Abstract
The release of Non-Steroidal Anti-Inflammatory drugs (NSAIDs) such as Ibuprofen (Ibu), Naproxen (Nab) and Diclofenac (Dic) to the aquatic system cause serious environmental problems. In this study, green-synthesized copper nanoparticles (Cu NPs) have been used as nano-adsorbent for the removal of Ibu, Nab, and Dic from wastewater samples. Formation of Cu NPs was confirmed by different analytical techniques. The adsorption parameters such as temperature, pH, adsorbate concentration, adsorbent dose and contact time were studied. The best removal results were obtained at these conditions: temperature 298 K, pH = 4.5, 10.0 mg Cu NPs, 60 min. At these conditions, the removal percentage of Ibu, Nap, and Dic were found to be 74.4, 86.9 and 91.4% respectively. The maximum monolayer adsorption capacities were calculated as 36.0, 33.9 and 33.9 mg/g for Dic, Nap, and Ibu respectively. The kinetic studies conducted that the sorption process obeyed the second order kinetic model, while the thermodynamic results revealed that the adsorption process was spontaneous, endothermic (+23.8, +40.8 and +38.3 kJ/mol for Ibu, Nap and Dic respectively). The results revealed that green-synthesized copper nano-adsorbent may be used for the removal of the anti-inflammatory drugs from real wastewater efficiently.
Keywords: Environmental science, Analytical chemistry, Inorganic chemistry, Materials chemistry, Pharmaceutical chemistry, Physical chemistry, Nanotechnology, Adsorption, Naproxen, Copper nanoparticles, Diclofenac, Ibuprofen
1. Introduction
Currently, water contamination due to releasing of drugs and pharmaceuticals “new emerging pollutants” residues into aquatic systems is an emerging environmental problem. Among various water organic pollutants, Non-Steroidal Anti-Inflammatory drugs (NSAIDs) constitute a group of pharmaceuticals with analgesic, antipyretic and anti -inflammatory effects. The most abundant members of the NSAIDs family are Diclofenac (Dic), Naproxen (Nap) and Ibuprofen (Ibu). First, Ibu is found in water at a concentration of up to 24.6 μg/l, thus it represents hazardous pollutants for human health [1]. Ibu has also the properties of high consumption, slight water solubility, and high mobility in water. Second, Nap belongs to the aryl acetic acid group of NSAIDs. It also presents in water at a concentration with significant toxic effect towards ecosystems [2]. In addition, its photodegradation products are more toxic than Nap itself. Third, Dic was detected in wastewater, due to its high level of consumption and resistance for biodegradation. Due to their properties of hydrophilicity and stability, they can be detected in the aqueous phase. Thus, removal of NSAIDs from the aquatic environment is essential.
Various techniques were applied for the treatment of NSAIDs pollutants such as photocatalytic degradation [3], microextraction [4], oxidation [5], biodegradation [6], chlorination [7], bio filtration [8], electrocoagulation-flotation [9] and electrochemical oxidation [10]. However, the majority of these methods are associated with certain limitations such as cost un-effectiveness, limited scope, and time consumption. An alternative treatment, the adsorption phenomenon is considered the appropriate water treatment methodology due to the simplicity, low-cost and effective [11]. Further, adsorption technology exhibited an excellent removal efficiency towards NSAIDs [12]. A highly promising class of adsorbents used for this purpose is nanoparticles. Owing to their distinct features such as small particle size, catalytic potential, large surface area and a great number of active sites for the interaction with different pollutants, allowing high efficient removal. Nanoparticles “new generation nano-adsorbents” are used to remove these pollutants even at low concentrations i.e. μg/L.
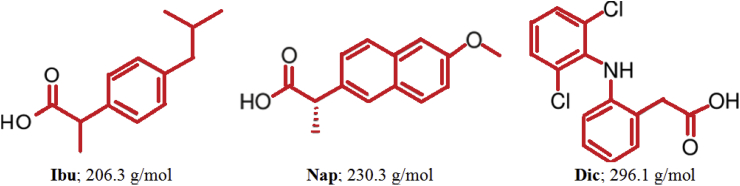
The present study describes the removal of new emerging pollutants using new generation nano-adsorbents by adsorption technology. In view of these facts, the green synthesized copper nanoparticles (Cu NPs) may provide an efficient and cost-effective way for the treatment of the pharmaceuticals contaminated wastewater. To the best of our knowledge, this work is the first to study the removal of non-steroidal anti-inflammatory drugs (Ibuprofen, naproxen, and diclofenac) by green synthesized metallic copper nanoparticles from aqueous solution. Important parameters such as solution pH, adsorbent dose and contact time that influence the adsorption process were studied. Thermodynamics, kinetics and equilibrium isotherms were studied to understand the behavior of NSAIDs/Cu NPs adsorption system. Structures of selected pharmaceuticals and their molecular weight are demonstrated in Fig. 1.
Fig. 1.
Chemical structure and molecular weight of the selected pharmaceutical compounds.
2. Materials and methods
2.1. Chemicals
All chemicals used in this work were analytical grade. Copper (II) sulfate pentahydrate salt, CuSO4 · 5H2O 99% purity and acetonitrile and methanol HPLC grade from (Merck). Tetraheptylammonium bromide and n-tetrabutylammonium hydroxide in methanol from (Sigma-Aldrich). NSAIDs drugs; Ibu, Nap, and Dic sodium salts were a gift from (Smart Pharma company, Egypt). Tilia leaves (Local market, Assuit, Egypt).
2.2. Adsorbent preparation
The detailed preparation method of copper nanoparticles (Cu NPs) was reported elsewhere [13]. In brief, Tilia aqueous extract was added to 0.01M CuSO4 · 5H2O (4:1 v/v) and heated to 90 °C with continuous stirring for 30 min. The mix was kept in a dark place for 24h and then centrifuged at 6000rpm. The separated precipitate washed with deionized water, then ethanol to remove any residual organic extract, then dried in an oven for 2 h at 100 °C.
2.3. Adsorbent characterization
The UV-vis absorption spectra of the prepared Cu NPs were performed using (PerkinElmer, USA); Lambda 750 UV/Vis/NIR Spectrophotometer while the X-ray diffraction (XRD) pattern was recorded using a Phillips; PW 1710 Diffractometer with Cu Kα radiation of wavelength 1.5418 A0. The morphology of the Cu NPs surfaces was investigated by transmission electron microscopy (TEM) using JEOL; JEM-100 CXII.
