Abstract
Osteoporosis is a chronic, progressive disease in which early diagnosis is very important. The neutrophil-to-lymphocyte ratio (NLR) and the platelet-to-lymphocyte ratio (PLR) have been reported as new predictors in inflammatory and immune diseases including osteoporosis. No studies have reported the relationship between monocyte-to-lymphocyte ratio (MLR) and osteoporosis patients.
To investigated the ability of MLR to predict osteoporosis.
Three hundred sixteen osteoporosis patients and 111 healthy control subjects were enrolled. Patients’ laboratory and clinical characteristics were recorded. MLR, NLR, and PLR levels were calculated. The differences were compared and the diagnostic values of MLR were analyzed.
There were 76 male and 105 female patients included, with a mean age of 56.57 ± 9.95 years. The levels of MLR, NLR, and PLR in osteoporosis patients were all higher than those in healthy control subjects. The area under the curve of MLR was higher than those of NLR and PLR. Multivariate linear regression analysis showed that T-score was affected by age and MLR. MLR was positively correlated with C-reactive protein, erythrocyte sedimentation rate, red blood cell distribution width, age, sex, and inversely with hemoglobin. MLR and PLR levels were significantly higher in osteoporosis patients than in osteopenia patients (P < .05).
The present study shows that MLR had a higher diagnostic value for osteoporosis. MLR may be a reliable, inexpensive, and novel potential predictor of osteoporosis.
Keywords: monocyte-to-lymphocyte ratio, neutrophil-to-lymphocyte ratio, osteoporosis, predictor
1. Introduction
Osteoporosis, a multifactorial process, is a bone metabolic disease characterized by normal bone tissue damage, increased bone fragility, and decreased bone mass per unit volume.[1,2] Osteoporosis is common in middle-aged and elderly people, especially postmenopausal women.[3] The onset of this disease is not obvious, lacking obvious disease characteristics, and is therefore difficult to diagnose early. Once a patient has a change in body shape or bone pain, the lesion has already entered an accelerated phase.[4] At present, clinical diagnostic methods mainly include osteoporosis screening tools, bone turnover marker, and bone mineral density (BMD). BMD detection technology can find bone loss, and is the most common methods to detect this disease.[5] An objective and noninvasive diagnostic predictor at an earlier disease stage is critically needed.
Peripheral blood neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) are newly proposed inflammatory factors, that have the advantages of simplicity, low cost, and noninvasiveness.[6] They can reflect the balance of the immune response, and have been reported to be independent and inexpensive predictors in many inflammatory and immune diseases, such as ankylosing spondylitis, coronary heart disease, malignant tumors, and acute pancreatitis among others.[7–10] Recently, a few studies reported that the monocyte-to-lymphocyte ratio (MLR) was also a new predictor of disease severity.
“Bone immunology” views the immune system and immune factors as important roles in the development of osteoporosis.[11,12] Research has not previously confirmed the correlation between MLR with osteoporosis. In this study, we evaluated the diagnostic value of MLR in osteoporosis.
2. Patients and methods
2.1. Patient characteristics
Three hundred sixteen inpatients who were treated at the Department of Orthopedics, Shenzhen Hospital of Traditional Chinese Medicine between January 1, 2015 and August 31, 2018 were included in this cross-sectional study. All patients diagnosed with osteoporosis fulfilled the 2015 Guidelines for the Diagnosis and Treatment of Osteoporosis issued by the Branch of Osteoporosis and Bone Mineral Salt Diseases, Chinese Medical Association. Those patients with hypertension (n = 52), acute inflammatory disease (n = 43), malignancy tumor (n = 4), autoimmune disease (n = 9), hematological disorders (n = 6) (n = 6), renal and liver failure (n = 8), thyroid or parathyroid disorders (n = 5), and infection (n = 8) were excluded. One hundred eleven healthy control subjects who were referred to our hospital for routine checkups with normal BMD levels were recruited. In this study, written or verbal consent was obtained from all the patients. The study was approved by the EC office of Guangzhou University of Chinese Medicine (Shenzhen Traditional Chinese Medicine Hospital 2018–66).
