Abstract
Arthropods often have obligate relationships with symbiotic microbes, and recent investigations have demonstrated that such host-microbe relationships could be exploited to suppress natural populations of vector carrying mosquitos. Strategies that target the interplay between agricultural pests and their symbionts could decrease the burden caused by agricultural pests; however, the lack of comprehensive genomic insights into naturally occurring microbial symbionts presents a significant bottleneck. Here we employed amplicon surveys, genome-resolved metagenomics, and scanning electron microscopy to investigate symbionts of the wheat stem sawfly (Cephus cinctus), a major pest that causes an estimated $350 million dollars or more in wheat yield losses in the northwestern United States annually. Through 16S rRNA gene sequencing of two major haplotypes and life stages of wheat stem sawfly, we show a novel Spiroplasma species is ever-present and predominant, with phylogenomic analyses placing it as a member of the ixodetis clade of mollicutes. Using state-of-the-art metagenomic assembly and binning strategies we were able to reconstruct a 714 Kb, 72.7%-complete Spiroplasma genome, which represents just the second draft genome from the ixodetis clade of mollicutes. Functional annotation of the Spiroplasma genome indicated carbohydrate-metabolism involved PTS-mediated import of glucose and fructose followed by glycolysis to lactate, acetate, and propionoate. The bacterium also encoded biosynthetic pathways for essential vitamins B2, B3, and B9. We identified putative Spiroplasma virulence genes: cardiolipin and chitinase. These results identify a previously undescribed symbiosis between wheat stem sawfly and a novel Spiroplasma sp., availing insight into their molecular relationship, and may yield new opportunities for microbially-mediated pest control strategies.
Keywords: Spiroplasma, Scanning electron microscopy, Wheat stem sawfly, Metagenomics, Microbial genomics, Pangenomics, Phylogenomics
Introduction
The wheat stem sawfly (Cephus cinctus) is a hymenopteran insect native to the Western U.S. and Canada. Currently, wheat stem sawflies (further referred to as WSS) are some of the worst pests of wheat production (Shanower & Hoelmer, 2004). WSS causes losses in excess of $350 million US annually (Beres et al., 2011). Females oviposit into wheat stems and, after hatching the larvae consume the internal structures lining the stem, both diminishing photosynthetic capacity of the plant and making it more susceptible to lodging (Shanower & Hoelmer, 2004; Beres et al., 2011). Current methods used by growers to reduce crop losses due to WSS include operational control measures (e.g., crop rotation, tillage, and swathing), infrequent use of insecticides, and biological control agents (e.g., parasitic wasps) (Shanower & Hoelmer, 2004; Beres et al., 2011). Most research efforts aimed at curbing this problem are to develop WSS-resistant wheat cultivars, including solid-stemmed varieties that provide less suitable habitat for WSS reproduction, but many of these wheat varieties have lower yield potentials and inconsistent pith expression, so the identification of alternative controls and other host plant resistance is desirable (Beres, Cárcamo & Byers, 2007; Beres et al., 2011; Knodel et al., 2009; Portman et al., 2018).
One option may be to manipulate symbioses between WSS and any symbiotic microbe(s) to negatively affect the insects’ fitness. Metabolic contributions of symbiotic microbes are often critical to the nutritive, physiological, immunological, reproductive, and developmental health of animals (Torrazza & Neu, 2011; Flint et al., 2012; Thaiss et al., 2016; Borgogna & Yeoman, 2017). Insects, in particular, have many well-described long-term relationships with their microbiota that include obligate-(endo)symbioses necessary for host (and microbe) survival and reproductive success (Moran & Telang, 1998; Wernegreen, 2002). Wolbachia and Spiroplasma are the most prevalent and characterized genera of insect-associated symbionts. Wolbachia are Gram-negative, intracellular, members of the Rickettsial family that are maternally transmitted in at least 40% of arthropod species (Zug & Hammerstein, 2012). Many Wolbachia-insect interactions are parasitic, reducing host lifespan and affecting reproductive phenotypes, causing outcomes such as feminization and male killing of larvae, parthenogenesis, and cytoplasmic incompatibility (Werren, Baldo & Clark, 2008). Because Wolbachia have also been reported to reduce viral replication in their hosts they have been explored as a potential biocontrol for virus-vectoring mosquito populations (Teixeira, Ferreira & Ashburner, 2008; Mousson et al., 2012). A specimen of WSS from Alberta assessed for presence of Wolbachia tested negative (Floate, Kyei-Poku & Coghlin, 2007). Spiroplasma, conversely, are small, helical, motile bacteria lacking cell walls (Anbutsu & Fukatsu, 2011). Most Spiroplasma species are considered commensal, but others have been shown to induce male-killing in their insect host or cause diseases in plants (e.g., Corn Stunt) after transfer by the vector (Chen & Liao, 1975; Regassa & Gasparich, 2006; Haselkorn, 2014; Bolaños, Servín-Garcidueñas & Martínez-Romero, 2015). Some Spiroplasma spp. have also been reported to be mutualistic, improving host defenses against fungal pathogens (Łukasik et al., 2012), parasitic nematodes (Jaenike et al., 2010), and parasitoid wasps (Xie, Vilchez & Mateos, 2010; Xie et al., 2013). Spiroplasma species are grouped into three phylogenetic clades: Citri-Chrysopicola-Mirum (CCM), Apis, and Ixodetis (Gasparich et al., 2004).
Herein, we sought to identify the symbiotic microbes of WSS and obtain insight into the nature of the insect-microbe relationships so that we could begin to determine if these insect-microbial relationships could be exploited as alternate measures to control WSS damage in crops.
Materials and Methods
Insect collection
Midseason haplodiploid larval (n = 10) and early-adult WSS (n = 10) representatives were collected from highland grasses (Flesher pass, Lincoln, MT; n = 10) and lowland wheat crops (Three Forks, MT; n = 10) over the 2013, 2015, and 2016 growing seasons. Together, the highland grass (Mountain) and lowland wheat (Northern) populations represent two of the three major haplotype clades of WSS (Lesieur et al., 2016). Northern sample collection was carried out on private lands with permission from the land owners and Mountain samples on public lands in compliance with existing regulations for insects defined as non-commercial, as determined by local regulatory offices.
Sample preparation and DNA extraction
Specimens were surfaced sterilized by washing samples in 1% bleach and rinsing with an excess of sterile water under aseptic conditions. Twenty adult females and whole larvae were individually processed through a Mo-Bio Power-Soil kit (Mo-Bio, Carlsbad, CA, USA) following the manufacturers protocol with inclusion of a 1 min bead-beating step performed in a Mini-Beadbeater-96 (Biospec products; Bartlesville, OK, USA) at 2,400 oscillations/min and using the 0.7-mm garnet beads supplied with the Mo-Bio kit instead of the 10 min vortex. An additional collection of 50 lowland WSS larvae collected in 2016 were processed exclusively for metagenomic sequencing to increase the representation of the Spiroplasma sp. in the DNA preparation and final sequencing data. This was deemed necessary because 98.9 ± 1.0% of each sample’s reads from whole processed individuals were classified as being eukaryotic, and included host reads (Cephus cinctus) and reads belonging to wheat (Triticum aestivum). The protocol proceeded as follows: Following washing, as above, the 50 lowland WSS larvae were lightly crushed with a mortar and pestle in 3 ml sterile PBS under aseptic conditions. The entire solution was filtered through 450 nm nitrocellulose filters to enrich the bacteria in the cell lysate for Spiroplasma and remove other bacterial species larger than 450 nm, as per Nai et al. (2014). The filtrate was then centrifuged at 16,000× g for 30 min at 4 °C in order to pellet the cells that passed through the filter. The pellet was resuspended, filtered, and centrifuged again. The final pellet was processed through a Mo-Bio Power-Soil kit (Mo-Bio), as described above. This extraction procedure yielded 15 ng total DNA that, following sequencing and retrospective analyses, was found to have reduced the total eukaryotic DNA to 89.9%. This sequence data was used for metagenomic assembly but did not provide Spiroplasma sp. genomic data that was not otherwise captured in metagenomes of individual insects or larvae.
16S rRNA gene sequencing
The V3 and V4 hypervariable regions of the 16S rRNA gene was PCR amplified with previously described dual indexed primers (Yeoman et al., 2018). The PCR was performed using KAPA HiFi DNA Polymerase (Kapa Biosystems, Wilmington, MA, USA) and a PCR protocol with initial denaturation of 98 °C for 45 s, followed by 25 cycles of 15 s denaturation at 98 °C, 30 s annealing step at 60 °C, and 30 s elongation step at 72 °C, and then a final extension at 72 °C for 2 min. The PCR reaction was limited to 25 cycles to minimize the potential for chimera formation and error (Smyth et al., 2010). PCR amplicon concentrations and quality were checked on a Bioanalyzer TapeStation using D1000 Screentapes (both Agilent Technologies, Santa Clara, CA, USA). Resulting amplicons were pooled at equimolar concentrations, cleaned up using the AxyPrep MagTM PCR Clean-up Kit (Corning, NY, USA), and then gel purified from a 1.5% agarose gel using the QIAquick gel extraction kit (QIAGEN, Valencia, CA, USA) according to kit instructions. The 16S rRNA gene library was then quantified using the KAPA Library Quantification Kit (Kapa Biosystems), diluted to 15 pM, mixed with 10% phiX and sequenced on an Illumina MiSeq using a 2 × 250 V2 sequencing kit (Illumina, San Diego, CA, USA). On average, each sample yielded 4,125 reads with one sample only yielding 151 reads. Because the low yielding sample provided the same result as other samples, no further sequencing was attempted, and those results were retained.
