Abstract
Objective:
Exposure to high levels of fine particle air pollution (PM2.5) is associated with adolescent pathophysiology. It is unclear, however, if PM2.5 is associated with physiology within psychosocial contexts, such as social stress, and whether some adolescents are particularly vulnerable to PM2.5 -related adverse effects. This study examined the association between PM2.5 and autonomic reactivity to social stress in adolescents and tested whether symptoms of anxiety and depression moderated this association.
Methods:
Adolescents from Northern California (n=144) participated in a modified Trier Social Stress Test (TSST) while providing high-frequency heart rate variability (HRV) and skin conductance level (SCL) data. PM2.5 data were recorded from CalEnviroScreen. Adolescents reported on their own symptoms of anxiety and depression using the Youth Self-Report, which has been used in prior studies and has good psychometric properties (Cronbach’s alpha in this sample was 0.86).
Results:
Adolescents residing in neighborhoods characterized by higher concentrations of PM2.5 demonstrated greater autonomic reactivity (i.e., indexed by lower HRV and higher SCL) (β=.27; b=0.44, p=.001, 95% CI [0.19, 0.68]) in response to social stress; this association was not accounted for by socioeconomic factors. In addition, adolescents who reported more severe anxiety and depression symptoms showed the strongest association between PM2.5 and autonomic reactivity to social stress (β=.53; b=0.86, p<.001, 95% CI [0.48, 1.23]).
Conclusions:
Exposure to PM2.5 may heighten adolescent physiological reactivity to social stressors. Moreover, adolescents who experience anxiety and depression may be particularly vulnerable to the adverse effects of PM2.5 on stress reactivity.
Keywords: air pollution, particulate matter, autonomic reactivity, social stress, adolescence, internalizing symptoms
INTRODUCTION
Air pollution is currently considered the greatest environmental threat to public health (1). Short- and long-term exposure to high levels of air pollution exacerbates and increases risk for health problems such as autoimmune disorders, lung cancer, and cardiovascular disease (2–4). Many of the health risks of air pollution have been attributed specifically to fine particle pollution, or particulate matter (PM2.5). PM2.5 refers to air-suspended mixtures of solid and liquid particles smaller than or equal to 2.5 micrometers in diameter. PM2.5 may be particularly detrimental to health given that these particles are composed of mixtures of harmful chemicals (e.g., sulfates, nitrates, metals), and remain in the air for longer periods of time, and penetrate the lungs more deeply, than more coarse particles (i.e., PM10–2.5) (3). Scientists have posited that inhalation and ingestion of PM2.5 leads to cardiopulmonary inflammation and oxidative stress which, in turn, may contribute to alterations in autonomic nervous system functioning (2,4). In fact, PM2.5 has been associated with elevated heart rate (5), increased arterial blood pressure (6), and reduced heart rate variability (7), which is often interpreted as an indicator of poor autonomic control and decreased flexibility (8). Taken together, these associations suggest that PM2.5 increases autonomic imbalance characterized by relatively greater sympathetic than parasympathetic nervous system activity (5). This pattern of autonomic imbalance is involved in negative affective states (9) and, over time, places excessive energy demands on bodily systems that can lead to health problems such as hypertension, diabetes, metabolic syndrome, and cardiovascular disease (8).
To date, studies of PM2.5 have used measures of autonomic functioning that involve baseline or resting state tasks (sitting, standing, or supine position), 24 hour ambulatory monitoring, and paced breathing tasks. It is unclear, however, whether PM2.5 is associated with autonomic responding in psychosocial challenge contexts that have been implicated in health and negative affect, such as social stress.
Adolescence is a key developmental window during which to investigate the effects of PM2.5 on reactivity to social stress. Adolescents are particularly susceptible to the adverse effects of air pollution because they spend considerable time outdoors and are physically active, both of which increase exposure to PM2.5 (10). Physiological systems involved in responding to stress are also developing rapidly during adolescence, which can increase vulnerability to environmental insults. Importantly, greater exposure to PM2.5 has been negatively associated with adolescent development and functioning of the immune (11), metabolic (12), and pulmonary systems (13). Adolescence is also a period of increased sensitivity to social stress (14). Adolescents have higher physiological reactivity to social stress inductions than do children (15); moreover, adolescent physiological reactivity to social-evaluative stressors increases with age and pubertal development (16,17). Although the link between adolescent stress reactivity and social environmental factors such as early caregiving experiences is well documented, researchers have not examined whether physical environmental factors like PM2.5 also influence stress reactivity in adolescence.
A growing body of research suggests that psychological factors can moderate vulnerability to the negative effects of environmental pollutants on health and physiology (18–20). In a cross-sectional study, Hicken et al. (21) found that depression heightens vulnerability to the effects of lead on high blood pressure in African-American adults. Similarly, in a cross-sectional study Cakmak, Dales, and Blanco (22) found evidence of stronger associations between air pollution and both higher blood pressure and reduced lung functioning in adults who reported lower levels of happiness. These findings suggest that the combination of physical pollutant exposure and psychosocial risk factors is most detrimental to health, as stress may amplify adverse effects of physical pollutants (19,20). Psychosocial stress and PM2.5 affect health through similar pathways that contribute directly (e.g., neural mechanisms) and indirectly (e.g., oxidative stress, inflammation) to autonomic imbalance. Thus, exposure to both psychosocial stress and PM2.5 may compound risk for impaired physiological functioning (19,20).
