Abstract
Pulmonary tuberculosis (PTB) continues to be one of the significant public health threats with significant morbidity and mortality. The present study was aimed to assess the clinical characteristics and chest computed tomography (CT) findings of smear-positive and smear-negative PTB in hospitalized adult patients.
Hospitalized adult patients diagnosed with PTB by positive Mycobacterium tuberculosis growth on acid-fast bacilli culture from bronchial aspiration or sputum from 2015 to 2017 were reviewed. Only the patients who had chest CT within 14 days of the diagnosis of PTB were included. Medical records and CT images were analyzed.
A total of 189 patients was enrolled. The median age was 62 years, and there were 118 males (62.4%). More than half of the patients had underlying chronic medical conditions (55.5%). The most common CT finding was nodular lesions (96.8%). The patients were categorized in 2 groups of smear-positive (n = 94, 49.7%) and smear-negative (n = 95, 50.3%). Between 2 groups, there was no difference in underlying medical conditions. However, there were more lesions of cavity, consolidation, bronchiectasis, upper lobe involvement, multiple lobe involvement, and lymphadenopathy in the smear-positive group. A predictive model for smear-positive tuberculosis was created based on the comparison analysis that had an area of 0.724 under the receiver operating characteristic curve. In a multivariate logistic regression analysis, CT findings of consolidation (odds ratio [OR] 2.521, 95% confidence interval [CI] 1.175–5.408, P = .02), lymphadenopathy (OR 1.947, 95% CI 1.025–3.696, P = .04), and multi-lobe involvement (OR 2.795, 95% CI 1.084–7.205, P = .03) were associated with smear-positive PTB.
PTB patients who have chest CT findings of cavity, consolidation, bronchiectasis, upper lobe involvement, multiple lobe involvement, and lymphadenopathy may be at higher risk for smear-positive TB. A predictive model may be helpful for further assessment.
Keywords: computed tomography, pulmonary tuberculosis, smear-negative, smear-positive
1. Introduction
Tuberculosis (TB) is a specific infection caused by Mycobacterium tuberculosis, which continues to pose significant challenges to global public health. According to the World Health Organization, 10.4 million people were estimated to have developed TB in 2015.[1] The annual incidence of TB in the Republic of Korea (80 per 100,000 population) is higher than those reported among high-income countries such as in Japan (17 per 100,000 population) and in the United States of America (3.2 per 100,000 population).[1] The risk for TB is related to exposure of M tuberculosis and the degree of immunodeficiencies, which include human immunodeficiency virus (HIV), transplantation, chronic renal failure, and rheumatoid arthritis.[2] There are increasing elderly population with immunocompromised condition(s), latent TB infection, diabetes as well as an influx of immigrants from countries with high TB burden in the Republic of Korea.[3] Thus, the risk of TB infection may continue to be present.
The lungs are the most common site for TB infection. Prompt diagnosis of pulmonary TB (PTB) is critical for appropriate therapeutic intervention and infection control.[4] For the diagnosis of PTB, smear microscopy, and culture of the respiratory specimen (sputum or bronchial fluid) have been recommended. While culture has a higher diagnostic yield than that of smear microscopy, its turn-around time is approximately 2 to 8 weeks, which limits usefulness as an initial diagnostic test. Smear microscopy test for acid-fast bacilli (AFB) stain is useful for the initial diagnostic test based on its quicker turnaround time. Also, AFB stain is crucial for infection control assessment. Transmission risk and infectivity of PTB is directly related to smear positivity since smear-positive TB cases are more infectious than those with smear-negative.[5] Therefore, patients with smear-positive TB should be placed in an airborne infection isolation room to prevent transmission.[6] This mandatory air-borne isolation infection control practice contributes to an increased financial burden associated with smear-positive TB.
