Abstract
Purpose
Vitamin D deficiency has been found in children with chronic tic disorders (CTDs). Our previous data showed that serum 25-hydroxyvitamin D [25(OH)D] level in children with CTDs was lower than that of the healthy controls and lower serum 25(OH)D level was associated with increased severity of the tic disorder. Thus, we intend to further verify this phenomenon and examine the effect of vitamin D3 on CTDs.
Patients and methods
In total, 120 children with CTDs and 140 normal controls were enrolled in this study, with 36/120 of those in the CTD group receiving vitamin D3 treatment for 3 months. The Yale Global Tic Severity Scale (YGTSS) and Clinical Global Impression of Severity of Illness (CGI-SI) were, respectively, used to evaluate the tic severity. High-performance liquid chromatography and tandem mass spectrometry were used to measure serum 25(OH)D level.
Results
Those children with CTDs exhibited significantly lower 25(OH)D levels than did healthy controls, and these reduced 25(OH)D levels were linked to increasing severity of tic symptoms. After treatment with supplemental vitamin D3, serum 25(OH)D level and scores of YGTSS total, motor tics, phonic tics, total tic, impairment, and CGI-SI improved significantly in children with CTDs without any adverse reactions.
Conclusion
Supplementation vitamin D3, given its low cost and excellent safety, may be an effective means of improving symptoms in certain children with CTDs.
Keywords: vitamin D, chronic tic disorders, 25(OH)D, dopamine, child
Introduction
Chronic tic disorders (CTDs) are neurodevelopmental disorders, which commonly begin in childhood and are characterized by the presence of repeated, stereotyped, discrete, and nonrhythmic movements and/or vocalizations, which are present for at least 1 year.1 According to the DSM-5, the idiopathic CTDs are mainly divided into Tourette’s syndrome (TS) and chronic motor or vocal tic disorders.2 In the latter two cases, motor and phonic tics are not necessary to coexist. TS is the most severe CTD, presenting with a range of motor tics as well as one or more vocal tic. The prevalence of CTDs is estimated at 2–3% and that of TS is approximate 1% of the school-age children, with a ratio of three to four times more common in boys than girls.3,4 The majority (approximately 80%) of individuals with CTDs has at least one comorbid behavioral problem, of which obsessive-compulsive disorder (OCD) and attention deficit hyperactivity disorder (ADHD) are most common.5,6
Factors responsible for CTDs have not been clarified yet. Genetics, neurotransmitters, as well as psychological and environmental factors are believed to contribute to causing these disorders. It has been suggested that the dysfunction of the cortico-basal ganglia pathways is associated with the pathology of CTDs and dopaminergic input from the substantia nigra pars compacta modulate the activity throughout the basal ganglia.7 Furthermore, CTDs are complex, with factors including dietary constituents, toxins, sleep, and exercise are probably also involved, which interplay with genetic and neurotransmitters factors and may increase susceptibility to CTDs.
Vitamin D is a steroid with key roles in maintaining normal brain physiology and neurodevelopment, regulating >200 genes.8 At the cellular level, vitamin D regulates gene expression in a manner protective of the dopamine system,9 and it further has a direct role in regulating the key enzymes such as tyrosine hydroxylase and catechol-O-methyltransferase, which are essential for dopamine production and metabolism, as well as that of norepinephrine and epinephrine.10,11
Our previous data showed that children with tic disorders exhibit reduced serum 25-hydroxyvitamin D [25(OH)D] levels, with a direct negative correlation between these levels and tic severity.12,13 Similarly, Stagi et al, found that in children with streptococcal-associated pediatric autoimmune neuropsychiatric disorders, serum 25(OH)D levels were reduced relative to healthy individuals, and these levels were again negatively correlated with the severity of symptoms including tics and obsessive-compulsive behaviors.14 Additionally, vitamin D deficiency is linked to psychiatric conditions including ADHD, autism, depression, and schizophrenia.15–18
These clinical findings warrant further study. Based on our previous findings, we hypothesized that vitamin D insufficiency or deficiency may be contributive to the increased susceptibility of CTDs, and therefore vitamin D3 supplementation will reduce the severity of tic symptoms in children with CTDs. We, therefore, sought to evaluate how vitamin D3 supplementation affected 120 children with CTDs.
Materials and methods
Study design
This case-control and randomized-control study (in CTDs group) received approval from the Research Ethics Committee of the First Hospital of Jilin University. Prior to enrollment, parents or legal guardians provided written informed consent for all participants and this was conducted in accordance with the Declaration of Helsinki. This clinical trial was registered in the Chinese Clinical Trial Registry (ChiCTR) (registration number: ChiCTR-OPC-17013502).
