Abstract
Background:
Orexin A, a small-molecule peptide, can regulate female hormones, but limited evidence for its mechanism of activity exists in ovine.
Aims:
The objective of this study was to investigate the effect of orexin A on progesterone (P4) secretion in cultured granulosa of sheep follicles.
Methods:
Sheep ovarian granulosa were isolated and identified, pre-incubated with luteinizing hormone (LH) (2.5 IU/ml), follicle-stimulating hormone (FSH) (2.5 IU/ml), or oestrogen (1 µg/ml); and cultured in vitro. The pretreated sheep ovarian granulosa were subsequently cultured with different concentrations (1 nM, 10 nM, 58 nM, 100 nM, and 145 nM) of orexin A for varying amounts of time (0 h, 24 h, 48 h, and 72 h). Then, the expression levels of P4, steroidogenic acute regulatory protein (StAR), 3β-Hydroxysteroid dehydrogenase (3β-HSD) and cytochrome P450 (CYP11) were determined.
Results:
The results showed that the sheep ovarian granulosa were correctly identified. The different concentrations of orexin A promoted the secretion of P4 from granulosa in the ovine ovary compared with that in the control. The expression of StAR, 3β-HSD and P450 (CYP11) gradually increased, and then decreased with increasing concentrations of orexin A, but the expression of P450 (CYP11) decreased with the increase of time.
Conclusion:
These results revealed that orexin A promotes the secretion of P4 by regulating the expression of StAR, 3β-HSD, and P450 (CYP11). Understanding the mechanism underlying the promotion of P4 by orexin A could open new therapeutic possibilities in the treatment of hormone homeostasis.
Key Words: 3β-HSD, P450 (CYP11), Progesterone, Orexin A, StAR
Introduction
The neuropeptide, orexin A, is produced by neurons in posterior side of the hypothalamus (Cataldi et al., 2010 ▶). Orexin A is gaining increasing attention for its emerging role as a critical regulator of food intake, energy metabolism and gastrointestinal and reproductive functions (Cai et al., 1999 ▶; Ida et al., 2000 ▶; Piper et al., 2000 ▶; Russel et al., 2001 ▶; Muschamp et al., 2007 ▶; Aston-Jones et al., 2010 ▶; Puett et al., 2010 ▶). Orexin A modulates reproductive functions by regulating endocrine functions, including the hypothalamic-pituitary-adrenal (HPA), hypothalamic-pituitary-gonad (HPG), and hypothalamic-pituitary-thyroid (HPT) systems, as well as the growth hormone (GH) and prolactin axes (Li et al., 2014 ▶). A suppressive effect of orexin A on gonadotropin-releasing hormone (GnRH) in mice has been found (Gaskins and Moenter, 2012 ▶). Several hormonal interventions could affect the fate of the ovarian follicle within follicular wave (Hosseini and Niasari-Naslaji, 2018 ▶). Follicle-stimulating hormone (FSH) can promote proliferation and differentiation of preantral follicles, and thus induce follicular growth and maturation of ovarian follicles, resulting in the generation of mature eggs and the production of estrogens (Wei and Gong, 2017 ▶). Progesterone (P4), which is secreted by the ovary, is also an important hormone in females (Komatsu and Masubuchi, 2017 ▶). The orexin type 1 and type 2 receptors (OX1 and OX2) were shown to be expressed in a Chinese hamster ovarian cell line (Smart et al., 1999 ▶). Orexin A exerts its effect via the G protein-coupled receptors, OX1 and OX2. Thus, orexin A likely exerts its effect on P4 in the ovary.
The hormone P4 is a steroid produced primarily by the corpus luteum in the ovaries during the second half of the menstrual cycle or luteal phase (Diep et al., 2015 ▶) that maintains ovarian function. Steroidogenic acute regulatory protein (StAR), steroid synthetase P450 (CYP11), and 3β-Hydroxysteroid dehydrogenase (3β-HSD) play important roles in transforming cholesterol into steroid hormones, and these proteins regulate the production of P4. StAR regulates cholesterol transfer within the mitochondria, which is the rate-limiting step in the production of steroid hormones (Miller and Strauss, 1999 ▶). P450 (CYP11) participates in the biosynthesis of pregnenolone from cholesterol (Storbeck et al., 2007 ▶). 3β-HSD catalyzes the biosynthesis of P4 from pregnenolone (Cravioto et al., 1986 ▶). These three proteins are essential for the biosynthesis of all classes of hormonal steroids, including P4 (Lachance et al., 1992 ▶). Limited data have shown the effects of orexin A on the StAR, P450 (CYP11), and 3β-HSD.
Therefore, the effects of different concentrations (1 nM, 10 nM, 58 nM, 100 nM, and 145 nM) of orexin A on the P4, StAR, 3β-HSD and P450 (CYP11) in sheep ovarian granulosa were determined. Understanding the means by which orexin A promotes P4 production could open new therapeutic possibilities for the treatment of hormone homeostasis.
