Abstract
The application of nanotechnology to medicine promises a wide range of new tools and possibilities, from earlier diagnostics and improved imaging to better, more efficient, and more targeted therapies. This emerging field could help address obesity, with advances in drug delivery, nutraceuticals, and genetic and epigenetic therapeutics. Its application to obesity is still largely in the development phase. Here, we review the novel angle of nanotech applied to human consumable products, and their specific applications to addressing obesity through nutraceuticals, with respect to benefits and limitations of current nanotechnology methods. Further, we review potential future applications to deliver genetic and epigenetic-microRNA therapeutics. Finally, we discuss future directions, including theranostics, combinatory therapy, and personalized medicine.
Keywords: Diabetes, Obesity, Gene Therapy, Epigenetics, microRNA, Nanotechnology
Strategies to Fight Obesity
Obesity is an epidemic chronic disease affecting approximately 1 in 3 American adults and 1 in 5 American children [1]. Due to its related comorbidities—heart disease, diabetes, chronic liver disease, stroke, cancer, and Alzheimer’s disease—it incurs medical costs (in America ~$340 billion annually, or ~$3500 per person with obesity) [2] and lost workplace productivity due to factors like absenteeism (~$14 billion annually), disability, and premature mortality (~$30 billion annually) [3]. Nutritional quality is emphasized as a high priority goal to fight obesity [4]. Unfortunately, adherence to healthy eating has been low [5]. Innovative solutions to promote adherence to non-obesogenic dietary strategies are therefore needed.
There is also a need to identify pharmacological therapeutic approaches to treat or prevent obesity and metabolic syndrome. Another unexplored possibility lies in genetic therapeutics because there is a genetic component to these complex diseases [6]. A third and perhaps even more promising unexplored possibility is epigenetics, because population genetic changes alone cannot have caused the recent epidemic of metabolic syndrome [7]. Despite this potential, delivery of genetic and epigenetic therapies by traditional viral vector methods leaves them exposed to the body’s defense mechanisms which leads to diminished efficacy. There is a critical need for technology to encapsulate this material to protect it from degradation and uptake by the reticuloendothelial system in the circulation. These include physical methods that inject the genetic-epigenetic materials directly into the host cell (e.g., electroporation, gene guns) as well as chemical methods that surround the materials with protective molecules such as the nanotechnology methods discussed below (Box 1). These methods have successfully delivered DNA or small noncoding RNAs for treatment of cancer [8], hepatitis C [9], and skin or stomach wounds [10]. However, there is very little evidence for treating metabolic disease including obesity. In this review, we discuss progress toward the role of nanotechnology in this development and potential resolutions to the obesity epidemic.
Text Box #1: Nanotechnology and Chronic Diseases.
Nanotechnology has that potential to enhance current medicines and methods for preventing, controlling and mitigating diseases through drug delivery, sensors, and medical imaging because of its multifunctional aspects. Generally, biocompatible spherical nanomaterials act as carriers that can deliver the active agents located inside their hollow interiors with sensors on their surface which detect a diseased area or cell so that they release the active agents only at the targeted area for the therapeutic effect. In our opinion, this is one of the objectives when targeting fatty liver disease or fat tissues in obesity.
Nanotechnology
Nanotechnology shows one of the most promising functional avenues for the development of advanced drug and gene delivery vehicles in fighting this epidemic [11–15]. The first such function is that nanosized materials can encapsulate therapeutic and diagnostic moieties, enhancing their physiological stability from the body’s defense mechanisms. Compared to injected naked therapeutic agents, nanoparticle encapsulated ones have improved systemic half-life. Second, this encapsulation along with specific targeting efficacy enables therapeutic agents to accumulate into the local disease area, thus minimizing systemic toxicity. Otherwise these agents would be distributed into most body organs, requiring their significantly higher dosing that increases systemic toxicity. Third, nanotechnology-based biosensors or imaging devices may be able to isolate diseased areas from normal body conditions because their targeting efficacy can significantly accumulate them in the diseased area, leading to sensitivity of disease detection. Thus, nanotechnology may have strong potential to control diseases. Compared with cancer therapy-related nanotechnology, much less research has addressed anti-obesity nanotechnology. However, scientific interest in the latter has increased dramatically over the past decade as evidenced by the number of related publications annually. Numerous investigations have shown that the most beneficial application of nanotechnology is controlling both tissue and cell distribution profiles of drugs by their entrapment in nanoparticles [16–20]. The rationale behind this approach is to increase therapeutic efficacy while reducing systemic side-effects. We speculate that this can be used to our advantage to treat deregulated metabolic tissues.
Despite several advantages (Box 2), nanotechnology also has some limitations. To be effective in the ways just described, nanoparticles must be stable as an individual particle during storage and post-injection. However, due to their large surface-area-to-volume ratio, nanoparticles become settled and caked, alter size, and form clusters (i.e., aggregate themselves) [21]. Aggregation during systemic injection lessens the nanoparticle’s therapeutic advantage, while during storage limits shelf life, as nanoparticles settle and form cakes, rendering them ineffective. These stability issues require systemic toxic levels of excipients that synthesize or maintain the particles at the required size. Specifically, there remain some critical fundamental limitations of nanotechnology drug delivery systems despite their significant progress [22]. These include the inability to (a) precisely control shape, aspect ratios, size, and polydispersity of the nano-carriers and (b) integrate a variety of disease-specific triggered release mechanisms into the nanoparticle design. Overall, nanotechnology-driven drug and gene delivery systems have strong potential to control obesity effectively if researchers can overcome these limitations.
Text Box #2: Advantages of Nanotechnology.
The first advantage is increased dissolution rate. Most pharmaceutical entities are poorly water-soluble which limits their bioavailability and ability to reach therapeutic levels in the body. Drug designers have overcome this issue by decreasing the particle to nano-size (10 – 1,000 nm), increasing the surface area and surface curvature, thus reducing the width of the diffusion boundary layers and increasing the dissolution rate.
The second advantage of nanotechnology is improved solubility. For example, because Glimepiride (an antidiabetic agent) has low aqueous solubility (e.g., 0.3–6 μg/mL), it is typically co-administered with solubilizing agents that often cause severe side effects and drug fluctuations in the body {{6675 Seedher,N. 2008; 6674 Savjani,K.T. 2012; 6676 Taupitz,T. 2013}}. However, encapsulating Glimepiride in nanocapsules enhances its aqueous solubility by several orders of magnitude (e.g., ~2 mg/mL) without the toxic side effects of solubilizing agents and confers improved pharmacokinetic stability by protecting glimepiride from degrading {{6677 Du,B. 2013; 6678 Ahmed,O.A. 2016; 6679 Li,H. 2016; 6680 Thapa, R.J. 2017}}.
The third advantage of nanotechnology is the protection of agents against the host’s immune system by ‘PEGylation’ [92]. This protection prolongs their time in circulation by reducing renal clearance in the body. Thus, the agent is better able to permeate and be retained in body tissue, improving the ability to provide the drug to the disease area and increase its therapeutic potency. However, PEGylation is not the only protection against host immunity in nanotechnology. Several studies are ongoing to prevent immunogenicity of nanoparticles including hybridizing albumin with the nanoparticle [93], delivering cytokines or proteins that neutralize host immunogenicity [94], and manipulating agent geometry using the nanoparticle [95]. However, they were not approved by FDA, which prevents us to use them for clinical trials.
A fourth advantage is safety because nanotechnology can mitigate the initial rise in drug concentration for controlled and sustained drug release and reduce dose variability by controlling nanoparticle composition, types of nanoparticles, and formulation methods.
Finally, the fifth advantage of nanotechnology is specific organ and disease targeting capacity. Targeted drug delivery by a nanoparticle provides significant local levels of therapeutic compounds with minimum systemic levels. Thus, only a low dose of the drug is required to achieve its therapeutic efficacy. This is one of the primary characteristics required to treat non- alcoholic fatty liver which is one of the major dysregulations in obesity.
