Abstract
Objectives
To evaluate the hypothesis that chronic lung disease of prematurity (CLD) is a risk factor for asthma in children born extremely preterm, and the hypothesis that the risk factors for CLD are similar to those for asthma.
Methods
A retrospective analysis was performed using data collected prospectively from 882 children born before the 28th week of gestation between 2002–2004 who returned for follow-up at ages 12 and 24 months and 10 years. We created time-oriented logistic regression models to compare risk factors for CLD, defined as need for supplemental oxygen at 36 weeks postmenstrual age, and parent-reported asthma at 10 years of age.
Results
CLD diagnosed during neonatal admission was associated with bronchodilator use at 12 months and 24 months (p <0.001), but not with an asthma diagnosis at 10 years (Odds Ratio 1.3; 95% confidence interval 0.98–1.8). While risk factors for CLD include lower gestational age (OR 2.7; 1.5–4.7) and fetal growth restriction (OR 2.3; 1.4–3.7), risk factors for asthma include mother’s eligibility for public insurance (Medicaid) (OR 1.8; 1.1–2.8), and higher weight gain velocity during the first year (OR 1.5; 1.02–2.2) and between the 2nd and 10th year (OR 1.7; 1.2–2.4).
Conclusions
Among children born extremely preterm, the diagnosis of CLD and its antecedents were associated with transient preschool wheezing, but not with asthma. Post-NICU factors, such as growth velocity and socioeconomic disadvantage, appear to have stronger associations with asthma than exposures during NICU admission.
Keywords: Asthma and Early Wheeze, Bronchopulmonary dysplasia, Epidemiology, Extremely Low Birth Weight Infants, Socioeconomic Factors
Introduction
Infants born extremely preterm appear to be at increased risk of chronic lung disease of prematurity (CLD; also called bronchopulmonary dysplasia) during infancy, and also at increased risk of recurrent wheezing and asthma during childhood.1,2 CLD is defined as the need for supplemental oxygen at 36 weeks postmenstrual age, and as severe CLD if positive pressure is also required.3 Asthma is characterized by airway inflammation and reversible bronchospasm responsive to bronchodilator therapy. The frequency of CLD in extremely low gestational age newborns (ELGANs) has remained high (approximately 45%) for the past two decades; the frequency of asthma in ELGANs is not well characterized.4
Despite variations in methods, most studies suggest that CLD is associated with an increased risk of asthma later in life.5–14 However, whether airway reactivity is a result of lung injury sustained in the neonatal intensive care unit (NICU) or is due to intrinsic susceptibilities related to prematurity is not clear. Furthermore, environmental exposures following discharge from the NICU likely also contribute to airway reactivity in childhood.15
The objective of this study was to evaluate the hypothesis that CLD is a risk factor for childhood asthma, and the hypothesis that the risk factors for CLD are similar to the risk factors for asthma. The ELGAN Study provides a large sample of children born extremely preterm, with follow up at 12 and 24 months to obtain parent-reported data about use of bronchodilator therapy, and at 10 years to obtain parent-reported data about the use of bronchodilator therapy and asthma diagnosis. This allowed us to see if CLD and parent-report asthma shared risk factors. We also evaluated to what extent CLD is an antecedent of asthma.
Materials and Methods
The sample population
The ELGAN Study is a multi-center prospective, observational study of the risk of structural and functional neurologic disorders in children born before the 28th week of gestation between 2002 and 2004.16 Previous studies in this cohort have described early patterns of respiratory disease and identified fetal growth restriction as a risk factor for CLD.17–19 The families of all survivors at 12 and 24 months of age (n=1198) were contacted by mail or phone and invited to participate in follow-up assessments. At 10 years of age, we selected a subset (n=966) for further follow-up visits because we had measurements of inflammation-related proteins in their neonatal blood. Ninety-one percent of the children (n=882) returned for a follow-up evaluation (Figure). Enrollment and consent procedures for the ELGAN Study were approved by the institutional review boards of all participating institutions.
Figure 1. Description of study sample.
