Abstract
Previous adverse pregnancy outcomes (APO) in women with hereditary thrombophilia have emerged as new indications for prophylactic use of low-molecular-weight heparin (LMWH) during pregnancy. Recent meta-analysis conducted to establish if LMWH may prevent recurrent placenta-mediated pregnancy complications point to important therapeutic effect but these findings are absolutely not universal. Furthermore, previous studies regarding LMWH prophylaxis for APO in women with inherited thrombophilia were performed in high risk patients with previous adverse health outcomes in medical, family and/or obstetric history. Therefore, the aim of this study was to investigate the effects of LMWH prophylaxis on pregnancy outcomes in women with inherited thrombophilias regardless of the presence of previous adverse health outcomes in medical, family, and obstetric history.
Prospective analytical cohort study included all referred women with inherited thrombophilia between 11 and 15 weeks of gestation and followed-up to delivery. Patients were allocated in group with LWMH prophylaxis (study group) and control group without LWMH prophylaxis. The groups were compared for laboratory parameters and Doppler flows of umbilical artery at 28th to 30th, 32nd to 34th and 36th to 38th gestational weeks (gw), and for obstetric and perinatal outcomes.
The study group included 221 women and control group included 137 women. Mean resistance index of the umbilical artery Ri in 28 to 30, 32 to 34, and 36 to 38 gw were significantly higher in the control group compared to study group (0.71 ± 0.02 vs 0.69 ± 0.02; 0.67 ± 0.03 vs 0.64 ± 0.02; and 0.67 ± 0.05 vs 0.54 ± 0.08, respectively). Intrauterine fetal death (IUFD) and miscarriages were statistically significantly more frequent in control group compared to the patients in study (P < .001). The frequencies of fetal growth restriction (FGR) and APO were significantly higher in the control group compared to the study group (P = .008 and P < .001, respectively). In a multivariate regression model with APO as a dependent variable, only Ri was detected as a significant protective factor for APO, after adjusting for age and LMWH prophylaxis (P < .001).
We have demonstrated better perinatal outcomes in women with LMWH prophylaxis for APO compared to untreated women.
Keywords: adverse pregnancy outcomes, fetal growth restriction, low-molecular-weight heparin prophylaxis
1. Introduction
In recent years, hereditary thrombophilias are in the focus of research in all clinical fields, including obstetrics and gynecology. For many decades inherited thrombophilias have been linked with venous thromboembolism (VTE) in pregnancy and the puerperium[1] and recurrent miscarriages.[2] However, since recently they have also been recognized as risk factors for numerous adverse pregnancy outcomes (APO), such as preeclampsia,[3] intrauterine growth restriction,[4] placental abruption,[5] and stillbirth.[6] Kupfermink et al showed that as much as 65% of women with preeclampsia, unexpected still birth, placental abruption, and fetal growth restriction (FGR) had some form of inherited thrombophilias.[7]
Prerequisite for successful pregnancy is adequate development and function of placental circulation. Abnormal placentation alongside with placental vascular thrombosis is at least partly responsible for placenta-mediated pregnancy complications. Women with inherited trombophilias develop micro-thrombi in vascular placental bed more frequently compared to women without trombophilias.[8] Such thrombi in utero-placental circulation may lead to a reduction in trophoblast invasion, chronic hypoxia, and placental dysfunction, followed by APO.[9,10] Such observations have initiated the hypothesis that anticoagulants may prevent placental thrombosis and thus placenta-mediated pregnancy complications.[11] Recent meta-analysis conducted to establish if low-molecular-weight heparin (LMWH) may prevent recurrent placenta-mediated pregnancy complications point to important therapeutic effect but these findings are absolutely not universal.[11] Therefore, previous APO due to hereditary thrombophilia have emerged as potential new indication for use of anticoagulants during pregnancy.[12] Considering the biological plausibility of thrombotic mechanisms and the potential of LMWHs to influence it, it is important to consider if LMWHs may have a role not only in the prevention but also in the stopping progression of condition, especially after the reports from TIPPS and FRUITS studies.[13,14] There are data indicating the potential benefit of LMWH on the implantation and placental development. However, all above-mentioned studies have been performed in women with inherited trombophilias belonging to high risk population due to their uneventful medical, family, or obstetric history.
The absence of a strong and reliable evidence base to support clinical guidelines leads to conflicting recommendations in clinical guidelines even on thromboprophylaxis in pregnant women with inherited thrombophilias, such as from the Royal College of Obstetricians and Gynaecologists[15] compared with the American College of Chest Physicians.[16] The circumstances are even more confusing regarding LMWH prophylaxis of APO. Nevertheless, the prevalent use of prophylactic LMWH treatment for pregnant women with thrombophilia and a history of APO created on the logical relationship between thrombotic processes and these complications has prevailed the scarceness of data supporting this treatment. So far, there is no strong evidence supporting antenatal use of LMWH in pregnant women with inherited thrombophilia and previous placenta-mediated complications.[17] The situation is yet more unclear concerning pregnant women with inherited thrombophilia without previous adverse health outcomes in medical, family, and obstetric history. Consequently and not surprisingly, different approaches and protocols regarding LMWH prophylaxes of APO are applied sometimes even in the same clinic.[18] Approach regarding LMWH prophylaxis of APO in women with inherited thrombophilias in our clinic differs between various attending obstetricians. Therefore we aimed to evaluate prophylactic use of LWMH on pregnancy outcomes in women with inherited thrombophilias regardless of the presence of previous adverse health outcomes in medical, family, and obstetric history, thereby testing the hypothesis that prophylactic use of LMWH in such population could decrease the incidence of APO.
