY18 were cloned from a Col-0 cDNA library and cloned to the pDONR207 vector by BP reaction according to the manufacturer’s instructions. To obtain C-terminal GFP tag fusion proteins, WRKY6, WRKY18, WRKY36, TGA5, and TGA7 were cloned into pK7FWG2 by LR reaction. CDK8 was subcloned into pEARLEYGATE201 to obtain HA-CDK8. WRKYs and TGAs were cloned into pGADT7 and CDK8 was cloned into pGBKT7 for the Y2H assay. All the constructs were verified by sequencing.
Plant Infection and Treatment
Pseudomonas syringae pv maculicola strain ES4326 and Psm avrRpt2 were grown at 28°C in King’s B medium containing the appropriate antibiotics (100 mg L−1 streptomycin and 10 mg L−1 tetracycline). Bacteria were pelleted, washed three times with 10 mm MgCl2, resuspended, and diluted in 10 mm MgCl2 to the desired concentration. To induce SAR, two fully expanded leaves of each plant were infiltrated (primary inoculation) with a suspension containing Psm avrRpt2 at OD600 nm = 0.02. In parallel, control plants were similarly infiltrated with 10 mm MgCl2. Two days later, two upper uninfected systemic leaves were further infiltrated with the virulent Psm 4326 at OD600 nm = 0.0005. Bacterial titer in the upper or systemic leaves was measured 3 d post infection.
RNA Extraction and Reverse Transcription qPCR
Total RNA was isolated using the Trizol reagent (Invitrogen) according to the manufacturer’s instructions. RNA quality and quantity were determined using a spectrophotometer (Eppendorf). Genomic DNA was removed by DNase I (Thermo Fisher Scientific) treatment, and first-strand cDNA was synthesized using qScript cDNA SuperMix (Quanta Biosciences). qPCR was carried out using SYBR Green SuperMix (Quanta Biosciences). UBIQUITIN5 (UBQ5) was used as a control. Gene-specific primers were designed using Primer Premier 5 software, and their sequences are shown in Supplemental Table S1.
Western Blotting
Rosette leaves from 5-week-old plants or 14-d-old seedlings were treated with 0.5 mm SA at the indicated times. Samples were collected, frozen in liquid nitrogen, and homogenized in protein extraction buffer (50 mm Tris-HCl, pH 7.5, 150 mm NaCl, 5 mm EDTA, 0.1% (v/v) Triton X-100, 0.2% (v/v) Nonidet P-40, 1 mm phenylmethylsulfonyl fluoride, and 1× protease inhibitor cocktail). Homogenates were centrifuged (13,000g) for 15 min at 4°C twice. Proteins were then denatured at 70°C with 5× loading buffer supplemented with 500 mm dithiothreitol (DTT) for 15 min. Western blots were probed with an anti-GFP (Clontech) or anti-NPR1 antibody (a gift from Dr. Zhonglin Mou).
Y2H Assays
To test the interactions between CDK8 and WRKYs or NPR1, the Saccharomyces cerevisiae yeast strain AH109 was cotransformed with pGBKT7-CDK8 and pGADT7-EV, NPR1, npr1-1, npr1-2, WRKY6, WRKY18, or WRKY36 according to the Clontech yeast transformation protocol. To test the interactions between CDK8 and TGAs, yeast strains AH109 and Y187 were transformed with pGADT7-EV, pGADT7-TGAs, and pGBKT7-CDK8. Yeast strains were mated in Yeast Peptone Dextrose Adenine medium overnight, and diploid yeast colonies were selected on synthetic dextrose (SD)-Trp-Leu (DD) plates. Fresh single colonies were grown in the SD-Trp-Leu liquid medium overnight. The mated or cotransformed yeast cultures were diluted to OD600 nm = 1, 0.1, or 0.01 with sterilized distilled water. Ten microliters of yeast suspension was spotted on SD-Trp-Leu and SD-Trp-Leu-His (TD) plates with or without 3-AT. The interactions and yeast growth were observed after 4 to 7 d at 30°C.