Specific Methods
Several current nanoparticle formulation methods are known to achieve the advantages described above. Therefore, given that nanotech delivery of genetic and epigenetic drugs for obesity is a new concept, traditional nanotechnology methods should be the first steps to apply toward obesity prevention/treatment (Box 3).
Text Box #3: Nanotechnology Methods.
Nanotechnology methods fall into two categories: attrition and precipitation [96–100]. Attrition refers to breaking large particles into nanoparticles by physical means such as a high-power air blow or ultrasound while precipitation grows small particles from solutions until they reach the desired size. Besides its relative simplicity, precipitation is advantageous because it can achieve particle sizes <100nm through the choice of materials. Better growth rate control can be achieved using hydrophobic polymers, or hydrophobic-hydrophilic copolymer compounds that do not crystallize easily.
Since control of growth rate is paramount for effective drug delivery nanoparticles, which must be highly stable at small sizes (<50nm), it is best to use hydrophobic compounds or polymers that self-assemble and thus regulate the particle’s growth rate by thermodynamics. To initiate this self-assembly, compounds or polymers are dissolved together with the agent they are intended to carry in organic solvent then precipitated into water. The resulting molecule is called an emulsion. Besides their advantageous stability at small sizes, emulsions are easily and cheaply fabricated, and furthermore can be specifically engineered to deliver higher concentrations of pharmaceutical agents to desired locations. For these reasons, emulsions are ideal candidates for therapeutic agents against a specific disease such as obesity.
There are three specific types of emulsions: nanoemulsions (colloidal particles that are either an oil-in-water (O/W) or water-in-oil (W/O) dispersion, with emulsifying agents as surfactants to provide thermodynamic stability [101, 102]), liposomes (have a lipid bilayer [103, 104] and they can encapsulate either hydrophilic (e.g. RNA, hydrophobic agents, or both simultaneously), and micelles (composed of hydrophilic polar regions and hydrophobic inner regions).
Both nanoemulsions and liposomes use lipids originating from one of the cell membrane components, and both formulations tend to fuse with cells indiscriminately during systemic circulation [105, 106]. This non-specificity is sometimes not optimal for systemic application but can be overcome by attaching poly(ethylene glycol) (PEG) to their surface which induces a “stealth” property with minimal or no uptake by the reticuloendothelial system [107–109]. For example, a block copolymer of poly(Lactide-co-glycolide) (PLGA)-PEG tends to form a micelle but requires more chemical synthesis and purification [110, 111]. Micelles generally are optimal for the encapsulation of hydrophobic agents, their stability is better than nanoemulsions and liposomes, and the formulation process is also simpler. Encapsulation of hydrophilic agents (e.g. DNA, RNA) within a micelle requires additional pre-formulation, of an ionic complex between RNA and a cationic polymer that must be accomplished before or during the formulation of the micelle [112, 113].
Nanotechnology Applications to Obesity
Pharmaceuticals
Nanotechnology is much more advanced in cancer prevention than obesity and diabetes due to several possible reasons: infancy of biomarker discovery, multiplicity of pathways, requirement of sophisticated control of drug release, and difficulty of targeting access. Nanotech vehicles can deliver cytotoxic agents to cancerous cells at lower doses and greater bioavailability than chemotherapy. They can furthermore deliver diagnostic antibodies to the surface of individual cancer cells using distinctive enhanced permeability and retention effect-mediated tumor access, allowing for greater diagnostic sensitivity and specificity than gross tumor imaging [23–25]. On the other hand, one of the biggest challenges in obesity is the specific delivery of the drugs, genetic or epigenetic material to the targeted organ including adipose tissues. Recent evidence shows some potential where nanoparticles were used to: a) target the angiogenic vessels in adipose tissues [26]; b) coated with a peptide (CKGGRAKDC) to target white adipose tissue [27]; or c) coated with peptide (KGGRAKD) to target the adipose vascular marker prohibitin [28]. A PubMed search for terms related to nanotechnology and cancer revealed 43,690 articles but for obesity <1% of this total.
Nonetheless, the advantages of liposomes, micelles, and nanoemulsions can be exploited to deliver drugs addressing obesity and its metabolic syndrome consequences [11–15]. For example, the fat absorption inhibitor Orlistat blocks intestinal lipase but has very limited bioavailability since it is poorly water soluble. Nanoemulsions are hydrophilic but can encapsulate lipophilic agents thus making them more water soluble and have been successfully applied in vivo with orlistat [29]. Regarding metabolic syndrome consequences, toward treatment of type 2 diabetes, researchers have successfully delivered insulin orally (rather than the traditional subcutaneous pathway) by encapsulating it with liposomes. Since liposomes tend to fuse with any cells in the systemic circulation, the liposome-insulin complex fuses with cellular membranes and penetrates them more efficiently than insulin would by itself. This increases the biological action of insulin without the need for subcutaneous injection [11]. As a second example, toward treatment of hypertension, researchers have encapsulated the hydrophobic drugs candesartan cilexetil (an angiotensin II receptor blocker) and nimodipine (a calcium channel blocker) in micelles, which increased drug loading capacity and drug release, presumably due to increased aqueous solubility [12]. Additionally, the lipophilic anti-hypertensive drug Olmesartan medoxomil (an angiotensin II receptor blocker) has been encapsulated in oil-in-water nanoemulsions, which increased its thermodynamic stability and pharmacokinetic activity [30]. In summary, the advantages of nanotechnology methods discussed in the previous section including increased bioavailability, dissolution rate, control over drug release rate, and thermodynamic stability can enhance pharmacotherapies for metabolic syndrome.
Nutraceuticals
Nanotechnology holds several potential critical applications in the medical field. However, another field which can benefit is nutraceuticals. A nutraceutical is defined as a food or part of it that provides the body with medical or health benefits, including the prevention and treatment of a disease. This is an important area to explore in obesity prevention as nutrition plays a prominent role in this epidemic. The ability of nanoemulsions and micelles to increase the dissolution rate and aqueous solubility of bioactive molecules is therefore advantageous to delivery of not only drugs but also nutraceuticals, to increase diet quality. Many nutraceuticals, such as vitamins, minerals, antioxidants, fats, and proteins, are currently in common edible ingredients such as cooking oil. Some of them are frequently poorly absorbed during digestion, however, due to being more fat soluble than water soluble, thus hindering dietary benefit for the consumer [31]. These nutraceuticals with high fat solubility cannot be digested until fat has been digested, and during this delay are subject to degradation. Thus, nanoemulsions and micelles, which successfully increase the water solubility of nutraceuticals, increase the bioavailability of those nutraceuticals and absorbed nutritional quality.
Recent reviews [32] including two comprehensive lists of existing patents [33, 34], have listed foods that address this purpose of improving nutrient delivery through nanotechnology-enhanced water solubility. As discussed earlier in this review, the choice of encapsulating agent determines the type of nanoparticle formed. “Dried Formulations” [35] uses solubilizing agents such as polyoxyethanyl tocopheryl sebecate (PTS™) to form micelles around fat-soluble nutrients like coenzyme Q10 (CoQ10), omega fatty acids, vitamin B6, and resveratrol, thus increasing their water solubility and improving delivery. Resveratrol delivery can be further improved by solid lipid nanoparticles and nanostructured lipid carriers that increase its intestinal permeability [36]. Another patent “Nanosized Self-Assembled Liquid Dilutable Vehicles” [37] describes a multi-phase nanoemulsion including both aqueous and oil phases which the inventors claim increases water solubility 7–20 times above single-phase nanoemulsions. These techniques have applied the nanoencapsulation self-assembly procedures, due to their advantages of lower cost and better specificity of sizing and location of particle delivery compared to the attrition or precipitation techniques. Overall, the increased bioavailability of healthy nutrients using micelles and nanoemulsions addresses the problem of metabolic syndrome by improving dietary quality and thus reducing the caloric intake required to obtain adequate nutrition.
