Abstract
Children’s rights to autonomy of choice are differently expressed throughout Europe. We explored differences regarding expressions of respect for children’s autonomy throughout Europe, using the procedure of human papillomavirus (HPV) vaccination offer as indicator. We used a mixed methods approach, utilizing an expert survey within the frame of “Models of Child Health Appraised” (MOCHA), among all 30 European Union (EU) and European Economic Area states. A questionnaire was designed using vignettes regarding the vaccine provision. Thirty MOCHA country agents were invited to respond from June 2017 to April 2018. In total, 28 country agents responded. We studied the following themes: (i) provision of informed consent, (ii) parental and medical paternalism, (iii) relevance of the child’s chronological age or maturity, and (iv) vaccination programs targeting boys. These are being handled differently across the region. We explored associations of these implemented practices with the national vaccine coverage rate across Europe. We used the processes of HPV vaccination to study child’s autonomy, the paradigm change toward libertarian paternalism and issues of sex-equity. Interestingly, greater respect for children’s autonomy tends to be associated with medium or high vaccination coverage rates and lower respect with lower rates. Respect and empowerment seem to have practical as well as moral benefits. Identifying and transferring the most suitable ethical approaches is crucial and should be strengthened.
Keywords: Child health, Europe, papillomavirus vaccines, personal autonomy, vaccination, vaccination coverage
Introduction
Currently, there is no consensus in Europe regarding the ideal model for the provision of primary health care for children and adolescents. The majority of the different existing models of primary child health care throughout the European Union (EU) have never been appraised in terms of children’s health outcomes (van der Willik et al., 2016). It remains unclear, to what extent children may or may not be receiving optimal health care. Consequently, their entitlement to optimal health, as supported by the United Nations Convention on the Rights of the Child (UNICEF, 1989), is rather unknown. It is for this reason that the Models of Child Health Appraised (MOCHA) project has been instigated (Blair and Alexander, 2017).
Children’s rights to autonomy of choice may also be differently and unequally expressed or implemented throughout the EU. It is widely acknowledged that the developing autonomy of children in health care should be more respected and accepted (Gahr, 2015; Martakis et al., 2018). Inequalities in children’s autonomy can easily be identified within the primary health-care models for children and adolescents. For instance, differences in granting competence in decision-making based on the developmental or chronological age, differences in the process of informed consent, and the processes to be followed in cases that a health-care service is denied by children or their parents can raise significant ethical debates regarding the degree of respect to child’s developing autonomy (EU-FRA and Rights, 2017; Martakis et al., 2018) and moral equality across Europe (Wiesemann, 2016).
A major task of primary health care is to ensure vaccinations to prevent diseases in the population. Vaccination programs for HPV immunization have been offered across Europe since 2007 (Elfstrom et al., 2015) following recommendations from the European Centers for Disease Prevention and Control (ECDC) (Hamers and European Centre for Disease Prevention and Control, 2008). Unlike in many countries internationally, including the United States, in the majority of the European countries, HPV vaccines are commonly offered to girls in late childhood or adolescence. The implementation of the vaccination, however, is neither harmonized nor standardized across the EU (Elfstrom et al., 2015). There are several differences across the states including the type of applied vaccine (quadrivalent or bivalent), the age, and other characteristics of the target population, the vaccination delivery strategy, as well as the need for out-of-pocket payment of the vaccine (Elfstrom et al., 2015).
From the point of view of public health ethics, very little is known regarding the quality of the interaction between the child or adolescent receiving the HPV vaccine and the physician or nurse administering it. Thus, processes of vaccine provision may vary substantially across the national vaccination programs in Europe, from authoritarian paternalistic models, imposing a passive role on the child, to libertarian models, where mutual participation of both actors is needed (Martakis et al., 2018).
Study aims
Aim of this article is to explore differences regarding issues related to different expressions of respect for children’s developing autonomy throughout the EU. The procedure of HPV vaccination can be regarded as an indicator for developing autonomy. In Europe, the vaccine is commonly offered to girls in late childhood and adolescence, although boys may also benefit. Arguably, at this stage of the life course, cognitive development and decision-making competences of young people are approaching that of adulthood, and thus at least the consent of the person receiving the vaccine, next the consent of the legal guardians, should be requested (Hein et al., 2015). By this stage, young people should also be taking responsibility for their own health and salutogenic behavior. Furthermore, the vaccine protects against an infection that can also be sexually transmitted. Issues related to the right of sexual self-determination of children and adolescents and associated conflicts in the relationship with their parents can complicate the implementation of vaccination programs in different European settings. Finally, we aim to explore associations between expressions of respecting child’s autonomy and the HPV vaccine coverage rate (VCR) across Europe.