GST Pull-Down Assay
Escherichia coli strain C41, which was derived from E. coli BL21(DE3), expressing GST or GST-NPR1 was prepared according to a previous study (Fu et al., 2012). Five micrograms of GST or GST-NPR1 protein immobilized on the MagneGST Glutathione beads was combined with 1 μg of purified 6xHis-MBP-CDK8 in 1 mL of binding buffer (100 mm Tris-HCl, 1 mm EDTA, 300 mm NaCl, 0.5% Nonidet P-40, 1 mm DTT, 0.1 mg mL−1 bovine serum albumin, 1 mm phenylmethylsulfonyl fluoride, 50 μm MG115, and protease inhibitor cocktail) overnight. Beads were then washed five times with washing buffer (100 mm Tris-HCl, 1 mm EDTA, 300 mm NaCl, and 0.5% Nonidet P-40). Proteins were eluted with 2× loading buffer containing 100 mm DTT by boiling for 5 min. Eluted proteins were run on SDS-PAGE gels, and western blots were probed with an anti-6xHis or anti-GST antibody (Invitrogen).
GFP Pull-Down Assay
Agrobacterium tumefaciens strains carrying pK7FWG2-WRKY18 (WRKY18-GFP) and pMDC43-EV (GFP) were infiltrated into Nicotiana benthamiana leaves. After 48 h, leaves were harvested, frozen in liquid nitrogen, and homogenized with protein extraction buffer. Homogenates were centrifuged (20,000g) for 30 min at 4°C twice and then filtered through a 0.2-μm filter. GFP or GFP-tagged proteins were pulled down with GFP-Trap Magnetic Agarose beads (ChromoTek) at 4°C for 2 h. The beads with attached proteins were washed four times and resuspended in protein extraction buffer supplemented with 0.1 mg mL−1 bovine serum albumin and 1 μg of 6xHis-MBP-NPR1 with or without 200 μm SA. After 16 h, the beads were washed with washing buffer four times and eluted in 2× Laemmli sample buffer supplemented with 100 mm DTT, then boiled in a water bath at 95°C. Western blots were probed with an anti-GFP (Clontech) or anti-6xHis (Invitrogen) antibody.
Co-IP Assay
A. tumefaciens strains carrying pCB302-NPR1 (NPR1-GFP), pK7FWG2-WRKY6/WRKY18/TGA5/TGA7 (WRKY6/WRKY18/TGA5/TGA7-GFP), or pMDC43-EV (GFP) were coinfiltrated into N. benthamiana leaves with an A. tumefaciens strain carrying pEarlyGate201-CDK8 (HA-CDK8) and P19 silencer. After 48 h, leaves were harvested, frozen in liquid nitrogen, and homogenized with protein extraction buffer. Homogenates were centrifuged (20,000g) for 30 min at 4°C twice and then filtered through a 0.2-μm filter. GFP or GFP-tagged proteins were immunoprecipitated with GFP-Trap Magnetic Agarose beads (ChromoTek) at 4°C for 2 h. The immunoprecipitated proteins were washed four times and eluted in 2× Laemmli sample buffer supplemented with 100 mm DTT, then boiled in a water bath at 95°C. Western blots were probed with an anti-GFP (Clontech) or anti-HA (Roche) antibody. Fifty micrograms of protein extracts was used as an input control.
ChIP-qPCR Assay
Five-week-old plants or 14-d-old seedlings of GFP, NPR1-GFP npr1-2, Col-0, CDK8-MYC cdk8-1, and cdk8-4 were treated with SA at the indicated times. Three grams of each sample was harvested and cross-linked with 1% formaldehyde at room temperature for 10 min, followed by neutralization with 0.125 m Gly. The chromatin was isolated and sonicated according to a previous protocol (Saleh et al., 2008). The chromatin was then immunoprecipitated with an anti-MYC (Thermo Fisher Scientific) or anti-RNA polymerase II (Agrisera) antibody at 4°C overnight. The immunoprecipitated chromatin complex was incubated with Pierce protein A/G magnetic beads (Thermo Fisher Scientific) for 1 h at room temperature. For immunoprecipitation with GFP tag, the chromatin was immunoprecipitated with GFP-Trap or beads control for 4 h. Then the beads were washed with low-salt buffer, high-salt buffer, and LiCl wash buffer once and with Tris-EDTA buffer twice (Saleh et al., 2008). After washing, the immunoprecipitated chromatin was eluted with an elution buffer (1% SDS and 0.1 m NaHCO3). Protein-DNA cross-linking was reversed by incubating at 65°C overnight. DNA was recovered and analyzed by qPCR with gene-specific primers. Actin2 was used as a nonspecific target gene. ChIP enrichment was normalized to input control and converted to fold change (Zhu et al., 2014). Fold enrichment was calculated as the ratio of transgenic plant to Col-0 or control plant signal, which was set as 1. All primers are listed in Supplemental Table S1.