Furthermore, these nanoscale food processing techniques address the metabolic syndrome not only nutritionally but also behaviorally by improving the palatability of healthy foods through their flavor and texture. The Slim Shake Chocolate by Nanoceuticals™ (http://www.nanotechproject.org/cpi/products/nanoceuticalstm-slim-shake-chocolate/) adds pure Cocoa to its nanoencapsulations to enhance flavor without having to add pure sugar. Similarly, Nestle and Unilever used nanoemulsions to create ice cream with 1% instead of 16% fat content without compromising taste [38]. In sum, nanoencapsulation self-assembly techniques have the potential to increase not only the benefits of eating nutrient-rich foods but also the likelihood that people will prefer them to less nutritious and otherwise more palatable foods.
Another less explored nanotech approach to preventive nutrition for metabolic syndrome that may warrant further consideration is dietary supplementation with silver (Ag) nanoparticles. These are widely used in food storage, environmental sterilization, and animal husbandry due to antimicrobial properties. However, in the latter case a recent study reported they also enhanced muscle growth of the animals (poultry), along with decreased plasma cholesterol, triglyceride, and glucose but no change in fat growth [39]. These results were attributed to the Ag nanoparticles’ anti-inflammatory actions [40], digestion-stimulation [41], and promotion of growth factor transcription (IGF1, Glut1, Glut3). Similarly favorable metabolic changes from Ag nanoparticles were recently observed in vivo in a mouse model [42]. At the same time, other studies found dietary supplementation with Ag nanoparticles did not influence livestock growth [43–46]. In our opinion, more research is warranted and it is possible that Ag nanoparticle size, dose, exposure time, and/or preparation method may relate to these discrepancies.
In spite of the potential advantages, nanoscale food processing also poses potential health hazards that could cause adverse responses regarding metabolic syndrome phenotypes. The primary health concern we predict is the lack of knowledge about how the human body digests and absorbs these ingested nanoparticles. Molecular properties, such as concentration, particle size distribution, and electrical charge, are different on a nanoscale compared to traditional microscale nutraceuticals, which can affect digestion and absorption of the nanosized materials. This difference is concerning because some nanosized materials contain digestible obesogenic molecules (e.g., lipids in liposomes, carbohydrates in lipopolysaccharides). However, lower-energy nanoemulsions can be formed if co-solvents (e.g., propylene glycol) or co-surfactants (e.g., short and medium-chain alcohols) are added [32].
Another concern is that nanoemulsions also contain non-digestible inorganic nanoparticles including minerals and metals, which may influence biological pathways that contribute to metabolic syndrome such as inflammation. As recently reviewed by Siddiqi et al. [47], silver nanoparticles generate free radicals and reactive oxygen species that inhibit microbial growth. They can also block dietary supplements from otherwise scavenging these radicals [48]. It is unknown whether these free radicals could adversely affect human cells by reducing ATP content, increasing reactive oxygen species production, and/or damaging mitochondria and DNA. Such disruption of cellular signalling could exacerbate metabolic syndrome, if these free radicals and reactive oxygen species increased the inflammation that contributes to obesity-induced insulin resistance. These concerns are mitigated by animal models suggesting that silver nanoparticles undergo fecal elimination before accumulating in toxic doses [49].
Despite the growing number of patents supporting the benchtop efficacy of nanoscale food processing and the potential benefits of this technology as lifestyle therapy for metabolic syndrome, however, no human clinical trials exist to verify whether potential benefits outweigh risks. Nonetheless, these safety issues must be weighed thoughtfully when evaluating the risk-to-benefit ratio of using nanocarriers to deliver obesity therapeutics. Unlike malignant cancers more commonly treated by nanotechnology, obesity is a non-malignant condition and so the benefits of treating it do not necessarily outweigh the safety risks.
At the same time, it is important to recognize that nanocarriers can also improve the safety of both drugs and nutraceuticals by lowering their systemic toxicity through two mechanisms: 1) increased targeting efficacy, and 2) decreased drug carrier toxicity. Targeting efficacy of nano-encapsulated therapeutics can be enhanced by the choice of disease specific ligands coated on the nanoparticle. Additionally, the toxicity of the drug carrier is totally dependent on the nanoparticle toxicity. Currently, there are two FDA approved biomaterials, poly (lactic-co-glycolic acid) (PLGA) and polyethylene glycol (PEG). Nanoparticles composed of PLGA or PEG or a combination of both could minimize the systemic toxicity.
Besides safety considerations, we must consider whether nanocarriers can feasibly deliver drugs and nutraceuticals to have positive impact on these phenotypes. In particular, obesity and metabolic syndrome are chronic diseases that may require continuous delivery of therapeutics, in contrast to acute conditions such as cancer that can resolve with several courses of acute therapy. To determine if nanocarriers can address this challenge, their maximum tolerated dose must be determined because it guides the doses and even frequency of new therapeutics. Compared with naked therapeutics, the maximum tolerated dose of nano-encapsulated therapeutics is generally higher because the nanoparticle can protect the encapsulated therapeutics during systemic injection. It can consequently delay the release of therapeutics from nanoparticles, in turn decreasing the dosing frequency required to achieve continuous delivery. Thus, nanocarrier applications could achieve feasible effects in obesity and metabolic diseases.
Genetic Therapeutics
Beyond these applications of nanotechnology to deliver drugs and food, nanotechnology also bears the potential to deliver genetic material [14]. Nanotech methods can be used to encapsulate DNA so that it can be therapeutically delivered, without exposure to the host’s immune system as we see with traditional viral gene delivery. Nanotech delivery also averts the risk of recombination between the therapeutic gene and the vector, as can occur with viral gene delivery. It also confers other advantages over traditional viral gene delivery including unlimited length of the therapeutic DNA sequence, as well as increased target specificity and high delivery efficacy [14] just as we see with nanotech-delivered drugs and nutraceuticals.
As with drug delivery, DNA delivery by nanotech has been studied more extensively as therapy for cancer [14] than obesity or metabolic syndrome. Nonetheless, the approach bears future potential for obesity. Recently, Park et al. used nanoparticles to deliver an anti-obesity gene construct (pDNA) for anti-obesity in diet-induced obese mice [50].
By working at the genetic level, it is possible to impact multiple pathways, a concept known as synthetic biology, meaning the manipulation of an entire biological system as opposed to a single biological pathway. This approach could be particularly beneficial in the context of metabolic syndrome which is characterized by multiple abnormalities including obesity, hypertension, insulin resistance, and dyslipidemia that do not necessarily arise from a single underlying causal etiology [51]. This clustering of multiple abnormalities in patients with metabolic syndrome thwarts many common pharmaceutical therapies that only target one of them, for example antihypertensive drugs that lower blood pressure but do not impact lipid profile or insulin sensitivity.
However, recent experiments in mice support the possibility of gene therapy solutions to address multiple metabolic abnormalities concurrently. Ye et al. [52] demonstrated that by pharmacologically inducing cyclic adenosine monophosphate (cAMP) dependent signalling, they increased genetic expression of GLP-1-FcmIgG-Leptin, a polypeptide containing glucagon-like peptide 1 (GLP-1) and leptin. GLP-1 stimulates insulin secretion and leptin signals satiety, thus impacting insulin resistance and obesity respectively simultaneously. Thus, manipulation of GLP-1-FcmIgG-Leptin expression at the genetic level represents a potential future strategy to treat the metabolic syndrome at the biological system level, rather than treating a single component of the metabolic syndrome (e.g., insulin resistance) at the single biological pathway level. If future studies could use nanotech methods to deliver a grafted DNA sequence that increases GLP-1-FcmIgG-Leptin expression, this work may lead to the revolution of future genetic nanotech pharmacotherapies for metabolic syndrome.
Even such target genes that influence multiple metabolic syndrome components, however, are limited by their open-loop mechanism, meaning that no counter-regulatory mechanism exists to preserve homeostasis. Synthetic biology can address this limitation as well, however, by creating a closed-loop system in which auto-regulatory feedback controls metabolic abnormalities. For instance, Ye et al. [53] treated mice with obesity-induced insulin resistance by implanting a synthetic sensor-effector device capable of sensing heightened insulin levels and accordingly release the insulin-sensitizing hormone adiponectin to restore insulin sensitivity. The device worked by 1) adjusting mitogen-activated protein kinase (MAPK) signaling such that heightened insulin levels caused MAPK to activate a transcription factor (tetracycline repressor fused to ELK-1 derived transactivation domain, TetR-ELK1); and then 2) creating synthetic promoters such that TetR-ELK1 modulated gene expression to stimulate appropriate adiponectin release thus restoring insulin sensitivity, completing the closed-loop mechanism. Such a closed-loop glycemic regulatory system has also been achieved for human cells in vitro, by coupling glycolysis-mediated calcium entry to GLP-1 transcription, such that heightened glucose levels stimulated increased glucose uptake (i.e., “mimetic β-cells”) [54].
