Abstract
BACKGROUND
In a single-group, phase 1b trial, avelumab plus axitinib resulted in objective responses in patients with advanced renal-cell carcinoma. This phase 3 trial involving previously untreated patients with advanced renal-cell carcinoma compared avelumab plus axitinib with the standard-of-care sunitinib.
METHODS
We randomly assigned patients in a 1:1 ratio to receive avelumab (10 mg per kilogram of body weight) intravenously every 2 weeks plus axitinib (5 mg) orally twice daily or sunitinib (50 mg) orally once daily for 4 weeks (6-week cycle). The two independent primary end points were progression-free survival and overall survival among patients with programmed death ligand 1 (PD-L1)–positive tumors. A key secondary end point was progression-free survival in the overall population; other end points included objective response and safety.
RESULTS
A total of 886 patients were assigned to receive avelumab plus axitinib (442 patients) or sunitinib (444 patients). Among the 560 patients with PD-L1–positive tumors (63.2%), the median progression-free survival was 13.8 months with avelumab plus axitinib, as compared with 7.2 months with sunitinib (hazard ratio for disease progression or death, 0.61; 95% confidence interval [CI], 0.47 to 0.79; P<0.001); in the overall population, the median progression-free survival was 13.8 months, as compared with 8.4 months (hazard ratio, 0.69; 95% CI, 0.56 to 0.84; P<0.001). Among the patients with PD-L1–positive tumors, the objective response rate was 55.2% with avelumab plus axitinib and 25.5% with sunitinib; at a median follow-up for overall survival of 11.6 months and 10.7 months in the two groups, 37 patients and 44 patients had died, respectively. Adverse events during treatment occurred in 99.5% of patients in the avelumab-plus-axitinib group and in 99.3% of patients in the sunitinib group; these events were grade 3 or higher in 71.2% and 71.5% of the patients in the respective groups.
CONCLUSIONS
Progression-free survival was significantly longer with avelumab plus axitinib than with sunitinib among patients who received these agents as first-line treatment for advanced renal-cell carcinoma. (Funded by Pfizer and Merck [Darmstadt, Germany]; JAVELIN Renal 101 ClinicalTrials.gov number, .)
MOST PATIENTS WITH A DIAGNOSIS OF renal carcinoma have clear-cell renal-cell carcinoma, which harbors genetic abnormalities that lead to excessive production of vascular endothelial growth factor (VEGF), a key driver of angiogenesis.1,2 Although sunitinib is a standard-of-care first-line therapy for patients with advanced renal-cell carcinoma,3,4 many patients have inherent resistance to antiangiogenic drugs or they have progressive disease.
Immune checkpoint inhibitors include the anti–programmed death ligand 1 (PD-L1) anti-body avelumab. These agents have been shown to have acceptable safety and durable antitumor activity as first- and second-line treatments in patients with multiple tumor types, including advanced renal-cell carcinoma.5-10
In addition to antiangiogenic effects, VEGF receptor (VEGFR) inhibitors have immunomodulatory effects, including enhanced tumor infiltration of immune cells and reduced immuno-suppressive effects of myeloid-derived suppressor cells.11 We hypothesized that the combination of an immune checkpoint inhibitor with a VEGF-targeted antiangiogenic therapy might provide enhanced benefit through complementary mechanisms of action. Axitinib, a highly selective VEGFR inhibitor, is approved for the treatment of advanced renal-cell carcinoma after disease progression in patients receiving sunitinib,12,13 and we selected it over sunitinib for combination with avelumab because of its lower incidence of hepatic toxic effects. Preliminary data from a single-group, nonrandomized, phase 1b trial involving 55 patients with advanced renal-cell carcinoma showed that the combination of avelumab plus axitinib resulted in objective responses in 58% of patients and a rate of disease control of 78%, at a median follow-up of 52 weeks.14 A higher percentage of patients with PD-L1 expression on at least 1% of tumor-associated immune cells had objective responses than the percentage of those with PD-L1 expression on less than 1% of those cells.14 We report the primary efficacy and safety results of the phase 3 JAVELIN Renal 101 trial of avelumab plus axitinib as compared with sunitinib in patients with previously untreated advanced renal-cell carcinoma.
METHODS
PATIENTS
Eligible patients had previously untreated advanced renal-cell carcinoma with a clear-cell component. Additional key inclusion criteria were the presence of at least one measurable lesion according to the Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1; age of 18 years or older; Eastern Cooperative Oncology Group (ECOG) performance-status score of 0 or 1 (on a 5-point scale in which higher numbers indicate greater disability); a fresh or archival tumor specimen; and adequate renal, cardiac, and hepatic function. Patients across all Memorial Sloan Kettering Cancer Center (MSKCC) and International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) prognostic risk groups were included (see the Definitions of Selected Terms and End Points section in the Supplementary Appendix, available with the full text of this article at NEJM.org).15,16 Key exclusion criteria were active central nervous system metastases, autoimmune disease, and current or previous use of glucocorticoids or other immunosuppressants within 7 days before randomization.
TRIAL DESIGN
This was a multicenter, randomized, open-label, phase 3 trial comparing avelumab plus axitinib with sunitinib. Randomization (in a 1:1 ratio) was stratified according to ECOG performance-status score (0 vs. 1) and geographic region (United States vs. Canada and Western Europe vs. the rest of the world).
Avelumab was administered at a dose of 10 mg per kilogram of body weight as a 1-hour intravenous infusion every 2 weeks. An antihistamine and acetaminophen were administered approximately 30 to 60 minutes before each infusion. Axitinib was administered orally at a starting dose of 5 mg twice daily on a continuous dosing schedule. Sunitinib was administered at a dose of 50 mg orally once daily for 4 weeks of a 6-week cycle. Dose escalations and reductions of axitinib and dose reductions of sunitinib are described in the protocol (available at NEJM. org).17,18 Dose reductions of avelumab were not permitted, but subsequent infusions could be omitted in response to persisting toxic effects. The original primary objective was to show the superiority of avelumab plus axitinib over sunitinib in prolonging progression-free survival among patients with advanced renal-cell carcinoma, irrespective of PD-L1 expression. A June 2017 protocol amendment, while data were still masked, was based on new data from a single-group phase 1b trial14 and two trials of immune checkpoint inhibitors that showed an overall survival benefit among patients with renal-cell carcinoma.5,6 This amendment changed the primary objective of the trial to show the superiority of avelumab plus axitinib over sunitinib with respect to either progression-free or overall survival among patients with PD-L1–positive tumors.