Microbial community structure analyses
Raw sequencing data were obtained in FASTQ format and assembled into contigs using the default settings of PANDASEQ (Masella et al., 2012) but with FASTQ output (‘-F’) to retain quality scores. Assembled contigs were then trimmed at the 3′ end from the first nucleotide with a Q-score of <20 using the fastq_quality_trimmer tool from FastX-Toolkit (http://hannonlab.cshl.edu/fastx_toolkit/links.html). Retained contigs with a sequence length of <200 nt were subsequently removed. Sequences were then processed using mothur (Schloss et al., 2009) and aligned to the Silva v119 reference database (Pruesse et al., 2007). Sequences were removed if they had homopolymers >10 nt, an ambiguous nucleotide, did not align over the V3–V4 region of the 16S rRNA gene, or were determined using chimera.uchime to be a chimera. To reduce the effect of sequencing error, the sequences were then pre-clustered based on 2% nearest neighbor approach, previously described (Huse et al., 2010). Sequences were then clustered into phylotypes based on taxonomic classification using the “cluster.split” command (splitmethod = classify), and classified by RDP Naive Bayesian Classifier (Cole et al., 2009). Microbial composition was compared among samples using multivariate statistical approaches provided in the vegan package of R (Oksanen et al., 2007).
SEM sample preparation
Twenty-five larvae washed in 70% ethanol were lightly crushed with a mortar and pestle in 3 ml sterile PBS under aseptic conditions. The entire solution was filtered through a disposable 450 nm nitrocellulose filter (Nai et al., 2014), and pelleted by centrifugation at 16,000× g for 30 min at 4 °C as described above. The pellet was then eluted in 300 µl PBS. A volume of 40 µl of the pellet solution was pipetted onto a coverslip and left for one hour for bacteria to attach. For fixation, a modified protocol from Nai et al. (2014) was used where the cover slip was immersed in a 1.5% glutaraldehyde solution prepared in 0.1 M cacodylic acid buffer (pH 7.3) and incubated at 4 °C overnight. The coverslip was then rinsed in water for 20 min four times. The sample was then progressively dehydrated by placing the slip in solutions increasing by relative ethanol: 25% EtOH 30 min, 50% EtOH 30 min, 75% EtOH 30 min, 95% EtOH 30 min, 100% 60 min (three times to rinse coverslip off). Critical point drying, specimen mounting, and SEM imaging were performed at the Imaging and Chemical Analysis Laboratory (ICAL) at Montana State University. The coverslip was critically point dried in a Tousimis SAMDRI®-795 (Tousimis, Rockville, MD, USA) for thirty minutes, and mounted. The coverslip was then imaged on a Zeiss SUPRA 55VP (Carl Zeiss, Oberkochen, Germany) field emission scanning electron microscope.
Metagenomic sequencing
DNA was prepared using a Nextera XT kit as per manufacturer’s instructions and resulted in ∼250 bp insert sizes. Metagenomic libraries were pooled in equimolar concentrations with three libraries per pool and sequenced on Illumina MiSeq sequencing platform using a 2 × 150 V2 sequencing kit.
After a preliminary round of analysis, it was determined that WSS genome sequence data deposited in NCBI (Robertson et al., 2018), also included sequence data from the Spiroplasma sp. Therefore, in an effort to improve our Spiroplasma genome data and assembly, the raw sequence files from the WSS genome sequencing effort were also downloaded and used.
Metagenomic sequencing analysis
Raw sequencing data obtained in FASTQ format were filtered to remove low-quality reads using ‘iu-filter-quality-minoche’, a program in illumina-utils v1.4.1 (Eren et al., 2013). High-quality reads were assembled into longer contiguous segments of DNA (contigs) using MEGAHIT v1.0.6 (Li et al., 2015), and only contigs longer than 2,500 nt were kept for downstream analyses. Metagenome-assembled genomes (MAGs) were generated and curated using anvi’o v3 following a workflow outlined previously by Eren et al. (2015). Briefly, Bowtie2 (Langmead & Salzberg, 2012) mapped short metagenomic reads back to contigs using default parameters, and samtools (Li et al., 2009) converted resulting SAM files into sorted and indexed BAM files. We then used the program ‘anvi-gen-contigs-database’ to create a contigs database for the assembly, during which Prodigal v2.6.3 (Hyatt et al., 2010) predicted open-reading frames and HMMer identified of bacterial single-copy core genes among contigs. Then we used ‘anvi-profile’ to process BAM files from each metagenome, and merged resulting profile databases using ‘anvi-merge’. We then manually identified metagenomic bins in the anvi’o interactive interface using the hierarchical clustering of contigs based on patterns of tetra-nucleotide frequency and differential coverage patterns across metagenomes. The program ‘anvi-summarize’ generated a static HTML output, which gave access to FASTA files as well as coverage and detection statistics of each genome bin (and individual genes within them) across metagenomes. Comparative analysis of all bacterial single-copy core genes and their coverage were used to estimate genome completeness and redundancy, as previously described (Eren et al., 2015).
Three bins were determined, that phylogenomic analyses of all single copy core genes linked to either the host WSS, wheat, or to a bacterium of the order Entomoplasmatales, to which Spiroplasma belongs. Additional, phylogenetic and phylogenomic analyses described below confirmed this bin as belonging to a novel Sprioplasma sp. Reads that binned with the Spiroplasma sp. were annotated using the standard PATRIC pipeline (Wattam et al., 2013). Reads that binned with the WSS host were translated to their amino acid sequences in all frames using MEGA7 (Kumar, Stecher & Tamura, 2016) and then annotated to KEGG orthologies (KO) using BlastKOALA (Kanehisa, Sato & Morishima, 2016). Both WSS host and Spiroplasma sp. KO’s were then mapped to metabolic maps using iPATH v3 (Letunic et al., 2008) and analyzed to determine unique and overlapping pathways (Fig. S2). An explorable version of this pathway is available at https://pathways.embl.de/selection/ua1khKYbv9AKjZ8PimR.
Pangenomic and phylogenomic analyses
To compute the Spiroplasma pangenome, we used the anvi’o pangenomic workflow outlined by Delmont & Eren (2018) using the anvi’o version v5.5 (Eren et al., 2015). A reproducible workflow for this analysis is available at the URL http://merenlab.org/data/spiroplasma-pangenome. Briefly, we identified and downloaded all Spiroplasma genomes from NCBI using the program ncbi-genome-download (available from https://github.com/kblin/ncbi-genome-download) using flags “–assembly-level complete bacteria –genus Spiroplasma” (Genome accessions and information is listed in Table S1), and stored the information about these genomes that is also reported by ncbi-genome-download into a text file. We then used the anvi’o program anvi-script-process-genbank-metadata with the flag “–exclude-gene-calls-from-fasta-txt”, added three additional MAGs recently generated for members of the Ixodetis clade of mollicutes (EntAcro1 and EntAcro10 (Sapountzis et al., 2018) as well as our Spiroplasma sp. WSS MAG) into the resulting external genomes file. We then run the program anvi-gen-genomes-storage using the external genomes file, and finally the program anvi-pan-genome on the resulting genomes storage to compute the pangenome. To perform a phylogenomic analysis to infer evolutionary associations between genomes, we identified the Spiroplasma single-copy core genes revealed by the pangenome and recovered their aligned amino acid sequences using the program anvi-get-sequences-for-gene-clusters with parameters “–min-num-genomes-gene-cluster-occurs 31 –max-num-genes-from-each-genome 1 –concatenate-gene-clusters”. We then used trimAl v1.4.rev22 (Capella-Gutierrez, Silla-Martinez & Gabaldon, 2009) to remove positions that were gaps in more than 50% of the genes in the alignment using the parameter “-gt 0.50”, and IQ-TREE v1.5.5 (Nguyen et al., 2014) with the ‘WAG’ general matrix model (Whelan & Goldman, 2001) to infer the maximum likelihood tree. We used the program anvi-import-misc-data to import the resulting tree into the anvi’o pan database, and program anvi-display-pan to visualize the output.
Data availability
16S rRNA sequences were submitted to Sequence Read Archive and are available under accession number SRP108219. Metagenomic sequences were submitted to Sequence Read Archive under accession number SRP108220. Additional sequence data generated by Illumina HiSeq as part of the WSS genome project (Robertson et al., 2018) are available under accession number SRS694145. The metagenomic assembled genome is available in GenBank under bioproject PRJNA540284 and is available publicly at Patric with the genome ID 2132.146 (Spiroplasma sp. WSS).
Results
Wheat stem sawflies are colonized by a Spiroplasma sp
16S rRNA gene sequence profiles were obtained from individual whole-body WSS specimens representing two of the three major WSS haplotype clades, Northern and Mountain (Lesieur et al., 2016), and of both late stage larval and adult life stages. On average, each sample yielded 4,090 reads and across all samples these reads clustered into eight different phylotypes that each classified to unique bacterial genera. One phylotype that classified as a Spiroplasma spp., was found to predominate all individuals and life stages and represented 88.5 ± 8.3% of all reads (Table 1). Other phylotypes were much less abundant and their observed presence varied among samples. Each of the less prevalent and abundant phylotypes were classified as genera from the family Pasteurellaceae, including Nicoletella, Histophilus, Lonepinella, Actinobacillus, Basfia, Haemophilus, and Aggregatibacter genera (Table 1).
Table 1. Relative abundance of bacterial phylotypes from 16S rRNA survey.
