Abstract
Despite a well‐known behavioral finding of visual backward masking impairment in schizophrenia, its underlying neural mechanism remains obscure. This study examined neural correlates of a distinct type of visual backward masking, object substitution masking (OSM), in schizophrenia. Twenty schizophrenia patients and 26 healthy controls completed a 4‐Dot OSM task and three functional localizer tasks for the lateral occipital (LO), human motion‐sensitive (hMT+), and retinotopic areas in the scanner. In 4‐dot masking, subjects detected a target that was followed by a mask consisting of 4 dots that surrounded a target. Stimulus‐onset asynchrony (SOA) between target and mask was varied to examine the modulation of masking: (1) within three visual processing areas regions of interest (ROI) (i.e., ROI analysis) and (2) in brain regions outside the three visual processing areas (i.e., whole brain analysis). In the ROI analyses, LO and retinotopic areas showed increased peak amplitude when SOA become longer in both patients and controls. There was also an effect of ROI in that both groups showed higher activation in LO and hMT+ compared with the retinotopic areas. The whole brain analyses revealed a significantly activated area for longer SOAs vs. a short SOA in the occipital cortex in controls only, but the group contrast was not significant. Overall, this study did not find strong evidence for neural abnormalities of OSM in schizophrenia, suggesting that neural substrates of OSM in schizophrenia are not as compromised as those involved in the more common masking methods that rely on disruption of object formation. Hum Brain Mapp 35:4654–4662, 2014. © 2014 Wiley Periodicals, Inc.
Keywords: object substitution masking, schizophrenia, visual backward masking, fMRI
INTRODUCTION
Backward masking paradigms have been used extensively in schizophrenia to better understand early visual processing [Green et al., 2011a]. In a typical visual backward masking paradigm, a mask quickly following a visual target disrupts the visibility of a target. Schizophrenia patients have consistently shown impaired performance on visual backward masking and the field is beginning to understand the neural substrates of impaired visual backward masking in schizophrenia. In this study, we explored neural substrates of a distinct type of visual masking, object substitution masking, in schizophrenia using functional magnetic resonance imaging (fMRI).
Visual backward masking paradigms can be roughly divided into two types depending on which process a visual mask affects to reduce target visibility—masking associated with object formation and those associated with object substitution [Enns and Di Lollo, 2000]. In a typical task for object formation masking, a mask has a clear contour and spatially overlaps with a target. When this type of mask quickly follows a target, object formation masking occurs because representations of the target and the mask become joined together, forming an integrated target‐mask composite representation, and disrupt the visibility of the target. The closer the temporal proximity of a target and a mask, the more the integrated target‐mask composite representation can disrupt the identification of a target.
The majority of studies on visual backward masking in schizophrenia have focused on object formation masking. For example, we have previously shown that two visual processing areas, the lateral occipital complex (LO) and the human motion‐sensitive area (hMT+), were sensitive to object formation masking in healthy individuals such that neural activations in both regions became stronger as the target‐mask interval increased, which resulted in weaker masking [Green et al., 2005, 2009]. Further, schizophrenia patients showed reduced activations in the LO, regardless of the target visibility (i.e. a general LO blunting) [Green et al., 2009]. However, this type of masking provides only limited information regarding exactly how impaired backward masking arises in schizophrenia. Visual backward masking involves two distinct processes [Breitmeyer and Ogmen, 2000; Enns, 2004]: feed‐forward processes that involve the flow of visual information from retina to the visual cortex and re‐entrant processes that rely on cortical feedback to the visual cortex. Because both feed‐forward and re‐entrant processing are involved in object formation masking [Breitmeyer and Ogmen, 2000; Enns, 2004], findings of impaired object formation masking does not inform us whether impaired visual backward masking was due to impaired feed‐forward processing, deficit re‐entrant processing, or a combination of both.
In contrast to object formation masking that may involve both feed‐forward and re‐entrant processing, masking by object substitution is thought to rely more on re‐entrant processing [Di Lollo et al., 2000] although there is some debate on how central role a re‐entrant processing plays in object substitution masking [Dux et al., 2010; Goodhew et al., 2013]. Object substitution masking is typically assessed with the 4‐Dot Masking Task, a paradigm in which a mask consists of 4 dots that surround a target. Hence, the mask has no contour and does not overlap with the target. This mask disrupts the visibility of a target by replacing the representation of the target with the representation of the 4 dots before it reaches visual awareness (i.e., masking by object substitution) through reentrant processing [Di Lollo et al., 2000]. Object substitution masking may be initiated at relatively later stages in visual processing, after object representations are formed, but may affect earlier processing stages through “top‐down” or re‐entrant signals.