TRIAL OVERSIGHT
The trial was conducted in accordance with the ethics principles of the Declaration of Helsinki and the Good Clinical Practice guidelines, defined by the International Council for Harmonisation. All the patients provided written informed consent. The protocol, amendments, and informed-consent forms were approved by the institutional review board or independent ethics committee at each trial site. An independent external data monitoring committee reviewed efficacy and safety.
The trial was sponsored by Pfizer as part of an alliance between Pfizer and Merck (Darmstadt, Germany); both companies provided the trial drugs. The investigators worked with Pfizer on the trial design, collection and analysis of data, and interpretation of results. Data sets were reviewed by the authors, and all the authors participated fully in developing and reviewing the manuscript for submission for publication. A professional medical writer who was paid by the sponsor assisted in the preparation of the manuscript. All the authors had full access to all data, and the first author had final responsibility for the decision to submit the manuscript for publication. The authors vouch for the accuracy and completeness of the data and analyses and for the fidelity of the trial to the protocol and the statistical analysis plan (available at NEJM.org).
END POINTS AND ASSESSMENTS
The two independent primary end points were progression-free survival (as determined by blinded independent central review according to RECIST, version 1.1) and overall survival among patients with PD-L1–positive tumors (≥1% of immune cells staining positive within the tumor area of the tested tissue sample). PD-L1 expression was assessed at a central laboratory with the use of the Ventana PD-L1 (SP263) assay (Ventana Medical Systems).
Key secondary end points were progression-free survival as determined by blinded independent central review according to RECIST, version 1.1, and overall survival among patients in the overall population, irrespective of PD-L1 expression. Other secondary end points included progression-free survival as determined by investigator assessment, the objective response rate, adverse events, pharmacokinetic measures, tumor-tissue biomarkers, and patient-reported outcomes.19,20 All subgroup analyses were prespecified in the statistical analysis plan, except for body-mass index and smoking status, which were post hoc exploratory analyses.
Tumor assessments were performed with the use of computed tomography or magnetic resonance imaging at baseline, every 6 weeks after randomization for the first 18 months, and then every 12 weeks until confirmed disease progression. Adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03. Patients in each treatment group were permitted to continue therapy after RECIST-defined disease progression if the investigators determined that the therapy had benefit because the patients did not have clinical signs and symptoms associated with the radiographic findings.
STATISTICAL ANALYSIS
It was estimated that approximately 830 patients, including approximately 580 patients with PD-L1–positive tumors (70%), would undergo randomization. The overall type I error rate was maintained at or below a one-sided significance level of 0.025 by allocating an alpha level of 0.004 to the progression-free survival comparison and an alpha level of 0.021 to the overall survival comparison among the patients with PD-L1–positive tumors. A gatekeeping procedure to control for the overall type I error rate was used to allow further testing of progression-free and overall survival in the overall population (Fig. S1 in the Supplementary Appendix). The trial was considered to have met its success criteria if avelumab plus axitinib was superior to sunitinib in prolonging progression-free or over-all survival among the patients with PD-L1–positive tumors. Sensitivity analyses were also performed to explore the robustness of the primary analysis results.
For the primary analysis of progression-free survival among the patients with PD-L1–positive tumors, we estimated that 336 events would provide the trial with 90% power to detect a hazard ratio of 0.65 with the use of a one-sided log-rank test at a significance level of 0.004. A two-look group-sequential design with a Lan–DeMets (O’Brien–Fleming) alpha-spending function was used to determine the efficacy boundary.21
For the primary analysis of overall survival among the patients with PD-L1–positive tumors, we estimated that 368 events would provide the trial with 90% power to detect a hazard ratio of 0.70 with the use of a one-sided log-rank test at a significance level of 0.021. A four-look group-sequential design with a Lan–DeMets (O’Brien–Fleming) alpha-spending function was used to determine the efficacy boundary. This sample size would also allow assessment of progression-free and overall survival in the overall population. The preplanned interim analysis was based on a data-cutoff point of approximately 235 events of disease progression or death (70% information fraction) in the patients with PD-L1–positive tumors. The results of the interim analysis were reviewed by an external data monitoring committee on August 20, 2018. The committee reported that the efficacy boundaries for progression-free survival among the patients with PD-L1–positive tumors and in the overall population had been crossed. The trial continued to evaluate overall survival. All data reported here are based on the first interim analysis.
Efficacy end points were assessed in all patients who underwent randomization, and safety was evaluated in all patients who received at least one dose of a trial drug (avelumab, axitinib, or sunitinib). We calculated the objective response rate according to treatment group, along with corresponding exact two-sided 95% confidence intervals, using the Clopper–Pearson method.22 Progression-free and overall survival and duration of response were estimated with the use of the Kaplan–Meier method, and two-sided P values are reported.23 To account for the group-sequential design in this trial, the repeated confidence interval method24 was used for the hazard ratio at the interim analysis for progression-free survival and overall survival. In addition, the unadjusted 95% confidence interval for the hazard ratio was reported.
RESULTS
PATIENTS
From March 29, 2016, through December 19, 2017, at total of 886 patients were randomly assigned to treatment at 144 sites in 21 countries; 442 patients were assigned to the avelumab-plus-axitinib group and 444 were assigned to the sunitinib group (Fig. S2 in the Supplementary Appendix). A total of 873 patients received trial treatment (434 received avelumab plus axitinib and 439 received sunitinib), and 560 of 886 patients (63.2%) had PD-L1–positive tumors (69.0% of 812 patients for whom tumor-tissue samples were available for PD-L1 assessment). Baseline demographic and disease characteristics were balanced between the two treatment groups (among both patients with PD-L1–positive tumors and the overall population) (Table 1).
Table 1.