Materials and Methods
Reagents
Reagents used in this study included orexin A (Sigma), Dulbecco’s Modified Eagle’s Medium/F-12 (DMEM/F12) (Gibco, USA), fetal bovine serum (FBS) (Gibco, USA), penicillin-streptomycin solution (Gibco, USA), 0.25% pancreatic enzyme digestion (Gibco, USA), eosin Y solution (Tecenet, China, Shanghai), follicle-stimulating hormone receptor (FSHR) (China, Shanghai), Triton X-100 (Sigma), Alexa Fluor 647 donkey anti-rabbit (Sigma), 4',6-Diamidino-2-Phenylindole, (DAPI) (Sigma), phosphate buffer saline (PBS) (Gibco, USA), luteinizing hormone (LH) (China, Shanghai), FSH (China, Shanghai), estrogen 2 (E2, Sigma), sheep P4 enzyme-linked immunosorbent assay (ELISA) kits (Beyotime, China, Wuhan), and lysis buffer (RIPA:PMSF, 99:1).
In vitro culture of granulosa in the ovine ovary
The experimental protocol was approved by the Ethics Committee on the Use and Care of Animals, Inner Mongolia Agricultural University, China. The sheep were sacrificed with ether vapor. The ovary was rapidly removed and placed in a 0.9% sterile saline solution at 37°C. Then, the ovary was washed three times with 0.9% sterile saline solution on a clean bench. The liquid on the surface of the ovary was removed with sterile gauze. The ovine follicles were washed with serum-free DMEM/F12 medium via a 1 ml syringe. The follicular fluid was collected and cultured in DMEM/F12 (DMEM/F12) (Gibco, USA) supplemented with 10% FBS (Gibco, USA) and a 1% penicillin-streptomycin solution in a cell incubator (Amersham Corp., Arlington Heights, IL) in a humidified atmosphere of 5% CO2 at 37°C.
Identification of granulosa
Immunohistofluorescence method
Cell slides were immobilized with saline supplemented with 4% paraformaldehyde in 0.1 M phosphate buffer (PB) for 30 min in the 12-well plates, at pH = 7.4 (Fig. 1A). Cell slides were washed with PBS on a reciprocating decolorization shaking table, and treated with the 0.4% TritonX-100 for 20 min (pH=7.40). Then the cell slides were rinsed three times with PBS. Next, the cell sections were incubated in blocking buffer [PBS containing 3% normal donkey serum (D9663; Sigma) and 0.1% Triton X-100 (Sigma)] for 1 h at room temperature (RT) and then with FSHR (1:200) primary antibodies [Rabbit Anti-FSH Receptor Antibody, (Sangon Biotech, China, D151962)] prepared in blocking solution overnight at 4°C. The cell slides were rinsed thoroughly 4 to 5 times with PBS and exposed to Alexa Fluor 647 donkey anti-Rabbit (Jackson, USA, Alx647) (1:1000) secondary antibodies prepared in blocking solution for 2 h at RT. The cell slides were incubated in DAPI to stain nucleus for 10 min and rinsed thoroughly 4 to 5 times with PBS. After washing, the slides were mounted on glass slides and cover-slipped with 50% glycerin (China). Double-immunofluorescent images were acquired using a CLSM.Z1 ApoTome confocal laser scanning microscope (AxioCam MRm camera, AxioVision 4.6 software systems; Zeiss).
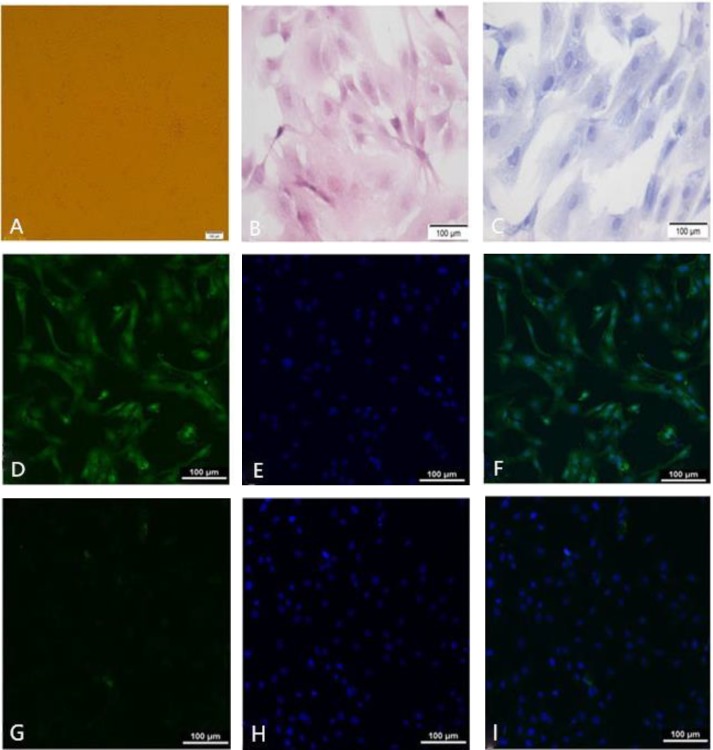
Fig. 1.