Methods
Study design
National vaccination programs principally utilize primary health-care facilities and services to achieve a universal vaccine offer. Currently, within the frame of the MOCHA project, an interprofessional network has been formed, linking scientific partners with a Country Agent in each one of 30 EU and European Economic Area member states, supplying data to answer precise questions. This is aimed at mapping and appraising the field of primary health-care services offered to children in Europe. This study was an expert survey performed within the framework of MOCHA, using a mixed methods approach, combining tools of quantitative and qualitative research. We finally explored associations between expressions of respect to child’s developing autonomy in the different vaccination approaches in Europe and the national HPV VCR, as currently reviewed by Sheikh et al. (2018).
Sampling
The recruited experts included the MOCHA country agents (http://www.childhealthservicemodels.eu/partnerlisting/country-agents/), a professional network with diverse professional qualifications, collaborating within the frame of the MOCHA project. This group of experts is a key methodological feature of the MOCHA project (Brenner et al., 2017; Kuhne et al., 2017). These individuals are local experts in child health in each country who have access to professional networks to answer research questions on a range of topics. The specific field of expertise included social pediatric experts and child health professionals, affiliated with 1 of the 30 participating academic institutions. In total, 30 MOCHA country agents from the following 30 EU and European Economic Area member states were invited to participate in the survey: Austria, Belgium, Bulgaria, Croatia, Cyprus, the Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Italy, Latvia, Lithuania, Luxemburg, Malta, the Netherlands, Norway, Poland, Portugal, Romania, Slovakia, Slovenia, Spain, Sweden, and the United Kingdom.
Data collection
Data collection and validation took place with the help of the MOCHA country agents. We designed a questionnaire, which underwent several rounds of revision based on scientific feedback from the MOCHA task working group, the project’s scientific managing team, and an independent expert advisory board, to confirm rationale, relevance, and clarity. The questionnaire included a combination of closed questions with specific response categories as well as deepening open-ended questions. MOCHA agents were asked to complete the questions on the basis of their expertise, or in other cases, to gather data from other national experts to respond to the study items. For the sake of transparency, the questionnaire is provided as supplement file (Kuhne et al., 2017). From June to December 2017, the MOCHA country agents from 30 European states were invited to respond to the questionnaire.
The questions related to national policies on respecting children’s choices and therefore their autonomy in the national primary health-care model. Concerning national law, the MOCHA country agents were asked to provide, wherever possible, links to the relevant pieces of legislation.
The country agents were further asked to refer to national policies or strong guidelines issued by a health professional or cross-sectoral body on the right of choice or refusal of treatment in childhood and adolescence and to provide the respective link if possible. Further, possible differences of legislation associated with the children’s chronological age or the child’s decision-making competences were documented. Regarding the issue of medical paternalism, we asked the MOCHA country agents, if the physician can overrule a child’s choice to receive or refuse to treatment in daily practice in their country.
We further explored and exemplified ethical issues regarding the respect for children’s developing autonomy, based on an ethical model developed by the lead author (Martakis et al., 2018), using a vignette referring to the provision of the HPV vaccine in girls. Finally, we included a similar vignette referring to the provision of the vaccination to a boy in countries where this was routinely offered, and who thus might wish to be immunized too.
All data were centrally collected and validated by the collaborating scientific partners of the MOCHA project. When clarifications were needed, the MOCHA coordinators directly contacted the country agents, who were asked to review and eventually revise their response.
Data analysis
We carried out a directorial qualitative content analysis (Hsieh and Shannon, 2005), examining concepts and models referring to the following study themes:
provision of written informed consent or assent from a child or adolescent or their legal guardians, to receive, request or refuse a treatment, and more specifically the HPV vaccine;
issues related to parental as well as medical paternalism;
association of the level of autonomy with the children’s chronological age or with their decision-making competence; and
reporting of HPV vaccination programs targeting boys.
We finally explored correlations between different practices applied throughout Europe regarding the themes (i)–(iii) and the national HPV VCRs. A quantitative descriptive analysis was further used to study the results. The maps in this article were created with mapchart.net. Atlas.ti—version 16.0 (ATLAS.ti Scientific Software Development GmbH, Berlin, Germany) was used for the analysis.
Results
Focal points of 28 European states: Austria, Bulgaria, Croatia, Cyprus, the Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Italy, Latvia, Lithuania, Malta, the Netherlands, Norway, Poland, Portugal, Romania, Slovakia, Slovenia, Spain, Sweden, and the United Kingdom responded by providing national data.