Statistical Analysis
Statistical analysis was carried out by Excel 2010 with two-tailed Student’s t test or PASW statistics 18 software with Duncan’s multiple range test.
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under the following accession numbers: NPR1 (AT1G64280), CDK8 (AT5G63610), MED12 (AT4G00450), MED13 (AT1G55325), CYCCa (AT5G48630), CYCCb (AT5G48640), WRKY6 (AT1G62300), WRKY18 (AT4G31800), WRKY36 (AT1G69810), TGA1 (AT5G65210), TGA2 (AT5G06950), TGA3 (AT1G22070), TGA4 (AT5G10030), TGA5 (AT5G06960), TGA6 (AT3G12250), and TGA7 (AT1G77920).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. NPR1 antibody recognizes both npr1-2 and npr1-1 proteins expressed in yeast.
Supplemental Figure S2. GFP and NPR1 protein levels in GFP npr1-2 and NPR1-GFP npr1-2 plants.
Supplemental Figure S3. PR1 gene expression levels in the local leaves of Col-0 wild-type, npr1-2, and cdk8-4 mutant plants infected with virulent Psm.
Supplemental Figure S4. CDK8 is required for NPR1 gene expression and tolerance to SA.
Supplemental Figure S5. CDK8 regulates NPR1 protein accumulation and PR1 gene expression in a kinase-independent manner.
Supplemental Table S1. Primers used in this study.
Acknowledgments
We thank Dr. Zhonglin Mou for providing us the NPR1 antibody and Dr. Beth Krizek for critical reading of the article.
Footnotes
This work was supported by the National Science Foundation (EAGER grant no. 1464527 and grant no. IOS-1758994 to Z.F.). J.C. was supported by a JAAS postdoc fellowship.
References
- An C, Mou Z (2013) The function of the Mediator complex in plant immunity. Plant Signal Behav 8: e23182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axtell MJ, McNellis TW, Mudgett MB, Hsu CS, Staskawicz BJ (2001) Mutational analysis of the Arabidopsis RPS2 disease resistance gene and the corresponding Pseudomonas syringae avrRpt2 avirulence gene. Mol Plant Microbe Interact 14: 181–188 [DOI] [PubMed] [Google Scholar]
- Bancerek J, Poss ZC, Steinparzer I, Sedlyarov V, Pfaffenwimmer T, Mikulic I, Dölken L, Strobl B, Müller M, Taatjes DJ, et al. (2013) CDK8 kinase phosphorylates transcription factor STAT1 to selectively regulate the interferon response. Immunity 38: 250–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block A, Li G, Fu ZQ, Alfano JR (2008) Phytopathogen type III effector weaponry and their plant targets. Curr Opin Plant Biol 11: 396–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle P, Le Su E, Rochon A, Shearer HL, Murmu J, Chu JY, Fobert PR, Després C (2009) The BTB/POZ domain of the Arabidopsis disease resistance protein NPR1 interacts with the repression domain of TGA2 to negate its function. Plant Cell 21: 3700–3713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canet JV, Dobón A, Roig A, Tornero P (2010) Structure-function analysis of npr1 alleles in Arabidopsis reveals a role for its paralogs in the perception of salicylic acid. Plant Cell Environ 33: 1911–1922 [DOI] [PubMed] [Google Scholar]
- Canet JV, Dobón A, Tornero P (2012) Non-recognition-of-BTH4, an Arabidopsis mediator subunit homolog, is necessary for development and response to salicylic acid. Plant Cell 24: 4220–4235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, Bowling SA, Gordon AS, Dong X (1994) Characterization of an Arabidopsis mutant that is nonresponsive to inducers of systemic acquired resistance. Plant Cell 6: 1583–1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, Glazebrook J, Clarke JD, Volko S, Dong X (1997) The Arabidopsis NPR1 gene that controls systemic acquired resistance encodes a novel protein containing ankyrin repeats. Cell 88: 57–63 [DOI] [PubMed] [Google Scholar]
