Abstract
Introduction:
Alkaptonuria (AKU) is a rare disease caused by deficiency of homogentisate 1,2-dioxygenase which results in deposition of homogentisic acid (HGA). Ochronotic arthritis, the deposition of excess oxidized HGA in the connective tissues, causes pigmentation and degeneration of the joint tissues ultimately resulting in chronic inflammation and osteoarthritis. The ochronotic arthritis has similar clinical features with osteoarthritis. There is currently no specific treatment for AKU and management is usually symptomatic. In severe cases, total joint arthroplasty is the major treatment approaches. It is rarely reported in China.
Patient concerns:
Here we reported a case of a patient with bilateral knee pain for more than 1 year. He complained of a 20-year history of chronic, nonspecific low back pain and stiffness. His urine was black since he was a child. Six years after the knee surgery, his Achilles tendon ruptured.
Diagnosis:
Specific radiographic and magnetic resonance imaging manifestations were observed. Darkly pigmented full-thickness cartilage and subchondral bone were found during the operation. Histological investigation also manifested dark stains in meniscus and synovial tissues. Black-denatured tendon tissue was also found during the operation. The patient was diagnosed as AKU.
Interventions:
Total knee arthroplasty and Achilles tendon repair were operated separately after the disease was diagnosed.
Outcomes:
The patient recovered very well after the second surgery. He returned to full activities, described no knee pain, and presented to the clinic walking without any aid. Physical examination revealed 0 to 20 of plantar flexion and 0 to 15 of dorsiflexion of the ankle.
Conclusions:
Ochronosis is a very rare disease in Asia. This paper supplies new information for study of this disease. The mechanism is still unknown right now. Further studies will be necessary.
Keywords: Achilles tendon rupture, alkaptonuria, ochronotic arthritis, total knee arthroplasty
1. Introduction
Alkaptonuria (AKU) is a rare hereditary autosomal recessive metabolic disease arising as a result of a defect in the gene coding for homogentisate 1,2-dioxygenase (HGD) and characterized by accumulation of homogentisic acid (HGA).[1] The condition is rare, affecting only 1 in 100,000 to 250,000 individuals. The HGA is excreted abundantly in the urine of these patients. Even so, there are elevated circulating levels of HGA in the blood and body tissues.[2] In tissues, under the effect of autoxidation, HGA-melanin-based polymers formed,[3] depositing in the connective tissues mainly in the joints, cardiovascular system, kidney, skin, and ligament. The polymer, named as ochronotic pigment which is also called ochronosis, affects the hyaline cartilages especially the weight bearing joints like knee.[4] The polymerization of deposited HGA eventually weakens the connective tissues, resulting in fragile and stiffer and ultimately leads to chronic inflammation, degeneration that resembles as osteoarthritis, called ochronotic arthritis.[5] Ochronotic arthritis often begins in the third decade. Ultimately joint replacement is needed, on average, this occurs at the age of 55 years.[6] Other manifestations of this disease include pigment deposition, aortic or mitral valve calcification or regurgitation, and occasionally aortic dilatation, renal stones, prostate stones, and tendon rupture.[7–9] Progressive ochronotic arthritis always shows articular space narrowing, bone sclerosis, and effusion.[10] Although many clinical features were reported on the AKU disease, there were still lack of physiopathological mechanism studies.
Management of joint pain should be tailored to the individual. Physical and occupational therapy may be adopted to help maintain muscle strength and flexibility. Analgesic treatment is essential for joint pain at early stage of ochronotic arthritis; however, timely joint arthroplasty is the major treatment for significant degenerative arthritis when needed.[11] Here we report a case of a 54-year-old male with a family history of parental consanguineous marriage. He was diagnosed with ochronotic arthritis and undertaken the right total knee arthroplasty (TKA) operation. Coincidentally, the patient was readmitted for the right Achilles tendon rupture 6 years after TKA, and the black Achilles tendon tissue was found during the operation.
2. Case report
A 54-year-old man was admitted to the joint department of our hospital complaining of bilateral knee pain for more than 1 year. He suffered pain particularly during walking and stair climbing and descending, with climbing being especially painful. He underwent bilateral knee arthroscopy 9 months ago, after being diagnosed as having a meniscus injury; however, his knee pain was not relieved. There was also fricative in both knees during active knee movement.
