Abstract
Angiogenesis, an essential component of a variety of physiological and pathological processes, offers attractive opportunities for therapeutic regulation. We hypothesized that matrix metalloproteinase-9 genetic deficiency (MMP-9−/−) will impair angiogenesis triggered by tissue ischemia, induced experimentally by femoral artery ligation in mice. To investigate the role of MMP-9, we performed a series of biochemical and histological analyses, including zymography, simultaneous detection of perfused capillaries, MMP-9 promoter activity, MMP-9 protein, and macrophages in MMP-9−/− and wild-type (WT) mice. We found that ischemia resulted in doubling of capillary density in WT and no change in the MMP-9−/− ischemic tissues, which translated into increased (39%) perfusion capacity only in the WT at 14 days after ligation. We also confirmed that capillaries in the MMP-9−/− presented significantly (P<0.05) less points of capillary intersections, interpreted by us as decreased branching. The combined conclusions from simultaneous localizations of MMP-9 expression, capillaries, and macrophages suggested that macrophage MMP-9 participates in capillary branching. Transplantation of WT bone marrow into the MMP-9−/−, restored capillary branching, further supporting the contribution of bone marrow–derived macrophages in supplying the necessary MMP-9. Our study indicates that angiogenesis triggered by tissue ischemia requires MMP-9, which may be involved in capillary branching, a potential novel role for this MMP that could be exploited to control angiogenesis.
Keywords: angiography, macrophage, imaging, microvessels, bone-marrow transplantation
Angiogenesis, the growth of new capillaries from existing ones, is a process necessary for regulation and repair during wound healing and after ischemic injury of tissue. However, angiogenesis also facilitates grave pathological processes such as tumor progression and metastasis,1 macular degeneration,2 and atheroma progression toward plaque instability.3 Oxygen deprivation, or tissue ischemia, is a potent stimulus of angiogenesis through both humoral and inflammatory processes.4
Angiogenesis is a complex process that involves the activation of vascular cells through a balance of pro- and antiangiogenic factors.4 The growth of new capillaries is thought to occur mainly through capillary splitting, also known as intussusception, or capillary budding leading to branching.5 As growth of capillaries likely necessitates invasion of existing tissues, the potential enabling action of matrix metalloproteinases (MMPs), a family of enzymes capable of degrading the extracellular matrix components, has come under scrutiny.6 In fact, the antiangiogenic action of nonspecific MMP inhibitors in vivo7 and in vitro8 is thought to be of major importance for their anticancer activity.9 However, these chemical agents have also undesirable effects.10
Two members of the MMP family, MMP-2 and MMP-9, known to degrade nonfibrillar collagens, a major component of the basement membrane, have been implicated in macular degeneration due to the angiogenic response to hyperoxia11 and ischemic reperfusion damage in a rat model of hindlimb ischemia.5 Genetic MMP-9 deficiency was found to result in retarded bone growth attributed to inhibition of angiogenesis.12 The potential involvement of MMPs in angiogenesis is further suggested by the discovery that MMPs have the ability to expose cryptic signaling sites on extracellular matrix components,13 and to release14 and activate matrix-bound growth factors.15 However, the specific processes controlled by MMP-9 remain unknown partially due to lack of adequate investigative methods.
Through using a combination of conventional and new technological tools, we were able to implicate the macrophage MMP-9 in the angiogenic response to ischemia resulting. We suggest its specific contribution to be enabling of capillary branching.
Materials and Methods
Mouse Strains
Several mouse strains were used in this study: MMP-9−/− mice12 in the 129 SvEv background (kindly provided by Drs Robert Senior and Mike Shipley, Washington University in St Louis, Mo) or in the C57BL6/J background and their strain-matched wild-type (WT) mice; Gelatinase B (GelB)/LacZ Tg mice, in which LacZ expression is driven by the MMP-9 promoter;16 and WT mice that express the rare CD45.1 allele (Jackson Labs, Bar Harbor, Maine), useful for in vivo leukocyte tracking.17
Ischemia-Induced Angiogenesis Murine Model
Mice underwent ligation and segmental resection of femoral vessels as described previously.18 The femoral artery and vein of the left hindlimb were exposed in mice anesthetized with ketamine (87 mg/kg) and xylazine (13 mg/kg). The superficial femoral artery and vein were ligated immediately distal to the deep and superficial femoral bifurcation, and the saphenous artery and vein were ligated just proximal to the tarsalis. To ensure complete cessation of flow, the artery and vein were resected between the ligatures, after which the wound was sutured. Experimental animals were euthanized without ligation (labeled as nonischemic) or at 1, 3, 7, or 14 days after surgery for either biochemical, histological, or fluorescence microangiographic analyses. For proliferation analysis, animals were injected intraperitoneally with 250 μg BrdU (Sigma) per day for 14 days.
Rescue experiments were done to assess the contribution of bone marrow–derived macrophages to angiogenesis. For tracking of donor bone marrow–derived leukocytes, we transplanted the bone marrow of WT C57BL6/J that express the rare leukocyte CD45.1 allele17 into the MMP-9−/− mice. Briefly, 2 days after irradiation with two doses of 5.5 Gy, bone-marrow transplantation was performed by retro-orbital injection of isolated bone marrow at 5 million cells per mouse. The level of bone-marrow engraftment determined by FACS17 4 weeks after transplantation indicated >90% engraftment in all transplanted mice. Thus, ischemic tissue was examined by double immunohistochemistry to confirm the bone marrow (donor) origin of leukocytes by detection of the rare CD45.1 allele expression, whereas the macrophage type was confirmed by detection of the specific Mac-3 antigen as previously described.17 The Emory University Institutional Animal Care and Use Committee approved all animal protocols.
