Supplemental Digital Content is available in the text
Keywords: β-blocker, catecholaminergic polymorphic ventricular tachycardia, flecainide, meta-analysis
Abstract
Background:
Owing to reports of recurrent cardiac events in some catecholaminergic polymorphic ventricular tachycardia (CPVT) patients using β-blockers, safer alternatives are being investigated. Flecainide is an alternative adjunctive anti-arrhythmic agent known to provide incomplete protection to CPVT patients.
Methods:
To investigate the efficacy and tolerability of flecainide, we searched 4 databases for retrospective cohort studies (RCs) and randomized controlled trials (RCTs) investigating the efficacy and safety of flecainide for CPVT patients. Data were extracted and analyzed (risk ratio [RR] or mean difference [MD]) using RevMan software. Seven RCs and 1 RCT (333 CPVT patients; 152 patients treated with flecainide) were identified.
Results:
Flecainide monotherapy was superior to standard therapy in alleviating the risk of arrhythmic events (RR = 0.46, confidence interval [CI] = [0.38, 0.56], P < .00001) and exercise-induced arrhythmia scores (MD = −0.39, CI = [−0.74, −0.05], P = .03). Combination therapy of flecainide and β-blockers was superior to β-blocker monotherapy in reducing the risk of arrhythmic and symptomatic events (RR = 0.29, CI = [0.13, 0.69], P = .005; RR = 0.36, CI = [0.20, 0.62], P = .0003, respectively), peak heart rate (MD = −16.81, CI = [−28.21, −5.41], P = .004), and exercise-induced arrhythmia scores (MD = −1.87, CI = [−2.71, 1.04], P < .0001). Flecainide did not increase the risk of all side effects (RR = 0.76, CI = [0.42, 1.40], P = .38) compared to that with β-blockers alone. No deaths were reported among patients treated with flecainide.
Conclusions:
Flecainide is an effective and safe anti-arrhythmic agent, and its use as a monotherapy might be a good alternative for CPVT patients with β-blocker intolerance. Combination therapy was superior to β-blocker monotherapy. More randomized clinical trials are required to explore the long-term efficacy and safety of flecainide in these patients.
1. Introduction
Catecholaminergic polymorphic ventricular tachycardia (CPVT) is a rare inherited arrhythmogenic disorder, and is a common cause of sudden cardiac death (SCD) in young and otherwise healthy patients.[1,2] Its exact prevalence is unknown, but has been estimated to affect 1 in 10,000 individuals.[3] Mutations in the RyR2gene encoding the cardiac ryanodine receptor Ca2+ release channel are the leading cause of this condition.[4,5] CPVT arrhythmic patterns can be reproduced using exercise stress tests,[6] and therefore, exercise testing is the most helpful clinical tool to diagnose CPVT.[3] Genetic testing is recommended for families affected by CPVT, as knowledge that someone in the family has a mutation can help to manage the disease better.[7] Currently, no treatment is completely effective or without risks for patients with CPVT. β-blockers reduce CPVT-induced ventricular arrhythmias and are indicated for use in all patients for symptom relief and the prevention of SCD as a first-line treatment. Previously, Leren et al revealed that nadolol, an unselective β-blocker, might be superior to β1-selective β-blockers in preventing arrhythmias in patients with CPVT.[8] Unfortunately, treatment failure is frequent, and side effects associated with β-blockade result in 8% of patients being taken off the drug or given a reduced subtherapeutic dose.[9] Flecainide is approved for use and works by blocking RyR2 channels, thereby preventing RyR2-mediated premature Ca2+ release, a clear trigger for ventricular arrhythmias. Studies describing the monotherapeutic use of flecainide have reported that it is at least as effective as β-blockers.[1,9] At present, the use of flecainide and β-blockers as a combined therapy has been tentatively reported to further reduce ventricular arrhythmias in CPVT patients compared to that using β-blockers alone, but definitive evidence is still lacking. To date, β-blocker monotherapy is still defined as standard therapy for CPVT. In addition, previous studies have also reported the use of β-blockers in conjunction with verapamil-type calcium channel antagonists for CPVT patients as standard therapy sometimes.[10] Data and the analysis of flecainide in CPVT are limited at the current time. In this study, we aimed to systematically review the published retrospective cohort studies (RCs) and randomized controlled trials (RCTs) and perform meta-analysis on the data.
2. Methods
The review was conducted and reported according to the preferred reporting items for systematic reviews and meta-analyses statement. An ethical approval was not necessary since meta-analysis was based on secondary data and not involved individual patients.
2.1. Eligibility criteria
Inclusion criteria were as follows:
-
(1)
RCs or RCTs;
-
(2)
baseline characteristics of patients with CPVT;
-
(3)
intervention of flecainide monotherapy or combination therapy at all doses;
-
(4)
controls of β-blocker monotherapy or standard therapy with head-to-head comparisons;
-
(5)
long-term follow-up and outcomes including efficacy and safety parameters related to the treatment.
The primary efficacy outcomes were arrhythmic events, symptomatic events, exercise-test results, and exercise-induced arrhythmia scores. Safety outcomes were defined as side effects or death events.
Exclusion criteria were as follows:
-
(1)
animal, cell, or molecular studies,
-
(2)
lack of a control group,
-
(3)
incomplete data, or
-
(4)
non-English publication.