Masking by object substitution is likely to involve neural regions that are distinct from those involved in object formation masking. However, less is known about which neural regions are associated with object substitution masking. Interestingly, one study reported decreased neural activations in the striate and extrastriate visual cortex with object substitution masking compared with object formation masking [Weidner et al., 2006], suggesting that different masking paradigms may show distinct patterns of activation in the visual cortex. On the other hand, a study of functional magnetic resonance (fMR) adaptation found that LO activation was modulated as a function of target visibility on 4‐dot masking [Carlson et al., 2007], similar to previous findings from object formation masking [Green et al., 2005, 2009].
We recently showed that schizophrenia patients have impaired performance on the 4‐Dot Masking task [Green et al., 2011b], indicating that impaired performance is present even when the masking effect does not involve feed‐forward processing. To extend this finding, this study was designed to examine neural correlates of masking by object substitution in schizophrenia. As in our previous studies on object formation masking (Green et al., 2009; Lee et al., 2010), we focused primarily on three key visual processing regions of interests (ROI): LO, hMT+ and early retinotopic cortex. In the 4‐Dot Masking Task, we varied the stimulus onset asynchronies (SOAs) between the onset of a target and the onset of a 4‐dot mask to create a range of masking effects (from weak to strong). We decided to vary SOAs instead of comparing a target without a mask with a target with a 4‐dot mask in order to examine the differences of the neural activity as a function of target visibility (i.e., masking effect) while keeping the visual input to the visual cortex constant. Consequently, the 4‐Dot Masking Task did not include trials without a 4‐dot mask. With this design, we addressed the following two research questions: (1) whether schizophrenia patients showed differential activation during the 4‐Dot Masking Task in the three key visual processing areas; and (2) whether group differences exist in brain regions outside the key visual processing areas were sensitive to masking by object substitution.
METHODS
Participants
Twenty‐three clinically stable outpatients with schizophrenia and 26 healthy controls participated in this study. Patients were recruited from outpatient clinics at the University of California, Los Angeles (UCLA), mental health clinics at the VA Greater Los Angeles Healthcare System (VAGLAHS) and local board and care facilities from the local community. Healthy control participants were recruited through website postings.
Diagnostic eligibility was confirmed with the Structured Clinical Interview for DSM‐IV (SCID) Axis I Disorders [First et al., 1997] for all participants and the SCID for Axis II disorders [First et al., 1996] for controls. Patients were included if they had: (1) a DSM‐IV diagnosis of schizophrenia, (2) no substance dependence in the last 6 months and no substance abuse in the past month, and (3) IQ > 70 based on review of medical records. Controls were included if they had (1) no history of schizophrenia, other psychotic disorders, recurrent major depressive disorder, substance dependence disorder, or substance abuse in the past month, (2) no family history of psychotic disorder among first‐degree relatives based on self‐report, and (3) none of the following Axis II disorders: avoidant, paranoid, schizoid, and schizotypal. Additional inclusion criteria for all participants were: no history of loss of consciousness for more than one hour due to head trauma, no significant neurological disorder, and sufficient fluency in English to understand the procedures based on clinician's judgment. Twenty patients were taking 2nd generation antipsychotic medications; one patient was taking both 1st generation and 2nd generation antipsychotic medications; and two were unmedicated at the time of testing. All participants had normal or corrected to normal vision (of at least 20/20).
All SCID interviewers were trained to a minimum kappa of 0.75 for key psychotic and mood items through the Treatment Unit of the Department of Veterans Affairs VISN 22 Mental Illness Research, Education, and Clinical Center (MIRECC). All participants were evaluated for the capacity to give informed consent and provided written informed consent after all procedures were fully explained, according to procedures approved by the Institutional Review Boards at UCLA and the VAGLAHS.
Procedures
All participants completed the 4‐Dot Masking task followed by three localizer tasks (retinotopic areas, hMT+, and LO) using MR‐compatible LCD goggles (Resonance Technology, Northridge, CA) in the MRI scanner. All tasks were presented using E‐prime software.
For the 4‐Dot Masking task, we employed a rapid event‐related design [Wager and Nichols, 2003]. Each trial started with two 100 ms flashes of a fixation point, followed by a 600‐ms blank period (Fig. 1). Then, a target array was presented for 33.3 ms, followed by a 66.6 ms mask at one of four possible SOAs: 0, 50, 100, 150 ms (SOA1, 2, 3, and 4, respectively). We reduced the number of SOAs in this study to reduce the total time in the scanner and the selection of these 4 SOAs was guided by our previous behavioral study on object substitution masking in schizophrenia [Green et al., 2011b]. The target array consisted of four squares, each with a gap on one of three sides (up, down, or left). The mask consisted of four dots that surround only one of four squares; it identified which of the four squares was the target on any given trial. Participants were instructed to identify the location of a gap in the target (up, bottom, or left) by pressing a corresponding button with their dominant hand. The only component that varied from trial to trial was the SOA between target and mask. The 4‐Dot masking task consisted of 6 runs, each with thirty 5‐s trials (i.e., 6 trials for each of the 4 SOAs and 6 null trials that included fixation but no stimuli).