2. Methods
This prospective analytical cohort study included all women with inherited thrombophilia between 11 and 15 weeks of gestation referred to Clinic for Gynecology and Obstetrics of the Clinical Centre of Serbia between 1st January 2016 and 1st August 2018 and followed-up to delivery. The study was approved by the Ethic Committee (decision No 2650/IV-13) of School of Medicine, University in Belgrade. Written informed consent was obtained from all study participants. After signing informed consent, detailed medical, family, and obstetric history have been obtained. When obtaining medical histories, particular attention was given to comorbidities that might imply obligatory routine thrombosis prophylaxis, for instance pulmonary embolism (PULME), deep venous thrombosis (DVT) and venous thromboembolism (VTE), and those comorbidities that are risk factors for APO, such as subclinical thyroid dysfunction (TD), insulin resistance (IR), and cigarette smoking. Also, we focused on those comorbidities that might imply obligatory routine thrombosis prophylaxis, such PULME, DVT, VTE, hypertension (HA), myocardial infarction (MI), and cerebrovascular insults (CVI). Detailed data were also obtained on previous conditions and events that could have effects on APO in current pregnancy, such as preterm birth, preeclampsia, placental abruption, and others. Furthermore, in order to identify potential other confounding factors, all participants were given a questionnaire on demographic characteristics (age, race, marital status, and educational level), environmental exposures and life style factors that may affect pregnancy outcomes (exposure to air pollution, environmental tobacco smoke, pesticides, solvents, metals, radiation, water contaminants such as disinfection by-products, arsenic, and nitrates, chemicals such as persistent organic pollutants, Bisphenol A, and phthalates, caffeine intake, alcohol, and marijuana consumption and exercise).
We have excluded women with age over 40 years, severe obesity (BMI over 40), acquired thrombophilia, transplanted organs, congenital anomalies of the uterus, previous gynecological surgeries, and chronic diseases that could influence the outcome of pregnancy (type 1 diabetes, chronic hypertension, chronic kidney diseases, overt hyperthyroidism, and hypothyroidism, comorbidities that require the use of anticoagulant therapy), pregnancy achieved by oocyte donation, and multiple pregnancy. Since the participants were followed-up to delivery, we have further excluded the patients with confirmed congenital fetal anomalies, abnormal fetal karyotype, placenta previa, and placenta accrete and confirmed perinatal infections.
All study participants were subjected in study group who underwent prophylactic LMWH treatment or in the control group with participants who were not subjected to prophylactic LMWH treatment. Allocation of participants into 1 of these 2 study groups was done by the decision of those attending physicians who were ultimately responsible for all aspects of patient care. Furthermore, the choice of the type of applied LMWH was made by the preference of attending physicians and by availability of different types of LMWHs at the time of patient treatment. The decision for this kind of allocation of study participants was based on the fact that this was an observational study, not interventional. Furthermore, as previously mentioned, since there is no consensus about LMWH prophylaxis of APO, different approaches exist worldwide. The same situation is in our clinic, where approaches regarding LMWH prophylaxis of APO in women with inherited thrombophilias, differ between various obstetricians.
All study participants underwent routine pregnancy check-ups such as second trimester ultrasound between 18th and 22nd week of gestation, evaluation of blood pressure, 24-hour urine protein test, and vaginal and cervical smear, and in all cases with suspicious infection the TORCH test was performed. Apart from these routine check-ups, all study participants underwent evaluation of ultrasound Doppler measurements of Resistance index (Ri) of Umbilical artery and laboratory parameters (platelet count, serum proteins, D-dimer) prospectively at 3 study time points: 28th to 30th, 32nd to 34th, and 36th to 38th gestational weeks.
The participants were followed up until delivery which enabled the evaluation of primary study objectives. These were the rates of: miscarriage, intrauterine fetal death (IUFD) defined as fetal demise after 20th gestational week, live birth, perinatal mortality, preterm birth, FGR, placental abruption, gestational hypertension, and preeclampsia. Perinatal mortality rate is defined as the sum of number of stillbirths and neonatal deaths divided by 1000 of total birth. FGR was defined as a weight below 10th percentile for the gestational age. Placental abruption was defined as a clinical triad composed of uterine hyper-contractility or hyperactivity, overt or obscure uterine bleeding. Gestational hypertension was defined as new-onset hypertension (≥140 mm Hg systolic or ≥90 mm Hg diastolic blood pressure) arising after 20 weeks’ gestation. Preeclampsia was defined as blood pressure of 140 mm Hg systolic or 90 mm Hg diastolic or higher that occurs after 20 week of gestation in a woman with previously normal blood pressure, together with proteinuria defined as urinary excretion of 0.3 g protein or higher in a 24 hours specimen.
Secondary study objective was to evaluate Ri of Umbilical artery, as well as laboratory parameters such as platelet count in order to diagnose or confirm thrombocytopenia, serum proteins and D-dimer. They were prospectively evaluated at 28th to 30th, 32nd to 34th, and 36th to 38th gestational week. Other secondary study objective was the estimation of the mode of delivery, gestational age at delivery, newborn body weights, and Apgar Scores.
