Abstract
Background:
Acute diarrhea is the 2nd highest prevalence disease among children under 5 years of age. It can cause malnutrition and even death in children, especially in developing country. Traditional Chinese medicine therapy has been applied and already in the guidelines for clinical practice of acute infectious diarrhea in children in China, but there is no specific methods or recommendations due to lacking of evidence. Zusanli acupoint injection as a form of acupuncture therapy, which is proved to be effective in randomised controlled trials (RCTs) and very suitable for children, has been used in acute diarrhea in children for a long time; therefore, a systematic review is necessary to provide available evidence for further study.
Methods:
Different studies from various databases will be involved in this study. Only RCTs of children under 5 years of age diagnosed with acute diarrhea using any recognized diagnostic criteria will be included. We will search manually the literature in the databases from China Conference Paper Database. Electronic database includes PubMed, Embase, Cochrane Library, Web of Science, China National Knowledge Internet, WanFang, Chongqing VIP, and China Biomedical Literature CDROM Database. Primary outcomes: clinical cure rate (clinical cure is defined as the frequency, timing and character of stool back to normal status, as well as disappearance of diarrhea symptoms), diarrhea duration (from admission to the cessation of diarrhea). Secondary outcomes: stool frequency within 24 hours, rate of adverse effect. Data will be extracted by 2 researchers independently; risk of bias of the meta-analysis will be evaluated based on the Cochrane Handbook for Systematic Reviews of Interventions. All data analysis will be conducted by data statistics software Review Manager V.5.3. and Stata V.12.0.
Results:
This study will synthesize and provide evidence based on the data of the currently published zusanli (ST36) acupoint injection for acute diarrhea in children under 5 years old, especially in terms of clinical efficacy and safety.
Conclusion:
This systematic review aims to evaluate the benefits and harms of zusanli acupoint injection for acute diarrhea in children under 5 years old reported in RCTs, and provide evidence reference in TCM field for Chinese guidelines on the treatment of acute diarrhea in children.
Ethics and dissemination:
This study is a systematic review; the outcomes are based on the published evidence, and hence examination and agreement by the ethics committee are not required in this study. We intend to publish the study results in a journal or conference presentations.
PROSPERO registration number:
PROSPERO 2019 CRD42019135275.
Keywords: acupoint injection, acute diarrhea, children, protocol, systematic review, Zusanli (ST36)
1. Introduction
1.1. Description of the condition
Acute diarrhea is the 2nd highest prevalence disease among children under 5 years of age (YOA).[1,2] It can lead to threatening dehydration and electrolytes disorder, and it is an important cause of malnutrition in children.[3] Acute diarrhea is not only one of the leading causes for hospital attendance of children (more than 50% of hospitalization of acute diarrhea was induced by rotavirus infection), but also one of the leading causes of morbidity and mortality in children younger than 5 YOA. About 577,508 children worldwide die of this disease every year, mainly in developing countries. China is still one of the countries with the highest diarrhea mortality rate. About 9072 children under 5 YOA die of diarrhea every year.[4]
1.2. Description of the intervention
Routine treatment for acute diarrhea in children, including fluid replacement therapy, dietary therapy, zinc supplementation therapy, drug treatment as probiotics, antibiotics, montmorillonite, Chinese medicine, etc. Acupoint injection involves the injection of medicine into specific acupuncture points to treat diseases or conditions, it is a synergetic effect of acupuncture and medication.[5,6] Acupoint injection also is a very successful treatment method combining traditional Chinese medicine (TCM) and western medicine. Clinical and experimental research shows that acupoint injection has the characteristics of low dosage and quick effect, and it is possible to magnify the pharmacologic effects of drugs by geometric order of magnitude,[7,8] but the mechanism of the effect is still unclear.[8]
1.3. How the intervention might work
Zusanli (ST36) is an acupoint located below the knee, on the tibialis anterior muscle, along the stomach meridian. We chose this acupoint because it is one of the most commonly used acupoints in Chinese medicine for diseases of spleen-stomach, and hence it is often applied to treat digestive system disease such as vomiting, diarrhea, colitis, dyspepsia, etc.[9,10] Studies have shown that acupuncture stimulation of zusanli can promote gastrointestinal motility by increasing the secretion of Motilin and Gastrin in treating functional gastrointestinal disorders-diarrhea (FGIDs-D),[11] and significantly improve gastrointestinal microcirculation by increasing the secretion of 5-HT in treating FGIDs,[12] and effectively improve the weight growth rate of irritable bowel syndrome, reduce the content of inflammatory factors in colonic mucosa, regulate the expression of nuclear factor kappa B protein in colonic tissue to reduce inflammatory injury.[13] Besides, there are randomized controlled trials (RCTs) published in China have indicated that zusanli acupoint injection as a complementary therapy combine with conventional treatment could improve the cure rate and shorten the course of acute diarrhea in children.[14–16]
1.4. Why it is important to this review
Zusanli acupoint injection is frequently used clinically in acute diarrhea in children as a complementary therapy, and prove to be more effective than using routine treatment only.[14–16] However, there is no critically appraised evidence as systematic review or meta-analysis of the potential benefits and harms of zusanli acupoint injection for acute diarrhea in children. If zusanli acupoint injection proved to be truly effective and safe in this study, and this method itself is simple to be operated, it could be much easier than ordinary acupuncture in application and promotion worldwide, therefore benefits more people.