Histological and Immunohistochemical Analyses
For paraffin embedded sections, mice were euthanized and perfused with saline followed by 10% buffered formalin (Fisher Scientific) through the left ventricle. The ischemic hindlimb was removed and decalcified in 5% acetic acid for 48 hours. Subsequently, the sample was dehydrated and embedded in paraffin. Sections were obtained for analysis from within 100 μm of the distal ligation. For frozen sections, mice were euthanized and perfused with saline. The excised adductor muscle was frozen in OCT (Sakura Finetek USA) in liquid nitrogen and stored at −80°C until sectioned. Affinity histology to identify endothelium was performed using biotinylated Griffonia simplicifolia lectin (Vector Labs) followed by FITC-conjugated streptavidin (Jackson ImmunoResearch Laboratories). Proliferating cells were identified using a rat anti-BrdU antibody (Abcam) followed by Rhodamine Red X–conjugated (RRX) goat anti-rat IgG (Jackson ImmunoResearch Laboratories). Macrophages were identified using a rat anti-mouse Mac-3 antibody (Pharmingen) followed by RRX-goat anti-rat IgG (Jackson ImmunoResearch Laboratories) or Alexafluor 488–conjugated goat anti-rat IgG (Molecular Probes). MMP-9 was identified using a rabbit anti-mouse MMP-9 antibody (Chemicon) and RRX-donkey anti-rabbit IgG (Jackson ImmunoResearch Laboratories). LacZ expression was detected using a rabbit anti-LacZ (Molecular Probes) and RRX-donkey anti-rabbit IgG (Jackson ImmunoResearch Laboratories). Donor-derived leukocytes were detected with RRX-mouse anti-CD45.1 (Pharmingen). Staining for mouse FLK-1 (A-3, Santa Cruz) and α-actin (1A4, Sigma), followed by Rhodamine Red X–conjugated goat anti-mouse (Jackson ImmunoResearch), was used for identification of endothelial cell precursor19 and pericytes,20 respectively. All histological samples were counterstained with Hoechst 33258 (Sigma) for nuclei. Capillary density was quantified from 7-mm thick paraffin sections as the number of lectin-positive capillary profiles per high-powered field. Three fields from the adductor muscle groups were taken for each animal (considered an independent sample), with four samples per time point per condition.
Fluorescence Microangiography
Mice were euthanized and perfused with 10 mL heparinized saline (10 U/mL) injected through the left ventricle. To identify capillaries with the capacity to be perfused through the circulatory system, we infused 0.1 μm Fluospheres (Molecular Probes) at a 1:20 dilution in heparinized saline. The hindlimb adductor muscle group was dissected and tissue was either examined as a whole mount specimen by confocal microscopy, or processed by homogenization for tissue fluorescence assays. Visualization of perfused microvasculature and quantification of capillary branching was done by fluorescence microangiography using a confocal microscope (Zeiss). Capillary intersection points were counted per 100× field for 4 to 6 mice per group. Simultaneous detection of MMP-9 promoter activation was done in GelB/LacZ Tg mice 3 days after ligation by injecting ImaGene Red C12RG LacZ substrate kit (Molecular Probes) retro-orbitally 24 hours before euthanasia. In this case, fluorescence microangiography was performed as above but with a 1:200 dilution of Fluospheres. For quantification of perfusion capacity, fluorescence was extracted from tissues of additional animals (4 per time point) by dissolving the Fluospheres contained in homogenized tissues using 100 μL of xylene (Fisher Scientific) for 48 hours, and fluorescence was measured with a CytoFluor 3000 plate reader (Applied Biosystems) and normalized by muscle weight to obtain an average perfusion capacity for 4 mice per time point.
Biochemical Analysis
Mice were euthanized using a lethal dose of CO2 and exsanguinated by perfusion with saline through the left ventricle. The adductor muscle group of the upper hindlimb between the ligations was excised, snap frozen in liquid nitrogen, and homogenized in lysis buffer.21 Protein concentration was determined using the Biorad DC protein assay kit. MMP-2 and MMP-9 activities were determined using SDS-PAGE gelatin zymography,22 using 15 μg protein per sample. The samples contained both latent and activated forms, revealed in the presence of SDS. After incubation, zymographies were imaged and quantified using GelDoc 1000 (Biorad). Gelatinolytic activities were separately normalized in regards to the standard loaded in each gel. Western blotting was performed using 15 μg protein per sample using a rabbit anti-mouse MMP-9 antibody (Chemicon). Mouse MMP standards were obtained by affinity chromatography separation: MMP-2 from the conditioned culture medium of mouse MMP-9−/− smooth muscle cells, and MMP-9 respectively from the conditioned culture medium of WT mouse macrophages; and used to standardize samples across gels. VEGF was assayed in tissue homogenates using western blotting with a goat anti-mouse antibody (Santa Cruz Biotechnology, Santa Cruz, CA).
Statistical Analysis
All quantifications were performed using 4 to 6 mice (each being considered an independent sample) per group. All values are given as mean±SEM. Comparisons were made using the Student’s t test or analysis of variance. Differences were considered significant if P<0.05.
Results
Tissue Ischemia Induces MMP-9
Protein Expression
Ligation of the femoral artery, an experimental model for peripheral occlusive vascular disease, creates upper hindlimb muscle ischemia resulting in an angiogenic reaction.18 The rapid (24 hour) postischemic induction of MMP-9 expression and activity in tissue homogenates of WT muscles (Figure 1), with activity peaking at 3 days and returning to baseline at 7 days, supported MMP-9 role in the early phase of ischemia-induced angiogenesis (0% for nonischemic versus 141±68% gelatinolytic activity of an MMP-9 standard at 3 days after ischemia, P<0.01, versus 14±4% at day 7 and 0% at day 14 after ischemia). As expected, MMP-9 was not expressed in any of the MMP-9−/− tissue homogenates, nor was there compensation by other gelatinases as illustrated in Figure 1. For a detailed separate analysis of MMP-2 and MMP-9 in WT and MMP-9−/− tissues see online Figure 1, available in the online data supplement at http://www.circresaha.org.
Figure 1.
