Abstract
Autosomal dominant polycystic kidney disease (ADPKD) is an inherited multisystem disorder, characterized by renal and extra-renal fluid-filled cyst formation and increased kidney volume that eventually leads to end-stage renal disease. ADPKD is considered the fourth leading cause of end-stage renal disease in the United States and globally. Care of patients with ADPKD was, for a long time, limited to supportive lifestyle measures, due to the lack of therapeutic strategies targeting the main pathways involved in the pathophysiology of ADPKD. As the first FDA approved treatment of ADPKD, Vasopressin (V2) receptor blocking agent, tolvaptan, is an urgently awaited advance for ADPKD patients. In our review, we also shed some lights on what is beyond Tolvaptan as there are other medications in the pipeline and many medications have been or are currently being studied in clinical trials such as Tesevatinib, Metformin and Pravastatin, with the goal of slowing the rate of progression of ADPKD by reducing the increase in total kidney volume or maintaining eGFR. Here, we review updates in the perspectives and management of ADPKD.
Keywords: vasopressin receptor antagonist, tolvaptan, metformin, total kidney volume, chronic kidney disease, hypertension
Overview
Autosomal Dominant Polycystic Kidney Disease (ADPKD) is the most common monogenic inherited kidney disease. It has an incidence of 1 in 500 to 1 in 1000 individuals.1 It affects over 600,000 individuals in the United States (US) and 12 million people worldwide.2 Approximately 70% of patients with ADPKD progress to end-stage renal disease (ESRD) at a median age of 58 years,3 making it the fourth leading cause of end-stage renal disease (ESRD) in the US and globally.4,5
In the United States, incidence rates of ESRD due to ADPKD are higher in men than in women (8.2 compared to 6.8 per million, respectively).5 Later onset of ESRD in ADPKD patients in recent years may be due to reduced cardiovascular mortality of older patients before reaching ESRD or increased access of older patients to kidney replacement therapy.5
ADPKD is characterized by the progressive development and growth of numerous bilateral renal cysts, resulting in numerous abnormalities (Table 1), the most important one being the loss of renal function.6 Despite the continuous destruction of renal parenchyma, compensatory hyperfiltration in surviving glomeruli maintains renal function within the normal range for decades.7 Only when the majority of nephrons have been destroyed does renal function decline, and ESRD eventually develops, typically after the fourth decade of life.8
Table 1.
How autosomal dominant polycystic kidney disease affects the kidneys
| 1. Hypertension |
| 2. Acute and chronic pain |
| 3. Cyst and urinary tract infections |
| 4. Hematuria |
| 5. Kidney stones |
| 6. Urine concentration defects |
| 7. Loss of renal function |
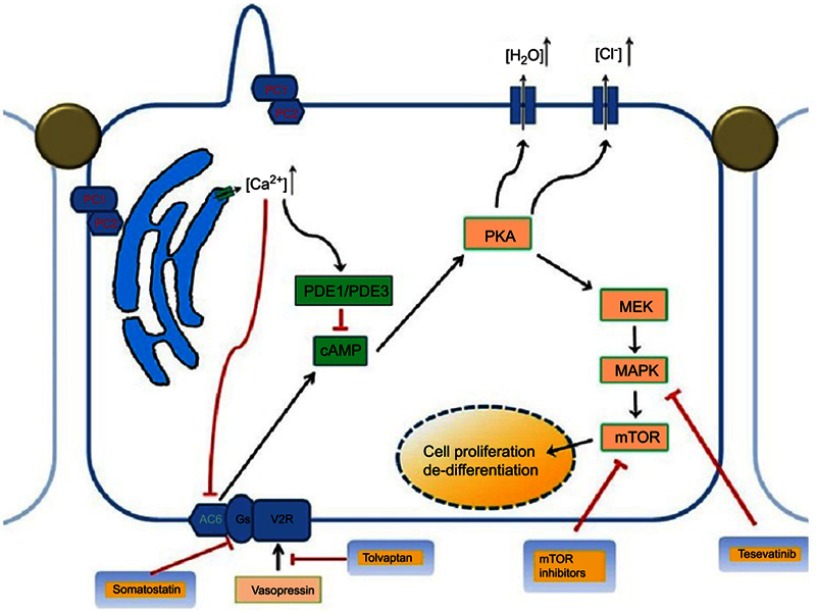
Mutations in the PKD1 and PKD2 genes coding for polycystin-1 (PC1) and polycystin-2 (PC2) are responsible for the 85 and 15% of ADPKD cases, respectively, in which many pathological pathways are involved (Figure 1).9 Novel genes, DNAJB11, and GANAB genes have been implicated in cases in which no mutation could be detected.10
Figure 1.
Pathophysiology and genetics of autosomal dominant polycystic kidney disease showing the multiple abnormal signaling pathways.
Abbreviations: cAMP, cyclic adenosine monophosphate; MEK/MAPK, mitogen-activated protein kinase enzymes; mTOR, mammalian target of rapamycin; PC1, polystin-1 receptor, PC2, polycystin-2 receptor; PKA, protein kinase A, PDE1, phosphodiesterase isoform 1; PDE3, phosphodiesterase isoform 3.
Diagnosis of ADPKD
ADPKD diagnosis is made on the basis of imaging.11 Ultrasonography is the imaging modality of choice for pre-symptomatic diagnosis, given its availability, safety, and low cost. Age-dependent ultrasound criteria for both diagnosis and disease exclusion have been established for patients with a positive family history.12 For PKD1 and PKD2 individuals, the presence of three or more renal cysts (unilateral or bilateral) for establishing the diagnosis for at-risk individuals 15–39 years; four (two or more cysts in each kidney) for individuals 40–59 years; and eight (four or more cysts in each) for individuals who are 60 years or older.13 In families of unknown genotype, Ravine et al established age-dependent ultrasound diagnostic criteria for PKD1 and PKD2 (Table 2).14
Table 2.
