Abstract
Intracranial metastatic disease (IMD) is a common and severe complication of primary cancers. Current treatment options for IMD include surgical resection and radiation therapy, although there has been recent interest in targeted therapy in the management of IMD. As of yet, insufficient data exist to support the recommendation of targeted therapies in the treatment of IMD. Paradoxically, targeted therapy has been hypothesized to play a role in the development of IMD in patients with primary cancers. This is based on the observations that patients who receive targeted therapy for primary cancer experience prolonged survival, and that prolonged survival has been associated with increased incidence of IMD. Few data exist to clarify if treatment of primary cancers with targeted therapies influences IMD incidence. Here, we discuss the role of targeted therapy in IMD management, review the current literature on IMD incidence and targeted therapy use in primary cancer, and propose the need for future studies to inform physicians in choosing treatment options and counseling patients.
Keywords: intracranial metastatic disease (IMD), brain metastases, targeted therapy, survival, incidence
Introduction
The development of intracranial metastatic disease (IMD) complicates the course of approximately 20% of patients with cancer, with the highest frequency of brain metastases arising in patients with melanoma (7–16%), breast cancer (5–20%), and lung cancer (20–56%) (1–3). The consequences of IMD are severe: across all cancers, patients with IMD have a 2-year survival of 8.1% (1). Prognosis is informed by patient age, Karnofksy performance status, extent of disease, and in recent years, molecular marker status, such as HER2/neu in breast cancer and EGFR in non-small cell lung cancer (4). Importantly, molecular marker status has also opened up the possibility for treatment of brain metastases with targeted therapies.
Targeted therapies are medications that inhibit cancer-specific driver mutations. For example, vemurafenib is a small molecule inhibitor of the B-raf/MEK pathway specific for cells possessing the V600E BRAF mutation. The B-raf/MEK pathway is a driver of cancer cell proliferation and survival in BRAF-mutant melanoma; inhibition of this pathway with vemurafenib results in programmed cell death in these melanoma cells (5). The arrival of targeted therapies has revolutionized cancer treatment and improved outcomes for many patients with cancer. However, little is known about role of targeted therapies in the treatment of patients with IMD, or if targeted therapies modify the risk of development of IMD in patients with systemic cancer. Some targeted therapies have been shown to improve survival in patients with brain metastases, a cohort deemed previously to harbor a uniformly poor survival (1).
Targeted Therapies and Survivorship in IMD
The therapeutic options that have historically been considered for treatment of IMD include surgical resection and radiation therapy; chemotherapies have not generally been useful in the treatment of brain metastases (6). Surgical resection has historically been reserved for patients with good Karnofsky performance status (KPS >70), well-controlled systemic disease, and a single or few accessible tumors (1, 7). Stereotactic radiosurgery (SRS), a therapy previously recommended for treating patients with up to four brain metastases (or >4 with cumulative volume <7 mL), is broadening its scope, and is now in clinical trial for patients with up to 20 brain metastases (NCT03075072) (8). Whole brain radiation therapy (WBRT) has historically been used as frontline therapy in patients with multiple brain metastases, but has been associated with neurocognitive decline in areas of episodic memory, executive function, processing speed, and fine motor control (6, 9, 10). Neuroprotective strategies adjunct to WBRT, such as memantine administration and hippocampal sparing, have been shown to reduce some of the deleterious neurocognitive effects of WBRT (6, 9, 10). Interest therefore exists in augmenting the treatment landscape, and replacing or delaying upfront radiotherapy with another treatment modality, such as targeted therapies (11).
Unfortunately, the data available in the literature on survival in patients with IMD treated with targeted therapies are limited and mixed. Existing studies support the hypothesis that patients who receive targeted therapies for the treatment of IMD experience prolonged survival (11–15). However, these studies have been limited by including only single study arms or too few patients, and have largely restricted their focus to IMD arising from single primary cancer subtypes. Some contradictory data also exist suggesting decreased overall survival (OS) with the use of targeted therapy for patients with IMD (16). Additionally, new-generation targeted therapies, such as alectinib and osimertinib, have been approved in only the last few years, and little is known about their outcomes on a population scale, although trial data suggest CNS efficacy (17, 18). At this time, the 2019 guidelines from the Congress of Neurological Surgeons cite insufficient evidence to recommend targeted therapies in treating IMD (19).