HPV vaccine is offered to girls in late childhood and early adolescence in all participating countries, starting from nine years of age in Austria and Germany. The study of different approaches regarding HPV vaccination in Europe revealed a series of emerging ethical issues. This vaccine is offered to girls through national vaccination programs; however, although generally accepted as safe and beneficial by regulatory authorities, it is sometimes not well accepted in certain population groups, and some public opinion has expressed concerns, including claims of short- or long-term adverse effects. For example, the HPV vaccine is part of the “National program primary prevention of cervical cancer in the Republic of Bulgaria 2017–2020”. After successful program start, the death of a teenager with a long-term systemic disorder two months after immunization leads to public skepticism and the program was terminated, despite there being no causal relation between the two events.
Our findings have been classified according to four study themes: ethics of provision of informed consent, issues related to parental or medical paternalism, issues associated with the children’s cognitive development or findings directly related with the respect for developing autonomy, and issues regarding HPV vaccination programs targeting boys. These results are presented in the following paragraphs.
Provision of informed consent
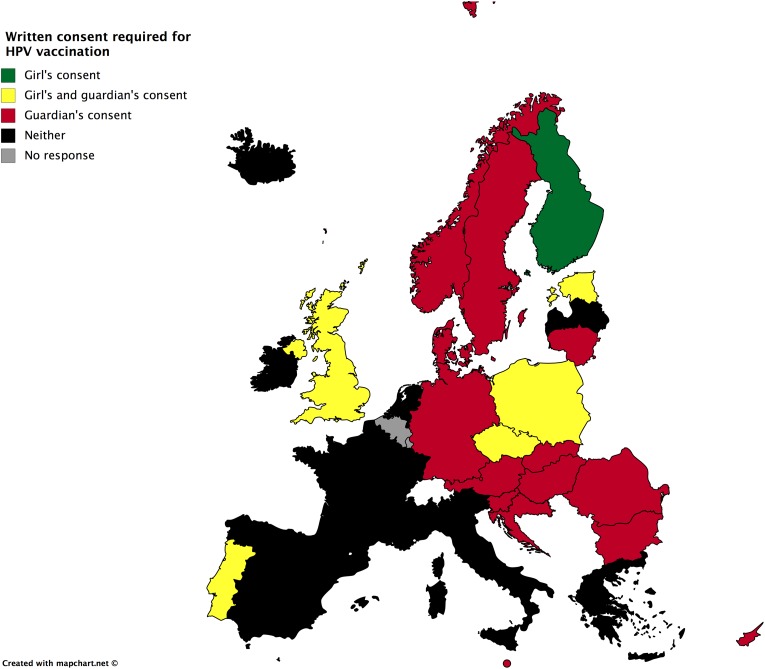
Regarding vaccine provision, written consent is required in the majority of the countries (Figure 1). Although written consent for the vaccine provision is required in the majority of the European states, the person who is responsible to provide informed consent, child or parents, differs substantially (Figure 1). Indeed, the girl’s sole written consent is sufficient for vaccination in Finland; an additional parental consent is needed in the Czech Republic, Estonia, Poland, Portugal, and the United Kingdom. Parental written consent, instead of the girl’s consent, is needed in Austria, Bulgaria, Croatia, Cyprus, Denmark, Hungary, Germany, Lithuania, Malta, Norway, Romania, Slovakia, and Sweden. Finally, in Greece, Iceland, Ireland, Italy, Latvia, the Netherlands, and Spain, the vaccine is provided without request of consent, but a parental refusal may deny application.
Figure 1.
Written consent required for HPV vaccination for girls in Europe. HPV: human papillomavirus.