2.1. Statistical analysis
Descriptive statistics on patients’ demographic characteristics were reported as mean with standard deviation. Categorical data were presented as numbers with percentages. Differences between groups were analyzed using Student t test and Mann–Whitney U test for numeric variables, and the Pearson Chi-Squared and Fisher exact test for categorical variables. The univariate and multivariate logistic regressions were used to determine the independent predictors for APO, adjusted for age and therapy. Adjustment variables were included in the multivariate regression analysis if they were significant at the P < .001 level according to the results of the univariate analysis. Results were expressed as odds ratios (OR), and their 95% confidence intervals (CI). Spearman correlation coefficients were calculated to explore the relationships between the routine measurement of Doppler flow artery umbilical presented with resistance index (Ri), D-dimmer level, weeks of gestation and birth weight. All tests were 2-tailed. P < .05 was considered statistically significant. All analyses were conducted using the Statistical Package for the Social Sciences (IBM SPSS, version 21). A sample size of 358 participants (221 in study and 137 in control group) would result in over 80% power to detect a 0.2 difference of proportion of APO between the groups at the 5% level of significance.
3. Results
After implementation of study inclusion and exclusion criteria, initial number of potentially eligible participants was 437. When examined for eligibility, 401 were confirmed as eligible, and initially included in the study. During follow-up of study participants, due to the confirmed congenital fetal anomalies, abnormal fetal karyotype, placenta previa and placenta accrete, and confirmed perinatal infections and as well lost patients during follow-up the total number of eligible subjects was 358. All qualified study participants were Caucasians and the average age of participants was 33.67 ± 4.01 years. The study group included 221 women and control group comprised 137 women. Following types of inherited thrombophilias were present in our study participants: methylene tetrahydrofolate reductase C677T (MTHFR), Plasminogen activator inhibitor (PAI) type 1 (PAI–1), Factor V Leiden (FVL), and Prothrombin gene mutation G20210 (PT). Besides, combined thrombophilia (more than 1 thrombophilia mutation in 1 patient) was present in 22 patients, 17 in study group, and 5 in control group. Significant difference was not found between those inherited thrombophilias with the highest odds related to APO (such as combined thrombophilia, PVL and PT) and those with lower odds (such as PAI and MTHFR) associated with APO (Table 1). Study groups were similar regarding demographic characteristics, environmental exposures, life style factors, and adverse health outcomes recorded in medical, family, and obstetric history. Significantly higher prevalence of subclinical thyroid dysfunction in medical history was present in study group compared to control group. A positive family history of HA and DVT was more frequent in the treated group, and these differences were statistically significant (P = .005 and P = .024, respectively). A large number of patients in both groups had previous APO but the differences between groups were not significant. The characteristics of study groups and distribution of different types of inherited thrombophilias are shown in Table 1.