Participants
From November 2016 to October 2018, we recruited 120 children aged from 5 to 14 years old with CTDs from the clinic of the First Hospital of Jilin University. The mean participant age in these children was 9±2 years old. In addition, we recruited 140 healthy controls (mean age: 9±2 years, range: 5–14 years), partly from the same clinic (mentioned above) and partly from a children’s activity center in Changchun. The control and CTD groups were matched with respect to age, sex, and season of blood collection.
Diagnose of CTDs was confirmed according to DSM-5 criteria by trained pediatricians who had received consensus training before study inclusion. Also, structured parent interviews by conducting Diagnostic Interview Schedule for Children Version Ⅳ (DISC-Ⅳ), and additional comorbidities were assessed using an internally generated behavioral screening questionnaire. We have previously published results pertaining to 56 cases and 60 healthy controls enrolled in this study.13
Exclusion criteria included the following conditions: co-morbidity with other neuropsychiatric disorders (such as autism, mental disorders, tuberous sclerosis, epilepsy), rheumatic fever, and common comorbidities such as ADHD and OCD that were more severe than the tic disorder itself. Children who were receiving any medications related to the treatment of tics and comorbidities, especially neuroleptics, SSRIs, or psychostimulants. We also excluded those children who underwent calcium or vitamin D therapy over the previous 3 months, as well as those with endocrine, liver, or kidney diseases and those taking any supplements or medications with the potential to disrupt vitamin D and calcium metabolism.
Evaluations and intervention
Initial clinical assessment
Self-made questionnaires, dietary intake of participants, and demographic data including sex, date of birth, age, place of residence, physical activity, vitamin supplements, recent diet, and sun exposure were recorded by physician. An electronic scale was used to record participant weight, while height was measured via stadiometer, with children in both groups being measured without shoes and while wearing only lightweight clothes. Body mass index (BMI) for age percentile was calculated as follows: BMI = weight (kg)/(height (m))2. Children were classified into normal weight (<85th percentile BMI), overweight (85–95th percentile BMI), and obesity (>95th percentile BMI) according to the age percentile of BMI.19 The Chinese version of the DISC-Ⅳ, which is known to have good reliability and validity,20 has been used to assess the comorbidities in our study. DISC is developed by the American working group for childhood disorders according to DSM-Ⅳ diagnostic criteria, including most major behavioral and emotional disorders in childhood, such as ADHD, oppositional defiant disorder, OCD, anxiety or depression, etc.
Laboratory investigations
Prior to the enrollment of CTDs group, each child with CTDs was required to receive blood routine test including blood cell count, anti-o antibody, rheumatoid factor, serum calcium level, liver, and kidney function. Serum 25(OH)D levels of both group at baseline were assessed via high-performance liquid chromatography in Guangzhou King Medical Center (Clinical Laboratory). The models of liquid chromatograph and mass spectrometer have been described in detail in our previous publication.13 Children who were enrolled after November 2017 and also completed vitamin D3 supplementation over a 3-month period needed to conduct the above laboratory test again at the end of the trial. Optimal vitamin D level is based on a serum 25(OH)D level of 30–90 ng/mL, whereas levels of 10–30 ng/mL indicate vitamin D insufficiency, and concentration below 10 ng/mL represents a vitamin D deficiency.21
In this study, blood samples have been collected in two periods of the year: winter season starting (November–May), and summer season (June–October). This approach is preferable according to the study result in Boston,22 and Changchun and Boston are both found between 42° and 45° of North latitude.
Intervention
Children with CTDs and vitamin D insufficiency/deficiency (enrolled after November 2017) were advised to receive vitamin D3 supplementation. Vitamin D3 treatment should be taken daily for 3 months, with a dose of 300 IU/kg/day (no more than 5000 IU/day). Vitamin D tablets (5000 IU) were supplied by NATURE’S BOUNTY, INC. Bohemia, NY 11716 USA.
Outcome measurements
Changes in symptoms according to the Yale Global Tic Severity Scale (YGTSS) from baseline to endpoint were the primary measured outcome. YGTSS is a semi-structured scale designed to assess both the impairment and severity of tic symptoms. The YGTSS total tic score is based on the combined severity of both vocal and motor tic scores with respect to the intensity, frequency, number, interference, and complexity of tics, with a possible score range of 0–50. In addition, the YGTSS incorporates an impairment rating scale aimed at gauging functional impairments associated with tics, such as psychosocial aspects with scores ranging from 0 to 50 points. The YGTSS scale has good psychometric properties,23 and a Chinese adaptation of this scale was utilized in this study.
The secondary efficacy outcome was measured with the improvement of Clinical Global Impression Severity of Illness (CGI-SI) score from baseline to 3 months after the trial. The CGI scales are widely used measurements to evaluate the efficacy in clinical psychiatric trials. The score of CGI-SI is rated 1–7 points from “normal” to “among the most extremely ill” according to the clinician’s overall impression of a patient’s condition.24 Treatment safety was evaluated based on a side effect questionnaire at the end of the third month.