He complained of a 20-year history of chronic, nonspecific lower back pain and stiffness, previously diagnosed as ankylosing spondylitis (AS) and was treated with the relevant drugs. When asked for his family history, he told us that his parents were a consanguineous marriage, his urine was black since childhood, but he never saw a doctor for this, and his brothers and children were all in good health. Interestingly, when he previously underwent bilateral knee arthroscopy, the doctors informed him that his knee joint was black but no follow-up work was done at that time.
On physical examination, there was obvious swelling of right knee but without elevated skin temperature. A floating patella test and McMurray sign were positive and there was apparent tenderness at the medial joint space of the right knee. The range of motion of the right knee was 5° (extension) to 90° (flexion), there was accompanying pain with extreme buckling, and fricative was palpated during right knee motion. There was mild tenderness at medial joint space of left knee and no limitation of motion. Both anterior and posterior drawer test and lateral stress test were all negative for bilateral lower limbs, and neurologic and myodynamic examinations were both negative. There was limitation of lumbar spine motion accompanied by lower back pain. No brownish pigmentation was observed on the ear auricle, the sclera, or any other part of the body.
Weight-bearing radiography of the bilateral limb showed narrowing of both knee joints, especially the medial femoral–tibial compartment. Mild osteophytosis of the distal femur, tibial plateau, and patella was observed (Fig. 1). The magnetic resonance imaging (MRI) of the right knee showed swollen, diffuse degenerative changes of cartilage and both meniscus and hydrops articuli, synovial hyperplasia, and anterior cruciate ligament (ACL) thickening at femoral side (Fig. 2).
Figure 1.
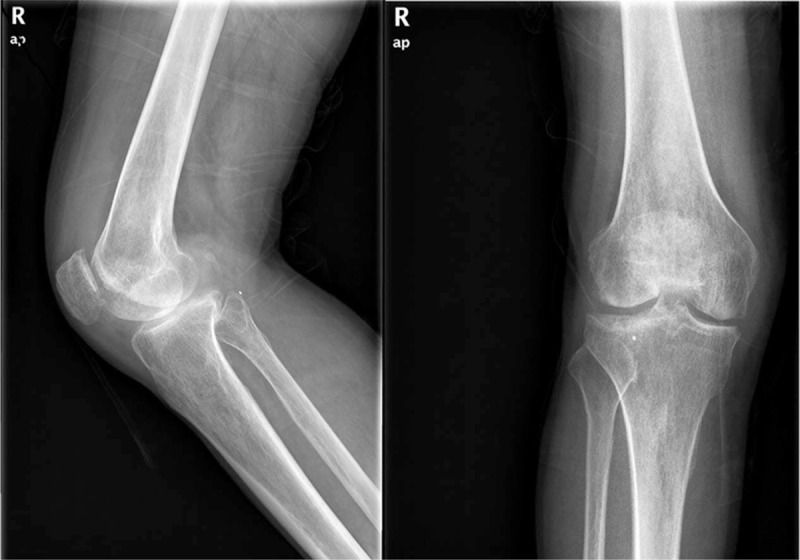
Preoperative X-rays of right knee. Narrowing joint space and rough of articular cartilage could be observed from the X-rays. Resemble as osteoarthritis.
Figure 2.
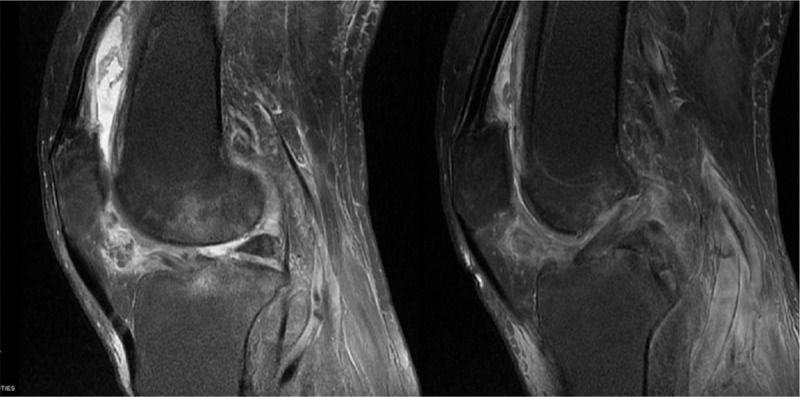
MRI of right knee. Diffuse patchy high signal, ground glass appearance, and synovial edema could be observed. MRI = magnetic resonance imaging.
Fresh urine was a normal color (buff), but when exposed to air for 4 to 6 hours, the color gradually changed to brown. When 10 drops of 10% NaOH were added to 10 mL of fresh urine, the color quickly changed to black (Fig. 3). The blood routine examination showed mild anemia and routine urine test showed that ketone was +1. AS associated cell cluster of differentiation molecules were negative and there were no other abnormalities in the results of hematological and biochemical laboratory investigations.