Targeted Therapies and IMD Incidence
One factor of import in addition to considering the effect of targeted therapies on survival in patients with IMD is the effect of these drugs on patient survival independent of the development of IMD. Targeted therapies have been shown to improve systemic disease control and prolong OS in patients with multiple cancer subtypes (20–23). Some literature supports the hypothesis that prolonged survival in patients with cancer is associated with increased incidence of IMD (3, 24, 25). In other words, targeted therapies for primary cancer may paradoxically be associated with increased incidence of brain metastases by extending patient survival through improved control of systemic disease, while relegating the brain as a “sanctuary” site in which undetected intracranial micrometastases are sheltered from systemic treatment that is unable to penetrate the “sanctuary” of the blood-brain barrier (BBB) (14, 24–29). For example, a meta-analysis of three randomized trials found that patients taking trastuzumab for HER2/neu-positive breast cancer had improved OS, but were 1.82 times more likely to develop IMD than non-trastuzumab comparators (29). Similarly, in patients with BRAF-mutant melanoma, one retrospective study found that 90 patients taking BRAF inhibitors were 30% more likely than a chemotherapy comparator group to develop IMD, although these results were not significant (p = 0.5129), nor did the study report data comparing OS in patients without IMD (14). In patients with EGFR-mutant non-small cell lung cancer, patients receiving first-line EGFR-targeted therapies had improved OS, but were 1.35 times more likely to develop IMD compared with patients receiving other therapies (28), although other analyses suggest the same first-line EGFR-targeted therapies decrease the incidence of IMD (30, 31).
Conversely, some have postulated that newer targeted therapies that are capable of crossing the BBB may decrease the incidence of IMD by overcoming the sanctuary effect. A randomized controlled trial of alectinib (BBB-penetrant) vs. crizotinib (less BBB-penetrant) for ALK-positive non-small cell lung cancer showed 12-month cumulative incidences of central nervous system progression of 9.4 and 41.4%, respectively (18, 32). Importantly, alectinib did not offer these patients a survival benefit beyond that gained by therapy with crizotinib: the 12-month survival rate was 84.3% (95% CI 78.4–90.2) for patients receiving alectinib, and 82.5% (95% CI 76.1–88.9) for patients receiving crizotinib. In contrast, targeted therapies for renal cell carcinoma (RCC) have been reported to decrease incidence of IMD compared to chemotherapy, despite minimal BBB penetration of these therapies due to active efflux by transporters P-glycoprotein and breast cancer resistance protein (33).
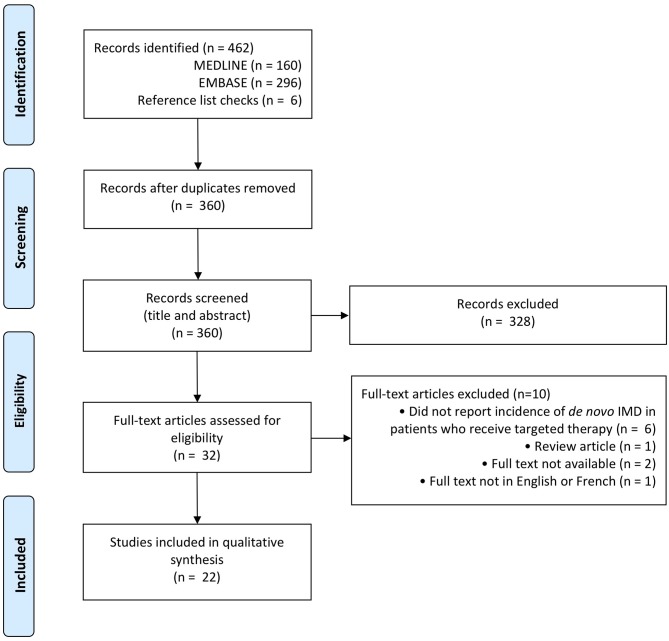
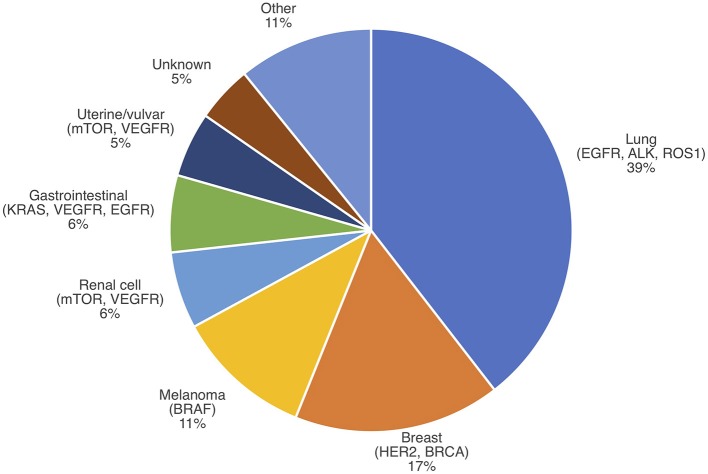
A snapshot of the current literature reveals that knowledge of the impact of targeted therapy on IMD incidence is sparse (Table 1, Figure 1, Appendix 1 in Supplementary Material). Few studies address the question of IMD incidence following targeted therapy in comparison to the volume of literature on IMD survival with targeted therapy. Notably, there appear to be more studies on IMD incidence from breast cancer and non-small cell lung cancer in comparison to melanoma, RCC, and hepatocellular carcinoma (HCC). This may be because targeted therapies for breast cancer and non-small cell lung cancer have existed longer, and in greater number, than for melanoma, RCC, and HCC. This is also consistent with the observed distribution of primary cancers that contribute to IMD prevalence, which attributes 56% of IMD cases to lung and breast cancers (Figure 2) (52). Regardless of primary disease type, most of the literature is comprised of retrospective cohort studies at single institutions, limited to several hundred patients, or lacking controls. Some studies are prospective or meta-analyses, but these form the minority.