2.2. Search strategy
A comprehensive literature search was performed for records published before February 1, 2019 using the Cochrane Library, PubMed, Embase, and Web of Science. Combinations of medical subject headings and free terms were used as a search strategy, including “CPVT,” “flecainide and CPVT,” “clinical management,” “therapeutic strategies.” All manuscripts were published in English. The most updated/inclusive data on each study were used for abstraction. Reference lists of relevant studies were also scanned.
2.3. Data extraction
Data were extracted by 2 independent reviewers. Extracted data included the following:
-
(1)
the main characteristics of the study design,
-
(2)
baseline data of enrolled patients,
-
(3)
risk of bias, and
-
(4)
study outcomes.
The events and total number of patients were extracted as dichotomous data within study groups, whereas continuous data were extracted as mean, standard deviations, and numbers of patients within study groups. Discrepancies were solved by discussion and consensus between reviewers. Figure 1 shows a flowchart for the study selection process.[1,9–15]
Figure 1.
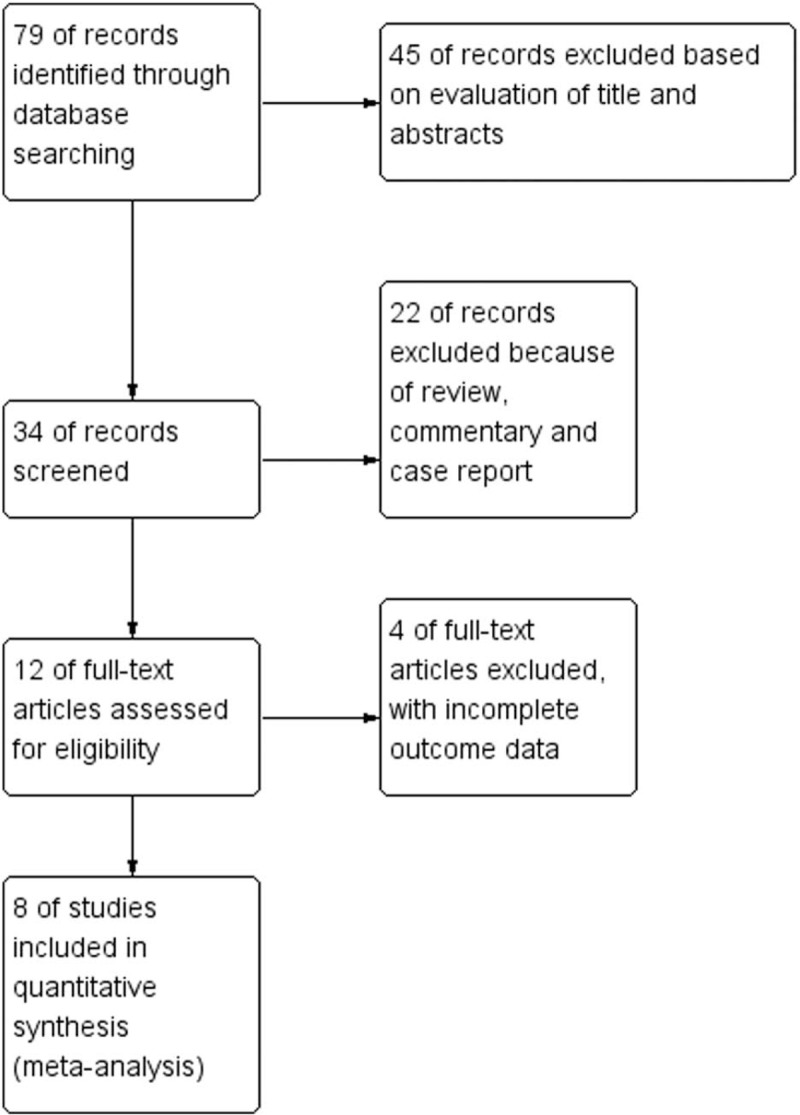
Study selection flowchart.
2.4. Quality assessment
Quality assessment was performed by 2 independent reviewers according to the Newcastle–Ottawa Scale,[16] which can be used for RCs (Fig. 2). Quality scores were assigned to each study after discussion and consensus among the reviewers. Seven cohort studies chosen for this meta-analysis were given quality scores above 6, constituting high methodological quality. The quality assessment of Kannankeril 2017 was conducted using QUADAS-2 tool criteria due to the presence of a RCT. A risk of bias assessment was also carried out for the work by Kannankeril 2017, and the study showed “low risk” as shown in Figure 2. Together, therefore, an independent evaluation of all pooled studies confirmed a low risk of bias.
Figure 2.