Figure 3.

Urine color at different times. (A) Fresh urine; (B) after adding 10% NaOH to fresh urine; and (C) fresh urine exposed to air for 4 hours. It can be observed that fresh urine darkens after adding 10% NaOH and after exposure to air for a period of time.
A right TKA was performed (Genesis SPC; Smith & Nephew, Inc, Memphis, TN) and intraoperative findings showed diffuse black pigment depositions in the cartilage and subchondral bone of the femoral condyle, trochlea, tibial plateau, and patella, (Fig. 4) and generalized degeneration throughout the knee was observed. Sporadic depositions were also found in the synovium. The medial and lateral meniscus showed extensive degenerative changes and samples of excised synovium, cartilage, and subchondral bone and meniscus were sent for analysis. The histological investigation of the resected material showed it to be degenerated and pigmented.
Figure 4.
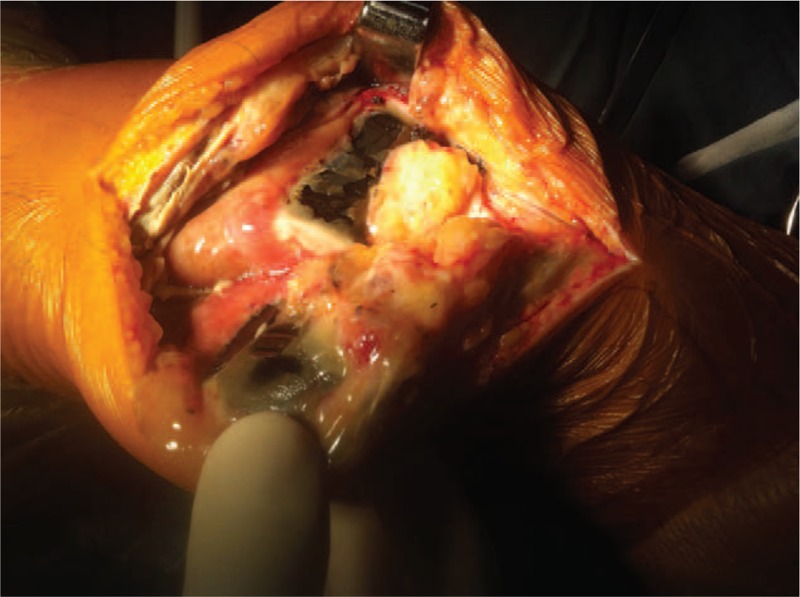
Intraoperative performance which showed black pigmented of cartilage.
The histological sections of removed bone demonstrated classical observations of ochronosis, including multiple pigmented areas, reactive giant cells, and thickened, inflamed synovium (Fig. 5). The patient progressed well postoperatively, regaining good range of motion and independent ambulation 6 weeks after surgery. At that time, active range of motion was from full extension to 110° of flexion. Postoperative radiographs showed total knee components were in good position and alignment. At 6 years following surgery, the patient had returned to full activities, described no knee pain, and was very satisfied with the outcome.
Figure 5.
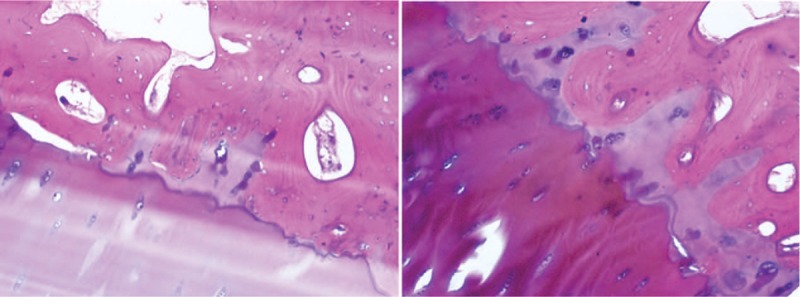
Postoperative pathological section of cartilage which shows the pigmented cartilage matrix and chondrocytes.