Issues related to parental or medical paternalism
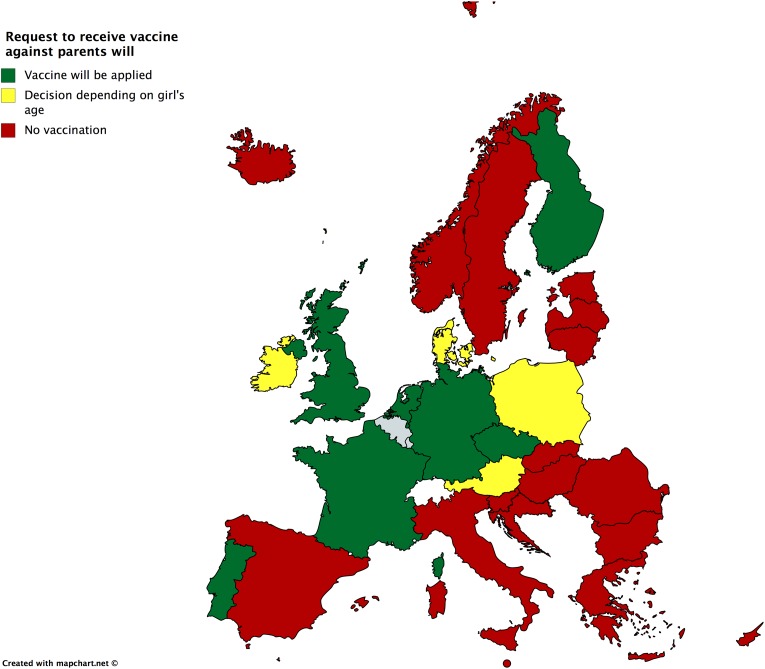
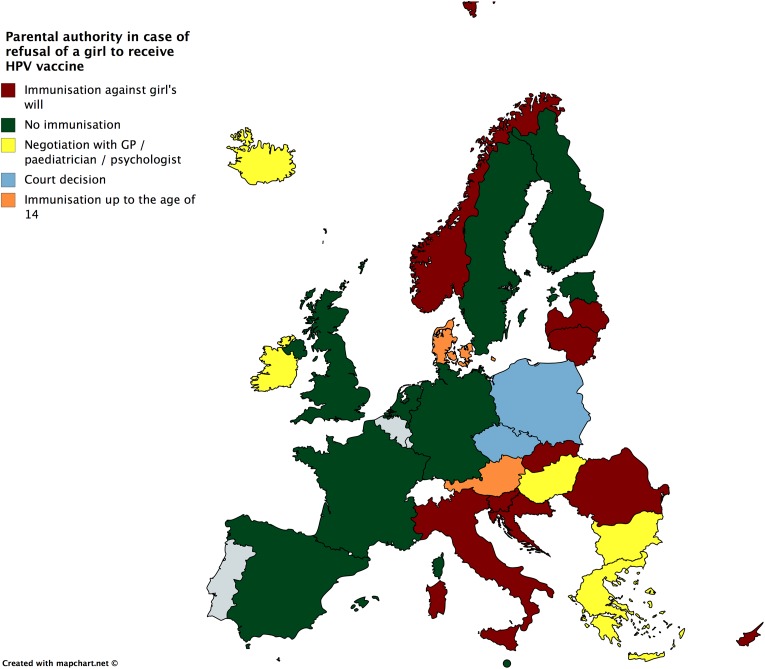
If the girl requests an offered HPV immunization but the parents refuse consent, she can be immunized in the Czech Republic, Finland, Germany, the Netherlands, Portugal, and the United Kingdom and in late adolescence in Austria, Denmark, Ireland, and Poland (Figure 2). On the other hand, if the parents or guardians request immunization, but the girl objects, she will still be obliged to receive vaccination in Croatia, Cyprus, Italy, Latvia, Lithuania, Norway, Romania, and Slovakia. A negotiation with the physician would be the rule in some states, such as Bulgaria, Greece, Hungary, Iceland, and Ireland, while a court decision is needed for being vaccinated in the Czech Republic and Poland (Figure 3).
Figure 2.
Request of a girl to receive vaccine against parents’ will.
Figure 3.
Parental authority in case of refusal of a girl to receive HPV vaccine. HPV: human papillomavirus.
Regarding medical paternalism, it seems that physicians may overrule a child’s choice without going to court in Austria, the Czech Republic, Denmark, Estonia, Ireland, and Portugal, in case, the child has not reached a chronological or developmental age threshold, and thus children may receive the treatment against their will.
Chronological age, maturity, and their association with the respect for autonomy
First, we examined the legal situation regarding the right of children to consent or assent in receiving or refusing the HPV vaccination in different European states. National law and policies on the right of children to choose or refuse treatment, even when this is advocated by the parents or their doctor, were reported in Austria, the Czech Republic, Denmark, Estonia, France, Finland, Germany, Ireland, the Netherlands, Norway, Poland, Portugal, Spain, Sweden, and the United Kingdom.
Regarding differences of legislation associated with chronological or developmental thresholds to grant children and adolescents a decision-making capacity, we identified a variety of approaches across Europe. Indeed, an age limit of 14 years of age to grant decision-making competence has been set in Austria and Portugal; 15 years of age in Denmark and Finland; 16 years of age in the Netherlands, Norway, Poland, and Spain; and 18 years of age in Estonia. On the contrary, the grade of development of decision-making competences is the relevant criterion in the Czech Republic, Germany, Sweden, the United Kingdom, and Spain (for ages 12–16 years). After confirmation of the maturity of the decision-making competences in an examination of mental competency, the child is granted the right to accept or refuse a medical treatment. This is often not the rule in life-threatening conditions.