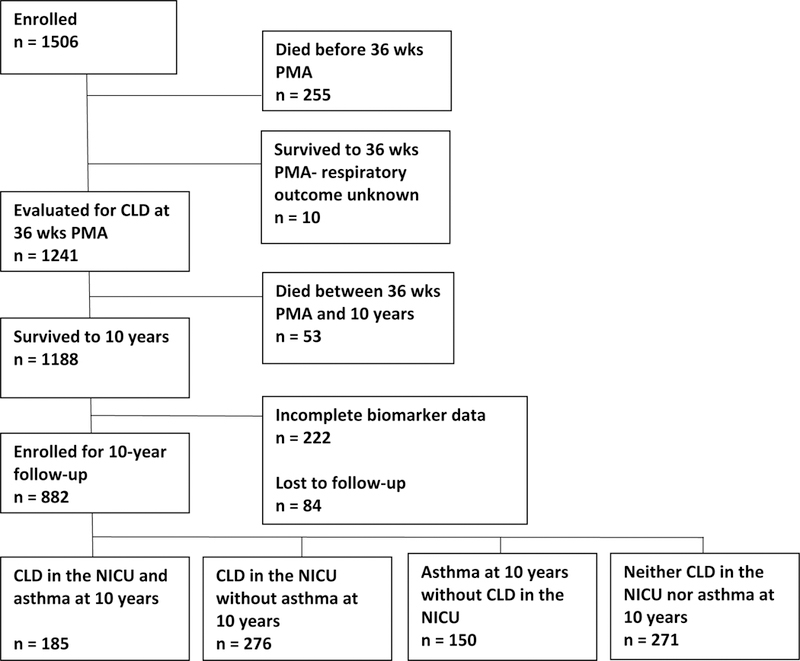
Abbreviations: PMA: postmenstrual age; CLD: chronic lung disease; NICU: neonatal intensive care unit
Data collection
We collected information on pregnancy characteristics, maternal demographics, and newborn characteristics including exposures and diagnoses during hospitalization. We recorded the form of ventilation assistance (e.g. none, increased ambient oxygen, nasal cannula, nasal continuous positive airway pressure, conventional mechanical ventilation, or high frequency ventilation) the child was receiving at 36 weeks post-menstrual age (PMA). 20 We defined chronic lung disease (CLD) as supplemental oxygen therapy with or without mechanical ventilation at 36 weeks PMA. Infants with CLD were subdivided into those who were dependent on mechanical ventilation and those who were not dependent on mechanical ventilation at the time of diagnosis. At the 12 month and 24 month visits, the parent or caregiver completed questionnaires that included whether their child was currently taking bronchodilator medication regularly or intermittently (yes/no). At the 10-year visit, the parent or caregiver completed questionnaires that asked whether their child has ever been given an asthma diagnosis by a health care provider since age 2 years. If the answer to this question was “yes”, we categorized the child as having asthma. We also inquired whether their child is currently taking a bronchodilator medication. The outcome of asthma, therefore, was based entirely on the results of the questionnaire and responses were not validated by a review of the child’s medical record. Maternal socioeconomic factors were obtained independently at each visit, including marital status, eligibility for public insurance (Medicaid), and highest level of education completed, as well as the presence of tobacco smokers at home. Growth parameters were obtained and used to calculate weight gain velocity (kilograms/day) by sex from the time of birth to 12 months, from 12 months to 24 months, and from 24 months to 10 years.
Data analysis
We evaluated the null hypothesis that the risk of asthma at age 10 years is not associated with any demographic, pregnancy, or perinatal characteristic. We created univariable analyses to search for potential confounders and effect modifiers. We followed the guidance that a variable should be considered a potential confounder if its associations with CLD and asthma have p values of < 0.25.21 Causal diagrams (also called directed acyclic graphs) were created using the web-based software DAGitty (www.dagitty.net) to delineate the relationships among the multiple variables using extreme prematurity as the exposure and chronic lung disease and asthma, separately and in aggregate, as outcomes‥22 (E-figures 1-3)
We performed a retrospective analysis using multivariable logistic regression models in which risk factors are ordered in a temporal pattern, so that the earliest occurring predictors/covariates of, first, CLD in the NICU and, second, asthma at age 10 years, are entered first and are not displaced by later occurring covariates. For these time-oriented logistic regression models (TORMs), we categorized sets of antecedents/covariates by the time they occur, or are identified. 20,23 We grouped demographic and pregnancy variables into the socioeconomic status(SES)/pregnancy epoch, delivery characteristics and exposures into the delivery epoch, all exposures and characteristics in the first week of life into the early neonatal epoch, and exposures and characteristics occurring or reported prior to discharge from the NICU into the late neonatal epoch. We created a separate model for each epoch that added variables for that period to the variables from previous epochs that contributed information about the risk of asthma. A final TORM was then created for CLD and asthma at 10 years of age using the same variables from the shared time periods (pregnancy through late neonatal epochs) to compare risk factors for each outcome. We used a step down procedure seeking a parsimonious solution without interaction terms. These models allowed us to calculate odds ratios and 95% confidence intervals. Finally, we created a TORM for asthma at 10 years of age with the addition of three post-NICU epochs, using variables collected at the 12-month, 24-month, and 10-year follow-up visits.
Results
The maternal and infant characteristics of the sample enrolled at birth (n = 1506), enrolled at 1–2 years (n = 1198), and enrolled at 10 years (n = 882) are listed in Table 1. Of the 882 children enrolled at 10 years, 461 developed CLD (52%) and 335 (38%) were reported by the parent/caregiver to have been given a diagnosis of asthma at any time since the follow-up visit at 24 months. CLD was associated with bronchodilator use at ages 12 and 24 months, but not at age 10 years. Bronchodilator use at all 3 ages (i.e., 12 and 24 months and 10 years), however, was associated with asthma at age 10 years. The results of the univariable analyses are included in E-tables 1–9.
Table 1:
Characteristics of the mothers and children at the child’s birth.