In addition, radiographic imaging is one of the most critical diagnostic evaluations of PTB. However, radiological manifestations of PTB can be varied. Upper lobe cavity lesion is typical of PTB in the immunocompetent adults while military TB, hilar lymphadenopathy, and pleural effusion are common findings of PTB in the immunocompromised adults.[7] Computed tomography (CT) is more sensitive than chest radiography for detection of subtle parenchymal TB or lymphadenopathy process.[8] However, there have been few studies reporting CT findings with regard to smear-positive and smear-negative PTB. Given the significant burden of TB, the present study was aimed to assess the clinical characteristics and CT findings of smear-positive and smear-negative PTB in hospitalized adult patients.
2. Methods
2.1. Patients
A hospital-based retrospective study was conducted at the Korea University Anam Hospital, Seoul, the Republic of Korea, which is a 1048-bed, university-affiliated tertiary care hospital. Hospitalized adult patients (≥19 years) diagnosed with PTB by positive M tuberculosis growth on AFB culture (solid2% Ogawa or liquid [Mycobacterium growth indicator tube] media) from bronchial aspiration sample or sputum sample from 2015 to 2017 were reviewed. Chest CT scan was performed for adult patients suspected of PTB or for adult patients diagnosed with PTB at the discretion of the treating physician. Only the patients who had chest CT within 14 days of the diagnosis of PTB were included. Clinical variables including patient demographic data, underlying medical conditions, AFB smear test results, and CT findings were collected and analyzed.
Underlying medical condition of immunocompromised status (IC) was defined as the presence of 1 or more of solid-organ or hematologic malignancy, congenital immunodeficiency, HIV, solid-organ or hematopoietic stem cell transplantation, functional or anatomical asplenia, immunosuppressive agents such as systemic steroids ≥20 mg/d of prednisone equivalent, alkylating agents, tumor necrosis factor inhibitors, or rituximab. Chronic medical conditions were defined as chronic liver disease (including liver cirrhosis and chronic hepatitis), chronic heart disease (including heart failure), chronic central nervous system disease (presence of 1 or more of stroke, epilepsy, Alzheimer disease, Parkinson disease, and other neurological diseases), chronic pulmonary disease (including asthma and chronic obstructive pulmonary disease), chronic kidney disease (including renal failure and nephrotic syndrome), and diabetes mellitus. Positive AFB smear test was defined by having positive stain results from both the fluorochrome stain and the Ziehl–Neelsen stain. This study was approved by the institutional review board at the Korea University Anam Hospital (IRB number 2017AN0409).
2.2. Details of CT
All the chest CT scans were performed by a 128-slice dual-source multi-detector CT (MDCT) scanner (SOMATOM Definition Flash; Siemens Healthcare, Forchheim, Germany). CT scans were performed using the spiral technique. Images were obtained during suspended full inspiration while the patients were in the supine position. CT parameters were 280-ms gantry rotation, 100 kVp, 175 mAs, 1-mm slice collimation, and 1 table pitch. In order to inject contrast material, an 18-gauge cannula was placed into a superficial vein of the antecubital fossa and 100 ml of iopromide (ProsureM300, LG Life Sciences, Seoul, Korea) was intravenously administered at 4 ml/s using a power injector (Envision CT; Medrad, Pittsburgh, PA). This was followed by the injection of a 30-ml saline solution at 4 ml/s. After the intravenous contrast material injection, the scanning delay was 20 seconds or less. Transverse and coronal CT images of chest were reconstructed at the scanner workstation by a commercially available software (Syngo; Siemens Medical Solutions). The reconstruction parameters were as follows: section thickness, 3 mm; increment, 3 mm. Then, the reconstructed CT images of the chest were transferred into a picture archiving and communication system (PACS) workstation (PiViewSTAR; Infinitt, Seoul, Korea) for the image analysis. Chest CT findings were reviewed and scored by 2 radiologists regarding various features of PTB including a pattern of parenchymal lesions and lung involvement, hilar or mediastinal or paratracheal lymphadenopathy (lymphadenopathy), pleural effusion, and pleural thickening. The final consensus CT image interpretation was rendered by a radiologist with 20 years of experience in chest CT image interpretation. CT images were reviewed on a PACS workstation in axial stack mode. All the assessments were performed by the measurement tools inherent to the PACS workstation.