Unified ravine criteria for autosomal dominant polycystic kidney disease
| Age | Number of cysts |
|---|---|
| 15–39 years | At least 3 unilateral or bilateral kidney cysts |
| 40–59 years | At least 2 cysts in each kidney |
| Above 60 years | At least 4 cysts in each kidney |
Note: Data from Pei et al.14
Advances in renal imaging (magnetic resonance imaging MRI and high-resolution ultrasound) might help with disease exclusion in at-risk individuals.15 A study suggested that MRI showing >10 cysts in patients younger than 30 years is 100% sensitive and specific for the diagnosis of ADPKD.15
Genetic testing is not done as part of standard care and is helpful to assess potential living related kidney donors with negative or equivocal scans and detect rare forms of ADPKD and other cystic diseases.16,17
Progression of ADPKD
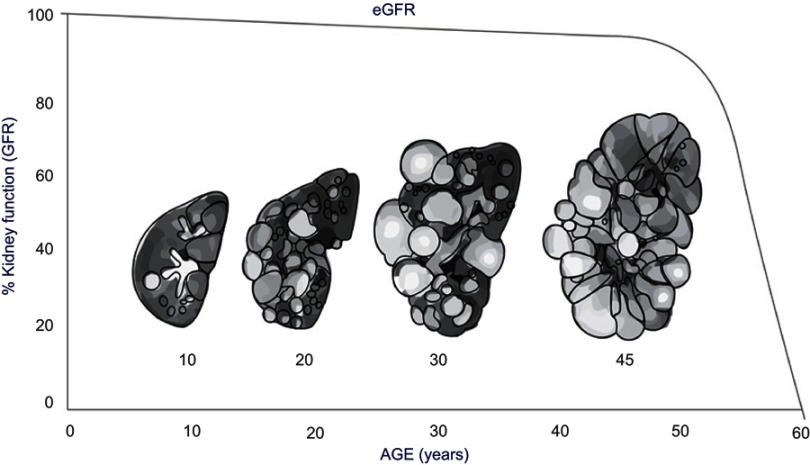
Typically, ADPKD progression is monitored through changes in serum creatinine levels and eGFR (Figure 2); however, this provides limited information especially early in disease progression, as serum creatinine levels do not typically rise and GFR typically remains in the normal range for 3–5 decades before there is a loss of kidney function.18 Thus, the use of a surrogate marker for disease progression is needed.
Figure 2.
Increase in kidney size and decrease in kidney function estimated by eGFR Correlated with age.
The gradual expansion of renal cysts in ADPKD can be measured as increased total kidney volume (TKV).18 Measurement of TKV is a valuable biomarker for treatment effect in clinical trials and more sensitive in disease progression than glomerular filtration rate (GFR) or serum creatinine.19
The US Consortium for Radiologic Imaging Studies in Polycystic Kidney Disease (CRISP) has shown that kidney enlargement is the first manifestation of the disease.20 The CRISP study showed that an increase in TKV is not only correlated to increased kidney cyst volume but also related to change in GFR.19 Multivariable analysis showed that a baseline height-adjusted TKV (htTKV) increase of 100 cc/m significantly predicted stage 3 CKD development within 8 years with a 1.48 odds ratio (95% confidence interval, 1.29–1.70). Baseline htTKV of more than 600 cc/m determined by MRI, predicted stage 3 CKD development within 8 years with an area under the curve of 0.84 (95% confidence interval, 0.79–0.90), qualifying htTKV as a prognostic biomarker in ADPKD.21
The CRISP study also emphasizes the association between large kidney volume and kidney complications and found that higher TKV is associated with several ADPKD complications, including proteinuria, microalbuminuria, hypertension, gross hematuria, and progressive loss of kidney function.18
Ultrasound measurement of kidney length have been found to be good predictors of GFR decline in cohorts of patients in a research setting, however, its utility for making treatment decisions is limited in clinical practice due to the inaccuracy of these measurements. Ultrasound overestimates true kidney size as it increases in advance stages, it requires appropriate measurement of depth and width for the assessment of TKV and is operator dependent.22
Several factors are associated with rapid ADPKD progression (Table 3).
Table 3.
Risk factors for rapid autosomal dominant polycystic kidney disease progression
| 1. PKD1 mutations (Truncating mutations has the worst outlook) |
| 2. Age |
| 3. Male gender |
| 4. Early decrease in GFR |
| 5. Early onset of hypertension |
| 6. High total kidney volume (Mayo Classification 1C-1E) |
| 7. Early onset or repeated episodes of gross hematuria |
| 8. Women with hypertension, three or more pregnancies |
| 9. Proteinuria, microalbuminuria and elevated serum copeptin levels |
| 10. Overweight/Obesity |
Pharmacologic management of ADPKD
Many medications have been or are currently being studied in clinical trials, with the goal of slowing the rate of progression of ADPKD by reducing the increase in TKV or maintaining eGFR (Table 4). Tolvaptan, an oral selective antagonist of the vasopressin V2 receptor, is the only medication for ADPKD that has demonstrated beneficial disease-modifying properties in adults.
Table 4.
Therapies slowing the progression of autosomal dominant polycystic kidney disease (ARPKD)
| Therapy | Mechanism of Action | Clinical Trial/Duration | Effect on eGFR (therapy vs placebo) | Effect on TKV Growth (therapy vs placebo) |
|---|---|---|---|---|
| Tolvaptan35 | Vasopressin V2 receptor antagonist | TEMPO ¾/ 3 years | −2.61 vs −3.81 mg/mL per year (P<0.001) | 2.8% vs 5.5% per year (P<0.001) |
| Octreotide90 | Somatostatin analogue | ALADIN/3 years | −3.9 vs −5.0 mL/min/1.73 m2 in 3 years (P= NS) | 46.2 mL vs 143.7 mL per year (P=0.03) |
| Lanreotide23 | Somatostatin analogue | DIPAK/2.5 years | −3.53 vs −3.46 mL/min/1.73 m2 per year (P = 0.81) | 4.15% vs 5.56% per year (P = 0.02) |
| Everolimus91 | mTOR inhibitor | Walz et al./2 years | −8.9 vs −7.7 mL/min/1.73 m2 at 2 years (P=0.15) | 102 mL vs 157 mL (P=0.02) at 1 year 230 mL vs 301 mL (P=0.06) at 2 years |
| Pravastatin76 | Statin-related inhibition of G proteins | Cadnapaphornchai et al/2 years | Creatinine clearance: 126 vs 126 mL/min (P=0.98) | 46% versus 68% per 2 years (P=0.03) |
| Metformin | AMPK activation, CFTR and mTOR suppression | NCT02656017/Started January 2016 | Ongoing phase 2 | Ongoing phase 2 |
| Tesevatinib (ARPKD) | Tyrosine kinase inhibitor | NCT03096080/Started March 2017 | Ongoing phase 1 | Ongoing phase 1 |
Abbreviation: TKV, total kidney volume.