Table 1.
Select studies reporting on IMD incidence in patients receiving targeted therapy.
| Disease | References | Therapy | Study type | Patients (n) | IMD incidence with targeted therapy | Findings |
|---|---|---|---|---|---|---|
| Breast Cancer | Berghoff et al. (34) | Trastuzumab, lapatinib | Retrospective cohort | 201 | — | IMD incidence trended toward lower in trastuzumab (38.2%) vs. no trastuzumab (57.1%, p = 0.058). IMD incidence trended toward lower in lapatinib (30.8%) vs. no lapatinib (39.6%, p = 0.530). |
| Swain et al. (35) | Pertuzumab vs. placebo (each with trastuzumab + docetaxel) | RCT | 808 | — | IMD incidence trended toward higher in pertuzumab arm (13.7%) vs. placebo arm (12.6%). But, median time-to-CNS-metastasis greater in pertuzumab arm (15.0 months) vs. placebo arm (12.9 months; HR, 0.58; 95% CI 0.39–0.85; p = 0.0049). | |
| Viani et al. (29)* | Trastuzumab vs. no trastuzumab | Meta-analysis | 6,738 | Higher | IMD incidence higher in trastuzumab arms by 1.82-fold (95% CI 1.89–3.16; p = 0.009). | |
| Bria et al. (36)* | Trastuzumab vs. no trastuzumab | Meta-analysis | 6,738 | Higher | IMD incidence higher in trastuzumab arms (RR, 1.57; 95% CI 1.03–2.37; p = 0.033). | |
| Okines et al. (37) | Ado-trastuzumab emtansine | Retrospective cohort | 39 | — | IMD incidence 18% in patients receiving ado-trastuzumab emtansine, with median time-to-IMD 7.5 months (95% CI 3.8–9.6). No control. | |
| Musolino et al. (38) | Trastuzumab vs. no trastuzumab | Retrospective cohort | 1,429 | Higher | IMD incidence higher in patients receiving trastuzumab (10.5%) vs. no trastuzumab (2.9%). HER2+ status and trastuzumab, together, predictive for CNS events (HR, 4.3; 95% CI 1.5–11.8; p = 0.005). | |
| Yau et al. (39) | Trastuzumab | Retrospective cohort | 87 | — | IMD risk not observed to be higher than disease-free population (RR, 1.0; 95% CI 0.4–2.2; p = 0.09). No control. | |
| Melanoma | Sloot et al. (14) | BRAF/MEK inhibitor vs. chemo | Retrospective cohort | 610 | — | IMD incidence not higher in BRAF inhibitor vs. chemotherapy (OR, 1.3; 95% CI 0.6–2.49; p = 0.5129). |
| Peuvrel et al. (40) | Vemurafenib | Retrospective cohort | 86 | — | IMD incidence 20% in patients receiving vemurafenib, with median time-to-IMD 5.3 months (±4.3). No control. | |
| NSCLC | Heon et al. (31) | EGFR inhibitor | Retrospective cohort | 81 | Lower | IMD incidence lower in EGFR inhibitor arms (25% at 42 months) vs. historical comparators (40–55% at 35–37 months). No study control. |
| Wang et al. (28) | EGFR inhibitor vs. other therapy | Retrospective cohort | 1,254 | Higher | IMD incidence higher in EGFR inhibitor vs. other therapy (HR,1.36; 95% CI 1.14–1.64; p = 0.001). | |
| Su et al. (41) | Gefitinib vs.Erlotinib vs.afatinib | Retrospective cohort | 219 | — | IMD incidences at 24 months for gefitinib (13.9%), erlotinib (9.3%), and afatinib (28.3%) were not significantly different (p = 0.80). Hazard ratio for IMD in afatinib vs. gefitinib 0.49 (95% CI 0.34–0.71; p = 0.001) | |
| Fu et al. (42) | Bevacizumab + chemo vs. chemo | Retrospective cohort | 159 | Lower | IMD incidence at 24 months lower in the bevacizumab + chemo arm (14.0%) vs. chemo arm (31%, p <0.01). | |
| Ilhan-Mutlu et al. (43) | Bevacizumab vs. chemo | Retrospective cohort | 1,043 | Lower | IMD incidence at 24 months lower for bevacizumab (2.6%) vs. chemo (5.8%, p = 0.01; HR, 0.36; 95% CI 0.19–0.68; p = 0.001). | |
| Gadgeel et al. (18) | Crizotinib vs. alectinib | RCT | 181 | — | IMD incidence at 12 months lower for alectinib (4.6%; 95% CI 1.5–10.6%) vs. crizotinib (31.5%; 95% CI 22.1–41.3%). Time-to-CNS progression longer in alectinib vs. crizotinib (csHR, 0.14; 95% CI 0.06–0.33; p < 0.0001). | |
| Nishio et al. (44) | Crizotonib vs. alectinib | Retrospective cohort | 164 | — | Time-to-CNS progression longer in alectinib vs. crizotinib (HR, 0.19; 95% CI: 0.07–0.53; p = 0.0004). | |