Numbers represent the average ± standard deviation of the relative abundance of each detected phylotype from five replicates of each life stage and haplotype. Where a phylotype was not detected in any replicate it is denoted as n.d.
| Genera | Mountain Larvae | Mountain Adult | Northern Larvae | Northern Adult |
|---|---|---|---|---|
| Spiroplasma | 85 ± 4.7% | 92 ± 9.6% | 92 ± 5.0% | 85 ± 12% |
| Actinobacillus | 0.3 ± 0.8% | 0.9 ± 0.9% | 0.4 ± 0.5% | n.d |
| Aggregatibacter | 2.1 ± 2.3% | 1.0 ± 0.7% | 0.2 ± 0.4% | 0.6 ± 1.3% |
| Basfia | 0.2 ± 0.5% | n.d. | 0.5 ± 0.7% | n.d. |
| Haemophilus | 0.2 ± 0.5% | 0.3 ± 0.6% | n.d. | n.d. |
| Histophilus | 4.9 ± 2.7% | 2.6 ± 4.7% | 0.8 ± 1.8% | 3.3 ± 4.1% |
| Lonepinella | 0.9 ± 1.3% | 0.7 ± 1.2% | 0.3 ± 0.7% | 0.6 ± 1.1% |
| Nicoletella | 6.2 ± 2.6% | 2.8 ± 3.2% | 6.0 ± 3.8% | 10 ± 7.7% |
An α-helical bacterium consistent with Spiroplasma is observable in larval lysates via SEM imaging
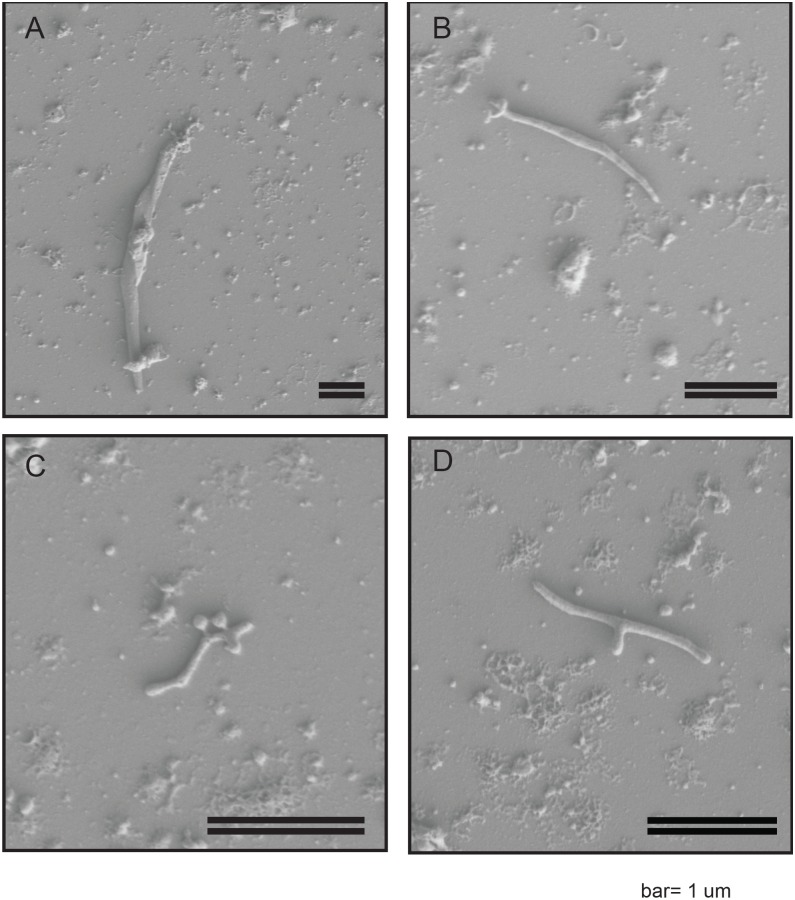
In order to confirm the presence of a Spiroplasma spp. in WSS, we employed a previously described protocol for the enrichment of Spiroplasma (Nai et al., 2014) to WSS larvae lysates and interrogated the resultant via Scanning Electron Microscopy (SEM). Cells were identified that had long filamentous shapes varying from 1–4 uM in length. These cells were not observed to be distinctly helical (Fig. 1) but were consistent with previously described Spiroplasma spp. morphologies. Some cells exhibited a y-shaped morphology (Fig. 1D), that has previously been observed in Spiroplasma poulsonii during division via longitudinal scission (Ramond et al., 2016).
Figure 1. SEM images of WSS reveal non-helical Spiroplasma-like morphotype.
Scanning Electron Microscopy Images of filtered WSS larvae lysates reveal a non-helical filamentous bacterium, consistent with Spiroplasma. Cells were non-helical and filamentous, ranging 1–4 µM in length (A–C). Some cells exhibited a y-shaped morphology, which may be cells replicating via longitudinal fission (D). The scale bar is 1 µM.
Metagenomic and genomic insight into the WSS Spiroplasma sp.
Attempts to cultivate the Spiroplasma sp. using previously described media for the cultivation of other Spiroplasma spp. were unsuccessful (for further discussion see Text S1). So, to gain insight into the genomic context of the WSS-associated Spiroplasma population, we used a genome-resolved metagenomics approach. On average sequencing of each individual WSS adult and larval specimen yielded ∼2.9 million reads per sample, which preliminary analyses determined to contain 98.9 ± 1.0% eukaryotic reads. A second separate preparation of 50 Northern WSS larvae following a previously described protocol to enrich for Spiroplasma spp. (Nai et al., 2014) yielded ∼20 million reads that were found to comprise 89.9% eukaryotic reads. Larvae were selected for this process because they were found to have greater DNA sequence representation of the Spiroplasma sp. in preliminary analyses of individual samples (0.21 ± 0.52% adults vs. 1.4 ± 0.83% larvae). The co-assembly of 12 metagenomes and the enriched preparation resulted in 14,217 contigs longer than 2,500 nt. The majority of these contigs matched to the host WSS genome, however, we were able to identify two additional genome bins among the rest of the contigs (Fig. S1). One of these bins matched to wheat DNA that was derived exclusively from cropland samples of WSS larvae. The second bin contained 145 contigs (max contig size of 15 kbp and an N50 of 5,160 bp) with a collective length of 713,566 bp. Analysis of all bacterial single-copy core genes also allowed us to estimate that this genome bin was 72.7% complete, and the sequence homology of the ribosomal proteins resolved it to Spiroplasma. The AT-rich Spiroplasma metagenome-assembled genome (MAG; subsequently referred to as Spiroplasma sp. WSS) had a GC% content of 24.56%, 754 open reading frames, components of a single ribosomal RNA operon, and 23 tRNA encoding genes (Full annotation at: https://www.patricbrc.org/view/Genome/2132.146 and NCBI GenBank accession VBWQ0100001 (Bioproject PRJNA540284)). Functional annotation of the 754 putative genes (427 of which were classified as hypothetical proteins) indicated that the bacterium is capable of importing and phosphorylating sugars via a phosphoenolpyruvate phosphotransferase (PTS) system (FCO83_02200 & FCO83_02960). Although specific substrates are not determinable, pathways for the utilization of glucose-6 phosphate (G6P), and fructose-6P are evident, including a complete glycolytic (FCO83_00750, FCO83_00765, FCO83_02365, FCO83_02370, FCO83_02375, FCO83_02525, FCO83_03295; Fig. S2) and a near-complete pentose phosphate pathway (FCO83_01715, FCO83_02845, & FCO83_03285; Fig. S2). Additionally, the bacterium encodes phosphomannomutase (FCO83_00995; EC 5.4.2.8) indicating mannose may also be a utilizable substrate, although no additional catabolic genes enabling the further processing of mannose-1P or mannose-6P were evident.
Genes for the production of lactate (FCO83_00760) and the short chain fatty acids acetate and propanoate suggest these are likely major fermentative end products. Additionally, a multi-functional formate efflux transporter (FCO83_02325 & FCO83_02330) may indicate formate is also a major end product. This transporter may also facilitate the export of each of these terminal acid products as has been seen in other bacterial species (Lü et al., 2012). The Spiroplasma sp. also encodes an F-type ATPase suggesting it is capable of oxidative phosphorylation and a low-affinity phosphate transport system (FCO83_00795, FCO83_00800, & FCO83_00805) for the scavenging of inorganic phosphate. The Spiroplasma sp. WSS MAG encodes near complete pathways for the biosynthesis of vitamins B2 (riboflavin), B3 (niacin & nicotinamide), and B9 (Folate). The presence of genes encoding thiamine-specific (ThiT; FCO83_03200) and folate-specific (FolT; FCO83_02260) ECF (energy coupling factor) importers (structural components include, FCO83_01665, FCO83_01670, FCO83_01675), and a ribosyl nicotinamide transporter (FCO83_00500) suggests the microbe can both biosynthesize and import folate and nicotinamide, as has been described in other bacterial species, but is likely dependent on extracellular levels of vitamin B1 (thiamine).
Seven ORFs that exhibited homology (39.7–53.9% amino acid identity) to the male-killing gene, Spaid of S. poulsonii (Harumoto & Lemaitre, 2018) were identified (Table 2). Each contained ankyrin repeat domains, as described for Spaid, but none possessed the deubiquitinase or N-terminal signal peptide domains also described by Harumoto & Lemaitre (2018). Finally, two additional potential virulence factors were identified, that included cardiolipin synthetase (FCO83_00175; EC 2.7.8.-) and chitinase (FCO83_02115; EC 3.2.2.27).
Table 2. Potential spaid homologues identified in the WSS-associated spiroplasma genome.