2.3. Statistical analysis
Comparison analyses of clinical variables and CT findings between smear-negative and smear-positive groups were performed. The Pearson χ2 test or the Fisher exact test was used for dichotomous variables. The Mann–Whitney U test was used for continuous variables. The Wilcoxon signed-rank test was used in smear-positive PTB prediction score between smear-positive and smear-negative groups. The sensitivity and specificity of the smear-positive PTB prediction model were calculated for each score value. Performance of the smear-positive PTB prediction model was evaluated using the receiver operating characteristic (ROC) curve[7,8] with the calculation of the area under the ROC curve. Variables with a P-value <.2 on comparison analysis were included in a multiple logistic regression analysis to determine risk factors associated with smear-positive PTB and to minimize the potential confounding. Odds ratio and 95% confidence intervals were calculated. A P-value <.05 was considered to be statistically significant. SPSS version 18.0 for Windows (SPSS Inc., Chicago, IL) was used for statistical analyses.
3. Results
3.1. Patients
A total of 189 patients was enrolled. The median age was 62 years with an interquartile range of 46 to 77 years. There were 118 males (62.4%). More than half of the patients had underlying chronic medical conditions (52.9%). Common chronic medical conditions were chronic heart disease (11.1%), chronic liver disease (10.6%), chronic kidney disease (9.0%), chronic pulmonary disease (10.6%), chronic central nervous system disease (4.8%), and diabetes mellitus (9.5%). However, the proportion of patients with IC was low (n = 20, 10.6%). The rate of long-term facility residence before the hospitalization was 24.3%.
3.2. CT findings
The interpretation of chest CT suggestive of PTB by radiologists was noted in 157 patients (83.1%). The most common CT finding was nodular lesions (centrilobular nodules) (96.8%), followed by consolidation (75.1%) and cavity (54.0%) lesions. The majority of the patients had upper lobe involvement (92.6%) and multi-lobe involvement (81.5%) of TB lesions. The patients were categorized in 2 groups of smear-positive (n = 94, 49.7%) and smear-negative (n = 95, 50.3%) patients. Despite a tendency toward more non-IC chronic medical conditions (chronic liver disease and diabetes mellitus) in the smear-positive patients, there were no significant differences between the 2 groups of patients regarding age, sex, and underlying medical conditions. Also, the interpretation of chest CT suggestive of PTB by radiologists did not differ significantly between the 2 groups (81.1% in smear-negative patients vs 85.1% in smear-positive patients, P = .46). However, smear-positive patients had more lung parenchymal lesions of cavity (61.7% vs 46.3%, P = .03), consolidation (85.1% vs 65.3%, P < .01), bronchiectasis (55.3% vs 35.8%, P = .01), upper lobe involvement (97.9% vs 87.4%, P = .01), and multi-lobe involvement (91.5% vs 71.6%, P < .01) compared to smear-negative patients. Also, lymphadenopathy was more frequent in smear-positive patients (62.8% vs 42.1%, P < .01) than in smear-negative patients (Table 1). Between 2 groups of patients with non-IC (n = 169, 89.4%) and IC (n = 20, 10.6%), CT findings were compared. There was a trend of a lower rate of the interpretation of chest CT suggestive of PTB by radiologists in the IC group than in the non-IC group without statistical significance (70.0% vs 84.6%, P = .12). Although there was a tendency toward more calcification in IC group than in non-IC group (40.0% vs 21.9%, P = .09), no significant differences in terms of CT findings were noted between these 2 groups (Table 2).
Table 1.
Clinical charateristics and computed tomography (CT) findings of pulmonary tuberculosis patients stratified to acid-fast bacilli (AFB) smear stain result.