| Zhao et al. (45) | Icotinib vs. chemo | Retrospective cohort | 396 | Lower | IMD incidence at 24 months lower for icotinib (10.2%) vs. chemotherapy (32.1%). Hazard ratio for IMD in chemotherapy vs. icotinib 3.32 (95% CI 1.89–5.82; p < 0.001). | |
| RCC | Verma et al. (46) | TKI vs. no TKI | Retrospective cohort | 338 | Lower | IMD incidence lower in TKI vs. no TKI (HR, 0.39; 95% CI 0.21–0.73; p = 0.003). |
| Dudek et al. (33) | TKI vs. no TKI | Retrospective cohort | 92 | Lower | IMD incidence lower in TKI vs. no TKI (per month incidence rate ratio 1.568; 95% CI 1.06–2.33). | |
| Massard et al. (47) | Sorafenib vs. placebo | Retrospective cohort | 139 | Lower | IMD incidence lower in sorafenib (3%) vs. placebo (12%, p < 0.05). | |
| Vanhuyse et al. (48) | Antiangiogenic** vs. other therapy | Retrospective cohort | 199 | — | IMD incidence in targeted therapy group (15.7%) lower than non-targeted therapy group (18.2%). However, targeted therapy was not associated with a lower cumulative rate of brain metastases (HR, 0.58; 95% CI 0.26–1.30; p = 0.18). | |
| HCC | Shao et al. (49) | Antiangiogenic therapy *** | Retrospective cohort | 158 | Higher | IMD incidence 7% in patients receiving antiangiogenic targeted therapies vs. 0.2–2.2% in historical comparators. Median time-to-IMD 9.6 months. |
- Incidence trends marked with a dash if study reports 1) insignificant results, 2) only comparison between multiple targeted therapies, or 3) no control.
Both Viani et al. and Bria et al. report on the same datasets.
Antiangiogenic therapies in Vanhuyse et al. study = sorafenib, sunitinib, bevacizumab, temsirolimus, or everolimus.
Antiangiogenic therapies in Shao et al. study = sorafenib, sorafenib plus tegafur/uracil, sunitinib, bevacizumab plus capecitabine, bevacizumab plus erlotinib, or thalidomide plus tegafur/uracil.
(cs)HR, (cause-specific) hazard ratio; RR, relative risk; RCT, randomized controlled trial; NSCLC, non-small cell lung cancer; RCC, renal cell carcinoma; HCC, hepatocellular carcinoma; TKI, tyrosine kinase inhibitor (sorafenib or sunitinib); EGFR inhibitor, gefitinib or erlotinib; BRAF/MEK inhibitor, BRAF, vemurafenib or dabrafenib; MEK, cobimetinib or trametinib.
Figure 1.
PRISMA flow diagram for IMD incidence with targeted therapy (50).
Figure 2.
IMD incidence by primary cancer type with select actionable mutations. Modified from Nussbaum et al. (51).
The current literature is also mixed on whether targeted therapies increase, decrease, or have any impact on the incidence of IMD incidence. Many studies report insignificant differences in IMD incidence between patients receiving a targeted therapy vs. a conventional chemotherapy (14, 34, 35, 39, 48). In breast cancer, most studies indicate that targeted therapy is associated with increase in IMD incidence (29, 35, 38). One study reports a prolonged median time-to-IMD in patients receiving targeted therapy vs. other therapies, supporting the “sanctuary” hypothesis that prolonged survival due to systemic disease control increases IMD risk (35). In RCC, targeted therapy is associated with a decrease in IMD incidence (33, 46, 47). In non-small cell lung cancer, some studies report an increase in IMD incidence with use of a targeted therapy, while others report an associated decrease (28, 31, 42, 43). One hypothesis to explain these differences between primary disease types is that the targeted therapies studied in breast cancer, such as the 140kDa+ monoclonal antibodies trastuzumab and pertuzumab, are less BBB-penetrant than available targeted therapies in RCC, such as the small molecule kinase inhibitors sunitinib and sorafenib, while there is a range of BBB-penetrability among the therapies used in non-small cell lung cancer. However, the arrival of novel BBB-penetrant agents may be anticipated to disrupt these trends.