| Sample, percent (N) | ||||
|---|---|---|---|---|
| Enrolled at birth (n=1506) |
Enrolled at 1–2 years (n=1198) |
Enrolled at 10 years (N=882) |
||
| Maternal characteristics | ||||
| Race | White | 59 (874) | 60 (714) | 63 (558) |
| Black | 29 (427) | 27 (322) | 26 (226) | |
| Other | 13 (187) | 13 (151) | 11 (96) | |
| Hispanic ethnicity | Yes | 12 (179) | 12 (147) | 10 (86) |
| Age, years | < 21 | 14 (218) | 14 (170) | 19 (113) |
| 21–35 | 68 (1017) | 67 (802) | 67 (591) | |
| >35 | 18 (269) | 19 (226) | 20 (178) | |
| Education | ≤ 12 | 45 (645) | 44 (518) | 41 (363) |
| 13–15 | 24 (348) | 24 (278) | 23 (209) | |
| ≥ 16 | 40 (434) | 32 (382) | 35 (310) | |
| Single marital status | Yes | 44 (658) | 44 (413) | 39 (348) |
| Public (Medicaid) insurance | Yes | 41 (594) | 40 (464) | 35 (302) |
| Smoked during pregnancy | Yes | 15 (212) | 14 (162) | 14 (118) |
| Infant characteristics | ||||
| Cesarean delivery | Yes | 65 (977) | 66 (795) | 66 (563) |
| Antenatal steroid course | Complete | 62 (937) | 64 (764) | 63 (556) |
| Partial | 27 (398) | 26 (309) | 26 (226) | |
| None | 11 (166) | 10 (122) | 11 (99) | |
| Initiator of preterm delivery | Preterm labor | 44 (665) | 45 (534) | 46 (405) |
| pPROM | 22 (327) | 22 (262) | 22 (191) | |
| Pre-eclampsia | 13 (197) | 13 (153) | 13 (113) | |
| Abruption | 10 (153) | 11 (128) | 10 (92) | |
| Cervical insufficiency | 6 (90) | 6 (72) | 5 (45) | |
| Fetal indication | 5 (74) | 4 (49) | 4 (36) | |
| Gestational age, weeks | 23–24 | 27 (409) | 20 (245) | 21 (186) |
| 25–26 | 44 (661) | 46 (553) | 45 (398) | |
| 27 | 29 (436) | 33 (400) | 34 (298) | |
| Birth weight, grams | ≤ 750 | 44 (660) | 36 (426) | 38 (331) |
| 751–1000 | 39 (586) | 43 (520) | 43 (377) | |
| >1000 | 17 (260) | 20 (242) | 10 (174) | |
| Birth weight Z-score | < −2 | 7 (110) | 5 (62) | 6 (53) |
| ≥ −2, < −1 | 14 (211) | 13 (153) | 13 (119) | |
| ≥ −1 | 79 (1185) | 82 (983) | 81 719) | |
| Male | Yes | 53 (799) | 52 (621) | 51 (452) |
| Singleton | Yes | 67 (1002) | 67 (805) | 65 (571) |
Time-Oriented Regression Models (Tables 2 and 3)
Table 2.
Time-oriented risk model (TORM) for chronic lung disease (CLD)
| Odds Ratios (95% Confidence Intervals) | ||||
|---|---|---|---|---|
| SES*/Pregnancy | Delivery | Early neonatal | Late neonatal | |
| Epoch 1: SES*/Pregnancy | ||||
| Trying to get pregnant | 1.4 (1.04, 1.8) | 1.3 (0.99, 1.7) | 1.5 (1.1, 2.1) | 1.6 (1.2, 2.3) |
| Increased syncytial knots | 1.7 (1.2, 2.4) | 1.5 (1.01, 2.1) | 1.6 (1.03, 2.4) | 1.5 (0.97, 2.4) |
| Epoch 2: Delivery | ||||
| No labor | 1.4 (1.02, 2.0) | 1.2 (0.8, 1.7) | 1.1 (0.7, 1.7) | |
| Maternal fever > 101.4F | 3.8 (1.9, 7.9) | 2.6 (1.2, 5.8) | 2.6 (1.1, 6.1) | |
| Epoch 3: Early neonatal | ||||
| Gestational age 23–24 weeks | 6.0 (3.7, 10) | 2.7 (1.5, 4.7) | ||
| Gestational age 25–26 weeks | 2.0 (1.4, 2.8) | 1.4 (0.9, 2.0) | ||
| Birth weight Z-score < −1 | 2.6 (1.6, 3.9) | 2.3 (1.4, 3.7) | ||
| SNAPII† 20–29 | 1.9 (1.3, 2.8) | 1.6 (1.1, 2.4) | ||
| SNAPII† ≥ 30 | 2.7 (1.8, 4.2) | 1.8 (1.1, 2.8) | ||
| Epoch 4: Late neonatal | ||||
| Any treatment for PDA | 1.8 (1.3, 2.6) | |||
| Transfusion in 3 of 4 weeks | 1.9 (1.3, 2.8) | |||
| Antibiotics weeks 2–4 | 1.6 (1.05, 2.5) | |||
| EPPD‡ | 1.6 (1.1, 2.2) | |||
| PIE§ | 2.3 (1.4, 3.9) | |||
Socioeconomic Status
Score for Neonatal Acute Physiology-II
Early Persistent Pulmonary Dysfunction
Pulmonary Interstitial Emphysema
Table 3.