Closed-loop genetic therapies have also been studied for regulation of satiety, lipid levels, and blood pressure in the presence of overfeeding and/or hyperlipidemia. Rossger et al. [55] treated mice via implanting cells containing the lipid receptor peroxisome proliferator-activated receptor-α (PPAR-α) grafted to bacterial DNA-binding repressor TtgR that stimulates expression of the satiety hormone pramlintide. This approach led to these cells functioning as lipid-sensing receptors causing the mice to experience increased satiety in the presence of hyperlipidemia, yielding overall reductions in caloric intake and lipid levels. In a separate study, Rossger et al. [56] genetically rewired the dopaminergic system such that the presence of food stimulus triggered the release of atrial natriuretic peptide, thus regulating blood pressure in hypertensive mice. In summary, researchers have developed genetic therapeutics that each address multiple metabolic syndrome components including obesity [52, 55], insulin resistance [52, 53], dyslipidemia, and hypertension [55]. If the synthetic genes for these methods could be delivered using nanotech methods, the advantages of nanotech could be harnessed including increased target specificity and high delivery efficacy and furthermore eliminate the need for viral DNA vectors that can recombine with the therapeutic gene. For example, an oligopeptide complex for targeted non-viral gene delivery to adipocytes showed regression of obesity and obesity-induced insulin resistance [57]. A polyionic complex containing a gene system engineered to promote energy expenditure through the conversion of white adipocytes into beige/brite adipocytes (i.e., browning) demonstrated similar results [58].
Traditional viral gene delivery also risks the possibility of recombination between the therapeutic gene and the viral vector, which is not a possibility with nanotech delivery. Nanotech gene delivery also confers other advantages over traditional viral gene delivery including delivery of unlimited length of DNA sequence, as well as increased target specificity and high delivery efficacy [14] just as we see with nanotech-delivered drugs and nutraceuticals.
Epigenetic and microRNA Therapeutics
Epigenetics, the interaction between genes and the environment is perhaps an even more promising unexplored possibility for obesity therapeutics than genetics because epigenetic changes happen before genetic changes, and they are reversible [59, 60]. Our previous review covers epigenetic modifying drugs that address obesity and metabolic syndrome by targeting histone acetylation, DNA methylation, and sirtuin activation [59]. In the present review we turn this discussion to the potential for small non-coding RNAs (another subset of the epigenetics concept) to be added to this list of therapeutic targets, since nanotech vehicles may turn out to be especially vital to this process.
Beyond the potential for delivery of DNA nanoemulsions, liposomes, and micelles have the potential to deliver RNA (also a hydrophilic agent). This is an important potential application of these nanotechnology vehicles because small non-coding RNAs bear strong therapeutic potential for metabolic syndrome, but delivery remains a major challenge. This section will overview the therapeutic potential of small non-coding RNAs, challenges that preclude using them as therapies for metabolic syndrome in humans, and potential nanotechnology solutions to these challenges.
The type of small non-coding RNAs most explored from a therapeutic perspective are micro RNAs (miRNAs): short (~22 nucleotides), single-stranded non-coding gene products which regulate gene expression. MiRNAs contribute to many essential biological processes like development, energy and lipid metabolism, insulin secretion, adipocyte differentiation, and arterial blood pressure regulation [61, 62]. They are aberrantly expressed in pathophysiological conditions like obesity, diabetes, and heart disease and can act as biomarkers for the diseased state [61, 62]. Thousands of unique miRNAs exist, and each miRNA can target multiple genes, and each gene can influence multiple metabolic pathways, suggesting the strong prognostic and therapeutic importance of miRNAs for human diseases.
Chakraborty et al. [63] conducted a systematic review and identified over 1,000 patents related to therapeutic use of miRNA. These technology developers are following the standard drug development process: discovery research, pharmacokinetics/pharmacodynamics testing, and large animal efficacy studies before moving to human clinical trials. The most popular molecular approach has been the construction of antisense oligonucleotides (anti-miRs) that bind specific miRNA transcripts, thus preventing those miRNA transcripts from regulating gene expression and achieving therapeutic impact on biological pathways involving those genes. Since miRNAs are short (21 base pairs) and highly conserved across species, anti-miRs have the potential to achieve much higher specificity than drugs that target DNA whose target sequence is likely to be longer and more variable between individuals.
Among all the miRNA patents Chakraborty et al. [63] identified, the disease most widely targeted is cancer. The single furthest developed therapeutic anti-miR to date, however, is anti-miR-122 which targets hepatitis C virus (HCV) and has reached stage 2 human clinical trials. Patients with HCV receiving anti-miR-122 injections for one month achieved prolonged dose-dependent reductions in both HCV RNA and miR-122 over four months [64]. The investigators also analyzed a panel of 179 other miRNA transcripts found in plasma for possible off-target pleiotropic side effects [65]. Although two non-targeted transcripts (miR-210 and miR-532–5p) changed acutely following the month of anti-miR-122 injections, the changes were much lower magnitude and much less prolonged in duration than the changes in the target transcript miR-122 [65]. These findings are encouraging regarding potential use of miRNA for obesity however cautions about side effects on other miRNAs.
The question is whether science can apply this therapeutic potential of anti-miRs exemplified by anti-miR-122 toward treatment for obesity and metabolic syndrome in humans, at which point these anti-miRs could be delivered via liposomes. There are several miRNAs that are: (1) dysregulated during obesity in vivo within adipocytes or other tissues (hepatocytes, pancreatic islets, hypothalamus, or circulating fluids); (2) associated with insulin resistance within adipocytes, skeletal muscle, and/or liver; and/or (3) associated with other aspects of obesity progression potentially influencing insulin resistance within adipocytes (adipogenesis, lipid metabolism, inflammation) or liver (lipid metabolism, steatosis).
These miRNAs represent promising therapeutic targets for new anti-miR drug technology that could address metabolic syndrome, but the standard concerns of drug discovery must be addressed, including both pharmacokinetics/ pharmacodynamics issues (tissue permeability, stability, target miRNA binding affinity) and off-target toxicity issues. A single miRNA can regulate genes in multiple biological pathways so even an anti-miR with high binding specificity to its target miRNA may induce unwanted pleiotropic side effects. Another concern is whether anti-miRs can achieve enough tissue permeability and in vivo stability to functionally block their target miRNA as intended.
Traditional strategies to alter the pharmacokinetics and pharmacodynamics of anti-MiRs include chemical modification of the sugar-phosphate backbone or the nucleotides themselves [66]. However, these concerns may be better addressed by nanotechnology. Encapsulation of an miRNA or anti-miR into a nanoparticle (e.g. liposome) can protect them from instability and their off-target tissues [67]. For example, nanographene oxides have been used to deliver let-7g mimics [68], as well as anti-miR-21 [69] to cancer cells.
Among all anti-miRs manufactured to date that have achieved enough stability in vivo to impact health outcomes among large animal models, the one that is pertinent to metabolic syndrome is 2’-fluoro/methoxyethyl modified anti-miR-33 (manufactured by Regulus Therapeutics, La Jolla, CA). Metabolic syndrome associates with lower plasma high-density lipoprotein cholesterol (HDL-C) and impaired reverse cholesterol efflux, leading to atherosclerotic plaque build-up. Studies in mice, as well as human cells in vitro, found miR-33 regulates this process by targeting genes involved in HDL-C biogenesis and/or cholesterol efflux (ABCA1, ABCG1) [70–74]. Whereas, administration of anti-miR-33 increases HDL-C and cholesterol efflux [70–72] while reducing atherosclerotic plaque [75, 76].