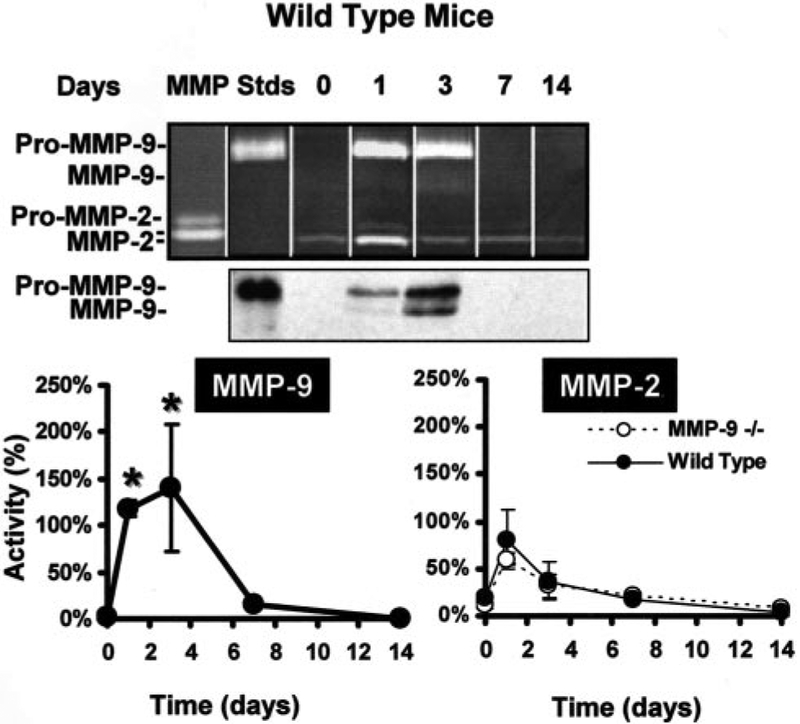
MMP-9 expression and activity are induced at early time points in hindlimb muscle tissues exposed to ischemia. MMP-9 protein expression was assayed in the nonischemic (day 0) or ischemic (1, 3, 7, and 14 days) WT adductor muscle tissue lysates using SDS-PAGE gelatin zymography (top) and Western blotting (middle). Both indicate early induction of MMP-9 (day 1), with levels peaking at 3 days and returning to baseline by 7 days after ischemia (latent “Pro” MMP-9 and activated MMP-9). Quantification of total gelatinolytic activity associated with MMP-9 or MMP-2 (both pro- and activated bands) in WT and MMP-9−/− tissue lysates, normalized using standards (“MMP Stds”) (n=4 animal for each time point), illustrates the significant early induction of MMP-9 in response to ischemia in the WT, and similar induction of MMP-2 in the WT and MMP-9−/−. *P<0.05 vs 0 day time point. For a detailed separate analysis of MMP-2 and MMP-9, see online Figure 1.
MMP-9 Deficiency Inhibits
Ischemia-Induced Angiogenesis
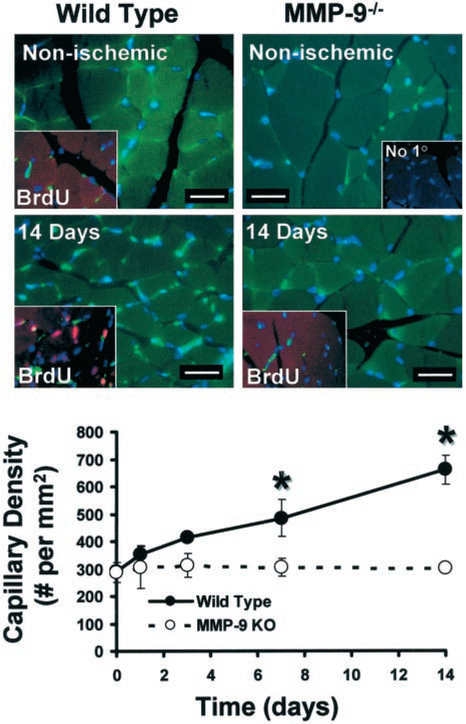
Quantification of capillary staining with endothelial specific Griffonia simplicifolia lectin (Figure 2) indicated similar baseline nonischemic capillary density in the nonischemic upper hindlimb of MMP-9−/− and WT mice (287±36 versus 289±11 capillaries/mm2 for MMP-9−/− versus WT). At 14 days after ischemia, capillary density was doubled in the upper hindlimb adductor muscle group of WT mice (128±18% increase at 14 days versus nonischemic; P<0.05), whereas capillary density was unchanged in MMP-9−/− ischemic tissue (5.5±3.1% increase at day 14 versus nonischemic; NS). Furthermore, identification of cell proliferation by incorporation of the thymidine analog, BrdU (Figure 2) in the WT, but not in the MMP-9−/− ischemic muscle was also consistent with the blunting of the angiogenic response due to MMP-9 deficiency. As a potential mediator of angiogenesis, we assayed for VEGF in ischemic tissue lysates; however, we did not find differences between levels of VEGF in the WT and MMP-9−/− (data not shown).
Figure 2.
Capillary histochemical affinity staining indicates lack of an ischemia-induced angiogenic response in MMP-9−/− mice. Examination of tissue cross-sections stained with endothelial specific Griffonia simplicifolia lectin (green) indicates that capillary density increases in WT, but not in MMP-9−/− tissues in response to ischemia (scale bar=20 μm). All nuclei counterstained with Hoechst (blue). Insets illustrate in addition BrdU-positive (red) immunodetection of proliferating cells (pink, due to localization to nuclei counterstained in blue) with capillary staining (green), as well as a negative control with no primary antibody (“No 10”). Proliferating cells were found only in ischemic WT tissues supporting the presence of a robust angiogenic response. Quantification of capillary density displays a significant increase in capillary number within WT but not within MMP-9−/− ischemic tissues (n=4 for each point, *P<0.05 vs MMP-9−/−), supporting MMP-9 role in ischemia-induced angiogenic response.