Future Directions
The questions of IMD incidence and survival are relevant today because the frequency of IMD is rising, while prognosis remains poor (3). As improvements are made in the early detection of IMD and the management of systemic disease, more clinicians will counsel patients on the risk and management of IMD. Additionally, the use of targeted therapies is expected to increase as the management of both primary systemic disease and IMD moves toward precision methods, raising the question of the impact of targeted therapies on IMD incidence and survival (11). Formal appraisal to date has found insufficient evidence for the use of targeted therapies in the treatment of IMD, and the question of IMD incidence following targeted therapy remains debated (19).
Future studies may address these gaps from multiple approaches. Trials of targeted therapies have historically excluded patients with baseline IMD, but more recent studies have done so, beginning the process of clarifying the role of targeted therapy in the management of this disease. Prospective collection of data on intracranial outcomes in patients treated with a targeted therapy will elucidate the risk of IMD and provide insight on the role of targeted therapy in treating IMD. Future retrospective studies interested in the question of IMD incidence may examine larger populations to more finely control for covariates like cancer mutation status, or compare the effects of targeted therapies across primary disease types. Meta-analyses will benefit from broader reporting of IMD incidence stratified by status of baseline CNS disease, and database studies will allow observation of longer-term outcomes across institutions as survival with IMD improves.
While the 2019 guidelines from the Congress of Neurological Surgeons do not make recommendations on the use of targeted therapy in the management of IMD, they note in their evidence review that therapies and studies since 2015 were not considered. Yet, targeted therapies in the field of IMD have undergone explosive development since that time, with new approvals in breast cancer, non-small cell lung cancer, melanoma, and RCC. New data will clarify the role of targeted therapy in the initial treatment of IMD, and clinicians will be required to make complex management decisions considering treatment sequencing, multimodal strategies with radiation and surgery, and weighing survival and quality of life for their patients with IMD. As survivorship in primary disease improves, more physicians may expect to discuss IMD risk with patients receiving targeted therapy, or to consider the implementation of focused surveillance imaging. Targeted therapy may replace the frontline modalities in the management of IMD, or it may occupy a prophylactic role for patients with primary disease. More immediately, targeted therapy may fill adjuvant or neoadjuvant roles alongside the current standard IMD treatments, and may vary between primary disease types.
Conclusion
Targeted therapies are emerging onto a dynamic treatment landscape for IMD, and future work will elucidate their place among current standards. Present data are few on IMD incidence among patients receiving targeted therapies for primary cancers, often limited to studies with single arms or small sample sizes. Future studies will stratify IMD incidence according to the BBB penetrance of targeted therapies in order to clarify the role of targeted therapies in preventing—or facilitating—the development of IMD. There is also a need for larger studies with higher power to elucidate the impact of targeted therapy on both incidence and survival in IMD. As more novel agents are developed, and the management of systemic disease improves, the treatment landscape for IMD may be expected to change, and physicians may anticipate considering IMD risk as they create management plans and counsel patients.
Author Contributions
AE and SD wrote and revised the manuscript. AE was responsible for review of the literature and drawing the table and figures. SD designed the article.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. SD is supported by an Early Researcher Award from the Province of Ontario. AE is supported by the Graduate Diploma in Health Research program at the University of Toronto.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2019.00797/full#supplementary-material
References
- 1.Achrol AS, Rennert RC, Anders C, Soffietti R, Ahluwalia MS, Nayak L, et al. Brain metastases. Nat Rev Dis Primers. (2019) 5:5. 10.1038/s41572-018-0055-y [DOI] [PubMed] [Google Scholar]