2.4. Adsorption study
All experiments were performed in batch technique. To determine the influencing parameters including pH, adsorbent dose, initial concentration, contact time and temperature on the adsorption process, all the parameters against the variable parameter under dedicate studying are fixed for the tested range. To study the adsorption isotherms, standard stock solutions (100 mg/L) of Ibu, Nap and Dic separately were prepared in methanol at 298K, and then serial dilutions were further diluted to the required concentrations. Initial concentrations of NSAIDs (10–40 mg/L), different pH (2.5–10.5), temperature range (293–308 K), contact time (0–60min) and adsorbent dose (5–20 mg/L) were studied for the determination of the effect of these parameters on the adsorption process of NSAIDs in aqueous solutions. Finally, the NSAIDs concentrations were measured using HPLC in triplicate and the mean value was reported in the analysis study. The measure repeatability in the same experiment was less than 1%. The NSAIDs adsorbed amount qe (mg/g) by the Cu NPs was calculated using the following Eq. (1):
| qe = (C0−Ce)V/m | (1) |
where C0 and Ce are the initial and equilibrium concentrations of Dic, Nap and Ibu (mg/L), respectively. V is the volume of the Dic, Nap and Ibu solution (mL); and m is the Cu NPs mass (mg).
For the calculations of the NSAIDs percentage adsorption (%), the following expression was used (Eq. 2):
| Adsorption (%) = [(C0−Ce)/C0] × 100 | (2) |
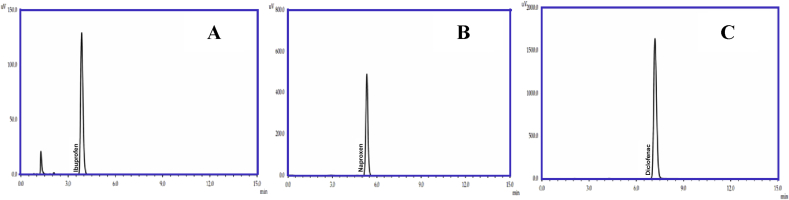
The concentrations of Ibu, Nap, and Dic were determined using LC-20A HPLC instrument with the photodiode array detector (Shimadzu, Japan). The HPLC chromatograms are presented in Fig. 2. The retention time of Dic, Nap and Ibu were found to be 3.8; 5.3 and 7.2 ± 0.2 min respectively, overall the experiments, as depicted in Fig. 2.
Fig. 2.
HPLC chromatograms for Ibuprofen (A), Naproxen (B) and Diclofenac (C).
2.5. Pharmaceutical wastewater samples
Three wastewater samples were utilized to explore the ability of the proposed adsorbent (Cu NPs) for NSAIDs removal. Wastewater samples were collected from an Egyptian pharmaceutical company located in Cairo Governorate, Egypt. Three wastewater samples named Dic-S, Nap-S, and Ibu-S were collected from the last phase of machine-washing wastewater after the production process. Each wastewater sample contaminated with only one single drug. The collected samples were filtered through 0.45μm filter paper and stored in clean vessels at 5 °C until use.
3. Results and discussion
3.1. Characterization of copper nano-adsorbents
Copper nanoparticles (Cu NPs) with high purity and stability were green synthesized using Tilia leaves extracts. The UV-vis spectrum of Cu-NPs suspension, Fig. S1 (Supplementary information), shows a plasmon peak of Cu NPs at 562nm. Fig. S2 shows the XRD pattern of Cu NPs, the diffraction peaks at 2θ = 35°, 50.50° and 74.21° were assigned to the (111), (200) and (220) planes of the faced center cubic (FCC) lattice of Cu respectively which found to be in a good agreement with reference to the unit cell of the FCC structure (JCPDS File No. 04-014-0265). TEM micrographs of Cu NPs in Fig. S3 shows a well-dispersed spherical and semispherical nanoparticle with particle diameters ranged from 4.7 to 17.4nm.
3.2. Adsorption studies
3.2.1. Effect of pH on the removal of NSAIDs
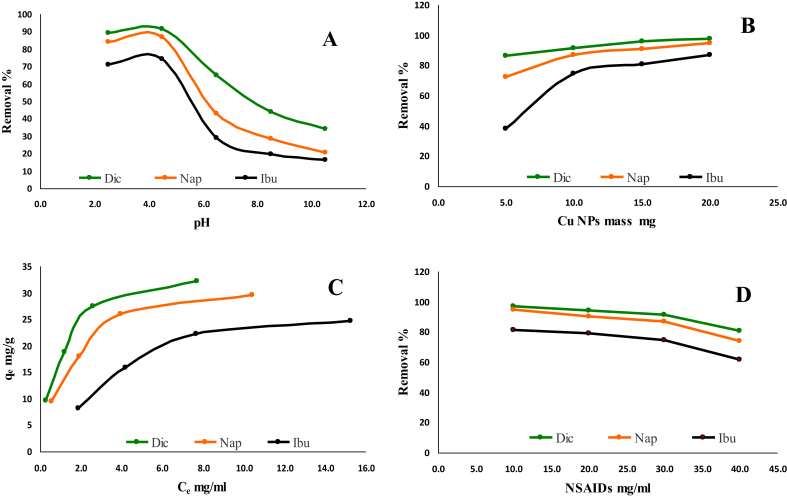
Fig. 3A shows that the highest removal percentages of NSAIDs were achieved at a pH value of 4.5. At lower pH, the increase in the adsorption percentage may be attributed to the electrostatic attraction force with Cu NPs. At higher pH, the low removal percentage may be attributed to the electrostatic repulsion between Cu NPs surface and NSAIDs anions. However, at higher pH, the NSAIDs species are found in anionic form, while at lower pH range NSAIDs ions were presented as cations according to their pKa values; 4.00, 4.19 and 4.91 for Dic, Nap, and Ibu respectively.
Fig. 3.
Effect of pH (A), adsorbent dose (B), pollutant concentration (C & D) on the removal of Dic, Nap and Ibu by Cu NPs.