- Chai J, Liu J, Zhou J, Xing D (2014) Mitogen-activated protein kinase 6 regulates NPR1 gene expression and activation during leaf senescence induced by salicylic acid. J Exp Bot 65: 6513–6528 [DOI] [PubMed] [Google Scholar]
- Chi Y, Huddleston MJ, Zhang X, Young RA, Annan RS, Carr SA, Deshaies RJ (2001) Negative regulation of Gcn4 and Msn2 transcription factors by Srb10 cyclin-dependent kinase. Genes Dev 15: 1078–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke JD, Liu Y, Klessig DF, Dong X (1998) Uncoupling PR gene expression from NPR1 and bacterial resistance: Characterization of the dominant Arabidopsis cpr6-1 mutant. Plant Cell 10: 557–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney TP, Uknes S, Vernooij B, Friedrich L, Weymann K, Negrotto D, Gaffney T, Gut-Rella M, Kessmann H, Ward E, et al. (1994) A central role of salicylic acid in plant disease resistance. Science 266: 1247–1250 [DOI] [PubMed] [Google Scholar]
- Després C, DeLong C, Glaze S, Liu E, Fobert PR (2000) The Arabidopsis NPR1/NIM1 protein enhances the DNA binding activity of a subgroup of the TGA family of bZIP transcription factors. Plant Cell 12: 279–290 [PMC free article] [PubMed] [Google Scholar]
- Després C, Chubak C, Rochon A, Clark R, Bethune T, Desveaux D, Fobert PR (2003) The Arabidopsis NPR1 disease resistance protein is a novel cofactor that confers redox regulation of DNA binding activity to the basic domain/leucine zipper transcription factor TGA1. Plant Cell 15: 2181–2191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Sun T, Ao K, Peng Y, Zhang Y, Li X, Zhang Y (2018) Opposite roles of salicylic acid receptors NPR1 and NPR3/NPR4 in transcriptional regulation of plant immunity. Cell 173: 1454–1467.e1415 [DOI] [PubMed] [Google Scholar]
- Falk A, Feys BJ, Frost LN, Jones JDG, Daniels MJ, Parker JE (1999) EDS1, an essential component of R gene-mediated disease resistance in Arabidopsis has homology to eukaryotic lipases. Proc Natl Acad Sci USA 96: 3292–3297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan W, Dong X (2002) In vivo interaction between NPR1 and transcription factor TGA2 leads to salicylic acid-mediated gene activation in Arabidopsis. Plant Cell 14: 1377–1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu ZQ, Dong X (2013) Systemic acquired resistance: Turning local infection into global defense. Annu Rev Plant Biol 64: 839–863 [DOI] [PubMed] [Google Scholar]
- Fu ZQ, Yan S, Saleh A, Wang W, Ruble J, Oka N, Mohan R, Spoel SH, Tada Y, Zheng N, et al. (2012) NPR3 and NPR4 are receptors for the immune signal salicylic acid in plants. Nature 486: 228–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbraith MD, Donner AJ, Espinosa JM (2010) CDK8: A positive regulator of transcription. Transcription 1: 4–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbraith MD, Allen MA, Bensard CL, Wang X, Schwinn MK, Qin B, Long HW, Daniels DL, Hahn WC, Dowell RD, et al. (2013) HIF1A employs CDK8-mediator to stimulate RNAPII elongation in response to hypoxia. Cell 153: 1327–1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Gómez L, Boller T (2000) FLS2: An LRR receptor-like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis. Mol Cell 5: 1003–1011 [DOI] [PubMed] [Google Scholar]
- Hirst M, Kobor MS, Kuriakose N, Greenblatt J, Sadowski I (1999) GAL4 is regulated by the RNA polymerase II holoenzyme-associated cyclin-dependent protein kinase SRB10/CDK8. Mol Cell 3: 673–678 [DOI] [PubMed] [Google Scholar]
- Jeronimo C, Robert F (2017) The mediator complex: At the nexus of RNA polymerase II transcription. Trends Cell Biol 27: 765–783 [DOI] [PubMed] [Google Scholar]