Figure 1.
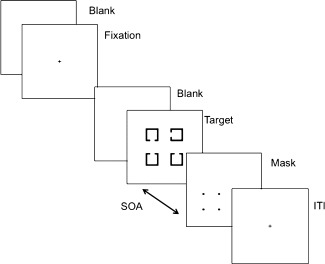
Schematic diagram of a single trial in the 4‐Dot Masking Task. The beginning of each visual backward masking trial was signaled by two 100 ms flashes of a fixation point, followed by a 600‐ms blank period. A target array of four squares, each with a gap on one of three sides, was presented for 33.3 ms, followed by a 66.6 ms mask at one of four possible SOAs: 0, 50, 100, 150 ms (SOA 1, 2, 3, and 4, respectively). The mask consisted of four dots that surrounded only one of four squares of the target array: it identified which of the four squares was the target on any given trial. Participants were asked to identify the location of a gap in the target (top, bottom, or left) by pressing a corresponding button with their dominant hand.
Three types of functional localization tasks were used: retinotopic areas, hMT+, and LO. Full descriptions of the three functional localizer tasks are provided elsewhere [Green et al., 2009; Lee et al., 2010] and are summarized briefly here. To identify retinotopic areas, participants viewed slowly rotating wedges of a contrast‐reversing checkerboard [Engel et al., 1997]. The wedge made five rotations, with one rotation every 30 s. For motion sensitive areas (hMT+) alternating blocked presentations of moving rings and stationary rings were presented, with each block presented for 15 s. There were five blocks each of moving and stationary rings. The LO localizer task consisted of alternating blocked presentations of pictures of abstract objects (i.e., sculptures) and scrambled pictures of objects, with each block containing 10 images presented for a total of 12.5 s [Grill‐Spector and Malach, 2004; Malach et al., 1995]. There were six blocks each of abstract objects and scrambled objects.
fMRI Data Acquisition
All scanning was conducted on a 3T Trio scanner (Siemens Trio, Erlangen, Germany) located in the UCLA Ahmanson Lovelace Brain Mapping Center. For anatomical reference, a high‐resolution echo planar axial T2‐weighted series was obtained for each subject prior to functional scanning (TR = 5,000 ms, TE = 30 ms, flip angle = 90°, 33 slices, FOV 22 cm). A T2*‐weighted gradient‐echo sequence was used to detect blood‐oxygen level‐dependent (BOLD) signal (TR = 2,000 ms, TE = 30 ms, flip angle = 75°, voxel size of 3.4 × 3.4 × 4.00 mm3), acquiring 33 slices parallel to the AC‐PC plane.
fMRI Data Analysis
Data were analyzed using the FMRIB Software Library [FSL, Smith et al., 2004]. The prestatistics processing included motion correction [Jenkinson et al., 2002], nonbrain removal [Smith, 2002], spatial smoothing using a Gaussian kernel of the full width at half maximum (FWHM) 5 mm, and high pass temporal filtering. To facilitate multisubject analyses, statistical images created for each subject were normalized into a standard space using Montreal Neurological Institute (MNI) coordinates. To examine neural activations associated with 4‐Dot Masking in schizophrenia, we approached the fMRI data analyses in two complementary ways: an ROI‐based approach and a whole brain analysis.
In the ROI analysis, we first identified the three key visual processing ROIs using the functional localizer tasks and then extracted response‐amplitude within each ROI during the 4‐Dot Masking Task. Retinotopic areas were defined as a group of contiguously activated voxels within the occipital cortex that were temporally correlated with a sinusoid at the stimulus frequency with a predefined threshold (P < 0.001, uncorrected) [Engel et al., 1997]. Area hMT+ was identified based on contiguously activated voxels within the occipital cortex bilaterally using the statistical parametric map of t‐values for the contrast of moving rings versus stationary rings with a predefined threshold (P < 0.001, uncorrected). LO was identified as a group of contiguously activated voxels within the lateral occipital cortex bilaterally (P < 0.001, uncorrected).