MMP-9 Deficiency Results in Diminished
Microvascular Perfusion Capacity
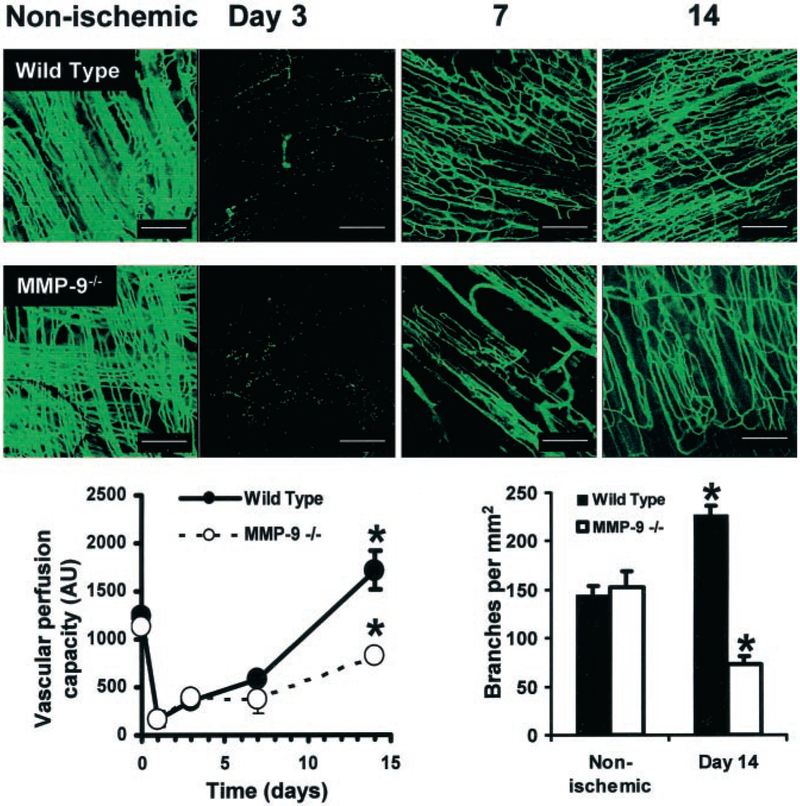
Histological analysis of the ischemic areas suggested effects of MMP-9 deficiency on the angiogenic response, but could not provide information regarding perfusion of microvascular structures. Perfusion of fluorescent microspheres postmortem indicated that both WT and MMP-9−/− nonischemic muscle had a high microvascular perfusion capacity, as illustrated by fluorescence micrographs and fluorescence levels recovered from tissue homogenates (Figure 3). After onset of ischemia, capillary perfusion capacity decreased significantly in both WT and MMP-9−/− with partial recovery by 7 days in WT tissues and actually exceeded baseline capacity after 14 days (1715±198 AU at day 14 versus 1238±34 AU for nonischemic; P<0.05), indicating an efficient angiogenic response. Fluorescence microangiography additionally indicated an increased number of points of intersection in the capillary structures, which we considered to be a measure of branching (226±10 branches per mm2 at 14 days versus 144±10 branches per mm2 for nonischemic; P<0.01) and increased tortuosity in the WT ischemic tissues. On the other hand, perfusion capacity of MMP-9−/− tissues was only partially recovered after 14 days (821±47 AU at day 14 versus 1126±125 AU for nonischemic; P=0.088). Furthermore, visualization of the microstructural capillary characteristics suggested a drop in capillary branching in the MMP-9−/− tissues compared nonischemic tissue (73±7 branches per mm2 at day 14 versus 153±15 branches per mm2 for nonischemic; P<0.05) as well as WT tissues, all confirmed by quantification (Figure 3).
Figure 3.
MMP-9 deficiency decreases restoration of microvascular perfusion capacity in response to ischemia. Time course of perfusion capacity imaged on whole-mount tissue specimens using fluorescence microangiography (scale bars=200 μm). Nonischemic muscle contains numerous perfused capillaries with no apparent differences between WT and MMP-9−/− mice. The number of perfused capillaries drops dramatically after femoral artery ligation, illustrated at day 3 in both WT and in MMP-9−/− tissues, in spite of existing anatomical structures (illustrated by specific staining in Figure 2). Perfusion capacity is significantly better restored in WT tissues compared with the MMP-9−/− tissues (day 7), and actually becomes higher than the baseline at day 14, whereas it remains deficient in MMP-9−/− tissue, supporting the role of MMP-9 for proper revascularization. In addition, note the increased number of points of capillary intersections, indicative of branching, and increased tortuosity in WT compared with MMP-9−/− ischemic tissue (day 14) or compared with nonischemic tissues. Vascular perfusion capacity of adductor muscle (left graph), quantified as fluorescence extracted from tissue homogenates (n=4 animals per point), dropped dramatically after femoral artery ligation in both WT and MMP-9−/− tissue. By 14 days, WT vascular perfusion capacity surpassed initial capacity, indicating robust revascularization and angiogenesis, whereas in the MMP-9−/− ischemic tissues, perfusion capacity remained lower than initial capacity (*P<0.05 vs WT 14 day), illustrating blunted revascularization. Quantification of capillary branching density (from confocal microscope images of tissues, n=4 animals for each point) indicated a significant increase in branching density in the WT after 14 days compared with normal nonischemic tissues (*P<0.05 vs nonischemic). On the other hand, MMP-9−/− ischemic tissues had a decreased density of branches at 14 days (*P<0.05 vs WT), suggesting that lack of capillary branching contributed to decreased perfusion capacity.
Macrophage MMP-9 Likely Contributes to Ischemia-Induced Angiogenesis
The association of MMP-9 expression and capillary branching was further supported by the detection of MMP-9 promoter activity in GelB/LacZ transgenic mice localized perivascularly at early time points (day 3) after onset of ischemia (Figure 4). Macrophages were detected in the extracapillary space in the WT but not in the MMP-9−/− ischemic muscle, suggesting an association with the ischemia-induced angiogenic capillary branching. We specifically investigated the macrophages as the source of MMP-9, by combining observations from cell-specific immunohistochemistry, LacZ detection of promoter activity, and confirmation of MMP-9 protein production by macrophages using double immunohistochemistry in ischemic WT femoral tissues (Figure 4).