At 6 years after TKA surgery, the patient was readmitted with a right Achilles tendon rupture due to minor trauma (Fig. 6) and magnetic resonance imaging confirmed a complete rupture of the right Achilles tendon. Also at this time, pigmentation was observed on the ear auricle (Fig. 7). There are a few reports in the literature of Achilles tendon rupture in AKU patients.[9,12] During the operation, as expected, the patient's Achilles tendon tissue was found to be black (Fig. 8), the black necrotic tissue was removed (Fig. 9), and right long flexor tendon transposition was performed. After the operation, a pathological examination was performed and it was found that the black sputum tissue showed a glassy change (Fig. 10). The patient was discharged from the hospital 5 days after surgery with a cast fitted in a slight plantar flexion. At the 4-week follow-up, the cast was changed to have a slightly greater plantar flexion position and then removed 2 weeks later. The patient was sent home for gentle ankle motion physiotherapy and at the 16 weeks postoperative follow-up, presented to the clinic walking without any aid. Physical examination revealed 0 to 20 of plantar flexion and 0 to 15 of dorsiflexion
Figure 6.
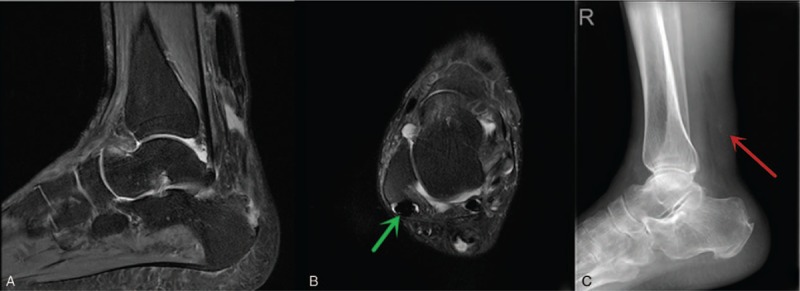
(A) The Achilles tendon rupture; edema could be found around the rupture tendon. (B) The shrinking and dense tendon tissue around the ankle joint (green arrow). (C) The calcification shadows around the Achilles tendon rupture position (red arrow).
Figure 7.
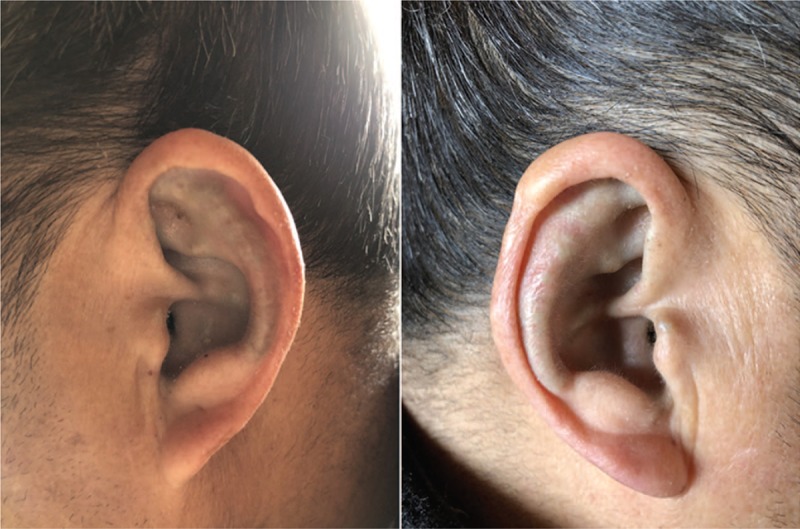
Pigmented bilateral auricle.
Figure 8.
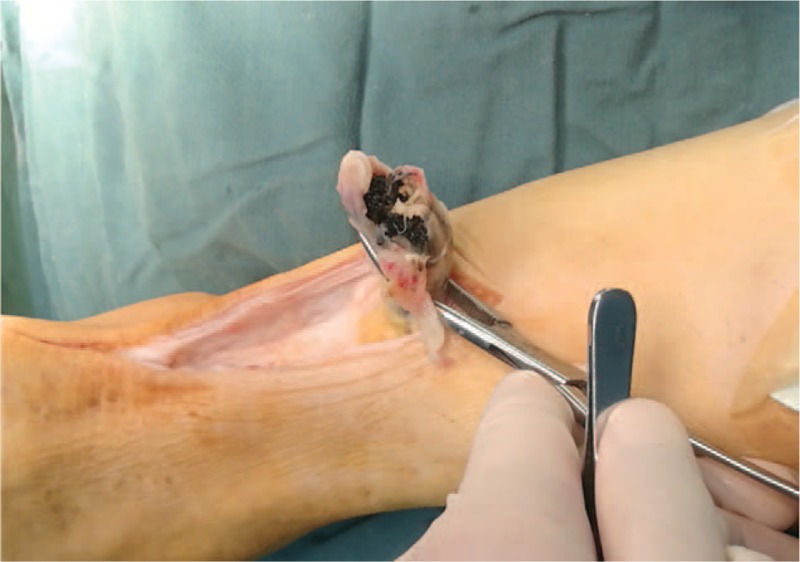
Black-stained rupture Achilles tendon tissue observed during surgery.