Baseline Demographic and Clinical Characteristics of the Patients Who Underwent Randomization.*
| Characteristic | Patients with PD-L1–Positive Tumors | Overall Population | ||
|---|---|---|---|---|
| Avelumab plus Axitinib (N = 270) |
Sunitinib (N = 290) |
Avelumab plus Axitinib (N = 442) |
Sunitinib (N = 444) |
|
| Median age (range) — yr | 62.0 (29.0–83.0) | 60.5 (27.0–88.0) | 62.0 (29.0–83.0) | 61.0 (27.0–88.0) |
| Sex — no. (%) | ||||
| Male | 203 (75.2) | 224 (77.2) | 316 (71.5) | 344 (77.5) |
| Female | 67 (24.8) | 66 (22.8) | 126 (28.5) | 100 (22.5) |
| MSKCC prognostic risk group — no. (%)† | ||||
| Favorable | 52 (19.3) | 60 (20.7) | 96 (21.7) | 100 (22.5) |
| Intermediate | 180 (66.7) | 201 (69.3) | 283 (64.0) | 293 (66.0) |
| Poor | 33 (12.2) | 24 (8.3) | 51 (11.5) | 45 (10.1) |
| Not reported | 5 (1.9) | 5 (1.7) | 12 (2.7) | 6 (1.4) |
| IMDC prognostic risk group — no. (%)‡ | ||||
| Favorable | 52 (19.3) | 59 (20.3) | 94 (21.3) | 96 (21.6) |
| Intermediate | 173 (64.1) | 191 (65.9) | 271 (61.3) | 276 (62.2) |
| Poor | 44 (16.3) | 39 (13.4) | 72 (16.3) | 71 (16.0) |
| Not reported | 1 (0.4) | 1 (0.3) | 5 (1.1) | 1 (0.2) |
| Geographic region — no. (%) | ||||
| United States | 75 (27.8) | 82 (28.3) | 128 (29.0) | 130 (29.3) |
| Canada and Western Europe | 80 (29.6) | 81 (27.9) | 128 (29.0) | 128 (28.8) |
| Rest of the world | 115 (42.6) | 127 (43.8) | 186 (42.1) | 186 (41.9) |
| Previous nephrectomy — no. (%) | ||||
| Yes | 233 (86.3) | 252 (86.9) | 352 (79.6) | 355 (80.0) |
| No | 37 (13.7) | 38 (13.1) | 90 (20.4) | 89 (20.0) |
| RECIST-defined tumor sites at baseline, according to independent review — no. (%) | ||||
| 0 | 8 (3.0) | 11 (3.8) | 11 (2.5) | 16 (3.6) |
| 1 | 120 (44.4) | 118 (40.7) | 181 (41.0) | 174 (39.2) |
| 2 | 85 (31.5) | 101 (34.8) | 148 (33.5) | 151 (34.0) |
| 3 | 40 (14.8) | 50 (17.2) | 67 (15.2) | 79 (17.8) |
| ≥4 | 17 (6.3) | 10 (3.4) | 35 (7.9) | 24 (5.4) |
Percentages may not total 100 because of rounding. RECIST denotes Response Evaluation Criteria in Solid Tumors.
Patients with favorable risk had a Memorial Sloan Kettering Cancer Center (MSKCC) score of 0, those with intermediate risk had a score of 1 or 2, and those with poor risk had a score of 3 or more. MSKCC risk scores are defined according to the number of the following risk factors present: a Karnofsky performance-status score of less than 80 (on a scale from 0 to 100, with lower scores indicating greater disability; patients with a performance-status score of <70 were excluded from the trial), less than 1 year from the time of initial diagnosis to the start of therapy, a hemoglobin level below the lower limit of the normal range, a lactate dehydrogenase level more than 1.5 times the upper limit of the normal range, and a corrected serum calcium concentration of more than 10 mg per deciliter (2.5 mmol per liter).
Patients with favorable risk had an International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) score of 0, those with intermediate risk had a score of 1 or 2, and those with poor risk had a score of 3 to 6. IMDC risk scores are defined according to the number of the following risk factors present: a Karnofsky performance-status score of less than 80, time from initial diagnosis to randomization of less than 1 year, hemoglobin level below the lower limit of the normal range, corrected serum calcium level above the upper limit of the normal range, absolute neutrophil count above the upper limit of the normal range, and platelet count above the upper limit of the normal range.
Data cutoff occurred on June 20, 2018. On that date, of 442 patients who had been randomly assigned to the combination group, 230 (52.0%) were still receiving avelumab and 246 (55.7%) were still receiving axitinib; 221 patients (50.0%) continued to receive avelumab plus axitinib, 9 patients (2.0%) continued to receive avelumab alone, and 25 patients (5.7%) continued to receive axitinib alone. Of the 444 patients who had been randomly assigned to the sunitinib group, 167 (37.6%) continued to receive treatment.
EFFICACY
Primary End Points among Patients with PD-L1–Positive Tumors
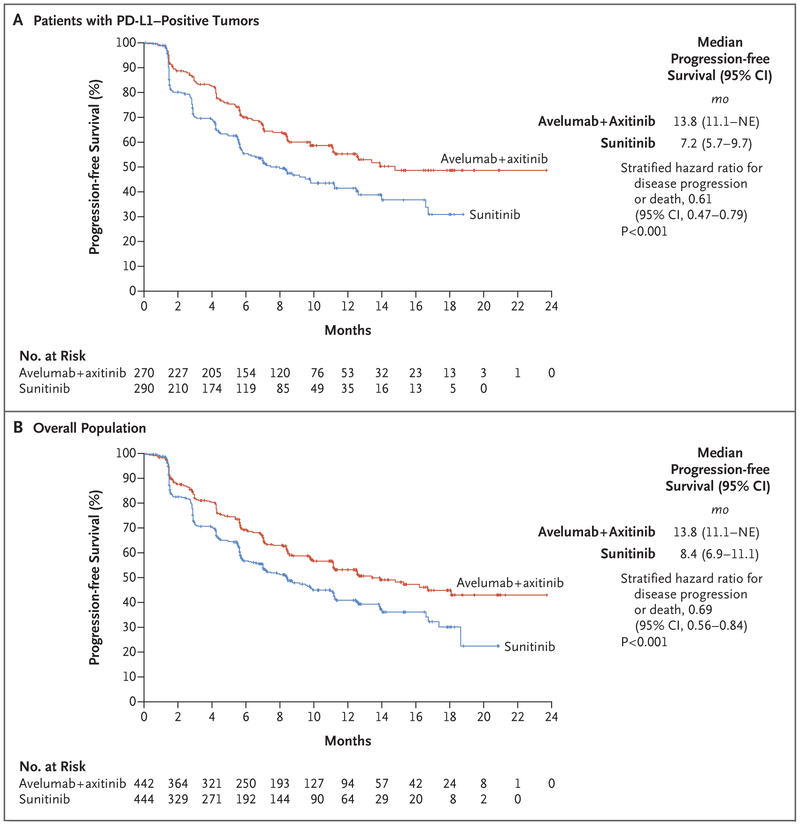
Among the patients with PD-L1–positive tumors, progression-free survival was significantly longer among patients who received avelumab plus axitinib than among those who received sunitinib; the median progression-free survival was 13.8 months (95% confidence interval [CI], 11.1 to could not be estimated) with avelumab plus axitinib, as compared with 7.2 months (95% CI, 5.7 to 9.7) with sunitinib (stratified hazard ratio for disease progression or death, 0.61; 95% CI, 0.47 to 0.79; P<0.001; repeated confidence interval, 0.43 to 0.92) (Fig. 1A). The median follow-up was 9.9 months with avelumab plus axitinib and 8.4 months with sunitinib. Among the patients with PD-L1–positive tumors, deaths from any cause were observed in 37 patients (13.7%) who received avelumab plus axitinib and in 44 patients (15.2%) who received sunitinib (stratified hazard ratio for death, 0.82; 95% CI, 0.53 to 1.28; P=0.38; repeated confidence interval, 0.46 to 2.40). The median follow-up was 11.6 months and 10.7 months, respectively.