Figure 4.
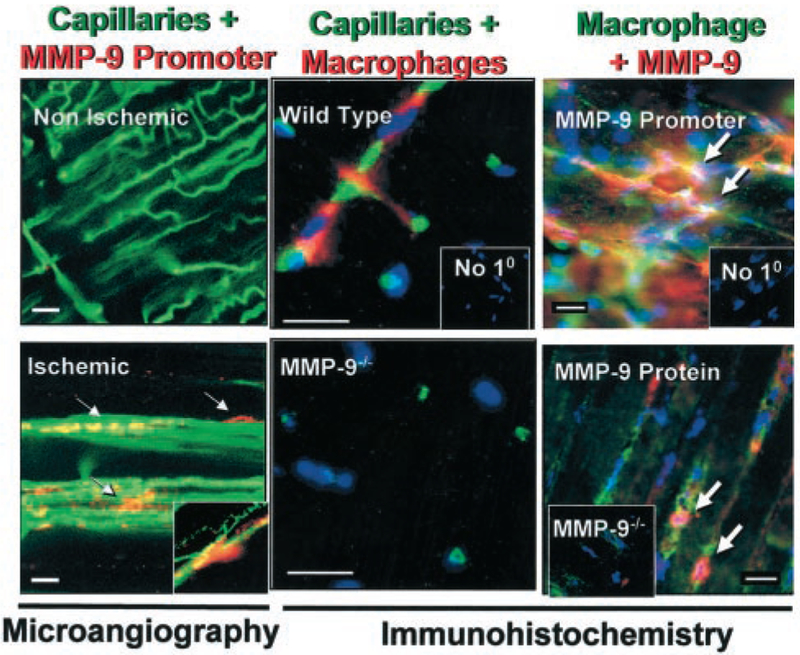
Detection of capillaries, MMP-9 promoter activity and protein, as well as macrophages suggests a role for macrophage MMP-9 in the early angiogenic response to ischemia. Left, Visualization by fluorescence microangiography of perfused capillaries (green) and MMP-9 promoter activity (red) in the GelB/LacZ Tg mice in nonischemic and ischemic tissue (day 3), indicates a perivascular localization of cells actively transcribing MMP-9 (arrows) in ischemic tissues. Inset, Typical finding of MMP-9 promoter activity at branching points. Middle, Positive detection for macrophages (red) in ischemic WT tissues was frequently observed at points of capillary (green) intersections. On the other hand, no macrophages were detected in MMP-9−/− ischemic tissues. Inset, Negative control for staining with no primary antibody (No 10). All nuclei are counterstained with Hoechst (blue). Right, Double immunohistochemistry illustrates colocalization (arrows) of (top) macrophages (green) and MMP-9 promoter activity (red, GelB/LacZ Tg mouse), as well as (bottom) macrophages (green) and MMP-9 protein (red, WT mouse). Insets demonstrate the specificity of immunostaining, ie, consecutive sections processes without primary antibody (No 10) and the lack of MMP-9–positive signal in ischemic tissues harvested from the MMP-9−/− mice. All scale bars=20 μm. Taken together, the results of these analyses suggest a link between MMP-9, macrophages, and angiogenic branching in ischemic tissues.
WT Bone Marrow Rescues Capillary Branching in MMP-9−/−
To confirm our observations suggesting a role for macrophage-derived MMP-9 in capillary branching, we tested the possibility of rescuing the angiogenic response in the MMP-9−/− mice by transplantation of WT bone marrow. Three days after inducing ischemia in the MMP-9−/− mice with engrafted WT bone marrow, we were able to identify MMP-9–positive donor macrophages in the ischemic tissue (Figure 5). Importantly, fluorescent microangiography in these transplanted mice confirmed the rescue of the angiogenic response, as reflected by the recovery of branching density to levels similar to those found in WT mice after ischemia (355±22 branches per mm2 for MMP-9−/− with WT bone marrow versus 355±41 branches per mm2 for WT, NS, versus 163±11 branches per mm2 for MMP-9−/−; P<0.01). Because other bone marrow–derived cells may contribute to the process of angiogenesis, we also performed staining for Flk-1 (online Figure 2), one of the markers used for endothelial cell progenitors.19 However, the level of staining was too low to draw any conclusions regarding potential differences between the WT and MMP-9−/− ischemic tissues. We also stained for pericytes20 but did not observe any differences between WT and MMP-9−/− tissues.
Figure 5.