The somatostatin analogue lanreotide has been recently studied in a randomized clinical trial that included 309 patients with ADPKD.23 Among patients with later-stage ADPKD, treatment with lanreotide compared with standard care did not slow the decline in kidney function over 2.5 years of follow-up.23 Other medications are still in ongoing clinical trials (Table 4). Strong evidence exists suggesting that early assessment and treatment of patients with high-risk ADPKD improves the outcomes of these patients.24
Tolvaptan (V2R antagonists)
The US Food and Drug Administration (FDA) approved tolvaptan as the first drug treatment to slow kidney function decline in adults at risk of rapidly progressing ADPKD.25 Tolvaptan is a vasopressin-2-receptor (V2R) antagonist commonly used in the treatment of hyponatremia. This drug has shown to have a high affinity and strength for the V2 receptor, inhibiting ERK pathway, cAMP production, Cl– secretion and the in vitro cyst growth of three-dimensionally cultured ADPKD cells.26
Studies in the last decade have shown that circulating vasopressin acting on vasopressin V2 receptors in the basolateral cell membranes and/or, less likely, urine vasopressin acting on V2 receptors in the primary cilia exert a strong modulatory effect on the development of ADPKD.27 Administration of the V2 receptor agonist increases the renal levels of cAMP and aggravates the development of ADPKD rodent models orthologous to autosomal recessive PKD (ARPKD, PCK rat) and ADPKD caused by PKD1 (Pkd1RC/RC mouse) or PKD2 (Pkd2WS25/− mouse) mutations.28 Peripheral resistance to the antidiuretic hormone vasopressin has been suggested and high vasopressin levels were observed in ADPKD patients at baseline and after hypertonic saline infusion.29 An explanation for such peripheral resistance to vasopressin is the existence of cystic lesions impairing the establishment of the interstitial osmotic gradient driving water reabsorption in the distal nephron.30
Clinical trials have assessed the pharmacokinetics and safety of the tolvaptan in adult patients with ADPKD.31 A phase 2, open-label, 3-year trial showed beneficial changes in TKV and eGFR in 63 patients compared to controls, and all adverse events were related to water excretion.32 Another following study has shown that short-term treatment with Tolvaptan was associated with a decrease of eGFR, a reduced clearance in uric acid and a 3.1% reduction in kidney volume as assessed by MRI.33
Tolvaptan Phase 3 Efficacy and Safety Study in ADPKD (TEMPO 3:4), which included 1445 adult patients with preserved renal excretory function (eGFR >60 mL/min) and enlarged kidneys (TKV >750 mL, as measured by MRI) evaluated the effect of tolvaptan treatment on the change in TKV and eGFR in comparison to placebo.34 It demonstrated that tolvaptan delayed the rate of TKV growth by 45% (from 5.5% to 2.8% per year), while the rate of eGFR loss on treatment was reduced by 26% (from 3.70 to 2.72 mL/min per 1.73 m2/year) during 3 years of follow-up,35 and a 36% lower risk of kidney pain.36
In the cross-sectional analysis of baseline data of the TEMPO 3:4 trial where a history of kidney pain was observed in 50.9% of participants, tolvaptan decreased the incidence of kidney pain events independent of patient characteristics possibly due to reductions in ADPKD-related complications.36 Another study has investigated the baseline urine osmolality (Uosm) as a marker for severity of ADPKD has shown that Tolvaptan reduced Uosm by 200–300 mOsm kg−1 over 36 months and the Uosm response to Tolvaptan depended on baseline eGFR and Uosm.37
In the TEMPO 4:4 Study, which is an open label extension study, all patients from the TEMPO 3:4 Study were treated with tolvaptan for additional 2 years.38 The results support a sustained disease-modifying effect of tolvaptan on eGFR compared with the placebo group and the safety profile was similar to that observed in TEMPO 3:4.38
The results of the REPRISE (Replicating Evidence of Preserved Renal Function: an Investigation of Tolvaptan Safety and Efficacy in ADPKD) trial, which was a 12-month randomized with an 8-week pre-randomization phase, multicenter, placebo-controlled, double-blind trial, confirm the beneficial effect of tolvaptan on disease progression in ADPKD.39,40 Tolvaptan led to a slower rate of decline in kidney function than placebo over a period of up to 12 months in patients with chronic kidney disease of stage 2 to stage 4 (eGFR of the participants, 25–65 mL/minute per 1.73 m2).41 The mean decline in eGFR from baseline to 12 months was 35%.42 In both the REPRISE and TEMPO 3:4 trials, the greatest beneficial effect of tolvaptan on eGFR decline was seen in patients with a baseline eGFR >45 mL/min/1.73 m2 (CKD Stage 3a).43
The FDA-approved indication for tolvaptan in ADPKD is to slow the decline of kidney function in adults with rapidly progressive ADPKD.44 The predictive value of htTKV was confirmed by the ADPKD Outcomes Consortium and led to qualification of htTKV along with age and eGFR as prognostic markers for rapid progression of the disease.45 Physicians prescribing tolvaptan should consider these parameters to identify individuals at the highest risk of rapid progression.
Imaging is important in the classification setting, as well as to rule out other contributing factors including genetic mutations other than PSC1 and PSC 2 genes mutations.44 The Mayo imaging classification uses htTKV and age to identify the highest risk patients for progression independent of renal function using CT scan (including contrast enhancement in patients with eGFR>60 mL/min per 1.73 m2) or MRI scan without contrast (in patients with reduced eGFR).46,47 About 95% of ADPKD patients have typical diffuse cystic disease (class 1) and is stratified into five classes (A, B, C, D, E) based on the growth rates (<1.5%, 1.5–3%, 3–4.5%, 4.5–6%, or >6% per year respectively).48 Patients class 1C, 1D or 1E are the most likely to benefit from treatment, and the benefit is predicted to be greater for young patients.39 Another classification that determines the rapid progression of ADPKD is the European Renal Association-European Dialysis and Transplant Association algorithm which puts emphasis first on eGFR indexed for age.49 This algorithm can distinguish rapidly from slowly progressive disease in the majority of patients with ADPKD, although it has some limitations in patients aged 18–30 years.44 The PROPKD score uses genetics, urological complications, hypertension, and sex to create a model predicting disease progression.50 While it seems to be useful with patients aged >35 years, its value is limited in patients aged <35 years without complications or in patients with missing clinical information, and genetic information alone could be used for prognosis for these patients.51
Tolvaptan blocking of V2 receptors stimulates aquaresis, increases afferent arterioles vasoconstriction, and lowers eGFR.52 Thus, subjects taking tolvaptan develop more frequent adverse events related to water excretion: polyuria, thirst, nocturia; and fewer adverse events related to ADPKD: pain, hematuria, UTI.31,35,39 Slight initial reduction in eGFR by 5–10% is expected with tolvaptan prompting adequate hydration, and 20% reduction in eGFR is an indication for the medication withhold.44 Patients treated with tolvaptan also showed an increase in serum sodium and uric acid levels, and in the frequency of gout.35 Further analysis revealed that tolvaptan decreased albuminuria compared with placebo, independent of blood pressure.53