Table 1.
Characteristics of study participants.
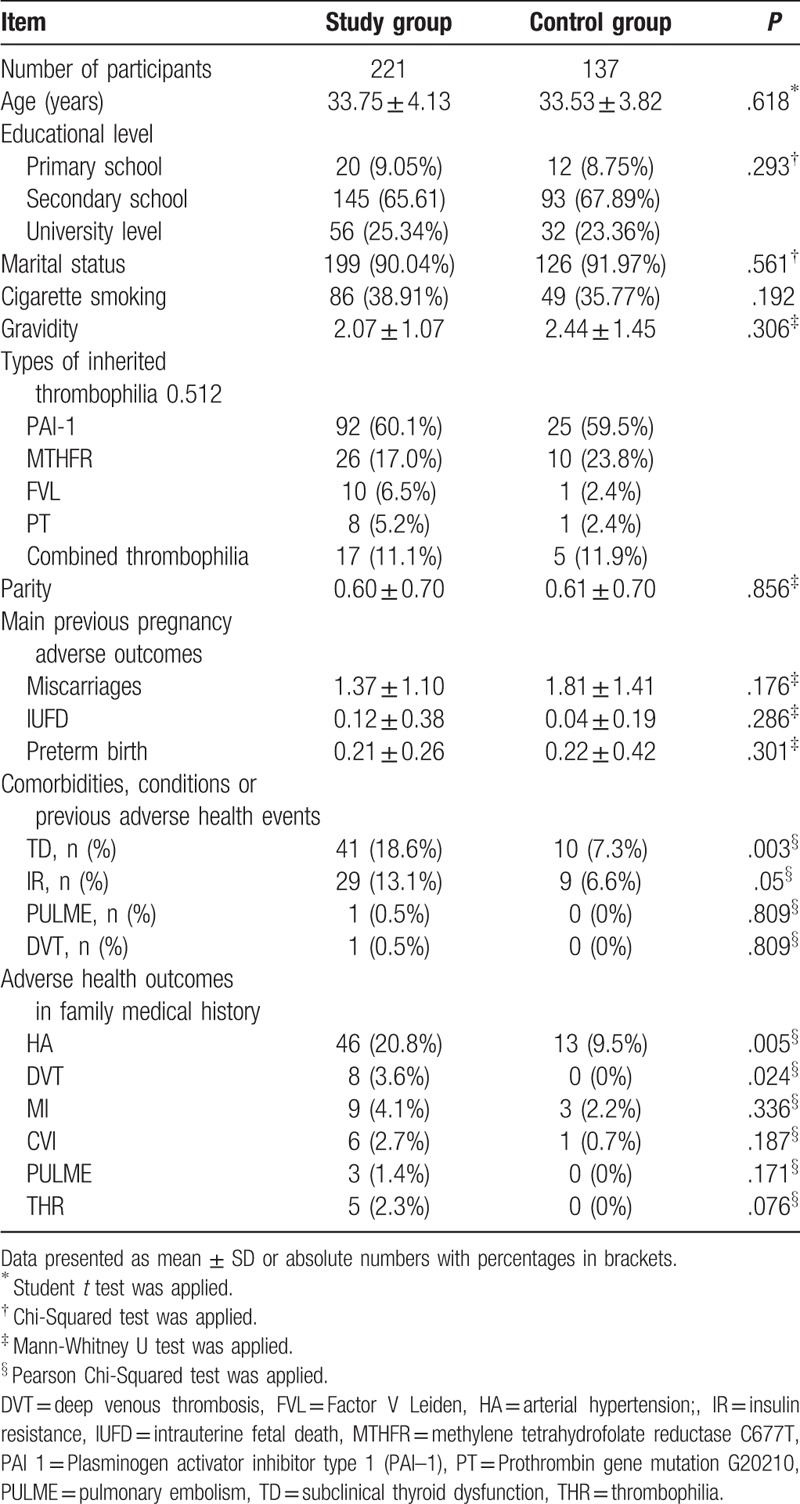
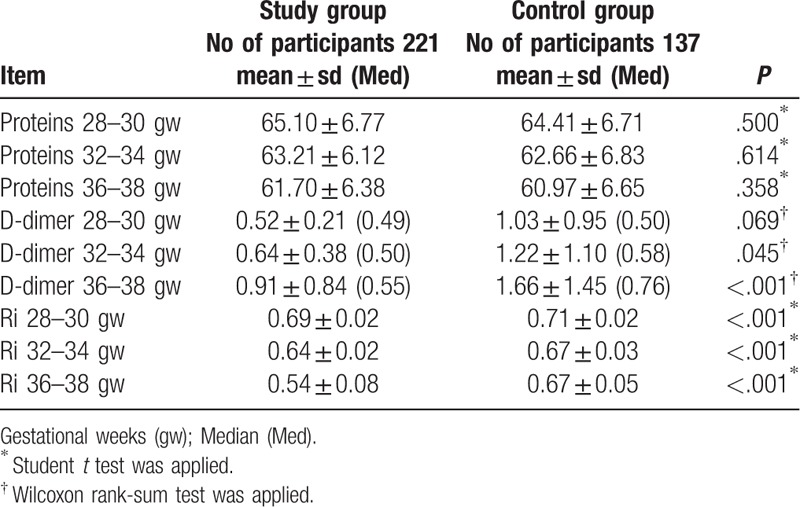
The largest number of participants in study group received nadroparin (71.3%), dalteparin (17.8%), enoxaparin (10.9%). All cases of thrombocytopenia, 1 in study group (0.45%) and 2 in control group (1.46%), were observed only at gestational age ranged between 36th and 38th weeks of gestation, without significant difference in frequency between study groups (P = .341) and with OR 0.33 in 95% CI ranging from 0.03 to 3.66. Significant differences were not found in the mean serum protein levels at each study time points and D-dimer at 28th to 30th weeks of gestation between the groups. However, D-dimer at 32nd to 34th week's gestation and 36th to 38th weeks of gestation as well Doppler indices of Umbilical arteries at each study time points were significantly different between study groups (Table 2).
Table 2.
Laboratory parameters and Doppler indices of Umbilical artery.
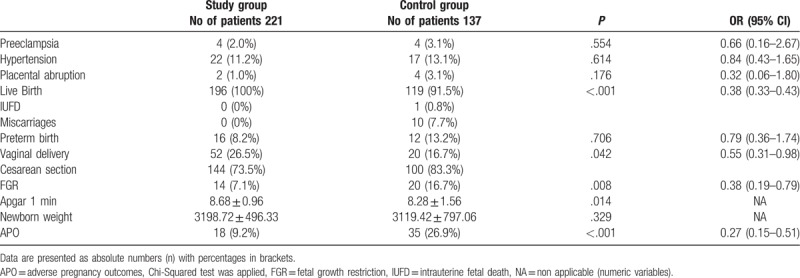
Preeclampsia, hypertension, placental abruption, and thrombocytopenia were more common in patients without LMWH prophylaxis compared to the group with LMWH prophylaxis, but the differences were not significant (Table 3). The majority of patients with anticoagulation therapy (91.8%) delivered at 38/39 weeks of gestation while there were 8.2% of premature deliveries. In study group IUFD and miscarriages have not been recorded. In control group 1 IUFD and 10 miscarriages have been recorded, 82.3% participants gave birth at 38/39 weeks of gestation, while 13.2% had preterm birth. Significantly higher prevalence of IUFD and miscarriages were present in control group compared to the study group (P < .001). In the study group, 26.5% of patients had vaginal delivery while 16.7% of patients in the control group had vaginal delivery. Vaginal delivery was significantly more frequent in the study group (P = .042).
Table 3.
Pregnancy perinatal and delivery outcomes.
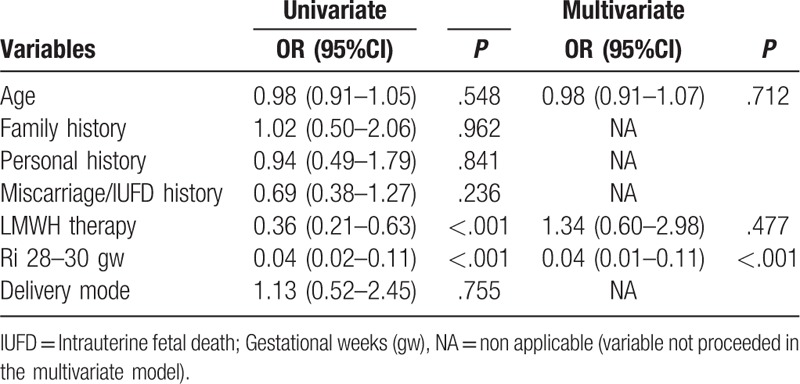
According to the results of the univariate analysis (Table 4), LMWH prophylaxis and Ri were significant protective factors for APO (Table 4). Women with LMWH prophylaxis were 64% less likely to have APO than patients without prophylaxis (OR = 0.36; 95% CI: 0.21–0.63). Women with normal Ri were 96% less likely to have APO than patients with elevated Ri (OR = 0.04; 95% CI: 0.02–0.11). In a multivariate regression model with APO as a dependent variable, only Ri was detected as a significant protective factor, after adjusting for age and LMWH prophylaxis (P < .001). Women with normal Ri were 96% less likely to have APO than patients with elevated Ri (OR = 0.04; 95% CI: 0.01–0.11).