Statistical analysis
SPSS v22.0 (IBM Corp., Armonk, NY) was used for data analysis. The Kolmogorov–Smirnov test was used to assess distribution normality. Independent t-tests and Mann–Whitney tests were used to compare the means and medians of 2+ continuous variables per group, respectively. Chi-squared and Mann–Whitney tests were utilized in order to assess differences in categorical variables. Differences within groups were assessed via paired t-tests and Wilcoxon tests. Pearson’s correlation test was used for correlation analysis of variables with normal distribution, and Spearman test was used for those not conform to the normal distribution. All comparisons were two-sided, and p<0.05 was the significance threshold.
Results
This study enrolled 120 children with CTDs. Clinical classifications were TS in 67 patients (67/120, 56%), chronic motor tic disorder in 43 patients (43/120, 36%), and chronic vocal tic disorder in 10 patients (10/120, 8%). Besides, 38 patients (32%) had ADHD, 22 patients (18%) had OCD, and 13 patients (11%) had anxiety problems.
Table 1 shows the demographic data and vitamin D conditions of the two groups. There were no differences in sex, age, BMI, or season of blood sample isolation between children with CTDs and healthy controls. Other variables including activity level, diet, and residence area were also comparable between groups (Data not shown). The children with CTDs exhibited significantly lower serum 25(OH)D levels than did the control group, and the difference was found both during winter and summer period. Groups differed significantly with respect to overall frequencies of children with 25(OH)D levels that were optimal, insufficient, and deficient, with significantly higher rates of insufficiency and deficiency in the children with CTDs relative to the healthy controls.
Table 1.
Patient demographic data
| Characteristics | CTDs | Controls | t/χ2 (Z) | p |
|---|---|---|---|---|
| Number of participants | 120 | 140 | ||
| Sex | 0.83 | 0.36 | ||
| Male | 102 (85%) | 113 (81%) | ||
| Female | 18 (15%) | 27 (19%) | ||
| Age. y | 9 (8–11) | 9 (7–10) | (–1.42) | 0.16 |
| BMI | 0.56 | 0.76 | ||
| <85th | 87 (72%) | 102 (73%) | ||
| 85–95th | 26 (22%) | 27 (19%) | ||
| >95th | 7 (6%) | 11 (8%) | ||
| Season of blood collection | 0.39 | 0.53 | ||
| Winter | 98 (82%) | 110 (79%) | ||
| Summer | 22 (18%) | 30 (21%) | ||
| 25 (OH)D, (ng/mL) | 19±7a | 29±7 | 10.5 | <0.001 |
| During winter | 19±8a | 28±7 | 9.37 | <0.001 |
| During summer | 21±6a | 30±8 | 4.68 | <0.001 |
| Vitamin D status | 49.3 | <0.001 | ||
| Optimal | 8 (7%)a | 62 (44%) | ||
| Insufficient | 98 (81%)a | 74 (53%) | ||
| Deficient | 14 (12%)a | 4 (3%) |
Notes: ap<0.05 vs controls. Data represent the percentages of, mean ± SD, or medians (P25–P75). Independent t test, χ2 test, and Mann–Whitney test were employed for comparing control children to those with CTDs.
Abbreviations: CTDs, Chronic tic disorders (referred as case group); Controls, normal healthy controls.
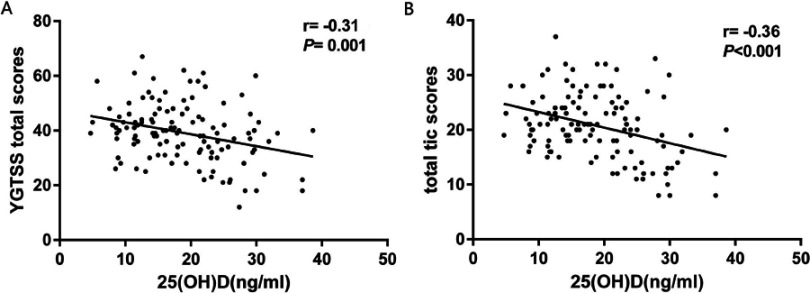
We examined the relationship between 25(OH)D levels and YGTSS scores in the whole CTDs group (n=120) at baseline, revealing a negative Pearson’s correlation between these levels and total YGTSS scores (r= −0.31, p=0.001, Figure 1A), and between these values and YGTSS total tic scores (r= −0.36, p<0.001, Figure 1B). There was additionally a significant negative Spearman’s correlation between 25(OH)D levels and motor tics scores (rs = −0.24, p=0.01). No significant correlations were found between the levels of 25(OH)D and Phonic tics scores (rs= −0.14, p=0.14) and Impairment scores (rs = −0.17, p=0.06).
Figure 1.