Tolvaptan led to increased liver enzyme levels due to hepatocellular injury, although abnormalities resolved after the discontinuation of the drug.54 Because of the potential hepatocellular toxicity, a risk evaluation including liver function testing before initiation and at specific intervals (after 2 and 4 weeks, then monthly for 18 months, and every 3 months thereafter), is required for tolvaptan treatment in all patients with ADPKD.44 Tolvaptan was approved by the FDA with a boxed warning that mandates frequent monitoring of liver function tests after treatment initiation as part of the risk evaluation and mitigation strategy (REMS).44 Tolvaptan must be discontinued in patients with ALT or AST enzymes are elevated >3 times upper normal limit unless explained by other eitiologies.44
Monitoring the efficacy of Tolvaptan treatment can be achieved by obtaining CT or MRI to measure TKV growth rate every 3–5 years.44 Concomitant use of other drugs with tolvaptan such as CYP3A inhibitors and diuretics is not recommended, although some studies suggest that a thiazide may increase the tolerability to tolvaptan by reducing the polyuria.55,56
The initiation of tolvaptan treatment include strict blood pressure control, increase water consumption to reach urine osmolarity below 280 mOsm/Kg, and restrict sodium intake to 2.3–3 g/day.13,57 Maintaining adequate hydration is necessary to prevent vasopressin elevation and V1 receptors activation.44
Supportive management of ADPKD
Supportive measures, which were the only therapeutic options available until recently, aim to alleviate eGFR loss and reduce morbidity and mortality associated with ADPKD disease manifestations.8,58
Hypertension
Hypertension is the most common early manifestation of ADPKD.50 It occurs in 50–70% of cases prior to any significant decline in kidney function, with an average age of onset of 30 years.5 Hypertensive patients with ADPKD show defects in primary cilium causing endothelial dysfunction and the increased activation of the renin-angiotensin-aldosterone system (RAAS) compared to patients with essential hypertension of the same age, renal function, and level of blood pressure.59,60 Some studies have reported activation of the sympathetic nervous system as a mechanism responsible for the development of elevated BP in ADPKD.61 In fact, renal volume clearly correlates with BP when hypertension is already diagnosed: hypertensive ADPKD patients have constantly shown larger kidney volumes than those with normotension.62
HALT-PKD trial stressed the importance of very good control of blood pressure.63 In the HALT-PKD study, a combination of an angiotensin-converting enzyme (ACE) inhibitor and an angiotensin II receptor blocker (ARB) were used on 1000 ADPKD patients for 5.5 years. The group of patients with low target blood pressure (95/60–110/75 mmHg) showed a delayed increase in TKV, decrease in left ventricular hypertrophy and proteinuria.64 HALT-PKD results revealed that rigorous blood-pressure control diminished the annual rate of increase in TKV by 14.2% and was associated with decreased urinary albumin excretion.63 Additionally, it noted that dual renin-angiotensin-aldosterone system blockade was safe in patients with ADPKD with an eGFR of 30 mL/min/1·73 m2 or higher.65 However, there were no differences in renal function (eGFR) between groups.64
Cardiovascular abnormalities in ADPKD are evident from a young age onwards.66 Patients with ADPKD may develop signs or symptoms of hypertension during childhood. In a retrospective multicenter study conducted on 310 children with ADPKD to collect ambulatory BP monitoring recordings, results revealed that the prevalence of children with hypertension was 35%, 52% of children lacked a physiologic nocturnal BP dipping, and 18% had isolated nocturnal hypertension.67 It is recommended to have children with a family history of ADPKD screened for hypertension from the age of 5 years onward, with an interval of 3 years in cases in which no hypertension is found. Diagnosis and treatment of hypertension in the pediatric population should follow prevailing pediatric guidelines, with the exception that RAAS blockade is preferred as the first line treatment.68
Systolic Blood Pressure Intervention Trial (SPRINT) compared the outcome of intensive antihypertensive treatment (SBP target of <120) versus Standard antihypertensive treatment (SBP target of <140) in 9361 hypertensive adults ≥50 years of age with average SBP of 130–180 mm Hg.69 The trial concluded a reduced outcome of cardiovascular events (myocardial infarction, non–myocardial infarction acute coronary syndrome, stroke, acute decompensated heart failure, and CVD death) by 25% in the intensive treatment group compared with the standard treatment group.69 It is important to mention that hypertensive patients with ADPKD were excluded from SPRINT because of other ongoing National Institutes of Health–funded trials.69
The initial antihypertensive agent should be an ACE inhibitor in hypertensive ADPKD patients with careful monitoring of renal function and serum potassium levels, and an ARB is considered when there is intolerance to ACE inhibitors.59 The use of ACE inhibitors or ARBs is also indicated in normotensive ADPKD patients with left ventricular hypertrophy (LVH), albuminuria, or masked hypertension.59
Hydration
Maintaining hydration plays a particular role in ADPKD.70 Vasopressin is a key factor in cyst growth and its secretion is primarily regulated by serum osmolality, and lowering vasopressin concentration by increasing water intake is an interesting treatment option.70 ADPKD patients are encouraged to increase daily spread-out water intake to maintain average urine osmolarity ≤280 mOsm/Kg.13,58 Measurement of first morning urine osmolality can be a good indicator of adequate vasopressin suppression.13 PREVENT-ADPKD is an ongoing 3-year controlled clinical trial aiming to study the potential effect of increased water intake on decreasing vasopressin levels.71
Drinking up to 3000 mL of water per day, spread throughout waking hours and before bed time, is recommended to prevent urine supersaturation for lithogenic salts and to reduce stone formation. However, long term renal consequences of prolonged water diuresis need further analysis.30
Dietary sodium
High dietary sodium is known to increase arterial blood pressure, urine albumin excretion, and cardiovascular mortality in the general population.66 The importance of dietary sodium restriction in ADPKD has received attention since persistent high sodium level stimulates vasopressin secretion, promotes plasma levels of endogenous cardiotonic steroids and promotes fluid retention and arterial hypertension.63,72 Available study review on 1787 patients supports an association between high sodium intake (>4.6 g/day) and eGFR decline.73 Another randomized clinical trial on 500 patients with CKD receiving RAAS-inhibiting treatment showed that higher dietary sodium was associated with increased risk of progression to ESRD.74 In the HALT PKD study analysis, study A and B showed that dietary sodium was associated with notable rates of TKV increase and eGFR decline, respectively.72 Therefore, moderate dietary sodium restriction (2.3–3 g/day) for patients with ADPKD is generally recommended.13
Dyslipidemia
Although this analysis of the HALT PKD trials does not demonstrate a benefit of statin therapy,75 a small trial in children and adolescents with pravastatin administration for 3 years was associated with reduced renal cyst growth.76 Another retrospective study on 14,497 CKD patients, where 858 statin users were paired with non-users, suggested the beneficial effects of statins on renal progression and all-cause mortality only for the patients with early stage CKD.77 KDIGO guidelines recommend the use of statins in all patients with chronic kidney disease who are older than 50 years and not receiving dialysis, regardless of serum cholesterol levels, for the prevention of cardiovascular disease.42 Therefore, it is recommended that a serum LDL cholesterol level ≤100 mg/dl in all ADPKD patients.13 On the other hand, concomitant use of tolvaptan could raise the levels of OATP1B1/3 and OAT3 transporter substrates including statins, potentiating the statins effect, which necessitates a closer monitor to optimize the management.44