2.2. Laboratory and clinical assessment
Recorded BMD, sex, age, neutrophil, monocyte, lymphocyte, and platelet counts, red blood cell (RBC), white blood cell (WBC), red blood cell distribution width (RDW), hemoglobin (HGB), C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), alanine aminotransferase (ALT), aspartate aminotransferase (AST), fasting plasma glucose (FPG), and creatinine (CREA) were recorded. MLR, NLR, and PLR levels were calculated. BMD was measured by DEXA (Hologic Discovery A S/N 88788; Marlborough, MA) at the femoral neck (FN) and lumbar spine (L2–L4). Patients were divided into 2 groups by BMD score, as follows: −2.5 standard deviation (SD) < T-score < −1 SD was the osteopenia group, and T-score ≤−2.5 SD with no fractures was the osteoporosis group.
2.3. Statistical analysis
SPSS 19.0 software (SPSS Inc, Chicago, IL) was applied for statistical analysis. Continuous data were expressed as the mean ± SD, the non-normal distribution index was taken as the natural logarithm, and the comparison between the 2 groups was performed by Mann–Whitney U tests. Normally distributed data were analyzed by independent samples using the Student t test. Categorical data was expressed as a percentage and comparisons between groups were performed using Chi-squared tests. Correlation analysis was done using Pearson correlation and multivariate linear regression analysis. Areas under the receiver operating characteristic (ROC) curves were computed to determine the diagnostic value of MLR, NLR, and PLR. P < .05 was considered statistically significant.
3. Results
3.1. Patient characteristics and MLR, NLR, and PLR levels
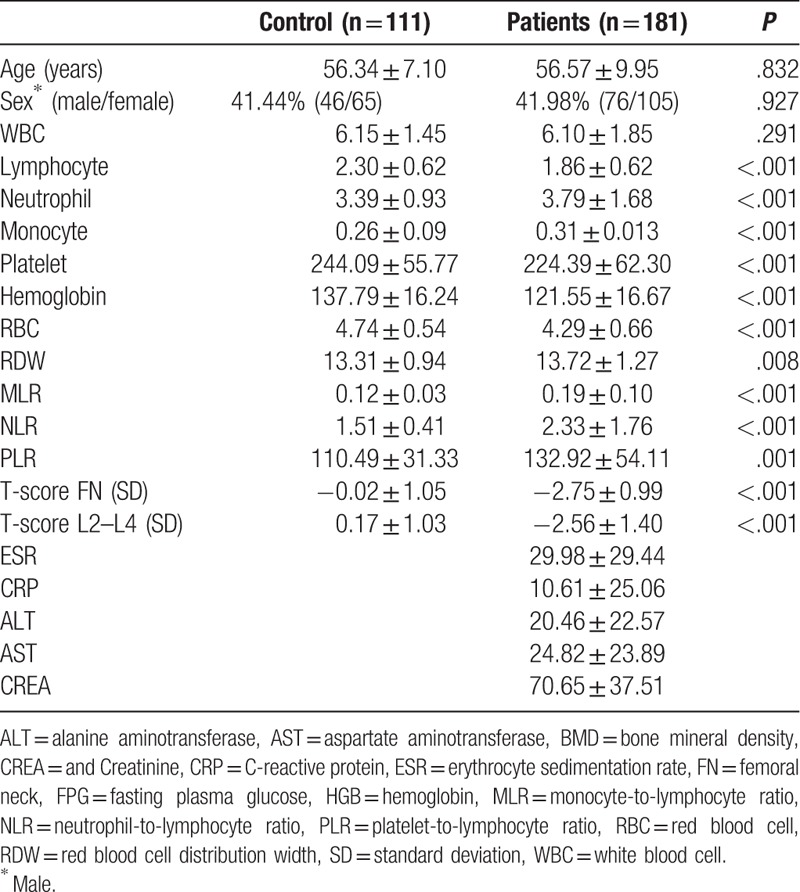
In this cross-sectional study, 181 osteoporosis patients and 111 healthy control subjects were included. Table 1 provides the main laboratory and clinical characteristics. There was no significant difference in sex and age between the 2 groups. The counts of neutrophil, monocyte, and RDW were higher, and the counts of lymphocyte, neutrophil, platelet, HGB, and RBC were lower in the osteoporosis patients than healthy control subjects. The levels of MLR, NLR, and PLR were all higher than those in healthy control subjects.