Correlation of 25(OH)D levels with YGTSS scores. Associations between 25(OH)D levels and YGTSS scores in the whole CTDs group (n=120) at baseline were analyzed via Pearson’s correlation test. (A) Total YGTSS score correlation with 25(OH)D levels. (B) Total YGTSS tic score correlation with 25(OH)D.
Abbreviations: 25(OH)D, 25-hydroxyvitamin D; YGTSS, Yale Global Tic Severity Scale; CTDs, chronic tic disorders.
All children with CTDs who were vitamin D insufficient or deficient (enrolled after November 2017, n=60) were given the advice to intake supplemental vitamin D3. Only 36 (mean age: 8±2 years old, male to female ratio was 5:1) of the 60 CTDs children with vitamin D insufficiency or deficiency had finished 3 months of consistent vitamin D3 supplementation (Table 2). Lack of compliance in other children was attributable to factors including parental doubt regarding treatment efficacy, worries about vitamin D poisoning, or failing to attend regular follow-up visits.
Table 2.
Follow-up treatment in children with CTDs and vitamin D deficiency or insufficiency
| Vitamin D deficiency(<10 ng/mL) | Vitamin D insufficiency(10–30 ng/mL) | Total number | |
|---|---|---|---|
| Be advised to receive vitamin D3 supplementation | 11 | 49 | 60 |
| Taking vitamin D3 supplementation | 11 | 43 | 54 |
| Loss of contact or unable to conduct evaluations | 4 | 14 | 18 |
| Finished vitamin D3 supplementation | 7 | 29 | 36 |
Rating scores and serum 25(OH)D levels of those with CTDs who were vitamin D insufficient or deficient, before and after vitamin D3 supplementation are shown in Table 3. After 3 months of vitamin D3 supplementation, there was a significant elevation in 25(OH)D levels in the serum compared to the baseline. The total scores and sub-scores of YGTSS, CGI-SI scores were significantly reduced relative to pretreatment values. The percentage of children with a CGI-SI score of 1–2 points (less severe tics) was significantly higher and the percentage of children with a score of 5–6 points (more severe tics) was significantly reduced relative to baseline.
Table 3.
Rating scores and 25(OH)D levels in children that have CTDs before and after vitamin D3 supplementation
| Variable | Baseline | After 3 months | t (Z) | p |
|---|---|---|---|---|
| YGTSS total scores | 41±11 | 26±13a | 9.68 | <0.001 |
| Total tics scores | 23±5 | 13±7a | 10.15 | <0.001 |
| Motor tics | 16 (13–18) | 10 (7–12)a | (−5.0) | <0.001 |
| Phonic tics | 8 (5–10) | 5 (2–7)a | (−4.44) | <0.001 |
| Impairment | 20 (10–20) | 10 (10–20)a | (−3.83) | <0.001 |
| CGI-SI scores | 4 (4–5) | 3 (2–4)a | (−3.79) | <0.001 |
| CGI-SI (%) | (−3.38) | 0.001 | ||
| 1–2 scores | 1 (3%) | 9 (25%)a | ||
| 3–4 scores | 23 (64%) | 24 (67%) | ||
| 5–6 scores | 12 (33%) | 3 (8%)a | ||
| 25(OH)D(ng/mL) | 21(15–23) | 51(43–58)a | (−5.23) | <0.001 |
Note: Data are means ± SD or medians (P25–P75), percentage of children. Paired t-tests, Wilcoxon tests, and Mann–Whitney tests were used to compare before and after therapy in children with CTDs. aSignificantly different from the baseline (p<0.05).
Abbreviations: 25(OH)D, 25-hydroxyvitamin D; CTDs, chronic tic disorders; YGTSS, Yale Global Tic Severity Scale.
In general, children were able to readily tolerate the high-dose vitamin D3 supplementation without negative side effects. The side effects questionnaire found that only one patient had transient abdominal pain after 1 month of vitamin D3 supplementation. The serum level of urea nitrogen, calcium, creatinine, alanine transaminase, and aspartate transaminase was all in the normal range before and after vitamin D3 treatment.
Discussion
Firstly, we observed significantly reduced in serum 25(OH)D levels in children with CTDs, who were also significantly more likely to be vitamin D insufficient/deficient compared to healthy controls. Secondly, serum 25(OH)D levels were found to be negatively correlated with tic severity. Thirdly, the vitamin D3 supplementation at a dose of 300 IU/kg/d (no more than 5000 IU/d) reduced tic severity in children with CTDs after 3 months of treatment. To the best of our knowledge, this is the first trial assessing the efficacy of vitamin D3 supplementation in children with CTDs.