To extract response amplitude of 4‐Dot Masking, we modeled the hemodynamic response functions (HRFs) at each SOA during the 4‐Dot Masking task using 7 finite impulse response (FIR) functions, one for each peristimulus time point (total window = 14 s). With fewer assumptions about the exact shape of the HRF [Ollinger et al., 2001a,b], the FIR model makes it possible to selectively average each trial type for a fast‐event related design. After fitting the FIR function, response amplitudes were calculated by averaging event‐related responses across trials, separately for each SOA, within each ROI. These response amplitudes were then used in a repeated‐measure ANOVA (rmANOVA) with group as a between‐subject variable and time point and SOA as within‐subject factors.
For the whole‐brain analyses, we completed a first‐level analysis in which fMRI data for each SOA were convolved with a canonical HRF in a multiple regression analysis for each run and each subject. The main contrast of interest was SOA1 vs. SOAs 2, 3, and 4 (combined) because the 4‐Dot masking effect is typically weakest at SOA 1 and then performance dips and plateaus. In our previous behavioral study, we also did not observe differences among SOAs 2, 3, and 4 [Green et al., 2011b]. Six motion parameters were included as covariates of no interest to control for activation coming from motion artifact. For second‐level analysis, we averaged across the 6 runs for each subject using a fixed‐effects model in FLAME (FMRIB's Local Analysis of Mixed Effects) [Beckmann et al., 2003; Woolrich et al., 2004]. Finally for third‐level analysis, we performed a mixed‐effects model (FLAME 1+2) [Beckmann et al., 2003; Woolrich et al., 2004] to compare neural response between the two groups on contrasts of interest. We also examined neural activation pattern on the contrasts of interest within each group separately. The resulting statistical images were thresholded using the cluster threshold of z ≥ 2.3 and P ≤ 0.05, corrected for multiple comparisons using Gaussian random field theory [Worsley, 2001].
RESULTS
Three patients were excluded from analyses due to excessive movement artifacts. Therefore, 20 schizophrenia patients (4 females) and 26 healthy controls (7 females) were included in the analyses.
Demographic Information and Performance Data
Table 1 shows demographic characteristics and behavioral performance of the 4‐Dot Masking Task in the scanner. Schizophrenia patients and controls were comparable in terms of age, gender and parental education, but not personal education (personal education, t 44 = 2.20, P = 0.03). For the behavioral data in the 4‐Dot task, an rmANOVA with SOAs as a within‐group factor and group as a between‐group factor showed a significant main effect of SOA (F 3,132 = 17.10, P < 0.001). No other effect was significant. Post‐hoc analyses confirmed that both groups showed highest accuracy at the shortest SOA (SOA1) compared with other SOAs (SOA1 vs. SOA2, P < 0.001; SOA1 vs. SOA3 < p.001; and SOA1 < SOA4, P < 0.001). This pattern of performance is consistent with previous findings, including our own [Green et al., 2011b]. Thus, in all subsequent analyses, we combined the responses for SOAs 2, 3, and 4.
Table 1.
Demographic characteristics and behavioral performance of the 4‐Dot Masking Task
| Healthy controls | Schizophrenia patients | |
|---|---|---|
| Age | 41.1 (8.5) | 38.9 (11.2) |
| Parental Education (yrs.) | 14.1 (2.5) | 14.2 (3.1) |
| Personal Education (yrs.) | 14.3 (1.5) | 13.3 (1.7) |
| Gender (Female/Male) | 7/19 | 4 /16 |
| 4‐Dot Masking Taska | ||
| SOA1 | 0.52 (0.13) | 0.52 (0.16) |
| SOA2 | 0.42 (0.11) | 0.40 (0.11) |
| SOA3 | 0.40 (0.09) | 0.41 (0.12) |
| SOA4 | 0.46 (0.13) | 0.44 (0.14) |
*Values are presented as mean (standard deviation). The SOAs between the onset of a target and the onset of a 4‐dot mask were 0, 50, 100, and 150 ms for SOAs 1, 2, 3, and 4. The SOA234 indicates the combined responses for SOAs 2,3,4.
Percent accuracy of the 4‐Dot Masking Task.
ROI Analyses
Figure 2 presents the time series of percent signal change for each ROI during the 4‐Dot Masking Task. We conducted ROI analyses in two ways: (1) using percent signal change over 7 time points, and (2) and using the peak of the time series. For the time series, we examined whether the masking effect across SOAs modulated neural activations in the three ROIs using a rmANOVA with ROI (retinotopic areas, LO, hMT+), SOA (SOA1 versus SOA234), and time (7 time points) as within subject factors, and group as between‐subject factor. This analysis showed a significant main effect of time and a significant interaction of ROI by time (F 6,144 = 23.69, P < 0.001; and F 12,288 = 3.77, P < 0.001, respectively). No other effect was significant. To further understand ROI by time effect, post‐hoc analyses were conducted. At time point 4, both patients and controls showed lower activation in the retinotopic areas compared to LO and hMT+ (retinotopic vs. LO, P < 0.01; and retinotopic areas vs. hMT+, P < 0.05), which did not differ from each other (hMT+ vs. LO, P = 0.78).