1.5. Objectives
This review aims to systematically evaluate the benefits and harms of zusanli acupoint injection for acute diarrhea in children under 5 years old reported in RCTs. We look forward to provide evidence reference in TCM field for Chinese guidelines on the treatment of acute diarrhea in children.
2. Methods
2.1. Study registration
The protocol of the systematic review has been registered in International Prospective Register of Systematic Reviews (PROSPERO), and the registration number is CRD42019135275. This systematic review protocol will be conducted and reported strictly according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)[17] statement guidelines, and the important protocol amendments will be documented in the full review.
2.2. Criteria for considering studies for this review
We will strictly screen studies that meet the following inclusion criteria.
2.2.1. Type of included studies
Only RCTs (except quasi-RCTs and cluster RCTs) will be included. Animal mechanism studies and nonrandomized clinical trials will be excluded. Article that substantially overlaps with another published article in print or electronic media will be excluded. Duplicate publications produced by a single experiment and published as separate papers with different criteria for measuring results, priority will be given to original publications and other publications will be excluded. The language and time of publication will not be restricted.
2.2.2. Participants
Children under 5 YOA diagnosed with acute diarrhea using any recognized diagnostic criteria, dysentery, and drug-relationship diarrhea will be excluded, regardless of sex and types of acute diarrhea.
2.2.3. Interventions and controls
Interventions include zusanli acupoint injection as a complementary therapy combined with conventional treatment. Conventional treatment refers to comprehensive treatment, which is based on the guideline[18] and mainly about fluid replacement therapy to maintain acid-base balance of water and electrolyte; diet therapy; zinc supplementation therapy; and pharmacotherapy like probiotics, montmorillonite, antibiotics, etc. Controls use only conventional treatment. The choice of routine treatment in each RCT does not have to be exactly consistent, but zusanli acupoint injection should be the only difference between interventions and controls. Any type of injected medication will be included.
2.2.4. Type of outcome measures
Primary outcomes: clinical cure rate (clinical cure is defined as the frequency, timing and character of stool back to normal status, as well as disappearance of diarrhea symptoms[19]), diarrhea duration (from admission to the cessation of diarrhea).
Secondary outcomes: stool frequency within 24 hours, rate of adverse effect.
The most severe threat posed by diarrhea is dehydration, which is closely related to the time and frequency of diarrhea, and hence we record and contrast diarrhea duration (from admission to the cessation of diarrhea) and stool frequency within 24 hours to measure the curative effect. As for clinical cure rate and rate of adverse effect, they are the most intuitive result evaluation.
2.3. Search methods
2.3.1. Search resources
This review will include grey literature sourced from China Conference Paper Database, manual searching. Electronic database includes PubMed, Embase, Cochrane Library, Web of Science, China National Knowledge Internet, WanFang, Chongqing VIP, and China Biomedical Literature CDROM Database will also be searched. We will simply present the search process of the PubMed (Table 1). The data will be searched in English and Chinese databases from their inception to April 2019.
Table 1.
Example of PubMed search strategy.

2.3.2. Search strategies
The following MeSH terms and their combinations will be searched: zusanli OR ST36 OR point ST36; acupuncture points OR acupoints OR points; injections; diarrhea OR diarrheas OR diarrhea; and children OR child OR infant OR baby.