Time-oriented risk model (TORM) for asthma
| Odds Ratios (95% Confidence Intervals) | |||||||
|---|---|---|---|---|---|---|---|
| SES*/ Pregnancy |
Delivery | Early neonatal |
Late neonatal |
12 months | 24 months | 10 years | |
| Epoch 1: SES*/Pregnancy | |||||||
| Race other than white | 1.5 (1.1, 2.0) | 1.5 (1.1, 2.0) | 1.5 (1.1, 2.0) | 1.5 (1.1, 2.0) | 1.4 (0.97, 1.9) | 1.3 (0.9, 1.9) | 1.3 (0.9, 1.8) |
| Public (Medicaid) insurance | 1.9 (1.4, 2.6) | 1.9 (1.4, 2.6) | 1.9 (1.4, 2.7) | 1.9 (1.4, 2.7) | 1.9 (1.3, 2.9) | 1.9 (1.3, 3.0) | 1.7 (1.1, 2.7) |
| First trimester bleeding | 1.4 (1.1, 1.9) | 1.4 (1.1, 1.9) | 1.4 (1.1, 1.9) | 1.4 (1.1, 1.9) | 1.6 (1.2, 2.2) | 1.7 (1.3, 2.4) | 1.7 (1.2, 2.4) |
| Decidual hemorrhage | 1.6 (1.1, 2.3) | 1.6 (1.1, 2.3) | 1.7 (1.1, 2.5) | 1.7 (1.1, 2.5) | 1.7 (1.1, 2.5) | 1.5 (1.02, 2.4) | 1.4 (0.9, 2.2) |
| Epoch 2: Delivery | |||||||
| No variables added | ----- | ----- | ----- | ----- | ----- | ----- | |
| Epoch 3: Early neonatal | |||||||
| Definite tracheal colonization | 0.6 (0.4, 0.9) | 0.6 (0.4, 0.9) | 0.6 (0.4, 0.9) | 0.6 (0.4, 0.9) | 0.6 (0.4, 0.9) | ||
| Epoch 4: Late neonatal | |||||||
| Chronic lung disease | 1.3 (0.98, 1.8) | ----- | ----- | ----- | |||
| Epoch 5: 12 Months | |||||||
| 3rd quartile WV† (0–12 mons) | 1.6 (1.1, 2.3) | 1.7 (1.2, 2.6) | 1.6 (1.1, 2.4) | ||||
| 4rd quartile WV† (0–12 mons) | 1.7 (1.2, 2.4) | 1.7 (1.2, 2.5) | 1.5 (1.02, 2.2) | ||||
| Public (Medicaid) insurance | 1.5 (1.1, 2.3) | 1.1 (0.7, 1.8) | 1.0 (0.6, 1.6) | ||||
| Mother is single | 0.7 (0.4, 0.99) | 1.6 (0.7, 3.6) | 1.4 (0.6, 3.1) | ||||
| Epoch 6: 24 Months | |||||||
| Public (Medicaid) insurance | 1.9 (1.1, 3.2) | 1.6 (0.9, 2.8) | |||||
| Mother is single | 0.3 (0.1, 0.7) | 0.3 (0.1, 0.7) | |||||
| Epoch 7: 10 years | |||||||
| 4rd quartile WV† (2–10 yrs) | 1.7 (1.2, 2.4) | ||||||
| Public (Medicaid) insurance | 1.8 (1.1, 2.8) | ||||||
Socioeconomic Status
Weight Gain Velocity, g/kg/day
In the TORM for CLD (Table 2), the predominant risk factors for CLD are indicators/correlates of low gestational age, including maternal fever during labor (Odds Ratio 2.6; 95% confidence interval 1.1–6.1), gestational age 23–24 weeks (OR 2.7; 1.5–4.7), birth weight Z-score < −1 (OR 2.3; 1.4–3.7), and a diagnosis of pulmonary interstitial emphysema (OR 2.3; 1.4–3.9).
In the TORM for asthma (Table 3), variables related to low SES, including public insurance eligibility at the time of delivery (OR 1.7; 1.1–2.7) and at 10 years (OR 1.8; 1.1–2.8), are the predominant predictors of asthma. In the early neonatal epoch, definite tracheal colonization is associated with a lower odds of childhood asthma (OR 0.6; 0.4–0.9). When CLD is added to the final TORM, the CLD association with asthma is not significant (OR 1.3; 0.98–1.8). In the final model (last column of Table 3), the top two quartiles of weight gain velocity during the first year (OR 1.6; 1.1–2.4 and OR 1.5; 1.02–2.2) and the top quartile between the 2nd and 10th year (OR 1.7; 1.2–2.4) are associated with increased risk of asthma at age 10 years.
Discussion
In this prospective cohort study of 882 infants born before the 28th week of gestation, we found that the risk factors for CLD are not the risk factors of parent-reported asthma, and that CLD is not a risk factor for childhood asthma. Risk factors for CLD include maternal fever, lower gestational age and birth weight Z-score, physiologic instability in the first 12 hours, and a variety of neonatal complications, such as pulmonary interstitial emphysema, whereas indicators of lower socioeconomic status and higher weight gain velocity at 12 months and 10 years are risk factors for asthma. As a whole, these findings suggest that, at least in the 21st century, the long-term pulmonary outcome of individuals born extremely premature is influenced more by socioeconomic resources available to the family than by neonatal exposures.