Spiroplasma sp. WSS ORFs displaying homology to the S. poulsonii gene, Spaid, determined to be involved in the male-killing phenotype. Only Ankyrin repeat domains (IPR002110) were identified and the number of their domain repeats are indicated.
| Gene locus tag (Patric feature ID) | E-value | % AA identity | Coverage of Spaid | Domains Identified |
|---|---|---|---|---|
| FCO83_00980 (PEG.209) | 2e−16 | 47.8% | 100% | Ankyrin repeats1 (×5) |
| FCO83_02170 (PEG.469) | 1e−15 | 50.8% | 100% | Ankyrin repeats1 (×11) |
| FCO83_02755 (PEG.596) | 1e−14 | 49.1% | 100% | Ankyrin repeats1 (×8) |
| FCO83_00085 (PEG.21) | 2e−13 | 53.9% | 91% | Ankyrin repeats1 (×1) |
| FCO83_01420 (PEG.305) | 5e−13 | 47.5% | 100% | Ankyrin repeats1 (×7) |
| FCO83_00005 (PEG.1) | 1e−12 | 52.6% | 89% | Ankyrin repeats1 (×2) |
| FCO83_01210 (PEG.255) | 3e−07 | 39.7% | 91% | Ankyrin repeats1 (×3) |
Phylogenomic and phylogenetic analyses place the Spiroplasma sp. WSS in the Ixodetis Clade of Spiroplasma species
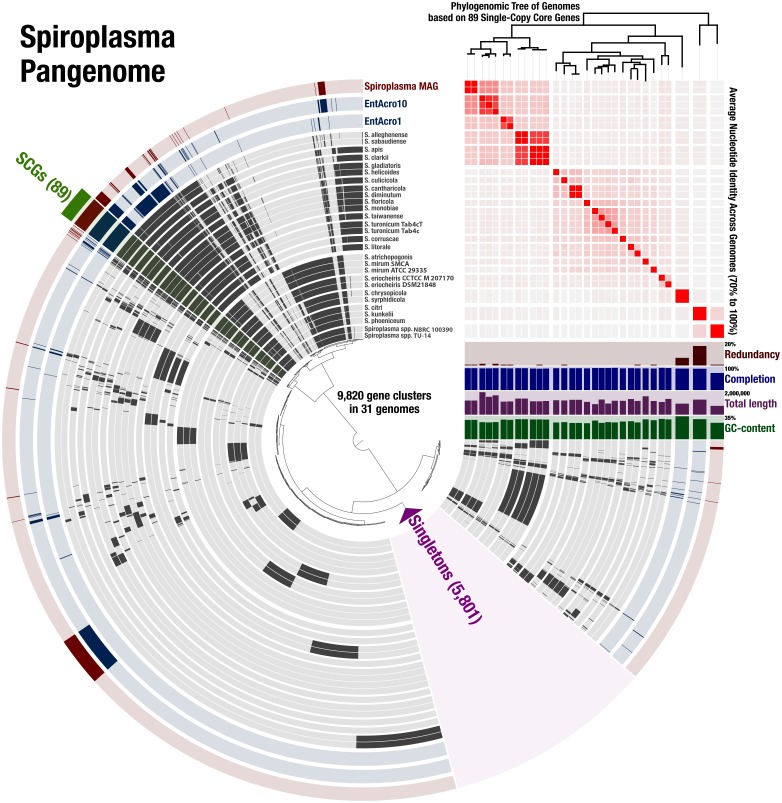
To determine the relationship of the Spiroplasma sp. WSS to previously published Spiroplasma genomes, we used a total of 31 genomes that included our MAG, two recently published Entomoplasmatales genomes (Sapountzis et al., 2018), and 28 Spiroplasma genomes available from NCBI (Table S1). The Spiroplasma pangenome (Fig. 2) revealed a total of 9,820 gene clusters. 89 of these gene clusters were those that represented single-copy core genes of the Spiroplasma pangenome (where each genome contributed precisely a single gene), and 5,801 of those were singletons (gene clusters and alignments are reported in Supplemental Information 2). Our phylogenomic analysis using a maximum likelihood model with the 89 single-copy core genes revealed the three distinct clades that corresponded to the known major mollicute clades (Citri-Chrysopicola-Mirum (CCM), Apis, and Ixodetis) (Fig. 3). The distinction of these clades was also supported by differences in average nucleotide identity values between genomes (Fig. 2, Table S2). Spiroplasma sp. WSS was most closely related to the recently described Ixodetis member, EntAcro10 (Sapountzis et al., 2018), with both being distinct from all other Spiroplasma spp. that were instead distributed in the CCM and Apis clades. Average nucleotide identity (ANI) between Spiroplasma sp. WSS and EntAcro10 was just 73% over 34% of the total alignment (Table S2).
Figure 2. Spiroplasma. spp. Have a Large Pangenome.
Pangenomic analysis of 28 sequenced Spiroplasma spp., and 3 mollicute MAGs (including the WSS-associated Spiroplasma MAG) revealing 89 (<1%) single copy core gene clusters, and 5,801 (59%) species-specific gene clusters among 9,820 total gene clusters along with their distribution and average nucleotide identities.
Figure 3. Phylogeny of Spiroplasma. sp. WSS shows it belongs to the Ixodetes clade of Mollicutes.
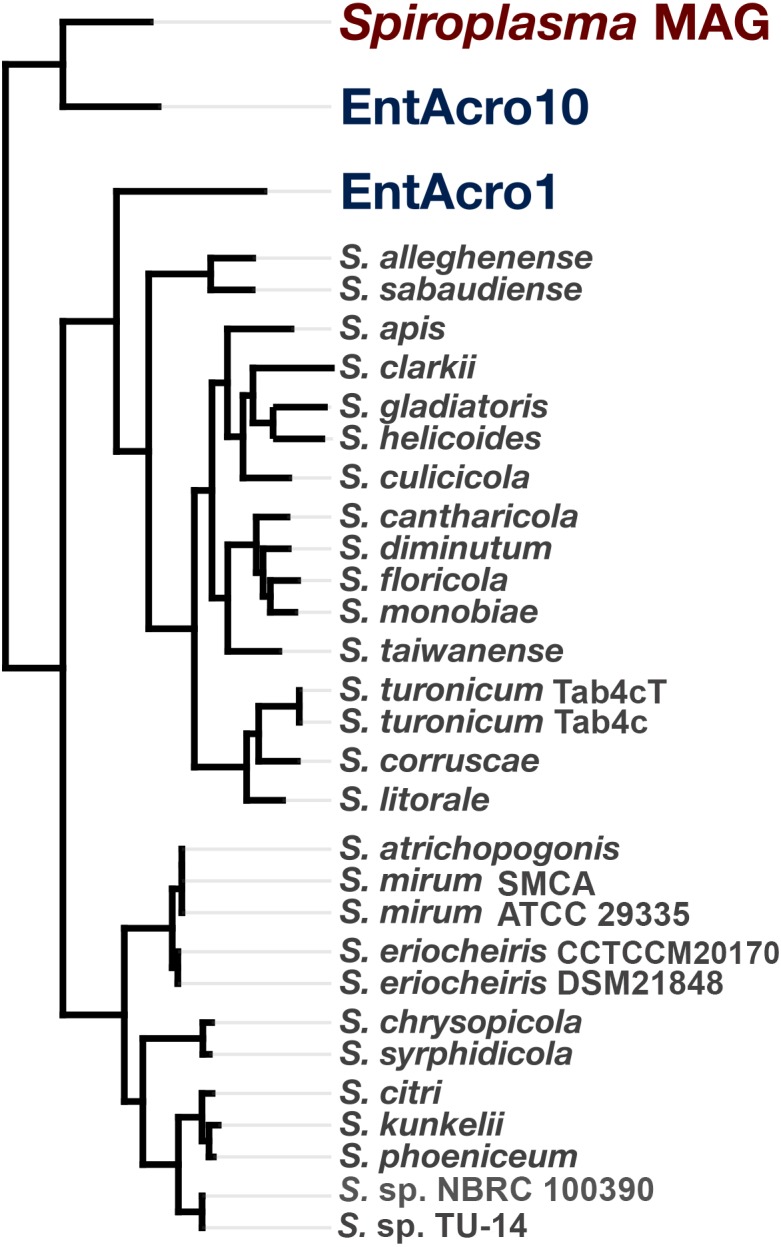
A maximum likelihood tree constructed from the 89 single-copy core Spiroplasma genes revealed from a pangenomic analysis showing the phylogenomic relationship of 28 sequenced Spiroplasma spp., and three mollicute MAGs (including the WSS-associated Spiroplasma MAG) and their distribution among the three phylogentic clades: Apis, Citri-Chrysopicola-Mirum (CCM), and Ixodetis.
Discussion
This is the first work to describe and characterize the microbiota of the wheat stem sawfly (WSS; Cephus cinctus). We show by 16S rRNA gene sequencing, metagenomic data, and SEM imaging that WSS is symbiotically colonized by a novel Spiroplasma species of the Ixodetis clade. The Spiroplasma species was seen in both adults and larvae that had not yet emerged from wheat stems suggesting the bacterium is obtained early in life either vertically from the female before oviposition or is picked up horizontally from feeding on the inner wheat stem lining. Several less abundant members of the Pasteurellaceae family were also detected, however, due to our whole-WSS processing approach to obtain DNA, it is not clear if these microbes are co-located in the WSS with Spiroplasma sp. WSS.