2.4. Data collection and analysis
2.4.1. Studies selection
There will be 2 researchers (TY and ZY) carry out the selection of research literature independently using endnote x9 software. We will 1st make the preliminary selection by screening titles and abstracts. Secondly, we will download full text of the relevant studies for further selection according to the inclusion criteria. If there is any different opinion, 2 researchers will discuss and reach an agreement. If a consensus could not be reached, there will be a 3rd researcher (HHC) who makes the final decision. Details of the selection process were shown in the flow chart (Fig. 1).
Figure 1.
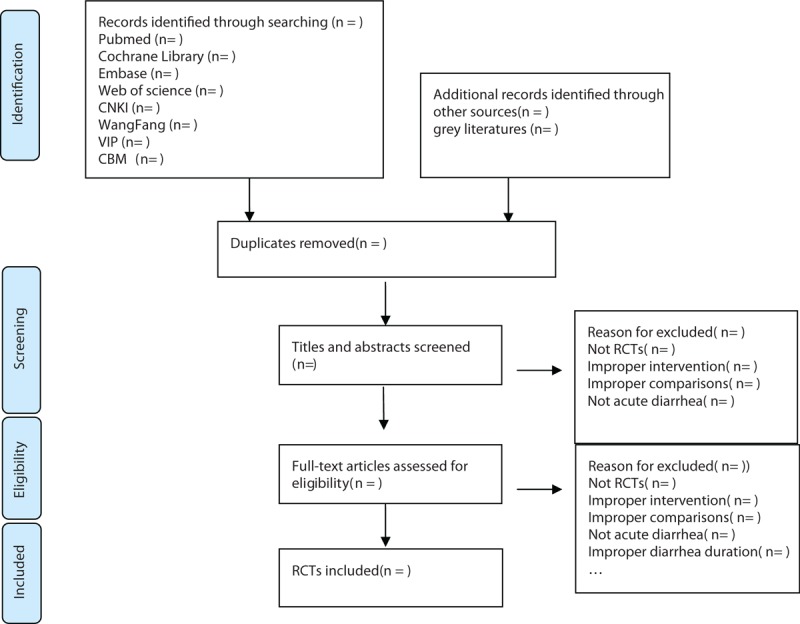
Flow chart of the study selection.
2.4.2. Data extraction
Two researchers (WLZ and ZLY) will read all the included text in full, and independently extract the following information: basic information (year of publication, the 1st authors name), study method (design, blinding), information of participants (number of children, gender, age, stool frequency within 24 hours, course of disease), information of treatment (interventions and controls, medicine, dose, frequency, duration), outcomes (clinical cure rate, diarrhea duration, stool frequency within 24 hours, rate of adverse effect), and P-value. If we could not reach an agreement, a 3rd researcher (HHC) would make the final decision. One researcher (WLZ) would contact the corresponding author by telephone or e-mail for more information when the reported data were insufficient or ambiguous.
2.4.3. Assessment of risk of bias
All the included studies will be evaluated based on the guidelines of Cochrane Handbook for Systematic Reviews of Interventions.[20] The quality of each trial will categorized into “low,” “unclear,” or “high” risk of bias according to the following items: adequacy of generation of the allocation sequence, allocation concealment, blinding of participants and personal, blinding of outcome assessors, incomplete outcome data, selected reporting the results, and other sources of bias (such as comparable baseline characteristic, inclusion and exclusion criteria).
2.4.4. Assessment of reporting biases
Reporting biases and small-study effects will be detected by funnel plot and Egger test if there are 10 more studies included in this meta-analysis. For Egger test, P-value of <.10 was considered to indicate the existence of reporting biases and small study effects.
2.4.5. Data analysis
We used RevMan 5.3 software provided by the Cochrane collaboration to analyze the data. Binary outcomes will be summarized using risk ratio with 95% confidence interval (CI) for relative effect. Continuous outcomes will be summarized by using weighted mean difference with 95% CI. We will use random-effect model for meta-analysis in this review according to research recommendations[21].
Statistical heterogeneity will be assessed by Chi-squared and I2 statistical tests. Where P-value ≥.1 and I2 ≤50%, there is no obvious statistical heterogeneity among the studies. On the contrary, where P-value <.1 or I2 >50% indicates a considerable heterogeneity. Meta-analysis will be performed when the statistical heterogeneity is acceptable (P-value ≥.1 and I2 ≤50%); otherwise, subgroup analysis will be applied to explore the influence of potential factors on the outcome measures. We will conduct subgroup analyses by different medication of injection (654-2, vitamin B, Chinese patent medicine, etc). We will conduct sensitivity analyses by omitting studies one by one to probe the impact of an individual study. If a meta-analysis cannot be performed, we will conduct descriptive analysis instead.