Table 4.
Logistic regression analysis.
4. Discussion
This study compared 2 approaches in management of pregnant women with inherited thrombophilias regarding APO. One is conventional approach without use of LMWH, and the other 1 uses LMWH for targeting potential prophylaxis of APO. Our study provides further evidence that supports the usefulness of the LMWH in pregnant women with inherited thrombophilia for prevention of APO. Evaluation of primary study outcomes has revealed better perinatal outcomes in women with LMWH prophylaxis compared to women managed with conventional approach. An important finding among secondary study outcomes was significantly higher umbilical artery Ri in control group compared to study group in all 3 study time points. After adjusting for LMWH prophylaxis and age as potential confounding factors, multivariate regression analysis revealed increased Ri as an independent predictor for APO.
Significant differences in rate of FGR between the groups and calculated OR could indicate the beneficial use of LMWHs in preventing FGR. Maternal malnutrition, low serum protein levels, and smoking are risk factors for FGR. Absence of significant differences in mean serum protein levels and frequencies of smokers between the study groups highlight the strength of assumed association between use of LMWHs and reduction of incidence FGR. Moreover, it could be assumed that one of the reasons for FGR in women with inherited thrombophilias is impaired utero-placental circulation. This was pointed by significantly higher values of D-dimer from 32nd gw and umbilical artery Ri at all 3 study time points in control group comparing to the study group. Women with FGR and/or preeclampsia have serious changes in the fibrinolytic activity comparing to healthy pregnant women.[19] Furthermore, increase of D-dimer may be associated with APO, such as FGR and preeclampsia.[20,21,22] Additionally, a case-controlled study that compared the incidence of FGR between healthy women and women with inherited thrombophilias without LMWH therapy revealed that LMWH untreated inherited thrombophilias represents risk factors for FGR.[4]
Mean values of umbilical artery Ri measured on all 3 study time points (28th to 30th, 32nd to 34th and 36th to 38th weeks of gestation) were significantly higher in the control group compared to LMWH treated group. This is in accordance with the study performed in non-thrombophilic women with history of preeclampsia.[23] All these findings suggest the role of LMWHs in the reduction of resistance of utero-placental flow. Umbilical artery resistance reflects the resistance in placental blood vessels and constantly decreases as result of placental development.[24] Insufficient placental development, followed by the failure in reduction of umbilical artery resistance, is usually associated with FGR, preeclampsia, and other APO.[24] Furthermore, placentas of women with inherited thrombophilias show features of abnormal placental development and maternal placental vascular under-perfusion.[8] This was further supported by Trudinger and Giles, who have demonstrated higher Ri of umbilical artery in women with inherited thrombophilias and pregnancy complications. This was caused by increased resistance of small placental vessels due to thrombotic lesions observed on the pathologic examination of the placenta in those women.[25] Both in vitro[26] and in vivo[27] experiments have demonstrated that LMWHs decrease vascular resistance by direct anti-inflammatory and antithrombotic effects on utero-placental vessels.[28] Inflammatory mechanisms are at play at the maternal–fetal interface and have influence on placental development. Natural killer (NK) cells are producing several angiogenic factors essential for angiogenesis, placentation, remodeling of decidual vessels, and uterine spiral arteries and therefore are important for satisfactory placental perfusion in normal pregnancy.[29] On the other hand, excessively increased NK cell fractions decrease uterine blood flow through pro-inflammatory actions of NK cells, leading to inflammation and thrombosis in decidual vessels. Koo and colleagues have confirmed a positive correlation between NK cell fractions with Ri of utero-placental vessels in pregnant women with history of recurrent miscarriages.[30] Increased resistance was especially evident in women with elevated NK cell fractions. Moreover, mean Ri of utero-placental vessels was significantly decreased 1 week after LMWH treatment.[30] LMWHs durably improve uterine blood flow and pregnancy outcomes particularly in women with decreased uterine blood flow and poor obstetric histories,[31] while others report that LMWHs only transitorily improve utero-placental circulation in women with thrombophilia at risk of severe APO.[32]
Preeclampsia, hypertension, and placental abruption were more common in patients without LMWH prophylaxis, which is in line with other studies,[14,23,33,34] but in contrast with the study of Martinelli and colleagues who have also evaluated all types of preeclampsia.[35] Surprisingly, our results regarding all preeclampsia types are in line with the study of Gris et al who have included only women with severe or early onset preeclampsia.[34]
LMWH prophylaxis resulted in significantly lower rates of miscarriages and IUFD compared with control group, which is in accordance with other studies.[33,34,36] While Aracic et al demonstrated that regardless the type of thrombophilia, LMWHs significantly improved pregnancy outcomes by decreasing the rate of miscarriages,[36] Foka and colleagues found that FVL and PT mutations, but not MTHFR, were associated with miscarriages rates.[37]
No differences were found in preterm birth rates between study groups, which is in line with Abheiden and colleagues,[38] but in contrast to the results of Aracic et al[39] and to meta-analysis performed by Roger and colleagues.[11] Vaginal deliveries were more common in study group compared to controls, which is in contrast to the results of Martinelli and colleagues.[35] However, they have prematurely stopped the study because of funding issues and slow recruitment rate, which could influence the results regarding study outcomes.