Figure 9.
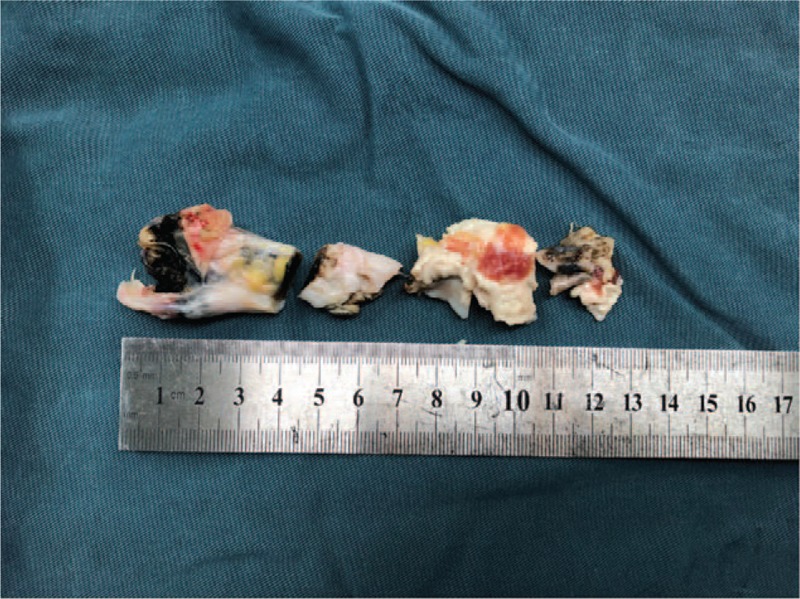
Necrotic Achilles tendon tissue resected during surgery.
Figure 10.
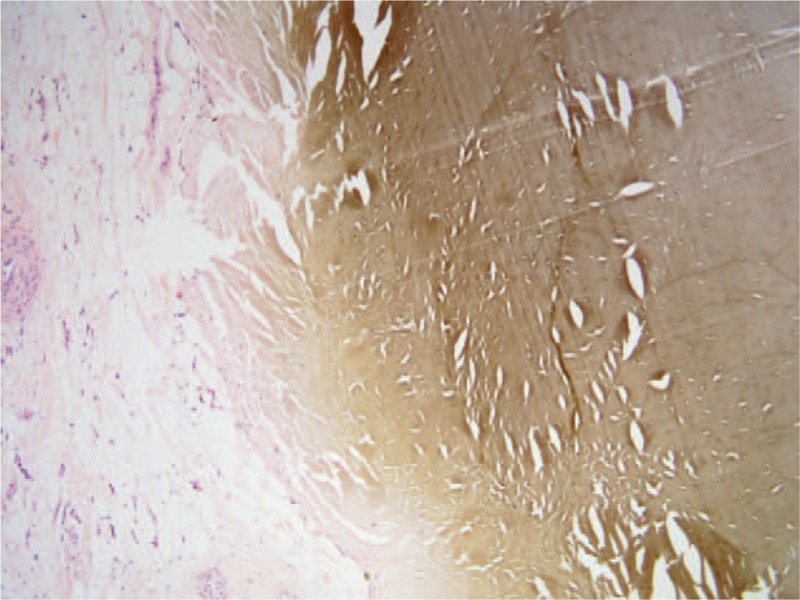
Postoperative pathological section of Achilles tendon which shows the pigmented and normal Achilles tendon tissue.