2.4.6. Patient and public involvement
This is a meta-analysis study based on previously published data, and hence patient and public involvement will not be included in this study.
2.4.7. Ethics and dissemination
Ethical approval will not be required as this is a protocol for systematic review and meta-analysis. The findings of this study will be disseminated to a peer-reviewed journal and presented at a relevant conference.
2.4.8. Evidence assessed
The quality of evidence for this study will be assessed by Grades of Recommendations Assessment, Development and Evaluation (GRADE) standard established by the World Health Organization and international organizations.[22] To achieve transparency and simplification, the quality of evidence is divided into 4 levels in GRADE system: high, medium, low, and very low. We will employ GRADE profiler 3.2 for analysis.
3. Discussion
Nowadays, acupuncture and moxibustion are considered to be effective in treating gastrointestinal dysfunction. In recent years, more and more studies have found that acupuncture and moxibustion play an important role in regulating gastrointestinal motility,[23] such as diabetic gastroparesis,[24] bowel preexcitation syndrome,[25] etc. especially IBS (diarrhea-predominant irritable bowel syndrome referred to as diarrhea in TCM[26]). Recent studies have shown that acupuncture and moxibustion have a definite effect on IBS,[27] acupoints and methods are various,[28–29] and the curative effect is better than that of conventional drugs.[30] Acupuncture and moxibustion treatment of diarrhea-predominant irritable bowel syndrome has obvious advantages in enhancing clinical efficacy, shortening course of treatment, improving main clinical symptoms and serum biochemical indicators more quickly and ideally, and assisting in regulating patients’ mood.[26]
Zusanli acupoint has always been the preferred acupoint for the acupuncture therapy of gastrointestinal diseases; therefore, widely used in modern clinical practice in treating gastritis, gastralgia, functional dyspepsia, vomiting, hiccup, abdominal pain, diarrhea, abdominal distention, and other gastrointestinal diseases, with remarkable effective.[31] Studies have proved that acupuncture stimulation of zusanli can enhance gastric motility,[32,33] protect gastric mucosa,[34] and improve intestinal function.[11–13] Acupoint injection therapy can not only exert the function of acupoints, but also exert the pharmacologic function of western medicine; it is a double stimulation with acupuncture and medicine liquid pairs in acupoints.[4,5,35,36] It is difficult for children to adopt acupuncture therapy, and it is also inconvenient for therapist to operate; however, because of the application of acupoint injection, we can achieve the same purpose as acupuncture, which is easy for parents and kids to accept. Therefore, acupoint injection is especially suitable for children.[37]
Zusanli acupoint injection combines the triple function of acupuncture, zusanli acupoint, and medicine[36]; it was applied in acute diarrhea in children was 1st reported in 1979,[38] and has been used until now. But there is no any systematic review or meta-analysis of the potential benefits and harms on zusanli acupoint injection for acute diarrhea in children, and although TCM treatment is listed in the guidelines for the treatment of acute diarrhea in children, there is also no specific method or recommendation due to lack of evidence.[18] Hence other than providing evidence for acupoint injection of acute diarrhea in children, this study is anticipated to provide evidence reference in TCM field for Chinese guidelines on the treatment of acute diarrhea in children.
In summary, this systematic review and meta-analysis will help to determine potential benefits and harms on zusanli acupoint injection for acute diarrhea in children. Furthermore, the findings of this study may not only provide reference basis for the guideline, but also might promote acupuncture treatment and application of acupuncture points which would benefit more patients in the future.
Author contributions
Conceptualization: Yuan Tian.
Data curation: Yuan Zhang, Linyue Zhou, Lizhen Wang.
Formal analysis: Yuan Tian, Hengchang Hu.
Methodology: Yuan Tian, Hengchang Hu.
Project administration: Chunguang Xie.
Resources: Yuan Tian, Chunguang Xie.
Software: Yuan Tian, Chunguang Xie.
Writing – original draft: Yuan Tian.
Writing – review & editing: Chunguang Xie.
Footnotes
Abbreviations: CI = confidence interval, FGIDs-D = functional gastrointestinal disorders-diarrhea, IBS = irritable bowel syndrome, GAS= Gastrin, IBS = irritable bowel syndrome, MTL= Motilin, PRISMA-P = Preferred Reporting Items for Systematic Reviews and Meta-Analysis Protocol, RCT = randomized controlled trial, TCM = traditional Chinese medicine, YOA = years of age.