SEM imaging indicated a characteristically long and filamentous but non-helical structure. Spiroplasma cells have been shown to vary in morphology based on conditions and age of in vitro culture, with older cells or those that have been cultured in sub-optimal conditions tending to lack helical structure (Itoh, Pan & Koshimizu, 1989). Thus, the non-helical structure observed may be an artifact of our inability to cultivate Spiroplasma sp. WSS (see supplementary material for further discussion on cultivation efforts). Alternatively, strains of Spiroplasma lacking helical structure, regardless of culture conditions have been identified, including a non-helical strain of S. citri (Townsend, Burgess & Plaskitt, 1980) and S. culilicola, which is also reported to predominantly have a non-helical morphology (Hung et al., 1987). Known morphologies of Spiroplasma from the Ixodetis clade, in particular, have been reported to vary widely. For example, S. platyhelix exhibits a “kinked” morphology (Townsend, Burgess & Plaskitt, 1980). Nevertheless, further research is necessary to optimize growth conditions for the Spiroplasma sp. WSS and subsequently confirm or refute its non-helical morphology.
SEM images also revealed some cells with a Y-shaped morphology. This Y-shape has previously been observed among S. poulsonii cells as characteristic of their longitudinal scission method of cell division (Ramond et al., 2016). This observation for Spiroplasma sp. WSS therefore adds support to the hypothesis that longitudinal scission is the most prevalent method of cell-division utilized by this bacterial genus (Ramond et al., 2016).
Using advanced metagenomic sequencing, assembly, and binning processes, we were able to obtain genomic insights into Spiroplasma sp. WSS. Spiroplasma genome sequencing and assembly is notoriously difficult due to an A-T rich nucleotide composition, which for some Spiroplasma genomes can be further complicated by repetitive transposon-like elements and plectiviral sequences (Bolaños, Servín-Garcidueñas & Martínez-Romero, 2015; Lo, Gasparich & Kuo, 2015). Additional challenges experienced in completing the Spiroplasma sp. WSS genome included its recalcitrance to cultivation and limitations when attempting to enrich Spiroplasma DNA from the WSS host and wheat DNA. Nevertheless, the 714 KB draft sequence provides important insight into the WSS symbiont, as well as more broadly into the Ixodetis clade (Bolaños, Servín-Garcidueñas & Martínez-Romero, 2015).
Spiroplasma sp. WSS is only the second member of the Ixodetis clade for which genomic data has been obtained, and although phylogenomic data supports the relationship between this genome and the other Ixodetis MAG, EntAcro10, it is clear that there are substantial genetic differences between the two with just 73% ANI between the two genomes. Given the suggested 95% ANI for species boundaries (Konstantinidis, Ramette & Tiedje, 2006), these data suggest that Spiroplasma sp. WSS and EntAcro10 represent relatively closely related yet distinct taxa.
Our data indicate Spiroplasma sp. WSS has a complete genome size of ∼0.98 Mb, consistent with the small genome size predicted for EntAcro10 (Sapountzis et al., 2018) and the typically small Spiroplasma genomes described to date that have ranged from 0.78–2.2 Mb, the upper and lower bounds of which belonging to members of the Ixodetis clade (Bolaños, Servín-Garcidueñas & Martínez-Romero, 2015; Lo, Huang & Kuo, 2016). The draft genome includes evidence for only a single rRNA operon, consistent with all previous Spiroplasma genomes, except S. sabaudiense, and 23 tRNA genes, approximately the number expected from this level of genome completeness, given other Spiroplasma genomes house 29-33 tRNA genes (Ku et al., 2013; Bolaños, Servín-Garcidueñas & Martínez-Romero, 2015; Lo, Gasparich & Kuo, 2015). The Spiroplasma genome provides preliminary insight into the nutritional requirements for the bacterium that we hope will help guide future cultivation efforts necessary for taxonomic description and to advance our ability to elaborate the extent of interaction between Spiroplasma sp. WSS and the WSS host. Although the genome is only ∼73% complete, its gene content indicates the bacterium can import and phosphorylate sugars via a phosphoenolpyruvate phosphotransferase (PTS) system with pathways for the utilization of G6P, and fructose-6P. Consistently, the utilization of glucose is a universal characteristic of all Spiroplasma spp. described to date and fructose utilization is well described in the CCM and Citri clades (Gaurivaud et al., 2000; Whitman, 2011). Genes for the production of lactate and the short chain fatty acids acetate and propanoate suggest these are likely the major fermentative end products. This is also consistent, with lactate and acetate having both previously been observed as the major fermentative end products of S. citri (Gaurivaud et al., 2000). Additional carbohydrate substrate utilization pathways may exist within the uncaptured regions of the genome as would be consistent with previous studies that have found horizontal gene transfer events have led to a high diversity of carbohydrate transporters among Spiroplasma (Chang et al., 2014; Lo, Gasparich & Kuo, 2015; Lo & Kuo, 2017).
Of potential importance to the symbiosis of Spiroplasma sp. WSS with the insect host is the bacterium’s encoded ability to biosynthesize the B vitamins riboflavin (B2), Niacin (B3), and folate (B9). Currently, the available genome sequence for WSS lacks genes necessary for the biosynthesis of most B-vitamins, with only genes for the biosynthesis of pyridoxine (B6) and a partial folate biosynthetic pathway evident ( Text S2 and Fig. S2). The ability of Spiroplasma sp. WSS to both biosynthesize and import folate and nicotinamide suggests these vitamins may be of particular importance especially in adult life stages following emergence from the wheat stem, where these B-vitamins are abundant (Kumar et al., 2011). The presence of pathways for the biosynthesis and uptake of folate have been described in several bacterial species, including several pathogens, where the additional ability to uptake folate has been found to confer resistance to antifolate drugs that target the folate biosynthetic and thymidylate pathways (Wang et al., 2004; De Crécy-Lagard et al., 2007; Eudes et al., 2008). Yet the potential benefit of such resistance to either WSS or its Spiroplasma symbiont is unclear.
Additionally, the Spiroplasma sp. WSS genome was found to encode cardiolipin synthetase and chitinase. Cardiolipin is a component of the Spiroplasma membrane and is also found in eukaryotic mitochondria (Paredes et al., 2015). When produced in excess in eukaryotic cells, cardiolipin can promote apoptosis (Gonzalvez & Gottlieb, 2007), and consequently it has been hypothesized that it may play a role in insect defense against nematodes and parasitic wasps due to its toxic nature (Paredes et al., 2015). Chitinase may also defend against parasitic wasps by degrading their chitin-based cuticles, as well as facilitating effective digestion of WSS larva consumed by internecine cannibalism (Buteler et al., 2015). In addition, this ability would also extend to digestion after predation upon vulnerable parasitoids feeding on the paralyzed host, which is an expectation in the population dynamics across trophic levels in this system (Weaver et al., 2005). To date, neither cardiolipin or chitinase have demonstrably been shown to be actively involved in insect defenses against parasitoid wasps or nematodes (for a review of current knowledge on these processes see Ballinger & Perlman (2019). Nevertheless, the putative roles of cardiolipin and chitinase in protecting against parasitic wasps is intriguing because the WSS is commonly parasitized by at least two congeneric species of parasitic wasps: Bracon cephi and B. lissogaster (Morrill, Kushnak & Gabor, 1998). For each species, WSS larvae are the only known hosts; B. lissogaster is more frequently associated with WSS living in native grass stems than B. cephi, which primarily parasitizes WSS colonizing wheat (Morrill, Kushnak & Gabor, 1998). The role of this metabolite may be of further importance in consideration of inability of an exotic parasitoid that had not co-evolved with the host to successfully develop on native WSS despite being a strong candidate (Rand et al., 2016; Rand, Waters & Shanower, 2016). We hope these findings will motivate future studies investigating the role of Spiroplasma sp. WSS symbiosis in host resistance to Bracon spp. wasp parasitism.
The identification of several genes showing homology to the male-killing Spaid gene of S. poulsonii may warrant further analysis to determine if Spiroplasma sp. WSS may be capable of affecting WSS sex ratios. However, it is noteworthy that all gene-candidates within the Spiroplasma sp. WSS genome lacked the deubiquitinase domain that was determined by Harumoto & Lemaitre (2018) to be essential to effective male-killing activity. Further, although a previous study on the sex ratio of WSS larvae determined a female larvae bias in the preferred larger-stemmed wheat, it also found a male-bias of larvae oviposited in smaller-stemmed wheat (Morrill et al., 2000). Nevertheless, beyond Spaid, there appears few examples of ankyrin-repeat containing genes among Spiroplasma spp. and so these genes are worthy of further characterization.
Other symbiotic attributes reported among various other Spiroplasma spp. described to date, such as improving host defenses against fungal pathogens (Łukasik et al., 2012) cannot be determined because the genetic determinants are yet to be described and phylogeny is not indicative of these traits. For example, this might include defense against a complex of Fusarium spp. commonly occurring in wheat that have been shown to kill WSS larvae (Wenda-Piesik et al., 2009). Likewise, the potential for Spiroplasma sp. WSS to contribute to the wheat damage associated with WSS, as has been described in corn and citrus (Chen & Liao, 1975; Regassa & Gasparich, 2006; Haselkorn, 2014; Bolaños, Servín-Garcidueñas & Martínez-Romero, 2015) is unknown but deserves attention.
Overall, these findings identify the presence of a previously undescribed Spiroplasma symbiont of the WSS, a major wheat pest. Although we have been, thus far, unable to cultivate the bacterium, metagenomic sequencing and binning provided genetic insight into the lifestyle of Spiroplasma sp. WSS The genome sequence also provides the first opportunity for comparative genomic analyses of members of the Ixodetis clade of mollicutes and highlights substantial genomic variation among its earliest genome resolved members.