Fig. 3A, shows also when the sorption solution pH was increased from 2.5 to 4.5, the removal percentage increased from 89.4, 70.8 and 83.9% to 91.4, 74.4 and 86.9% for Dic, Ibu and Nap respectively. By increasing in pH from 4.5 to 6.5, the removal percentage decreased sharply from 91.4, 74.4 and 86.9% to 64.9, 29.0 and 43.0% for Dic, Ibu, and Nap, respectively. Further, an increase in solution pH by two units, a decreasing in the removal percentage was observed until it reached pH 10.5. The removal percentage decreases from 43.8, 19.8 and 28.6% at pH 8.5 to 34.2, 16.3 and 20.4% at pH 10.5. Hence, it is concluded that the NSAIDs removal by Cu NPs adsorbent is completely a pH-dependent as the surface charge of Cu NPs were affected by the pH value of the sorption. In addition, hydrolysis degree of NSAIDs; Dic, Ibu, and Nap in aqueous solution is pH-dependent.
3.2.2. Effect of adsorbent dose on NSAIDs removal
The effect of adsorbent dose is an important parameter affecting the NSAIDs removal by green- synthesized Cu NPs adsorbent, Fig. 3B displays the adsorption efficiency of NSAIDs increases with increasing the dose of Cu NPs adsorbent until of 0.01g. This increase could be attributed to the surface area of Cu NPs which increases the available large number of active sites for the adsorption process [14]. When the adsorbent dose was small, the binding sites on the adsorbent surface were also small so the adsorption efficiency was low [15]. The increasing in Cu NPs dose leads to the increase the active sites for NSAIDs binding which means more NSAIDs species are adsorbed on Cu NPs surfaces. After 0.01g adsorbent mass, any increase in the mass is accompanied by a slight increase in the removal efficiency.
3.2.3. Effect of initial NSAIDs concentrations
Fig. 3C shows an increase in the adsorption capacity of Cu NPs with the increase of NSAIDs concentrations; the maximum adsorption capacity reached to 24.8, 29.6 and 32.3 mg/g for Ibu, Nap, and Dic respectively. It was clear that the harmony of the NSAIDs compounds towards the Cu NPs followed the order Ibu < Nap < Dic and a sudden decreasing in the drug removal percentage occurred after the concentration of 30 mg/L (Fig. 3D). After the concentration of 30 mg/L, the removal percentage was decreased from 74.4 to 61.9%; from 86.9 to 74.0% and from 91.4 to 80.7% for Ibu, Nap and Dic, respectively. This affinity may be attributed to the drugs molecular structures (Fig. 1), where Dic and Nap contain two benzene rings, i.e., more in hydrophobicity, while Ibu contains only one benzene ring which makes the interaction between NSAIDs and Cu NPs via π-π interaction [16]. Another factor, the molecular weight of the drug and subsequently the molecule size; the molecule with higher molecular weight; has the priority on the surface of the Cu NPs [17]. The molecular weights are 206.3, 230.3 and 296.1 g/mol for Ibu, Nap and Dic, respectively arranged in the following order: Ibu < Nap < Dic.
3.3. Adsorption isotherms
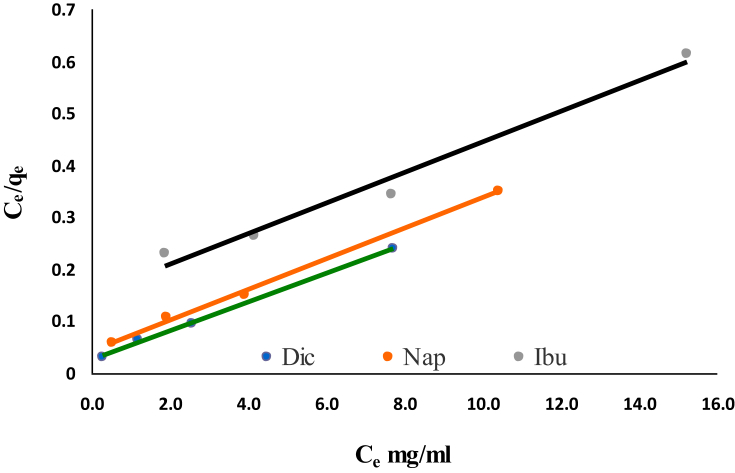
It is an important aim to determine the adsorption isotherms to explore the relationship between the surface of an adsorbent material; Cu NPs, and the transferred amount of drug molecules onto active sites in an aqueous medium. The adsorption data were analyzed by different isotherm equations of Dubinin–Radushkevich (D-R), Freundlich, Langmuir and Temkin, and their constants were calculated. Langmuir model equation [18, 19, 20] was expressed as Eq. (3):
| Ce/qe = (1/qLKL) + (1/qL)Ce | (3) |
where Ce is equilibrium concentration (mg/L) of Cu NPs, qe is the amount of NSAIDs (mg/g), qL is the monolayer adsorption capacity of Cu NPs (mg/g) and KL is Langmuir energy of adsorption constant (L/mg) as in Fig. 4. Experimental and calculated data were listed in Table 1. Langmuir isotherm model can be described by a sensitive equilibrium parameter or separation factor, (RL) which calculated by the following Eq. (4):
| RL = 1/(1 + KLCmax) | (4) |
where Cmax is the highest initial drug concentration in the solution (mg/L).
Fig. 4.
Langmuir isotherm model for removal of Dic, Nap and Ibu by Cu NPs.
Table 1.
Parameters values of different isothermal adsorption models.
| Model | Parameter | Diclofenac | Ibuprofen | Naproxen |
|---|---|---|---|---|
| Langmuir | qL | 36.0 | 33.9 | 33.9 |
| KL | 1.123 | 0.197 | 0.693 | |
| RL | 0.022 | 0.113 | 0.035 | |
| R2 | 0.998 | 0.977 | 0.998 | |
| Freundlich | n | 2.7 | 1.8 | 2.5 |
| 1/n | 0.375 | 0.542 | 0.400 | |
| KF | 16.8 | 6.5 | 13.1 | |
| R2 | 0.955 | 0.917 | 0.943 | |
| Temkin | B | 7.080 | 8.222 | 7.120 |
| bT | 0.35 | 0.301 | 0.348 | |
| A | 13.972 | 1.575 | 7.244 | |
| R2 | 0.979 | 0.964 | 0.971 | |
| D-R | β | 0.1 × 10−6 | 1 × 10−6 | 0.2 × 10−6 |
| E | 2.5 | 0.7 | 1.8 | |
| qm | 27.9 | 23.7 | 26.2 | |
| R2 | 0.914 | 0.971 | 0.922 |
The adsorption isotherm model is favorable (0 < RL < 1), unfavorable (RL > 1), linear (RL = 1) and irreversible (RL = 0), which depends on equilibrium parameter RL. In this investigation, the RL values were found to be 0.022, 0.035 and 0.113 for Dic, Nab and Ibu, respectively, indicating favorable adsorption for NSAIDs concentration at 298 K.