3. Discussion
AKU progresses more rapidly in men than in women.[13] So far, HGD gene mutations in AKU patients have been found mainly in Eastern Europe[14]; however, studies on the genetic basis of AKU have only become clearer in the past 2 decades.[15] All over Asia, including China, the HGD mutation is very rarely reported. In the Chinese population, AKU has been observed in recent years[16] and our report describes 1 of these few cases. HGD is an enzyme which catalyzes the conversion of HGA to 4-maleylacetoacetate. HGA has high affinity for collagenous tissues such as vessel walls, cardiac valves,[17] cartilage tendons, intervertebral discs, skin, and sclera.[2] HGA oxidizes to become benzoquinone which in turn can develop into amyloidogenic polymers[18] and ultimately results in tissue damage, which includes tendon rupture. Consequently, dark cartilage discoloration, meniscus degeneration, and chondromalacia can all be observed during an operation on an AKU patient.[19]
The main clinical manifestation of AKU is the premature degeneration of articular cartilage, leading to osteoarthritis and fusion of the vertebrae.[10,20,21] The rupture of muscle, ligaments, and tendons are all important features of AKU.[22] Ochronotic pigment is deposited in all connective tissues, but cartilage is the preferential site for ochronosis. Ochronotic arthritis is a very uncommon disease that can be potentially misdiagnosed as osteoarthritis. Meanwhile, the musculoskeletal disorders in AKU patients always resemble AS in regards to their damage to the spine and large joints but differ in their damage to the sacroiliac joint. The most characteristic radiographical finding is the calcification of the intervertebral discus then the narrowing of the intervertebral disc space, resembling bamboo.[13] Over time, the weight-bearing joints show similar radiologic changes to those seen in osteoarthritis with the articular space narrowing and sclerosis. Magnetic resonance imaging shows thickened tendons, meniscus deformation, asymptomatic tears, and bone resorption in knee joint.[23] In our case, the initial onset of lower back pain symptoms in this patient occurred at his third decade and he was misdiagnosed with AS and treated for it for more than 20 years; however, his bilateral knee pain persisted and progressed, and finally, the mystery of his underlying condition has been determined.
The knee is the most frequently affected joint, followed by the hip.[5,24] The HGA-amyloidogenic polymer that leads to fragile articular cartilage eventually becomes fragmented, forming microshards, which leads to joint degeneration.[14] Numerous shards of ochronotic cartilage are then embedded in the synovium, with an associated inflammatory response.[25] Meanwhile, the synovium itself and meniscus also become pigmented by the colored polymer. Tendons and ligaments also are heavily pigmented due to their collagen content and become brittle,[26,27] brownish-blackish rods and pigmented cartilage fragments with fibrillar connective tissue can be observed in the synovial effusion.[28,29] These floating pigmented particles stimulate the synovium, resulting in synovitis and so, joint degeneration can generate serious synovial inflammation. A synovectomy relieves symptoms to some extent and delays arthroplasty, not only in the knee joint but also in the hip and shoulder.
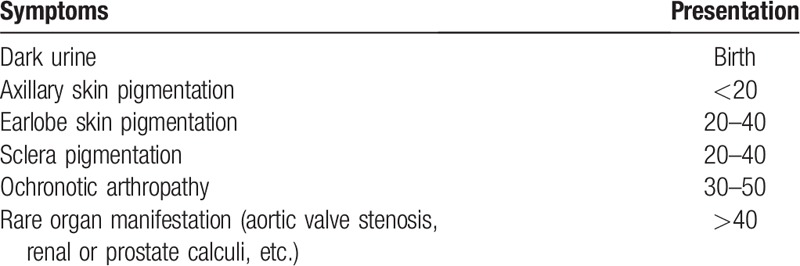
No specific examination is currently used in solely diagnosing ochronotic arthritis, but it can be confirmed according to the physician's comprehensive judgment. In childhood, dark coloration of diapers or urine can be observed following exposure to air[23] and as patients grow older, many common manifestations present as described in Table 1. These common clinical presentations are of great importance in diagnosing ochronotic arthritis. Plain film, computed Tomography, and MRI are suggested as part of a routine examination and aspiration of synovial effusion can also be used in diagnosis and recently, arthroscopic diagnosis has been reported in a few cases.[19,30,31] Before our patient came in to our hospital, when he underwent a knee arthroscopy, dark joint structure was observed.
Table 1.
Clinical manifestations of alkaptonuria patients at different age stages.
There is limited data on the MRI appearance of tendons in ochronosis. In our patient, we found some characteristic features by MRI such as dense, shrinking, and carbonized tendon tissue with an unclear fiber bundle. A small amount of calcification can be seen on the X-ray film. Rupture of the Achilles and patellar tendons have been reported in a few published case reports. Most cases reported were from India and Middle East[9,32] and to date, no case of ochronotic arthritis and ochronotic Achilles tendon rupture occurring in the same patient has been reported in China.
Early management of ochronosis can be challenging and is limited to controlling the patient's symptoms with nonsteroidal anti-inflammatory drugs. The treatment of glucosamine and chondroitine for degenerative joint disease is also effective for ochronotic arthritis. Ascorbic acid inhibits oxidation and polymerization of HGA in vitro, but the efficacy of this form of treatment has not yet been established.[33] More advanced cases of ochronotic arthritis necessitate surgical intervention and as we have reported, total knee replacement has had excellent clinical outcomes in a patient with significant degenerative arthritis due to ochronosis.