HPV vaccination programs targeting boys
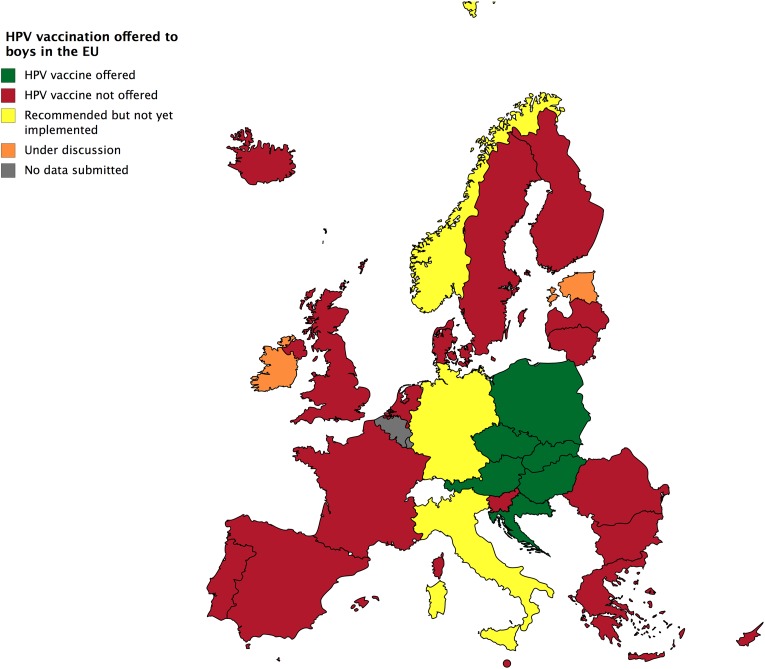
Unlike some countries such as the United States, vaccinating boys against HPV is still not the norm in Europe, although this is increasing. In the second semester of 2017, the vaccine was offered to boys regularly in Austria, Croatia, the Czech Republic, Poland, and Slovakia (Figure 4). The ethical issues referring to the children’s rights to receive or refuse treatment, as well as the grade of parental or medical paternalism did not differ from the ones referring to girls of the same age in these states (Figure 4). In June 2018, the Standing Committee on Vaccinations of the Robert Koch Institute announced a plan of launching a vaccination program targeting boys in Germany (RKI, 2018).
Figure 4.
HPV vaccination offered to boys in Europe. Data as in June 2018. HPV: human papillomavirus.
Correlation with the national HPV VCR
We explored associations of the studied vaccine practices with the HPV VCR across Europe, as reported by Sheikh et al. (2018). The VCR data of only 11 countries, of the 28 recruited in our study, were available (Table 1). We classified the countries according to their HPV VCR in three categories: low rate (<40%), medium rate (40–75%), and high rate (>75%). Due to the small sample, an analysis using inferential statistics was not possible. However, as Table 1 depicts, higher VCR was often achieved in countries with less paternalistic approaches (not vaccinating against child’s will), such as Spain, Sweden, and the United Kingdom.
Table 1.
Association of the HPV VCR, according to Sheikh et al. (2018), and the procedures related to respect of the child’s developing autonomy across Europe.
| Country | HPV vaccine rate | Written informed consent needed? | If the child provides consent and the parents don’t (child autonomy) | If the parents provide consent and the child doesn’t (paternalism) |
|---|---|---|---|---|
| Austria | NR | Guardian’s | Vaccination | Court decision |
| Belgium | 55.5% M | NR | NR | NR |
| Bulgaria | 14.1% L | Guardian’s | No vaccination | Vaccination |
| Croatia | NR | Guardian’s | No vaccination | Vaccination |
| Cyprus | NR | Guardian’s | No vaccination | Vaccination |
| Czech Republic | NR | Both | Vaccination | Court decision |
| Denmark | NR | Guardian’s | Vaccination, after 14 y. | Vaccination, until 14 years |
| Estonia | NR | Both | No vaccination | No vaccination |
| Finland | 68.8% M | Child’s | Vaccination | No vaccination |
| France | 19.1% L | No | Vaccination | No vaccination |
| Germany | 42.5% M | Guardian’s | Vaccination | No vaccination |
| Greece | 27.0% L | No | No vaccination | Negotiation |
| Hungary | NR | Guardian’s | No vaccination | Negotiation |
| Iceland | NR | No | No vaccination | Negotiation |
| Ireland | NR | No | Depending on chronological age | Negotiation |
| Italy | 70.1% H | No | No vaccination | Vaccination |
| Latvia | NR | No | No vaccination | Vaccination |
| Lithuania | NR | Guardian’s | No vaccination | Vaccination |
| Malta | NR | Guardian’s | No vaccination | No vaccination |
| Netherlands | 53.0% M | No | Vaccination | No vaccination |
| Norway | NR | Guardian’s | No vaccination | Vaccination |
| Poland | 23.0% L | Both | Vaccination, after 14 years | Court decision |
| Portugal | NR | Both | Vaccination | NR |
| Romania | NR | Guardian’s | No vaccination | Vaccination |
| Slovakia | NR | Guardian’s | No vaccination | Vaccination |
| Slovenia | NR | Guardian’s | No vaccination | Vaccination |
| Spain | 79.0% H | No | No vaccination | No vaccination |
| Sweden | 80.0% H | Guardian’s | No vaccination | No vaccination |
| UK | 85.9% H | Both | Vaccination | No vaccination |
Note: VCR: vaccine coverage rate; HPV: human papillomavirus; L: low VCR (<40%); M: medium VCR (40–75%); H: high VCR (>75%); NR: not reported; NA: not applicable (no national program).