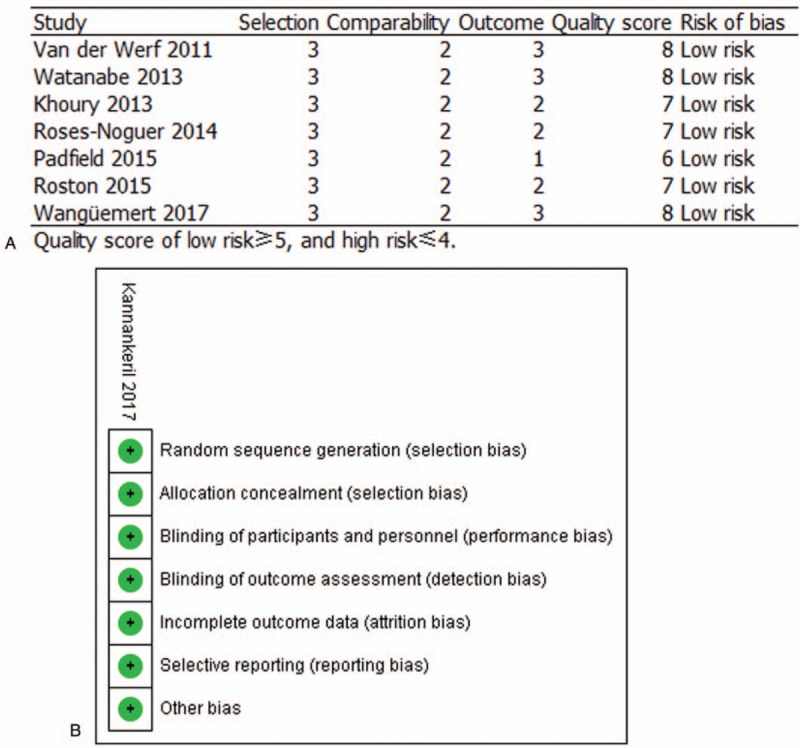
Quality assessment of the included studies using the NOS and QUADAS-2 tool criteria in A and B, respectively. NOS = Newcastle–Ottawa Scale.
2.5. Data analysis
Dichotomous data were pooled as risk ratios using the Mantel–Haenszel method. Continuous data were pooled as mean differences (MDs) using the inverse-variance method. Missing or unreported standard deviations were calculated from the standard error or 95% confidence interval (CI) according to the Cochrane handbook for systematic review of interventions. The cut-off value for statistical significance was P < .05. We used Review Manager (RevMan version 5.3.5 for Windows) to conduct meta-analyses and generate forest plots.
2.6. Assessment of heterogeneity
Heterogeneity was first assessed by visual inspection of the forest plots and then measured using I-square and Chi-square tests. We assessed and interpreted heterogeneity according to the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions (Chapter 9). When significant heterogeneity was present (I-square ≥50% and Chi-square P < .1), we performed the analysis under the random effect model. Otherwise, the fixed-effect model was adopted (I-square <50% and Chi-square P ≥ .1). Subgroup analysis was performed based on therapeutic strategies, symptomatic events, and side effects to reduce heterogeneity, and 4 subgroups were divided from included studies. In addition, to identify the heterogeneity caused by different methods of scoring, we performed sensitivity analysis using the leave-one-out method.
2.7. Publication bias
Funnel plots were constructed to assess publication bias, and none of the subgroups showed significant bias (Fig. 3). According to the Egger regression test, publication bias assessment is not reliable for fewer than 10 pooled studies per outcome.[17] Therefore, we could not conduct the Egger test.
Figure 3.
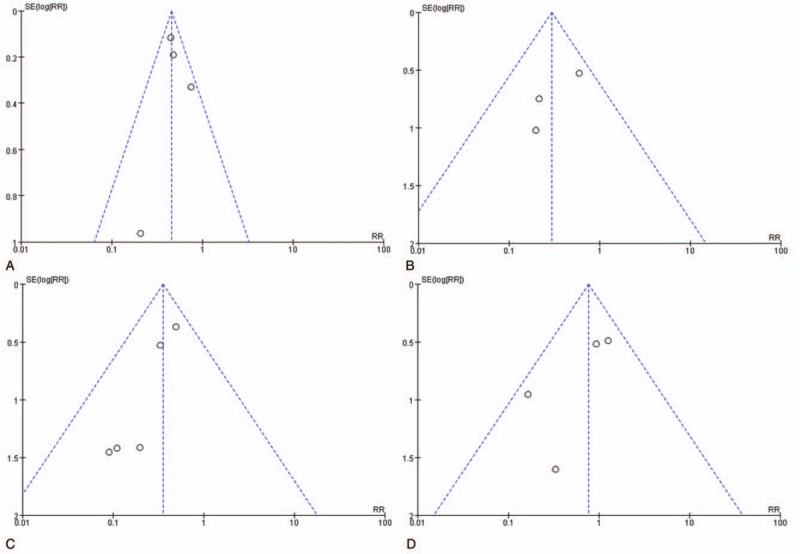
Funnel plots showing no significant publication bias in the different subgroups. (A) Arrhythmic events: flecainide monotherapy versus standard therapy; (B) arrhythmic events: combination therapy versus β-blocker monotherapy; (C) symptomatic events: combination therapy versus β-blocker monotherapy; (D) side effects: flecainide versus β-blocker.
3. Results
A total of 7 RCs and 1 RCT met the eligibility criteria and were included for quantitative analysis. The clinical characteristics of patients with CPVT are shown in Table 1. A total of 333 CPVT patients and 161 patients with RyR2 mutations were included and the majority were severely symptomatic and genotype RyR2-positive young female patients (≤20 years of age) treated with β-blockers, which might have been associated with the secretion of sex hormones. In addition, a total of 152 CPVT patients treated with flecainide were identified, as shown in Table 2. The qualitative scoring system proposed by Van der Werf et al was applied to quantify exercise-induced ventricular arrhythmias.[10] Exercise-induced arrhythmic events were analyzed and scored using the following predefined parameters: 0 = no ventricular ectopy; 1 = isolated premature ventricular contractions (<10 per minute); 2 = bigeminal and/or frequent premature ventricular contractions (>10 per minute); 3 = a single couplet or couplets; 4 = nonsustained bidirectional and/or polymorphic ventricular tachycardia. We quantified all exercise-induced results and listed them in Table 3.