Ochronosis is a very rare disease in Asia with only 1 or 2 cases having been reported in China. This paper supplies new information critical for study of this disease and as its mechanism is still unknown, further studies will be necessary to understand and uncover it.
Acknowledgments
We are very grateful to the patient for providing urine samples and pictures, and also very grateful to the pathologists Baizhou Li and Jiabin Lai for providing pathological pictures.
Author contributions
Conceptualization: Lifeng Jiang, Xudong Miao.
Data curation: Lifeng Jiang.
Formal analysis: Lifeng Jiang.
Funding acquisition: Xudong Miao, Xuesong Dai.
Methodology: Le Cao.
Project administration: Xinning Yu.
Resources: Jinghua Fang.
Software: Xinning Yu.
Validation: Xudong Miao.
Visualization: Le Cao.
Writing – original draft: Lifeng Jiang.
Writing – review & editing: Lifeng Jiang.
Footnotes
Abbreviations: ACL = anterior cruciate ligament, AKU = alkaptonuria, AS = ankylosing spondylitis, HGA = homogentisic acid, HGD = homogentisate 1,2-dioxygenase, TKA = total knee arthroplasty.
Informed written consent was obtained from the patient for publication of this case report and accompanying images.
Authors LJ and LC contributed equally to this work and should be considered co-first authors.
This study was supported by the National Natural Science Foundation of China (No. 81801371).
The authors have no conflicts of interest to disclose.
References
- [1].Introne WJ, Kayser MA, Gahl WA. Pagon RA, Adam MP, Bird TD, Dolan CR, Fong CT, Stephens K. Alkaptonuria. University of Washington, Seattle, GeneReviews. Seattle, WA: 1993. [Google Scholar]
- [2].Helliwell TR, Gallagher JA, Ranganath L. Alkaptonuria: a review of surgical and autopsy pathology. Histopathology 2008;53:503–12. [DOI] [PubMed] [Google Scholar]
- [3].Martin JP, Jr, Batkoff B. Homogentisic acid autoxidation and oxygen radical generation: implications for the etiology of alkaptonuric arthritis. Free Radic Biol Med 1987;3:241–50. [DOI] [PubMed] [Google Scholar]
- [4].Taylor AM, Boyde A, Wilson PJ, et al. The role of calcified cartilage and subchondral bone in the initiation and progression of ochronotic arthropathy in alkaptonuria. Arthritis Rheum 2011;63:3887–96. [DOI] [PubMed] [Google Scholar]
- [5].Abimbola O, Hall G, Zuckerman JD. Degenerative arthritis of the knee secondary to ochronosis. Bull NYU Hosp Jt Dis 2011;69:331–4. [PubMed] [Google Scholar]
- [6].Gil JA, Wawrzynski J, Waryasz GR. Orthopedic manifestations of ochronosis: pathophysiology, presentation, diagnosis, and management. Am J Med 2016;129:536.e1–6. [DOI] [PubMed] [Google Scholar]
- [7].Wauthy P, Seghers V, Mathonet P, Deuvaert FE. Cardiac ochronosis: not so benign. Eur J Cardiothorac Surg 2009;35:732–3. [DOI] [PubMed] [Google Scholar]
- [8].Nanda SK, Suresh DR, Vamseedhar A, et al. Cerebro-spinal and renal ochronosis: a rare case report. Indian J Clin Biochem 2010;25:213–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Alajoulin OA, Alsbou MS, Ja’afreh SO, Kalbouneh HM. Spontaneous Achilles tendon rupture in alkaptonuria. Saudi Med J 2015;36:1486–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Mannoni A, Selvi E, Lorenzini S, et al. Alkaptonuria, ochronosis, and ochronotic arthropathy. Semin Arthritis Rheum 2004;33:239–48. [DOI] [PubMed] [Google Scholar]
- [11].Kefeli M, Tomak Y, Can B, et al. Arthroplasty for the treatment of joint degeneration caused by ochronosis in two cases. Acta Orthop Traumatol Turc 2008;42:139–44. [DOI] [PubMed] [Google Scholar]
- [12].Tanoglu O, Arican G, Ozmeric A, et al. Calcaneal avulsion of an ochronotic Achilles tendon: a case report. J Foot Ankle Surg 2018;57:179–83. [DOI] [PubMed] [Google Scholar]