Therefore, Rayner et al. [77] extended this testing to primates, comparing changes in cholesterol profile between six African green monkeys receiving anti-miR-33 versus six receiving a placebo mismatched anti-miR over 12 weeks. Like the mice, monkeys receiving anti-miR-33 increased hepatic ABCA1 as well as plasma HDL-C indicating reduced susceptibility to metabolic syndrome [78]. However, the investigators expected more pleiotropic effects of the drug among monkeys than mice because while both species express miR-33a only monkeys not mice express the sibling transcript miR-33b. MiR-33b originates from the same seed sequence as miR-33a but differs by two nucleotides. Therefore, the investigators also examined whether anti-miR-33 influenced target genes of miR-33b including those related to fatty acid oxidation (CROT, CPT1A, HADHB and PRKAA1) and synthesis (SREBF1, FASN, ACLY and ACACA). Notably, the former increased expression whereas the latter decreased expression, resulting in lower plasma very low-density lipoprotein (VLDL)-associated triglycerides. In summary, the benefits of anti-MiR-33 for metabolic syndrome risk were enhanced even more among large versus small animals. These benefits could be reaped to a greater degree using delivery of miR-33b via liposomes to increase stability and target gene specificity in humans.
At the same time, caution is warranted because anit-miR-33 and/or genetic ablation of miR-33 in mice has led to detrimental effects over long-term experiments (~12 weeks) related to metabolic syndrome including hypertriglyceridemia, hepatic steatosis, body weight gain, and insulin resistance [79–83]. Thus, efficacy trials over a longer portion of lifespan in non-human primates are required before moving to human clinical trials, as is improved target gene specificity that could be achieved using liposome delivery vehicles (Figure 1).
Figure 1. Proposed Steps of Delivering Nano-encapsulated Anti-miR-33 Targeting Liver.
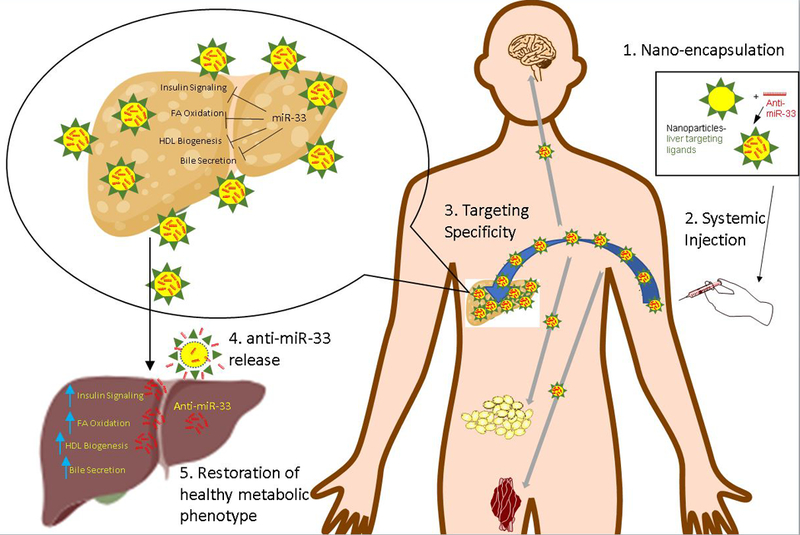
Nano encapsulated anti-miR-33 rescues the fatty liver when delivered to the body. Increased miR-33 inhibits expression of genes in the liver which would otherwise stimulate insulin signaling, FA oxidation, HDL biogenesis, and bile secretion. Anti-miR-33 reverses the adverse metabolic effect and rescues the fatty liver and its downstream effect. Due to the liver specific coating, a significant portion would go to the liver and very little to other organs. FA-fatty acid. HDL-high density lipoprotein. WAT-white adipose tissue.
A search of clinicaltrials.gov for “microRNA AND (obesity OR dyslipidemia OR hypertension OR diabetes)” revealed 112 trials but none pertained to human trials of miRNA-targeting drugs. All pertained to miRNAs as disease biomarkers or biomarkers assessed for change in response to a standard clinical intervention for metabolic syndrome either pharmacological (i.e., oral antihyperglycemic for diabetes) or behavioral lifestyle (i.e., diet or exercise). Progression toward FDA-approved nanotechnology for metabolic syndrome utilizing anti-miRs appears slow, likely due to not only the safety concerns but also the stability challenges described above. Thus, the new nanotech approach of encapsulation with liposomes to enhance stability holds promise to aid this process. As proof of this concept, a mimic of the oncogene inhibitor miR-34 has been successfully encapsulated in liposomes and injected into human cancer patients (MRX34, Regulus Therapeutics) [84], although the phase 1 clinical trial of this agent was terminated early due to immune-related serious adverse events (https://clinicaltrials.gov/ct2/show/NCT01829971).
It is also noteworthy that beyond miRNAs, there are other small non-coding RNAs including short-hairpin RNAs (shRNAs) and small inhibitory RNAs (siRNAs) that are also hydrophilic and can be carried by liposomes [85]. Supporting the possibility that these other small non-coding RNAs could be therapeutic for obesity, Won et al. [57] designed an shRNA that successfully silenced the fatty-acid-binding 4 gene (shFABP4) that otherwise facilitates the storage of lipid droplets in white adipocytes contributing to fat accumulation, which led to weight loss in obese mice. However, this shRNA has not been tested in larger mammals so, compared to miRNA therapies for obesity, the challenges in translation that will arise and the specific role that nanotechnology could play is less well known.
Concluding Remarks and Future Perspectives
Nanotechnology has shown promising efficacy in animal models to achieve more effective in vitro delivery of drugs addressing metabolic syndrome complications of obesity including type 2 diabetes and hypertension. It can achieve nutraceutical delivery in humans to increase diet quality and the techniques could be harnessed to deliver genetic and epigenetic therapeutics for obesity and metabolic syndrome.
In summary, we suggest several future directions (Figure 2, Key Figure). The first direction is “theranostics” that combine therapy and diagnosis within a nanoparticle. Thus, the diagnostic and therapeutic agents are encapsulated and delivered to the disease area. This leads to guided delivery that can identify the disease targeting area and also can track the progress of therapy. For example, magnetoliposomes loaded with polyunsaturated fatty acids have been demonstrated to both detect and attenuate inflammatory biomarkers in mice [86]. Along similar lines, it may be possible, for example, to encapsulate anti-miR-33 which can treat dyslipidemia, into a liposome also containing a detection agent for dyslipidemia.
Figure 2 (Key Figure). Nanotechnology Approach to Obesity.
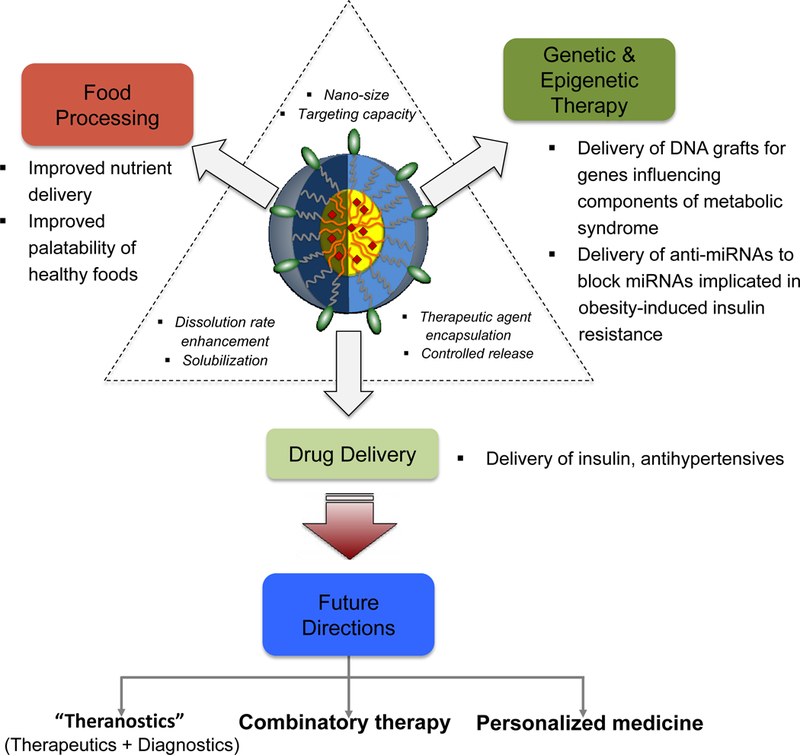
Schematic diagram for control of obesity by the unique properties of nanoparticles such as targeting capacity, dissolution rate enhancement, and efficient encapsulation of various types of agents. Due to these properties, the nanoparticle can be applicable for food processing, genetic & epigenetic therapeutics, and drug delivery. Future applications include theranostics, combinatory therapy, and personalized medicine.