Table 1.
Baseline characteristics of patients with catecholaminergic polymorphic ventricular tachycardia in the included studies.
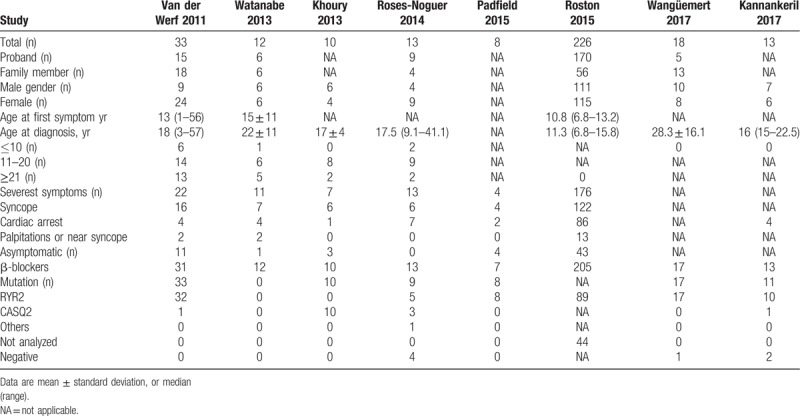
Table 2.
Clinical characteristics of catecholaminergic polymorphic ventricular tachycardia patients treated with flecainide in the included studies.
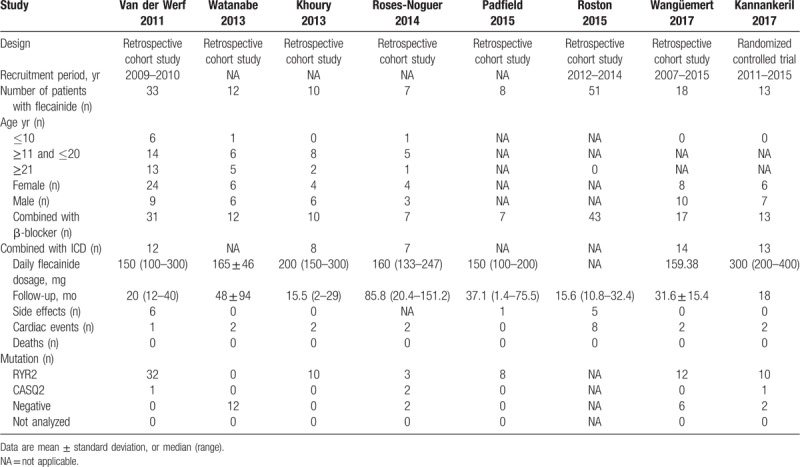
Table 3.
Exercise test results of catecholaminergic polymorphic ventricular tachycardia patients treated with flecainide and β-blockers in the included studies.
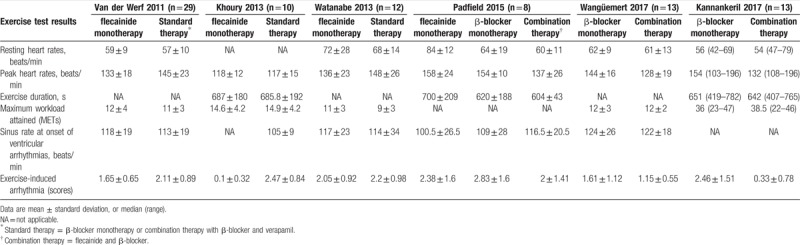
First, our meta-analysis was directed towards measuring the efficacy of flecainide. According to the subgroup analysis, flecainide monotherapy was associated with a significant decrease in the risk of arrhythmic events (RR = 0.46, CI = [0.38, 0.56], P < .00001) unlike standard therapy, as was the case with combination therapy (RR = 0.29, CI = [0.13, 0.69], P = .005) (Fig. 4). In addition, flecainide monotherapy reduced the risk of arrhythmic events compared to that with standard therapy in CPVT patients with and without genetic mutations (RR = 0.45, CI = [0.36, 0.57], P < .00001; P = .0001) (see Fig. 1, Supplemental Content, which illustrates the comparison between flecainide monotherapy and standard therapy for the risk of arrhythmic events in CPVT patients with genetic mutations and without genetic mutations). However, there was no significant difference between the efficacy of combination therapy and flecainide monotherapy (P = .7). Analysis of the incidence of symptomatic events showed that there was a significant difference between combination therapy and β-blocker monotherapy (RR = 0.36, CI = [0.20, 0.62], P = .0003) (Fig. 5). Especially, combination therapy could obviously reduce the incidence of symptomatic events compared to that with β-blocker monotherapy in patients with genetic mutations (RR = 0.13, CI = [0.02, 0.92], P = .04) (see Fig. 2, Supplemental Content, which illustrates the comparison between combination therapy and β-blocker monotherapy for the risk of symptomatic events in CPVT patients with genetic mutations and without genetic mutations).
Figure 4.