Figure 1. Progression-free Survival.
Progression-free survival among patients with programmed death ligand 1 (PD-L1)–positive tumors (Panel A) and among patients in the overall population (Panel B) is shown. NE denotes could not be estimated.
Key Secondary End Points
In the overall population, progression-free survival was also significantly longer with avelumab plus axitinib than with sunitinib; the median progression-free survival was 13.8 months (95% CI, 11.1 to could not be estimated) with avelumab plus axitinib, as compared with 8.4 months (95% CI, 6.9 to 11.1) with sunitinib (stratified hazard ratio for disease progression or death, 0.69; 95% CI, 0.56 to 0.84; P<0.001) (Fig. 1B). The median follow-up was 10.8 months and 8.6 months, respectively. Deaths from any cause were observed in 63 patients (14.3%) who received avelumab plus axitinib and 75 patients (16.9%) who received sunitinib (stratified hazard ratio for death, 0.78; 95% CI, 0.55 to 1.08; P=0.14) (Fig. S3 in the Supplementary Appendix). The median follow-up was 12.0 months and 11.5 months, respectively.
Sensitivity analyses were performed to explore the robustness of the primary analysis results for progression-free survival; the results of these analyses were similar to those of the primary analysis. The model assumption of proportional hazards was assessed, and an analysis of restricted mean survival time showed similar results (see the Sensitivity Analyses section and Fig. S4 and S5 in the Supplementary Appendix).
Other Secondary End Points
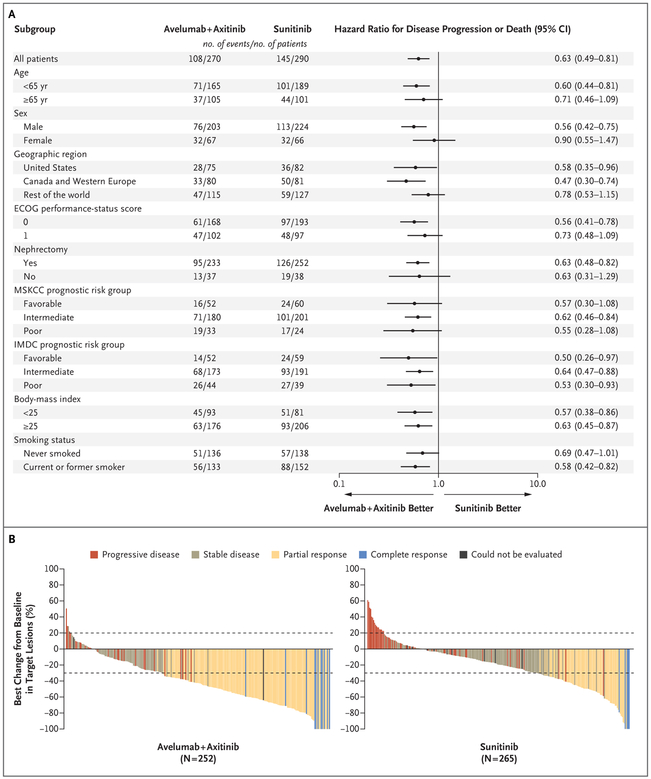
Among the patients with PD-L1–positive tumors, the confirmed objective response rate was 55.2% (95% CI, 49.0 to 61.2) with avelumab plus axitinib and 25.5% (95% CI, 20.6 to 30.9) with sunitinib; confirmed complete response rates were 4.4% and 2.1%, respectively (Table 2, and Fig. S6 in the Supplementary Appendix). In the overall population, confirmed objective response rates were similar to those observed among the patients with PD-L1–positive tumors (Table 2). Progression-free survival and objective response rates favored avelumab plus axitinib in all sub-groups assessed, including those based on PD-L1 status and all MSKCC and IMDC prognostic risk groups (Fig. 2A, and Figs. S7 through S9 and Table S1 in the Supplementary Appendix). Among patients in the overall population with IMDC favorable, intermediate, and poor risk who received avelumab plus axitinib, 68.1%, 51.3%, and 30.6%, respectively, had objective responses as compared with 37.5%, 25.4%, and 11.3% of patients who received sunitinib (Fig. S9 in the Supplementary Appendix). The best percentage change in the sum of target-lesion diameters is shown in Figure 2B, and in Figure S10 in the Supplementary Appendix.
Table 2.
Antitumor Activity among Patients with PD-L1–Positive Tumors and in the Overall Population.*
| Variable | Patients with PD-L1–Positive Tumors | Overall Population | ||
|---|---|---|---|---|
| Avelumab plus Axitinib (N = 270) |
Sunitinib (N = 290) |
Avelumab plus Axitinib (N = 442) |
Sunitinib (N = 444) |
|
| Confirmed objective response rate (95% CI) — % | 55.2 (49.0–61.2) | 25.5 (20.6–30.9)† | 51.4 (46.6–56.1) | 25.7 (21.7–30.0)‡ |
| Confirmed best overall response — no. (%) | ||||
| Complete response | 12 (4.4) | 6 (2.1) | 15 (3.4) | 8 (1.8) |
| Partial response | 137 (50.7) | 68 (23.4) | 212 (48.0) | 106 (23.9) |
| Stable disease | 72 (26.7) | 125 (43.1) | 131 (29.6) | 202 (45.5) |
| Progressive disease | 30 (11.1) | 63 (21.7) | 51 (11.5) | 83 (18.7) |
| Could not be evaluated | 12 (4.4)§ | 21 (7.2)¶ | 25 (5.7)‖ | 35 (7.9)** |
| Other†† | 7 (2.6) | 7 (2.4) | 8 (1.8) | 10 (2.3) |
| Median time to response (range) — mo | 1.6 (1.2–10.1) | 3.0 (1.2–11.6) | 2.6 (1.2–13.8) | 3.2 (1.2–11.6) |
| Median duration of response (95% CI) — mo | NR (NE) | NR (10.9–NE) | NR (NE) | NR (11.2–NE) |
| Patients with ongoing response — no./total no. (%) | 108/149 (72.5) | 48/74 (64.9) | 158/227 (69.6) | 81/114 (71.1) |
NE denotes could not be estimated, and NR not reached. Percentages may not total 100 because of rounding.