Funded by the 2nd batch of scientific research projects for the construction of the national traditional Chinese medicine clinical research base (no: JDZX2015222).
The authors have no conflicts of interest to disclose.
References
- [1]. [Accessed June 9, 2019]. Diarrhea disease, WHO website, 2016. Available at: http://www.who.int/mediacentre/factsheets/fs330/en/ [Google Scholar]
- [2].Pinzon-Rondon AM, Zarate-Ardila C, Hoyos-Martinez A, et al. Country characteristics and acute diarrhea in children from developing nations: a multilevel study. BMC Public Health 2015;15:811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Tette EM, Sifah EK, Nartey ET. Factors affecting malnutrition in children and the uptake of interventions to prevent the condition. BMC Pediatr 2015;15:189–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Liu L, Oza S, Hogan D, et al. Global, regional, and national causes of child mortality in 2000-13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet (London, England) 2015;385:430–40. [DOI] [PubMed] [Google Scholar]
- [5].Sha T, Gao LL, Zhang CH, et al. An update on acupuncture point injection. QJM 2016;109:639–41. [DOI] [PubMed] [Google Scholar]
- [6].Li P. Acupoint Injection Therapy in Clinical Situations [in Chinese]. 2003;Beijing: Military Medical Science Press, 15–18. [Google Scholar]
- [7].Wang QY. Research progress on clinical effect and mechanism of acupoint injection. Zhongguo Zhongyiyao Xiandai Yuancheng Jiaoyu 2013;11:160–1. [Google Scholar]
- [8].Li YS, Xu B. Analysis of the mechanism of Zusanli point injection in the treatment of digestive system diseases. Zhenjiu Linchuang Zazhi 2014;30:70–2. [Google Scholar]
- [9].Chen XL, Yue ZH, Liu L, et al. Ancient and modern application and research of zusanli point. Zhenjiu Linchuang Zazhi 2016;32:80–3. [Google Scholar]
- [10].Shi XM. Science of Acupuncture and Moxibustion [in Chinese]. 2007;Beijing: China Press of Traditional Chinese Medicine, 221–222. [Google Scholar]
- [11].Li F, Gao F. Effect of acupuncture at zusanli point on gastrointestinal hormones GAS and MTL levels in rats with gastrointestinal dysfunction-diarrhea model. Zhejiang Zhongyiyao Daxue Xuebao 2017;41:271–3+277. [Google Scholar]
- [12].Liu YL, Tan WS, Zhu Q, et al. Effect of electroacupuncture at Zusanli on gastrointestinal microcirculation and 5-HT in FGIDs rats. Liaoning Zhongyiyao Daxue Xuebao 2018;20:79–81. [Google Scholar]
- [13].Ran GP, Liu JY, Yang DY, et al. Mechanisms of electro-acupuncture Zusanli on inflammatory injury in DIARRHEA-TYPE rats with irritable bowel syndrome. Zhongguo Zhongxiyi Jiehe Zazhi 2019;39:342–6. [Google Scholar]
- [14].Hao Y. Observation of the therapeutic effect of recombinant human interferon alpha 1b Zusanli blockade on 56 cases of infantile diarrhea in autumn and winter. Zhongguo Zhongxiyi Jiehe Erkexue 2012;4:149–50. [Google Scholar]
- [15].Huang ZA. Efficacy and safety of 654-2 Zusanli acupoint injection in the treatment of infantile autumn diarrhea. Zhongguo Yiyao Kexue 2016;6:63–5. [Google Scholar]
- [16].Qu SZ, Lin YQ, Ji X. Observation on the effect of Zusanli acupoint flow to compound vitamin B injection in treating infantile diarrhea. Guoji Yiyao Weisheng Daobao 2005;11:133–4. [Google Scholar]
- [17].Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 2015;350:g7647. [DOI] [PubMed] [Google Scholar]
- [18].Digestive Group, Society of Pediatrics, Chinese Medical Association. Guidelines for Clinical Practice of Acute Infectious Diarrhea in Children in China. Zhonghua Erke Zazhi 2016;54:483–8.27412736 [Google Scholar]
- [19].Fang HS, Wei CY, Duan SC, et al. Supplementary suggestion for the standard of judging the effect of diarrhea. Chin J Pract Pediatrics 1998;13:64. [Google Scholar]