Our finding of an association between maternal eligibility for public insurance (Medicaid) and asthma is consistent with several studies identifying low socioeconomic status as a risk factor for asthma in children born at term.24–26 Several mechanisms for this association have been proposed, including higher prevalence of obesity and increased exposure to indoor allergens and environmental pollutants in children from families with low socioeconomic status.27,28 Furthermore, a longitudinal study of household socioeconomic status found a protective effect against asthma among children in families with upward social mobility.29
We did not find a statistically-significant association between maternal smoking or passive smoke exposure and childhood asthma at 10 years. In a population-based cohort study of late preterm infants (34 to 36 6/7 weeks gestational age), maternal smoking was identified as an environmental exposure which accounted for the association of late prematurity and childhood asthma.30 Maternal smoking was previously found to be associated with an increased risk of prelabor premature rupture of membranes and placental abruption in the ELGAN cohort.31 Consequently, the relationship between maternal smoking and asthma in our population might have been confounded by the association of maternal smoking with premature birth. Because cigarette smoking is associated with lower social class, the lack of a direct association between maternal exposure to tobacco smoke and asthma risk in our sample might reflect nothing more than total greater effects of the variables conveying low socioeconomic status already in the TORM.32 In other words, maternal smoking may not confer additional risk to the development of asthma when other parameters of socioeconomic status, such as race/ethnicity and eligibility for public insurance (Medicaid), are accounted for in the model. Additionally, all of the infants in our sample were born extremely preterm, while previous studies examining the risk of maternal smoking in pregnancy and later childhood wheezing have almost exclusively included late preterm or term infants.30,33–35 It is possible that the contribution of prenatal tobacco exposure to subsequent wheezing is weaker in the extremely premature population. We found that higher weight gain velocity during the first postnatal year and between 2 and 10 years of age were associated with a higher prevalence of asthma. Our findings are consistent with a meta-analysis of 31 European cohort studies that identified that a higher infant weight gain in premature infants was associated with preschool wheezing and childhood asthma.36 Obesity during puberty has been linked to increased growth velocity in the first years of life 37 and obesity is associated with childhood asthma.38–40 A proposed mechanism for this association is the release of immunomodulatory cytokines from adipose tissue leading to airway hyper-responsiveness or through mechanical restriction of the chest wall, however the association between asthma and obesity is often confounded by deconditioning.39
We found that tracheal colonization in the neonatal period was associated with a decreased risk of asthma. The relationship between lung and gut microbiota and childhood asthma appears to be complex.41–45 Nevertheless, our results can be viewed as lending support to the hypothesis that reduced exposure to bacteria in early life may increase the risk of asthma and other atopic diseases.46
In our study, the diagnosis of asthma was based on the results of questionnaires completed by parents. While parental report of asthma is commonly used for epidemiologic studies, use of this is potentially subject to recall bias. A recent case-control study of 203 children ages 9–12 born at term found that 45% of children with parent-reported asthma were over-diagnosed based on more rigorous diagnostic criteria using spirometry, methacholine challenge, and allergy skin prick testing.47 However, children included in the sample were from a predominantly high socioeconomic strata, whereas low socioeconomic status has been associated with an under-diagnosis of asthma.48 While parent-reported asthma might overestimate the true frequency of asthma in our cohort, the resulting measurement bias is more likely to lead to rejection of the null hypothesis (i.e. in favor of finding an association between CLD and asthma) since parents of infants with existing lung disease might be more likely to over-report symptoms.
In an individual participant data meta-analysis for 147,252 children enrolled in 31 birth cohort studies, younger gestational age at birth was associated with preschool wheezing (1–4 years) and school-age asthma (5–10 years).36 Yet that is not what we found. The discrepancy might reflect our narrow gestational age range (23–27 weeks) and therefore the inability to compare extremely preterm to term deliveries. Another possible explanation is that our time-oriented models begin with prenatal variables. In our model of asthma, the first risk factor is “race other than white,” which conveys information about preterm delivery.49 The second risk factor is eligibility for “public insurance (Medicaid),” which also conveys information about the risk of preterm delivery.50 Consequently, by introducing these variables to the time-oriented regression model before any pregnancy or delivery variable, which we consider appropriate, we diminish the opportunity to identify low gestational age as an asthma risk factor. Additionally, our findings of an association between CLD and bronchodilator use at 12 and 24 months, which may be a marker of preschool wheezing, but not CLD and childhood asthma lends supports that early transient wheezing and asthma in school-age children are distinct entities with unique risk factors.
The strengths and weaknesses of our study are those inherent in the observational nature of the study design. The large number of infants in our sample allowed us to control for a number of confounding variables in the prenatal, early neonatal, and late neonatal epochs with sufficient statistical power. However, the variables collected in the post-NICU epochs were less robust and were limited by the subjectivity of parental reporting. In addition, while we had a relatively high percentage of follow-up for a cohort study of this size (74% of survivors), we do not know the prevalence of asthma in the children not followed.
In conclusion, we found that while CLD was associated with bronchodilator use at 12- and 24-months, CLD was not associated with parent-reported asthma diagnosis at 10 years of age in the ELGAN cohort. The strongest predictors for childhood asthma in our cohort were eligibility for public insurance (Medicaid) and increased weight gain velocity after the neonatal period. Our findings suggest that post-NICU factors, such as growth velocity and socioeconomic disadvantage, could be more important contributors than neonatal complications to the development of asthma in infants born extremely premature.