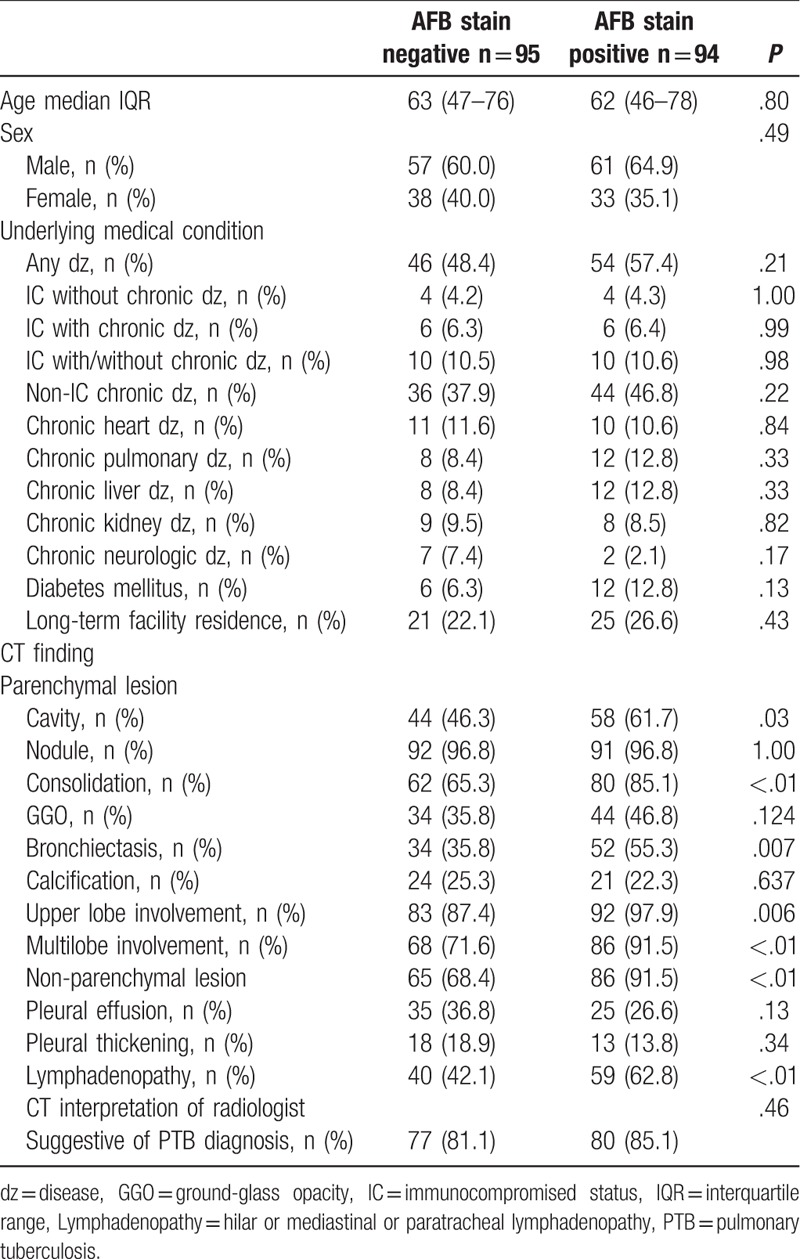
Table 2.
Computed tomography (CT) findings of pulmonary tuberculosis patients stratified to immunocompromised status (IC).