Discussion
Developing autonomy, parental, and medical paternalism
The study of HPV vaccine practices in Europe revealed significant differences in practices in primary health-care services targeting children throughout the EU. The child’s developing autonomy, parental authority, and medical paternalism are differently weighed and respected.
Children’s developing autonomy is a dynamic process that should be facilitated throughout their life (Martakis et al., 2018). In practice, as seen from the legislation and practices regarding the provision of the HPV vaccine in the different states, a large number of European children are assessed as competent or incompetent to meet health-care choices usually according to their chronological and sometimes developmental age. The chronological threshold also varies across Europe, because adolescents are often legally entitled to decision-making in different ages. The developmental age is currently primarily taken into consideration in a few countries, but such approaches are not standardized yet.
The grade of parental authority deriving from more or less paternalistic paradigms of parenting also emerges in the HPV vaccine case. It is clear (Figure 1) that with the exception of Finland, parents are the ones expected to decide in Europe if a child shall be immunized or not, either through processes of informed consent or simply not choosing to opt-out of this option, in countries where written informed consent is not required.
Furthermore, in cases where there is disagreement between the girl and her parents, the expected outcome differs throughout Europe. In the European South, parental refusal would be prioritized, ignoring the child’s will to receive the HPV vaccine (Figure 2), while the girl’s refusal would only be respected in some countries, such as Malta and Spain (Figure 3). In Western Europe, the norm would favor the girl’s will. A well-defined legal and ethical framework guiding the interaction of all actors (parents, child, professional offering the vaccine) should safeguard that loss of parental trust in this unique case would not substantially influence the interaction of the actors for the child’s medical good. The situation is more diverse in Northern and Eastern Europe.
Medical paternalism is a third force and a further emerging ethical issue regarding vaccine administration in Europe. First, we discovered that providing information to patients and parents and expecting written informed consent to provide a vaccine is not always necessary in some European states, primarily in the European south (Figure 1). Second, overruling a child’s decision, even of one who is competent to meet a decision based on developmental criteria, is still acceptable in a large part of the EU. Third, the physician may function as a negotiator in cases of disagreement between children and their parents. This may actually facilitate a solution to the problem, because the physician is required to provide valid information regarding the vaccination to both children and parents by facilitating discussions among all actors. Deriving from libertarian paternalism theories, such an approach not only respects but can also constructively boost the children’s developing autonomy (Martakis et al., 2018).
The age at which the vaccine is offered introduces a further ethical issue, regarding the level of parental or medical paternalism in the different European states. Thus, the vaccine is offered already with the ninth year of life in Austria and Germany, two states that show one of the most and least paternalistic patterns, respectively. On the other hand, in the United Kingdom, a country where the level of maturity and not the chronological age signalizes the decision-making competence, the vaccine is only offered with the 14th year of age, and thus indirectly enabling girls to consent for the vaccine application regardless of parental consent. Offering the vaccine with a delay of a couple of years may seem to be boosting children’s autonomy, however. We should keep in mind though, that the vaccination shall be provided before the treated individuals are sexually active. Thus, a delayed application in late teenage years, an age in which many adolescents are already sexually active, may indeed jeopardize the effect of the vaccine and the child’s medical good.
Immunizing boys against HPV
From a public health ethics perspective, the case of the HPV vaccine raises issues of cost-effectiveness, as well as sex-equity issues. Meanwhile, it is scientifically clear that the application of the vaccine to male adolescents is to protect against HPV-related forms of penile, oropharyngeal as well as anal carcinomas (Gulland, 2016). This intervention is especially protective among men who have sex with men (Wise, 2017). Additionally, the increase of herd immunity may also be an additional motive to be immunized against HPV. However, issues of cost-effectiveness and arguments, the vaccination of females is adequate to protect men, have led to recommendations against the universal vaccination of teenage boys. Such recommendations are not based on scientific evidence (Wise, 2017).