Although much of the research in this area has focused on adults, it is likely that psychological factors earlier in development contribute to the emergence of this vulnerability. In this context, given that anxious and depressed youth experience high levels of psychosocial stress (23), these adolescents may be particularly vulnerable to the adverse effects of environmental pollutants. Some longitudinal studies have found evidence that traffic-related air pollution exposure predicts children’s asthma and respiratory functioning, but primarily in children with a greater history of social stress (24,25). Conversely, Chen et al. (18) found that a history of chronic family stress was associated concurrently with more inflammation, and longitudinally predicted increases in asthma symptoms over 6 months, but only for adolescents who were exposed to less traffic-related air pollution. It is important to note that these studies focused on family stress and exposure to violence rather than on mental health status as the moderator of the link between air pollution and outcome. In the only study to date that examined mental health status as a moderator of the concurrent association between air pollution exposure and physiology in a pediatric sample, Dales and Cakmak (26) found that living in neighborhoods with greater air pollution was associated with higher resting blood pressure and reduced lung function in children and adolescents with mood disorders.
In the present study we assessed whether levels of PM2.5 were associated with physiological responses to a social stress induction in adolescence. We expected that adolescents living in neighborhoods with higher concentrations of PM2.5 would exhibit greater autonomic reactivity to a social stress task, indexed by higher sympathetic reactivity and stronger withdrawal of parasympathetic activity (i.e., autonomic imbalance). We then examined the potential moderating role of symptoms of anxiety and depression on the relation between PM2.5 and autonomic reactivity. Based on previous work suggesting that psychosocial-stress related factors can increase vulnerability to physical pollutants, we expected that the association between PM2.5 and autonomic reactivity would be strongest in adolescents who reported the most severe symptoms of anxiety and depression. Finally, given previously demonstrated associations among air pollution exposure, neighborhood poverty, race/ethnicity (27,28), family socioeconomic status and adolescent health and functioning (29), and body mass index (BMI) and autonomic measures (30), we controlled for family and neighborhood socioeconomic factors, ethnic/racial minority status, and BMI to examine the specificity to PM2.5 of aberrant autonomic reactivity.
Methods
Participants
Participants were 144 adolescents (79 girls, 65 boys) from Northern California (primarily from the San Francisco Bay Area; mean age=12.20, SD=1.39, range=9.56–15.82) who were part of a larger study on stress, neurodevelopment, and risk for psychopathology over the course of puberty. The participants included in the current analyses were ethnically diverse (42% European-American; 23% biracial; 12% Asian-American; 8% African-American; 9% Hispanic/Latino; 5% Other; 1% did not report) and came from a wide range of socioeconomic backgrounds (mean annual household income=$75,000-$100,000, range from less than $5,000 to greater than $150,000). The design of the larger study included matching boys and girls on pubertal stage using the Tanner Staging self-report questionnaire (31). Because girls typically reach sexual maturity earlier than boys, boys in our sample were slightly older than girls (mean difference=0.67 years, t(142)=2.95, p=.004). Online advertisements and locally distributed flyers were used to recruit families for this study. Families who were interested in participating underwent a telephone eligibility screening in which they received more information about study aims and requirements. Contact information, which included California street addresses for each participant, was obtained during this initial interview. To be eligible for participation, adolescents had to be fluent in English and not have a history of major medical illness or neurological disorder. Given that the larger study involved a neuroimaging component, inability to participate in an MRI session was also an exclusion criterion. Most of the data for the current analyses were collected in our laboratory from 2013–2017. Our measure of PM2.5 was based on census tract-level data collected from 2012–2014 (see below). There was no significant clustering of participants by census tract; our sample included 122 unique census tracts, and the maximum number of participants living within the same census tract was 3. Participants who provided autonomic nervous system data were included in the current analyses. These participants did not differ significantly from participants who were excluded because they did not provide autonomic nervous system data with respect to family income (t(201)=0.02), pubertal stage (t(214)=1.77), sex (X2 (1) = 0.24), ethnicity (X2 (5) = 5.26), neighborhood socioeconomic disadvantage (t(204)=0.66), or PM2.5 levels (t(204)=1.29) (all p>.077). All participants signed assent forms, and their parents signed consent forms, to participate in this study, which was approved by the Stanford University Institutional Review Board.
PM2.5
The Office of Environmental Health Hazard Assessment created CalEnviroScreen as an online tool that maps various environmental indicators for pollution and population characteristics across California neighborhoods at the census tract level. We used the latest iteration of CalEnviroScreen 3.0 (updated in June, 2018). We recorded CalEnviroScreen 3.0 census tract scores for PM2.5 for each participant’s address. Using air quality monitoring station data from the California Air Resources Board, CalEnviroScreen 3.0 provides PM2.5 measurements based on the mean level of PM2.5 from each quarter of a year, which were averaged from 2012–2014 (μg/m3) (32). In our sample, percentiles of the concentration of PM2.5 relative to other neighborhoods in California ranged from 18 to 94 (for more information about the methods used in the CalEnviroScreen 3.0 calculation of PM2.5 for each census tract, see the CalEnviroScreen report for PM2.5 at https://oehha.ca.gov). One participant was missing PM2.5 data due to living in a neighborhood that was missing census tract data in Calenviroscreen 3.0.