Freundlich model equation [19, 21] presumed for the heterogeneous surface of adsorbent and distributed adsorption sites of adsorbate. The linear equation was written as (Eq. 5):
| Log qe = Log KF + (1/n) Log Ce | (5) |
Where, Ce is the equilibrium concentration (mg/L) of Cu NPs, in the solution, qe is the amount of NSAIDs adsorbed at equilibrium time (mg/g), KF is Freundlich adsorption capacity of Cu NPs (mg/g) and n is Freundlich constant characteristics of the system, indicating the adsorption intensity. The values of Freundlich parameters (KF, n, and R2) are presented in Table 1 indicates that the favorable adsorption of Ibu, Nap, and Dic at 298 (K) temperature adsorbed onto Cu NPs surface [19, 22]. The obtained KF and n values were found to be increased in the following order Ibu < Nap < Dic which assured the above-mentioned fact related to adsorption affinity in removal percentage [15, 17, 23]. According to Freundlich model results; the values of 1/n were found to be 0.54, 0.40 and 0.38 for Ibu, Nap and Dic respectively. This indicated that NSAIDs/Cu NPs adsorption system was not heterogeneous [20].
Temkin isotherm model [15, 19, 23, 24] was also used to analyze the obtained experimental data. This model assumed that the adsorption heat would decrease by increasing the adsorbent mass. The relation applied for contaminant adsorption onto the heterogeneous surface is (Eq. 6) [25]:
| qe = B Ln A + B Ln Ce | (6) |
Where A is the binding constant (L/mg) and it was related to the maximum binding energy while B isTemkin adsorption constant that is related to the sorption heat. If the value of B parameter is lesser than 8 kJ/mol, this will be meant a weak interaction between NSAIDs/Cu NPs surfaces was formed. The results in Table 1 reveals that the value of B constant was found to be in the range 7.1–8.2 kJ/mol proved a weak interaction between Ibu, Nap and Dic molecules and Cu NPs. Another Temkin isotherm constant, bT, could be determined via the following relation (Eq. 7):
| B = RT/bT | (7) |
where, R is the gas constant (8.314J/mol K) and T is absolute temperature, 298K. The constant bT has importance significant in the determination of the adsorption process type. If bT value is <80; this means that the adsorption process is physically in nature [19]. The results revealed that the removal of NSAIDs by Cu NPs occurred through physisorption process.
D-R model equation [15, 19, 23, 24] was also used to analyze the experimental data and approached the following linearized relation form (Eq. 8):
| Ln qe = Ln qm−β ε2 | (8) |
where β is a coefficient related to the mean free energy; E; (kJ/mol) of sorption per molecule of the sorbate (NSAIDs) when it transferred to the surface of the solid Cu NPs from infinity in the solution. The parameter ε is Polanyi potential given as (Eq. 9):
| ε = RT(1 + 1/Ce) | (9) |
D-R model can be used to predict adsorption type with a calculation of adsorption energy; E; as (Eq. 10):
| E = (−2β)−1/2 | (10) |
In this work, the values of adsorption energy, E, were found to be 0.7, 1.8 and 2.5 kJ/mol, for Ibu, Nap, and Dic, respectively. Such finding assures the physical nature of the NSAIDs/Cu NPs adsorption system.
All calculated parameters of adsorption isotherms (Langmuir, D-R, Freundlich, Temkin model) are tabulated in Table 1. The linear square regression correlation coefficient; R2, intercept and the slope of each adsorption isotherm was calculated. Comparing of R2 values showed that the higher values for the Langmuir isotherm model and the minimum for the D-R adsorption isotherm model. Therefore, Langmuir isotherm was the best fitted in sorption. As listed in Table 1, the adsorption isotherm models are followed in a manner: Langmuir > Temkin > Freundlich > D –R Model.
To evaluate the utility of Cu NPs as a remover for NSAIDs from water, the maximum adsorption capacity of Cu NPs towards Dic, Nap, and Ibu should be compared with previous studies. However, the literature on the evaluation of NSAIDs removal using adsorption technique is relatively scarce. Table 2 summarizes a comparison of the maximum capacities of Nap, Dic, and Ibu by different adsorbents including Cu NPs for the present study. The comparison conducted that Cu NPs could be used as an efficient adsorbent agent for Dic, Nap and Ibu removal from environment wastewater. Green-synthesized Cu NPs display a similar maximum adsorption capacity of NSAIDs as like as other reported adsorbents, indicating a promising utility for Cu NPs utilization in NSAIDs removal from contaminated water bodies.
Table 2.
Maximum adsorption capacities mg/g of NSAIDs; Dic, Nap and Ibu for different adsorbents.