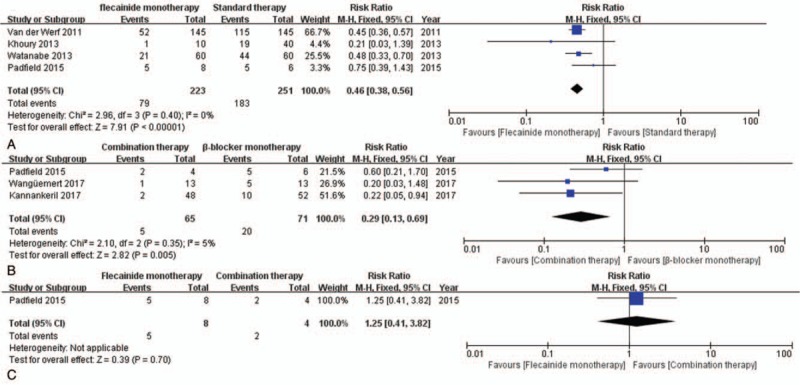
Forest plots of comparisons (A) between flecainide monotherapy and standard therapy, (B) between combination therapy and β-blocker monotherapy, and (C) between flecainide monotherapy and combination therapy for the risk of arrhythmic events.
Figure 5.
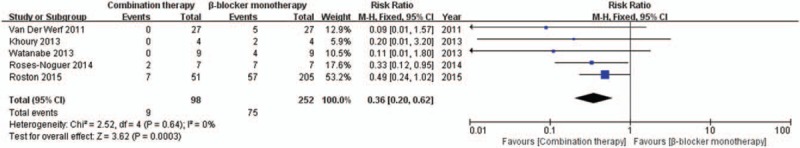
Forest plot of the comparison between combination therapy and β-blocker monotherapyin the risk of symptomatic events.
In the exercise test results, mean peak heart rates were lower with combination therapy than with β-blocker monotherapy (MD = −16.81, CI = [−28.21, −5.41], P = .004) (Fig. 6B). However, there was no significant difference in resting heart rate in patients treated with combination therapy compared to that with β-blocker monotherapy (MD = −1.68, CI = [−8.13, 4.78], P = .61). The same was true of the sinus rate at the onset of ventricular arrhythmias (MD = 0.16, CI = [−14.96, 15.27], P = .98) (Fig. 6A and C). In comparing patients treated by flecainide monotherapy versus standard β-blocker monotherapy, there was no significant different in the resting heart rates (MD = 3.38, CI = [−1.18, 7.93], P = .15), peak heart rates (MD = −5.53, CI = [−12.36, 1.30], P = .11), or sinus rates at onset of ventricular arrhythmias (MD = 3.75, CI = [−4.93, 12.44], P = .40) (Fig. 7).
Figure 6.
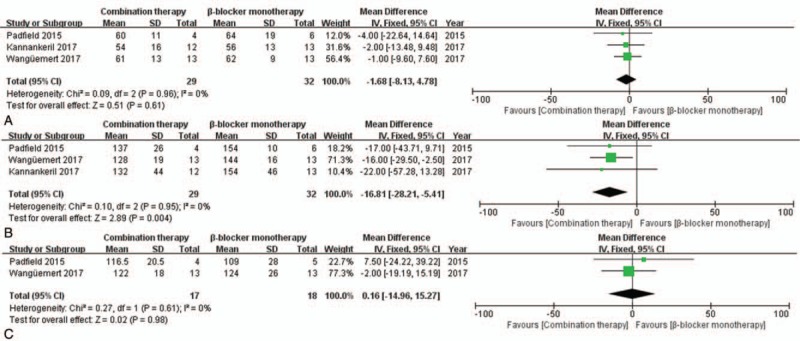
Forest plots of the comparison between combination therapy and β-blocker monotherapy in terms of mean differences in (A) resting heart rate, (B) peak heart rate, and (C) sinus rate at the onset of ventricular arrhythmias during exercise testing.
Figure 7.
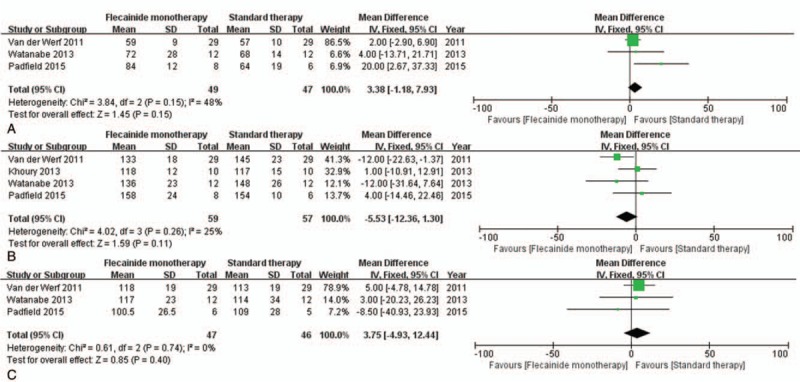
Forest plots of the comparison between flecainide monotherapy and standard therapy in terms of mean differences in (A) resting heart rate, (B) peak heart rate, and (C) sinus rate at onset of ventricular arrhythmias during exercise testing.