- Johnson C, Boden E, Arias J (2003) Salicylic acid and NPR1 induce the recruitment of trans-activating TGA factors to a defense gene promoter in Arabidopsis. Plant Cell 15: 1846–1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JD, Dangl JL (2006) The plant immune system. Nature 444: 323–329 [DOI] [PubMed] [Google Scholar]
- Kesarwani M, Yoo J, Dong X (2007) Genetic interactions of TGA transcription factors in the regulation of pathogenesis-related genes and disease resistance in Arabidopsis. Plant Physiol 144: 336–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HS, Delaney TP (2002) Over-expression of TGA5, which encodes a bZIP transcription factor that interacts with NIM1/NPR1, confers SAR-independent resistance in Arabidopsis thaliana to Peronospora parasitica. Plant J 32: 151–163 [DOI] [PubMed] [Google Scholar]
- Kinkema M, Fan W, Dong X (2000) Nuclear localization of NPR1 is required for activation of PR gene expression. Plant Cell 12: 2339–2350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinboelting N, Huep G, Kloetgen A, Viehoever P, Weisshaar B (2012) GABI-Kat SimpleSearch: New features of the Arabidopsis thaliana T-DNA mutant database. Nucleic Acids Res 40: D1211–D1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Park YJ, Seo PJ, Kim JH, Sim HJ, Kim SG, Park CM (2015) Systemic immunity requires SnRK2.8-mediated nuclear import of NPR1 in Arabidopsis. Plant Cell 27: 3425–3438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Pang Z, Trivedi P, Zhou X, Ying X, Jia H, Wang N (2017) ‘Candidatus Liberibacter asiaticus’ encodes a functional salicylic acid (SA) hydroxylase that degrades SA to suppress plant defenses. Mol Plant Microbe Interact 30: 620–630 [DOI] [PubMed] [Google Scholar]
- Li M, Chen H, Chen J, Chang M, Palmer IA, Gassmann W, Liu F, Fu ZQ (2018) TCP transcription factors interact with NPR1 and contribute redundantly to systemic acquired resistance. Front Plant Sci 9: 1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao SM, Zhang J, Jeffery DA, Koleske AJ, Thompson CM, Chao DM, Viljoen M, van Vuuren HJ, Young RA (1995) A kinase-cyclin pair in the RNA polymerase II holoenzyme. Nature 374: 193–196 [DOI] [PubMed] [Google Scholar]
- Lindermayr C, Sell S, Müller B, Leister D, Durner J (2010) Redox regulation of the NPR1-TGA1 system of Arabidopsis thaliana by nitric oxide. Plant Cell 22: 2894–2907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishina TE, Zeier J (2007) Pathogen-associated molecular pattern recognition rather than development of tissue necrosis contributes to bacterial induction of systemic acquired resistance in Arabidopsis. Plant J 50: 500–513 [DOI] [PubMed] [Google Scholar]
- Mou Z, Fan W, Dong X (2003) Inducers of plant systemic acquired resistance regulate NPR1 function through redox changes. Cell 113: 935–944 [DOI] [PubMed] [Google Scholar]
- Nelson C, Goto S, Lund K, Hung W, Sadowski I (2003) Srb10/Cdk8 regulates yeast filamentous growth by phosphorylating the transcription factor Ste12. Nature 421: 187–190 [DOI] [PubMed] [Google Scholar]
- Nemet J, Jelicic B, Rubelj I, Sopta M (2014) The two faces of Cdk8, a positive/negative regulator of transcription. Biochimie 97: 22–27 [DOI] [PubMed] [Google Scholar]
- Ng S, Giraud E, Duncan O, Law SR, Wang Y, Xu L, Narsai R, Carrie C, Walker H, Day DA, et al. (2013) Cyclin-dependent kinase E1 (CDKE1) provides a cellular switch in plants between growth and stress responses. J Biol Chem 288: 3449–3459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobuta K, Okrent RA, Stoutemyer M, Rodibaugh N, Kempema L, Wildermuth MC, Innes RW (2007) The GH3 acyl adenylase family member PBS3 regulates salicylic acid-dependent defense responses in Arabidopsis. Plant Physiol 144: 1144–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiró S, Escrivà M, Puig I, Barberà MJ, Dave N, Herranz N, Larriba MJ, Takkunen M, Francí C, Muñoz A, et al. (2006) Snail1 transcriptional repressor binds to its own promoter and controls its expression. Nucleic Acids Res 34: 2077–2084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieterse CM, Leon-Reyes A, Van der Ent S, Van Wees SC (2009) Networking by small-molecule hormones in plant immunity. Nat Chem Biol 5: 308–316 [DOI] [PubMed] [Google Scholar]