Neighborhood-Level Socioeconomic Disadvantage
We created a composite measure of neighborhood-level socioeconomic disadvantage using census tract measures of poverty, educational attainment, unemployment, and housing burden provided by CalEnviroScreen 3.0. Poverty, educational attainment, and unemployment for each census tract were calculated using data from The American Community Survey and taking the 5-year estimate (2011–2015) of the percent of the population in the census tract living below twice the Federal Poverty Level, over the age of 25 without a high school education, and over the age of 16 and unemployed, respectively. Housing burden for each census tract was calculated using data from multiple nationally representative datasets and taking the 5-year estimate (2009–2013) of the percent of households that are both low income and spending more than half of their income on housing costs. CalEnviroScreen 3.0 converts the poverty, educational attainment, unemployment, and housing burden data into percentiles representing the amount of socioeconomic disadvantage relative to other neighborhoods in California. These percentile values were positively correlated with each other (all r>.41, p<.001) and were averaged to form a measure of overall neighborhood socioeconomic disadvantage. The same participant who was missing PM2.5 data was also missing neighborhood-level socioeconomic data due to living in a census tract that was not included in the CalEnviroScreen 3.0 tool.
Family Income
Parents reported on their annual family income before taxes using a 10-point scale ranging from “Less than $5,000” to “$150,000 or greater.”
Trier Social Stress Test
Participants completed a modified version of the Trier Social Stress Test (TSST; 33). The TSST is one of the most widely used and reliable laboratory protocols for inducing physiological stress related to social evaluation and uncontrollability (34). Shortly after arriving at the laboratory, electrodes were placed on adolescents to assess autonomic nervous system activity. Specifically, electrodes were placed on adolescents’ two lower ribs and underneath the collarbone to collect electrocardiogram (ECG) data. Two electrodes were placed on adolescents’ non-dominant hand to assess skin conductance level. After all electrodes were attached, adolescents had a resting baseline period in which they were instructed to sit quietly and relax for five minutes. This baseline period was followed by a modified TSST. An examiner began a story and instructed the participants to prepare an exciting ending to the story over the next five minutes. The participant was told that a judge would come in to evaluate the end of their story based on content and memorization. After the 5 min speech preparation period, adolescents presented their story to a “judge” who maintained a neutral expression while appearing to take notes about the presentation. After the five minutes had passed, the judge instructed the participant to complete a five-minute subtraction task. The judge took notes during this final task, interrupting participants when they made a mistake and instructing them to start over. The judge maintained a neutral expression throughout the subtraction task. After participants completed the baseline task, they reported on how upset and stressed they felt using scales ranging from 1 (e.g., “not at all upset”) to 7 (e.g., “very upset”). Participants reported on their feelings again immediately following the TSST using the same rating scales. Overall, as expected, adolescents reported feeling more upset and stressed after the TSST than after the baseline procedure (mean difference=1.19, t(143)=10.59, p<.001); thus, the TSST was effective in increasing negative affect. Participants were debriefed after the conclusion of the TSST. Participants were sitting throughout the TSST.
Autonomic Reactivity to Stress
High frequency heart rate variability (HRV) refers to beat-to-beat fluctuations in heart rate and is a measure of parasympathetic nervous system activity. Skin conductance level (SCL) represents the overall conductivity (i.e., sweat) at the palms and is a measure of sympathetic nervous system activity. We inspected and edited the ECG and skin conductance data using ANSlab software. For HRV, we set the high-frequency bandpass to range from 0.15 to 0.40. We computed HRV and SCL within the baseline task and the speech preparation and subtraction portions of the TSST (5 min each). For HRV and SCL, the values in the two TSST tasks were averaged and subtracted from the baseline task to create change scores. Overall, adolescents had lower HRV (mean difference=−.40, t(141)=5.65, p<.001) and higher SCL (mean difference=3.81, t(140)=12.00, p<.001) during the TSST than during the baseline task; thus, the TSST was effective in eliciting autonomic reactivity indexed by increasing sympathetic nervous system dominance. We integrated these change scores to create an index of autonomic imbalance using an approach similar to that described by Berntson and colleagues (35). First, we standardized the HRV and SCL change scores using z-transformation. Then, because decreases in HRV represent greater parasympathetic reactivity to the TSST, we multiplied the standardized HRV change score values by −1 so that higher values indexed greater parasympathetic withdrawal (i.e., parasympathetic reactivity). We then summed the inverse standardized HRV and standardized SCL change scores to create an index of shift to sympathetic nervous system dominance from the baseline to the TSST. Three participants were missing autonomic reactivity values because they did not provide useable HRV or SCL data.
Anxiety and Depression
Adolescents reported on their anxiety and depression symptoms over the preceding six months using the anxious/depressed subscale of the Youth Self-Report (YSR; 36). The anxious/depressed subscale included 13 items (e.g., “I feel that no one loves me,” “I am too fearful or anxious”) that were rated on a 3-point scale (0-Note true, 1-Somewhat or sometimes true, and 2-Very often or often true) (Cronbach’s alpha=0.86). To deal with any missing item responses, these ratings were averaged and multiplied by 13 (the total number of items) to generate a total score for the severity of symptoms of anxiety and depression. Six participants were missing YSR data due to experimenter error.