Figure 2.
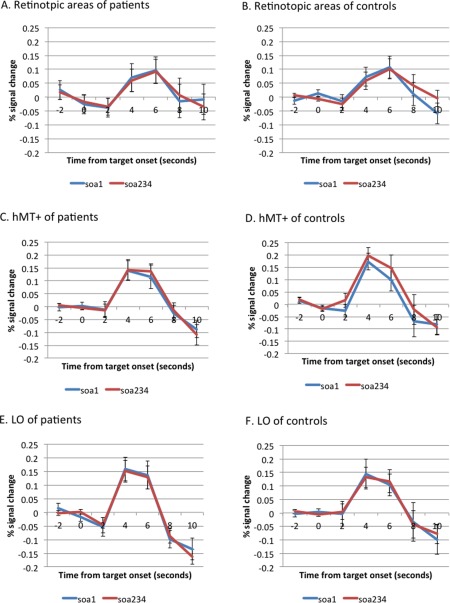
Time series of the visual processing regions of interest. These figures show the time series of percent signal change for each region of interest in schizophrenia patients (the left panel) and siblings (the right panel). The abscissa reflects the time since target onset and the ordinate indicates percent signal change. A (patients) and B (controls), retinotopic areas. C (patients) and D (controls), the human motion‐sensitive cortex (hMT+). E (patients) and F (controls), the lateral occipital complex (LO). Values are presented as mean (SE). The SOAs between the onset of a target and the onset of a 4‐dot mask were 0, 50, 100, and 150 ms for SOAs 1, 2, 3, and 4. The SOA234 indicates the combined responses for SOAs 2,3,4. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
For the peak amplitude of the time series, a rmANOVA with ROI and SOA as within‐subject factor and group as between‐subject factor showed a significant ROI by SOA interaction (F 2,48 = 3.19, P = 0.05) (Fig. 3). Both groups showed a higher peak for SOA1 vs. SOA234 in LO and retinotopic areas but a reversed pattern in the hMT+, but direct comparisons of SOA1 vs. SOA234 within each ROI were not significant. Finally, we explored the association between behavioral performance and peak amplitude for SOA1 and SOA234 within each ROI. The correlation between performance at SOA234 and peak amplitude of SOA234 in the hMT+ (r = 0.33, P < 0.05) was significant, indicating that higher accuracy at SOA234 was positively correlated with higher amplitude in the hMT+. There was no other significant correlation.
Figure 3.
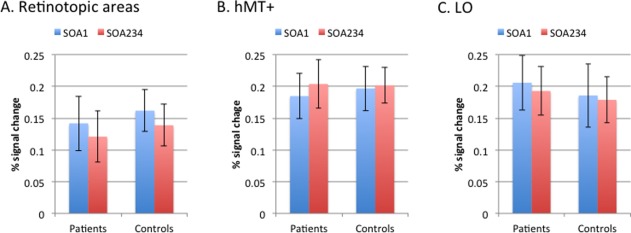
Peak amplitude of percent signal change for regions of interest. This figure shows the peak amplitude of percent signal change for both schizophrenia patients and controls separated by SOA. The left panel reflects retinotopic areas; the middle panel reflects the human motion‐sensitive cortex (hMT+); and the right panel reflects the lateral occipital complex. The SOAs between the onset of a target and the onset of a 4‐dot mask were 0, 50, 100, and 150 ms for SOAs 1, 2, 3, and 4. The SOA234 indicates the combined responses for SOAs 2,3,4. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Whole Brain Analyses
We examined brain areas outside the three visual ROIs that were modulated with masking by object substitution (that is, showed decreased activation in SOAs 234 versus SOA1). We found significantly increased activations in SOA1 in the bilateral middle occipital gyri when patients and controls were combined (x = 34, y = −86, z = 6, maximum z statistics = 4.48, 460 voxels; x = −38, y = −90, z = 4, maximum z statistics = 4.29, 397 voxels). Within the control group, we observed increased activation in the right middle occipital gyrus (x = 34, y = −88, z = −4, maximum z statistic = 3.4, 471 voxels; Fig. 4), and we observed no significantly activated areas in patients. Direct comparison between the two groups did not show any regions with significant activation difference.
Figure 4.