Significant heterogeneity was observed in only 2 outcomes upon subgroup analysis (I-square = 75% and Chi-square P = .02; I-square = 91% and Chi-square P < .00001) (Fig. 8A, C). Analysis of these outcomes was therefore conducted using the random-effects model. After sensitivity analysis, it was decided that the heterogeneity would be best resolved by removing the studies by Khoury 2013 and Wangüemert 2017. After their removal, the effect estimate remained significant in the 2 outcomes.
Figure 8.
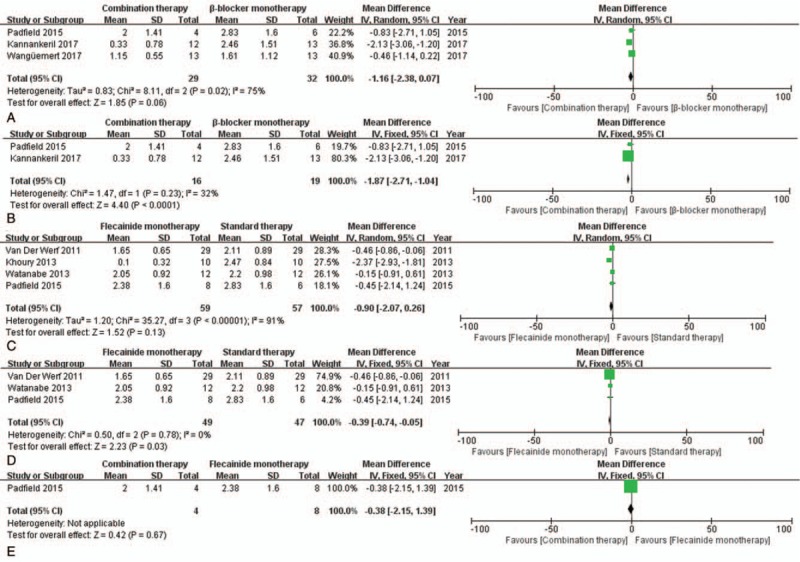
Forest plots of the comparisons of mean differences in exercise-induced arrhythmia scores. (A, B) show combination therapy and β-blocker monotherapy; (C, D) show flecainide monotherapy and standard therapy; (E) shows combination therapy and flecainide monotherapy.
Next, we measured the MD in exercise-induced arrhythmia scores, and our analysis showed that combination therapy was favored over β-blocker monotherapy (MD = −1.87, CI = [−2.71, 1.04], P < .0001) (Fig. 8B). The same was true of flecainide monotherapy, which was favored over standard therapy (MD = −0.39, CI = [−0.74, −0.05], P = .03) (Fig. 8D). There was no significant difference between combination therapy and flecainide monotherapy (P = .67) (Fig. 8E). Further, flecainide did not increase the risk of side effects (RR = 0.76, CI = [0.42, 1.40], P = .38) compared to that with β-blockers, as shown in Figure 9. No deaths were reported in CPVT patients treated with flecainide, as shown in Table 2.
Figure 9.
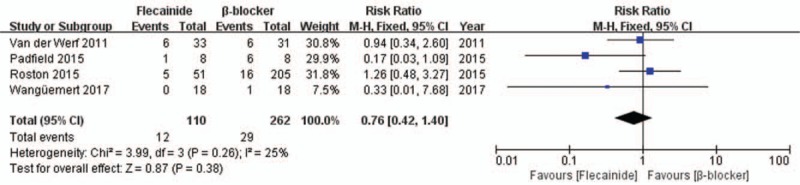
Forest plot of the comparison between flecainide and β-blocker for the risk of side effects.
4. Discussion
In this systematic review and meta-analysis, we investigated the efficacy and safety of flecainide, a class 1c anti-arrhythmic agent, for CPVT patients. Major findings included the following:
-
(1)
in accordance with previous studies, genotype RyR2-positive young patients with severe symptoms have an increased risk of arrhythmic events;
-
(2)
flecainide monotherapy, compared with β-blocker monotherapy, reduces the risk of arrhythmic events as measured by arrhythmia scores from exercise testing, including patients with and without genetic mutations;
-
(3)
combination therapy is superior to β-blocker monotherapy in reducing the risk of arrhythmic as well as symptomatic events, especially in patients with genetic mutations;
-
(4)
the effectiveness of flecainide might partially be due to a marked reduction in peak heart rates during exercise, but no other metrics in the exercise test were altered;
-
(5)
there is no significant difference between flecainide and β-blockers in terms of drug safety.
Pharmacologic treatment of CPVT is often unavoidably lifelong.[6] β-blockers have long been used effectively and remain the first-line therapy, but problems do exist. The most important problems associated with β-blocker treatment in CPVT are frequently observed sinus bradycardia, noncompliance at high doses of β-blockers, and inadequate therapy where the dose is reduced to prevent side effects.[18] Current clinical practice with regards CPVT treatment is to use flecainide as an adjunct therapy in cases where incomplete protection is evident at optimal doses of β-blockers. Based on current literature, the use of flecainide as monotherapy should also be recommended for CPVT patients intolerant to β-blockers. Flecainide exerts anti-arrhythmic effects independent of β-blockade and has particular advantages in this regard, as it exerts a lesser effect on sinus rate during exercise testing. Importantly, flecainide is effective for CPVT patients with or without genetic mutations, suggesting that spontaneous calcium release from ryanodine channels plays a role in arrhythmia susceptibility.[12] In our study, none of the reported serious adverse events were found to be related to flecainide, and the side-effects of flecainide were found to be mild. According to clinical guidelines, flecainide has a 2a recommendation for CPVT patients with breakthrough arrhythmias who are already receiving active β-blocker treatment.[19]
Flecainide has a dual effect, influencing both Na+ and Ca2+ ion channels. Compelling preclinical evidence indicates that flecainide suppresses delayed afterdepolarizations responsible for ventricular arrhythmias in CPVT. Experimental studies also demonstrate successful suppression of atrial activity and atrial fibrillation with flecainide.[20,21] The precise mechanism underlying the specificity of flecainide to CPVT is unclear, but current data support a model of flecainide action in which Na+-dependent modulation of intracellular Ca2+ handling attenuates RyR2 dysfunction.[22,23] Further study is therefore required to fully elucidate the anti-arrhythmic mechanism of flecainide in CPVT.