Table 1.
Basic characteristics of patients and controls.
3.2. MLR diagnostic value was higher in osteoporosis patients
We used ROC curves to explore the diagnostic value of MLR, NLR, and PLR for osteoporosis patients. The area under the curve (AUC) of MLR was 0.75 (95% confidence interval [CI]: 0.698–0.808). The optimal cutoff value was 0.14, with a sensitivity of 64.1% and specificity of 78.4%. The AUC of NLR and PLR was respective 0.70 (95% CI: 0.641–0.759) and 0.619 (95% CI: 0.556–0.682). MLR yielded a higher AUC value than NLR and PLR (Fig. 1).
Figure 1.
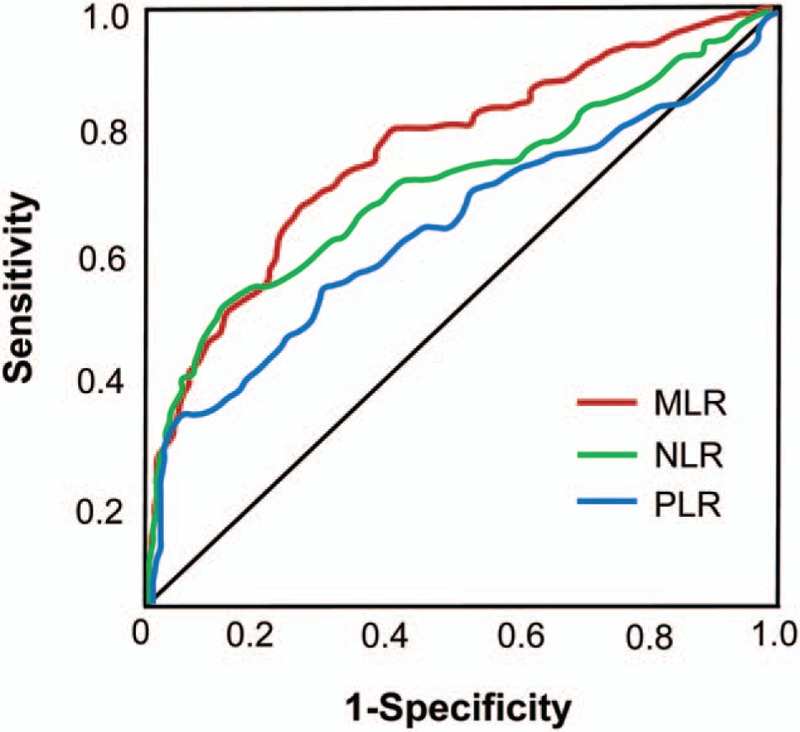
ROC curves to explore the diagnostic value of MLR, NLR, and PLR. MLR = monocyte to lymphocyte ratio, NLR = neutrophil to lymphocyte ratio, PLR = platelet to lymphocyte ratio.
3.3. Correlation of MLR, NLR, and PLR levels with clinical variables
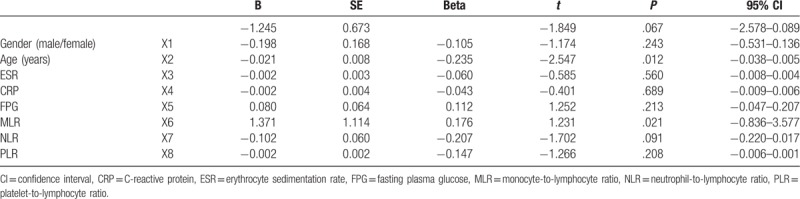
Analysis of the correlation between the variables showed that MLR was positively correlated with CRP, ESR, RDW, age, sex, and inversely correlated with HGB. NLR was positively correlated with CRP, sex, and inversely correlated with HGB. PLR was only correlated with sex (Table 2). Multivariate linear regression analysis showed that T-score FN was affected by age and MLR (P = .012 and .021, respectively, Table 3).