Statistical Analyses
We conducted regression analyses to examine whether PM2.5 was associated with autonomic reactivity to social stress after adjusting for sex, age, pubertal stage, BMI, minority status, family income, and neighborhood socioeconomic disadvantage, and to test whether anxiety and depression symptoms moderated this relation. All predictors were centered prior to forming interaction terms in the regression analysis. Significant interactions were probed by examining the effect of PM2.5 on autonomic reactivity at 1 SD above and below the mean level of anxiety and depression symptoms. Extreme outlier values for all variables were identified using boxplots (values greater or less than the mean by 3 times the interquartile range). This is considered a better method for outlier detection than using standard deviations, which can miss outliers due to extreme values increasing the sample mean and variance (37). Two outliers were identified for SCL reactivity and were winsorized prior to analyses. All analyses were conducted using the lavaan package in R. To account for missing data, all model parameters were estimated using full information maximum likelihood estimation. To correct for multiple testing in the regression model, we used a Bonferroni-corrected threshold for statistical significance at p<.005. Our analyses did not have issues related to multicollinearity (all tolerance and variance inflation factor values < .44 and 2.27, respectively), and visual inspection suggested normality of residuals and homoscedacity.
Results
Descriptive statistics and bivariate correlations are presented in Table 1. Baseline measures of HRV, SCL, and autonomic imbalance were not associated with PM2.5 (all p>.327) and are not presented in the table. As hypothesized, adolescents who resided in neighborhoods characterized by higher concentrations of PM2.5 exhibited greater overall autonomic reactivity in response to the TSST (r=.24, p=.004). PM2.5 was also associated with greater decreases in HRV (r=−.19, p=.022), but not SCL reactivity (r=.15, p=.064), in response to the TSST.
Table 1.
Descriptive Statistics and Zero-Order Correlations.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Sex (female=1)a | 1 | |||||||||||
| 2. Agea | −.24** | 1 | ||||||||||
| 3. Pubertal stageb | .10 | .67*** | 1 | |||||||||
| 4. BMIc | .05 | .21* | .34*** | 1 | ||||||||
| 5. Minority status (1=racial/ethnic minority)a | −.07 | −.08 | −.16 | .23** | 1 | |||||||
| 6. Family incomed | −.05 | .05 | −.02 | −.17 | −.32*** | 1 | ||||||
| 7. Neighborhood disadvantage (% ranking)e | .02 | −.18* | −.03 | .06 | .23** | −.43*** | 1 | |||||
| 8. PM2.5 (μg/m3)e | .03 | −.07 | −.10 | .04 | .00 | −.01 | .00 | 1 | ||||
| 9. Anxiety/Depressionb | .07 | −.18* | −.10 | −.06 | .17* | −.03 | −.10 | .03 | 1 | |||
| 10. Δ in ANS imbalanceb | .15 | −.04 | −.03 | −.14 | −.12 | .09 | .04 | .24** | .02 | 1 | ||
| 11. Δ in HRVf | −.12 | .04 | .03 | .18* | .11 | −.03 | −.01 | −.19* | .09 | −.72*** | 1 | |
| 12. Δ in SCLb | .10 | −.02 | −.03 | −.01 | −.07 | .10 | .04 | .15 | .13 | .71*** | −.09 | 1 |
| Mean or % | 55% | 12.20 | 2.55 | 19.42 | 58% | 8.33 | 28.86 | 9.38 | 5.12 | 0.00 | −0.40 | 3.89 |
| SD | 1.39 | 1.09 | 4.54 | 1.96 | 18.16 | 0.88 | 4.42 | 1.43 | 0.84 | 3.29 | ||
| Range | 9.56–15.82 | 1.00–5.00 | 6.57–35.98 | 1.00–10.00 | 0.75–85.75 | 7.86–13.44 | 0.00–21.00 | −5.20–3.58 | 3.89–26.00 | −7.72–13.67 |
Note.
p<.001,
p<.01,
p<.05.
PM2.5= particulate matter < 2.5 micrometers; BMI=body mass index; ANS=autonomic nervous system; HRV=heart rate variability; SCL=skin conductance level. Boys were coded as 0 and girls were coded as 1. European American participants were coded as 0 and all other participants were coded as 1. Full information maximum likelihood was used to take advantage of the full sample (N=144) to estimate all correlations.
n=144;
n=141;
n=135;
n=133;
n=143;
n=142.