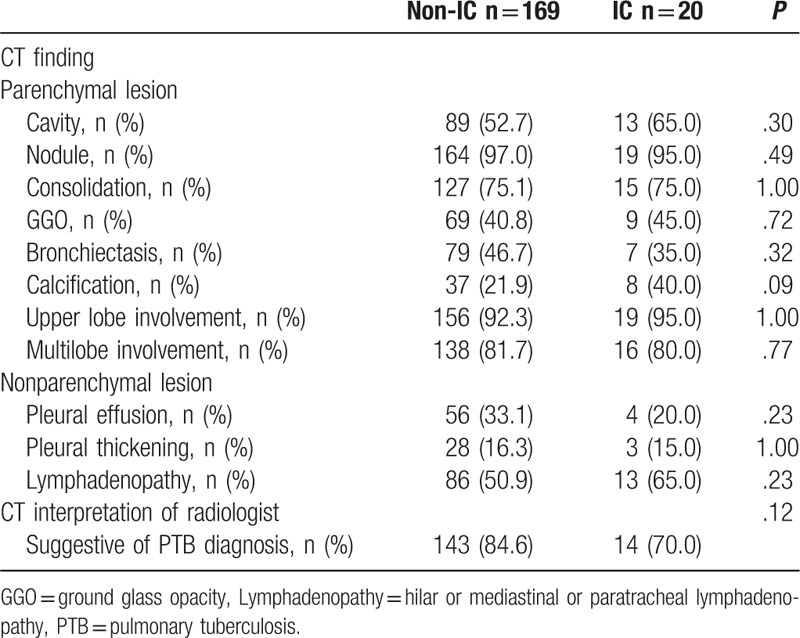
Each of the 6 significant variables (cavity, consolidation, bronchiectasis, upper lobe involvement, multi-lobe involvement, and lymphadenopathy) identified from the comparison analysis was given a point score for the creation of the smear-positive PTB prediction model. Smear-positive patients had higher scores when tested by the Wilcoxon signed-rank test (P < .01) (Table 3). A ROC curve had an area under the curve of 0.724 for the smear-positive PTB prediction model (Fig. 1). The ideal threshold score of 4 was identified from the ROC curve with a sensitivity of 84.0% and a specificity of 52.6%.[8,9]
Table 3.
Acid-fast bacilli (AFB) smear-positive tuberculosis prediction score.
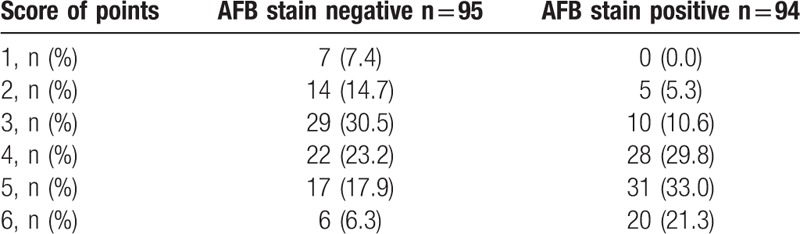
Figure 1.
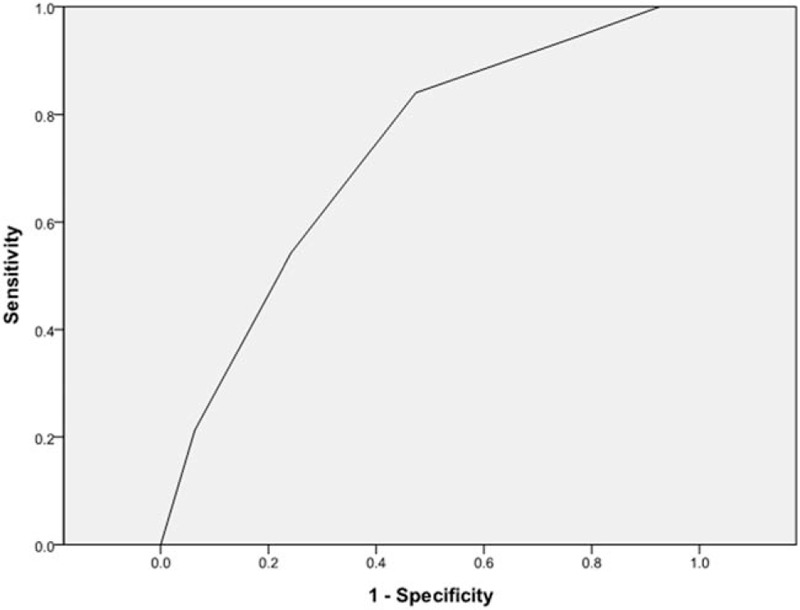
AFB smear-positive tuberculosis prediction model: the ROC curve. AFB = acid-fast bacilli, ROC = receiver operating characteristics.