Table 2.
Analysis of the correlation between variables.
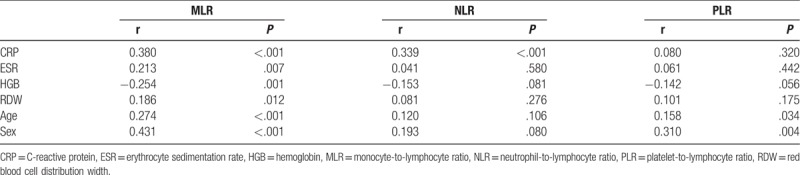
Table 3.
Multivariate linear regression analysis of factors influencing T-score femoral neck.
3.4. MLR and NLR were elevated in osteoporosis patients
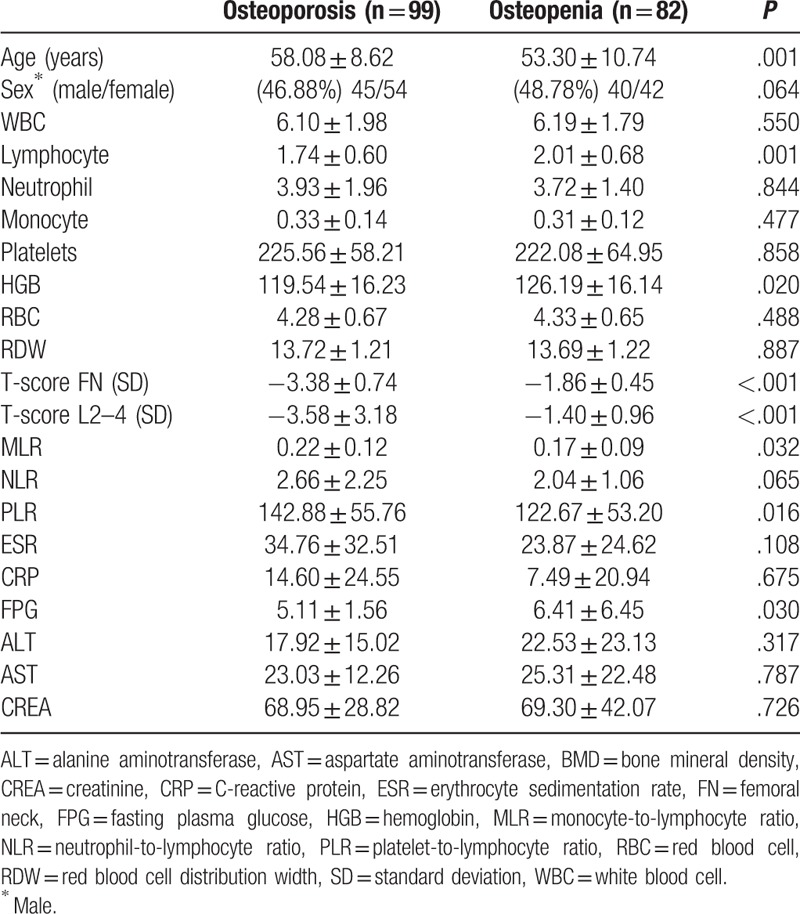
According to their BMD scores, we divided patients into osteoporosis group and osteopenia group. There were 99 patients belonging to the osteoporosis group, and 82 patients belonging to the osteopenia group. In the osteopenia group, MLR and PLR levels were lower than osteoporosis group (P < .05). The levels of lymphocyte, HGB, and FPG were higher in the osteopenia patients than in the osteoporosis group. NLR, age, sex, neutrophil count, platelet count, RDW, RBC, CRP, ESR, ALT, AST, and CREA levels showed no significant difference between the 2 groups (Table 4).
Table 4.
Comparison of variables between groups.