Elfstrom et al. (2015), based on a questionnaire-based data collection conducted from May 2012 until May 2014, reported no organized vaccination programs targeting boys in Europe. The vaccine was available to males from high-risk populations, who actively asked for an immunization, and was usually expected to finance it out-of-pocket, raising sex-equity issues (Elfstrom et al., 2015). In 2017, however, such programs have already been implemented in Eastern Europe, as depicted in Figure 4, while the vaccination has already been recommended in Italy and Norway, and recently also in Germany. Extended discussions among all relevant actors, including youth organizations and the vaccine manufacturers, shall be held on an international and regional level, to explore realistic alternatives regarding the financial coverage of the intervention, as well as the facilitation of other determinants of diffusion, dissemination, and implementation of the treatment in the rest of the EU (Greenhalgh et al., 2004).
Identifying and transferring best practices
UNICEF presents an ethical frame in which access to health-care services shall be facilitated and preventive measures shall be offered in childhood and adolescence (UNICEF and Rights, 1991). According to the convention, the right of children to access primary health care, including preventive health-care services, is indisputable (Article 24), while educating parents and children regarding child health is essential (Article 24). Further significant ethical conditions include steering the parenting style toward more libertarian patterns (Article 14 of the Convention), empowering children through offering health education (Article 17), and treating children and adolescents with disabilities, or chronic conditions equivalently to healthy ones (Article 23), while distinctions of any kind based on sex are not acceptable (Preamble).
From an ethics perspective, the transferability of good ethics practices in child health care and the harmonization of policies with respect to the child’s developing autonomy are crucial within the EU (Schloemer and Schroder-Back, 2018). To enable transferability, it is important to consider contextual conditions in the different countries, particularly the characteristics of the target populations, such as health education and literacy of families and their usual way of cooperation with providers of the vaccination. For some countries, the transfer of good ethics practices will need changes of the procedures of vaccination. This requires the analysis of environmental conditions, such as available resources for service delivery and the expertise of providers with regard to ethical practices. However, there are several facilitating aspects for transferability of good practices in the EU. Providing health education regarding a vaccine to be offered to children and their parents and requesting written consent or assent are already common practices in many national vaccination programs across Europe and could be easily spread throughout the EU. The emerging role of the pediatrician as negotiator in cases of disagreement between the child and the parents also reflects a paradigm change, framing a potential standardized ethical role of the physicians treating children and adolescents.
The exploration of associations of the level of respect for the child’s autonomy and the HPV VCR across Europe revealed that average or above performing countries tend to follow less authoritative and paternalistic approaches for the vaccine provision (Table 1). Interestingly, the country with the best VCR performance, the United Kingdom, also follows most autonomy respectful paradigms (vaccination of a consenting child, even without parental consent).
In the case of the HPV vaccination offer in Europe, it seems ethically most appropriate to implement an approach including the following elements:
– Educate children and parents regarding the vaccine provision and involve them all in informed consent processes.
– Grant decision-making competence to children and adolescents depending on their maturity.
– In cases of refusal to treat, consider offering the vaccine to older children and adolescents, who have most probably reached maturity. However, the vaccine should be applied before the individuals are sexually active.
– Involve the pediatrician as negotiator in cases of disagreement between children and parents.
– Do not restrict the provision of health education to children with disabilities, including diseases affecting cognition.
– Offer the vaccine to children and adolescents of both sexes.
Limitations
Expert surveys may be helpful for national data collection, however, are associated with a probable bias and thus a relevant limitation for the study (Collins and Evans, 2007). Thus, such data could partially be not representative for a population of a country or could not reveal differences between different regions of a country. However, the study of data of the European Union Agency for Fundamental Rights for the EU countries, published in November 2017, mapping the minimum age requirements concerning the right of the child to consent to medical treatment without parental consent in the EU, did not show significant differences, when compared to the results of our expert survey (EU-FRA and Rights, 2017). Thus, we regard the validity and reliability grade of the collected data as satisfying.
Such a study design regarding an understudied topic presents important advantages, because the expertise and experience of the recruited professionals provide valuable insight for understanding the diversity among the European states, for future research and for developing recommendations worth transferring regionally and internationally.
Conclusions
The procedure of HPV vaccination is an interesting indicator for studying emerging ethical issues in European public health, such as the child’s developing autonomy and the paradigm change toward more libertarian forms of parental and medical paternalism, as well as issues of sex equity. Interestingly, but not surprisingly, greater respect for children’s autonomy tends to be associated with medium or high vaccination coverage rates and lower respect with lower rates. Respect and empowerment seem to have practical as well as moral benefits. Identifying and transferring the most suitable ethical approaches is crucial and should be strengthened.