The safety of flecainide is as important as its protective effect against arrhythmias. In patients encountering side effects associated with β-blockers, the addition of flecainide might facilitate a reduction in the β-blocker dose.[24] Combined high-dose flecainide (5 mg/kg) and low-dose β-blockers, applied to minimize adverse effects and improve treatment adherence, has previously been shown to increase clinical efficacy and ventricular arrhythmia suppression.[25] In most cases, treatment failure has been associated with low flecainide dosing and/or noncompliance. Adequate dosing of flecainide seems to be critical for the suppression of CPVT. However, flecainide and β-blocker combination doses have not been characterized in terms of their adverse effects, and therefore, they should be optimized with serial exercise tests to limit dosage as much as possible.
In this study, we employed a comprehensive literature search of 4 major databases to retrieve all relevant studies related to flecainide in CPVT. The low risk of bias as measured by 2 different quality assessment tools added to the strength of our findings, and the assessment outcomes showed an acceptable level of reliability.
Our study had several limitations. First, the majority of the chosen studies were RCs and few randomized trials are included in the meta-analysis. Also, the number of CPVT patients treated with combination therapy or flecainide monotherapy was small. Second, results from exercise testing have high variability, especially with respect to exercise-induced arrhythmias. Hayashi et al showed that a reduction in arrhythmias during exercise testing did not equate to a reduction in clinical events,[26] and that the predictive ability of ventricular arrhythmia based on exercise testing was not clear. Third, left cardiac sympathetic denervation and/or implantable cardioverter-defibrillator implantation might play an important role in patients who remain symptomatic or continue to have persistent arrhythmias despite treatment with combination therapy. In addition, combination therapy has not been adequately tested in terms of possible side effects or efficacy. Therefore, we are cautious about drawing conclusions based on these data.
Based on our meta-analysis, future randomized controlled trials should directly compare flecainide to placebo in CPVT patients treated with β-blockers to prove its efficacy and safety. Moreover, compared to flecainide monotherapy and standard therapy, the impact of combination therapy on clinical events and side effects in CPVT patients with and without genetic mutations should be investigated in future longitudinal studies.
In summary, our meta-analysis revealed that flecainide is an effective and safe anti-arrhythmic agent and that using flecainide as a monotherapy might be a good alternative for patients with β-blocker intolerance. This is the first meta-analysis examining the efficacy and safety of flecainide in CPVT patients, especially in patients with and without genetic mutations. Moreover, our analysis indicates that combination therapy could offer better anti-arrhythmic effects than β-blocker monotherapy, especially for patients with genetic mutations. Future studies require more randomized clinical trials, which should make head-to-head comparisons between flecainide and β-blockers, and investigate the benefits of replacing or supplementing flecainide with β-blockers.
Author contributions
Conceptualization: Guangqiang Wang.
Data curation: Guangqiang Wang, Na Zhao, Shu Zhong, Yingrong Wang.
Formal analysis: Guangqiang Wang, Na Zhao, Shu Zhong.
Investigation: Shu Zhong, Yingrong Wang.
Methodology: Guangqiang Wang.
Software: Guangqiang Wang, Na Zhao.
Supervision: Na Zhao, Shu Zhong, Jianping Li.
Validation: Jianping Li.
Visualization: Yingrong Wang.
Writing – original draft: Guangqiang Wang, Na Zhao.
Writing – review and editing: Guangqiang Wang, Na Zhao.
Supplementary Material
Footnotes
Abbreviations: CPVT = catecholaminergic polymorphic ventricular tachycardia, MD = mean difference, RC = retrospective cohort, RCT = randomized controlled trial, RR = risk ratio, RyR2 = cardiac ryanodine receptor, SCD = sudden cardiac death.