- 2.Cagney DN, Martin AM, Catalano PJ, Redig AJ, Lin NU, Lee EQ, et al. Incidence and prognosis of patients with brain metastases at diagnosis of systemic malignancy: a population-based study. Neuro Oncol. (2017) 19:1511–21. 10.1093/neuonc/nox077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol. (2004) 22:2865–72. 10.1200/JCO.2004.12.149 [DOI] [PubMed] [Google Scholar]
- 4.Sperduto PW, Kased N, Roberge D, Xu Z, Shanley R, Luo X, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol. (2012) 30:419–25. 10.1200/JCO.2011.38.0527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ascierto PA, Kirkwood JM, Grob JJ, Simeone E, Grimaldi AM, Maio M, et al. The role of BRAF V600 mutation in melanoma. J Transl Med. (2012) 10:85. 10.1186/1479-5876-10-85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kotecha R, Gondi V, Ahluwalia MS, Brastianos PK, Mehta MP. Recent advances in managing brain metastasis. (2018) 7:F1000Res. 10.12688/f1000research.15903.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soffietti R, Abacioglu U, Baumert B, Combs SE, Kinhult S, Kros JM, et al. Diagnosis and treatment of brain metastases from solid tumors: guidelines from the European Association of Neuro-Oncology (EANO). Neuro Oncol. (2017) 19:162–74. 10.1093/neuonc/now241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Graber JJ, Cobbs CS, Olson JJ. Congress of neurological surgeons systematic review and evidence-based guidelines on the use of stereotactic radiosurgery in the treatment of adults with metastatic brain tumors. Neurosurgery. (2019) 84:E168–70. 10.1093/neuros/nyy543 [DOI] [PubMed] [Google Scholar]
- 9.Tsao MN, Xu W, Wong RK, Lloyd N, Laperriere N, Sahgal A, et al. Whole brain radiotherapy for the treatment of newly diagnosed multiple brain metastases. Cochrane Database Syst Rev. (2018) 1:CD003869. 10.1002/14651858.CD003869.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaspar LE, Prabhu RS, Hdeib A, McCracken DJ, Lasker GF, McDermott MW, et al. Congress of neurological surgeons systematic review and evidence-based guidelines on the role of whole brain radiation therapy in adults with newly diagnosed metastatic brain tumors. Neurosurgery. (2019) 84:E159–62. 10.1093/neuros/nyy541 [DOI] [PubMed] [Google Scholar]
- 11.Martinez P, Mak RH, Oxnard GR. Targeted therapy as an alternative to whole-brain radiotherapy in EGFR-mutant or ALK-positive non-small-cell lung cancer with brain metastases. JAMA Oncol. (2017) 3:1274–5. 10.1001/jamaoncol.2017.1047 [DOI] [PubMed] [Google Scholar]
- 12.Sperduto PW, Yang TJ, Beal K, Pan H, Brown PD, Bangdiwala A, et al. Estimating survival in patients with lung cancer and brain metastases: an update of the graded prognostic assessment for lung cancer using molecular markers (Lung-molGPA). JAMA Oncol. (2017) 3:827–31. 10.1001/jamaoncol.2016.3834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Juloori A, Miller JA, Parsai S, Kotecha R, Ahluwalia MS, Mohammadi AM, et al. Overall survival and response to radiation and targeted therapies among patients with renal cell carcinoma brain metastases. J Neurosurg. (2019) 18:1–9. 10.3171/2018.8.JNS182100 [DOI] [PubMed] [Google Scholar]
- 14.Sloot S, Chen YA, Zhao X, Weber JL, Benedict JJ, Mule JJ, et al. Improved survival of patients with melanoma brain metastases in the era of targeted BRAF and immune checkpoint therapies. Cancer. (2018) 124:297–305. 10.1002/cncr.30946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ha FJ, Spain L, Dowling A, Kwan EM, Pezaro C, Day D, et al. Timing of brain metastases development in metastatic renal cell cancer patients treated with targeted therapies and survival outcomes: an Australian multicenter study. Asia Pac J Clin Oncol. (2019). 10.1111/ajco.13109. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 16.Sperduto PW, Wang M, Robins HI, Schell MC, Werner-Wasik M, Komaki R, et al. A phase 3 trial of whole brain radiation therapy and stereotactic radiosurgery alone versus WBRT and SRS with temozolomide or erlotinib for non-small cell lung cancer and 1 to 3 brain metastases: Radiation Therapy Oncology Group 0320. Int J Radiat Oncol Biol Phys. (2013) 85:1312–8. 10.1016/j.ijrobp.2012.11.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goss G, Tsai CM, Shepherd FA, Ahn MJ, Bazhenova L, Crino L, et al. CNS response to osimertinib in patients with T790M-positive advanced NSCLC: pooled data from two phase II trials. Ann Oncol. (2018) 29:687–93. 10.1093/annonc/mdx820 [DOI] [PubMed] [Google Scholar]