4. Discussion
As has been reported as noninvasive and cost-effective markers in various systemic inflammatory diseases, we evaluate the diagnostic value of MLR, NLR, and PLR in osteoporosis patients in this cross-sectional study. In this study, osteoporosis patients showed higher MLR, NLR, and PLR levels than did the control subjects. The diagnostic ability of MLR was higher than NLR, PLR, and other parameters. Multivariate linear regression analysis showed that T-score FN was affected by age and MLR. MLR was positively correlated with ESR, CRP, WBC, and fibrinogen. We divided patients into osteoporosis group and osteopenia group. In the osteopenia group, MLR and PLR levels were lower than osteoporosis group. Therefore, we come to the conclusion that MLR had a high diagnostic value for osteoporosis patients.
With the rapid development of modern biochemistry, molecular biology, immunology, and radiography, great progress has been made in the diagnosis of metabolic bone diseases, especially osteoporosis in recent years. New experimental techniques and special inspection provide more specific and sensitive evidence for clinical diagnosis and identification of osteoporosis.[4,5] Early diagnosis is very important; however, none of the current examination methods can detect osteoporosis at an early stage. Leukocyte subpopulation tests are commonly used to indicate inflammatory disease[13]; however in recent years, studies have shown that NLR, MLR, and PLR may be more appropriate indicators of inflammation than the leukocyte subpopulation. They all are less likely influenced by various physiological conditions, and all are inexpensive, simple, useful prognosis in systemic inflammatory disease, had been investigated in acute limb ischemia, cardiovascular, rheumatoid arthritis, and neoplastic diseases.[6,8,14,15] This reflects of 2 important and opposite immune pathways. Thus, we speculated MLR may be appropriate inflammation indicators in osteoporosis.
In this study, the main finding was that MLR was superior in the prediction value of osteoporosis. ROC curves showed the diagnostic value of MLR was higher than NLR, as reported by Zeynel et al that NLR levels were found to be elevated in osteoporosis group compared to osteopenia and control group.[17] NLR represents a ratio of 2 different immune pathways, that a high N is responsible for active nonspecific inflammation and a decrease L is responsible for poor physiological stress.[17] Interestingly, this study also found that MLR was positively correlated with CRP, ESR, RDW, age, and inversely with HGB and sex. These correlations were not very strong but higher than NLR and PLR with variables. According to the BMD scores, we divided the patients into osteoporosis group and osteopenia group. In the osteoporosis group, MLR and PLR levels were significantly higher than osteopenia group. NLR levels were also higher in the osteoporosis, but there is no statistical significance. Previous studies demonstrate that NLR levels of patients with osteoporosis were significantly higher than osteopenia and control subjects.[16] In osteoporosis, immune response occurs later than inflammatory response, which may resolve the inflammatory process.
The exact mechanism of how MLR was increased in osteoporosis remains unclear and requires further investigation. Higher MLR indicated higher monocyte or lower lymphocyte levels in the peripheral blood. In a few studies, peripheral blood MLR has been recognized as a significant predictor of advanced stages, involved in immune escape, and immune regulation. Lymphocytes can suppress tumor cell proliferation and migration, and monocytes can differentiate into tumor-associated macrophages which can promote solid-tumor progression and metastasis.[18,19] A few immune disease studies reported the diagnose values of MLR, announced that it might reflect systemic inflammation and the severity of immune injury.[7,10] The immune system and immune factors play important roles in the development of osteoporosis. Bone immunology is a new interdisciplinary subject, providing new pathogenetic and clinical interpretations for osteoporosis.[11,20,21] Many immune cells, signaling factors, and inflammatory cytokines are involved in this process.[22] Factors secreted by macrophages or T cells strongly influence bone growth or degradation, depending on the bone microenvironment. T lymphocyte cells can regulate bone cells and hematopoietic function in the immune system, through secreting inflammatory factors and Wnt ligands.[23,24] The bone marrow niches consist of osteoblasts, osteoclasts, mesenchymal cells, endothelial cells, and macrophage. Some studies have shown that macrophages in the bone marrow niches play an important role, can influence the migration of osteoblasts and osteoclasts from the bone marrow into the blood.[25–27]
Some limitations exist in this study and should be noted. First, this was a single center study, and the number of patients included is small. This study also lacks research on the pathogenetic mechanism of elevated MLR and NLR. More patients from different centers for investigate such a topic. Longitudinal and molecular biology studies are needed to explore the mechanism of elevated MLR and NLR levels.