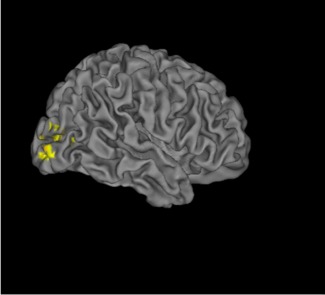
Whole brain analyses. This figure shows increased activations in the right middle occipital gyrus in controls for SOA1 vs. SOA234 in the whole brain analyses. The coordinates of the area are presented in the text. The SOAs between the onset of a target and the onset of a 4‐dot mask were 0, 50, 100, and 150 ms for SOAs 1, 2, 3, and 4. The SOA234 indicates the combined responses for SOAs 2,3,4.
DISCUSSION
This study examined the neural correlates of masking by object substitution in schizophrenia and healthy adults. During the 4‐Dot Masking Task, we found neural activations in LO and retinotopic areas were stronger when a mask and a target were simultaneously presented and the masking effect was weakest (i.e., SOA1). Thus, neural activity in LO and retinotopic areas was stronger when the target representation was more likely to reach visual awareness, and became weaker when target visibility was likely disrupted by re‐entrant processing. It should be noted that the effect was small and only seen with both groups combined. Further, we did not observe any significant group differences in LO and retinotopic areas during the 4‐Dot Masking Task. When examining neural regions in the whole brain analyses, we found increased activation in the right visual cortex in controls, but no significantly activated regions in patients. The absence of significant group difference in the whole brain analyses is further evidence that object substitution masking operates similarly in patients and controls at the neural level.
In this study, at the longer SOAs, the target visibility decreased and neural activity in LO became weaker. This finding is consistent with a recent study that examined object substitution masking with fMR adaptation and found a lack of adaptation in LO when the mask effectively interfered with the recognition of a target [Carlson et al., 2007]. It is also consistent with the results of our whole brain analysis in controls, in which visual cortex showed greater activity at shorter SOAs. Given the relationship between object substation masking and re‐entrant processing, these results collectively indicate that LO is likely to be involved in this type of visual processing.
Re‐entrant processing of visual information occurs on at least two different levels. One is early re‐entrant processing between striate and extrastriate cortex within the visual cortex [Haynes et al., 2005; Pascual‐Leone and Walsh, 2001]. The other is a later stage re‐entrant processing over longer distances between visual and higher brain regions (including frontal, parietal, and cingulate cortices) [Dehaene et al., 2006; Haynes et al., 2005; Lamme, 2003]. Although not tested in the current study, the modulation of LO and the retinotopic areas by object substitution masking in this study may be related to early re‐entrant processing.
Object substitution masking, such as 4‐Dot masking, is fundamentally different from object formation masking. For example, this study did not find blunted LO activation in patients during masking by object substitution masking, in contrast to our previous fMRI study of object formation masking in schizophrenia [Green et al., 2009]. In this study, LO activation in both groups was lower than what we previously observed using object formation masking. The reasons for the lower LO activation are not entirely clear, but they may be related to specific features of 4‐Dot Masking. The 4‐Dot Masking Task requires distributed spatial attention and this feature may have lowered overall LO activation. In fact, masking in the 4‐Dot Masking Task does not occur without distributed spatial attention, because the identity of a target needs to remain ambiguous until the mask appears. However, divided attention, as required in 4‐Dot Masking, is also known to reduce brain activation in the visual cortex [McMains and Somers, 2005; Scalf and Beck, 2010]. Aside from divided spatial attention, another possible reason for lower LO activation may be related to behavioral performance of both patients and controls in this study, which is lower than in our previous masking studies. Better object recognition has been associated with higher LO activation [Grill‐Spector et al., 2000]. Perhaps the representation of a target in the current 4‐dot masking study may have been weaker than with other types of masking, resulting in overall lower LO activation in both groups.
In the whole brain analyses, controls showed greater activation in an occipital area (including the middle occipital gyrus) when object substitution masking was weaker. This finding is consistent with previous studies showing that the middle occipital gyrus is related to object recognition [James et al., 2002; Ishai et al., 2000; Wilson and Farah, 2006]. One may wonder why this did not show up in the ROI analyses. We operationally defined LO as contiguously activated voxels that showed significant activation for abstract sculptures vs. scrambled objects. Thus, if other areas in the lateral occipital regions were activated but not part of contiguously activated voxels, these areas (e.g., the middle occipital gyrus) would not have been included in the defined LO.