Kidney stones
The prevalence of kidney stones is 2 times higher in patients with ADPKD than that of the general population and 10–35% of patients with ADPKD are reported to have nephrolithiasis.43 It can be explained by increased intra-renal anatomic obstruction, as well as lower levels of such urinary inhibitors of stones as magnesium and citrate.78 Half of the ADPKD patients with kidney stones have symptoms, that include pain, obstruction, hematuria and urinary tract infection exacerbation.79,80 CT scan is the imaging modality of choice for evaluation of kidney stones in ADPKD patients.79 Management requires team approach and includes correction of electrolytes imbalance, treating the urinary tract infection, urgent de-obstruction and careful selection of the endourological procedure.79
Other general measures
A daily protein intake of 0.8–1.0 g/kg of body weight is recommended in ADPKD.13 In the CRISP study analysis, some data suggested that protein intake is associated with TKV increase and GFR decline during a period of 6 years.77 However, more data are needed in this regard. Additionally, patients with ADPKD are advised to avoid smoking, limit dietary phosphate intake, avoidance of weight gain, and increase physical activity.8
Cyst infection
Cyst infection is a challenging complication of ADPKD. A single center study involving 389 identified patients with ADPKD was conducted during an 11-yr period, and the results showed that cyst infections were relatively infrequent with an incidence estimated at 0.01 episode per patient per year, and that cyst infections accounted for only approximately 10% of causes that led to hospitalization of patients with ADPKD.81 Fluoroquinolones and third-generation cephalosporins remain the standard treatment for cyst infections in patients with ADPKD and were used in 95% of the patients included in this study. Data suggest that fluoroquinolones may be superior to β-lactamine and biotherapy superior to monotherapy in treating cyst infections in patients with ADPKD. In larger infected cysts (>5 cm diameter), including hepatic cysts, data suggests that drainage and antibiotics are more efficacious than antibiotics alone.81,82
Cerebral aneurysm
Intracranial aneurysms (ICAs) are important extra-renal manifestations of ADPKD and are usually asymptomatic. They have been detected in approximately 20% and 6% of ADPKD patients with and without a family history respectively.83 The risk of rupture of an aneurysm in patients with ADPKD is equivalent to the general population and it has shown to cluster in families with significant interfamilial heterogeneity.84 The results of many studies recommend MRA-based screening all patients with ADPKD over the age of 30 for ICAs who have a family history of ICA with or without rupture.85 In ADPKD patients without family history, screening is recommended in patients with neurological symptoms, high-risk occupation, anxiety, any history of ruptured aneurysm, and patients preparing for surgery or receiving long-term anticoagulation therapy.85
Kidney transplant
Kidney transplantation is the preferred type of renal replacement therapy for patients with ADPKD and ESRD, and the rates of patient and graft survival following kidney transplantation are excellent.86 Native nephrectomy prior to transplantation is indicated only in patients with symptomatic recurrent bleeding, infection, stones, severe pain, or massive kidney size preventing allograft placement.87 ADPKD patients with liver involvement require pretransplant imaging using CT or MRI and measurement of baseline serum CA19-9 levels.87 Combined liver–kidney transplantation should be considered in cases of symptomatic hepatomegaly or recurrent cholangitis if the glomerular filtration rate is ≤30 mL/min/1.73 m2.88 Pre-transplant MRI screening of intracranial aneurysms in patients with ADPKD should follow general recommendations to identify high risk patients, and control of hypertension and hyperlipidaemia is strongly recommended.89 Following transplantation, the volume of native ADPKD kidneys usually decreases markedly while liver cysts continue to grow, and somatostatin analogue therapy should be considered in patients with massive symptomatic liver disease.87
Disclosure
Dr. Rastogi has received research grants from Otsuka, Kadmon, Reata, Sanofi-Genzyme and honorarium and travel supports from Kadmon, Otsuka, Sanofi-Genzyme. Dr. Rastogi was part of the Speaker Bureau and Advisory Board/Consultant for Otsuka. He received grants from Sanofi, Kadmon, and Reata Pharmaceuticals, during the conduct of the study. Dr. Kamgar has received honorarium and travel support from Otsuka. The authors report no other conflicts of interest in this work.
References
- 1.Igarashi P, Somlo S, Editor F. Genetics and pathogenesis of polycystic kidney disease. J Am Soc Nephrol. 2002;13(9):2384–2398. doi: 10.1097/01.ASN.0000028643.17901.42 [DOI] [PubMed] [Google Scholar]
- 2.Helal I. Autosomal dominant polycystic kidney disease: new insights into treatment. Saudi J Kidney Dis Transpl. 2013;24(2):230–234. doi: 10.4103/1319-2442.109561 [DOI] [PubMed] [Google Scholar]
- 3.Spithoven EM, Kramer A, Meijer E, et al. Analysis of data from the ERA-EDTA registry indicates that conventional treatments for chronic kidney disease do not reduce the need for renal replacement therapy in autosomal dominant polycystic kidney disease. Kidney Int. 2014;86(6):1244–1252. doi: 10.1038/ki.2014.120 [DOI] [PubMed] [Google Scholar]
- 4.Collins AJ, Foley RN, Chavers B, et al. US renal data system 2011 annual data report. Am J Kidney Dis. 2012;59(1):A7. doi: 10.1053/j.ajkd.2011.11.015 [DOI] [PubMed] [Google Scholar]
- 5.Chebib FT, Torres VE. Autosomal dominant polycystic kidney disease: core curriculum 2016. Am J Kidney Dis. 2016;67(5):792–810. doi: 10.1053/j.ajkd.2015.07.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grantham JJ. Autosomal dominant polycystic kidney disease. N Engl J Med. 2008;359(14):1477–1485. doi: 10.1056/NEJMcp0804458 [DOI] [PubMed] [Google Scholar]
- 7.Grantham JJ, Chapman AB, Torres VE. Volume progression in autosomal dominant polycystic kidney disease: the major factor determining clinical outcomes. Clin J Am Soc Nephrol. 2005;1(1):148–157. doi: 10.2215/CJN.00330705 [DOI] [PubMed] [Google Scholar]
- 8.Chapman AB, Devuyst O, Eckardt K-U, et al. Autosomal-dominant polycystic kidney disease (ADPKD): executive summary from a Kidney Disease: improving Global Outcomes (KDIGO) controversies conference. Kidney Int. 2015;88(1):17–27. doi: 10.1038/ki.2015.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aguiari G, Catizone L, Del Senno L. Multidrug therapy for polycystic kidney disease: a review and perspective. Am J Nephrol. 2013;37(2):175–182. doi: 10.1159/000346812 [DOI] [PubMed] [Google Scholar]
- 10.Cornec-Le Gall E, Torres VE, Harris PC. Genetic complexity of autosomal dominant polycystic kidney and liver diseases. J Am Soc Nephrol. 2018;29(1):13–23. doi: 10.1681/ASN.2017050483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chapman AB, Guay-Woodford LM, Grantham JJ, et al. Renal structure in early autosomal-dominant polycystic kidney disease (ADPKD): the Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP) cohort1. Kidney Int. 2003;64(3):1035–1045. doi: 10.1046/j.1523-1755.2003.00185.x [DOI] [PubMed] [Google Scholar]