Next, we suggest combinatory therapy: the delivery of both drugs and genes in the same nanotech delivery vehicle. This combinatory therapy could result in a synergistic effect that will yield benefits including enhanced therapeutic effects, diminished dose, and reduced side toxicity. For example, a single liposome could be used to encapsulate both insulin and synthetic DNA altering PPAR-γ expression to increase insulin sensitivity.
The final suggested direction is personalized medicine. Researchers and clinicians could tailor the targeted delivery of genetic and epigenetic therapeutics by nanotech vehicles to the genomic and epigenomic profile of the individual. For instance, anti-miR-33 may be more effective for individuals with higher levels of miR-33 and/or polymorphisms of its target gene ABCA1 that increase binding with miR-33, whereas individuals with lower levels of miR-33 and/or not carrying those polymorphisms may benefit from different therapies. As science gains further knowledge of genomic variation and function pertinent to metabolic syndrome, more such possibilities will emerge.
In conclusion, we propose that the novel idea of nanotherapeutics in obesity could benefit a broad spectrum of research in the near future (see Outstanding Questions) aiming to prevent obesity and its comorbidities.
Acknowledgements
GIA was supported during writing by NIH/NIDDK (T32DK097718). MC is supported by Morris L Lichtenstein Jr Medical Research Foundation.
References
- 1.Benjamin EJ et al. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. (2018) Circulation 137, e67–e492 [DOI] [PubMed] [Google Scholar]
- 2.Cawley J and Meyerhoefer C The medical care costs of obesity: an instrumental variables approach. (2012) J Health Econ 31, 219–230 [DOI] [PubMed] [Google Scholar]
- 3.Lehnert T et al. Economic costs of overweight and obesity. (2013) Best Pract Res Clin Endocrinol Metab 27, 105–115 [DOI] [PubMed] [Google Scholar]
- 4.United Nations Department of Economic and Social Affairs. Global Sustainable Development Report 2016, https://sustainabledevelopment.un.org/index.php?page=view&type=400&nr=2328&menu=1515 (2016).
- 5.Rehm CD et al. Dietary Intake Among US Adults, 1999–2012. (2016) JAMA 315, 2542–2553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ingelsson E and McCarthy MI Human Genetics of Obesity and Type 2 Diabetes Mellitus: Past, Present, and Future. (2018) Circ Genom Precis Med 11, e002090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Speakman JR Evolutionary perspectives on the obesity epidemic: adaptive, maladaptive, and neutral viewpoints. (2013) Annu Rev Nutr 33, 289–317 [DOI] [PubMed] [Google Scholar]
- 8.Zhou Z et al. Nonviral cancer gene therapy: Delivery cascade and vector nanoproperty integration. (2017) Adv Drug Deliv Rev 115, 115–154 [DOI] [PubMed] [Google Scholar]
- 9.Weiland O et al. Therapeutic DNA vaccination using in vivo electroporation followed by standard of care therapy in patients with genotype 1 chronic hepatitis C. (2013) Mol Ther 21, 1796–1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu P et al. Non-viral gene delivery systems for tissue repair and regeneration. (2018) J Transl Med 16, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kesharwani P et al. Nanotechnology based approaches for anti-diabetic drugs delivery. (2018) Diabetes Res Clin Pract 136, 52–77 [DOI] [PubMed] [Google Scholar]
- 12.Sharma M, Sharma R and Jain DK Nanotechnology Based Approaches for Enhancing Oral Bioavailability of Poorly Water Soluble Antihypertensive Drugs. (2016) Scientifica (Cairo) 2016, 8525679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Y et al. Nanomedicine for obesity treatment. (2018) Sci China Life Sci 61, 373–379 [DOI] [PubMed] [Google Scholar]
- 14.Prasad M et al. Nanotherapeutics: An insight into healthcare and multi-dimensional applications in medical sector of the modern world. (2018) Biomed Pharmacother 97, 1521–1537 [DOI] [PubMed] [Google Scholar]
- 15.Martin Gimenez VM, Kassuha DE and Manucha W Nanomedicine applied to cardiovascular diseases: latest developments. (2017) Ther Adv Cardiovasc Dis 11, 133–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sridhar R et al. Electrosprayed nanoparticles and electrospun nanofibers based on natural materials: applications in tissue regeneration, drug delivery and pharmaceuticals. (2015) Chem Soc Rev 44, 790–814 [DOI] [PubMed] [Google Scholar]
- 17.Rafiei P and Haddadi A Pharmacokinetic Consequences of PLGA Nanoparticles in Docetaxel Drug Delivery. (2017) Pharm Nanotechnol 5, 3–23 [DOI] [PubMed] [Google Scholar]
- 18.Ulbrich K et al. Targeted Drug Delivery with Polymers and Magnetic Nanoparticles: Covalent and Noncovalent Approaches, Release Control, and Clinical Studies. (2016) Chem Rev 116, 5338–5431 [DOI] [PubMed] [Google Scholar]
- 19.Rafiei P and Haddadi A Docetaxel-loaded PLGA and PLGA-PEG nanoparticles for intravenous application: pharmacokinetics and biodistribution profile. (2017) Int J Nanomedicine 12, 935–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.K SJ et al. Evaluation of in-vitro cytotoxicity and cellular uptake efficiency of zidovudine-loaded solid lipid nanoparticles modified with Aloe Vera in glioma cells. (2016) Mater Sci Eng C Mater Biol Appl 66, 40–50 [DOI] [PubMed] [Google Scholar]
- 21.Piazza R Settled and unsettled issues in particle settling. (2014) Rep Prog Phys 77, 056602 [DOI] [PubMed] [Google Scholar]
- 22.Liu D et al. The Smart Drug Delivery System and Its Clinical Potential. (2016) Theranostics 6, 1306–1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sielaff CM and Mousa SA Status and future directions in the management of pancreatic cancer: potential impact of nanotechnology. (2018) J Cancer Res Clin Oncol 144, 1205–1217 [DOI] [PubMed] [Google Scholar]
- 24.Avitabile E et al. How can nanotechnology help the fight against breast cancer? (2018) Nanoscale 10, 11719–11731 [DOI] [PubMed] [Google Scholar]
- 25.Chen XJ et al. Nanotechnology: a promising method for oral cancer detection and diagnosis. (2018) J Nanobiotechnology 16, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xue Y et al. Preventing diet-induced obesity in mice by adipose tissue transformation and angiogenesis using targeted nanoparticles. (2016) Proc Natl Acad Sci U S A 113, 5552–5557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kolonin MG et al. Reversal of obesity by targeted ablation of adipose tissue. (2004) Nat Med 10, 625–632 [DOI] [PubMed] [Google Scholar]
- 28.Hossen N et al. A comparative study between nanoparticle-targeted therapeutics and bioconjugates as obesity medication. (2013) J Control Release 171, 104–112 [DOI] [PubMed] [Google Scholar]
- 29.Sangwai M, Sardar S and Vavia P Nanoemulsified orlistat-embedded multi-unit pellet system (MUPS) with improved dissolution and pancreatic lipase inhibition. (2014) Pharm Dev Technol 19, 31–41 [DOI] [PubMed] [Google Scholar]
- 30.Gorain B et al. Nanoemulsion strategy for olmesartan medoxomil improves oral absorption and extended antihypertensive activity in hypertensive rats. (2014) Colloids Surf B Biointerfaces 115, 286–294 [DOI] [PubMed] [Google Scholar]
- 31.Lee VHL (1986) Non-Viral Delivery Systems in Gene Therapy and Vaccine Development In Delivery Systems for Peptide Drugs (Davis SS, Illum L & Tomlinson, eds), pp. 87–104, Springer. [Google Scholar]
- 32.Thiruvengadam M, Rajakumar G and Chung IM Nanotechnology: current uses and future applications in the food industry. (2018) 3 Biotech 8, 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sonkaria S, Ahn SH and Khare V Nanotechnology and its impact on food and nutrition: a review. (2012) Recent Pat Food Nutr Agric 4, 8–18 [DOI] [PubMed] [Google Scholar]