The stratified odds ratio for the objective response rate among the patients with PD-L1–positive tumors was 3.73 (95% CI, 2.53 to 5.37).
The stratified odds ratio for the objective response rate in the overall population was 3.10 (95% CI, 2.30 to 4.15).
No assessments were performed after baseline because of early death or other reasons such as withdrawal of consent or start of new anti-cancer therapy (in 10 patients) or stable disease less than 6 weeks after randomization (in 2 patients).
No assessments were performed after baseline because of early death or other reasons such as withdrawal of consent or start of new anti-cancer therapy (in 9 patients), stable disease less than 6 weeks after randomization (in 9 patients), or new anticancer therapy started before the first postbaseline assessment (in 2 patients), or the overall response in all assessments after baseline could not be evaluated (in 1 patient).
No assessments were performed after baseline because of early death or other reasons such as withdrawal of consent or start of new anti-cancer therapy (in 18 patients), stable disease less than 6 weeks after randomization (in 5 patients), or no adequate baseline assessment (in 2 patients).
No assessments were performed after baseline because of early death or other reasons such as withdrawal of consent or start of new anti-cancer therapy (in 14 patients), stable disease less than 6 weeks after randomization (in 15 patients), new anticancer therapy started before first postbaseline assessment (in 3 patients), or no adequate baseline assessment (in 2 patients), or the overall response in all assessments after baseline could not be evaluated (in 1 patient).
This category includes patients who did not have target lesions at baseline according to independent review and whose response was categorized as “non–complete response” or “non–progressive disease.”
Figure 2. Subgroup Analyses of Progression-free Survival and Best Percentage Change in Target Lesions among Patients with PD-L1–Positive Tumors.
Panel A shows the results of a subgroup analysis of progression-free survival among the patients with PD-L1–positive tumors. Eastern Cooperative Oncology Group (ECOG) performance-status scores range from 0 to 5,with higher numbers reflecting greater disability. Patients with favorable risk had a Memorial Sloan Kettering Cancer Center (MSKCC) score of 0, those with intermediate risk had a score of 1 or 2, and those with poor risk had a score of 3 or more. MSKCC risk scores are defined according to the number of the following risk factors present: a Karnofsky performance-status score of less than 80 (on a scale from 0 to 100, with lower scores indicating greater disability; patients with a performancestatus score of <70 were excluded from the trial), less than 1 year from the time of initial diagnosis to the start of therapy, a hemoglobin level below the lower limit of the normal range, a lactate dehydrogenase level more than 1.5 times the upper limit of the normal range, and a corrected serum calcium concentration of more than 10 mg per deciliter (2.5 mmol per liter). Patients with favorable risk had an International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) score of 0, those with intermediate risk had a score of 1 or 2, and those with poor risk had a score of 3 to 6. IMDC risk scores are defined according to the number of the following risk factors present: a Karnofsky performance-status score of less than 80, time from initial diagnosis to randomization of less than 1 year, hemoglobin level below the lower limit of the normal range, corrected serum calcium level above the upper limit of the normal range, absolute neutrophil count above the upper limit of the normal range, and platelet count above the upper limit of the normal range. Body-mass index is the weight in kilograms divided by the square of the height in meters. Panel B shows the best percentage change from baseline in the sum of the longest diameters of target lesions in the patients with PD-L1–positive tumors. Dotted lines indicate Response Evaluation Criteria in Solid Tumors (RECIST)–defined progressive disease (≥20% increase in the sum of target-lesion diameters, with baseline as the reference) and partial response (≥30% decrease in the sum of target-lesion diameters, with baseline as the reference).
Results according to investigator assessment were consistent with those according to blinded independent central review: among the patients with PD-L1–positive tumors, the median progression-free survival was 13.3 months in the avelumab-plus-axitinib group and 8.2 months in the sunitinib group (stratified hazard ratio for disease progression or death, 0.51), and in the overall population, the median progression-free survival was 12.5 months and 8.4 months, respectively (stratified hazard ratio, 0.64) (Fig. S11 in the Supplementary Appendix). Among the patients with PD-L1–positive tumors, the objective response rates were 61.9% (95% CI, 55.8 to 67.7) in the avelumab-plus-axitinib group and 29.7% (95% CI, 24.5 to 35.3) in the sunitinib group, and among patients in the overall population, the objective response rates were 55.9% (95% CI, 51.1 to 60.6) and 30.2% (95% CI, 25.9 to 34.7), respectively (Table S2 in the Supplementary Appendix).
Exposure and Safety in the Overall Population
The median duration of treatment was 8.6 months (range, 0.5 to 25.3) in patients who received avelumab, 9.0 months (range, 0.02 to 24.9) in patients who received axitinib, and 7.3 months (range, 0.2 to 23.0) in patients who received sunitinib. The median relative dose intensity was 91.5%, 89.4%, and 83.9% among patients who received avelumab, axitinib, and sunitinib, respectively. Among patients who received axitinib in the combination group, 183 (42.2%) had at least one reduction in the dose of axitinib and 47 (10.8%) had at least one escalation in the dose of axitinib. Of the patients who received sunitinib, 187 (42.6%) had at least one dose reduction.
Adverse events of any grade during treatment occurred in 432 of 434 patients (99.5%) who received avelumab plus axitinib and in 436 of 439 patients (99.3%) who received sunitinib; adverse events of grade 3 or higher during treatment occurred in 309 patients (71.2%) and 314 patients (71.5%) in the respective groups (Table 3). Adverse events that occurred during treatment led to discontinuation of both avelumab and axitinib in 33 patients (7.6%) who received the combination and led to discontinuation of sunitinib in 59 patients (13.4%) who received sunitinib. Treatment-related adverse events are detailed in Table S3 in the Supplementary Appendix. Death due to toxicity of trial treatment that occurred in 3 patients in the avelumab-plus-axitinib group (0.7%) was attributed to sudden death, myocarditis, and necrotizing pancreatitis, and death due to toxicity of trial treatment in 1 patient in the sunitinib group (0.2%) was attributed to intestinal perforation.