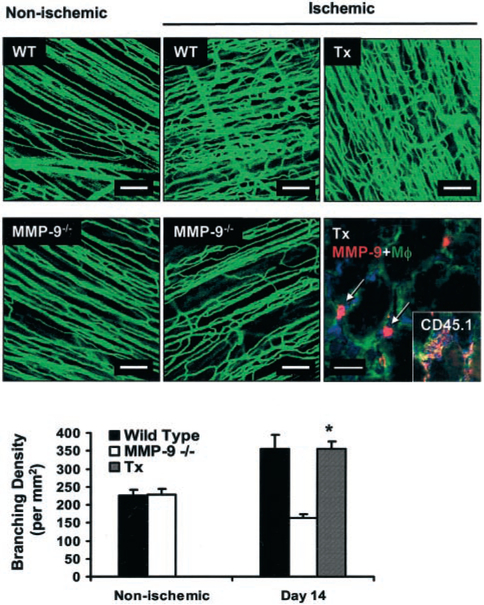
Transplantation with WT bone marrow rescues ischemia-induced angiogenic capillary branching in the MMP-9−/− mice. Nonischemic tissues illustrate similar levels of capillary perfusion capacity in the WT or MMP-9−/−. Left, Fluorescence microangiography. Middle, Increased perfusion capacity was detected in WT ischemic tissues, whereas MMP-9−/− ischemic tissues showed an impaired perfusion (day 14), consistent with inadequate angiogenesis. Transplantation (Tx) of WT bone marrow to MMP-9−/− mice (right) rescued perfusion capacity, as suggested by the high capillary tortuosity and branching in the MMP-9−/− ischemic tissue (top right). All scale bars=100 μm. Bottom right, MMP-9 (red)–positive macrophages (green) were detected (arrows) at early time points in the MMP-9−/− ischemic tissues of mice transplanted with WT marrow by double immunohistochemistry (scale bar=20 μm). Inset, Demonstration that macrophages (green) are bone marrow–derived because they are positive for the donor rare allele CD45.1 (red). All nuclei are counterstained with Hoechst (blue). Graph, Quantification of capillary branching by fluorescence microangiography confirms increased branching in WT (solid bar) ischemic tissue (P<0.05 vs nonischemic) and decreased branching in MMP-9−/− (white bar) ischemic tissue (P<0.05 vs nonischemic) and demonstrates that transplantation (Tx) of WT bone marrow into MMP-9−/− mice (striped) increases capillary branching in MMP-9−/− ischemic tissues vs MMP-9−/− nonischemic tissues (*P<0.05), as well as ischemic tissues of nontransplanted MMP-9−/− (*P<0.05), bringing it to levels comparable to WT ischemic tissues (P=NS).
Discussion
Previous studies suggested multiple potential mechanisms through which MMPs may assists angiogenesis, but the nature of the MMPs involved and their specific roles remain uncertain. Impaired bone growth in MMP-9−/− mice was attributed to decreased release of matrix-bound VEGF.12 Similarly, MMP-9 ability to cleave latent tumor growth factor-β was related to induction of angiogenesis and tumor growth.15 Other work investigating tumor angiogenesis indicated that both MMP-2 and MMP-9 are upregulated in tumor growth,23 whereas retarded growth of implanted tumors likely due to decreased angiogenesis was reported in MMP-9−/−, but not in MMP-2−/− mice.7,24 On the other hand, lentiviral delivery of PEX, a peptide fragment mimicking a noncatalytic fragment of both MMP-2 and MMP-9, suppressed angiogenesis.25 Other recent investigations support the involvement of membrane-type 1 MMP in the upregulation of VEGF induction of angiogenesis.26 In our experimental model, we did not find differences between levels of VEGF in the WT and MMP-9−/− ischemic tissues.
Using a novel method that provides both quantitative and visual information about the capillary perfusion capacity, we have found that MMP-9 is necessary for ischemia-induced angiogenesis. Our results support the conclusion that the recovery of the infiltration of bone marrow–derived macrophages into ischemic tissues may be the source of MMP-9 and appears to be related to restoration of points of intersections in microvasculature in the MMP-9−/− with transplanted WT bone marrow. The effect of MMP-9 is reminiscent of previous observations implicating MMPs in the budding and branching of other systems, eg, MMP-3 during in vitro branching of mammary epithelial cells,27 and MMP-2 and MT-1-MMP during in vivo and in vitro morphogenesis of lung tissues. An investigation of the ex vivo sprouting and outgrowth of capillaries from arterial rings embedded in collagen gels also supported the role of MMPs through the use of a nonspecific MMP inhibitor.28 We attribute the in vivo loss of points of capillary intersections in our experiments to inhibition of capillary branching due to MMP-9 deficiency. Although branching is most likely a necessary event in angiogenesis, and thus may be turned off by other factors that impair angiogenesis, our study suggests a connection between MMP-9 availability in the ischemic tissue, as well as its specific localization in the initial stages of capillary branching. We found MMP-2 levels to be similar in WT and MMP-9−/− tissues, both at baseline and after ischemia, with no compensatory increase in MMP-9−/− tissues, whereas the angiogenic inhibitory effect was significant in the MMP-9−/− tissues, suggesting that the observed effect was not associated with MMP-2 levels. However, our data do not exclude the possibility that MMP2 may also contribute to ischemia-induced angiogenesis, but its individual function remains to be determined using genetic or other type of specific inhibition.
We additionally questioned the potential cellular source of MMP-9 at the time before branching in ischemia-induced angiogenesis. Investigation of potential candidates, such as pericytes or endothelial cell progenitors, did not reveal conclusive differences between WT and MMP-9−/− tissues (online Figure 2). On the other hand, the combined results from our experiments using fluorescent microangiography in a transgenic mouse carrying an MMP-9 promoter-LacZ reporter system, immunohistochemistry to identify donor bone marrow–derived leukocytes and macrophages, and the ability to rescue branching in the MMP-9−/− by WT bone-marrow transplantation all are consistent with the notion that the necessary MMP-9 activity was provided by bone marrow–derived macrophages. In fact, the inflammatory response to hypoxia has come under recent scrutiny for its ability to facilitate angiogenesis. Administration of monocyte chemoattractant protein-1 in mice was found to increase ischemia-induced angiogenesis and atherosclerotic plaque growth.29 Macrophages, as well as mast cells and neutrophils produce MMP-9,30 and are thought to facilitate angiogenesis.23,24,31 Matrix degradation by secreted proteases may besides directly facilitate migration of endothelial or precursors cells, potentially by “drilling tunnels,”32 also expose cryptic sites enhancing angiogenesis,13 or release matrix bound proangiogenic growth factors.14 Reconstitution of WT splenic macrophages23 or bone marrow24 increased tumor angiogenesis and growth in MMP-9−/− mice. Downregulation of MMP-9 expression was also implicated as an explanation for the antiangiogenic effect of the antiinflammatory cytokine interleukin-10 (IL-10), found to decrease angiogenesis in response to hypoxia.33 Proangiogenic collagen type IV cryptic sites13 could be exposed by MMP-9 derived from macrophages infiltrating the subendothelial capillary space, as suggested by experiments with macular degeneration in response to hyperoxia in neonatal MMP-9−/− mice.11
Interestingly, through these experiments, we also found strain-related differences in the extent of capillary branching. Specifically, both WT and MMP-9−/− mice in the C57BL6/J background (Figure 5) had higher capillary branching density compared with mice in the 129SvEv background (Figure 3).