- 34.Pradhan N et al. Facets of Nanotechnology as Seen in Food Processing, Packaging, and Preservation Industry. (2015) Biomed Res Int 2015, 365672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berl V Zymes Inc. Dried Formulations, US20100080785. [Google Scholar]
- 36.Neves AR et al. Nanoscale Delivery of Resveratrol towards Enhancement of Supplements and Nutraceuticals. (2016) Nutrients 8, 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garti N et al. NutraLease Ltd. Nano-sized self-assembled liquid dilutable vehicles, US7182950B2 [Google Scholar]
- 38.Silva HD, Cerqueira MA and Vicente AA Nanoemulsions for Food Applications: Development and Characterization. (2012) Food Bioprocess Technol 5, 854–867 [Google Scholar]
- 39.Saleh AA and El-Magd MA Beneficial effects of dietary silver nanoparticles and silver nitrate on broiler nutrition. (2018) Environ Sci Pollut Res Int 25, 27031–27038 [DOI] [PubMed] [Google Scholar]
- 40.Nadworny PL et al. Anti-inflammatory activity of nanocrystalline silver-derived solutions in porcine contact dermatitis. (2010) J Inflamm (Lond) 7, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ahmadi F and Rahimi F The effect of different levels of nano silver on performance and retention of silver in edible tissues of broilers. (2011) World Appl Sci J 12, 1–4 [Google Scholar]
- 42.Jarak I et al. From the Cover: Metabolism Modulation in Different Organs by Silver Nanoparticles: An NMR Metabolomics Study of a Mouse Model. (2017) Toxicol Sci 159, 422–435 [DOI] [PubMed] [Google Scholar]
- 43.Ahmadi F and Kurdestani A The impact of silver nano particles on growth performance, lymphoid organs and oxidative stress indicators in broiler chicks. (2010) Global Vet 5, 366–370 [Google Scholar]
- 44.Ognik K et al. The effect of chemicallysynthesized silver nanoparticles on performance and the histology and microbiological profile of the jejunum in chickens. (2016) Ann Anim Sci 22, 439–446 [Google Scholar]
- 45.Pineda L et al. Effect of silver nanoparticles on growth performance, metabolism and microbial profile of broiler chickens. (2012) Arch Anim Nutr 66, 416–429 [DOI] [PubMed] [Google Scholar]
- 46.Sawosz E et al. Influence of hydrocolloidal silver nanoparticles on gastrointestinal microflora and morphology of enterocytes of quails. (2007) Arch Anim Nutr 61, 444–451 [DOI] [PubMed] [Google Scholar]
- 47.Siddiqi KS, Husen A and Rao RAK A review on biosynthesis of silver nanoparticles and their biocidal properties. (2018) J Nanobiotechnology 16, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang H et al. Effects of noble metal nanoparticles on the hydroxyl radical scavenging ability of dietary antioxidants. (2018) J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 36, 84–97 [DOI] [PubMed] [Google Scholar]
- 49.Bergin IL et al. Effects of particle size and coating on toxicologic parameters, fecal elimination kinetics and tissue distribution of acutely ingested silver nanoparticles in a mouse model. (2016) Nanotoxicology 10, 352–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Park H et al. Combinatorial gene construct and non-viral delivery for anti-obesity in diet-induced obese mice. (2015) J Control Release 207, 154–162 [DOI] [PubMed] [Google Scholar]
- 51.Alberti KG et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. (2009) Circulation 120, 1640–1645 [DOI] [PubMed] [Google Scholar]
- 52.Ye H et al. Pharmaceutically controlled designer circuit for the treatment of the metabolic syndrome. (2013) Proc Natl Acad Sci U S A 110, 141–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ye H et al. Self-adjusting synthetic gene circuit for correcting insulin resistance. (2017) Nat Biomed Eng 1, 0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xie M et al. Beta-Cell-Mimetic Designer Cells Provide Closed-Loop Glycemic Control. (2016) Science 354, 1296–1301 [DOI] [PubMed] [Google Scholar]
- 55.Rossger K, Charpin-El-Hamri G and Fussenegger M A closed-loop synthetic gene circuit for the treatment of diet-induced obesity in mice. (2013) Nat Commun 4, 2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rossger K, Charpin-El Hamri G and Fussenegger M Reward-based hypertension control by a synthetic brain-dopamine interface. (2013) Proc Natl Acad Sci U S A 110, 18150–18155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Won YW et al. Oligopeptide complex for targeted non-viral gene delivery to adipocytes. (2014) Nat Mater 13, 1157–1164 [DOI] [PubMed] [Google Scholar]
- 58.Park H et al. Enhanced thermogenic program by non-viral delivery of combinatory browning genes to treat diet-induced obesity in mice. (2015) Biomaterials 73, 32–41 [DOI] [PubMed] [Google Scholar]
- 59.Arguelles AO et al. Are epigenetic drugs for diabetes and obesity at our door step? (2016) Drug Discov Today 21, 499–509 [DOI] [PubMed] [Google Scholar]
- 60.Sun X et al. From genetics and epigenetics to the future of precision treatment for obesity. (2017) Gastroenterol Rep (Oxf) 5, 266–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rupaimoole R and Slack FJ MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. (2017) Nat Rev Drug Discov 16, 203–222 [DOI] [PubMed] [Google Scholar]
- 62.Deiuliis JA MicroRNAs as regulators of metabolic disease: pathophysiologic significance and emerging role as biomarkers and therapeutics. (2016) Int J Obes (Lond) 40, 88–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chakraborty C et al. Therapeutic miRNA and siRNA: Moving from Bench to Clinic as Next Generation Medicine. (2017) Mol Ther Nucleic Acids 8, 132–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Janssen HL et al. Treatment of HCV infection by targeting microRNA. (2013) N Engl J Med 368, 1685–1694 [DOI] [PubMed] [Google Scholar]
- 65.van der Ree MH et al. Miravirsen dosing in chronic hepatitis C patients results in decreased microRNA-122 levels without affecting other microRNAs in plasma. (2016) Aliment Pharmacol Ther 43, 102–113 [DOI] [PubMed] [Google Scholar]
- 66.Stenvang J et al. Inhibition of microRNA function by antimiR oligonucleotides. (2012) Silence 3, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Adams BD et al. Targeting noncoding RNAs in disease. (2017) J Clin Invest 127, 761–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang HW et al. Gadolinium-functionalized nanographene oxide for combined drug and microRNA delivery and magnetic resonance imaging. (2014) Biomaterials 35, 6534–6542 [DOI] [PubMed] [Google Scholar]
- 69.Wang F et al. Imaging Dendrimer-Grafted Graphene Oxide Mediated Anti-miR-21 Delivery With an Activatable Luciferase Reporter. (2016) ACS Appl Mater Interfaces 8, 9014–9021 [DOI] [PubMed] [Google Scholar]
- 70.Marquart TJ et al. miR-33 links SREBP-2 induction to repression of sterol transporters. (2010) Proc Natl Acad Sci U S A 107, 12228–12232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Najafi-Shoushtari SH et al. MicroRNA-33 and the SREBP host genes cooperate to control cholesterol homeostasis. (2010) Science 328, 1566–1569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rayner KJ et al. MiR-33 contributes to the regulation of cholesterol homeostasis. (2010) Science 328, 1570–1573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Allen RM et al. miR-33 controls the expression of biliary transporters, and mediates statin- and diet-induced hepatotoxicity. (2012) EMBO Mol Med 4, 882–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li T et al. Regulation of cholesterol and bile acid homeostasis by the cholesterol 7alpha-hydroxylase/steroid response element-binding protein 2/microRNA-33a axis in mice. (2013) Hepatology 58, 1111–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rayner KJ et al. Antagonism of miR-33 in mice promotes reverse cholesterol transport and regression of atherosclerosis. (2011) J Clin Invest 121, 2921–2931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rotllan N et al. Therapeutic silencing of microRNA-33 inhibits the progression of atherosclerosis in Ldlr−/− mice--brief report. (2013) Arterioscler Thromb Vasc Biol 33, 1973–1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rayner KJ et al. Inhibition of miR-33a/b in non-human primates raises plasma HDL and lowers VLDL triglycerides. (2011) Nature 478, 404–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.de Haan W et al. Hepatic ABCA1 expression improves beta-cell function and glucose tolerance. (2014) Diabetes 63, 4076–4082 [DOI] [PubMed] [Google Scholar]