Conclusion
We set out to identify the symbiotic microbe(s) of WSS and obtain insight into the nature of the insect-microbe relationships so that we could begin to determine if these insect-microbial relationships could be exploited as alternate measures to control WSS damage in crops. Although our ability to determine if the WSS-Spiroplasma symbiosis may be manipulated to impact the WSS fitness requires further experimentation, the identification of Spiroplasma sp. WSS and greater genetic insight into its metabolism provide a critical first step toward our pursuit of a novel biocontrol approach.
Supplemental Information
Descriptions of Attempts to Isolate and Culture Spiroplasma sp. and Metabolic Pathway Analysis Of Draft Wheat Stem Sawfly and Spiroplasma sp. Genomes
The 9,820 gene clusters determined from pangenomic analyses of 28 Spiroplasma spp. and 3 MAGs and their alignments.
A list of the 28 Spiroplasma species and 3 MAGs downloaded and utilized in our comparative analyses along with their NCBI accession information
Average nucleotide identities from alignments of 89 single copy core genes identified in the pangenome of Spiroplasma.
Longitudinal metabolomic analyses of cultivation efforts showing metabolites whose concentrations were deferentially altered (highlighted green) and others that were detected.
Shows the recruitment of all contigs by sample to each of the three bins, WSS host, wheat, and the Spiroplasma sp. symbiont.
Metabolic pathways encoded by genes found exclusively in the draft WSS (green) or Spiroplasma sp. (red) genome and those in both (orange) are shown on a KEGG map as created by iPATH.
Acknowledgments
Insect specimens were generously provided by students in the Wheat Stem Sawfly Laboratory. The authors express gratitude to Alexander Ulanov at the Roy J. Carver Biotechnology center for associated metabolomic analyses of cultures.
Funding Statement
This work was supported by the Montana Wheat and Barley Committee and through the Montana Agricultural Experiment Station project MONB00113. Laura Brutscher was also partially funded by the Molecular Biosciences Program (MBSP) and Montana State University. There was no additional external funding received for this study. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
A. Murat Eren is an Academic Editor for PeerJ.
Author Contributions
Carl J. Yeoman conceived and designed the experiments, analyzed the data, contributed reagents/materials/analysis tools, authored or reviewed drafts of the paper, approved the final draft.
Laura M. Brutscher performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Özcan C. Esen and Furkan Ibaoglu performed the experiments, analyzed the data, prepared figures and/or tables, approved the final draft.
Curtis Fowler performed the experiments, approved the final draft.
A. Murat Eren performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Kevin Wanner contributed reagents/materials/analysis tools, approved the final draft.
David K. Weaver performed the experiments, contributed reagents/materials/analysis tools, authored or reviewed drafts of the paper, approved the final draft.
Field Study Permissions
The following information was supplied relating to field study approvals (i.e., approving body and any reference numbers):
Permission to collect the Highland population was obtained from the Lincoln Ranger District. There are no formal permits required for this because it is an incidental population of a highly abundant agricultural pest.
The lowland populations were collected on land cultivated by Wheat Montana Farms with permissions obtained from Dean Folkvord and on land cultivated by Matt Flikkema.
DNA Deposition
The following information was supplied regarding the deposition of DNA sequences:
16S rRNA sequences are available in the Sequence Read Archive: SRP108219. Metagenomic sequences are available in the Sequence Read Archive: SRP108220. Additional sequence data generated by Illumina HiSeq as part of the WSS genome project (Robertson et al., 2018) are available at: SRS694145. The metagenomic assembled genome is available in GenBank under bioproject PRJNA540284 and is available publicly at Patric with the genome ID 2132.146 (Spiroplasma WSS).
References
- Anbutsu & Fukatsu (2011).Anbutsu H, Fukatsu T. Spiroplasma as a model insect endosymbiont. Environmental Microbiology Reports. 2011;3:144–153. doi: 10.1111/j.1758-2229.2010.00240.x. [DOI] [PubMed] [Google Scholar]
- Ballinger & Perlman (2019).Ballinger MJ, Perlman SJ. The defensive Spiroplasma. Current Opinion in Insect Science. 2019;32:36–41. doi: 10.1016/j.cois.2018.10.004. [DOI] [PubMed] [Google Scholar]
- Beres, Cárcamo & Byers (2007).Beres BL, Cárcamo HA, Byers JR. Effect of wheat stem sawfly damage on yield and quality of selected Canadian spring wheat. Journal of Economic Entomology. 2007;100:79–87. doi: 10.1093/jee/100.1.79. [DOI] [PubMed] [Google Scholar]
- Beres et al. (2011).Beres BL, Dosdall LM, Weaver DK, Cárcamo HA, Spaner DM. Biology and integrated management of wheat stem sawfly and the need for continuing research. The Canadian Entomologist. 2011;143:105–125. doi: 10.4039/n10-056. [DOI] [Google Scholar]
- Bolaños, Servín-Garcidueñas & Martínez-Romero (2015).Bolaños LM, Servín-Garcidueñas LE, Martínez-Romero E. Arthropod-Spiroplasma relationship in the genomic era. FEMS Microbiology Ecology. 2015;91:1–8. doi: 10.1093/femsec/fiu008. [DOI] [PubMed] [Google Scholar]
- Borgogna & Yeoman (2017).Borgogna J-LC, Yeoman CJ. The application of molecular methods towards an understanding of the role of the vaginal microbiome in health and disease. In: Harwood C, editor. The human microbiome. Vol. 44. Academic Press; Oxford: 2017. pp. 37–91. [DOI] [Google Scholar]
- Buteler et al. (2015).Buteler M, Peterson RKD, Hofland ML, Weaver DK. A multiple decrement life table reveals that host plant resistance and parasitism are major causes of mortality for the wheat stem sawfly. Environmental Entomology. 2015;44:1571–1580. doi: 10.1093/ee/nvv128. [DOI] [PubMed] [Google Scholar]
- Capella-Gutierrez, Silla-Martinez & Gabaldon (2009).Capella-Gutierrez S, Silla-Martinez JM, Gabaldon T. trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics. 2009;25:1972–1973. doi: 10.1093/bioinformatics/btp348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang et al. (2014).Chang T-H, Lo W-S, Ku C, Chen L-L, Kuo C-H. Molecular evolution of the substrate utilization strategies and putative virulence factors in mosquito-associated Spiroplasma species. Genome Biology and Evolution. 2014;6:500–509. doi: 10.1093/gbe/evu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen & Liao (1975).Chen TA, Liao CH. Corn stunt Spiroplasma: isolation, cultivation, and proof of pathogenicity. Science. 1975;188:1015–1017. doi: 10.1126/science.188.4192.1015. [DOI] [PubMed] [Google Scholar]
- Cole et al. (2009).Cole JR, Wang Q, Cardenas E, Fish J, Chai B, Farris RJ, Kulam-Syed-Mohideen AS, McGarrell DM, Marsh T, Garrity GM, Tiedje JM. The Ribosomal Database Project: improved alignments and new tools for rRNA analysis. Nucleic Acids Research. 2009;37:D141–D145. doi: 10.1093/nar/gkn879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Crécy-Lagard et al. (2007).De Crécy-Lagard V, Yacoubi El B, La Garza de R, Noiriel A, Hanson AD. Comparative genomics of bacterial and plant folate synthesis and salvage: predictions and validations. BMC Genomics. 2007;8:245. doi: 10.1186/1471-2164-8-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmont & Eren (2018).Delmont TO, Eren AM. Linking pangenomes and metagenomes: the Prochlorococcus metapangenome. PeerJ. 2018;6:e4320-23. doi: 10.7717/peerj.4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eren et al. (2015).Eren AM, Esen ÖC, Quince C, Vineis JH, Morrison HG, Sogin ML, Delmont TO. Anvi’o: an advanced analysis and visualization platform for ‘omics data. PeerJ. 2015;3:e1319-29. doi: 10.7717/peerj.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eren et al. (2013).Eren AM, Vineis JH, Morrison HG, Sogin ML. A filtering method to generate high quality short reads using illumina paired-end technology. PLOS ONE. 2013;8:e66643-6. doi: 10.1371/journal.pone.0066643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eudes et al. (2008).Eudes A, Erkens GB, Slotboom DJ, Rodionov DA, Naponelli V, Hanson AD. Identification of genes encoding the folate- and thiamine-binding membrane proteins in firmicutes. Journal of Bacteriology. 2008;190:7591–7594. doi: 10.1128/JB.01070-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint et al. (2012).Flint HJ, Scott KP, Louis P, Duncan SH. The role of the gut microbiota in nutrition and health. Nature Reviews Gastroenterology & Hepatology. 2012;9:577–589. doi: 10.1038/nrgastro.2012.156. [DOI] [PubMed] [Google Scholar]
- Floate, Kyei-Poku & Coghlin (2007).Floate KD, Kyei-Poku GK, Coghlin PC. Overview and relevance of Wolbachia bacteria in biocontrol research. Biocontrol Science and Technology. 2007;16:767–788. doi: 10.1080/09583150600699606. [DOI] [Google Scholar]