Identification of granulosa cells. A: Granulosa cells were cultured at 24 h, B: Granulosa cells were stained with haematoxylin and eosin staining, C: Granulosa cells were stained with giemsa staining, D: FSHR positive stained green fluorescence in the cytoplasm, E: Granulosa cells nucleus were stained with DAPI, F: The merge between D and E, G: Control groups stained green fluorescence in the cytoplasm, H: Control groups nucleus were stained with DAPI, and I: The merge between G and H
Hematoxylin and eosin staining of cell slides: the cells were cultured in a humidified atmosphere under 5% CO2 for 4 days. The cells were digested by 0.25% pancreatic enzyme and the cell slides were cultured in the cell plates for 24 h. The cell slides were immobilized with saline followed by 4% paraformaldehyde in 0.1 M PB for 20 min and then were immersed in PBS with agitation. The cell slides were dipped into a Coplin jar containing Mayer and hematoxylin and agitated for 2 min. The cell slides were rinsed in water, and then dehydrated with acid alcohol, after rapid rinsing in water for 3 min. The cell slides were stained with a 5% eosin Y solution for 3 min, and then rinsed in water. The cell slides were rehydrated in ether alcohol, and made transparent with xylene. One or two drops of mounting medium was added and the coverslips were covered. The cell morphology was examined using a light-inverted microscope (Nikon, Japan).
Giemsa staining
The immobilization method was the same as the haematoxylin and eosin staining method. The cell slides were dipped into a coplin jar containing giemsa for 30 min. The cell slides were rinsed in water. One or two drops of mounting medium was added and coverslips were applied. The cell morphology was examined using a light-inverted microscope (Nikon, Japan).
Granulocyte luteinization treatment and identification
Cells were divided into no added FSH, LH and E2 (no-FLE) group and FLE group. The no-FLE group was granulosa cells while the FLE group was luteinized granulosa cells that were pre-incubated with LH (2.5 IU/ml), FSH (2.5 IU/ml), and E2 (1 ug/ml) for different time periods (0 h, 24 h, 48 h, and 72 h), after which the cell supernatant was collected. The supernatant was used to determine the total P4 concentration with a sheep P4 ELISA kit (Beijing Bayer Diagnostic Technology Co., Ltd.).
ELISA assay
The granulose cells were cultured and treated with different concentrations of orexin A (1 nM, 10 nM, 58 nM, 100 nM, and 145 nM) and pre-incubated for varying amounts of time (0 h, 24 h, 48 h, and 72 h). After centrifugation at 2000 × g for 10 min, specimens were obtained from the upper layer and stored at -80°C. The P4 levels were measured with sheep P4 ELISA kits (Beijing Bayer Diagnostic Technology Co., Ltd.).
Western blotting
The granulosa cells were cultured and treated with different concentrations of orexin A (1 NM, 10 NM, 58 NM, 100 NM, and 145 NM groups), and pre-incubated for varying amounts of time (0 h, 24 h, 48 h, and 72 h). Protein extracts were prepared from each sample in lysis buffer (RIPA:PMSF, 99:1) using an ultrasonic instrument (VCX130, Shanghai) to thoroughly break the cells (70 v, 15 s). The samples were embedded in ice, and the process was repeated three times. The cells lysates were cleared by centrifugation at 12,000 × g for 3 min at 4°C. The protein content was determined using the bicinchoninic acid (BCA) method (Thermo). The BSA concentration diagram gradient standard liquid was prepared according to the manufacturer’s instruction. Protein samples of 25 μL were transferred into each well of a 96-well plate, and 200 μL of BCA working liquid was then added to each well and mixed. The protein concentrations of each sample was determined using a microplate reader (Molecular Devices, Shanghai, China).
To make all the protein sample concentrations homogeneous, the actual concentrations of the protein samples were calculated according to the optical density (OD) value. Then, 72 μL of each sample was added to 19.2 μL of 5x sodium alkyl sulfate twelve (SDS) protein sample buffer. The samples were boiled for 5 min and stored at -80°C. The cells lysates were electrophoresed for 20 min at 120 V in 3.9% Tris-acetate, and then for 60 min at 200 V in precast 12% MES SDS-polyacrylamide gels using an SDS-PAGE gel kit (CWBIO, Beijing). Then, the proteins were transferred onto polyvinylidene difluoride membranes (LC2002; Invitrogen) for 25 min at 25 V. The membranes were blocked for 1 h in blocking buffer [Tris-buffered saline and Tween 20 (TBST) and 5% nonfat milk] on a reciprocating decolorization shaking table at RT, and then incubated overnight at 4°C with the appropriate primary antibody diluted in blocking buffer. The membrane was washed three times with TBST, the following day, the membrane was washed three times with TBST before exposure to fluorescent-conjugated secondary antibodies diluted in blocking buffer for 1 h at RT. The membrane was scanned using a scanner (Odyssey) and processed with Adobe Photoshop (Adobe Systems Incorporated, USA). Values were considered to be statistically significant at confidence levels of 95% (P<0.05) and 99% (P<0.01).