- 79.Allen RM et al. Control of very low-density lipoprotein secretion by N-ethylmaleimide-sensitive factor and miR-33. (2014) Circ Res 115, 10–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Goedeke L et al. Long-term therapeutic silencing of miR-33 increases circulating triglyceride levels and hepatic lipid accumulation in mice. (2014) EMBO Mol Med 6, 1133–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Price NL et al. Genetic Dissection of the Impact of miR-33a and miR-33b during the Progression of Atherosclerosis. (2017) Cell Rep 21, 1317–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Horie T et al. MicroRNA-33 regulates sterol regulatory element-binding protein 1 expression in mice. (2013) Nat Commun 4, 2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Price NL et al. Genetic Ablation of miR-33 Increases Food Intake, Enhances Adipose Tissue Expansion, and Promotes Obesity and Insulin Resistance. (2018) Cell Rep 22, 2133–2145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Agostini M and Knight RA miR-34: from bench to bedside. (2014) Oncotarget 5, 872–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hong CA and Nam YS Functional nanostructures for effective delivery of small interfering RNA therapeutics. (2014) Theranostics 4, 1211–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Calle D et al. Magnetoliposomes loaded with poly-unsaturated fatty acids as novel theranostic anti-inflammatory formulations. (2015) Theranostics 5, 489–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Singla AK, Garg A and Aggarwal D Paclitaxel and its formulations. (2002) Int J Pharm 235, 179–192 [DOI] [PubMed] [Google Scholar]
- 88.Singer JW Paclitaxel poliglumex (XYOTAX, CT-2103): a macromolecular taxane. (2005) J Control Release 109, 120–126 [DOI] [PubMed] [Google Scholar]
- 89.Yang T et al. Liposome Formulation of Paclitaxel with Enhanced Solubility and Stability. (2007) Drug Deliv 14, 301–308 [DOI] [PubMed] [Google Scholar]
- 90.Yang T et al. (2007) Enhanced solubility and stability of PEGylated liposomal paclitaxel: In vitro and in vivo evaluation. International Journal of Pharmaceutics 338, 317–326 [DOI] [PubMed] [Google Scholar]
- 91.Marupudi NI et al. Paclitaxel: a review of adverse toxicities and novel delivery strategies. (2007) Expert Opinion on Drug Safety 6, 609–621 [DOI] [PubMed] [Google Scholar]
- 92.Suh J et al. PEGylation of nanoparticles improves their cytoplasmic transport. (2007) Int J Nanomedicine 2, 735–741 [PMC free article] [PubMed] [Google Scholar]
- 93.Chen CC et al. Evaluation of the Biological Behavior of a Gold Nanocore-Encapsulated Human Serum Albumin Nanoparticle (Au@HSANP) in a CT-26 Tumor/Ascites Mouse Model after Intravenous/Intraperitoneal Administration. (2019) Int J Mol Sci 20, E217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mazor R et al. Tolerogenic nanoparticles restore the antitumor activity of recombinant immunotoxins by mitigating immunogenicity. (2018) Proc Natl Acad Sci U S A 115, E733–E742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Roberts RA et al. Towards programming immune tolerance through geometric manipulation of phosphatidylserine. (2015) Biomaterials 72, 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.DeCastro CL & Mitchell BS Nanoparticles from Mechanical Attrition In Synthesis, Functionalization, and Surface Treatment of Nanoparticles: (Baraton MI, ed.), pp. 1–15, American Scientific Publishers. [Google Scholar]
- 97.Govender T et al. PLGA nanoparticles prepared by nanoprecipitation: drug loading and release studies of a water soluble drug. (1999) J Control Release 57, 171–185 [DOI] [PubMed] [Google Scholar]
- 98.Bilati U, Allemann E and Doelker E Development of a nanoprecipitation method intended for the entrapment of hydrophilic drugs into nanoparticles. (2005) Eur J Pharm Sci 24, 67–75 [DOI] [PubMed] [Google Scholar]
- 99.Anton N, Benoit JP and Saulnier P Design and production of nanoparticles formulated from nano-emulsion templates-a review. (2008) J Control Release 128, 185–199 [DOI] [PubMed] [Google Scholar]
- 100.Zambaux MF et al. Influence of experimental parameters on the characteristics of poly(lactic acid) nanoparticles prepared by a double emulsion method. (1998) J Control Release 50, 31–40 [DOI] [PubMed] [Google Scholar]
- 101.Jaiswal M, Dudhe R and Sharma PK Nanoemulsion: an advanced mode of drug delivery system. (2015) 3 Biotech 5, 123–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Singh Y et al. Nanoemulsion: Concepts, development and applications in drug delivery. (2017) J Control Release 252, 28–49 [DOI] [PubMed] [Google Scholar]
- 103.Akbarzadeh A et al. Liposome: classification, preparation, and applications. (2013) Nanoscale Res Lett 8, 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bozzuto G and Molinari A Liposomes as nanomedical devices. (2015) Int J Nanomedicine 10, 975–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yang J et al. Drug Delivery via Cell Membrane Fusion Using Lipopeptide Modified Liposomes. (2016) ACS Cent Sci 2, 621–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Huang RTC and et al. Fusion between cell membrane and liposomes containing the glycoproteins of influenza virus. (1980) Virology 104, 294–302 [DOI] [PubMed] [Google Scholar]
- 107.Lentz BR and Lee JK Poly(ethylene glycol) (PEG)-mediated fusion between pure lipid bilayers: a mechanism in common with viral fusion and secretory vesicle release? (1999) Mol Membr Biol 16, 279–296 [DOI] [PubMed] [Google Scholar]
- 108.Szoka F et al. Use of lectins and polyethylene glycol for fusion of glycolipid-containing liposomes with eukaryotic cells. (1981) Proc Natl Acad Sci U S A 78, 1685–1689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Li SD and Huang L Nanoparticles evading the reticuloendothelial system: role of the supported bilayer. (2009) Biochim Biophys Acta 1788, 2259–2266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ashjari M et al. Self-assembled nanomicelles using PLGA-PEG amphiphilic block copolymer for insulin delivery: a physicochemical investigation and determination of CMC values. (2012) J Mater Sci Mater Med 23, 943–953 [DOI] [PubMed] [Google Scholar]
- 111.Emami J et al. PLGA-PEG-RA-based polymeric micelles for tumor targeted delivery of irinotecan. (2018) Pharm Dev Technol 23, 41–54 [DOI] [PubMed] [Google Scholar]
- 112.Zhang CG et al. Novel polymer micelle mediated co-delivery of doxorubicin and P-glycoprotein siRNA for reversal of multidrug resistance and synergistic tumor therapy. (2016) Sci Rep 6, 23859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kataoka K et al. Smart polymeric micelles as nanocarriers for oligonucleotides and siRNA delivery. (2005) Nucleic Acids Symp Ser (Oxf) (49):17–18. [DOI] [PubMed] [Google Scholar]