| Adsorbent | Ibu | Nap | Dic | Reference |
|---|---|---|---|---|
| Nanographene | 11.9 | 17.8 | 19.3 | [16] |
| Mugwort leaves | 16.9 | - | - | [26] |
| AC | - | 18.87 | - | [27] |
| Magnetic MWCNT | - | 20.75 | - | [28] |
| Purified MWCNT | - | - | 19.9 | [29] |
| Magnetic MW | - | - | 33.37 | [30] |
| Natural clay | 50 | 30.87 | - | [31] |
| Rice Hull AC | 100 | - | - | [32] |
| Copper nanoparticles | 33.9 | 33.9 | 36.0 | Present study |
| N-biochar | 311 | 290 | 372 | [33] |
| O-biochar | 286 | 228 | 214 | |
| AC cloths | 492 | - | - | [34] |
| Micelle-clay complex | - | 71.42 | - | [27] |
| AC from apricot waste | - | 106.38 | - | [2] |
| AC fibers [textile waste] | - | 294.11 | - | [35] |
| Sawdust– polyaniline | - | - | 89.28 | [36] |
| Montmorillonite clay | - | - | 497 | [37] |
3.4. Kinetic study
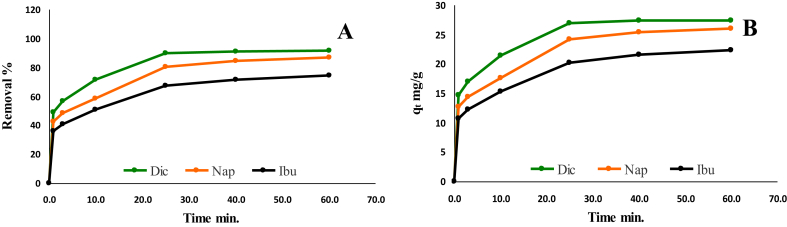
The kinetic profile of NSAIDs; Dic, Nap and Ibu adsorption onto Cu NPs surface at different time intervals as depicted in Fig. 5 A and B. The results revealed that NSAIDs adsorption onto Cu NPs surface was clearly time-dependent. It was observed that most of Dic, Nap and Ibu species uptake occurred within a time of 30 min for the three selected drugs. After 30 min, the less increasing and approximately practically stability in plateau has occurred. The adsorption uptake was increased sharply in the first 20 min due to the presence of more free active adsorption sites on the Cu NPs surface and the high concentration of the solution. After that period, a few free active sites on the adsorbent surface were available. Subsequently, a slow rate of increase in the removal of Dic, Nap, and Ibu was observed.
Fig. 5.
Adsorption of Dic, Ibu and Nap onto Cu NPs as a function of time, removal % (A) and adsorption capacity mg/g (B).
Various kinetic models were tested to analyze the kinetic data in order to provide valuable information on the adsorption kinetic mechanism of Dic, Ibu and Nap adsorption onto Cu NPs surface.
Pseudo-first-order model is expressed by Lagergren [19, 38] as (Eq. 11):
| Log(qe−qt) = Log qe−(K1/2.303)t | (11) |
Where qe is the amount of NSAIDs contaminant adsorbed by Cu NPs (mg/g) at equilibrium, qt is the amount of NSAIDs adsorbed by Cu NPs (mg/g) at predetermined time interval t and K1 is rate constant of pseudo-first-order adsorption process (min−1).
Pseudo-second-order model has given by McKay and Ho [19, 39] is given by the following Eq. (12):
| (t/qt) = 1/(K2qe2) + (1/qe)t | (12) |
where K2 is the rate constant of pseudo-second order adsorption process (g/mg min).
Another kinetic model was used to evaluate the experimental data; Elovich model (Eq. 13); [15, 17, 23]:
| qt = (1/β)(Ln αβ) + (1/β)Ln(t) | (13) |
where β is constant related to surface coverage and the activation energy for chemisorptions (g/mg) and α is the initial sorption rate constant (mg/g min).
Weber's and Moris's model also were explored to investigate the intraparticle diffusion mechanism, it was symbolized as (Eq. 14) [15, 17, 23, 40]:
| qt = C + Kint(t)1/2 | (14) |
where Kint is the intraparticle rate constant (mg/g min1/2). C value gives information about the boundary thickness, as the larger the intercept, the greater is the boundary layer effect [15, 17, 23]. Higher values of Kint reflect an enhancement in the adsorption rate of NSAIDs onto Cu NPs surface.
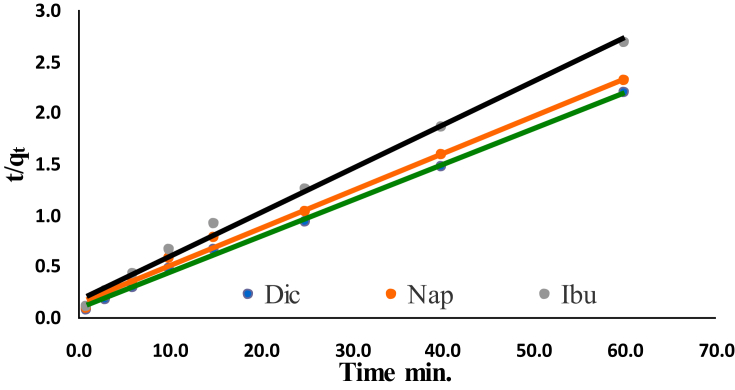
Table 3 summarizes the obtained results of various applied kinetic models. For various kinetic models, the obtained kinetic data are supported to a pseudo-second-order kinetic model, Fig. 6 and Table 3. Pseudo-first order model shows less linearity in the regression coefficient R2; which were found to be 0.9626, 0.9739 and 0.9844. On the contrary, the correlation coefficient values for a pseudo-second-order kinetic model were closed to the unit (0.9978, 0.9935 and 0.9939 for Dic, Nap, and Ibu respectively) and greater than all adsorption kinetic models. The calculated qe values were found to be 28.5, 27.5 and 23.4 mg/g for Dic, Nap and Ibu, respectively, which were very close to the obtained experimental values, Table 3.
Table 3.
Parameters values of different kinetic models for the NSAIDs adsorption onto Cu NPs.
| Model | Parameter | Dic | Ibu | Nap |
|---|---|---|---|---|
| Lagergren 1st order | R2 | 0.963 | 0.984 | 0.974 |
| K1 | 0.132 | 0.069 | 0.078 | |
| qe calculated | 17.6 | 13.1 | 16.0 | |
| McKay- Ho 2nd order | R2 | 0.998 | 0.994 | 0.994 |
| K2 | 0.016 | 0.011 | 0.010 | |
| qe calculated | 28.5 | 23.4 | 27.5 | |
| qe expermintal | 27.4 | 22.3 | 26.1 | |
| Elovich | R2 | 0.960 | 0.945 | 0.930 |
| α | 207.653 | 65.923 | 77.625 | |
| β | 0.290 | 0.327 | 0.278 | |
| Weber and Moris | R2 | 0.885 | 0.960 | 0.948 |
| Kint | 1.953 | 1.821 | 2.143 | |
| C | 14.6 | 9.5 | 11.2 |
Fig. 6.