Even though there are a large number of studies, as well as protocols, the impression is that a joint consensus about the administration of LMWH prophylaxis in women with inherited thrombophilias has not yet achieved. It is obvious that we need joint contribution and summation of insights of physicians’ specialized in different area of medicine, together with individual, tailored-made approach to each patient. Our study demonstrates that conventional approach without LMWH prophylaxis and the increase of umbilical artery Ri were predictors of APO. Multivariate regression model demonstrated that the increase of umbilical artery Ri is the independent predictor of APO in women with inherited thrombophilias.
The present study is prospective observational cohort study, not randomized clinical trial. Therefore, LMWH prophylaxes for APO as exposure and pregnancy outcomes were ascertained almost concurrently. Furthermore, these types of studies have in general low internal validity. Moreover, our control group was not comparable to the study group in all ways. Subclinical thyroid dysfunction was more prevalent in participants in study group compared to those in control group. Furthermore, arterial hypertension and deep venous thrombosis in family history were more prevalent in participants of study group. Deep venous thrombosis in family history had impact on decision of attending obstetrician for LMWH prophylaxis according to present guidelines for thrombophrophylaxis in pregnancy.[15,16] Similarly, subclinical thyroid dysfunction could affect the outcome of pregnancy. All above mentioned facts we acknowledge as the limitations of study. However, we have used sophisticated techniques, such as multivariate regression analysis, to account for confounding.
Antenatal screening for plasminogen, Protein C and S deficiency is expensive and in our health care system reserved only for high risk patients. Consequently, we were not able to screen these thrombophilias in all participants and therefore they have not been evaluated in our study and we acknowledged this issue as the limitation of the study.
This study aimed to draw implications about the effect of LMWH prophylaxis for APO in women with inherited thrombophilias, where the assignment of participants to groups is observed instead of influenced through randomization by the investigator. In order to answer research question, we have used primary data, collected by the investigation team for the purpose of the study, instead of already collected data for another purpose but used to examine a research question, which we consider as the strength of the study. We were able to control data collection and follow-up methods. The prospective design of study, with thorough personal, family, reproductive histories obtained before the follow-up of the participants lessened recall bias. Moreover, the prospective nature of the study meant it was also possible to estimate the time course of events, or to be precise, to determine whether LMWH prophylaxis influenced succeeding pregnancy outcomes. Therefore, we were able to identify the population at risk for APO among our study participants. Furthermore, to the best of our knowledge, this is the first study that evaluated LMWH prophylaxis for APO in women with hereditary thrombophilias regardless to previous adverse health outcomes in personal, family or reproductive history. External validity is achieved by a relatively large number of study participants obtained from a population from multiple geographical localities, instead of single geographic location. Our clinic is the tertiary health care center and the biggest clinic in this region of Europe with high risk patients referred not only from all parts of Serbia, but also from neighboring countries, such as Montenegro, Republic of North Macedonia, Bosnia, and Herzegovina. Because of this, we could assume that the assumptions drawn about cause-effect relationships apply to people in other geographic locations.
Presented results demonstrate better pregnancy, perinatal and delivery outcomes in women with inherited thrombophilia managed with LMWH prophylaxis compared to those with conventional approach, which implies medical surveillance alone. Conventional approach and increase of Umbilical artery Ri were predictors of APO. However, since the umbilical artery Ri is independent predictor of APO, we believe that Doppler evaluation should be considered as a possible tool for redefining approach to women with inherited thrombophilias with LMWH prophylaxis for APO. However, randomized clinical trials are needed to evaluate this relationship, and to confirm the role of LMWH prophylaxis for APO. We believe the future research could also focus on the possible role of increased Doppler resistance indices of umbilical artery in the guidance of management of women with inherited thrombophilia and LMWH prophylactic usage.
Author contributions
Conceptualization: Stefan Dugalic, Milos Petronijevic, Milan Perovic.
Data curation: Stefan Dugalic, Aleksandar Stefanovic, Katarina Stefanovic, Svetlana Vrzic Petronijevic, Nemanja Milincic.
Formal analysis: Stefan Dugalic, Dejana Stanisavljevic, Nemanja Milincic.
Investigation: Stefan Dugalic, Milos Petronijevic, Aleksandar Stefanovic, Katarina Stefanovic, Svetlana Vrzic Petronijevic, Sonja Perkovic Kepeci, Nemanja Milincic.
Methodology: Stefan Dugalic, Milos Petronijevic, Igor Pantic, Milan Perovic.
Resources: Stefan Dugalic, Milos Petronijevic, Aleksandar Stefanovic, Katarina Stefanovic, Svetlana Vrzic Petronijevic.
Software: Dejana Stanisavljevic, Sonja Perkovic Kepeci.
Supervision: Milos Petronijevic, Igor Pantic, Milan Perovic.
Validation: Milos Petronijevic, Aleksandar Stefanovic, Milan Perovic.
Visualization: Stefan Dugalic, Svetlana Vrzic Petronijevic, Dejana Stanisavljevic, Sonja Perkovic Kepeci.
Writing – original draft: Stefan Dugalic, Aleksandar Stefanovic, Katarina Stefanovic.
Writing – review & editing: Igor Pantic, Milan Perovic.