Our results are consistent with our previous research and other research results: Vitamin D insufficiency and deficiency are common in children with tic disorders. Theoretically, a sustained drop in 25(OH)D levels may drive provisional tic disorders to transform into CTDs and might be a biological marker for the course of tic disorder.12–14
Vitamin D can act on the nervous system as a neuroactive steroid via regulation of genes expression, neurotrophic factor synthesis, calcium transition, and antioxidant properties for both brain development and mature brain function.25,26 The mechanism governing lower vitamin D levels of CTDs is not clear, though it could be linked to eating habits or limited sunshine exposure. Up to now, little data are available on assessing how tic disorders relate to vitamin D, although there are multiple plausible explanations for why vitamin D3 supplementation improves tic symptoms in those with CTDs.
One of them is that vitamin D is related to the dopamine system. Cortico-basal ganglia dopaminergic dysfunction is associated with the pathology of CTDs.27,28 Through immunohistochemical staining, 1,25-dihydroxyvitamin D3 receptor (VDR) and 1alpha-hydroxylase can be found in the hypothalamus and in the large neurons of substantia nigra, presumably dopaminergic neurons, which help to form active vitamin D.29 Cui et al, confirmed that VDR exists in the nucleus of TH+ neurons in the substantia nigra of both human and rats, and VDR appears in the early brain development of rats.30 Jiang et al, showed that after 6 weeks of calcitriol given to rats at the dose of 50 or 100 ng/kg/d, the expression of TH was induced, a rate-limiting enzyme for the conversion of tyrosine into dopamine.31 An in vitro study by Cui et al, also confirmed that vitamin D regulates the expression of tyrosine hydroxylase.10 Hawes et al, found that the expression of brain tyrosine hydroxylase gene in female fetuses deficient in vitamin D of the BALB/c mouse was decreased.32 In addition, vitamin D is able to control the production of glial cell line-derived neurotrophic factor (GDNF) and a range of other neurotrophic factors.33 Vitamin D could protect the dopamine system by modulating GDNF receptor, C-Ret, which is greatly important for cell differentiation and survival of dopaminergic neurons.34 These studies suggested that vitamin D deficiency in fetuses or early brain development might be contributing to the etiology of tic disorders.
Another explanation for the effectiveness of vitamin D in treating CTDs is the link between vitamin D and γ-aminobutyric acid (GABA) system. Lerner et al, observed impaired GABA-A receptor binding in many regions in Tourette patients, and particularly, that abnormal insular and cerebellar GABA-A binding is likely associated with tic symptoms.35 In mice, selectively disrupting GABA-A receptor signaling or blocking the activation of GABAergic striatal interneurons can lead to the development of tic-like movements.36,37 Vitamin D is an active controller of the genetic regulation of the synthesis of GABA.38 Vitamin D could significantly reduce the activity of glutamic acid decarboxylase, a critical enzyme in GABAergic interneurons.39 The GABA levels of the prefrontal cortex and the striatum in rats with vitamin D deficiency were significantly increased.39 The increased level of γ-GABA also found in vitamin D deficient BALB/c mice.40 These studies indicate that one of the reasons vitamin D may effectively treat CTDs is by regulating the concentration of GABA.
An ideal 25(OH)D level of the biological effect of vitamin D in promoting intestinal calcium absorption for bone metabolism is 34–38 ng/mL.41,42 It is possible that higher levels of 25(OH)D will enable vitamin D to play a role in neuro-immunomodulation, neurotransmission, antioxidation, and gene regulation in behavioral and neuropsychiatric disorders.43–45 Cannell et al , recommended the ideal level of 25(OH)D ranged from 55 to 70 ng/mL for supplemental treatment of chronic disorders with vitamin D deficiency.43 Therefore, our study chose to give vitamin D3 supplementation at a dose of 300 IU/kg/d (no more than 5000 IU/d) in order to reach the ideal 25(OH)D level of 55–70 ng/mL. In this study, vitamin D3 supplementation improved the symptoms of CTDs children, possibly because the level of 25(OH)D was close to the ideal concentration, thus making vitamin D plays a role in neuro-modulation.
Since sunlight exposure will influence the production of vitamin D, we divided the children into summer and winter group according to the blood collecting season, but we found comparable vitamin D levels in both groups, with slightly but not significantly higher levels in the summer (p>0.05). This might be related to the higher percentage of the children who were enrolled in the winter compared to the summer. In addition, the majority of the children in our study were school-age children and usually have little outdoor physical activity, which might undermine the significant seasonal influence on 25(OH)D levels.
Limitations
Although our results are promising, the study does have two serious limitations. Firstly, many of the CTD children with vitamin D insufficiency or deficiency did not finish 3 months of vitamin D3 supplementation or lost contact during the follow-up period. This large attrition rate might have biased the study to positive findings as “non-responders” would be more likely to drop out. Secondly, this study is an open-label study, and thus susceptible to patient and rater biases.