- 12.Gabow P. Autosomal dominant polycystic kidney disease. N Engl J Med. 2008;359(14):1477–1485. doi: 10.1136/bmj.i679 [DOI] [PubMed] [Google Scholar]
- 13.Chebib FT, Torres VE. Recent advances in the management of autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2018;13(11):1765–1776. doi: 10.2215/CJN.03960318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pei Y, Obaji J, Dupuis A, et al. Unified criteria for ultrasonographic diagnosis of ADPKD. J Am Soc Nephrol. 2009;20(1):205–212. doi: 10.1681/ASN.2008050507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pei Y, Hwang Y-H, Conklin J, et al. Imaging-based diagnosis of autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2015;26(3):746–753. doi: 10.1681/ASN.2014030297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nguyen A-NT, Wallace DP, Blanco G. Ouabain binds with high affinity to the Na,K-ATPase in human polycystic kidney cells and induces extracellular signal-regulated kinase activation and cell proliferation. J Am Soc Nephrol. 2007;18(1):46–57. doi: 10.1681/ASN.2006010086 [DOI] [PubMed] [Google Scholar]
- 17.Cornec-Le Gall E, Chebib FT, Madsen CD, et al. The value of genetic testing in polycystic kidney diseases illustrated by a family with PKD2 and COL4A1 mutations. Am J Kidney Dis. 2018;72(2):302–308. doi: 10.1053/j.ajkd.2017.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grantham JJ, Torres VE, Chapman AB, et al. Volume progression in polycystic kidney disease. N Engl J Med. 2006;354(20):2122–2130. doi: 10.1056/NEJMoa054341 [DOI] [PubMed] [Google Scholar]
- 19.Tangri N, Hougen I, Alam A, Perrone R, Mcfarlane P, Pei Y. Total kidney volume as a biomarker of disease progression in autosomal dominant polycystic kidney disease. Can J Kidney Health Dis. 2017;4:2054358117693355. doi: 10.1177/2054358117693355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kühn EW, Walz G. The treatment of autosomal dominant polycystic kidney disease. Dtsch Arztebl Int. 2015;112(51–52):884. doi: 10.3238/arztebl.2015.0884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chapman AB, Bost JE, Torres VE, et al. Kidney volume and functional outcomes in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2012;7(3):479. doi: 10.2215/cjn.09500911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhutani H, Smith V, Rahbari-Oskoui F, et al. A comparison of ultrasound and magnetic resonance imaging shows that kidney length predicts chronic kidney disease in autosomal dominant polycystic kidney disease. Kidney Int. 2015;88(1):146–151. doi: 10.1038/ki.2015.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meijer E, Visser FW, van Aerts RMM, et al. Effect of lanreotide on kidney function in patients with autosomal dominant polycystic kidney disease. JAMA. 2018;320(19):2010. doi: 10.1001/jama.2018.15870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lanktree MB, Chapman AB. New treatment paradigms for ADPKD: moving towards precision medicine. Nat Rev Nephrol. 2017;13(12):750–768. doi: 10.1038/nrneph.2017.127 [DOI] [PubMed] [Google Scholar]
- 25.Torres VE. Pro: tolvaptan delays the progression of autosomal dominant polycystic kidney disease. Nephrol Dial Transplant. 2019;34(1):30–34. doi: 10.1093/ndt/gfy297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reif GA, Yamaguchi T, Nivens E, Fujiki H, Pinto CS, Wallace DP. Tolvaptan inhibits ERK-dependent cell proliferation, Cl- secretion, and in vitro cyst growth of human ADPKD cells stimulated by vasopressin. AJP Ren Physiol. 2011;301(5):F1005–F1013. doi: 10.1152/ajprenal.00243.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chebib FT, Sussman CR, Wang X, Harris PC, Torres VE. Vasopressin and disruption of calcium signalling in polycystic kidney disease. Nat Rev Nephrol. 2015;11(8):451–464. doi: 10.1038/nrneph.2015.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hopp K, Wang X, Ye H, Irazabal MV, Harris PC, Torres VE. Effects of hydration in rats and mice with polycystic kidney disease. Am J Physiol Renal Physiol. 2015;308(3):F261–F266. doi: 10.1152/ajprenal.00345.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zittema D, Boertien WE, van Beek AP, et al. Vasopressin, copeptin, and renal concentrating capacity in patients with autosomal dominant polycystic kidney disease without renal impairment. Clin J Am Soc Nephrol. 2012;7(6):906–913. doi: 10.2215/CJN.11311111 [DOI] [PubMed] [Google Scholar]
- 30.Torres VE, Bankir L, Grantham JJ. A case for water in the treatment of polycystic kidney disease. Clin J Am Soc Nephrol. 2009;4(6):1140–1150. doi: 10.2215/CJN.00790209 [DOI] [PubMed] [Google Scholar]
- 31.Clark WF, Devuyst O, Roussel R. The vasopressin system: new insights for patients with kidney diseases. J Intern Med. 2017;282(4):310–321. doi: 10.1111/joim.12654 [DOI] [PubMed] [Google Scholar]
- 32.Higashihara E, Torres VE, Chapman AB, et al. Tolvaptan in autosomal dominant polycystic kidney disease: three years’ experience. Clin J Am Soc Nephrol. 2011;6(10):2499–2507. doi: 10.2215/CJN.03530411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Irazabal MV, Torres VE, Hogan MC, et al. Short-term effects of tolvaptan on renal function and volume in patients with autosomal dominant polycystic kidney disease. Kidney Int. 2011;80(3):295–301. doi: 10.1038/ki.2011.119 [DOI] [PubMed] [Google Scholar]
- 34.Torres VE, Meijer E, Bae KT, et al. Rationale and design of the TEMPO (Tolvaptan Efficacy and Safety in Management of Autosomal Dominant Polycystic kidney disease and its outcomes) 3–4 study. Am J Kidney Dis. 2011;57(5):692–699. doi: 10.1053/j.ajkd.2010.11.029 [DOI] [PubMed] [Google Scholar]
- 35.Torres VE, Chapman AB, Devuyst O, et al. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2012;367(25):2407–2418. doi: 10.1056/NEJMoa1205511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Casteleijn NF, Blais JD, Chapman AB, et al. Tolvaptan and kidney pain in patients with autosomal dominant polycystic kidney disease: secondary analysis from a randomized controlled trial. Am J Kidney Dis. 2017;69(2):210–219. doi: 10.1053/j.ajkd.2016.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Devuyst O, Chapman AB, Gansevoort RT, et al. Urine osmolality, response to tolvaptan, and outcome in autosomal dominant polycystic kidney disease: results from the TEMPO 3:4 trial. J Am Soc Nephrol. 2017;28(5):1592–1602. doi: 10.1681/ASN.2016040448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Torres VE, Chapman AB, Devuyst O, et al. Multicenter, open-label, extension trial to evaluate the long-term efficacy and safety of early versus delayed treatment with tolvaptan in autosomal dominant polycystic kidney disease: the TEMPO 4:4 trial. Nephrol Dial Transplant. 2018;33(3):477–489. doi: 10.1093/ndt/gfx043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Torres VE, Chapman AB, Devuyst O, et al. Tolvaptan in later-stage autosomal dominant polycystic kidney disease. N Engl J Med. 2017:NEJMoa1710030. doi: 10.1056/NEJMoa1710030 [DOI] [PubMed] [Google Scholar]
- 40.Gy ECOLO. News & views: tolvaptan slows disease progression in late-stage ADPKD. Nat Publ Gr. 2015;1–2. doi: 10.1038/nature16329 [DOI] [Google Scholar]
- 41.Rizvi NA, Peters S. Tolvaptan and autosomal dominant polycystic kidney disease. N Engl J Med. 2017;377(20):1986–1988. doi: 10.1056/NEJMe1711430 [DOI] [PubMed] [Google Scholar]
- 42.Kidney Disease Improving Global Outcomes (KDIGO) Lipid Work Group. Clinical practice guideline for lipid management in chronic kidney disease. Kidney Int. 2013;3(3):259–305. doi: 10.7326/M13-2453 [DOI] [Google Scholar]
- 43.Torres VE, Wilson DM, Hattery RR, Segura JW. Renal stone disease in autosomal dominant polycystic kidney disease. Am J Kidney Dis. 1993;22(4):513–519. doi: 10.1016/S0272-6386(12)80922-X [DOI] [PubMed] [Google Scholar]
- 44.A Practical Guide for Treatment of Rapidly Progressive (1).pdf.