Supplementary Material
Acknowledgements:
The authors gratefully acknowledge the contributions of their subjects, and their subjects’ families, as well as those of their colleagues: Janice Ware, Taryn Coster, Brandi Henson, Rachel Wilson, Kirsten McGhee, Patricia Lee, Aimee Asgarian, Anjali Sadhwani, Karl Kuban, Ellen Perrin, Emily Neger, Kathryn Mattern, Jenifer Walkowiak, Susan Barron, Jean Frazier, Lauren Venuti, Beth Powers, Ann Foley, Brian Dessureau, Molly Wood, Jill Damon-Minow, Richard Ehrenkranz, Jennifer Benjamin, Elaine Romano, Kathy Tsatsanis, Katarzyna Chawarska, Sophy Kim, Susan Dieterich, Karen Bearrs, Nancy Peters, Patricia Brown, Emily Ansusinha, Ellen Waldrep, Jackie Friedman, Gail Hounshell, Debbie Allred, Stephen C. Engelke, Nancy Darden-Saad, Gary Stainback, Diane Warner, Janice Wereszczak, Janice Bernhardt, Joni McKeeman, Echo Meyer, Steve Pastyrnak, Wendy Burdo-Hartman, Julie Rathbun, Sarah Nota, Teri Crumb, Madeleine Lenski, Deborah Weiland, Megan Lloyd, Scott Hunter, Michael Msall, Rugile Ramoskaite, Suzanne Wiggins, Krissy Washington, Ryan Martin, Barbara Prendergast, Megan Scott, Judith Klarr, Beth Kring, Jennifer DeRidder, and Kelly Vogt.
Sources of financial assistance: This study was supported by The National Institute of Neurological Disorders and Stroke (5U01NS040069–05; PI: Alan Leviton and 2R01NS040069–06A2; PI: Karl Kuban), the National Institute of Child Health and Human Development (5P30HD018655; PI Scott Pomeroy), and the Office of the National Institutes of Health Director (1UG3OD023348–01; PI T. Michael O’Shea). The authors have no conflicts of interest to disclose.
References
- 1.Bhandari A, Panitch HB. Pulmonary outcomes in bronchopulmonary dysplasia. Semin Perinatol 2006;30(4):219–226. [DOI] [PubMed] [Google Scholar]
- 2.Goncalves C, Wandalsen G, Lanza F, Goulart AL, Sole D, Dos Santos A. Repercussions of preterm birth on symptoms of asthma, allergic diseases and pulmonary function, 6–14 years later. Allergol Immunopathol (Madr) 2016;44(6):489–496. [DOI] [PubMed] [Google Scholar]
- 3.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med 2001;163(7):1723–1729. [DOI] [PubMed] [Google Scholar]
- 4.Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, Laptook AR, Sanchez PJ, Van Meurs KP, Wyckoff M, Das A, Hale EC, Ball MB, Newman NS, Schibler K, Poindexter BB, Kennedy KA, Cotten CM, Watterberg KL, D’Angio CT, DeMauro SB, Truog WE, Devaskar U, Higgins RD, Eunice Kennedy Shriver National Institute of Child H, Human Development Neonatal Research N. Trends in Care Practices, Morbidity, and Mortality of Extremely Preterm Neonates, 1993–2012. JAMA 2015;314(10):1039–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fawke J, Lum S, Kirkby J, Hennessy E, Marlow N, Rowell V, Thomas S, Stocks J. Lung function and respiratory symptoms at 11 years in children born extremely preterm: the EPICure study. Am J Respir Crit Care Med 2010;182(2):237–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palta M, Sadek-Badawi M, Sheehy M, Albanese A, Weinstein M, McGuinness G, Peters ME. Respiratory symptoms at age 8 years in a cohort of very low birth weight children. Am J Epidemiol 2001;154(6):521–529. [DOI] [PubMed] [Google Scholar]
- 7.Hennessy EM, Bracewell MA, Wood N, Wolke D, Costeloe K, Gibson A, Marlow N, Group EPS. Respiratory health in pre-school and school age children following extremely preterm birth. Arch Dis Child 2008;93(12):1037–1043. [DOI] [PubMed] [Google Scholar]
- 8.Teune MJ, van Wassenaer AG, van Buuren S, Mol BW, Opmeer BC, Dutch PCSG. Perinatal risk-indicators for long-term respiratory morbidity among preterm or very low birth weight neonates. Eur J Obstet Gynecol Reprod Biol 2012;163(2):134–141. [DOI] [PubMed] [Google Scholar]
- 9.Anand D, Stevenson CJ, West CR, Pharoah PO. Lung function and respiratory health in adolescents of very low birth weight. Arch Dis Child 2003;88(2):135–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doyle LW, Victorian Infant Collaborative Study G. Respiratory function at age 8–9 years in extremely low birthweight/very preterm children born in Victoria in 1991–1992. Pediatr Pulmonol 2006;41(6):570–576. [DOI] [PubMed] [Google Scholar]
- 11.Cazzato S, Ridolfi L, Bernardi F, Faldella G, Bertelli L. Lung function outcome at school age in very low birth weight children. Pediatr Pulmonol 2013;48(8):830–837. [DOI] [PubMed] [Google Scholar]
- 12.Vom Hove M, Prenzel F, Uhlig HH, Robel-Tillig E. Pulmonary outcome in former preterm, very low birth weight children with bronchopulmonary dysplasia: a case-control follow-up at school age. J Pediatr 2014;164(1):40–45 e44. [DOI] [PubMed] [Google Scholar]
- 13.Lodha A, Ediger K, Rabi Y, Lodha S, Tang S, Bhandari A, Sauve R, Bhandari V . Does chronic oxygen dependency in preterm infants with bronchopulmonary dysplasia at NICU discharge predict respiratory outcomes at 3 years of age? J Perinatol 2015;35(7):530–536. [DOI] [PubMed] [Google Scholar]