In conclusion, our study is the first demonstration that MLR and NLR were higher in osteoporosis patients compared with healthy controls; especially MLR had a higher diagnostic value for osteoporosis and had a close relationship with ESR and CRP levels. Importantly, MLR may be a reliable, inexpensive, and novel predictor for osteoporosis.
Author contributions
Data curation: Wenxiu Zhu, Yong Zhang, Weidong Liu, Heng Li, Quan Li.
Funding acquisition: Yafei Cao.
Investigation: Kun Gao.
Project administration: Kun Gao, Weiji Yu, Yafei Cao.
Writing – original draft: Kun Gao.
Writing – review & editing: Kun Gao.
Yafei Cao orcid: 0000-0001-9193-376X.
Footnotes
Abbreviations: ALT = alanine aminotransferase, AST = aspartate aminotransferase, AUC = area under the curve, BMD = bone mineral density, CREA = and Creatinine, CRP = C-reactive protein, EC = ethics committee, ESR = erythrocyte sedimentation rate, FN = femoral neck, FPG = fasting plasma glucose, HGB = Hemoglobin, MLR = monocyte-to-lymphocyte ratio, NLR = neutrophil-to-lymphocyte ratio, PLR = platelet-to-lymphocyte ratio, RBC = red blood cell, RDW = red blood cell distribution width, WBC = white blood cell.
The study was supported by Sanming Project of Medicine in Shenzhen (No. SZSM201812066).
The study was approved by the EC office of Guangzhou University of Chinese Medicine (Shenzhen Traditional Chinese Medicine Hospital 2018–66).
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
The authors have no conflicts of interest to disclose.
References
- [1].van den Bergh JP, van Geel TA, Geusens PP. Osteoporosis, frailty and fracture: Implications for case finding and therapy. Nat Rev Rheumatol 2012;8:163–72. [DOI] [PubMed] [Google Scholar]
- [2].Zhao H, Zhao N, Zheng P, et al. Prevention and treatment of osteoporosis using Chinese medicinal plants: special emphasis on mechanisms of immune modulation. J Immunol Res 2018;2018:6345857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Akkawi I, Zmerly H. Osteoporosis: current concepts. Joints 2018;6:122–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Vlot MC, den Heijer M, de Jongh RT, et al. Clinical utility of bone markers in various diseases. Bone 2018;114:215–25. [DOI] [PubMed] [Google Scholar]
- [5].Schultz K, Wolf JM. Emerging technologies in osteoporosis diagnosis. J Hand Surg Am 2019;44:240–3. [DOI] [PubMed] [Google Scholar]
- [6].Uthamalingam S, Patvardhan EA, Subramanian S, et al. Utility of the neutrophil to lymphocyte ratio in predicting long-term outcomes in acute decompensated heart failure. Am J Cardiol 2011;107:433–8. [DOI] [PubMed] [Google Scholar]
- [7].Huang Y, Deng W, Zheng S, et al. Relationship between monocytes to lymphocytes ratio and axial spondyloarthritis. Int Immunopharmacol 2018;57:43–6. [DOI] [PubMed] [Google Scholar]
- [8].Ommen SR, Gibbons RJ, Hodge DO, Thomson SP. Usefulness of the lymphocyte concentration as a prognostic marker in coronary artery disease. Am J Cardiol 1997;79:812–4. [DOI] [PubMed] [Google Scholar]
- [9].Azab B, Jaglall N, Atallah JP, et al. Neutrophil-lymphocyte ratio as a predictor of adverse outcomes of acute pancreatitis. Pancreatology 2011;11:445–52. [DOI] [PubMed] [Google Scholar]