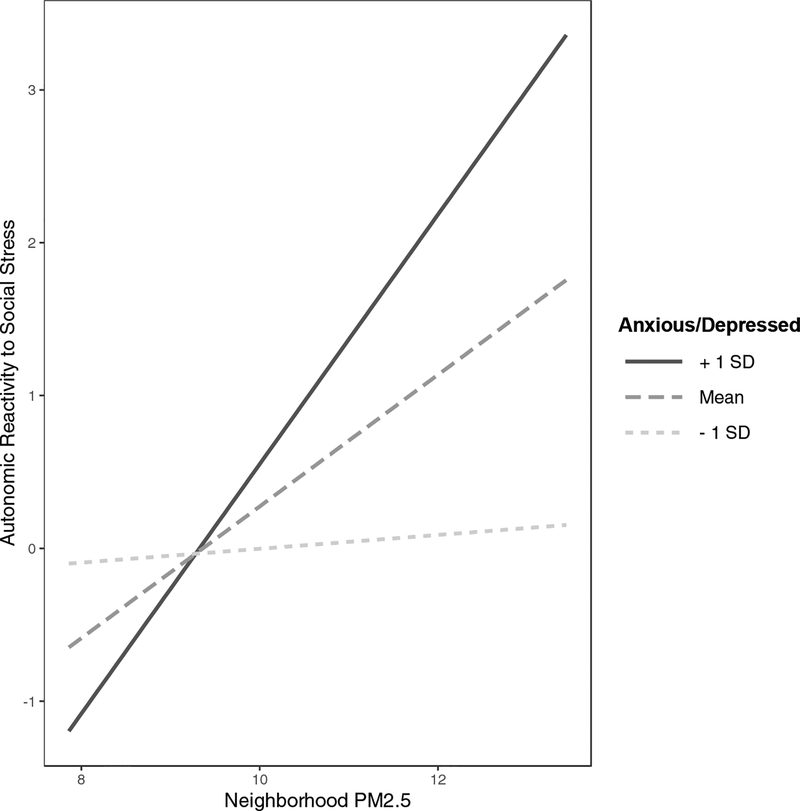
We conducted a regression analysis to determine whether the association between PM2.5 and autonomic reactivity to stress was still significant after adjusting for other variables, and whether the association between PM2.5 and autonomic reactivity was moderated by symptoms of anxiety and depression. The full regression models are presented in Table 2. In this model, the main effect of PM2.5 on autonomic reactivity, or imbalance, was still significant (β=.27; b=0.44, p=.001, 95% CI [0.19, 0.68]) after adjusting for sex, age, pubertal stage, BMI, minority status, family income, and neighborhood socioeconomic disadvantage. Neighborhood-level socioeconomic disadvantage was also associated with greater autonomic reactivity (β=.18; b=0.01, p=.048, 95% CI [0.00, 0.03]), but this effect did not survive the more conservative Bonferroni corrected threshold of p<.005. The interaction of PM2.5 with symptoms of anxiety and depression in predicting autonomic reactivity was also statistically significant (β=.25; b=0.09, p=.002, 95% CI [0.04, 0.15]). Figure 1 presents the simple slopes of the interaction effect. The positive association between PM2.5 and autonomic reactivity to stress was approximately twice as strong in adolescents who reported more severe symptoms of anxiety and depression (β=.53; b=0.86, p<.001, 95% CI [0.48, 1.23]) than it was for adolescents with average levels of symptom severity (β=.27, p=.001). Conversely, there was no association between PM2.5 and autonomic reactivity to stress in adolescents who reported the least severe symptoms of anxiety and depression (β=.01; b=.02, p=.910, 95% CI [−0.32, 0.36]). None of the other covariates were significantly associated with autonomic imbalance in the context of the regression model (all p>.081).
Table 2.
Regression Model Predicting Autonomic Reactivity to Stress
| Δ in ANS imbalance | ||||
|---|---|---|---|---|
| B | SE | β | p | |
| Intercept | .22 | |||
| Sex | .39 | .24 | .14 | .102 |
| Age | .11 | .12 | .11 | .352 |
| Pubertal stage | −.10 | .15 | −.08 | .527 |
| BMI | −.03 | .03 | −.10 | .292 |
| Minority status | −.37 | .25 | −.13 | .149 |
| Family income | .12 | .07 | .16 | .082 |
| Neighborhood disadvantage | .01 | .01 | .18 | .048 |
| PM2.5 | .44 | .13 | .27 | .001 |
| Anxiety/Depression | .02 | .03 | .05 | .521 |
| PM2.5 x Anxiety/Depression | .09 | .03 | .25 | .002 |
Note. ANS=autonomic nervous system; BMI=body mass index; PM2.5=particulate matter < 2.5 micrometers. Full information maximum likelihood was used to take advantage of the full sample (N=144) to estimate regression model parameters.
Figure 1.
Simple slopes demonstrating the association between PM2.5 and autonomic reactivity to stress at different levels of anxiety and depression symptoms. Higher autonomic reactivity to stress values indicate greater shifts to sympathetic nervous system dominance in response to the Trier Social Stress Test. Given that the measure of autonomic reactivity was based on aggregating standardized scores, the zero value represents the mean shift to sympathetic nervous system dominance (i.e., increasing skin conductance and decreasing heart rate variability).
Discussion
PM2.5 is a well-documented environmental threat to public health. Although PM2.5 has been linked to autonomic imbalance at rest, no study has examined the relation between PM2.5 and autonomic reactivity to social stress. Our study provides novel evidence that adolescents residing in neighborhoods with higher concentrations of PM2.5 exhibit more sympathetic nervous system dominance in response to social stress. Moreover, our findings suggest that anxious and depressed youth are particularly vulnerable to the effects of PM2.5 on autonomic reactivity to social stress. Importantly, these associations were not accounted for by other demographic or socioeconomic factors.
General activation of the peripheral stress response, which includes systemic inflammation and oxidative stress, has been implicated in the adverse effects of PM2.5 on health (2,4). Our findings are the first, however, to demonstrate that PM2.5 exposure may contribute to activation of a stress response in adolescents that is specific to a psychosocial context. This finding warrants particular attention given that adolescence is already a period of heightened sensitivity to social stress and evaluation (14,15). Exposure to high levels of PM2.5 may exacerbate adolescents’ physiological responses to these stressors, potentially culminating in greater negative affect.