Conclusion
Our study confirmed again that children with CTDs are more likely to be vitamin D insufficient or deficient, with significantly reduced 25(OH)D levels relative to healthy controls. Furthermore, decreased 25(OH)D levels were linked to worse severity of tic symptoms. After 3 months of vitamin D3 supplementation, severity of tic symptoms in children with CTDs was reduced without adverse effects. Although these are promising results, large randomized-controlled clinical Trails are still needed to further verify our conclusion and unlock the exact mechanism of vitamin D in the etiology of CTDs.
Acknowledgments
The authors thank the patients and their parents for their participation.
The National Key Research and Development Program of China (No: 2016YFC1306204) and Natural Science Foundation of China (No: 81602847) supported this work.
Abbreviations
CTDs, chronic tic disorders; TS, Tourette syndrome; OCD, obsessive-compulsive disorder; ADHD, attention deficit hyperactivity disorder; 25(OH)D, 25-hydroxyvitamin D; DISC, IV-Diagnostic Interview Schedule for Children Version IV; BMI, body mass index; YGTSS, Yale Global Tic Severity Scale; CGI-SI, Clinical Global Impression Severity of Illness; VDR-1, 25-dihydroxyvitamin D3 receptor; GDNF, glial cell line-derived neurotrophic factor; GABA, γ-aminobutyric acid.
Data sharing statement
Starting 3 months after the publication of the paper, and within 5 years after the publication, the researcher shall provide a methodological and reasonable proposal and send it to honghua_li1986@jlu.edu.cn. After signing the data acquisition agreement, the researcher can share the individual deidentified participant data and other documents related to the study, such as the study protocol and statistical analysis plan.
Disclosure
The authors have no conflicts of interest relevant to this article to disclose.
References
- 1.Sanger TD, Chen D, Fehlings DL, et al. Definition and classification of hyperkinetic movements in childhood. Mov Disord. 2010;25(11):1538–1549. doi: 10.1002/mds.23088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013:81–85. [Google Scholar]
- 3.Knight T, Steeves T, Day L, Lowerison M, Jette N, Pringsheim T. Prevalence of tic disorders: a systematic review and meta-analysis. Pediatr Neurol. 2012;47(2):77–90. doi: 10.1016/j.pediatrneurol.2012.05.002 [DOI] [PubMed] [Google Scholar]
- 4.Yang C, Zhang L, Zhu P, Zhu C, Guo Q. The prevalence of tic disorders for children in China: a systematic review and meta-analysis. Medicine (Baltimore). 2016;95(30):e4354. doi: 10.1097/MD.0000000000004864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eapen V, Robertson MM. Are there distinct subtypes in Tourette syndrome? Pure-Tourette syndrome versus Tourette syndrome-plus, and simple versus complex tics. Neuropsychiatr Dis Treat. 2015;11:1431–1436. doi: 10.2147/NDT.S72284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lebowitz ER, Motlagh MG, Katsovich L, et al. Tourette syndrome in youth with and without obsessive compulsive disorder and attention deficit hyperactivity disorder. Eur Child Adolesc Psychiatry. 2012;21(8):451–457. doi: 10.1007/s00787-012-0278-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yael D, Vinner E, Bar-Gad I. Pathophysiology of tic disorders. Mov Disord. 2015;30(9):1171–1178. doi: 10.1002/mds.26304 [DOI] [PubMed] [Google Scholar]
- 8.Cannell JJ, Grant WB. What is the role of vitamin D in autism? Dermatoendocrinol. 2013;5(1):199–204. doi: 10.4161/derm.24356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Y, Li YW, Tang YL, et al. Vitamin D: preventive and therapeutic potential in parkinson’s disease. Curr Drug Metab. 2013;14(9):989–993. [DOI] [PubMed] [Google Scholar]
- 10.Cui X, Pertile R, Liu P, Eyles DW. Vitamin D regulates tyrosine hydroxylase expression: N-cadherin a possible mediator. Neuroscience. 2015;304:90–100. doi: 10.1016/j.neuroscience.2015.07.048 [DOI] [PubMed] [Google Scholar]
- 11.Pertile RA, Cui X, Eyles DW. Vitamin D signaling and the differentiation of developing dopamine systems. Neuroscience. 2016;333:193–203. doi: 10.1016/j.neuroscience.2016.07.020 [DOI] [PubMed] [Google Scholar]