GW and NZ contributed equally to this work.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Padfield GJ, AlAhmari L, Lieve KV, et al. Flecainide monotherapy is an option for selected patients with catecholaminergic polymorphic ventricular tachycardia intolerant of beta-blockade. Heart Rhythm 2016;13:609–13. [DOI] [PubMed] [Google Scholar]
- [2].Sy RW, Gollob MH, Klein GJ, et al. Arrhythmia characterization and long-term outcomes in catecholaminergic polymorphic ventricular tachycardia. Heart Rhythm 2011;8:864–71. [DOI] [PubMed] [Google Scholar]
- [3].Lieve KV, van der Werf C, Wilde AA. Catecholaminergic polymorphic ventricular tachycardia. Circ J 2016;80:1285–91. [DOI] [PubMed] [Google Scholar]
- [4].Priori SG, Napolitano C, Tiso N, et al. Mutations in the cardiac ryanodine receptor gene (hRyR2) underlie catecholaminergic polymorphic ventricular tachycardia. Circulation 2001;103:196–200. [DOI] [PubMed] [Google Scholar]
- [5].Laitinen PJ, Brown KM, Piippo K, et al. Mutations of the cardiac ryanodine receptor (RyR2) gene in familial polymorphic ventricular tachycardia. Circulation 2001;103:485–90. [DOI] [PubMed] [Google Scholar]
- [6].Napolitano C. Flecainide monotherapy for catecholaminergic polymorphic ventricular tachycardia: perspectives and limitations. Heart Rhythm 2016;13:614–5. [DOI] [PubMed] [Google Scholar]
- [7].Kozlovski J, Ingles J, Connell V, et al. Delay to diagnosis amongst patients with catecholaminergic polymorphic ventricular tachycardia. Int J Cardiol 2014;176:1402–4. [DOI] [PubMed] [Google Scholar]
- [8].Leren IS, Saberniak J, Majid E, et al. Nadolol decreases the incidence and severity of ventricular arrhythmias during exercise stress testing compared with beta1-selective beta-blockers in patients with catecholaminergic polymorphic ventricular tachycardia. Heart Rhythm 2016;13:433–40. [DOI] [PubMed] [Google Scholar]
- [9].Roston TM, Vinocur JM, Maginot KR, et al. Catecholaminergic polymorphic ventricular tachycardia in children: analysis of therapeutic strategies and outcomes from an international multicenter registry. Circ Arrhythm Electrophysiol 2015;8:633–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].van der Werf C, Kannankeril PJ, Sacher F, et al. Flecainide therapy reduces exercise-induced ventricular arrhythmias in patients with catecholaminergic polymorphic ventricular tachycardia. J Am Coll Cardiol 2011;57:2244–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Khoury A, Marai I, Suleiman M, et al. Flecainide therapy suppresses exercise-induced ventricular arrhythmias in patients with CASQ2-associated catecholaminergic polymorphic ventricular tachycardia. Heart Rhythm 2013;10:1671–5. [DOI] [PubMed] [Google Scholar]
- [12].Watanabe H, van der Werf C, Roses-Noguer F, et al. Effects of flecainide on exercise-induced ventricular arrhythmias and recurrences in genotype-negative patients with catecholaminergic polymorphic ventricular tachycardia. Heart Rhythm 2013;10:542–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Roses-Noguer F, Jarman JW, Clague JR, et al. Outcomes of defibrillator therapy in catecholaminergic polymorphic ventricular tachycardia. Heart Rhythm 2014;11:58–66. [DOI] [PubMed] [Google Scholar]
- [14].Wanguemert PF, Hernandez AJS, Groba MMDV, et al. Flecainide reduces ventricular arrhythmias in patients with genotype RyR2-positive catecholaminergic polymorphic ventricular tachycardia. Rev Esp Cardiol 2018;71:185–91. [DOI] [PubMed] [Google Scholar]
- [15].Kannankeril PJ, Moore JP, Cerrone M, et al. Efficacy of flecainide in the treatment of catecholaminergic polymorphic ventricular tachycardia: a randomized clinical trial. JAMA Cardiol 2017;2:759–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [17].Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Biernacka EK, Hoffman P. Efficacy of flecainide in a patient with catecholaminergic polymorphic ventricular tachycardia. Europace 2011;13:129–30. [DOI] [PubMed] [Google Scholar]
- [19].Lieve KV, Wilde AA, van der Werf C. The role of flecainide in the management of catecholaminergic polymorphic ventricular tachycardia. Arrhythm Electrophysiol Rev 2016;5:45–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Salvage SC, Chandrasekharan KH, Jeevaratnam K, et al. Multiple targets for flecainide action: implications for cardiac arrhythmogenesis. Br J Pharmacol 2018;175:1260–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Salvage SC, King JH, Chandrasekharan KH, et al. Flecainide exerts paradoxical effects on sodium currents and atrial arrhythmia in murine RyR2-P2328S hearts. Acta Physiol 2015;214:361–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bannister ML, Thomas NL, Sikkel MB, et al. The mechanism of flecainide action in CPVT does not involve a direct effect on RyR2. Circ Res 2015;116:1324–35. [DOI] [PubMed] [Google Scholar]
- [23].Bannister ML, Alvarez-Laviada A, Thomas NL, et al. Effect of flecainide derivatives on sarcoplasmic reticulum calcium release suggests a lack of direct action on the cardiac ryanodine receptor. Br J Pharmacol 2016;173:2446–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].van der Werf C, Lieve KV. Beta-blockers in the treatment of catecholaminergic polymorphic ventricular tachycardia. Heart Rhythm 2016;13:441–2. [DOI] [PubMed] [Google Scholar]
- [25].Steinfurt J, Dechant MJ, Bockelmann D, et al. High-dose flecainide with low-dose beta-blocker therapy in catecholaminergic polymorphic ventricular tachycardia: a case report and review of the literature. J Cardiol Cases 2015;11:10–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hayashi M, Denjoy I, Extramiana F, et al. Incidence and risk factors of arrhythmic events in catecholaminergic polymorphic ventricular tachycardia. Circulation 2009;119:2426–34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