Footnotes
Abbreviations: APO = adverse pregnancy outcomes, CVI = cerebrovascular insults, DVT = deep venous thrombosis, FGR = fetal growth restriction, FVL = Factor V Leiden, HA = hypertension, IR = insulin resistance, IUFD = intrauterine fetal death, LMWH = low-molecular-weight heparin, MI = myocardial infarction, MTHFR = methylene tetrahydrofolate reductase C677T, NK= Natural killer, PAI–1= Plasminogen activator inhibitor (PAI) type 1, PT = prothrombin gene mutation G20210, PULME = pulmonary embolism, Ri = resistance index, TD = subclinical thyroid dysfunction, VTE = venous thromboembolism, VTE = venous thromboembolism.
Stefan Dugalic is a member of Research Project 175025 of The Ministry of Education and Science, Republic of Serbia; Milan Perovic, Igor Pantic, and Stefan Dugalic are members of Research Project 62013 of the Mediterranean Society for Metabolic Syndrome, Diabetes and Hypertension in Pregnancy.
The data used to support the findings of this study are available from the corresponding author upon request.
The authors do not have any conflicts of interest to report.
References
- [1].Zotz RB, Gerhardt A, Scharf RE. Inherited thrombophilia and gestational venous thromboembolism. Best Pract Res Clin Haematol 2003;16:243–59. [DOI] [PubMed] [Google Scholar]
- [2].Middeldorp S. Thrombophilia and pregnancy complications: cause or association? J Thromb Haemost 2007;5Suppl 1:276–82. [DOI] [PubMed] [Google Scholar]
- [3].Deveer R, Engin-Ustun Y, Akbaba E, et al. Association between pre-eclampsia and inherited thrombophilias. Fetal Pediatr Pathol 2013;32:213–7. [DOI] [PubMed] [Google Scholar]
- [4].Dugalić S, Petronijevic M, Stefanovic A, et al. The association between IUGR and maternal inherited thrombophilias: a case-control study. Medicine (Baltimore) 2018;97:e12799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Procházka M, Lubuský M, Slavík L, et al. Frequency of selected thrombophilias in women with placental abruption. Aust N Z J Obstet Gynaecol 2007;47:297–301. [DOI] [PubMed] [Google Scholar]
- [6].Simchen MJ, Ofir K, Moran O, et al. Thrombophilic risk factors for placental stillbirth. Eur J Obstet Gynecol Reprod Biol 2010;153:160–4. [DOI] [PubMed] [Google Scholar]
- [7].Kupferminc MJ, Rimon E, Many A, et al. Low molecular weight heparin treatment during subsequent pregnancies of women with inherited thrombophilia and previous severe pregnancy complications. J Matern Fetal Neonatal Med 2011;24:1042–5. [DOI] [PubMed] [Google Scholar]
- [8].Franco C, Walker M, Robertson J, et al. Placental infarction and thrombophilia. Obstet Gynecol 2011;117:929–34. [DOI] [PubMed] [Google Scholar]
- [9].Reynold L, Borowicz P, Caton J, et al. Uteroplacental vascular development and placental function: an update. Int J Dev Biol 2010;54:355–66. [DOI] [PubMed] [Google Scholar]
- [10].Brosens I, Pijnenborg R, Vercruysse L, et al. The “Great Obstetrical Syndromes” are associated with disorders of deep placentation. Am J Obstet Gynecol 2011;204:193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Rodger MA, Carrier M, Le Gal G, et al. Low-Molecular-weight heparin for placenta-mediated pregnancy complications study group. Meta-analysis of low-molecular-weight heparin to prevent recurrent placenta-mediated pregnancy. Blood 2014;123:822–8. [DOI] [PubMed] [Google Scholar]
- [12].Montavon C, Hoesli I, Holzgreve W, et al. Thrombophilia and anticoagulation in pregnancy: indications, risks and management. J Matern Fetal Neonatal Med 2008;21:685–96. [DOI] [PubMed] [Google Scholar]
- [13].Rodger MA, Hague WM, Kingdom J, et al. Antepartum dalteparin versus no antepartum dalteparin for the prevention of pregnancy complications in pregnant women with thrombophilia (TIPPS): a multinational open-label randomised trial. Lancet 2014;384:1673–83. [DOI] [PubMed] [Google Scholar]
- [14].de Vries JI, van Pampus MG, Hague WM, et al. FRUIT Investigators. Low-molecular-weight heparin added to aspirin in the prevention of recurrent early-onset pre-eclampsia in women with inheritable thrombophilia: The FRUIT-RCT. J Thromb Haemost 2012;10:64–72. [DOI] [PubMed] [Google Scholar]
- [15].Green-Top Guideline No. 37a. London: Royal College of Obstetricians and Gynaecologists; Reducing the Risk of Venous Thromboembolism during Pregnancy and the Puerperium. 2015. https://www.rcog.org.uk/globalassets/documents/guidelines/gtg-37a.pdf Accessed February 30, 2019. [Google Scholar]
- [16].Bates SM, Greer IA, Middeldorp S, et al. VTE, thrombophilia, antithrombotic therapy, and pregnancy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;1412 Suppl:e691S–736S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ormesher L, Simcox L, Tower C, et al. Management of inherited thrombophilia in pregnancy. Womens Health (Lond) 2016;12:433–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Roeters van Lennep JE, Meijer E, Klumper FJ, et al. Prophylaxis with low-dose low-molecular-weight heparin during pregnancy and postpartum: is it effective? J Thromb Haemost 2011;9:473–80. [DOI] [PubMed] [Google Scholar]