Statistical analysis
A column diagram was drawn with the GraphPad Prism version 5.0 software program (GraphPad Software, Inc., USA). The figures were organized by Adobe Photoshop CS (Adobe Systems Incorporated, USA). Statistical analysis was performed using the SPSS 20.0 package programmer (SPSS Inc., Chicago, IL, USA). The data are expressed as the mean±standard deviation (SD, bar on the top of each column). A P-value of less than 0.05 was considered significant, and a P-value of less than 0.01 was considered highly significant.
Results
The identification of granulosa cells
The cell morphology of the granulosa cells is shown in the Fig. 1A. Haematoxylin and eosin staining of cell sections and giemsa staining showed that the cells form was complete, and the edge was clear, resembling a triangular cone or an irregular star. The cytoplasm was pink, and the nucleus was dyed blue (Figs. 1A-C).
Follicle-stimulating hormone receptor immuno-fluorescence chemical dyeing and DAPI staining was specific for ovarian granulosa, and we found the FSHR positive green-stained fluorescence in the cytoplasm, and positive DAPI staining indicated by blue fluorescence in the nucleus. The positive rate was more than 90% identical to the control group. Therefore, that the sheep ovarian granulosa cells in vitro culture were more than 90% pure (Figs. 1D-I).
Luteinizing granulosa cells secreted P4
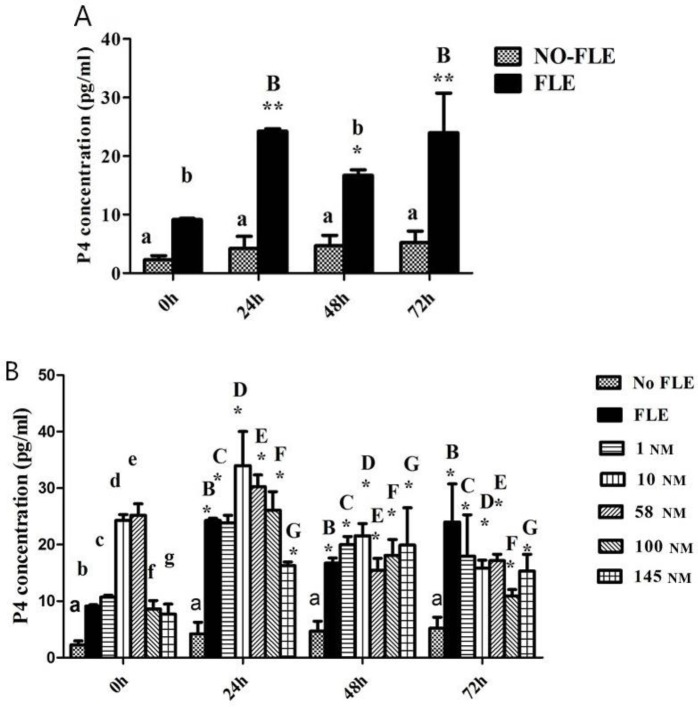
The ELISA results indicated that the luteinizing group significantly increased the P4 concentration in the FLE group compared to that in the no-FLE group at each time point (P<0.05; P<0.01). Over time, the P4 concentration gradually increased and then decreased in the FLE group. P4 was higher at 24, 48, and 72 h than at 0 h (P<0.05; P<0.01) (Fig. 2A).
Fig. 2.
P4 concentration. A: Identification of corpus luteum granulosa. A and a indicate significant difference, and B and b indicate significant difference. B: P4 concentrations were influenced by concentrations of Orexin A and different time. The same letter with different case indicates significant difference compared with the P4 concentration expression in no-FLE group at 0 h (P<0.05). * indicates significant difference compared with the P4 concentration expression in no-FLE group at 0, 24, 48, and 72 h (P<0.05)
Orexin A promotes the secretion of P4 by granulosa
The P4 concentration in the FLE group increased from 0 to 24 h, decreased from 24 h to 48 h, and then increased from 48 h to 72 h (P<0.05) compared with that at 0 h. The P4 concentrations in the 1, 10, 58 and 100 NM groups increased from 0 to 24 h, and then decreased from 24 h to 72 h (P<0.05) compared with that at 0 h. The P4 concentration in the 145 NM group increased from 0 to 48 h, and then decreased from 48 h to 72 h compared with that at 0 h (P<0.05) (Fig. 2B).