Pseudo second order plots of Dic, Nap and Ibu adsorption by Cu NPs.
3.5. Thermodynamic study
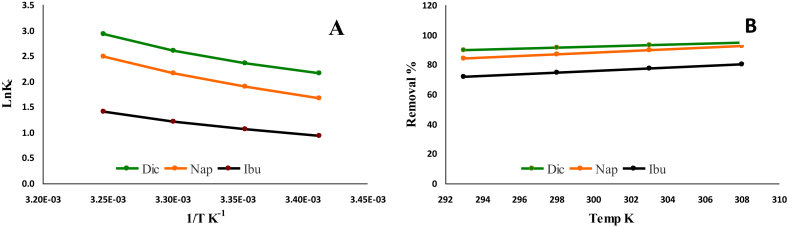
The effect of temperature on the removal % is illustrated in Fig. 7. Thermodynamic studies for NSAIDs uptake by Cu NPs were carried out at four different temperatures for understanding the nature of the adsorption process and its feasibility. The highest in temperature is the highest in removal % of NSAIDs, which indicates an endothermic adsorption process. This outcome was confirmed after the analysis of the experimental thermodynamic data via the following Eqs. (15) and (16):
| ΔG° = −RT Ln Kc | (15) |
| Ln Kc = −ΔG°/RT = −(ΔH°/RT) + (ΔS°/R) | (16) |
where ΔG° is the free energy change (J/mol), R is the gas constant (8.314 J/mol K), T is the absolute temperature (K), ΔH° is the enthalpy change (J/mol), ΔS° is the entropy change (J/mol K) and Kc is the thermodynamic equilibrium constant which can be expressed as (Eq. 17):
| Kc = C∂/Ce | (17) |
where C∂ is the concentration (mg/L) of NSAIDs contaminant present in solution and Ce is the equilibrium concentration (mg/L) of drugs in the sorption solution. The values of Δ H° and Δ S° calculated from the slope and intercept values of the graph plot of Ln KC versus 1/T, Fig. 7A.
Fig. 7.
Plot of Ln Kc versus 1/T (A) and effect of temperature on the removal % of NSAIDs by Cu NPs (B).
Results in Table 4 were confirmed the previous findings regarding the endothermic nature of the adsorption process. The positive signs of ΔH° and ΔS° indicated that the adsorption process reactions were obeyed an endothermic path. This explains the increase in removal percentage with increasing in solution temperature; Fig. 7B. According to adsorption thermodynamics; the magnitude of ΔH° determines whether the adsorption process is chemical or physical. Generally, the heat of chemisorption generally falls into a higher range of 80–200 kJ/mol [15]. As revealed in Table 4, the ΔH°values of Dic, Nap and Ibu were determined as 38.3, 40.8 and 23.8 kJ/mol, respectively. These results indicate that NSAIDs adsorption onto Cu NPs is a purely physical process and confirmed by the mean free energy. Physisorption process occurs because of a long range of weak Van der Waals forces between adsorbent (Cu NPs) and NSAIDs adsorbates. The negative values of ΔG° verify favored and spontaneous reaction nature.
Table 4.
Thermodynamic parameters values for adsorption of Dic, Ibu and Nap by Cu NPs.
| Items | Dic | Ibu | Nap | |
|---|---|---|---|---|
| ΔH° kJ/mol | +38.3 | +23.8 | +40.8 | |
| ΔS° J/mol K | +148.5 | +88.7 | +153.0 | |
| ΔG° kJ/mol | 293 K | -5.25 | -2.27 | -4.05 |
| 298 K | -5.85 | -2.64 | -4.69 | |
| 303 K | -6.53 | -3.06 | -5.46 | |
| 308 K | -7.50 | -3.62 | -6.35 | |
3.6. Treatment of the pharmaceutical wastewater samples
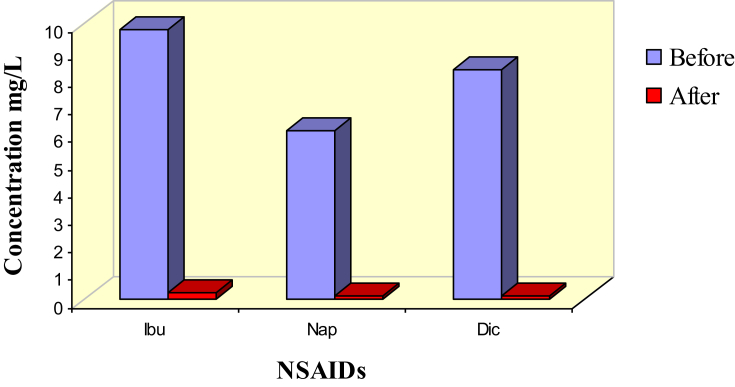
The uptake of Nap, Ibu, and Dic by Cu NPs from real wastewater samples has been explored to determine the efficiency of the green-synthesized Cu NPs adsorbent. Before treatment, the initial contaminant concentrations in the wastewater samples namely; Dic-S, Nap-S, and Ibu-S, were measured by HPLC. In addition, water characteristics, such as pH, conductivity and total dissolved solids (TDS) were measured and listed in Table 5. Then 10 mL of raw wastewater was added to 20 mg of Cu NPs and agitated for 3 h. The adsorbed amount of contaminants was studied by comparing the adsorbed amount of Ibu, Nap and Dic compounds before and after the adsorption process. After application of Cu NPs into real wastewater, the physicochemical parameters of wastewater were measured. From Table 5, it noticed that the alkalinity of raw wastewater was neutralized to some extent after the remediation with Cu NPs adsorbent. Furthermore, both conductivity and TDS values decreased after treatment with Cu NPs. The obtained results were revealed in Fig. 8. It is obvious that most of the Ibu, Nap and Dic contaminants were removed from the wastewater samples. This confirmed the high efficiency and applicability of Cu NPs in the treatment of aquatic bodies contaminated with NSAIDs such as Ibu, Nap, and Dic.
Table 5.