Table 3.
Adverse Events of Any Grade That Occurred during Treatment in 10% or More of Patients or Adverse Events of Grade 3 or Higher That Occurred in 5% or More of Patients in the Overall Population of 873 Patients.
| Variable | Avelumab plus Axitinib (N = 434) |
Sunitinib (N = 439) |
||
|---|---|---|---|---|
| All Grades | Grade ≥3 | All Grades | Grade ≥3 | |
| number of patients (percent) | ||||
| Patients with any events | 432 (99.5) | 309 (71.2) | 436 (99.3) | 314 (71.5) |
| Diarrhea | 270 (62.2) | 29 (6.7) | 209 (47.6) | 12 (2.7) |
| Hypertension | 215 (49.5) | 111 (25.6) | 158 (36.0) | 75 (17.1) |
| Fatigue | 180 (41.5) | 15 (3.5) | 176 (40.1) | 16 (3.6) |
| Nausea | 148 (34.1) | 6 (1.4) | 172 (39.2) | 7 (1.6) |
| Palmar–plantar erythrodysesthesia syndrome | 145 (33.4) | 25 (5.8) | 148 (33.7) | 19 (4.3) |
| Dysphonia | 133 (30.6) | 2 (0.5) | 14 (3.2) | 0 |
| Decreased appetite | 114 (26.3) | 9 (2.1) | 126 (28.7) | 4 (0.9) |
| Hypothyroidism | 108 (24.9) | 1 (0.2) | 61 (13.9) | 1 (0.2) |
| Stomatitis | 102 (23.5) | 8 (1.8) | 103 (23.5) | 4 (0.9) |
| Cough | 100 (23.0) | 1 (0.2) | 83 (18.9) | 0 |
| Headache | 89 (20.5) | 1 (0.2) | 71 (16.2) | 1 (0.2) |
| Dyspnea | 86 (19.8) | 13 (3.0) | 57 (13.0) | 7 (1.6) |
| Arthralgia | 85 (19.6) | 4 (0.9) | 50 (11.4) | 2 (0.5) |
| Decreased weight | 85 (19.6) | 12 (2.8) | 30 (6.8) | 4 (0.9) |
| Vomiting | 80 (18.4) | 4 (0.9) | 87 (19.8) | 7 (1.6) |
| Back pain | 77 (17.7) | 2 (0.5) | 65 (14.8) | 8 (1.8) |
| Constipation | 77 (17.7) | 0 | 64 (14.6) | 0 |
| Increased alanine aminotransferase level | 74 (17.1) | 26 (6.0) | 50 (11.4) | 11 (2.5) |
| Chills | 69 (15.9) | 1 (0.2) | 33 (7.5) | 0 |
| Asthenia | 64 (14.7) | 11 (2.5) | 72 (16.4) | 13 (3.0) |
| Increased aspartate aminotransferase level | 63 (14.5) | 17 (3.9) | 52 (11.8) | 9 (2.1) |
| Rash | 62 (14.3) | 2 (0.5) | 49 (11.2) | 2 (0.5) |
| Mucosal inflammation | 61 (14.1) | 5 (1.2) | 61 (13.9) | 5 (1.1) |
| Pruritus | 61 (14.1) | 0 | 22 (5.0) | 0 |
| Abdominal pain | 59 (13.6) | 5 (1.2) | 43 (9.8) | 8 (1.8) |
| Dysgeusia | 57 (13.1) | 0 | 142 (32.3) | 0 |
| Pyrexia | 56 (12.9) | 0 | 62 (14.1) | 1 (0.2) |
| Infusion-related reaction | 53 (12.2) | 7 (1.6) | 0 | 0 |
| Pain in extremity | 52 (12.0) | 1 (0.2) | 46 (10.5) | 3 (0.7) |
| Dizziness | 51 (11.8) | 2 (0.5) | 47 (10.7) | 3 (0.7) |
| Oropharyngeal pain | 44 (10.1) | 0 | 27 (6.2) | 0 |
| Dry skin | 43 (9.9) | 0 | 44 (10.0) | 0 |
| Edema, peripheral | 39 (9.0) | 2 (0.5) | 45 (10.3) | 1 (0.2) |
| Epistaxis | 37 (8.5) | 0 | 49 (11.2) | 0 |
| Dyspepsia | 35 (8.1) | 0 | 83 (18.9) | 0 |
| Anemia | 26 (6.0) | 7 (1.6) | 101 (23.0) | 36 (8.2) |
| Thrombocytopenia | 15 (3.5) | 1 (0.2) | 85 (19.4) | 27 (6.2) |
| Decreased platelet count | 8 (1.8) | 0 | 63 (14.4) | 22 (5.0) |
| Neutropenia | 6 (1.4) | 1 (0.2) | 83 (18.9) | 35 (8.0) |
| Decreased neutrophil count | 1 (0.2) | 0 | 45 (10.3) | 25 (5.7) |
Of the 434 patients who received avelumab plus axitinib, 166 patients (38.2%) had adverse events that were categorized as immune-related adverse events according to a prespecified case definition; 39 patients (9.0%) had events of grade 3 or higher. The most frequent immune-related adverse events were immune-related thyroid disorders, which were observed in 107 patients (24.7%) who received avelumab plus axitinib. High-dose glucocorticoids (≥40 mg total daily dose of prednisone or equivalent) were administered to 48 patients (11.1%) who had an immune-related adverse event with avelumab plus axitinib.
SUBSEQUENT THERAPY
A smaller percentage of patients in the combination group than in the sunitinib group received subsequent anticancer drug therapies: 92 patients (20.8%) and 174 patients (39.2%), respectively.In the combination group, the most frequently used subsequent anticancer drug therapy (in ≥5% of patients) was cabozantinib (in 9.5%); in the sunitinib group, the most frequently used subsequent anticancer drug therapies were nivolumab, abozantinib, and sunitinib (in 24.1%, 6.3%, and 5.2%, respectively) (Table S4 in the Supplementary Appendix). In the sunitinib group, 116 of 174 patients who received subsequent anticancer therapy (66.7%) were known to have been treated with an anti–PD-1 or anti–PD-L1 agent.