At 24 h, the P4 concentration increased from the FLE group to the 1 NM group, increased from 1 group to 10 NM group, and decreased from 10 to 145 NM compared with that in the no-FLE group (P<0.05). At 48 h, the P4 concentration increased from the FLE group to the 10 NM group, decreased from 10 to 58 NM, and increased from 58 to 145 NM compared with that in the no-FLE group (P<0.05). At 72 h, the P4 concentration decreased from the FLE group to the 10 NM group, increased from 10 to 58 NM, decreased from 58 to 100 NM group, and increased from 100 to 145 NM compared with that in the no-FLE group (P<0.05) (Fig. 2B).
Effects of orexin A on StAR, 3β-HSD, and P450 (CYP11) expression
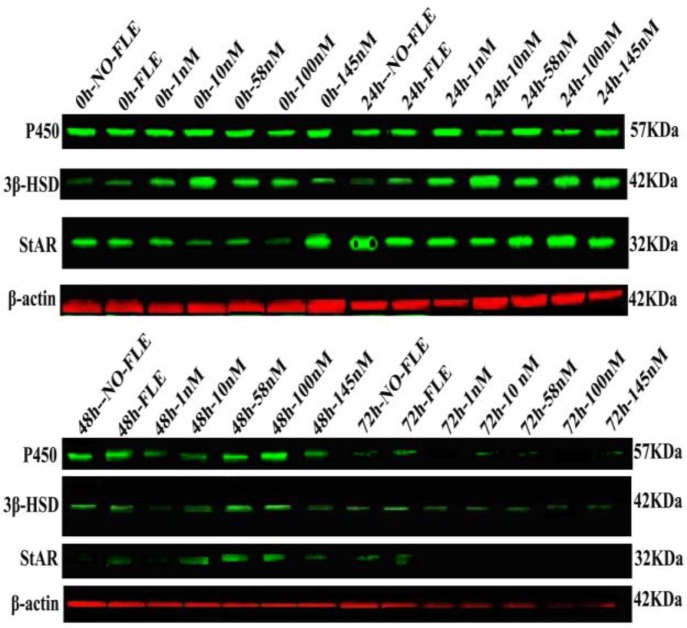
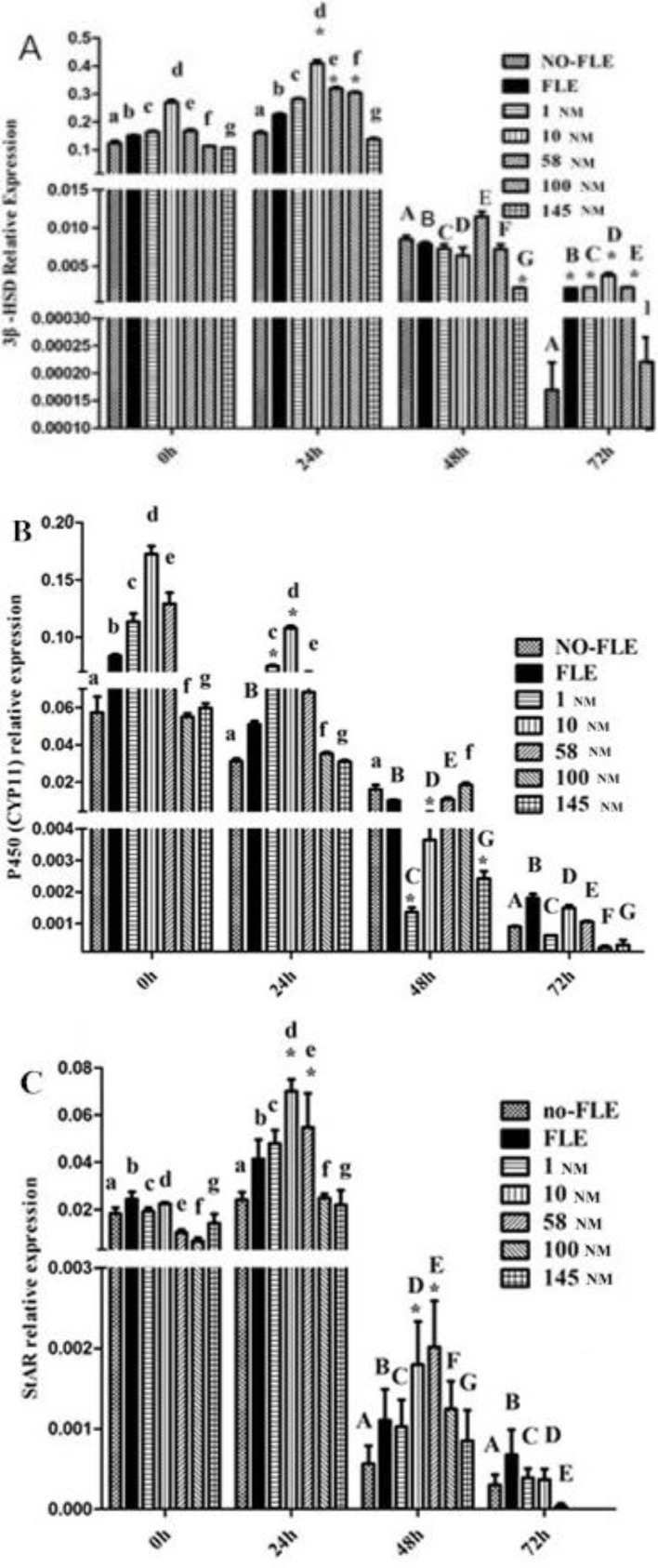
The StAR, 3β-HSD, and P450 (CYP11) expression levels are shown in Fig. 3. From the NO-FLE group to the 145 NM group, the expressions of the 3β-HSD at 48 and 72 h was lower than that at 0 h (P<0.05). At 24 h, the expressions of the 3β-HSD in the 10, 58, and 100 NM groups were higher than that in the no-FLE group (P<0.05). At 48 h, the expression of 3β-HSD in the 145 NM group was lower than that in the no-FLE group (P<0.05). At 72 h, the expression of the 3β-HSD in the FLE, 1, 10, and 58 NM groups was higher than that in the no-FLE group (P<0.05) (Fig. 4A). In the no-FLE group, the P450 (CYP11) expression at 72 h was lower than that at 0 h. In the FLE group, the P450 (CYP11) expression at 24, 48 and 72 h was lower than that at 0 h (P<0.05). In the 1, 10, 58, 100, and 145 NM groups, the P450 (CYP11) expression at 48 and 72 h group was lower than that at 0 h (P<0.05). At 24 h, the P450 (CYP11) expression levels in the 1 and 10 NM groups were higher than that in the no-FLE group (P<0.05). At 48, the P450 (CYP11) expression at 1, 10, and 145 NM was lower than that in the no-FLE group (P<0.05). At 72 h, no significant difference was found (Fig. 4B). From the no-FLE group to the 145 nM group, the StAR expression at 48 and 72 h was lower than that at 0 h (P<0.05). At 24 h and 48 h, the StAR expression in the 10 and 58 NM groups was higher than that in the no-FLE group (P<0.05). At 72 h, no significant difference was found (Fig. 4C).
Fig. 3.
StAR, 3β-HSD, P450 (CYP11) expression were influenced by concentrations of Orexin A and different time
Fig. 4.
3β-HSD (A), P450 (CYP11) (B), and StAR (C) expression were influenced by concentrations of orexin A and different time. The same letter with different case indicates significant difference compared with the A, B and C expression in no-FLE group at 0 h (P<0.05). * Indicates significant difference with the A, B and C expression in NO-FLE group at 0, 24, 48 and 72 h (P<0.05)