Identifying and transferring the ethically most suitable approached in European models of health care is crucial and shall be strengthened in the coming years. Educating children and their parents regarding vaccines and implementing written consent approaches that would include and respect the child’s autonomy are already existing practices that should be further spread throughout Europe. This would also facilitate a paradigm change in the physician’s role, evolving into an advocate for the child’s autonomy development and empowerment, and a negotiator in cases of disagreement between children and their parents.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This project has received funding from the European Union’s Horizon 2020 research and innovation program under grant agreement no. 634201.
ORCID iDs: Kyriakos Martakis  https://orcid.org/0000-0003-3982-0914
https://orcid.org/0000-0003-3982-0914
Peter Schröder-Bäck  https://orcid.org/0000-0003-4496-3936
https://orcid.org/0000-0003-4496-3936
References
- Blair MRM, Alexander D. (2017) Final Report on Current Models of Primary Care for Children, Including Sections on Context, Operation and Effects, and Related Business Models. February 2017, Commission Deliverable D6 (D1.2). [Google Scholar]
- Brenner M, O’Shea M, J Larkin P, et al. (2017) Exploring integration of care for children living with complex care needs across the european union and european economic area. International Journal of Integrated Care 17: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins H, Evans R. (2007) Rethinking Expertise. Chicago: University of Chicago Press. [Google Scholar]
- Elfstrom KM, Dillner J, Arnheim-Dahlstrom L. (2015) Organization and quality of HPV vaccination programs in Europe. Vaccine 33: 1673–1681. [DOI] [PubMed] [Google Scholar]
- EU-FRA and Rights EUAfF (2017) Consenting to Medical Treatment Without Parental Consent. Available at: http://fra.europa.eu/en/publication/2017/mapping-minimum-age-requirements/consent-medical-treatments (accessed 1 July 2017).
- Gahr M. (2015) Patientenverfügungen von minderjährigen. Monatsschrift Kinderheilkunde 163: 375–378. [Google Scholar]
- Greenhalgh T, Robert G, Macfarlane F, et al. (2004) Diffusion of innovations in service organizations: systematic review and recommendations. Milbank Memorial Fund Quarterly-Health and Society 82: 581–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulland A. (2016) Boys should receive HPV vaccination, doctors urge government. BMJ 353: i3372. [DOI] [PubMed] [Google Scholar]
- Hamers FF. and European Centre for Disease Prevention and Control (2008) European Centre for Disease Prevention and Control issues guidance for the introduction of human papillomavirus (HPV) vaccines in European Union countries. Eurosurveillance 13: pii: 8022. [PubMed] [Google Scholar]
- Hein IM, De Vries MC, Troost PW, et al. (2015) Informed consent instead of assent is appropriate in children from the age of twelve: policy implications of new findings on children’s competence to consent to clinical research. BMC Medical Ethics 16: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh HF, Shannon SE. (2005) Three approaches to qualitative content analysis. Qualitative Health Research 15: 1277–1288. [DOI] [PubMed] [Google Scholar]
- Kuhne G, Rigby MJ, Majeed A, et al. (2017) Towards safe and efficient child primary care - gaps in the use of unique identifiers in europe. Studies in Health Technology and Informatics 235: 53–57. [PubMed] [Google Scholar]
- Martakis K, Brand H, Schroder-Back P. (2018) Developing child autonomy in pediatric healthcare: towards an ethical model. Archivos Argentinos De Pediatria 116: e401–e408. [DOI] [PubMed] [Google Scholar]
- RKI (2018) Vorabinformation: STIKO empfiehlt HPV-Impfung für Jungen. Available at: https://www.rki.de/DE/Content/Kommissionen/STIKO/Empfehlungen/Vorabinformation_HPV_Jungen.html (accessed 2 August 2018).
- Schloemer T, Schroder-Back P. (2018) Criteria for evaluating transferability of health interventions: a systematic review and thematic synthesis. Implementation Science 13: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh S, Biundo E, Courcier S, et al. (2018) A report on the status of vaccination in Europe. Vaccine 36: 4979–4992. [DOI] [PubMed] [Google Scholar]
- UNICEF (1989) Convention on the rights of the child. Child Labor 8. [Google Scholar]
- UNICEF and United Nations Centre for Human Rights (1991) Convention on the Rights of the Child: Information Kit. Geneva: United Nations Centre for Human Rights. [Google Scholar]
- van der Willik J, Reijneveld S, Michaud P, et al. (2016) The primary care indicator set for adolescents: the EU MOCHA project: Danielle jansen. The European Journal of Public Health 26: ckw164. 049. [Google Scholar]
- Wiesemann C. (2016) Moral Equality, Bioethics, and the Child. Berlin: Springer. [Google Scholar]
- Wise J. (2017) Teenage boys shouldn’t be given HPV vaccine, says joint committee. BMJ 358: j3523. [DOI] [PubMed] [Google Scholar]