DISCUSSION
In this phase 3 trial, patients with PD-L1–positive, clear-cell, advanced renal-cell carcinoma who received first-line avelumab plus axitinib had significantly longer progression-free survival than patients who received sunitinib. The efficacy benefit was also observed in the overall population.
At the time of the data cutoff, patients continued to be followed for overall survival, and 81 of 368 deaths (22.0%) that had to have occurred for the final analysis were observed among the patients with PD-L1–positive tumors. The objective response rate among patients who received avelumab plus axitinib was double that among patients who received sunitinib, both among the patients with PD-L1–positive tumors and in the overall population (55.2% vs. 25.5% and 51.4% vs. 25.7%, respectively). These results were similar to those determined by investigator assessment.
The population enrolled in this trial consisted of patients in all three prognostic risk groups (favorable, intermediate, and poor risk) according to two sets of published criteria (MSKCC and IMDC)15,16 and patients with positive, negative, or unknown PD-L1 expression status. In this analysis, the longer progression-free survival and higher objective response rate among patients who received avelumab plus axitinib than among those who received sunitinib were observed both among the patients with PD-L1–positive tumors and in the overall population, as well as across prognostic risk groups in both populations.
The frequency and severity of adverse events observed with the combination of avelumab plus axitinib were generally consistent with the known safety profiles of avelumab17 and axitinib18 when administered as monotherapy or in combination.14 Although hypothyroidism was classified as an immune-related event in this trial, it has been recognized as an adverse event that is associated with both avelumab and axitinib,17,18 and distinguishing between possible causes of this condition is challenging. Overall, the frequency of adverse events that occurred during treatment, including events of grade 3 or higher, was similar in the two treatment groups. Hypertension and skin toxic effects were among the more common adverse events; the investigators attributed them to the VEGF inhibitor. Axitinib was combined with avelumab in this trial, rather than with the global standard-of-care sunitinib,25 because axitinib is associated with survival rates and response rates that are similar to those of other single-agent VEGFR inhibitors in the first-line treatment of renal-cell carcinoma.2,26 Also, the use of axitinib plus avelumab reduces the risk of potential adverse events, including the high incidence of hepatic toxic effects that have been observed with sunitinib and pazopanib plus immune checkpoint inhibitor–based combinations.27
In this trial, as assessed by independent review, the median progression-free survival in the overall population among patients who received sunitinib was 8.4 months (95% CI, 6.9 to 11.1). This rate was similar to or lower than that observed in other phase 3 trials of sunitinib: 11 months (95% CI, 11 to 13),28 9.5 months (95% CI, 8.3 to 11.1),29 8.3 months (95% CI, 7.0 to 9.7),30 and 12.3 months (95% CI, 9.8 to 15.2).6 Studies comparing axitinib directly with sunitinib as first-line treatment are limited.13 In a phase 3 trial comparing first-line axitinib with sorafenib, the objective response rate was 32% and the median progression-free survival was 10.1 months (95% CI, 7.2 to 12.1).31 Although the efficacy benefit for the combination could be attributed in part to a higher level of activity with axitinib than with sunitinib, the magnitude of benefit with respect to objective response and progression-free survival associated with avelumab plus axitinib as compared with sunitinib supports at least additive if not synergistic effects of the VEGF tyrosine kinase inhibitor–immune checkpoint inhibitor combination.32
In conclusion, the JAVELIN Renal 101 trial evaluated first-line therapy in patients with advanced renal-cell carcinoma. Patients who received a combination of avelumab plus axitinib had longer progression-free survival and a higher objective response rate than those who received sunitinib.
Supplementary Material
Acknowledgments
Supported by Pfizer and an alliance between Pfizer and Merck (Darmstadt, Germany). The conduct of the trial at the Memorial Sloan Kettering Cancer Center was supported in part by Memorial Sloan Kettering Cancer Center Support Grant/Core Grant (P30 CA008748).
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
We thank the patients and their families, and the investigators, coinvestigators, and trial teams at each of the centers for their participation in this trial and Shaun Rosebeck of ClinicalThinking for medical writing support.
Presented in part at the European Society for Medical Oncology Congress 2018, Munich, Germany, October 19–23, 2018, and at the American Society of Clinical Oncology Genitourinary Cancers Symposium 2019 Congress, San Francisco, February 14–16, 2019.
Contributor Information
Robert J. Motzer, Memorial Sloan Kettering Cancer Center, New York
Konstantin Penkov, Private Medical Institution Euromedservice, St. Petersburg, Russia
John Haanen, Netherlands Cancer Institute, Amsterdam
Brian Rini, Cleveland Clinic, Cleveland
Laurence Albiges, Institut Gustave Roussy, Villejuif, France
Matthew T. Campbell, University of Texas M.D. Anderson Cancer Center, Houston
Balaji Venugopal, University of Glasgow, Beatson West of Scotland Cancer Centre, Glasgow, United Kingdom
Christian Kollmannsberger, British Columbia Cancer Agency, Vancouver, Canada
Sylvie Negrier, Centre Léon Bérard, University of Lyon, Lyon, France
Motohide Uemura, Osaka University Hospital, Osaka, Japan
Jae L. Lee, University of Ulsan College of Medicine, Asan Medical Center, Seoul, South Korea
Aleksandr Vasiliev, Nonstate Health Institution Road Clinical Hospital–Russian Railways, St. Petersburg, Russia
Wilson H. Miller, Jr., Lady Davis Institute and Jewish General Hospital, McGill University, Montreal, Canada
Howard Gurney, Macquarie University, Sydney
Manuela Schmidinger, Department of Medicine I, Clinical Division of Oncology and Comprehensive Cancer Center, Medical University of Vienna, Vienna
James Larkin, Royal Marsden NHS Foundation Trust, London, United Kingdom
Michael B. Atkins, Georgetown Lombardi Comprehensive Cancer Center, Washington, DC
Jens Bedke, Department of Urology, University of Tübingen, Tübingen, Germany
Boris Alekseev, Moscow Scientific Research Oncology Institute, Moscow, Russia
Jing Wang, Pfizer, Cambridge, Massachusetts
Mariangela Mariani, Pfizer, Milan
Paul B. Robbins, Pfizer, San Diego, CA
Aleksander Chudnovsky, Pfizer, Cambridge, Massachusetts
Camilla Fowst, Pfizer Italia, Milan
Subramanian Hariharan, Pfizer, New York
Bo Huang, Pfizer, Groton, CT
Alessandra di Pietro, Pfizer, Milan
Toni K. Choueiri, Lank Center for Genitourinary Oncology at Dana–Farber Cancer Institute, and Brigham and Women’s Hospital, Boston, Massachusetts
REFERENCES
- 1.American Cancer Society. Survival rates for kidney cancer by stage. 2017. (https://www.cancer.org/cancer/kidney-cancer/detection-diagnosis-staging/survival-rates.html#references).