- [13].Phornphutkul C, Introne WJ, Perry MB, et al. Natural history of alkaptonuria. N Engl J Med 2002;347:2111–21. [DOI] [PubMed] [Google Scholar]
- [14].Gaines JJ., Jr The pathology of alkaptonuric ochronosis. Hum Pathol 1989;20:40–6. [DOI] [PubMed] [Google Scholar]
- [15].Zatkova A. An update on molecular genetics of alkaptonuria (AKU). Inherit Metab Dis 2011;34:1127–36. [DOI] [PubMed] [Google Scholar]
- [16].Yang YJ, Guo JH, Chen WJ, et al. First report of HGD mutations in a Chinese with alkaptonuria. Gene 2013;518:467–9. [DOI] [PubMed] [Google Scholar]
- [17].Hannoush H, Introne WJ, Chen MY, et al. Aortic stenosis and vascular calcifications in alkaptonuria. Mol Genet Metab 2012;105:198–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Millucci L, Spreafico A, Tinti L, et al. Alkaptonuria is a novel human secondary amyloidogenic disease. Biochim Biophys Acta 2012;1822:1682–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Raaijmaakers M, Steenbrugge F, Dierickx C. Ochronosis, arthroscopy of a black knee: a case report and review of the literature. Knee Surg Sports Traumatol Arthrosc 2008;16:182–4. [DOI] [PubMed] [Google Scholar]
- [20].Castagna A, Giombini A, Vinanti G, et al. Arthroscopic treatment of shoulder ochronotic arthropathy: a case report and review of literature. Knee Surg Sports Traumatol Arthrosc 2006;14:176–81. [DOI] [PubMed] [Google Scholar]
- [21].Chakraverty JK, Lawson TM, Herdman G. Not just simple degenerative disc disease (alkaptonuria). Spine J 2013;13:985–6. [DOI] [PubMed] [Google Scholar]
- [22].Abate M, Schiavone C, Salini V, et al. Occurrence of tendon pathologies in metabolic disorders. Rheumatology (Oxford) 2013;52:599–608. [DOI] [PubMed] [Google Scholar]
- [23].Keller JM, Macaulay W, Nercessian OA, Jaffe IA. New developments in ochronosis: review of the literature. Rheumatol Int 2005;25:81–5. [DOI] [PubMed] [Google Scholar]
- [24].Siavashi B, Zehtab MJ, Pendar E. Ochronosis of hip joint; a case report. Cases J 2009;2:9337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Gaines JJ, Jr, Tom GD, Khankhanian N. An ultrastructural and light microscopic study of the synovium in ochronotic arthropathy. Hum Pathol 1987;18:1160–4. [DOI] [PubMed] [Google Scholar]
- [26].Manoj Kumar RV, Rajasekaran S. Spontaneous tendon ruptures in alkaptonuria. J Bone Joint Surg Br 2003;85:883–6. [PubMed] [Google Scholar]
- [27].Chua SY, Chang HC. Bilateral spontaneous rupture of the quadriceps tendon as an initial presentation of alkaptonuria: a case report. Knee 2006;13:408–10. [DOI] [PubMed] [Google Scholar]
- [28].Bhangle S, Panush RS, Berman EL, Schumacher HR. Clinical images: synovial fluid clues to ochronosis. Arthritis Rheum 2012;64:473. [DOI] [PubMed] [Google Scholar]
- [29].Stiehl P, Kluger KM. Joint effusion findings in alkaptonuric arthropathy (ochronosis). Z Rheumatol 1994;53:150–4. [PubMed] [Google Scholar]
- [30].Chen AL, Rose DJ, Desai P. Arthroscopic diagnosis and management of ochronotic arthropathy of the knee. Arthroscopy 2001;17:869–73. [DOI] [PubMed] [Google Scholar]
- [31].Thacker M, Garude S, Puri A. Ochronotic arthropathy: arthroscopic findings in the shoulder and the knee. Arthroscopy 2003;19:E14–7. [DOI] [PubMed] [Google Scholar]
- [32].Jebaraj I, Rao A. Achilles tendon enthesopathy in ochronosis. J Postgrad Med 2006;52:47–8. [PubMed] [Google Scholar]
- [33].Braconi D, Laschi M, Taylor AM, et al. Proteomic and redox-proteomic evaluation of homogentisic acid and ascorbic acid effects on human articular chondrocytes. J Cell Biochem 2010;111:922–32. [DOI] [PubMed] [Google Scholar]