Although our experiments demonstrated the participation of macrophages to ischemia-induced angiogenesis, the potential contribution of other bone marrow–derived cells, including endothelial cell progenitors, cannot be ruled out. Interestingly, a recent report indicates that progenitor cells including endothelial precursor and hematopoietic stem cells require MMP-9 for release from the bone marrow and processing of a differentiation factor.34 This novel function of MMP-9 suggests potential impairment of macrophage recruitment from the bone marrow in the MMP-9−/− mice, also consistent with our finding that macrophages populated WT but not MMP-9−/− tissues soon after the onset of ischemia.
Quantification of microangiography indicated that revascularization after ischemia resulted in moderate resolution of perfusion capacity, but not in increased vascular capacity in the MMP-9−/− as in the WT ischemic tissue. Furthermore, microvascular pattern visualization suggested that blood flow was reestablished in the MMP-9−/− most likely through recanalization of existing capillary beds.
Taken together our results support the notion that MMP-9 is necessary for capillary branching and suggest the macrophages to be a likely source. This novel function for MMP-9 may be exploited for the therapeutic control of angiogenesis.
Supplementary Material
Acknowledgments
Funding for these studies was provided through the NIH RO1 HL64689, the American Heart Association Established Investigator Award No. 0040087N, and the National Science Foundation (NSF) Award EEC-9731643 to Dr Zorina Galis and through the NIH RO1 EY12651, P30 EY13078, and Research to Prevent Blindness awards to Dr M. Elizabeth Fini. Dr Susan Lessner was supported through NRSA No. 1 F32 HL68449-01, and Dr Chad Johnson was supported through the NSF Award EEC-9731643.
Contributor Information
Chad Johnson, Wallace H. Coulter Department of Biomedical Engineering, Georgia Institute of Technology/Emory University School of Medicine, Atlanta, Ga.
Hak-Joon Sung, Wallace H. Coulter Department of Biomedical Engineering, Georgia Institute of Technology/Emory University School of Medicine, Atlanta, Ga.
Susan M. Lessner, Division of Cardiology, Emory School of Medicine, Atlanta, Ga
M. Elizabeth Fini, McKnight Vision Research Center, Bascom Palmer Eye Institute, University of Miami School of Medicine, Miami, Fla..
Zorina S. Galis, Wallace H. Coulter Department of Biomedical Engineering, Georgia Institute of Technology/Emory University School of Medicine, Atlanta, Ga; Division of Cardiology, Emory School of Medicine, Atlanta, Ga
References
- 1.Folkman J Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29:15–18. [DOI] [PubMed] [Google Scholar]
- 2.Aiello LP. Clinical implications of vascular growth factors in proliferative retinopathies. Curr Opin Ophthalmol. 1997;8:19–31. [DOI] [PubMed] [Google Scholar]
- 3.Ross JS, Stagliano NE, Donovan MJ, Breitbart RE, Ginsburg GS. Atherosclerosis: a cancer of the blood vessels? Am J Clin Pathol. 2001; 116(suppl):S97–S107. [DOI] [PubMed] [Google Scholar]
- 4.Carmeliet P Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–395. [DOI] [PubMed] [Google Scholar]
- 5.Rivilis I, Milkiewicz M, Boyd P, Goldstein J, Brown MD, Egginton S, Hansen FM, Hudlicka O, Haas TL. Differential involvement of MMP-2 and VEGF during muscle stretch- versus shear stress-induced angiogenesis. Am J Physiol Heart Circ Physiol. 2002;283:H1430–H1438. [DOI] [PubMed] [Google Scholar]
- 6.Pepper MS. Role of the matrix metalloproteinase and plasminogen activator-plasmin systems in angiogenesis. Arterioscler Thromb Vasc Biol. 2001;21:1104–1117. [DOI] [PubMed] [Google Scholar]
- 7.Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z, Hanahan D. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2:737–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fisher C, Gilbertson-Beadling S, Powers EA, Petzold G, Poorman R, Mitchell MA. Interstitial collagenase is required for angiogenesis in vitro. Dev Biol. 1994;162:499–510. [DOI] [PubMed] [Google Scholar]
- 9.Tosetti F, Ferrari N, De Flora S, Albini A. Angioprevention: angiogenesis is a common and key target for cancer chemopreventive agents. FASEB J. 2002;16:2–14. [DOI] [PubMed] [Google Scholar]
- 10.Levitt NC, Eskens FA, O’Byrne KJ, Propper DJ, Denis LJ, Owen SJ, Choi L, Foekens JA, Wilner S, Wood JM, Nakajima M, Talbot DC, Steward WP, Harris AL, Verweij J. Phase I and pharmacological study of the oral matrix metalloproteinase inhibitor, MMI270 (CGS27023A), in patients with advanced solid cancer. Clin Cancer Res. 2001;7:1912–1922. [PubMed] [Google Scholar]
- 11.Lambert V, Munaut C, Jost M, Noel A, Werb Z, Foidart JM, Rakic JM. Matrix metalloproteinase-9 contributes to choroidal neovascularization. Am J Pathol. 2002;161:1247–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vu TH, Shipley JM, Bergers G, Berger JE, Helms JA, Hanahan D, Shapiro SD, Senior RM, Werb Z. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell. 1998;93:411–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hangai M, Kitaya N, Xu J, Chan CK, Kim JJ, Werb Z, Ryan SJ, Brooks PC. Matrix metalloproteinase-9-dependent exposure of a cryptic migratory control site in collagen is required before retinal angiogenesis. Am J Pathol. 2002;161:1429–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Engsig MT, Chen QJ, Vu TH, Pedersen AC, Therkidsen B, Lund LR, Henriksen K, Lenhard T, Foged NT, Werb Z, Delaisse JM. Matrix metalloproteinase 9 and vascular endothelial growth factor are essential for osteoclast recruitment into developing long bones. J Cell Biol. 2000; 151:879–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-β and promotes tumor invasion and angiogenesis. Genes Dev. 2000;14:163–176. [PMC free article] [PubMed] [Google Scholar]