- 14.Skromme K, Vollsaeter M, Oymar K, Markestad T, Halvorsen T. Respiratory morbidity through the first decade of life in a national cohort of children born extremely preterm. BMC Pediatr 2018;18(1):102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blanken MO, Rovers MM, Molenaar JM, Winkler-Seinstra PL, Meijer A, Kimpen JL, Bont L, Dutch RSVNN. Respiratory syncytial virus and recurrent wheeze in healthy preterm infants. N Engl J Med 2013;368(19):1791–1799. [DOI] [PubMed] [Google Scholar]
- 16.O’Shea TM, Allred EN, Dammann O, Hirtz D, Kuban KC, Paneth N, Leviton A. The ELGAN study of the brain and related disorders in extremely low gestational age newborns. Early Hum Dev 2009;85(11):719–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bose C, Van Marter LJ, Laughon M, O’Shea TM, Allred EN, Karna P, Ehrenkranz RA, Boggess K, Leviton A, Extremely Low Gestational Age Newborn Study I. Fetal growth restriction and chronic lung disease among infants born before the 28th week of gestation. Pediatrics 2009;124(3):e450–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laughon M, Allred EN, Bose C, O’Shea TM, Van Marter LJ, Ehrenkranz RA, Leviton A, Investigators ES. Patterns of respiratory disease during the first 2 postnatal weeks in extremely premature infants. Pediatrics 2009;123(4):1124–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laughon M, Bose C, Allred EN, O’Shea TM, Ehrenkranz RA, Van Marter LJ, Leviton A, Investigators ES. Antecedents of chronic lung disease following three patterns of early respiratory disease in preterm infants. Arch Dis Child Fetal Neonatal Ed 2011;96(2):F114–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laughon M, O’Shea MT, Allred EN, Bose C, Kuban K, Van Marter LJ, Ehrenkranz RA, Leviton A. Chronic lung disease and developmental delay at 2 years of age in children born before 28 weeks’ gestation. Pediatrics 2009;124(2):637–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dales LG, Ury HK. An improper use of statistical significance testing in studying covariables. Int J Epidemiol 1978;7(4):373–375. [DOI] [PubMed] [Google Scholar]
- 22.Williamson EJ, Aitken Z, Lawrie J, Dharmage SC, Burgess JA, Forbes AB. Introduction to causal diagrams for confounder selection. Respirology 2014;19(3):303–311. [DOI] [PubMed] [Google Scholar]
- 23.Allred EN, Dammann O, Kuban K, Leviton A, Pagano M. Neonatal risk factors for cerebral palsy in very preterm babies. Time oriented analyses of risk are useful. BMJ 1997;314(7094):1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gong T, Lundholm C, Rejno G, Mood C, Langstrom N, Almqvist C. Parental socioeconomic status, childhood asthma and medication use--a population-based study. PLoS One 2014;9(9):e106579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Almqvist C, Pershagen G, Wickman M. Low socioeconomic status as a risk factor for asthma, rhinitis and sensitization at 4 years in a birth cohort. Clin Exp Allergy 2005;35(5):612–618. [DOI] [PubMed] [Google Scholar]
- 26.Flores G, Tomany-Korman SC. Racial and ethnic disparities in medical and dental health, access to care, and use of services in US children. Pediatrics 2008;121(2):e286–298. [DOI] [PubMed] [Google Scholar]
- 27.Stevenson LA, Gergen PJ, Hoover DR, Rosenstreich D, Mannino DM, Matte TD. Sociodemographic correlates of indoor allergen sensitivity among United States children. J Allergy Clin Immunol 2001;108(5):747–752. [DOI] [PubMed] [Google Scholar]
- 28.von Mutius E, Schwartz J, Neas LM, Dockery D, Weiss ST. Relation of body mass index to asthma and atopy in children: the National Health and Nutrition Examination Study III. Thorax 2001;56(11):835–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kozyrskyj AL, Kendall GE, Jacoby P, Sly PD, Zubrick SR. Association between socioeconomic status and the development of asthma: analyses of income trajectories. Am J Public Health 2010;100(3):540–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Voge GA, Carey WA, Ryu E, King KS, Wi CI, Juhn YJ. What accounts for the association between late preterm births and risk of asthma? Allergy Asthma Proc 2017;38(2):152–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McElrath TF, Hecht JL, Dammann O, Boggess K, Onderdonk A, Markenson G, Harper M, Delpapa E, Allred EN, Leviton A. Pregnancy disorders that lead to delivery before the 28th week of gestation: an epidemiologic approach to classification. . Am J Epidemiol 2008;168:980–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haustein KO. Smoking and poverty. Eur J Cardiovasc Prev Rehabil 2006;13(3):312–318. [DOI] [PubMed] [Google Scholar]