Discussion
Since the discovery of the orexinergic system, the involvement of orexins in the hypothalamic-pituitary-ovarian axis and the hormonal modulation of the orexinergic system have been documented, but the mechanisms and basic functions of orexins in the female reproductive system have remained unclear. Orexin A promotes the secretion of gonadal hormone and release hormone for the control of the gonad axis, and it is also regulated by the central nervous system to promote the secretion of LH (Pu et al., 1998 ▶; Kok et al., 2004 ▶). P4, a natural hormone secreted by the ovarian corpus luteum, is essential for maintaining pregnancy. P4 abnormality has been considered the clinical reason for precursor sex amenorrhea or amenorrhea, such as abortion, habitual abortion, reactivity of diagnosis, etc. Exploration of the relationship between oxexin and P4 is important. In this study, the sheep ovarian luteinizing granulosa cells were stimulated by different concentrations of orexin A for varying amounts of time to detect P4 secretion, as well as StAR, 3β-HSD, and P450 (CYP11) protein expression.
In this study, the secretion of P4 in the presence of orexin A was high, which indicated that orexin A played a positive regulatory role in sheep ovarian granulosa cells that secrete P4. It appears that orexin A can play various roles in P4 production depending on the concentration and treatment time. Orexin A can affect P4 secretion in sheep by changing the concentration and time, and can change the sheep reproductive status and the energy metabolism. Orexin A has been clinically used to treat polycystic ovary syndrome caused by low P4 levels, precursor abortion, habitual abortion, sexual reproduction, and energy metabolism disease. In this study, the P4 concentration first increased and then decreased.
Cholesterol in the mitochondrial outer membrane conveys the mitochondrial membrane in the P4 process. StAR transports cholesterol (Stocco, 2001 ▶). A StAR precursor protein activates StAR by interacting with the mitochondrial outer membrane via the c-terminus, and transports cholesterol to the mitochondrial membrane. The cholesterol transport process immediately stops after mature StAR enters the mitochondrial membrane (Stocco, 2000 ▶). Thus, StAR regulates the P4 production. In the present study, StAR and P4 showed a similar trend, which indicates that orexin A regulates P4 via StAR.