Physicochemical parameters of pharmaceutical wastewater samples contaminated with NSAIDs before and after treatment.
| Item | Dic |
Nap |
Ibu |
|||
|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | |
| Concentration (mg/L) | 8.31 | 0.12 | 6.10 | 0.09 | 9.76 | 0.24 |
| pH | 8.7 | 8.1 | 8.6 | 7.6 | 8.4 | 8.1 |
| Conductivity (μS/cm) | 350 | 316 | 487 | 463 | 177 | 164 |
| TDS (mg/L) | 178.5 | 161.2 | 248.4 | 236.1 | 90.3 | 83.6 |
Fig. 8.
Ibu, Nap and Dic concentrations in wastewater samples before and after treatment with Cu NPs.
4. Conclusions
In this study, green-synthesized Cu NPs was used for the removal of three selected pharmaceutical drugs namely: Dic, Ibu, and Nap. The adsorption data were fitted to Langmuir isotherm model with regression coefficient, R2, the value of 0.998, 0.977 and 0.998 for Dic, Ibu and Nap respectively, the maximum monolayer adsorption capacities were found to be 36.0, 33.9 and 33.9 mg/g for Dic, Ibu and Nap respectively. Moreover, the kinetic studies fitted well with the pseudo-second-order kinetic model. The sorption process described as spontaneous, endothermic and physical in nature. The results confirmed the efficiency and applicability of green-biosynthesized Cu NPs in the treatment of the aquatic systems contaminated with NSAIDs such as Ibuprofen, Naproxen, and Diclofenac.
Declarations
Author contribution statement
D. Z. Husein: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
R. Hassanien: Conceived and designed the experiments; contributed reagents, materials, analysis tools or data.
M. F. Al-Hakkani: Performed the experiments.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors present great thanks to New Valley University for supporting this work under its scientific projects and also thank Smart Pharma Company.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- 1.Guedidi H., Reinert L., Lévêque J.-M., Soneda Y., Bellakhal N., Duclaux L. The effects of the surface oxidation of activated carbon, the solution pH and the temperature on adsorption of ibuprofen. Carbon. 2013;54:432–443. [Google Scholar]
- 2.Önal Y., Akmil-Başar C., Sarıcı-Özdemir C. Elucidation of the naproxen sodium adsorption onto activated carbon prepared from waste apricot: kinetic, equilibrium and thermodynamic characterization. J. Hazard Mater. 2007;148(3):727–734. doi: 10.1016/j.jhazmat.2007.03.037. [DOI] [PubMed] [Google Scholar]
- 3.Kaur A., Umar A., Kansal S.K. Sunlight-driven photocatalytic degradation of non-steroidal anti-inflammatory drug based on TiO2 quantum dots. J. Colloid Interface Sci. 2015;459:257–263. doi: 10.1016/j.jcis.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 4.Manzo V., Honda L., Navarro O., Ascar L., Richter P. Microextraction of non-steroidal anti-inflammatory drugs from waste water samples by rotating-disk sorptive extraction. Talanta. 2014;128:486–492. doi: 10.1016/j.talanta.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Rodríguez-Álvarez T., Rodil R., Quintana J.B., Triñanes S., Cela R. Oxidation of non-steroidal anti-inflammatory drugs with aqueous permanganate. Water Res. 2013;47(9):3220. doi: 10.1016/j.watres.2013.03.034. [DOI] [PubMed] [Google Scholar]
- 6.Koumaki E., Mamais D., Noutsopoulos C. Environmental fate of non-steroidal anti-inflammatory drugs in river water/sediment systems. J. Hazard Mater. 2017;323:233–241. doi: 10.1016/j.jhazmat.2016.03.026. [DOI] [PubMed] [Google Scholar]
- 7.Noutsopoulos C., Koumaki E., Mamais D., Nika M.-C., Bletsou A.A., Thomaidis N.S. Removal of endocrine disruptors and non-steroidal anti-inflammatory drugs through wastewater chlorination: the effect of pH, total suspended solids and humic acids and identification of degradation by-products. Chemosphere. 2015;119:S109–S114. doi: 10.1016/j.chemosphere.2014.04.107. [DOI] [PubMed] [Google Scholar]
- 8.Binelli A., Magni S., Soave C., Marazzi F., Zuccato E., Castiglioni S., Parolini M., Mezzanotte V. The biofiltration process by the bivalve D. polymorpha for the removal of some pharmaceuticals and drugs of abuse from civil wastewaters. Ecol. Eng. 2014;71:710–721. [Google Scholar]
- 9.Liu Y.-J., Lo S.-L., Liou Y.-H., Hu C.-Y. Removal of nonsteroidal anti-inflammatory drugs (NSAIDs) by electrocoagulation–flotation with a cationic surfactant. Separ. Purif. Technol. 2015;152:148–154. [Google Scholar]
- 10.Feng L., van Hullebusch E.D., Rodrigo M.A., Esposito G., Oturan M.A. Removal of residual anti-inflammatory and analgesic pharmaceuticals from aqueous systems by electrochemical advanced oxidation processes. A review. Chem. Eng. J. 2013;228:944–964. [Google Scholar]
- 11.Suriyanon N., Permrungruang J., Kaosaiphun J., Wongrueng A., Ngamcharussrivichai C., Punyapalakul P. Selective adsorption mechanisms of antilipidemic and non-steroidal anti-inflammatory drug residues on functionalized silica-based porous materials in a mixed solute. Chemosphere. 2015;136:222–231. doi: 10.1016/j.chemosphere.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 12.Vona A., di Martino F., Garcia-Ivars J., Picó Y., Mendoza-Roca J.-A., Iborra-Clar M.-I. Comparison of different removal techniques for selected pharmaceuticals. J. Water Process Eng. 2015;5:48–57. [Google Scholar]