- [10].Xiang J, Zhou L, Li X, et al. Preoperative monocyte-to-lymphocyte ratio in peripheral blood predicts stages, metastasis, and histological grades in patients with ovarian cancer. Transl Oncol 2017;10:33–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Moschen AR. The RANKL/OPG system is activated in inflammatory bowel disease and relates to the state of bone loss. Gut 2005;54:479–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Limmer A, Wirtz DC. Osteoimmunology: influence of the immune system on bone regeneration and consumption. Z Orthop Unfall 2017;155:273–80. [DOI] [PubMed] [Google Scholar]
- [13].Weitzmann MN, Ofotokun I. Physiological and pathophysiological bone turnover: role of the immune system. Nat Rev Endocrinol 2016;12:518–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bozan N, Alpayci M, Aslan M, et al. Mean platelet volume, red cell distribution width, platelet-to-lymphocyte and neutrophil-to-lymphocyte ratios in patients with ankylosing spondylitis and their relationships with high-frequency hearing thresholds. Eur Arch Otorhinolaryngol 2016;273:3663–72. [DOI] [PubMed] [Google Scholar]
- [15].Mercan R, Bitik B, Tufan A, et al. The association between neutrophil/lymphocyte ratio and disease activity in rheumatoid arthritis and ankylosing spondylitis. J Clin Lab Anal 2016;30:597–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Tasoglu I, Cicek OF, Lafci G, et al. Usefulness of neutrophil/lymphocyte ratio as a predictor of amputation after embolectomy for acute limb ischemia. Ann Vasc Surg 2014;28:606–13. [DOI] [PubMed] [Google Scholar]
- [17].Öztürk ZA, Yesil Y, Kuyumcu ME, et al. Inverse relationship between neutrophil lymphocyte ratio (NLR) and bone mineral density (BMD) in elderly people. Arch Gerontol Geriat 2013;57:81–5. [DOI] [PubMed] [Google Scholar]
- [18].Azab B, Zaher M, Weiserbs KF, et al. Usefulness of neutrophil to lymphocyte ratio in predicting short- and long-term mortality after non-ST-Elevation myocardial infarction. Am J Cardiol 2010;106:470–6. [DOI] [PubMed] [Google Scholar]
- [19].Song S, Li C, Li S, et al. Derived neutrophil to lymphocyte ratio and monocyte to lymphocyte ratio may be better biomarkers for predicting overall survival of patients with advanced gastric cancer. Onco Targets Ther 2017;10:3145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Du J, Chen S, Shi J, et al. The association between the lymphocyte-monocyte ratio and disease activity in rheumatoid arthritis. Clin Rheumatol 2017;36:2689–95. [DOI] [PubMed] [Google Scholar]
- [21].Takayanagi H. Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat Rev Immunol 2007;7:292–304. [DOI] [PubMed] [Google Scholar]
- [22].Terashima A, Takayanagi H. Overview of osteoimmunology. Calcif Tissue Int 2018;102:503–11. [DOI] [PubMed] [Google Scholar]
- [23].Ginaldi L, De Martinis M. Osteoimmunology and beyond. Curr Med Chem 2016;23:3754–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Li JY, Tawfeek H, Bedi B, et al. Ovariectomy disregulates osteoblast and osteoclast formation through the T-cell receptor CD40 ligand. Proc Natl Acad Sci U S A 2011;108:768–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bozec A, Zaiss MM. T regulatory cells in bone remodelling. Curr Osteoporos Rep 2017;15:121–5. [DOI] [PubMed] [Google Scholar]
- [26].Winkler IG, Sims NA, Pettit AR, et al. Bone marrow macrophages maintain hematopoietic stem cell (HSC) niches and their depletion mobilizes HSCs. Blood 2010;116:4815–28. [DOI] [PubMed] [Google Scholar]
- [27].Méndez-Ferrer S, Michurina TV, Ferraro F, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 2010;466:829–34. [DOI] [PMC free article] [PubMed] [Google Scholar]