- 12.Li HH, Wang B, Shan L, Wang CX, Jia FY. Serum levels of 25-hydroxyvitamin D in children with tic disorders. Zhongguo Dang Dai Er Ke Za Zhi. 2017;19(11):1165–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li HH, Shan L, Wang B, Du L, Xu ZD, Jia FY. Serum 25-hyroxyvitamin d levels and tic severity in Chinese children with tic disorders. Psychiatry Res. 2018;267:80–84. doi: 10.1016/j.psychres.2018.05.066 [DOI] [PubMed] [Google Scholar]
- 14.Stagi S, Lepri G, Rigante D, Matucci CM, Falcini F. Cross-sectional evaluation of plasma vitamin D levels in a large cohort of Italian Patients with pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections. J Child Adolesc Psychopharmacol. 2018;28(2):124–129. doi: 10.1089/cap.2016.0159 [DOI] [PubMed] [Google Scholar]
- 15.Anglin RE, Samaan Z, Walter SD, McDonald SD. Vitamin D deficiency and depression in adults: systematic review and meta-analysis. Br J Psychiatry. 2013;202:100–107. doi: 10.1192/bjp.bp.111.106666 [DOI] [PubMed] [Google Scholar]
- 16.Berridge MJ. Vitamin D deficiency. infertility and neurodevelopmental diseases (attention deficit hyperactivity disorder, autism and schizophrenia). Am J Physiol Cell Physiol. 2018;314(2):C135–C151. doi: 10.1152/ajpcell.00188.2017 [DOI] [PubMed] [Google Scholar]
- 17.Khoshbakht Y, Bidaki R, Salehiabargouei A. Vitamin D status and attention deficit hyperactivity disorder: a systematic review and meta-analysis of observational studies. Adv Nutr. 2018;9(1):9–20. doi: 10.1093/advances/nmx002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saad K, Abdel-Rahman AA, Elserogy YM, et al. Vitamin D status in autism spectrum disorders and the efficacy of vitamin D supplementation in autistic children. Nutr Neurosci. 2016;19(8):346–351. doi: 10.1179/1476830515Y.0000000019 [DOI] [PubMed] [Google Scholar]
- 19.Li H, Zong XN, Ji CY, Mi J. Body mass index cut-offs for overweight and obesity in Chinese children and adolescents aged 2-18 years. Zhonghua Liu Xing Bing Xue Za Zhi. 2010;31(6):616–620. [PubMed] [Google Scholar]
- 20.Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version Ⅳ(NIMHDISC- Ⅳ): description,differencesfrom previous versions, and reliability of some common diagnoses. J Am Acad Child Adolesc Psychiatry. 2000;39(1):28–38. doi: 10.1097/00004583-200001000-00014 [DOI] [PubMed] [Google Scholar]
- 21.Canadian Paediatric Society. Vitamin D supplementation: recommendations for Canadian mothers and infants. Paediatr Child Health. 2007;12(7):583–598. [PMC free article] [PubMed] [Google Scholar]
- 22.Webb AR, Pilbeam C, Hanafin N, Holick MF. An evaluation of the relative contributions of exposure to sunlight and of diet to the circulating concentrations of 25-hydroxyvitamin D in an elderly nursing home population in Boston. Am J Clin Nutr. 1990;51(6):1075–1081. doi: 10.1093/ajcn/51.6.1075 [DOI] [PubMed] [Google Scholar]
- 23.Leckman JF, Riddle MA, Hardin MT, et al. The Yale Global Tic Severity Scale: initial testing of a clinician-rated scale of tic severity. J Am Acad Child Adolesc Psychiatry. 1989;28(4):566–573. doi: 10.1097/00004583-198907000-00015 [DOI] [PubMed] [Google Scholar]
- 24.Dunlop BW, Gray J, Rapaport MH. Transdiagnostic clinical global impression scoring for routine clinical settings. Behav Sci (Basel). 2017;7(4):pii E40. doi: 10.3390/bs7030040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cannell JJ. Autism and vitamin D. Med Hypotheses. 2008;70(4):750–759. doi: 10.1016/j.mehy.2007.08.016 [DOI] [PubMed] [Google Scholar]
- 26.Muscogiuri G, Altieri B, Annweiler C, et al. Vitamin D and chronic diseases: the current state of the art. Arch Toxicol. 2017;91(1):97–107. doi: 10.1007/s00204-016-1804-x [DOI] [PubMed] [Google Scholar]
- 27.Buse J, Schoenefeld K, Münchau A, Roessner V. Neuromodulation in tourette syndrome: dopamine and beyond. Neurosci Biobehav Rev. 2013;37(6):1069–1084. doi: 10.1016/j.neubiorev.2012.10.004 [DOI] [PubMed] [Google Scholar]
- 28.Müller-Vahl KR, Loeber G, Kotsiari A, Müller-Engling L, Frieling H. Gilles de la tourette syndrome is associated with hypermethylation of the dopamine D2 receptor gene. J Psychiatr Res. 2016;86:1–8. doi: 10.1016/j.jpsychires.2016.11.004 [DOI] [PubMed] [Google Scholar]