- 18.Gadgeel S, Peters S, Mok T, Shaw AT, Kim DW, Ou SI, et al. Alectinib versus crizotinib in treatment-naive anaplastic lymphoma kinase-positive (ALK+) non-small-cell lung cancer: CNS efficacy results from the ALEX study. Ann Oncol. (2018) 29:2214–22. 10.1093/annonc/mdy405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elder JB, Nahed BV, Linskey ME, Olson JJ. Congress of neurological surgeons systematic review and evidence-based guidelines on the role of emerging and investigational therapties for the treatment of adults with metastatic brain tumors. Neurosurgery. (2019) 84:E201–E3. 10.1093/neuros/nyy547 [DOI] [PubMed] [Google Scholar]
- 20.Coppin C, Kollmannsberger C, Le L, Porzsolt F, Wilt TJ. Targeted therapy for advanced renal cell cancer (RCC): a Cochrane systematic review of published randomised trials. BJU Int. (2011) 108:1556–63. 10.1111/j.1464-410X.2011.10629.x [DOI] [PubMed] [Google Scholar]
- 21.Moja L, Tagliabue L, Balduzzi S, Parmelli E, Pistotti V, Guarneri V, et al. Trastuzumab containing regimens for early breast cancer. Cochrane Database Syst Rev. (2012) 4:CD006243 10.1002/14651858.CD006243.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen KS, Neal JW, Wakelee H. Review of the current targeted therapies for non-small-cell lung cancer. World J Clin Oncol. (2014) 5:576–87. 10.5306/wjco.v5.i4.576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eggermont AMM, Robert C, Ribas A. The new era of adjuvant therapies for melanoma. Nat Rev Clin Oncol. (2018) 15:535–6. 10.1038/s41571-018-0048-5 [DOI] [PubMed] [Google Scholar]
- 24.Gampa G, Vaidhyanathan S, Resman BW, Parrish KE, Markovic SN, Sarkaria JN, et al. Challenges in the delivery of therapies to melanoma brain metastases. Curr Pharmacol Rep. (2016) 2:309–25. 10.1007/s40495-016-0072-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bartsch R, Berghoff A, Pluschnig U, Bago-Horvath Z, Dubsky P, Rottenfusser A, et al. Impact of anti-HER2 therapy on overall survival in HER2–overexpressing breast cancer patients with brain metastases. Br J Cancer. (2012) 106:25–31. 10.1038/bjc.2011.531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Puhalla S, Elmquist W, Freyer D, Kleinberg L, Adkins C, Lockman P, et al. Unsanctifying the sanctuary: challenges and opportunities with brain metastases. Neuro Oncol. (2015) 17:639–51. 10.1093/neuonc/nov023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nolan C, Deangelis LM. Overview of metastatic disease of the central nervous system. Handb Clin Neurol. (2018) 149:3–23. 10.1016/B978-0-12-811161-1.00001-3 [DOI] [PubMed] [Google Scholar]
- 28.Wang BX, Ou W, Mao XY, Liu Z, Wu HQ, Wang SY. Impacts of EGFR mutation and EGFR-TKIs on incidence of brain metastases in advanced non-squamous NSCLC. Clin Neurol Neurosurg. (2017) 160:96–100. 10.1016/j.clineuro.2017.06.022 [DOI] [PubMed] [Google Scholar]
- 29.Viani GA, Afonso SL, Stefano EJ, De Fendi LI, Soares FV. Adjuvant trastuzumab in the treatment of her-2–positive early breast cancer: a meta-analysis of published randomized trials. BMC Cancer. (2007) 7:153. 10.1186/1471-2407-7-153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heon S, Yeap BY, Lindeman NI, Joshi VA, Butaney M, Britt GJ, et al. The impact of initial gefitinib or erlotinib versus chemotherapy on central nervous system progression in advanced non-small cell lung cancer with EGFR mutations. Clin Cancer Res. (2012) 18:4406–14. 10.1158/1078-0432.CCR-12-0357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heon S, Yeap BY, Britt GJ, Costa DB, Rabin MS, Jackman DM, et al. Development of central nervous system metastases in patients with advanced non-small cell lung cancer and somatic EGFR mutations treated with gefitinib or erlotinib. Clin Cancer Res. (2010) 16:5873–82. 10.1158/1078-0432.CCR-10-1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peters S, Camidge DR, Shaw AT, Gadgeel S, Ahn JS, Kim DW, et al. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med. (2017) 377:829–38. 10.1056/NEJMoa1704795 [DOI] [PubMed] [Google Scholar]
- 33.Dudek AZ, Raza A, Chi M, Singhal M, Oberoi R, Mittapalli RK, et al. Brain metastases from renal cell carcinoma in the era of tyrosine kinase inhibitors. Clin Genitourin Cancer. (2013) 11:155–60. 10.1016/j.clgc.2012.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berghoff AS, Bago-Horvath Z, Dubsky P, Rudas M, Pluschnig U, Wiltschke C, et al. Impact of HER-2–targeted therapy on overall survival in patients with HER-2 positive metastatic breast cancer. Breast J. (2013) 19:149–55. 10.1111/tbj.12070 [DOI] [PubMed] [Google Scholar]