In the process of P4 regulation, P450 (CYP11) is mainly involved in three reactions. The first is the hydroxylation of cholesterol. P450 (CYP11) has an important physiological function in cholesterol transport and transformation. P450 (CYP11) modifies a side chain, and forms pregnenolone. The mitochondrial membrane is a fat-soluble membrane, and P450 (CYP11) targets the mitochondrial membrane surface. The mitochondrial membrane contains vesicles in which reactions occur. P450 (CYP11) functions importantly in P4 synthesis and is also the rate-limiting step of synthesis (Hu et al., 2004). P450 (CYP11) plays a key role in the formation of the placental P4, and P4 is necessary to maintain pregnancy. In the present study, P450 (CYP11) decreased with time and P4 was lower at the last two time points, which indicates that orexin A regulates P4 via P450 (CYP11). 3β-HSD is the key enzyme controlling the gonads and synthesizes the adrenal steroid hormone, and serves as the only synthesis pathway for cytochrome enzymes, catalyzing the transformation of pregnenolone to P4. 3β-HSD is necessary for steroid hormone biosynthesis (Kaynard et al., 1992 ▶). P4 and 3β-HSD showed similar behaviors in this study, which indicates that orexin A regulates P4 via 3β-HSD.
These results indicate that different exposure concentrations and times of orexin A influence the processing of P4 via StAR, 3β-HSD, and P450 (CYP11). Future studies are required to consider possible signaling pathways for orexin A that could influence the granulosa secretion of P4 and its mechanism of action.
Acknowledgements
We thank each of the authors for their contributions to the manuscript. This work was supported by the Natural Science Foundation Committee of Inner Mongolia (2017MS0362).
References
- Aston-Jones G, Smith RJ, Sartor GC, Moorman DE, Massi L, Tahsili-Fahadan P, Richardson KA. Lateral hypothalamic orexin/hypocretin neurons: a role in reward-seeking and addiction. Brain Res. J. Virol. 2010;1314:74–90. doi: 10.1016/j.brainres.2009.09.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai XJ, Widdowson PS, Harrold J, Wilson S, Buckingham RE, Arch JR, Tadayyon M, Clapham JC, Wilding J, Williams G. Hypothalamic orexin expression: modulation by blood glucose and feeding. Diabetes J. Virol. 1999;48:2132–2137. doi: 10.2337/diabetes.48.11.2132. [DOI] [PubMed] [Google Scholar]
- Cataldi NI, Lux-Lantos VA, Libertun C. Effects of orexins A and B on expression of orexin receptors and progesterone release in luteal and granulosa ovarian cells. Regul. Pept. 2010;178:56–63. doi: 10.1016/j.regpep.2012.06.008. [DOI] [PubMed] [Google Scholar]
- Cravioto MD, Ulloa-Aguirre A, Bermudez JA, Herrera J, Lisker R, Mendez JP, Perez-Palacios G. A new inherited variant of the 3 beta-hydroxysteroid dehydrogenase-isomerase deficiency syndrome: evidence for the existence of two isoenzymes. J. Clin. Endocrinol. Metab. 1986;63:360–367. doi: 10.1210/jcem-63-2-360. [DOI] [PubMed] [Google Scholar]
- Diep CH, Daniel AR, Mauro LJ, Knutson TP, Lange CA. Progesterone action in breast, uterine, and ovarian cancers. J. Mol. Endocrinol. 2015;54:R31–53. doi: 10.1530/JME-14-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskins GT, Moenter SM. Orexin a suppresses gonadotropin-releasing hormone (GnRH) neuron activity in the mouse. Endocrinology. 2012;153:3850–3860. doi: 10.1210/en.2012-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini A, Niasari-Naslaji A, Vojgani M, Gharagozloo F. Effect of time of eCG administration on the fate of ovarian follicle in Holstein heifers. Iran. J. Vet. Res. 2018;19:15–21. [PMC free article] [PubMed] [Google Scholar]
- Hu MC, Hsu HJ, Guo IC, Chung BC. Function of Cyp11a1 in animal models. Mol. Cell. Endocrinol. 2004;215:95–100. doi: 10.1016/j.mce.2003.11.024. [DOI] [PubMed] [Google Scholar]
- Ida T, Nakahara K, Murakami T, Hanada R, Nakazato M, Murakami N. Possible involvement of orexin in the stress reaction in rats. Biochem. Biophys. Res. Commun. 2000;270:318–323. doi: 10.1006/bbrc.2000.2412. [DOI] [PubMed] [Google Scholar]