- Qi G, Chen J, Chang M, Chen H, Hall K, Korin J, Liu F, Wang D, Fu ZQ (2018) Pandemonium breaks out: Disruption of salicylic acid-mediated defense by plant pathogens. Mol Plant 11: 1427–1439 [DOI] [PubMed] [Google Scholar]
- Raithatha S, Su TC, Lourenco P, Goto S, Sadowski I (2012) Cdk8 regulates stability of the transcription factor Phd1 to control pseudohyphal differentiation of Saccharomyces cerevisiae. Mol Cell Biol 32: 664–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryals J, Weymann K, Lawton K, Friedrich L, Ellis D, Steiner HY, Johnson J, Delaney TP, Jesse T, Vos P, et al. (1997) The Arabidopsis NIM1 protein shows homology to the mammalian transcription factor inhibitor I kappa B. Plant Cell 9: 425–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh A, Alvarez-Venegas R, Avramova Z (2008) An efficient chromatin immunoprecipitation (ChIP) protocol for studying histone modifications in Arabidopsis plants. Nat Protoc 3: 1018–1025 [DOI] [PubMed] [Google Scholar]
- Saleh A, Withers J, Mohan R, Marqués J, Gu Y, Yan S, Zavaliev R, Nomoto M, Tada Y, Dong X (2015) Posttranslational modifications of the master transcriptional regulator NPR1 enable dynamic but tight control of plant immune responses. Cell Host Microbe 18: 169–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah J. (2009) Plants under attack: Systemic signals in defence. Curr Opin Plant Biol 12: 459–464 [DOI] [PubMed] [Google Scholar]
- Shah J, Tsui F, Klessig DF (1997) Characterization of a salicylic acid-insensitive mutant (sai1) of Arabidopsis thaliana, identified in a selective screen utilizing the SA-inducible expression of the tms2 gene. Mol Plant Microbe Interact 10: 69–78 [DOI] [PubMed] [Google Scholar]
- Sims RJ III, Mandal SS, Reinberg D (2004) Recent highlights of RNA-polymerase-II-mediated transcription. Curr Opin Cell Biol 16: 263–271 [DOI] [PubMed] [Google Scholar]
- Song J, Durrant WE, Wang S, Yan S, Tan EH, Dong X (2011) DNA repair proteins are directly involved in regulation of gene expression during plant immune response. Cell Host Microbe 9: 115–124 [DOI] [PubMed] [Google Scholar]
- Spoel SH, Mou Z, Tada Y, Spivey NW, Genschik P, Dong X (2009) Proteasome-mediated turnover of the transcription coactivator NPR1 plays dual roles in regulating plant immunity. Cell 137: 860–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada Y, Spoel SH, Pajerowska-Mukhtar K, Mou Z, Song J, Wang C, Zuo J, Dong X (2008) Plant immunity requires conformational changes [corrected] of NPR1 via S-nitrosylation and thioredoxins. Science 321: 952–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uthe H, Vanselow JT, Schlosser A (2017) Proteomic analysis of the mediator complex interactome in Saccharomyces cerevisiae. Sci Rep 7: 43584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loon LC, Rep M, Pieterse CM (2006) Significance of inducible defense-related proteins in infected plants. Annu Rev Phytopathol 44: 135–162 [DOI] [PubMed] [Google Scholar]
- Vlot AC, Dempsey DA, Klessig DF (2009) Salicylic acid, a multifaceted hormone to combat disease. Annu Rev Phytopathol 47: 177–206 [DOI] [PubMed] [Google Scholar]