- 35.Swain SM, Baselga J, Miles D, Im YH, Quah C, Lee LF, et al. Incidence of central nervous system metastases in patients with HER2–positive metastatic breast cancer treated with pertuzumab, trastuzumab, and docetaxel: results from the randomized phase III study CLEOPATRA. Ann Oncol. (2014) 25:1116–21. 10.1093/annonc/mdu133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bria E, Cuppone F, Fornier M, Nisticò C, Carlini P, Milella M, et al. Cardiotoxicity and incidence of brain metastases after adjuvant trastuzumab for early breast cancer: the dark side of the moon? A meta-analysis of the randomized trials. Breast Cancer Res Treat. (2008) 109:231–9. 10.1007/s10549-007-9663-z [DOI] [PubMed] [Google Scholar]
- 37.Okines A, Irfan T, Khabra K, Smith I, O'Brien M, Parton M, et al. Development and responses of brain metastases during treatment with trastuzumab emtansine (T-DM1) for HER2 positive advanced breast cancer: A single institution experience. Breast J. (2018) 24:253–9. 10.1111/tbj.12906 [DOI] [PubMed] [Google Scholar]
- 38.Musolino A, Ciccolallo L, Panebianco M, Fontana E, Zanoni D, Bozzetti C, et al. Multifactorial central nervous system recurrence susceptibility in patients with HER2–positive breast cancer: epidemiological and clinical data from a population-based cancer registry study. Cancer. (2011) 117:1837–46. 10.1002/cncr.25771 [DOI] [PubMed] [Google Scholar]
- 39.Yau T, Swanton C, Chua S, Sue A, Walsh G, Rostom A, et al. Incidence, pattern and timing of brain metastases among patients with advanced breast cancer treated with trastuzumab. Acta Oncol. (2006) 45:196–201. 10.1080/02841860500486630 [DOI] [PubMed] [Google Scholar]
- 40.Peuvrel L, Saint-Jean M, Quéreux G, Brocard A, Khammari A, Knol AC, et al. Incidence and characteristics of melanoma brain metastases developing during treatment with vemurafenib. J Neurooncol. (2014) 120:147–54. 10.1007/s11060-014-1533-z [DOI] [PubMed] [Google Scholar]
- 41.Su PL, Wu YL, Chang WY, Ho CL, Tseng YL, Lai WW, et al. Preventing and treating brain metastases with three first-line EGFR-tyrosine kinase inhibitors in patients with EGFR mutation-positive advanced non-small cell lung cancer. Ther Adv Med Oncol. (2018) 10:1758835918797589. 10.1177/1758835918797589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fu Y, Hu J, Du N, Jiao S, Li F, Li X, et al. Bevacizumab plus chemotherapy versus chemotherapy alone for preventing brain metastasis derived from advanced lung cancer. J Chemother. (2016) 28:218–24. 10.1179/1973947815Y.0000000045 [DOI] [PubMed] [Google Scholar]
- 43.Ilhan-Mutlu A, Osswald M, Liao Y, Gommel M, Reck M, Miles D, et al. Bevacizumab prevents brain metastases formation in lung adenocarcinoma. Mol Cancer Ther. (2016) 15:702–10. 10.1158/1535-7163.MCT-15-0582 [DOI] [PubMed] [Google Scholar]
- 44.Nishio M, Nakagawa K, Mitsudomi T, Yamamoto N, Tanaka T, Kuriki H, et al. Analysis of central nervous system efficacy in the J-ALEX study of alectinib versus crizotinib in ALK-positive non-small-cell lung cancer. Lung Cancer. (2018) 121:37–40. 10.1016/j.lungcan.2018.04.015 [DOI] [PubMed] [Google Scholar]
- 45.Zhao X, Zhu G, Chen H, Yang P, Li F, Du N. Efficacy of icotinib versus traditional chemotherapy as first-line treatment for preventing brain metastasis from advanced lung adenocarcinoma in patients with epidermal growth factor receptor-sensitive mutation. J Cancer Res Ther. (2014) 10(Suppl.):C155–9. 10.4103/0973-1482.145851 [DOI] [PubMed] [Google Scholar]
- 46.Verma J, Jonasch E, Allen P, Tannir N, Mahajan A. Impact of tyrosine kinase inhibitors on the incidence of brain metastasis in metastatic renal cell carcinoma. Cancer. (2011) 117:4958–65. 10.1002/cncr.26138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Massard C, Zonierek J, Gross-Goupil M, Fizazi K, Szczylik C, Escudier B. Incidence of brain metastases in renal cell carcinoma treated with sorafenib. Ann Oncol. (2010) 21:1027–31. 10.1093/annonc/mdp411 [DOI] [PubMed] [Google Scholar]
- 48.Vanhuyse M, Penel N, Caty A, Fumagalli I, Alt M, Zini L, et al. Do anti-angiogenic therapies prevent brain metastases in advanced renal cell carcinoma? Bull Cancer. (2012) 99:100–6. 10.1684/bdc.2012.1672 [DOI] [PubMed] [Google Scholar]
- 49.Shao YY, Lu LC, Cheng AL, Hsu CH. Increasing incidence of brain metastasis in patients with advanced hepatocellular carcinoma in the era of antiangiogenic targeted therapy. Oncologist. (2011) 16:82–6. 10.1634/theoncologist.2010-0272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. (2009) 62:1006–12. 10.1016/j.jclinepi.2009.06.005 [DOI] [PubMed] [Google Scholar]
- 51.Nussbaum ES, Djalilian HR, Cho KH, Hall WA. Brain metastases. Histology, multiplicity, surgery, and survival. Cancer. (1996) 78:1781–8. [PubMed] [Google Scholar]
- 52.Nussbaum ES, Djalilian HR, Cho KH, Hall WA. Brain metastases. Histology, multiplicity, surgery, and survival. Cancer. (1996) 78:1781–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.