- [20].Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0: the Cochrane collaboration, 2011. Available at: Higgins JPT, Green S, eds. Cochrane handbook for systematic reviews of interventions version 5.1.0: the Cochrane collaboration, 2011. Available at: http://www.equator-network.org/reporting-guidelines/cochranehandbook-for-systematic-reviews-of-interventions-version-5-1-0/ Accessed June 10, 2019. [Google Scholar]
- [21].Furlan AD, Pennick V, Bombardier C, et al. 2009 updated method guidelines for systematic reviews in the Cochrane Back Review Group. Spine 2009;34:1929–41. [DOI] [PubMed] [Google Scholar]
- [22].Balshem H, Helfand M, Schunemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 2011;64:401–6. [DOI] [PubMed] [Google Scholar]
- [23].Li LF, Zheng SX, Xu JS. Effect of acupuncture and moxibustion on gastrointestinal function and its application. Yunnan Zhongyi Xueyuan Xuebao 2017;40:92–6. [Google Scholar]
- [24].Li G, Huang C, Zhang X, et al. The short-term effects of acupuncture on patients with diabetic gastroparesis: a randomized crossover study. Acupunct Med 2015;33:204–9. [DOI] [PubMed] [Google Scholar]
- [25].Xing L, Qu L, Chen H, et al. A clinical observation of irritable bowel syndrome treated by traditional Chinese spinal orthopedic manipulation. Complement Ther Med 2013;21:613–7. [DOI] [PubMed] [Google Scholar]
- [26].Li GY. Clinical observation of acupuncture and moxibustion in the treatment of diarrhea-predominant irritable bowel syndrome. Shanghai Zhenjiu Zazhi 2018;37:187–91. [Google Scholar]
- [27].Park JW, Lee BH, Lee H. Moxibustion in the management of irritable bowel syndrome: systematic review and meta-analysis. BMC Complement Altern Med 2013;13:247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Stuardi T, MacPherson H. Acupuncture for irritable bowel syndrome: diagnosis and treatment of patients in a pragmatic trail. J Altern Complement Med 2012;18:1021–7. [DOI] [PubMed] [Google Scholar]
- [29].Manheimer E, Cheng K, Wieland LS, et al. Acupuncture for treatment of irritable bowel syndrome. Cochrane Database Syst Rev 2012;5:CD005111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Manheimer E, Wieland LS, Cheng K, et al. Acupuncture for irritable bowel syndrome: systematic review and meta-analysis. Am J Gastroenterol 2012;107:835–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Lu FY, Wang YY, Xin JJ, et al. Discussion on the specificity of meridian-acupoint effect from “Dufu Sanli Liu”. Zhongguo Zhenjiu 2016;36:840–4. [DOI] [PubMed] [Google Scholar]
- [32].Chang XR, Yan J, Yi SX, et al. Effects of electroacupuncture on gastroelectrography and cerebroengial peptides in rats. Zhongguo Zhenjiu 2004;24:52–4. [Google Scholar]
- [33].Chang XR, Yan J, Lin YP, et al. Effects of acupuncture at Zuyangming meridian on the contents of motilin and gastrin in healthy human plasma. Zhongguo Zhongxiyi Jiehe Xiaohua Zazhi 2001;9:69–70. [Google Scholar]
- [34].Ji LX, YanP, Hao CY, et al. Study on the protective effect of acupoint prescription on gastric mucosa in rats with gastric mucosal injury. Zhongguo Zhenjiu 2002;22:35–8. [Google Scholar]
- [35].Luo RD. A comparative observation on the treatment of infantile autumn diarrhea by acupoint injection. Zhongguo Zhenjiu 2004;24:21–2. [Google Scholar]
- [36].Wang M, Zhang FX, Li YZ, et al. Clinical observation on the treatment of children's diarrhea in autumn and winter by acupoint injection of virazole and vitamin gum calcium. Zhongguo Zhenjiu 2001;21:15–6. [Google Scholar]
- [37].Tang JQ, Peng ZL, Fang F. Observation of therapeutic effect of acupoint injection on 256 cases of infantile diarrhea. Zhongguo Zhenjiu 1994;14:115–6+496. [Google Scholar]
- [38].Cui SQ. Vitamin B (12) acupoint blocking therapy for infantile diarrhea. Henan Chijiao Yisheng 1979;5:25. [Google Scholar]