In a multivariate logistic regression analysis using variables with a P-value <.2 on comparison analysis, CT findings of consolidation (odds ratio [OR] 2.521, 95% confidence interval [CI] 1.175–5.408, P = .02), lymphadenopathy (OR 1.947, 95% CI 1.025–3.696, P = .04), and multi-lobe involvement (OR 2.795, 95% CI 1.084–7.205, P = .03) were significantly associated with smear-positive PTB. This analysis revealed a borderline association between upper lobe involvement and smear-positive PTB (OR 5.096, 95% CI 0.955–27.192, P = .06). However, the presence of pleural effusion was inversely associated with smear-positive PTB (OR 0.488, 95% CI 0.249–0.953, P = .04).
4. Discussion
In our study, underlying medical conditions were not found to be associated with smear-positive PTB. However, specific CT findings of cavity, consolidation, bronchiectasis, upper lobe involvement, multi-lobe involvement, and lymphadenopathy were significantly more frequent in smear-positive PTB. These results suggest that specific CT findings are associated with infectivity of PTB. Based on these CT findings, a predictive model was created, which would be useful for health care providers in assessing the probability of smear-positive PTB for airborne isolation. Since CT is more sensitive than plain radiography in detecting parenchymal lesions and lymphadenopathy,[8,10] our predictive model may have improved sensitivity compared to a previous one based on plain radiography.[11] Thus, this predictive model might be applied to differentiate patients at high risk of smear-positive PTB from those at low risk during the medical evaluation process to reduce substantial hospital cost associated with airborne isolation for smear-positive PTB.[12]
CT findings of consolidation and multi-lobe involvement were significant factors associated with smear-positive PTB, which are in accordance with previous studies.[13,14] Caseous necrosis with inflammation is manifested as consolidation in PTB.[15] The numbers of AFB is closely correlated with the extent of consolidation, as necrotic material within the consolidation contain bacilli and drains through the airway.[14] Also, a previous study[13] showed that the number of lobes involving consolidation increased with the number of AFB, suggestive of the endobronchial spread of PTB. Therefore, our result reaffirms the significance of consolidation and multi-lobe involvement as predictors of smear-positive PTB. Lymphadenopathy was another significant predictor for smear-positive PTB. Although there is no direct communication between lymphadenopathy and bronchial secretion, lymph nodes (paratracheal, hilar, and mediastinal lymph nodes) are the initial sites of tuberculous infection spread from the lung parenchyma[16] and important sites of the persistence of significant numbers of tuberculous bacilli.[17] Thus, lymphadenopathy may serve as an indirect marker of PTB progression and infectivity. There was a borderline association between upper lobe involvement and smear-positive PTB in our study. Based on the demographic data and distribution of upper lobe involvement in our patients, the majority of PTB cases might be secondary to reactivation of TB. Upper lobe involvement is one of the significant characteristics of reactivation of TB, where there is increased oxygen tension, and this can facilitate replication of tuberculous bacilli.[18] Consequently, upper lobe involvement may have higher numbers of AFB, which could be related to infectivity as smear-positive PTB. Presence of pleural effusion was inversely associated with smear-positive PTB, which is in line with a previous study.[19] As tuberculous pleural effusion is usually caused by hypersensitivity to tuberculous protein[18,19] without direct tuberculous bacilli invasion, parenchymal lesions such as consolidation may have higher positive predictive values for smear-positive PTB. The most common CT finding of PTB was nodular lesions. However, the presence of nodular lesions did not correlate with smear-positive PTB. This result is in agreement with a previous study,[14] which showed that nodular lesions might be less infectious than consolidation due to a smaller amount of AFB within the nodular lesions and longer distance between the nodular lesions and central airway.