- 33.Robison RG, Kumar R, Arguelles LM, Hong X, Wang G, Apollon S, Bonzagni A, Ortiz K, Pearson C, Pongracic JA, Wang X. Maternal smoking during pregnancy, prematurity and recurrent wheezing in early childhood. Pediatr Pulmonol 2012;47(7):666–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hollams EM, de Klerk NH, Holt PG, Sly PD. Persistent effects of maternal smoking during pregnancy on lung function and asthma in adolescents. Am J Respir Crit Care Med 2014;189(4):401–407. [DOI] [PubMed] [Google Scholar]
- 35.Burke H, Leonardi-Bee J, Hashim A, Pine-Abata H, Chen Y, Cook DG, Britton JR, McKeever TM. Prenatal and passive smoke exposure and incidence of asthma and wheeze: systematic review and meta-analysis. Pediatrics 2012;129(4):735–744. [DOI] [PubMed] [Google Scholar]
- 36.Sonnenschein-van der Voort AM, Arends LR, de Jongste JC, Annesi-Maesano I, Arshad SH, Barros H, Basterrechea M, Bisgaard H, Chatzi L, Corpeleijn E, Correia S, Craig LC, Devereux G, Dogaru C, Dostal M, Duchen K, Eggesbo M, van der Ent CK, Fantini MP, Forastiere F, Frey U, Gehring U, Gori D, van der Gugten AC, Hanke W, Henderson AJ, Heude B, Iniguez C, Inskip HM, Keil T, Kelleher CC, Kogevinas M, Kreiner-Moller E, Kuehni CE, Kupers LK, Lancz K, Larsen PS, Lau S, Ludvigsson J, Mommers M, Nybo Andersen AM, Palkovicova L, Pike KC, Pizzi C, Polanska K, Porta D, Richiardi L, Roberts G, Schmidt A, Sram RJ, Sunyer J, Thijs C, Torrent M, Viljoen K, Wijga AH, Vrijheid M, Jaddoe VW, Duijts L. Preterm birth, infant weight gain, and childhood asthma risk: a meta-analysis of 147,000 European children. J Allergy Clin Immunol 2014;133(5):1317–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Simone M, Farello G, Palumbo M, Gentile T, Ciuffreda M, Olioso P, Cinque M, De Matteis F. Growth charts, growth velocity and bone development in childhood obesity. Int J Obes Relat Metab Disord 1995;19(12):851–857. [PubMed] [Google Scholar]
- 38.Ekstrom S, Magnusson J, Kull I, Andersson N, Bottai M, Besharat Pour M, Melen E, Bergstrom A. Body Mass Index Development and Asthma Throughout Childhood. Am J Epidemiol 2017;186(2):255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muc M, Mota-Pinto A, Padez C. Association between obesity and asthma - epidemiology, pathophysiology and clinical profile. Nutr Res Rev 2016;29(2):194–201. [DOI] [PubMed] [Google Scholar]
- 40.Castro-Rodriguez JA, Forno E, Rodriguez-Martinez CE, Celedon JC. Risk and Protective Factors for Childhood Asthma: What Is the Evidence? J Allergy Clin Immunol Pract 2016;4(6):1111–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamamoto-Hanada K, Yang L, Narita M, Saito H, Ohya Y. Influence of antibiotic use in early childhood on asthma and allergic diseases at age 5. Ann Allergy Asthma Immunol 2017;119(1):54–58. [DOI] [PubMed] [Google Scholar]
- 42.Chung KF. Airway microbial dysbiosis in asthmatic patients: A target for prevention and treatment? J Allergy Clin Immunol 2017;139(4):1071–1081. [DOI] [PubMed] [Google Scholar]
- 43.Stokholm J, Blaser MJ, Thorsen J, Rasmussen MA, Waage J, Vinding RK, Schoos AM, Kunoe A, Fink NR, Chawes BL, Bonnelykke K, Brejnrod AD, Mortensen MS, Al-Soud WA, Sorensen SJ, Bisgaard H. Maturation of the gut microbiome and risk of asthma in childhood. Nature communications 2018;9(1):141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bisgaard H, Hermansen MN, Buchvald F, Loland L, Halkjaer LB, Bonnelykke K, Brasholt M, Heltberg A, Vissing NH, Thorsen SV, Stage M, Pipper CB. Childhood asthma after bacterial colonization of the airway in neonates. N Engl J Med 2007;357(15):1487–1495. [DOI] [PubMed] [Google Scholar]
- 45.Vissing NH, Chawes BL, Bisgaard H. Increased risk of pneumonia and bronchiolitis after bacterial colonization of the airways as neonates. Am J Respir Crit Care Med 2013;188(10):1246–1252. [DOI] [PubMed] [Google Scholar]
- 46.Smits HH, Hiemstra PS, Prazeres da Costa C, Ege M, Edwards M, Garn H, Howarth PH, Jartti T, de Jong EC, Maizels RM, Marsland BJ, McSorley HJ, Muller A, Pfefferle PI, Savelkoul H, Schwarze J, Unger WW, von Mutius E, Yazdanbakhsh M, Taube C. Microbes and asthma: Opportunities for intervention. J Allergy Clin Immunol 2016;137(3):690–697. [DOI] [PubMed] [Google Scholar]
- 47.Yang CL, Simons E, Foty RG, Subbarao P, To T, Dell SD. Misdiagnosis of asthma in schoolchildren. Pediatr Pulmonol 2017;52(3):293–302. [DOI] [PubMed] [Google Scholar]
- 48.Yeatts K, Johnston Davis K, Peden D, Shy C. Health consequences associated with frequent wheezing in adolescents without asthma diagnosis. Eur Respir J 2003;22(5):781–786. [DOI] [PubMed] [Google Scholar]
- 49.Culhane JF, Goldenberg RL. Racial disparities in preterm birth. Semin Perinatol 2011;35(4):234–239. [DOI] [PubMed] [Google Scholar]
- 50.Hsieh VC, Shieh SH, Chen CY, Liou SH, Hsiao YC, Wu TN. Does Social Health Insurance Close the Gap: The Case of Socioeconomic Status and Preterm Low-Birth-Weight Survival. Asia Pac J Public Health 2015;27(5):497–508. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