- Gasparich et al. (2004).Gasparich GE, Whitcomb RF, Dodge D, French FE, Glass J, Williamson DL. The genus Spiroplasma and its non-helical descendants: phylogenetic classification, correlation with phenotype and roots of the Mycoplasma mycoides clade. International Journal Of Systematic and Evolutionary Microbiology. 2004;54:893–918. doi: 10.1099/ijs.0.02688-0. [DOI] [PubMed] [Google Scholar]
- Gaurivaud et al. (2000).Gaurivaud P, Danet JL, Laigret F, Garnier M, Bové JM. Fructose utilization and phytopathogenicity of Spiroplasma citri. Molecular Plant-Microbe Interactions. 2000;13:1145–1155. doi: 10.1094/MPMI.2000.13.10.1145. [DOI] [PubMed] [Google Scholar]
- Gonzalvez & Gottlieb (2007).Gonzalvez F, Gottlieb E. Cardiolipin: setting the beat of apoptosis. Apoptosis. 2007;12:877–885. doi: 10.1007/s10495-007-0718-8. [DOI] [PubMed] [Google Scholar]
- Harumoto & Lemaitre (2018).Harumoto T, Lemaitre B. Male-killing toxin in a bacterial symbiont of Drosophila. Nature. 2018;557:252–255. doi: 10.1038/s41586-018-0086-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haselkorn (2014).Haselkorn TS. The Spiroplasma heritable bacterial endosymbiont of Drosophila. Fly. 2014;4:80–87. doi: 10.4161/fly.4.1.10883. [DOI] [PubMed] [Google Scholar]
- Hung et al. (1987).Hung SHY, Chen TA, Whitcomb RF, Tully JG, Chen YX. Spiroplasma culicicola sp. nov. from the salt marsh mosquito Aedes sollicitans. International Journal of Systematic Bacteriology. 1987;37:365–370. doi: 10.1099/00207713-37-4-365. [DOI] [Google Scholar]
- Huse et al. (2010).Huse SM, Welch DM, Morrison HG, Sogin ML. Ironing out the wrinkles in the rare biosphere through improved OTU clustering. Environmental microbiology. 2010;12:1889–1898. doi: 10.1111/j.1462-2920.2010.02193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyatt et al. (2010).Hyatt D, Chen G-L, Locascio PF, Land ML, Larimer FW, Hauser LJ. Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinformatics. 2010;11:119. doi: 10.1186/1471-2105-11-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh, Pan & Koshimizu (1989).Itoh K, Pan IJ, Koshimizu K. A proposed life cycle model of Spiroplasma mirum based on scanning electron microscopical observations of growth in liquid culture. Microbiology and Immunology. 1989;33:821–832. doi: 10.1111/j.1348-0421.1989.tb00968.x. [DOI] [PubMed] [Google Scholar]
- Jaenike et al. (2010).Jaenike J, Unckless R, Cockburn SN, Boelio LM, Perlman SJ. Adaptation via symbiosis: recent spread of a Drosophila defensive symbiont. Science. 2010;329:212–215. doi: 10.1126/science.1188235. [DOI] [PubMed] [Google Scholar]
- Kanehisa, Sato & Morishima (2016).Kanehisa M, Sato Y, Morishima K. BlastKOALA and GhostKOALA: KEGG tools for functional characterization of genome and metagenome sequences. Journal of Molecular Biology. 2016;428:726–731. doi: 10.1016/j.jmb.2015.11.006. [DOI] [PubMed] [Google Scholar]
- Knodel et al. (2009).Knodel JJ, Beauzay PB, Eriksmoen ED, Pederson JD. Pest management of wheat stem maggot (Diptera: Chloropidae) and wheat stem sawfly (Hymenoptera: Cephidae) using insecticides in spring wheat. Journal of Agricultural and Urban Entomology. 2009;26:183–198. doi: 10.3954/1523-5475-26.4.183. [DOI] [Google Scholar]
- Konstantinidis, Ramette & Tiedje (2006).Konstantinidis KT, Ramette A, Tiedje JM. The bacterial species definition in the genomic era. Philosophical Transactions of the Royal Society B: Biological Sciences. 2006;361:1929–1940. doi: 10.1098/rstb.2006.1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku et al. (2013).Ku C, Lo W-S, Chen L-L, Kuo C-H. Complete genomes of two dipteran-associated Spiroplasmas provided insights into the origin, dynamics, and impacts of viral invasion in Spiroplasma. Genome Biology and Evolution. 2013;5:1151–1164. doi: 10.1093/gbe/evt084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar et al. (2011).Kumar P, Yadava RK, Gollen B, Kumar S, Verma RK, Yadav S. Nutritional contents and medicinal properties of wheat: a review. Life Sciences and Medicine Research. 2011;2011 Article LSMR-22. [Google Scholar]
- Kumar, Stecher & Tamura (2016).Kumar S, Stecher G, Tamura K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead & Salzberg (2012).Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nature Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesieur et al. (2016).Lesieur V, Martin J-F, Weaver DK, Hoelmer KA, Smith DR, Morrill WL, Kadiri N, Peairs FB, Cockrell DM, Randolph TL, Waters DK, Bon M-C. Phylogeography of the wheat stem sawfly, Cephus cinctus Norton (Hymenoptera: Cephidae): implications for pest management. PLOS ONE. 2016;11(12):e0168370-19. doi: 10.1371/journal.pone.0168370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letunic et al. (2008).Letunic I, Yamada T, Kanehisa M, Bork P. iPath: interactive exploration of biochemical pathways and networks. Trends in Biochemical Sciences. 2008;33:101–103. doi: 10.1016/j.tibs.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Li et al. (2009).Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R, 1000 Genome Project Database Processing Subgroup The sequence alignment/map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2015).Li D, Liu C-M, Luo R, Sadakane K, Lam T-W. MEGAHIT: an ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics. 2015;31:1674–1676. doi: 10.1093/bioinformatics/btv033. [DOI] [PubMed] [Google Scholar]
- Lo, Gasparich & Kuo (2015).Lo W-S, Gasparich GE, Kuo C-H. Found and lost: the fates of horizontally acquired genes in arthropod-symbiotic Spiroplasma. Genome Biology and Evolution. 2015;7:2458–2472. doi: 10.1093/gbe/evv160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo, Huang & Kuo (2016).Lo W-S, Huang Y-Y, Kuo C-H. Winding paths to simplicity: genome evolution in facultative insect symbionts. FEMS Microbiology Reviews. 2016;40:855–874. doi: 10.1093/femsre/fuw028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo & Kuo (2017).Lo W-S, Kuo C-H. Horizontal acquisition and transcriptional integration of novel genes in mosquito-associated Spiroplasma. Genome Biology and Evolution. 2017;9:3246–3259. doi: 10.1093/gbe/evx244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lü et al. (2012).Lü W, Du J, Schwarzer NJ, Gerbig-Smentek E, Einsle O, Andrade SLA. The formate channel FocA exports the products of mixed-acid fermentation. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:13254–13259. doi: 10.1073/pnas.1204201109/-/DCSupplemental/sapp.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Łukasik et al. (2012).Łukasik P, Van Asch M, Guo H, Ferrari J, Charles J, Godfray H. Unrelated facultative endosymbionts protect aphids against a fungal pathogen. Ecology Letters. 2012;16:214–218. doi: 10.1111/ele.12031. [DOI] [PubMed] [Google Scholar]
- Masella et al. (2012).Masella AP, Bartram AK, Truszkowski JM, Brown DG, Neufeld JD. PANDAseq: paired-end assembler for illumina sequences. BMC Bioinformatics. 2012;13:31. doi: 10.1186/1471-2105-13-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran & Telang (1998).Moran NA, Telang A. Bacteriocyte-associated symbionts of insects. JSTOR. 1998;48:295–304. [Google Scholar]
- Morrill et al. (2000).Morrill WL, Gabor JW, Weaver DK, Kushnak GD, Irish NJ. Effect of host plant quality on the sex ratio and fitness of female wheat stem sawflies (Hymenoptera: Cephidae) Environmental Entomology. 2000;29(2):195–199. doi: 10.1603/0046-225X(2000)029[0195:EOHPQO]2.0.CO;2. [DOI] [Google Scholar]
- Morrill, Kushnak & Gabor (1998).Morrill WL, Kushnak GD, Gabor JW. Parasitism of the wheat stem sawfly (Hymenoptera: Cephidae) in Montana. Biological Control. 1998;12:159–163. doi: 10.1006/bcon.1998.0629. [DOI] [Google Scholar]
- Mousson et al. (2012).Mousson L, Zouache K, Arias-Goeta C, Raquin V, Mavingui P, Failloux A-B. The native Wolbachia symbionts limit transmission of dengue virus in Aedes albopictus. PLOS Neglected Tropical Diseases. 2012;6(12):e1989-10. doi: 10.1371/journal.pntd.0001989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nai et al. (2014).Nai Y-S, Su P-Y, Hsu Y-H, Chiang C-H, Kim JS, Chen Y-W, Wang C-H. A new Spiroplasma isolate from the field cricket (Gryllus bimaculatus) in Taiwan. Journal of Invertebrate Pathology. 2014;120:4–8. doi: 10.1016/j.jip.2014.04.006. [DOI] [PubMed] [Google Scholar]
- Nguyen et al. (2014).Nguyen L-T, Schmidt HA, Haeseler von A, Minh BQ. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Molecular Biology and Evolution. 2014;32:268–274. doi: 10.1093/molbev/msu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oksanen et al. (2007).Oksanen J, Kindt R, Legendre P, O’Hara B. The vegan package. Community Ecology Package. 2007;10:631–637. [Google Scholar]
- Paredes et al. (2015).Paredes JC, Herren JK, Schüpfer F, Marin R, Claverol S, Kuo C-H, Lemaitre B, Béven L. Genome sequence of the Drosophila melanogaster male-killing Spiroplasma Strain MSRO endosymbiont. mBio. 2015;6:677–12. doi: 10.1128/mBio.02437-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portman et al. (2018).Portman SL, Jaronski ST, Weaver DK, Reddy GVP. Advancing biological control of the wheat stem sawfly: New strategies in a 100-yr struggle to manage a costly pest in the northern great plains. Annals of the Entomological Society of America. 2018;111:85–91. doi: 10.1093/aesa/say002. [DOI] [Google Scholar]