Our study has several limitations. First, this was a retrospective study at the single medical center. Thus, sampling and selection bias introduced by the small number of patients with heterogeneity and possible information bias from an interpretation of radiologists may have affected our analysis. Also, there might be additional effects from unmeasured variables. Our results did not show the significance of underlying medical conditions nor cavity lesions in a multivariate analysis for association with smear-positive PTB. Cavity lesions and medical conditions that may affect immune statuses such as malnutrition and HIV have been associated with smear-positive PTB in other studies.[20–22] These inconsistent findings might be due to the difference in clinical characteristics or due to the limitation of the analysis by the small sample size and low prevalence of cavity lesions and IC conditions in our patients. Furthermore, our results of no significant differences in terms of CT findings between 2 groups of patients with non-IC and IC might be due to a small number of patients with IC. The tendency of more calcification and a lower rate of the interpretation of chest CT suggestive of PTB by radiologists in patients with IC might suggest reactivation of PTB with less typical CT findings of PTB in patients with IC. However, these findings warrant further investigation with a larger number of patients with IC. Second, high-resolution CT which may be more sensitive for evaluating PTB than conventional CT[23] was not performed for all PTB patients. However, the improved resolution of MDCT used in our study may be able to overcome this.[24]
5. Conclusions
Hospitalized PTB patients who have chest CT findings of cavity, consolidation, bronchiectasis, upper lobe involvement, multiple lobe involvement, and lymphadenopathy may be at higher risk for smear-positive TB. A predictive model using CT findings may be helpful for timely assessment of infectivity of PTB. Prospective studies with a more significant number of patients are necessary to define the optimal predictive models in the future.
Author contributions
Conceptualization: Jong Hun Kim, Min Ja Kim, Soo-Youn Ham.
Data curation: Jong Hun Kim, Min Ja Kim.
Formal analysis: Jong Hun Kim.
Supervision: Min Ja Kim, Soo-Youn Ham.
Writing – original draft: Jong Hun Kim.
Writing – review and editing: Jong Hun Kim, Soo-Youn Ham.
Soo-Youn Ham orcid: 0000-0002-5333-9839.
Footnotes
Abbreviations: AFB = acid-fast bacilli, CT = computed tomography, IC = immunocompromised status, MD = multi-detector, PTB = pulmonary tuberculosis, ROC = receiver operating characteristic, TB = tuberculosis.
This study was approved by the institutional review board at the Korea University Anam Hospital (IRB number 2017AN0409).
This research was supported by a grant of the Korea Health Technology R&D Project (grant number: HI18C0673) through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea.
The authors have no funding and conflicts of interest to disclose.
References
- [1].World Health Organization. Global Tuberculosis Report 2016. Geneva: World Health Organization; c2017. Available at: http://www.who.int/tb/publications/global_report/en/ [Accessed on Jun 8, 2017] [Google Scholar]
- [2].Sester M, van Leth F, Bruchfeld J, et al. Risk assessment of tuberculosis in immunocompromised patients. A TBNET study. Am J Respir Crit Care Med 2014;190:1168–76. [DOI] [PubMed] [Google Scholar]
- [3].Kim JH, Yim JJ. Achievements in and challenges of tuberculosis control in South Korea. Emerg Infect Dis 2015;21:1913–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lewinsohn DM, Leonard MK, LoBue PA, et al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention clinical practice guidelines: diagnosis of tuberculosis in adults and children. Clin Infect Dis 2017;64:e1–33. [DOI] [PubMed] [Google Scholar]