- 29.Eyles DW, Smith S, Kinobe R, Hewison M, Mcgrath JJ. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J Chem Neuroanat. 2005;29(1):21–30. doi: 10.1016/j.jchemneu.2004.08.006 [DOI] [PubMed] [Google Scholar]
- 30.Cui X, Pelekanos M, Liu PY, Burne TH, McGrath JJ, Eyles DW. The vitamin D receptor in dopamine neurons; its presence in human substantia nigra and its ontogenesis in rat midbrain. Neuroscience. 2013;236:77–87. doi: 10.1016/j.neuroscience.2013.01.035 [DOI] [PubMed] [Google Scholar]
- 31.Jiang P, Zhang LH, Cai HL, et al. Neurochemical effects of chronic administration of calcitriol in rats. Nutrients. 2014;6(12):6048–6059. doi: 10.3390/nu6126048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hawes JE, Tesic D, Whitehouse AJ, Zosky GR, Smith JT, Wyrwoll CS. Maternal vitamin D deficiency alters fetal brain development in the BALB/c mouse. Behav Brain Res. 2015;286:192–200. doi: 10.1016/j.bbr.2015.03.008 [DOI] [PubMed] [Google Scholar]
- 33.Brown J, Bianco JI, Mcgrath JJ, Eyles DW. 1, 25-dihydroxyvitamin D3 induces nerve growth factor, promotes neurite outgrowth and inhibits mitosis in embryonic rat hippocampal neurons. Neurosci Lett. 2003;343(2):139–143. doi: 10.1016/s0304-3940(03)00303-3 [DOI] [PubMed] [Google Scholar]
- 34.Pertile RAN, Cui X, Hammond L, Eyles DW. Vitamin D regulation of GDNF/Ret signaling in dopaminergic neurons. FASEB J. 2018;32(2):819–828. doi: 10.1096/fj.201700713R [DOI] [PubMed] [Google Scholar]
- 35.Lerner A, Bagic A, Simmons JM, et al. Widespread abnormality of the γ-aminobutyric acid-ergic system in Tourette syndrome. Brain. 2012;135(pt 6):1926–1936. doi: 10.1093/brain/aws104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bronfeld M, Yael D, Belelovsky K, Bar-Gad I. Motor tics evoked by striatal disinhibition in the rat. Front Syst Neurosci. 2013;7:50. doi: 10.3389/fnsys.2013.00039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gittis AH, Leventhal DK, Fensterheim BA, Pettibone JR, Berke JD, Kreitzer AC. Selective inhibition of striatal fast-spiking interneurons causes dyskinesias. J Neurosci. 2011;31(44):15727–15731. doi: 10.1523/JNEUROSCI.3875-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garcion E, Wion-Barbot N, Montero-Menei CN, Berger F, Wion D. New clues about vitamin D functions in the nervous system. Trends Endocrinol Metab. 2002;13(3):100–105. [DOI] [PubMed] [Google Scholar]
- 39.Byrne JH, Voogt M, Turner KM, Eyles DW, McGrath JJ, Burne TH. The impact of adult vitamin D deficiency on behavior and brain function in male Sprague-Dawley rats. PLoS One. 2013;8(8):e71593. doi: 10.1371/journal.pone.0071593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Groves NJ, Kesby JP, Eyles DW, Mcgrath JJ, Mackay-Sim A, Burne TH. Adult vitamin D deficiency leads to behavioural and brain neurochemical alterations in C57BL/6J and BALB/c mice. Behav Brain Res. 2013;241:120–131. doi: 10.1016/j.bbr.2012.12.001 [DOI] [PubMed] [Google Scholar]
- 41.Bischoff-Ferrari HA, Dietrich T, Orav EJ, et al. Higher 25-hydroxyvitamin D concentrations are associated with better lower-extremity function in both active and inactive persons aged > or =60 y. Am J Clin Nutr. 2004;80(3):752–758. doi: 10.1093/ajcn/80.3.752 [DOI] [PubMed] [Google Scholar]
- 42.Heaney RP, Dowell MS, Hale CA, Bendich A. Calcium absorption varies within the reference range for serum 25-hydroxyvitamin D. J Am Coll Nutr. 2003;22(2):142–146. [DOI] [PubMed] [Google Scholar]
- 43.Cannell JJ, Hollis BW. Use of vitamin D in clinical practice. Altern Med Rev. 2008;13(1):6–20. [PubMed] [Google Scholar]
- 44.Eyles DW, Burne TH, Mcgrath JJ. Vitamin effects on brain development, adult brain function and the links between low levels of vitamin D and neuropsychiatric disease. Front Neuroendocrinol. 2013;34(1):47–64. doi: 10.1016/j.yfrne.2012.07.001 [DOI] [PubMed] [Google Scholar]
- 45.Groves NJ, Burne TH. The impact of vitamin D deficiency on neurogenesis in the adult brain. Neural Regen Res. 2017;12(3):393–394. doi: 10.4103/1673-5374.202936 [DOI] [PMC free article] [PubMed] [Google Scholar]