Our study also provides the first evidence that symptoms of anxiety and depression moderate the association between PM2.5 and autonomic reactivity to social stress in adolescence. The positive association between PM2.5 and autonomic reactivity was most pronounced for adolescents who reported the most severe anxiety and depression symptoms; in fact, there was not a significant association between PM2.5 and autonomic reactivity in adolescents who reported the least severe anxiety and depression symptoms. Previous work suggests that youth with mental health disorders are at greater risk for poor resting cardiorespiratory functioning related to high levels of air pollution (26). Our finding builds on this previous research by demonstrating that psychological traits may influence vulnerability to the effects of PM2.5 on autonomic reactivity to social stress.
One explanation for our finding that symptoms of anxiety and depression moderated the association between PM2.5 and autonomic reactivity is that these symptoms may contribute to a physiological milieu that increases and exacerbates exposure to PM2.5. Anxiety and depression in adolescents have been associated with physiological indicators of hyperarousal, such as low heart rate variability, high heart rate, and breathing irregularities (38–40). In much the same way that physical activity increases respiratory inhalation and deposition of PM2.5 (41), proneness to hyperarousal states characterized by elevated cardiorespiratory activity may increase the amount of PM2.5 that enters the lungs and blood stream. In addition, adolescent anxiety and depression have been shown to have a pro-inflammatory phenotype (42). Thus, these youth may have increased and chronic inflammatory reactivity to PM2.5 exposure that exacerbates other aspects of the stress response, including sympathetic dominance in the autonomic nervous system.
Although we were interested in examining anxiety and depression symptoms as a moderator of the relation between PM2.5 and stress reactivity, it is important to note that these symptoms have also been linked in previous studies to autonomic imbalance (38) and increased air pollution (43). Combined with the present findings, these associations raise the question of whether PM2.5-related increases in stress reactivity are implicated in the development of mental and physical health problems. One promising avenue for future research is to determine whether high autonomic reactivity to social stressors is a mechanism by which PM2.5 exposure compromises physical and mental health (i.e., longitudinal mediation model). It will be particularly important in this research to focus on adolescence, a developmental period that is characterized by more exposure to air pollution (10) and by increasing rates of anxiety and depression (44).
It is important to note limitations of this study. First, we used measures of community-level air pollution. Adolescents living within these communities vary in their exposure to ambient PM2.5 (e.g., spending more time outdoor or indoor), and we were unable to assess these differences. As a related point, the measure of PM2.5 was based on data from 2012 to 2014, and we do not know how long participants had lived in their current neighborhoods. That said, however, the fact that we found a significant association between adolescents’ exposure to PM2.5 and their autonomic reactivity suggests that our census tract data are valid. Nevertheless, future research should focus on determining whether the effects of PM2.5 on stress reactivity are due to exposure during specific developmental windows or to an accumulation of chronic exposure. Second, treating PM2.5 as the moderator, rather than symptoms, leads to a different interpretation of our findings – that more anxious and depressed youth are prone to social stress-related states of autonomic imbalance, but only when living in communities characterized by greater PM2.5 exposure. Given our cross-sectional design, we cannot rule out this interpretation. Third, participants in our study live in Northern California, primarily in the San Francisco Bay Area. According to a recent report from the American Lung Association (45), the San Francisco Bay Area is among the ten nationwide metropolitan areas with the worst particle air pollution. Thus, it is likely that PM2.5 exposure in our sample of adolescents was high relative to adolescents living in other parts of the United States. Future research should examine whether the association between PM2.5 and autonomic reactivity to social stress documented in this study generalizes to adolescents living in communities with lower levels of air pollution. Finally, recent research suggests that physiological reactivity to the TSST is correlated with responses to acute stressors in real life, such as taking an oral examination (46). Although exams and public speaking are common stressful events in adolescence, the extent to which the TSST is an ecologically valid measure of other stressors encountered by some youth, including safety concerns, peer conflict, and discrimination, is unclear. Research that combines ambulatory monitoring of physiology with ecological momentary assessments of stress is a promising avenue for examining the effects of PM2.5 on autonomic reactivity outside of the laboratory.
Despite these limitations, our study is important in providing the first evidence for an association between exposure to PM2.5 and stronger autonomic reactivity to social stress in adolescence. This increased autonomic reactivity was characterized by a shift to greater sympathetic and reduced parasympathetic nervous system activity. Furthermore, more severe symptoms of adolescent anxiety and depression appeared to potentiate the adverse effects of PM2.5 on reactivity to social stress. These findings contribute to a growing literature suggesting that physical pollutants play a significant role in psychosocial functioning. The results of this study may also have important policy and clinical implications. Limiting exposure to PM2.5 might help reduce adolescent reactivity to social stress and evaluation, which appears to be particularly helpful for youth who are experiencing symptoms of anxiety and depression.
Conflicts of Interest and Sources of Funding:
All authors declare no conflicts of interest. This research was supported by the National Institutes of Mental Health (R37MH101495 to IHG, T32MH019908 to Allan L. Reiss (funding JGM), T32MH019938 to Alan Schatzberg (funding EMM), and F32MH096385 to KK), the Brain and Behavior Research Foundation (Young Investigator Award 20814 to KK), and the Stanford University Precision Health and Integrated Diagnostics Center (PHIND to IHG).