- Wang W, Chen X (2004) HUA ENHANCER3 reveals a role for a cyclin-dependent protein kinase in the specification of floral organ identity in Arabidopsis. Development 131: 3147–3156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Yao J, Du X, Zhang Y, Sun Y, Rollins JA, Mou Z (2015) The Arabidopsis Mediator Complex Subunit16 is a key component of basal resistance against the necrotrophic fungal pathogen Sclerotinia sclerotiorum. Plant Physiol 169: 856–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Du X, Mou Z (2016) The mediator complex subunits MED14, MED15, and MED16 are involved in defense signaling crosstalk in Arabidopsis. Front Plant Sci 7: 1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Amornsiripanitch N, Dong X (2006) A genomic approach to identify regulatory nodes in the transcriptional network of systemic acquired resistance in plants. PLoS Pathog 2: e123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withers J, Dong X (2016) Posttranslational modifications of NPR1: A single protein playing multiple roles in plant immunity and physiology. PLoS Pathog 12: e1005707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Chen H, Curtis C, Fu ZQ (2014) Go in for the kill: How plants deploy effector-triggered immunity to combat pathogens. [Corrected]. Virulence 5: 710–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Zhang D, Chu JY, Boyle P, Wang Y, Brindle ID, De Luca V, Després C (2012) The Arabidopsis NPR1 protein is a receptor for the plant defense hormone salicylic acid. Cell Rep 1: 639–647 [DOI] [PubMed] [Google Scholar]
- Xie C, Zhou X, Deng X, Guo Y (2010) PKS5, a SNF1-related kinase, interacts with and phosphorylates NPR1, and modulates expression of WRKY38 and WRKY62. J Genet Genomics 37: 359–369 [DOI] [PubMed] [Google Scholar]
- Xu D, Li J, Gangappa SN, Hettiarachchi C, Lin F, Andersson MX, Jiang Y, Deng XW, Holm M (2014) Convergence of light and ABA signaling on the ABI5 promoter. PLoS Genet 10: e1004197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Li L, Qu LJ (2016) Plant Mediator complex and its critical functions in transcription regulation. J Integr Plant Biol 58: 106–118 [DOI] [PubMed] [Google Scholar]
- Yu D, Chen C, Chen Z (2001) Evidence for an important role of WRKY DNA binding proteins in the regulation of NPR1 gene expression. Plant Cell 13: 1527–1540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Chen S, Mou Z (2010) Nuclear localization of NPR1 is required for regulation of salicylate tolerance, isochorismate synthase 1 expression and salicylate accumulation in Arabidopsis. J Plant Physiol 167: 144–148 [DOI] [PubMed] [Google Scholar]
- Zhang X, Wang C, Zhang Y, Sun Y, Mou Z (2012) The Arabidopsis mediator complex subunit16 positively regulates salicylate-mediated systemic acquired resistance and jasmonate/ethylene-induced defense pathways. Plant Cell 24: 4294–4309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Yao J, Zhang Y, Sun Y, Mou Z (2013) The Arabidopsis Mediator complex subunits MED14/SWP and MED16/SFR6/IEN1 differentially regulate defense gene expression in plant immune responses. Plant J 75: 484–497 [DOI] [PubMed] [Google Scholar]
- Zhou JM, Trifa Y, Silva H, Pontier D, Lam E, Shah J, Klessig DF (2000) NPR1 differentially interacts with members of the TGA/OBF family of transcription factors that bind an element of the PR-1 gene required for induction by salicylic acid. Mol Plant Microbe Interact 13: 191–202 [DOI] [PubMed] [Google Scholar]
- Zhu Y, Schluttenhoffer CM, Wang P, Fu F, Thimmapuram J, Zhu JK, Lee SY, Yun DJ, Mengiste T (2014) CYCLIN-DEPENDENT KINASE8 differentially regulates plant immunity to fungal pathogens through kinase-dependent and -independent functions in Arabidopsis. Plant Cell 26: 4149–4170 [DOI] [PMC free article] [PubMed] [Google Scholar]