There are several limitations to this study. Notably, we did not find any performance differences between schizophrenia and controls in this study and both patients and controls showed slightly lower performance at SOA 1 compared with what we observed in our behavioral study (Green et al., 2011b). We modified our behavioral 4‐Dot Making Task to make it more appropriate for the scanner, which may have contributed to the lack of performance differences. Possibly, due to these modifications, the change in performance across SOAs was somewhat smaller and performance at SOA1 was lower than we had seen outside the scanner in the behavioral study. Another factor that might have affected this lack of performance difference is 2nd generation antipsychotic medications that, as antagonizers to serotonic 2A receptors, can restore impaired visual processing [Kometer et al., 2013]. However, most of the patients in our previous study [Green et al., 2011b] who showed impaired masking performance were also taking 2nd generation antipsychotic medications. Thus, it is unclear whether 2nd generation antipsychotic medication could explain the lack of performance difference between groups in this study. Additionally, to assess neural activations related to object substitution masking, we varied SOAs between a target and a mask instead of comparing a target with and without a 4‐dot mask. Thus, while it was possible to examine how target visibility modulates neural activity in the presence of a 4‐dot mask, it was not possible to examine how the 4‐dot mask affects neural responses in the brain. Finally, although the sample size of this study was comparable with those in our previous studies in schizophrenia [Green et al., 2009; Lee et al., 2010], a relatively small sample size might have made it difficult to detect subtle differences.
Overall, this study did not find evidence for neural abnormalities of object substitution masking in clinically stable outpatients with schizophrenia, in contrast to our previous fMRI study of object formation masking [Green et al., 2009]. Neural substrates of object substitution masking in schizophrenia may not be as compromised as those involved in the more common masking methods that rely on disruption of object formation. Further studies will be able to determine whether the current finding on neural substrates of masking by object substitution can be observed in patients with recent‐onset schizophrenia or individuals at risk for schizophrenia.
ACKNOWLEDGMENT
The authors would like to thank Poorang Nori and Mark McGee for assistance in data collection.
REFERENCES
- Beckmann CF, Jenkinson M, Smith SM (2003): General multilevel linear modeling for group analysis in fMRI. NeuroImage 20:1052–1063. [DOI] [PubMed] [Google Scholar]
- Breitmeyer BG, Ogmen H (2000): Recent models and findings in visual backward masking: A comparison, review, and update. Perception Psychophys 62:1572–1595. [DOI] [PubMed] [Google Scholar]
- Carlson TA, Rauschenberger R, Verstraten FA (2007): No representation without awareness in the lateral occipital cortex. Psychol Sci 18:298–302. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Changeux JP, Naccache L, Sackur J, Sergent C (2006): Conscious, preconscious, and subliminal processing: A testable taxonomy. Trends Cogn Sci 10:204–211. [DOI] [PubMed] [Google Scholar]
- Di Lollo V, Enns JT, Rensink RA (2000): Competition for consciousness among visual events: The psychophysics of reentrant visual processes. J Exp Psychol General 129:481–507. [DOI] [PubMed] [Google Scholar]
- Dux PE, Visser TA, Goodhew SC, Lipp OV (2010): Delayed reentrant processing impairs visual awareness: an object‐substitution‐masking study. Psychol Sci 21:1242–1247. [DOI] [PubMed] [Google Scholar]
- Engel SA, Glover GH, Wandell BA (1997): Retinotopic organization in human visual cortex and the spatial precision of functional MRI. Cerebral Cortex 7:181–192. [DOI] [PubMed] [Google Scholar]
- Enns JT (2004): Object substitution and its relation to other forms of visual masking. Vis Res 44:1321–1331. [DOI] [PubMed] [Google Scholar]
- Enns JT, Di Lollo V (2000): What's new in visual masking? Trends Cogn Sci 4:345–352. [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW, Benjamin LS (1996): Structured Clinical Interview for DSM‐IV Axis II Personality Disorders. Washington, D.C: American Psychiatric Press, Inc. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW (1997): Structured Clinical Interview for DSM‐IV Axis I Disorders—Patient Edition. Washington, D.C: American Psychiatric Press, Inc. [Google Scholar]
- Goodhew SC, Pratt J, Dux PE, Ferber S (2013): Substituting objects from consciousness: A review of object substitution masking. Psychon Bull Rev 20:859–877. [DOI] [PubMed] [Google Scholar]
- Green MF, Glahn D, Engel SA, Nuechterlein KH, Sabb F, Strojwas M, Cohen MS (2005): Regional brain activity associated with visual backward masking. J Cogn Neurosci 17:13–23. [DOI] [PubMed] [Google Scholar]