- 45.Perrone RD, Mouksassi M-S, Romero K, et al. Total kidney volume is a prognostic biomarker of renal function decline and progression to end-stage renal disease in patients with autosomal dominant polycystic kidney disease. Kidney Int Rep. 2017;2(3):442–450. doi: 10.1016/j.ekir.2017.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Irazabal MV, Rangel LJ, Bergstralh EJ, et al. Imaging classification of autosomal dominant polycystic kidney disease: a simple model for selecting patients for clinical trials. J Am Soc Nephrol. 2015;26(1):160–172. doi: 10.1681/ASN.2013101138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O’Neill WC, Robbin ML, Bae KT, et al. Sonographic assessment of the severity and progression of autosomal dominant polycystic kidney disease: the Consortium of Renal Imaging Studies in Polycystic Kidney Disease (CRISP). Am J Kidney Dis. 2005;46(6):1058–1064. doi: 10.1053/j.ajkd.2005.08.026 [DOI] [PubMed] [Google Scholar]
- 48.Girardat-Rotar L, Braun J, Puhan MA, Abraham AG, Serra AL. Temporal and geographical external validation study and extension of the mayo clinic prediction model to predict eGFR in the younger population of Swiss ADPKD patients. BMC Nephrol. 2017;18(1):241. doi: 10.1186/s12882-017-0654-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gansevoort RT, Arici M, Benzing T, et al. Recommendations for the use of tolvaptan in autosomal dominant polycystic kidney disease: a position statement on behalf of the ERA-EDTA working groups on inherited kidney disorders and European renal best practice. Nephrol Dial Transplant. 2016;31(3):337–348. doi: 10.1093/ndt/gfv456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cornec-Le Gall E, Audrézet M-P, Rousseau A, et al. The PROPKD score: a new algorithm to predict renal survival in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2016;27(3):942–951. doi: 10.1681/ASN.2015010016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cornec-Le Gall E, Audrezet M-P, Chen J-M, et al. Type of PKD1 mutation influences renal outcome in ADPKD. J Am Soc Nephrol. 2013;24(6):1006–1013. doi: 10.1681/ASN.2012070650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boertien WE, Meijer E, de Jong PE, et al. Short-term effects of tolvaptan in individuals with autosomal dominant polycystic kidney disease at various levels of kidney function. Am J Kidney Dis. 2015;65(6):833–841. doi: 10.1053/j.ajkd.2014.11.010 [DOI] [PubMed] [Google Scholar]
- 53.Gansevoort RT, Meijer E, Chapman AB, et al. Albuminuria and tolvaptan in autosomal-dominant polycystic kidney disease: results of the TEMPO 3:4 trial. Nephrol Dial Transplant. 2016;31(11):1887–1894. doi: 10.1093/ndt/gfv422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Watkins PB, Lewis JH, Kaplowitz N, et al. Clinical pattern of tolvaptan-associated liver injury in subjects with autosomal dominant polycystic kidney disease: analysis of clinical trials database. Drug Saf. 2015;38(11):1103–1113. doi: 10.1007/s40264-015-0327-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kramers BJ, van Gastel MDA, Meijer E, Gansevoort RT. Case report: a thiazide diuretic to treat polyuria induced by tolvaptan. BMC Nephrol. 2018;19(1):157. doi: 10.1186/s12882-018-0957-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang A, Hirose T, Ohsaki Y, et al. Hydrochlorothiazide ameliorates polyuria caused by tolvaptan treatment of polycystic kidney disease in PCK rats. Clin Exp Nephrol. 2018. doi: 10.1007/s10157-018-1669-9 [DOI] [PubMed] [Google Scholar]
- 57.Amro OW, Paulus JK, Noubary F, Perrone RD. Low-osmolar diet and adjusted water intake for vasopressin reduction in autosomal dominant polycystic kidney disease: a pilot randomized controlled trial. Am J Kidney Dis. 2016;68(6):882–891. doi: 10.1053/j.ajkd.2016.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Müller R-U, Benzing T. Management of autosomal-dominant polycystic kidney disease-state-of-the-art. Clin Kidney J. 2018;11(Suppl 1):i2–i13. doi: 10.1093/ckj/sfy103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rahbari-Oskoui F, Williams O, Chapman A. Mechanisms and management of hypertension in autosomal dominant polycystic kidney disease. Nephrol Dial Transplant. 2014;29(12):2194–2201. doi: 10.1093/ndt/gft513 [DOI] [PubMed] [Google Scholar]
- 60.Schrier RW. Decade in review—polycystic kidney disease: slowing progression of autosomal dominant polycystic kidney disease. Nat Rev Nephrol. 2015;11(11):638–639. doi: 10.1038/nrneph.2015.164 [DOI] [PubMed] [Google Scholar]
- 61.Cerasola G, Vecchi ML, Mulè G, et al. Sympathetic activity and blood pressure pattern in autosomal dominant polycystic kidney disease hypertensives. Am J Nephrol. 1998;18(5):391–398. doi: 10.1159/000013382 [DOI] [PubMed] [Google Scholar]
- 62.Chapman AB, Stepniakowski K, Rahbari-Oskoui F. Hypertension in autosomal dominant polycystic kidney disease. Adv Chronic Kidney Dis. 2010;17(2):153–163. doi: 10.1053/j.ackd.2010.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rysz J, Gluba-Brzózka A, Franczyk B, Banach M, Bartnicki P. Combination drug versus monotherapy for the treatment of autosomal dominant polycystic kidney disease. Expert Opin Pharmacother. 2016;17(15):2049–2056. doi: 10.1080/14656566.2016.1232394 [DOI] [PubMed] [Google Scholar]
- 64.Schrier RW, Abebe KZ, Perrone RD, et al. Blood pressure in early autosomal dominant polycystic kidney disease. N Engl J Med. 2014;371(24):2255–2266. doi: 10.1056/NEJMoa1402685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ong ACM, Devuyst O, Knebelmann B, Walz G. Autosomal dominant polycystic kidney disease: the changing face of clinical management. Lancet. 2015;385(9981):1993–2002. doi: 10.1016/S0140-6736(15)60907-2 [DOI] [PubMed] [Google Scholar]
- 66.Cadnapaphornchai MA, McFann K, Strain JD, Masoumi A, Schrier RW. Increased left ventricular mass in children with autosomal dominant polycystic kidney disease and borderline hypertension. Kidney Int. 2008;74(9):1192–1196. doi: 10.1038/ki.2008.397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Massella L, Mekahli D, Paripović D, et al. Prevalence of hypertension in children with early-stage ADPKD. Clin J Am Soc Nephrol. 2018;13(6):874–883. doi: 10.2215/CJN.11401017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004. 114(2 Suppl 4th Report):555–576. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15286277. Accessed February 10, 2017. [PubMed] [Google Scholar]