Abbreviations:
- PM2.5
particulate matter less than or equal to 2.5 micrometers
- TSST
Trier Social Stress Test
- HRV
heart rate variability
- SCL
skin conductance level
References
- 1.World Health Organization. Ambient air pollution: a global assessment of exposure and burden of disease, WHO, Geneva 2016. [Google Scholar]
- 2.Pope CA 3rd, Dockery DW. Health effects of fine particulate air pollution: lines that connect. J Air Waste Manag Assoc 2006;56:709–742. [DOI] [PubMed] [Google Scholar]
- 3.Seaton A, MacNee W, Donaldson K, Godden D. Particulate air pollution and acute health effects. Lancet 1995;345:176–178. [DOI] [PubMed] [Google Scholar]
- 4.Brook RD, Rajagopalan S, Pope CA 3rd, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA, Peters A, Siscovick D, Smith SC Jr, Whitsel L, Kaufman JD, American Heart Association Council on Epidemiology and Prevention, Council on the Kidney in Cardiovascular Disease, Council on Nutrition, Physical Activity and Metabolism. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation 2010;121:2331–2378. [DOI] [PubMed] [Google Scholar]
- 5.Pope CA 3rd, Verrier RL, Lovett EG, Larson AC, Raizenne ME, Kanner RE, Schwartz J, Villegas GM, Gold DR, Dockery DW. Heart rate variability associated with particulate air pollution. Am Heart J 1999;138:890–899. [DOI] [PubMed] [Google Scholar]
- 6.Fuks K, Moebus S, Hertel S, Viehmann A, Nonnemacher M, Dragano N, Möhlenkamp S, Jakobs H, Kessler C, Erbel R, Hoffman B; Heinz Nixdorf Recall Study Investigative Group. Long-term urban particulate air pollution, traffic noise, and arterial blood pressure. Environ Health Perspect 2011;119:1706–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He F, Shaffer ML, Li X, Rodriguez-Colon S, Wolbrette DL, Williams R, Cascio WE, Liao D. Individual-level PM2.5 exposure and the time course of impaired heart rate variability – the APACR study. J Expo Sci Environ Epidemiol 2011;21:65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thayer JF, Yamamoto SS, Brosschot JF. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int J Cardiol 2010;141:122–131. [DOI] [PubMed] [Google Scholar]
- 9.Kreibig SD. Autonomic nervous system activity in emotion: A review. Biol Psychol 2010;84:394–421. [DOI] [PubMed] [Google Scholar]
- 10.Sacks JD, Stanek LW, Luben TJ, Johns DO, Buckley BJ, Brown JS, Ross M. Particulate matter-induced health effects: who is susceptible? Environ Health Perspect 2011;119:446–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dobreva ZG, Kostadinova GS, Popov BN, Petkov GS, Stanilova SA. Proinflammatory and anti-inflammatory cytokines in adolescents from Southeast Bulgarian cities with different levels of air pollution. Toxicol Ind Health 2015;31:1210–1217. [DOI] [PubMed] [Google Scholar]
- 12.Kelishadi R, Mirghaffari N, Poursafa P, Gidding SS. Lifestyle and environmental factors associated with inflammation, oxidative stress and insulin resistance in children. Atherosclerosis 2009;203:311–319. [DOI] [PubMed] [Google Scholar]
- 13.Gauderman WJ, Avol E, Gilliland F, Vora H, Thomas D, Berhane K, McConnell R, Kuenzli N, Lurmann F, Rappaport E, Margolis H, Bates D, Peters J. The effect of air pollution on lung development from 10 to 18 years of age. N Engl J Med 2004;351:1057–1067. [DOI] [PubMed] [Google Scholar]
- 14.Somerville LH. The teenage brain: sensitivity to social evaluation. Curr Dir Psychol Sci 2014;22:121–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stroud LR, Foster E, Papandonatos GD, Handwerger K, Granger DA, Kivlighan KT, Niaura R. Stress response and the adolescent transition: performance versus peer rejection stressors. Dev Psychopathol 2009;21:47–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sumter SR, Bokhorst CL, Miers AC, Van Pelt J, Westenberg PM. Age and puberty differences in stress responses during a public speaking task: do adolescents grow more sensitive to social evaluation? Psychoneuroendocrinology 2010;35:1510–1516. [DOI] [PubMed] [Google Scholar]
- 17.van den Bos E, de Rooij M, Miers AC, Bokhorst CL, Westenberg PM. Adolescents’ increasing stress response to social evaluation: pubertal effects on cortisol and alpha-amylase during public speaking. Child Dev 2014;85:220–236. [DOI] [PubMed] [Google Scholar]
- 18.Chen E, Schreier HM, Strunk RC, Brauer M. Chronic traffic-related air pollution and stress interact to predict biologic and clinical outcomes in asthma. Environ Health Perspect 2008;116:970–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clougherty JE, Kubzansky LD. A framework for examining social stress and susceptibility to air pollution in respiratory health. Environ Health Perspect 2009;117:1351–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olvera Alvarez HA, Kubzansky LD, Campen MJ, Slavich GM. Early life stress, air pollution, inflammation, and disease: An integrative review and immunologic model of social-environmental adversity and lifespan health. Neurosci Biobehav Rev 2018;92:226–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hicken MT, Lee H, Morenoff J, House JS, Williams DR. Racial/ethnic disparities in hypertension prevalence: reconsidering the role of chronic stress. Am J Public Health 2014;104:117–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cakmak S, Dales RE, Blanco CV. Does emotional health influence susceptibility to the physiologic effects of air pollution on adults? Int J Sus Dev Plann 2016;11:537–545. [Google Scholar]