- Pruesse et al. (2007).Pruesse E, Quast C, Knittel K, Fuchs BM, Ludwig W, Peplies J, Glockner FO. SILVA: a comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Research. 2007;35:7188–7196. doi: 10.1093/nar/gkm864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramond et al. (2016).Ramond E, Maclachlan C, Clerc-Rosset S, Knott GW, Lemaitre B. Cell division by longitudinal scission in the insect endosymbiont Spiroplasma poulsonii. mBio. 2016;7:677–5. doi: 10.1128/mBio.00881-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand et al. (2016).Rand TA, Morrill WL, Runyon JB, Hoelmer KA, Shanower TG, Littlefield JL, Weaver DK. Assessing phenological synchrony between the Chinese sawfly, Cephus fumipennis (Hymenoptera: Cephidae), its egg-larval parasitoid, Collyria catoptron (Hymenoptera: Ichneumonidae), and the North American sawfly, Cephus cinctus: implications for biological control. The Canadian Entomologist. 2016;148:482–492. doi: 10.4039/tce.2015.64. [DOI] [Google Scholar]
- Rand, Waters & Shanower (2016).Rand TA, Waters DK, Shanower TG. Preliminary evaluation of the parasitoid wasp, Collyria catoptron, as a potential biological control agent against the wheat stem sawfly, Cephus cinctus, in North America. Biocontrol Science and Technology. 2016;26:61–71. doi: 10.1080/09583157.2015.1076377. [DOI] [Google Scholar]
- Regassa & Gasparich (2006).Regassa LB, Gasparich GE. Spiroplasmas: evolutionary relationships and biodiversity. Frontiers in Bioscience. 2006;11:2983–3002. doi: 10.2741/2027. [DOI] [PubMed] [Google Scholar]
- Robertson et al. (2018).Robertson HM, Waterhouse RM, Walden KKO, Ruzzante L, Reijnders MJMF, Coates BS, Legeai F, Gress JC, Biyiklioglu S, Weaver DK, Wanner KW, Budak H. Genome sequence of the wheat stem sawfly, Cephus cinctus, representing an early-branching lineage of the Hymenoptera, illuminates evolution of hymenopteran chemoreceptors. Genome Biology and Evolution. 2018;10:2997–3011. doi: 10.1093/gbe/evy232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapountzis et al. (2018).Sapountzis P, Zhukova M, Shik JZ, ELife MS. Reconstructing the functions of endosymbiotic Mollicutes in fungus-growing ants. eLIFE. 2018;7:e39209. doi: 10.7554/eLife.39209.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloss et al. (2009).Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, Sahl JW, Stres B, Thallinger GG, Van Horn DJ, Weber CF. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Applied and Environmental Microbiology. 2009;75:7537–7541. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanower & Hoelmer (2004).Shanower TG, Hoelmer KA. Biological control of wheat stem sawflies: past and future. Journal of Agricultural and Urban Entomology. 2004;21:197–221. [Google Scholar]
- Smyth et al. (2010).Smyth RP, Schlub TE, Grimm A, Venturi V, Chopra A, Mallal S, Davenport MP, Mak J. Reducing chimera formation during PCR amplification to ensure accurate genotyping. Gene. 2010;469:45–51. doi: 10.1016/j.gene.2010.08.009. [DOI] [PubMed] [Google Scholar]
- Teixeira, Ferreira & Ashburner (2008).Teixeira L, Ferreira Á, Ashburner M. The bacterial symbiont Wolbachia induces resistance to rna viral infections in Drosophila melanogaster. PLOS Biology. 2008;6(12):e1000002-11. doi: 10.1371/journal.pbio.1000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaiss et al. (2016).Thaiss CA, Zmora N, Levy M, Elinav E. The microbiome and innate immunity. Nature. 2016;535:65–74. doi: 10.1038/nature18847. [DOI] [PubMed] [Google Scholar]
- Torrazza & Neu (2011).Torrazza RM, Neu J. The developing intestinal microbiome and its relationship to health and disease in the neonate. Journal of Perinatology. 2011;31:S29–S34. doi: 10.1038/jp.2010.172. [DOI] [PubMed] [Google Scholar]
- Townsend, Burgess & Plaskitt (1980).Townsend R, Burgess J, Plaskitt KA. Morphology and ultrastructure of helical and nonhelical strains of Spiroplasma citri. Journal of Bacteriology. 1980;142:973–981. doi: 10.1128/jb.142.3.973-981.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (2004).Wang P, Nirmalan N, Wang Q, Sims PFG, Hyde JE. Genetic and metabolic analysis of folate salvage in the human malaria parasite Plasmodium falciparum. Molecular and Biochemical Parasitology. 2004;135:77–87. doi: 10.1016/j.molbiopara.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Wattam et al. (2013).Wattam AR, Abraham D, Dalay O, Disz TL, Driscoll T, Gabbard JL, Gillespie JJ, Gough R, Hix D, Kenyon R, Machi D, Mao C, Nordberg EK, Olson R, Overbeek R, Pusch GD, Shukla M, Schulman J, Stevens RL, Sullivan DE, Vonstein V, Warren A, Will R, Wilson MJC, Yoo HS, Zhang C, Zhang Y, Sobral BW. PATRIC, the bacterial bioinformatics database and analysis resource. Nucleic Acids Research. 2013;42:D581–D591. doi: 10.1093/nar/gkt1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver et al. (2005).Weaver DK, Nansen C, Runyon JB, Sing SE, Morrill WL. Spatial distributions of Cephus cinctus Norton (Hymenoptera: Cephidae) and its braconid parasitoids in Montana wheat fields. Biological Control. 2005;34:1–11. doi: 10.1016/j.biocontrol.2005.04.001. [DOI] [Google Scholar]
- Wenda-Piesik et al. (2009).Wenda-Piesik A, Sun Z, Grey WE, Weaver DK, Morrill WL. Mycoses of wheat stem sawfly (Hymenoptera: Cephidae) larvae by Fusarium spp. isolates. Environmental Entomology. 2009;38:387–394. doi: 10.1603/022.038.0211. [DOI] [PubMed] [Google Scholar]
- Wernegreen (2002).Wernegreen JJ. Genome evolution in bacterial endosymbionts of insects. Nature Reviews Genetics. 2002;3:850–861. doi: 10.1038/nrg931. [DOI] [PubMed] [Google Scholar]
- Werren, Baldo & Clark (2008).Werren JH, Baldo L, Clark ME. Wolbachia: master manipulators of invertebrate biology. Nature Reviews Microbiology. 2008;6:741–751. doi: 10.1038/nrmicro1969. [DOI] [PubMed] [Google Scholar]
- Whelan & Goldman (2001).Whelan S, Goldman N. A general empirical model of protein evolution derived from multiple protein families using a maximum-likelihood approach. Molecular Biology and Evolution. 2001;18:691–699. doi: 10.1093/oxfordjournals.molbev.a003851. [DOI] [PubMed] [Google Scholar]
- Whitman (2011).Whitman WB. Bergeys manual of systematic bacteriology. Springer; New York: 2011. [DOI] [Google Scholar]
- Xie et al. (2013).Xie J, Butler S, Sanchez G, Mateos M. Male killing Spiroplasma protects Drosophila melanogaster against two parasitoid wasps. Heredity. 2014;112:399–408. doi: 10.1038/hdy.2013.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, Vilchez & Mateos (2010).Xie J, Vilchez I, Mateos M. Spiroplasma bacteria enhance survival of Drosophila hydei attacked by the parasitic wasp Leptopilina heterotoma. PLOS ONE. 2010;5(8):e12149-7. doi: 10.1371/journal.pone.0012149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeoman et al. (2018).Yeoman CJ, Ishaq SL, Bichi E, Olivo SK, Lowe J, Aldridge BM. Biogeographical differences in the influence of maternal microbial sources on the early successional development of the bovine neonatal gastrointestinal tract. Scientific Reports. 2018;8 doi: 10.1038/s41598-018-21440-8. Article 3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zug & Hammerstein (2012).Zug R, Hammerstein P. Still a host of hosts for Wolbachia: analysis of recent data suggests that 40% of terrestrial arthropod species are infected. PLOS ONE. 2012;7(6):e38544-3. doi: 10.1371/journal.pone.0038544. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Descriptions of Attempts to Isolate and Culture Spiroplasma sp. and Metabolic Pathway Analysis Of Draft Wheat Stem Sawfly and Spiroplasma sp. Genomes
The 9,820 gene clusters determined from pangenomic analyses of 28 Spiroplasma spp. and 3 MAGs and their alignments.
A list of the 28 Spiroplasma species and 3 MAGs downloaded and utilized in our comparative analyses along with their NCBI accession information
Average nucleotide identities from alignments of 89 single copy core genes identified in the pangenome of Spiroplasma.
Longitudinal metabolomic analyses of cultivation efforts showing metabolites whose concentrations were deferentially altered (highlighted green) and others that were detected.
Shows the recruitment of all contigs by sample to each of the three bins, WSS host, wheat, and the Spiroplasma sp. symbiont.
Metabolic pathways encoded by genes found exclusively in the draft WSS (green) or Spiroplasma sp. (red) genome and those in both (orange) are shown on a KEGG map as created by iPATH.
Data Availability Statement
16S rRNA sequences were submitted to Sequence Read Archive and are available under accession number SRP108219. Metagenomic sequences were submitted to Sequence Read Archive under accession number SRP108220. Additional sequence data generated by Illumina HiSeq as part of the WSS genome project (Robertson et al., 2018) are available under accession number SRS694145. The metagenomic assembled genome is available in GenBank under bioproject PRJNA540284 and is available publicly at Patric with the genome ID 2132.146 (Spiroplasma sp. WSS).