- Green MF, Lee J, Cohen MS, Engel SA, Korb AS, Nuechterlein KH, Wynn JK, Glahn DC (2009): Functional neuroanatomy of visual masking deficits in schizophrenia. Arch Gen Psychiatry 66:1295–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, Lee J, Wynn JK, Mathis KI (2011a): Visual masking in schizophrenia: Overview and implications for theories of aberrant visual processing. Schizophrenia Bull 37:700–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, Wynn JK, Breitmeyer B, Mathis KI, Nuechterlein KH (2011b): Visual masking by object substitution in schizophrenia. Psychol Med 41:1489–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill‐Spector K, Kushnir T, Hendler T, Malach R (2000): The dynamics of object‐selective activation correlate with recognition performance in humans. Nat Neurosci 3:837–843. [DOI] [PubMed] [Google Scholar]
- Grill‐Spector K, Malach R (2004): The human visual cortex. Annual Rev Neurosci 27:649–677. [DOI] [PubMed] [Google Scholar]
- Haynes JD, Driver J, Rees G (2005): Visibility reflects dynamic changes of effective connectivity between V1 and fusiform cortex. Neuron 46:811–821. [DOI] [PubMed] [Google Scholar]
- Ishai A, Ungerleider LG, Martin A, Haxby JV (2000): The representation of objects in the human occipital and temporal cortex. J Cogn Neurosci 12:35–51. [DOI] [PubMed] [Google Scholar]
- James TW, Humphrey GK, Gati JS, Servos P, Menon RS, Goodale MA (2002): Haptic study of three‐dimensional objects activates extrastriate visual areas. Neuropsychologia 40:1706–1714. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S (2002): Improved optimisation for the robust and accurate linear registration and motion correction of brain images. NeuroImage 17:825–841. [DOI] [PubMed] [Google Scholar]
- Kometer M, Schmidt A, Jancke L, Vollenweider FX (2013): Activation of serotonin 2A receptors underlies the psilocybin‐induced effects on alpha oscillations, N170 visual‐evoked potentials, and visual hallucinations. J Neurosci 33:10544–10551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamme VA (2003): Why visual attention and awareness are different. Trends Cogn Sci 7:12–18. [DOI] [PubMed] [Google Scholar]
- Lee J, Cohen MS, Engel SA, Glahn D, Nuechterlein KH, Wynn JK, Green MF (2010): Regional brain activity during early visual perception in unaffected siblings of schizophrenia patients. Biol Psychiatry 68:78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malach R, Reppas JB, Benson RR, Kwong KK, Jiang H, Kennedy WA, Ledden PJ, Brady TJ, Rosen BR, Tootell RB (1995): Object‐related activity revealed by functional magnetic resonance imaging in human occipital cortex. Proc Natl Acad Sci USA 92:8135–8139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMains SA, Somers DC (2005): Processing efficiency of divided spatial attention mechanisms in human visual cortex. J Neurosci 25:9444–9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollinger JM, Corbetta M, Shulman GL (2001a): Separating processes within a trial in event‐related functional MRI. NeuroImage 13:218–229. [DOI] [PubMed] [Google Scholar]
- Ollinger JM, Shulman GL, Corbetta M (2001b): Separating processes within a trial in event‐related functional MRI. NeuroImage 13:210–217. [DOI] [PubMed] [Google Scholar]
- Pascual‐Leone A, Walsh V (2001): Fast backprojections from the motion to the primary visual area necessary for visual awareness. Science 292:510–512. [DOI] [PubMed] [Google Scholar]
- Scalf PE, Beck DM (2010): Competition in visual cortex impedes attention to multiple items. J Neurosci 30:161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM (2002): Fast robust automated brain extraction. Hum Brain Mapp 17:143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen‐Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM (2004): Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage 23:S208–S219. [DOI] [PubMed] [Google Scholar]
- Wager TD, Nichols TE (2003): Optimization of experimental design in fMRI: A general framework using a genetic algorithm. NeuroImage 18:293–309. [DOI] [PubMed] [Google Scholar]
- Weidner R, Shah NJ, Fink GR (2006): The neural basis of perceptual hypothesis generation and testing. J Cogn Neurosci 18:258–266. [DOI] [PubMed] [Google Scholar]
- Wilson KD, Farah MJ (2006): Distinct patterns of viewpoint‐dependent BOLD activity during common‐object recognition and mental rotation. Perception 35:1351–1366. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Behrens TE, Beckmann CF, Jenkinson M, Smith SM (2004): Multi‐level linear modeling for fMRI group analysis using Bayesian inference. NeuroImage 21:1732–1747. [DOI] [PubMed] [Google Scholar]
- Worsley KJ (2001): Statistical analysis of activation images In: Jezzard P, et al., editors. Functional MRI: An Introduction to Methods. USA: Oxford University Press. [Google Scholar]