- [19].Hunt BJ, Missfelder-Lobos H, Parra-Cordero M, et al. Pregnancy outcome and fibrinolytic, endothelial and coagulation markers in women undergoing uterine artery Doppler screening at 23 weeks. J Thromb Haemost 2009;7:955–61. [DOI] [PubMed] [Google Scholar]
- [20].Tchaikovski SN, Thomassen MC, Costa SD, et al. Role of protein S and tissue factor pathway inhibitor in the development of activated protein C resistance early in pregnancy in women with a history of preeclampsia. Thromb Haemost 2011;106:914–21. [DOI] [PubMed] [Google Scholar]
- [21].De Bonis M, Sabatini L, Galeazzi LR, et al. Maternal serum protein S forms in pregnancies complicated by intrauterine growth restriction. Eur J Obstet Gynecol Reprod Biol 2012;160:142–6. [DOI] [PubMed] [Google Scholar]
- [22].Hojo S, Tsukimori K, Kinukawa N, et al. Decreased maternal protein S activity is associated with fetal growth restriction. Thromb Res 2008;123:55–9. [DOI] [PubMed] [Google Scholar]
- [23].Mello G, Parretti E, Fatini C, et al. Low-molecular-weight heparin lowers the recurrence rate of preeclampsia and restores the physiological vascular changes in angiotensin-converting enzyme DD women. Hypertension 2005;45:86–91. [DOI] [PubMed] [Google Scholar]
- [24].Trudinger BJ, Giles WB. Clinical and pathologic correlations of umbilical and uterine artery waveforms. Clin Obstet Gynecol 1989;32:669–78. [DOI] [PubMed] [Google Scholar]
- [25].Weiner Z, Younis JS, Blumenfeld Z, et al. Assessment of uterine placental circulation in thrombophilic women. Semin Thromb Hemost 2003;29:213–8. [DOI] [PubMed] [Google Scholar]
- [26].Reantragoon S, Arrigo LM, Seoud MM, et al. Specific heparin fractions suppress endothelin-1 production in cultured human umbilical vein endothelial cells. Arch Biochem Biophys 1994;314:315–22. [DOI] [PubMed] [Google Scholar]
- [27].Torricelli M, Reis FM, Florio P, et al. Low-molecular-weight heparin improves the performance of uterine artery Doppler velocimetry to predict preeclampsia and small-for-gestational age infant in women with gestational hypertension. Ultrasound Med Biol 2006;32:1431–5. [DOI] [PubMed] [Google Scholar]
- [28].Mousavi S, Moradi M, Khorshidahmad T, et al. Anti-inflammatory effects of heparin and its derivatives: a systematic review. Adv Pharmacol Sci 2015;2015:507151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hanna J, Goldman-Wohl D, Hamani Y, et al. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med 2006;12:1065–74. [DOI] [PubMed] [Google Scholar]
- [30].Koo HS, Kwak-Kim J, Yi HJ, et al. Resistance of uterine radial artery blood flow was correlated with peripheral blood NK cell fraction and improved with low molecular weight heparin therapy in women with unexplained recurrent pregnancy loss. Am J Reprod Immunol 2015;73:175–84. [DOI] [PubMed] [Google Scholar]
- [31].Dolitzky M, Inbal A, Segal Y, et al. A randomized study of thromboprophylaxis in women with unexplained consecutive recurrent miscarriages. Fertil Steril 2006;86:362–6. [DOI] [PubMed] [Google Scholar]
- [32].Bar J, Mashiah R, Cohen-Sacher B, et al. Effect of thrombophylaxis on uterine and fetal circulation in pregnant women with a history of pregnancy complications. Thromb Res 2001;101:235–41. [DOI] [PubMed] [Google Scholar]
- [33].Gris JC, Chauleur C, Faillie JL, et al. Enoxaparin for the secondary prevention of placental vascular complications in women with abruptio placentae. The pilot randomised controlled NOH-AP trial. Thromb Haemost 2010;104:771–9. [DOI] [PubMed] [Google Scholar]
- [34].Gris JC, Chauleur C, Molinari N, et al. Addition of enoxaparin to aspirin for the secondary prevention of placental vascular complications in women with severe pre-eclampsia. The pilot randomised controlled NOH-PE trial. Thromb Haemost 2011;106:1053–61. [DOI] [PubMed] [Google Scholar]
- [35].Martinelli I, Ruggenenti P, Cetin I, et al. HAPPY Study Group. Heparin in pregnant women with previous placenta-mediated pregnancy complications: a prospective, randomized, multicenter, controlled clinical trial. Blood 2012;119:3269–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Aracic N, Roje D, Jakus IA, et al. The impact of inherited thrombophilia types and low molecular weight heparin treatment on pregnancy complications in women with previous adverse outcome. Yonsei Med J 2016;57:1230–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Foka ZJ, Lambropoulos AF, Saravelos H, et al. Factor V leiden and prothrombin G20210A mutations, but not methylenetetrahydrofolate reductase C677T, are associated with recurrent miscarriages. Hum Reprod 2000;15:458–62. [DOI] [PubMed] [Google Scholar]
- [38].Abheiden CN, Blomjous BS, Kroese SJ, et al. Low-molecular-weight heparin and aspirin use in relation to pregnancy outcome in women with systemic lupus erythematosus and antiphospholipid syndrome: a cohort study. Hypertens Pregnancy 2017;36:8–15. [DOI] [PubMed] [Google Scholar]
- [39].Aracic N, Roje D, Drmic Hofman I, et al. Low molecular weight heparin treatment and impact of inherited thrombophilia type in pregnancies with previous adverse outcome. J Matern Fetal Neonatal Med 2015;28:306–10. [DOI] [PubMed] [Google Scholar]