- Kaynard AH, Periman LM, Simard J, Melner MH. Ovarian 3 beta-hydroxysteroid dehydrogenase and sulfated glycoprotein-2 gene expression are differentially regulated by the induction of ovulation, pseudopregnancy, and luteolysis in the immature rat. Endocrinology. 1992;130:2192–2200. doi: 10.1210/endo.130.4.1547735. [DOI] [PubMed] [Google Scholar]
- Kok SW, Roelfsema F, Overeem S, Lammers GJ, Frolich M, Meinders AE, Pijl H. Pulsatile LH release is diminished, whereas FSH secretion is normal, in hypocretin-deficient narcoleptic men. Am. J. Physiol. Endocrinol. Metab. 2004;287:E630–6. doi: 10.1152/ajpendo.00060.2004. [DOI] [PubMed] [Google Scholar]
- Komatsu K, Masubuchi S. The concentration-dependent effect of progesterone on follicle growth in the mouse ovary. J. Reprod. Dev. 2017;63:271–277. doi: 10.1262/jrd.2016-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachance Y, Luu-The V, Labrie C, Simard J, Dumont M, de Launoit Y, Guerin S, Leblanc G, Labrie F. Characterization of human 3 beta-hydroxysteroid dehydrogenase/delta 5-delta 4-isomerase gene and its expression in mammalian cells. J. Biol. Chem. 1992;267:3551. [PubMed] [Google Scholar]
- Li J, Hu Z, de Lecea L. The hypocretins/orexins: integrators of multiple physiological functions. Br. J. Pharmacol. J. Virol. 2014;171:332–350. doi: 10.1111/bph.12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WL, Strauss JF. Molecular pathology and mechanism of action of the steroidogenic acute regulatory protein, StAR. J. Steroid. Biochem. Mol. Biol. 1999;69:131–141. doi: 10.1016/s0960-0760(98)00153-8. [DOI] [PubMed] [Google Scholar]
- Muschamp JW, Dominguez JM, Sato SM, Shen RY, Hull EM. A role for hypocretin (orexin) in male sexual behavior. J. Neurosci. 2007;27:2837–2845. doi: 10.1523/JNEUROSCI.4121-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper DC, Upton N, Smith MI, Hunter AJ. The novel brain neuropeptide, orexin-A, modulates the sleep-wake cycle of rats. Eur. J. Neurosci. 2000;12:726–730. doi: 10.1046/j.1460-9568.2000.00919.x. [DOI] [PubMed] [Google Scholar]
- Pu S, Jain MR, Kalra PS, Kalra SP. Orexins, a novel family of hypothalamic neuropeptides, modulate pituitary luteinizing hormone secretion in an ovarian steroid-dependent manner. Regul. Pept. 1998;78:133–136. doi: 10.1016/s0167-0115(98)00128-1. [DOI] [PubMed] [Google Scholar]
- Puett D, Angelova K, da Costa MR, Warrenfeltz SW, Fanelli F. The luteinizing hormone receptor: insights into structure-function relationships and hormone-receptor-mediated changes in gene expression in ovarian cancer cells. Mol. Cell. Endocrinol. 2010;329:47–55. doi: 10.1016/j.mce.2010.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell SH, Small CJ, Kennedy AR, Stanley SA, Seth A, Murphy KG, Taheri S, Ghatei MA, Bloom SR. Orexin A interactions in the hypothalamo-pituitary gonadal axis. Endocrinology. 2001;142:5294–5302. doi: 10.1210/endo.142.12.8558. [DOI] [PubMed] [Google Scholar]
- Smart D, Jerman JC, Brough SJ, Rushton SL, Murdock PR, Jewitt F, Elshourbagy NA, Ellis CE, Middlemiss DN, Brown F. Characterization of recombinant human orexin receptor pharmacology in a Chinese hamster ovary cell-line using FLIPR. Br. J. Pharmacol. 1999;128:1–3. doi: 10.1038/sj.bjp.0702780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocco DM. The role of the StAR protein in steroidogenesis: challenges for the future. J. Endocrinol. J. Virol. 2000;164:247–253. doi: 10.1677/joe.0.1640247. [DOI] [PubMed] [Google Scholar]
- Stocco DM. StAR protein and the regulation of steroid hormone biosynthesis. Annu. Rev. Physiol. J. Virol. 2001;63:193–213. doi: 10.1146/annurev.physiol.63.1.193. [DOI] [PubMed] [Google Scholar]
- Storbeck KH, Swart P, Swart AC. Cytochrome P450 side-chain cleavage: insights gained from homology modeling. Mol. Cell. Endocrinol. 2007;265-266:65–70. doi: 10.1016/j.mce.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Wei S, Gong Z, Guo H, Zhang T, Ma Z. FSH and eCG impact follicles development and expression of ovarian FSHR and caspase-9 in mice. Iran. J. Vet. Res. 2017;18:79–85. [PMC free article] [PubMed] [Google Scholar]