- 2.Choueiri TK, Motzer RJ. Systemic therapy for metastatic renal-cell carcinoma. N Engl J Med 2017;376:354–66. [DOI] [PubMed] [Google Scholar]
- 3.National Comprehensive Cancer Net-work. NCCN guidelines: kidney cancer. 2018. (https://www.nccn.org/professionals/physician_gls/PDF/kidney.pdf).
- 4.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 2007;356:115–24. [DOI] [PubMed] [Google Scholar]
- 5.Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 2015;373:1803–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med 2018;378:1277–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vaishampayan N, Schöffski P, Ravaud A, et al. First-line (1L) or second-line (2L) avelumab monotherapy in patients (pts) with advanced renal cell carcinoma (aRCC) enrolled in the phase 1b JAVELIN Solid Tumor trial. Ann Oncol 2018;29:Suppl 8: viii303–viii331 (https://oncologypro.esmo.org/Meeting-Resources/ESMO-2018-Congress/First-line-1L-or-second-line-2L-avelumab-monotherapy-in-patients-pts-with-advanced-renal-cell-carcinoma-aRCC-enrolled-in-the-phase-1b-JAVELIN-Solid-Tumor-trial). [Google Scholar]
- 8.Gulley JL, Rajan A, Spigel DR, et al. Avelumab for patients with previously treated metastatic or recurrent non-small-cell lung cancer (JAVELIN Solid Tumor): dose-expansion cohort of a multicentre, open-label, phase 1b trial. Lancet Oncol 2017;18:599–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaufman HL, Russell J, Hamid O, et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Lancet Oncol 2016; 17:1374–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel MR, Ellerton J, Infante JR, et al. Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN Solid Tumor): pooled results from two expansion cohorts of an open-label, phase 1 trial. Lancet Oncol 2018;19:51–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roland CL, Lynn KD, Toombs JE, Dineen SP, Udugamasooriya DG, Brekken RA. Cytokine levels correlate with immune cell infiltration after anti-VEGF therapy in preclinical mouse models of breast cancer. PLoS One 2009;4(11):e7669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rini BI, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet 2011;378:1931–9. [DOI] [PubMed] [Google Scholar]
- 13.Motzer RJ, Escudier B, Tomczak P, et al. Axitinib versus sorafenib as second-line treatment for advanced renal cell carcinoma: overall survival analysis and updated results from a randomised phase 3 trial. Lancet Oncol 2013;14:552–62. [DOI] [PubMed] [Google Scholar]
- 14.Choueiri TK, Larkin J, Oya M, et al. Preliminary results for avelumab plus axitinib as first-line therapy in patients with advanced clear-cell renal-cell carcinoma (JAVELIN Renal 100): an open-label, dose-finding and dose-expansion, phase 1b trial. Lancet Oncol 2018;19:451–60. [DOI] [PubMed] [Google Scholar]
- 15.Motzer RJ, Bacik J, Murphy BA, Russo P, Mazumdar M. Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol 2002;20:289–96. [DOI] [PubMed] [Google Scholar]
- 16.Heng DY, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol 2009;27:5794–9. [DOI] [PubMed] [Google Scholar]
- 17.Bavencio (avelumab) injection. Darmstadt, Germany:Merck KGaA, 2018. (package insert). [Google Scholar]
- 18.Inlyta (axitinib). New York:Pfizer, 2014. (package insert). [Google Scholar]
- 19.Rao D, Butt Z, Rosenbloom S, et al. A comparison of the Renal Cell Carcinoma-Symptom Index (RCC-SI) and the Functional Assessment of Cancer Therapy-Kidney Symptom Index (FKSI). J Pain Symptom Manage 2009;38:291–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rothrock NE, Jensen SE, Beaumont JL, et al. Development and initial validation of the NCCN/FACT Symptom Index for advanced kidney cancer. Value Health 2013;16:789–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lan KKG, DeMets DL. Discrete sequential boundaries for clinical trials. Biometrika 1983;70:659–63. [Google Scholar]
- 22.Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika 1934; 26:404–13. [Google Scholar]
- 23.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958;53:457–81. [Google Scholar]
- 24.Jennison C, Turnbull BW. Repeated confidence intervals for group sequential clinical trials. Control Clin Trials 1984;1: 33–45. [DOI] [PubMed] [Google Scholar]
- 25.Sutent (sunitinib). New York: Pfizer, 2011. (package insert). [Google Scholar]
- 26.Turajlic S, Swanton C, Boshoff C. Kidney cancer: the next decade. J Exp Med 2018;215:2477–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amin A, Plimack ER, Infante JR, et al. Nivolumab (anti-PD-1; BMS-936558, ONO-4538) in combination with sunitinib or pazopanib in patients (pts) with metastatic renal cell carcinoma (mRCC). J Clin Oncol 2014;32:5010 (http://ascopubs.org/doi/10.1200/jco.2014.32.15_suppl.5010). [Google Scholar]
- 28.Motzer RJ, Hutson TE, Tomczak P, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol 2009;27:3584–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Motzer RJ, Hutson TE, Cella D, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med 2013; 369:722–31. [DOI] [PubMed] [Google Scholar]
- 30.Motzer RJ, Powles T, Atkins MB, et al. IMmotion151: a randomized phase III study of atezolizumab plus bevacizumab vs sunitinib in untreated metastatic renal cell carcinoma (mRCC). J Clin Oncol 2018; 36:578 (http://ascopubs.org/doi/10.1200/JCO.2018.36.6_suppl.578). [Google Scholar]
- 31.Hutson TE, Lesovoy V, Al-Shukri S, et al. Axitinib versus sorafenib as first-line therapy in patients with metastatic renal-cell carcinoma: a randomised open-label phase 3 trial. Lancet Oncol 2013;14:1287–94. [DOI] [PubMed] [Google Scholar]
- 32.Yasuda S, Sho M, Yamato I, et al. Simultaneous blockade of programmed death 1 and vascular endothelial growth factor receptor 2 (VEGFR2) induces synergistic anti-tumour effect in vivo. Clin Exp Immunol 2013;172:500–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.