- [5].Sepkowitz KA. How contagious is tuberculosis? Clin Infect Dis 1996;23:954–62. [DOI] [PubMed] [Google Scholar]
- [6].Jensen PA, Lambert LA, Iademarco MF, et al. CDC. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Recomm Rep 2005;54:1–41. [PubMed] [Google Scholar]
- [7].Rozenshtein A, Hao F, Starc MT, et al. Radiographic appearance of pulmonary tuberculosis: dogma disproved. AJR Am J Roentgenol 2015;204:974–8. [DOI] [PubMed] [Google Scholar]
- [8].Centor RM, Schwartz JS. An evaluation of methods for estimating the area under the receiver operating characteristic (ROC) curve. Med Decis Making 1985;5:149–56. [DOI] [PubMed] [Google Scholar]
- [9].England WL. An exponential model used for optimal threshold selection on ROC curves. Med Decis Making 1988;8:120–31. [DOI] [PubMed] [Google Scholar]
- [10].Skoura E, Zumla A, Bomanji J. Imaging in tuberculosis. Int J Infect Dis 2015;32:87–9. [DOI] [PubMed] [Google Scholar]
- [11].Cohen R, Muzaffar S, Capellan J, et al. The validity of classic symptoms and chest radiographic configuration in predicting pulmonary tuberculosis. Chest 1996;109:420–3. [DOI] [PubMed] [Google Scholar]
- [12].Kellerman S, Tokars JI, Jarvis WR. The cost of selected tuberculosis control measures at hospitals with a history of Mycobacterium tuberculosis outbreaks. Infect Control Hosp Epidemiol 1997;18:542–7. [DOI] [PubMed] [Google Scholar]
- [13].Matsuoka S, Uchiyama K, Shima H, et al. Relationship between CT findings of pulmonary tuberculosis and the number of acid-fast bacilli on sputum smears. Clin Imaging 2004;28:119–23. [DOI] [PubMed] [Google Scholar]
- [14].Kosaka N, Sakai T, Uematsu H, et al. Specific high-resolution computed tomography findings associated with sputum smear-positive pulmonary tuberculosis. J Comput Assist Tomogr 2005;29:801–4. [DOI] [PubMed] [Google Scholar]
- [15].Im JG, Itoh H, Shim YS, et al. Pulmonary tuberculosis: CT findings – early active disease and sequential change with antituberculous therapy. Radiology 1993;186:653–60. [DOI] [PubMed] [Google Scholar]
- [16].Mohapatra PR, Janmeja AK. Tuberculous lymphadenitis. J Assoc Physicians India 2009;57:585–90. [PubMed] [Google Scholar]
- [17].Ganchua SKC, Cadena AM, Maiello P, et al. Lymph nodes are sites of prolonged bacterial persistence during Mycobacterium tuberculosis infection in macaques. PLoS Pathog 2018;14:e1007337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Nachiappan AC, Rahbar K, Shi X, et al. Pulmonary tuberculosis: role of radiology in diagnosis and management. Radiographics 2017;37:52–72. [DOI] [PubMed] [Google Scholar]
- [19].Wong KS, Huang YC, Lai SH, et al. Validity of symptoms and radiographic features in predicting positive AFB smears in adolescents with tuberculosis. Int J Tuberc Lung Dis 2010;14:155–9. [PubMed] [Google Scholar]
- [20].Lee CH, Jeong YJ, Heo EY, et al. Active pulmonary tuberculosis and latent tuberculosis infection among homeless people in Seoul, South Korea: a cross-sectional study. BMC Public Health 2013;13:720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Semunigus T, Tessema B, Eshetie S, et al. Smear positive pulmonary tuberculosis and associated factors among homeless individuals in Dessie and Debre Birhan towns, Northeast Ethiopia. Ann Clin Microbiol Antimicrob 2016;15:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ors F, Deniz O, Bozlar U, et al. High-resolution CT findings in patients with pulmonary tuberculosis: correlation with the degree of smear positivity. J Thorac Imaging 2007;22:154–9. [DOI] [PubMed] [Google Scholar]
- [23].Hatipoğlu ON, Osma E, Manisali M, et al. High resolution computed tomographic findings in pulmonary tuberculosis. Thorax 1996;51:397–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ko JM, Park HJ, Kim CH, et al. The relation between CT findings and sputum microbiology studies in active pulmonary tuberculosis. Eur J Radiol 2015;84:2339–44. [DOI] [PubMed] [Google Scholar]