- 13.Hassanien R., Husein D.Z., Al-Hakkani M.F. Biosynthesis of copper nanoparticles using aqueous Tilia extract: antimicrobial and anticancer activities. Heliyon. 2018;4 doi: 10.1016/j.heliyon.2018.e01077. e01077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghaedi M., Ansari A., Habibi M., Asghari A. Removal of malachite green from aqueous solution by zinc oxide nanoparticle loaded on activated carbon: kinetics and isotherm study. J. Ind. Eng. Chem. 2014;20(1):17–28. [Google Scholar]
- 15.Husein D.Z. Adsorption and removal of mercury ions from aqueous solution using raw and chemically modified Egyptian Mandarin peel. Desalin. Water Treat. 2013;51(34-36):6761–6769. [Google Scholar]
- 16.Al-Khateeb L.A., Hakami W., Salam M.A. Removal of non-steroidal anti-inflammatory drugs from water using high surface area nanographene: kinetic and thermodynamic studies. J. Mol. Liq. 2017;241:733–741. [Google Scholar]
- 17.Ahmed M.J. Adsorption of non-steroidal anti-inflammatory drugs from aqueous solution using activated carbons. J. Environ. Manag. 2017;190:274–282. doi: 10.1016/j.jenvman.2016.12.073. [DOI] [PubMed] [Google Scholar]
- 18.Langmuir I. The constitution and fundamental properties of solids and liquids. Part I. Solids. J. Am. Chem. Soc. 1916;38(11):2221–2295. [Google Scholar]
- 19.Erhayem M., Al-Tohami F., Mohamed R., Ahmida K. Isotherm, kinetic and thermodynamic studies for the sorption of mercury (II) onto activated carbon from Rosmarinus officinalis leaves. Am. J. Anal. Chem. 2015;6(01):1. [Google Scholar]
- 20.Langmuir I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918;40(9):1361–1403. [Google Scholar]
- 21.Freundlich H. About adsorption in solutions. Z. Phys. Chem. 1907;57(1):385–470. [Google Scholar]
- 22.Jodeh S., Abdelwahab F., Jaradat N., Warad I., Jodeh W. Adsorption of diclofenac from aqueous solution using Cyclamen persicum tubers based activated carbon (CTAC) J. Assoc. Arab Univ. Basic Appl. Sci. 2016;20:32–38. [Google Scholar]
- 23.Dada A., Olalekan A., Olatunya A., Dada O. Langmuir, Freundlich, Temkin and Dubinin–Radushkevich isotherms studies of equilibrium sorption of Zn2+ unto phosphoric acid modified rice husk. IOSR -JAC. 2012;3(1):38–45. [Google Scholar]
- 24.Temkin M. Kinetics of ammonia synthesis on promoted iron catalysts. Acta Physicochim. URSS. 1940;12:327–356. [Google Scholar]
- 25.Ebrahimian A., Saberikhah E., Emami M., Sotudeh M. Study of biosorption parameters: isotherm, kinetics and thermodynamics of basic blue 9 biosorption onto foumanat tea waste. Cellul. Chem. Technol. 2014;48:735–743. [Google Scholar]
- 26.Dubey S.P., Dwivedi A.D., Sillanpää M., Gopal K. Artemisia vulgaris-derived mesoporous honeycomb-shaped activated carbon for ibuprofen adsorption. Chem. Eng. J. 2010;165(2):6761–6769. [Google Scholar]
- 27.Qurie M., Khamis M., Scrano L., Bufo S.A., Mecca G., Karaman R. Removal of two NSAIDs: naproxen and diclofenac and a heavy metal Cr (VI) by advanced membranes technology. Case Stud. J. 2015;4:51–63. [Google Scholar]
- 28.İlbay Z., Şahin S., Kerkez Ö., Bayazit Ş. Isolation of naproxen from wastewater using carbon-based magnetic adsorbents. Int. J. Environ. Sci. Technol. 2015;12(11):3541–3550. [Google Scholar]
- 29.Hu X., Cheng Z., Sun Z., Zhu H. Adsorption of diclofenac and triclosan in aqueous solution by purified multi-walled carbon nanotubes. Pol. J. Environ. Stud. 2017;26(1):87–95. [Google Scholar]
- 30.XIONG Z.-H., WANG L., ZHOU J.-G., LIU J.-M. Thermodynamics and kinetics of adsorption of diclofenac on magnetic multiwalled carbon nanotubes in an aqueous solution. Acta Phys. - Chim. Sin. 2010;26(11):2890–2898. [Google Scholar]
- 31.Khazri H., Ghorbel-Abid I., Kalfat R., Trabelsi-Ayadi M. Removal of ibuprofen, naproxen and carbamazepine in aqueous solution onto natural clay: equilibrium, kinetics, and thermodynamic study. Appl. Water Sci. 2017;7(6):3031–3040. [Google Scholar]
- 32.Mukoko T., Mupa M., Guyo U., Dziike F. Preparation of rice hull activated carbon for the removal of selected pharmaceutical waste compounds in hospital effluent. J. Environ. Anal. Toxicol. 2015 [Google Scholar]
- 33.Jung C., Boateng L.K., Flora J.R., Oh J., Braswell M.C., Son A., Yoon Y. Competitive adsorption of selected non-steroidal anti-inflammatory drugs on activated biochars: experimental and molecular modeling study. Chem. Eng. J. 2015;264:1–9. [Google Scholar]
- 34.Guedidi H., Reinert L., Soneda Y., Bellakhal N., Duclaux L. Adsorption of ibuprofen from aqueous solution on chemically surface-modified activated carbon cloths. Arab. J. Chem. 2017;10:S3584–S3594. [Google Scholar]
- 35.Sarıcı-Özdemir Ç., Önal Y., Erdoğan S., Akmil-Başar C. Studies on removal of naproxen sodium by adsorption onto ACF in batch and column. Fresenius Environ. Bull. 2012;21:1–6. [Google Scholar]
- 36.Bajpai M., Rai N., Bajpai S. Equilibrium adsorption studies on removal of diclofenac sodium from aqueous solution using sawdust–polyaniline (SD–PAn) composites. J. Appl. Polym. Sci. 2012;125(2):1382–1390. [Google Scholar]
- 37.Kaur M., Datta M. Diclofenac sodium adsorption onto montmorillonite: adsorption equilibrium studies and drug release kinetics. Adsorpt. Sci. Technol. 2014;32(5):365–387. [Google Scholar]
- 38.Lagergren S.K. About the theory of so-called adsorption of soluble substances. Sven. Vetenskapsakad. Handingarl. 1898;24:1–39. [Google Scholar]
- 39.McKay G., Ho Y. Pseudo-second order model for sorption processes. Process Biochem. 1999;34(5):451–465. [Google Scholar]
- 40.Weber W.J., Morris J.C. Kinetics of adsorption on carbon from solution. J. Sanit. Eng. Div. Asce. 1963;89(2):31–60. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.