- 69.Cushman WC, Whelton PK, Fine LJ, et al. SPRINT trial results. Hypertension. 2015;67(2):263–265. doi: 10.1161/hypertensionaha.115.06722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Torres VE. Water for ADPKD? Probably, yes. J Am Soc Nephrol. 2006;17(8):2089–2091. doi: 10.1681/ASN.2006060603 [DOI] [PubMed] [Google Scholar]
- 71.Wong ATY, Mannix C, Grantham JJ, et al. Randomised controlled trial to determine the efficacy and safety of prescribed water intake to prevent kidney failure due to autosomal dominant polycystic kidney disease (PREVENT-ADPKD). BMJ Open. 2018;8(1):e018794. doi: 10.1136/bmjopen-2017-018794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Torres VE, Abebe KZ, Schrier RW, et al. Dietary salt restriction is beneficial to the management of autosomal dominant polycystic kidney disease. Kidney Int. 2016;91(2):493–500. doi: 10.1016/j.kint.2016.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Smyth A, O’Donnell MJ, Yusuf S, et al. Sodium intake and renal outcomes: a systematic review. Am J Hypertens. 2014;27(10):1277–1284. doi: 10.1093/ajh/hpt294 [DOI] [PubMed] [Google Scholar]
- 74.Cianciaruso B, Bellizzi V, Minutolo R, et al. Salt intake and renal outcome in patients with progressive renal disease. Miner Electrolyte Metab. 1998;24(4):296–301. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9554571. Accessed January 17, 2019. [DOI] [PubMed] [Google Scholar]
- 75.Brosnahan GM, Abebe KZ, Rahbari-Oskoui FF, et al. Effect of statin therapy on the progression of autosomal dominant polycystic kidney disease. a secondary analysis of the HALT PKD Trials. Curr Hypertens Rev. 2017;13(2):109–120. doi: 10.2174/1573402113666170427142815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cadnapaphornchai MA, George DM, McFann K, et al. Effect of pravastatin on total kidney volume, left ventricular mass index, and microalbuminuria in pediatric autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2014;9(5):889–896. doi: 10.2215/CJN.08350813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Torres VE, Grantham JJ, Chapman AB, et al. Potentially modifiable factors affecting the progression of autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2011;6(3):640–647. doi: 10.2215/CJN.03250410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Grampsas SA, Chandhoke PS, Fan J, et al. Anatomic and metabolic risk factors for nephrolithiasis in patients with autosomal dominant polycystic kidney disease. Am J Kidney Dis. 2000;36(1):53–57. doi: 10.1053/AJKD.2000.8266 [DOI] [PubMed] [Google Scholar]
- 79.Baishya R, Dhawan DR, Kurien A, Ganpule A, Sabnis RB, Desai MR. Management of nephrolithiasis in autosomal dominant polycystic kidney disease - A single center experience. Urol Ann. 2012;4(1):29–33. doi: 10.4103/0974-7796.91618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bhasin B, Alzubaidi M, Velez JCQ. Evaluation and management of gross hematuria in autosomal dominant polycystic kidney disease: a point of care guide for practicing internists. Am J Med Sci. 2018;356(2):177–180. doi: 10.1016/J.AMJMS.2017.11.013 [DOI] [PubMed] [Google Scholar]
- 81.Sallée M, Rafat C, Zahar J-R, et al. Cyst infections in patients with autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2009;4:1183–1189. doi: 10.2215/CJN.01870309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Telenti A, Torres VE, Gross JB, Van Scoy RE, Brown ML, Hattery RR. Hepatic cyst infection in autosomal dominant polycystic kidney disease. Mayo Clin Proc. 1990;65(7):933–942. doi: 10.1016/s0025-6196(12)65154-4 [DOI] [PubMed] [Google Scholar]
- 83.Pirson Y, Chauveau D, Torres V. Management of cerebral aneurysms in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2002;13(1):269–276. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11752048. Accessed May 22, 2019. [DOI] [PubMed] [Google Scholar]
- 84.Ring T, Spiegelhalter D. Risk of intracranial aneurysm bleeding in autosomal-dominant polycystic kidney disease. Kidney Int. 2007;72(11):1400–1402. doi: 10.1038/sj.ki.5002488 [DOI] [PubMed] [Google Scholar]
- 85.Luciano RL, Dahl NK. Extra-renal manifestations of autosomal dominant polycystic kidney disease (ADPKD): considerations for routine screening and management. Nephrol Dial Transplant. 2014;29(2):247–254. doi: 10.1093/ndt/gft437 [DOI] [PubMed] [Google Scholar]
- 86.Jacquet A, Pallet N, Kessler M, et al. Outcomes of renal transplantation in patients with autosomal dominant polycystic kidney disease: a nationwide longitudinal study. Transpl Int. 2011;24(6):582–587. doi: 10.1111/j.1432-2277.2011.01237.x [DOI] [PubMed] [Google Scholar]
- 87.Kanaan N, Devuyst O, Pirson Y. Renal transplantation in autosomal dominant polycystic kidney disease. Nat Rev Nephrol. 2014;10(8):455–465. doi: 10.1038/nrneph.2014.104 [DOI] [PubMed] [Google Scholar]
- 88.Yamamoto T, Watarai Y, Kobayashi T, et al. Kidney volume changes in patients with autosomal dominant polycystic kidney disease after renal transplantation. Transplantation. 2012;93(8):794–798. doi: 10.1097/TP.0b013e318246f910 [DOI] [PubMed] [Google Scholar]
- 89.Torres VE, Harris PC, Pirson Y. Autosomal dominant polycystic kidney disease. Lancet (London, England). 2007;369(9569):1287–1301. doi: 10.1016/S0140-6736(07)60601-1 [DOI] [PubMed] [Google Scholar]
- 90.Caroli A, Perico N, Perna A, et al. Effect of longacting somatostatin analogue on kidney and cyst growth in autosomal dominant polycystic kidney disease (ALADIN): a randomised, placebo-controlled, multicentre trial. Lancet. 2013;382(9903):1485–1495. doi: 10.1016/S0140-6736(13)61407-5 [DOI] [PubMed] [Google Scholar]
- 91.Walz G, Budde K, Mannaa M, et al. Everolimus in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2010;363(9):830–840. Available from: http://www.nejm.org/doi/abs/10.1056/NEJMoa1003491. [DOI] [PubMed] [Google Scholar]