- 23.Kim KJ, Conger RD, Elder GH Jr, Lorenz FO. Reciprocal influences between stressful life events and adolescent internalizing and externalizing problems. Child Dev 2003;74:127–143. [DOI] [PubMed] [Google Scholar]
- 24.Clougherty JE, Levy JI, Kubzanksy LD, Ryan PB, Suglia SF, Canner MJ, Wright RJ. Synergistic effects of traffic-related air pollution and exposure to violence on urban asthma etiology. Environ Health Perspect 2007;115:1140–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ranciere F, Bougas, Viola, Momas I. Early exposure to traffic-related air pollution, respiratory symptoms at 4 years of age, and potential effect modification by parental allergy, stressful family events, and sex: A prospective follow-up study of the PARIS birth cohort. Environ Health Perspect 2017;125:737–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dales RE, Cakmak S. Does mental health status influence susceptibility to the physiologic effects of air pollution? A population based study of Canadian children. PLoS One 2016;11:e0168931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Neill MS, Jerrett M, Kawachi I, Levy JI, Cohen AJ, Gouveia N, Wilkinson P, Fletcher T, Cifuentes L, Schwartz J; Workshop on Air Pollution and Socioeconomic Conditions. Health, wealth, and air pollution: advancing theory and methods. Environ Health Perspect 2003;111:1861–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones MR, Diez-Roux AV, Hajat A, Kershaw KN, O’Neill MS, Guallar E, Post WS, Kaufman JD, Navas-Acien A. Race/ethnicity, residential segregation, and exposure to ambient air pollution: The multi-ethnic study of artherosclerosis (MESA). Am J Public Health 2014;104:2130–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen E, Paterson LQ. Neighborhood, family, and sujective socioeconomic status: How do they relate to adolescent health. Health Psychol 2006;25:704–714. [DOI] [PubMed] [Google Scholar]
- 30.Koenig J, Jarczok MN, Warth M, Ellis RJ, Bach C, Hillecke TK, Thayer JF. Body mass index is related to autonomic nervous system activity as measured by heart rate variability – a replication using short term measurements. J Nutr Health Aging 2014;18:300–302. [DOI] [PubMed] [Google Scholar]
- 31.Morris NM, Udry JR. Validation of a self-administered instrument to assess stage of adolescent development. J Youth Adolesc 1980;9:271–280. [DOI] [PubMed] [Google Scholar]
- 32.OEHHA. Update to the California Communities Environmental Health Screening Tool, CalEnviroScreen 3.0. 2017.
- 33.Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’—a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology 1993;28:76–81. [DOI] [PubMed] [Google Scholar]
- 34.Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychol Bull 2004;130:355–391. [DOI] [PubMed] [Google Scholar]
- 35.Berntson GG, Norman GJ, Hawkley LC, Cacioppo JT. Cardiac autonomic balance versus cardiac regulatory capacity. Psychophysiology 2008;45:643–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Achenbach TM, Rescorla LA. Manual for the ASEBA school-age forms and profiles. 2001. Burlington, VT: University of Vermont, Research Center for Children, Youth, and Families. [Google Scholar]
- 37.Wilcox R Modern statistics for the social and behavioral sciences: A practical introduction. Boca Raton, FL: CRC Press; 2012. [Google Scholar]
- 38.Vazquez L, Blood JD, Wu J, Chaplin TM, Hommer RE, Rutherford HJ, Potenza MN, Mayes LC, Crowley MJ. High frequency heart-rate variability predicts adolescent depressive symptoms, particularly anhedonia, across one year. J Affect Disord 2016;196:243–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Greaves-Lord K, Ferdinand RF, Sondeijker FE, Dietrich A, Oldehinkel AJ, Rosmalen JG, Ormel J, Verhulst FC. Testing the tripartite model in young adolescents: is hyperarousal specific for anxiety and not depression? J Affect Disord 2007;102:55–63. [DOI] [PubMed] [Google Scholar]
- 40.Pine DS, Coplan JD, Papp LA, Klein RG, Martinez JM, Kovalenko P, Tancer N, Moreau D, Dummit ES 3rd, Shaffer D, Klein DF, Gorman JM. Ventilatory physiology of children and adolescents with anxiety disorders. Arch Gen Psychiatry 1998;55:123–129. [DOI] [PubMed] [Google Scholar]
- 41.Daigle CC, Chalupa DC, Gibb FR, Morrow PE, Oberdörster G, Utell MJ, Frampton MW. Ultrafine particle deposition in humans during rest and exercise. Inhal Toxicol 2003;15:539–552. [DOI] [PubMed] [Google Scholar]
- 42.Miller GE, Cole SW. Clustering of depression and inflammation in adolescents previously exposed to childhood adversity. Biol Psychiatry 2012;72:34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pun VC, Manjourides J, Suh H. Association of ambient air pollution with depressive and anxiety symptoms in older adults: Results from the NSHAP Study. Environ Health Perspect 2017;125:342–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thapar A, Collishaw S, Pine DS, Thapar AK. Depression in adolescence. Lancet 2012;379:1056–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.American Lung Association. State of the Air. 2016.
- 46.Henze G-I, Zankert S, Urschler DF, Hiltl TJ, Kudielka BM, Pruessner JC, Wust S. Testing the ecological validity of the Trier Social Stress Test: Association with real-life exam stress. Psychoneuroendocrinology 2017;75:52–55. [DOI] [PubMed] [Google Scholar]