- 16.Mohan R, Rinehart WB, Bargagna-Mohan P, Fini ME. Gelatinase B/lacZ transgenic mice, a model for mapping gelatinase B expression during developmental and injury-related tissue remodeling. J Biol Chem. 1998; 273:25903–25914. [DOI] [PubMed] [Google Scholar]
- 17.Lessner SM, Prado HL, Waller EK, Galis ZS. Atherosclerotic lesions grow through recruitment and proliferation of circulating monocytes in a murine model. Am J Pathol. 2002;160:2145–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Couffinhal T, Silver M, Zheng LP, Kearney M, Witzenbichler B, Isner JM. Mouse model of angiogenesis. Am J Pathol. 1998;152:1667–1679. [PMC free article] [PubMed] [Google Scholar]
- 19.Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–228. [DOI] [PubMed] [Google Scholar]
- 20.Morikawa S, Baluk P, Kaidoh T, Haskell A, Jain RK, McDonald DM. Abnormalities in pericytes on blood vessels and endothelial sprouts in tumors. Am J Pathol. 2002;160:985–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Godin D, Ivan E, Johnson C, Magid R, Galis ZS. Remodeling of carotid artery is associated with increased expression of matrix metalloproteinases in mouse blood flow cessation model. Circulation. 2000;102: 2861–2866. [DOI] [PubMed] [Google Scholar]
- 22.Galis ZS, Muszynski M, Sukhova GK, Simon-Morrissey E, Libby P. Enhanced expression of vascular matrix metalloproteinases induced in vitro by cytokines and in regions of human atherosclerotic lesions. Ann NY Acad Sci. 1995;748:501–507. [DOI] [PubMed] [Google Scholar]
- 23.Huang S, Van Arsdall M, Tedjarati S, McCarty M, Wu W, Langley R, Fidler IJ. Contributions of stromal metalloproteinase-9 to angiogenesis and growth of human ovarian carcinoma in mice. J Natl Cancer Inst. 2002;94:1134–1142. [DOI] [PubMed] [Google Scholar]
- 24.Coussens LM, Tinkle CL, Hanahan D, Werb Z. MMP-9 supplied by bone marrow–derived cells contributes to skin carcinogenesis. Cell. 2000;103: 481–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pfeifer A, Kessler T, Silletti S, Cheresh DA, Verma IM. Suppression of angiogenesis by lentiviral delivery of PEX, a noncatalytic fragment of matrix metalloproteinase 2. Proc Natl Acad Sci USA. 2000;97: 12227–12232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deryugina EI, Soroceanu L, Strongin AY. Up-regulation of vascular endothelial growth factor by membrane-type 1 matrix metalloproteinase stimulates human glioma xenograft growth and angiogenesis. Cancer Res. 2002;62:580–588. [PubMed] [Google Scholar]
- 27.Simian M, Hirai Y, Navre M, Werb Z, Lochter A, Bissell MJ. The interplay of matrix metalloproteinases, morphogens and growth factors is necessary for branching of mammary epithelial cells. Development. 2001; 128:3117–3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hata-Sugi N, Kawase-Kageyama R, Wakabayashi T. Characterization of rat aortic fragment within collagen gel as an angiogenesis model; capillary morphology may reflect the action mechanisms of angiogenesis inhibitors. Biol Pharm Bull. 2002;25:446–451. [DOI] [PubMed] [Google Scholar]
- 29.van Royen N, Hoefer I, Bottinger M, Hua J, Grundmann S, Voskuil M, Bode C, Schaper W, Buschmann I, Piek JJ. Local monocyte chemoattractant protein-1 therapy increases collateral artery formation in apolipoprotein E-deficient mice but induces systemic monocytic CD11b expression, neointimal formation, and plaque progression. Circ Res. 2003;92:218–225. [DOI] [PubMed] [Google Scholar]
- 30.Johnatty RN, Taub DD, Reeder SP, Turcovski-Corrales SM, Cottam DW, Stephenson TJ, Rees RC. Cytokine and chemokine regulation of proMMP-9 and TIMP-1 production by human peripheral blood lymphocytes. J Immunol. 1997;158:2327–2333. [PubMed] [Google Scholar]
- 31.Lindsey M, Wedin K, Brown MD, Keller C, Evans AJ, Smolen J, Burns AR, Rossen RD, Michael L, Entman M. Matrix-dependent mechanism of neutrophil-mediated release and activation of matrix metalloproteinase 9 in myocardial ischemia/reperfusion. Circulation. 2001;103:2181–2187. [DOI] [PubMed] [Google Scholar]
- 32.Moldovan NI, Goldschmidt-Clermont PJ, Parker-Thornburg J, Shapiro SD, Kolattukudy PE. Contribution of monocytes/macrophages to compensatory neovascularization: the drilling of metalloelastase-positive tunnels in ischemic myocardium. Circ Res. 2000;87:378–384. [DOI] [PubMed] [Google Scholar]
- 33.Silvestre JS, Mallat Z, Duriez M, Tamarat R, Bureau MF, Scherman D, Duverger N, Branellec D, Tedgui A, Levy BI. Antiangiogenic effect of interleukin-10 in ischemia-induced angiogenesis in mice hindlimb. Circ Res. 2000;87:448–452. [DOI] [PubMed] [Google Scholar]
- 34.Heissig B, Hattori K, Dias S, Friedrich M, Ferris B, Hackett NR, Crystal RG, Besmer P, Lyden D, Moore MA, Werb Z, Rafii S. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2002;109:625–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.