Abstract
Introduction:
The Waterhouse–Friderichsen syndrome (WFS), also known as purpura fulminans, is a potentially lethal condition described as acute hemorrhagic necrosis of the adrenal glands. It is often caused by infection. Classically, Neisseriae meningitidis represents the main microorganism related to WFS, although, infrequently, also other infectious agents are reported as a possible etiologic agent. The authors report the first case of death due to Proteus mirabilis infection, with postmortem evidence of WFS.
Patient concerns:
After a facial trauma that provoked a wound on the nose, the subject, a healthy 40-years old man, was conducted to the local hospital (in Sicily, Italy) after the primary care he was discharged. Subsequently, after 2 days of general malaise, he returned to the hospital due to the worsening of the clinical condition. During the hospitalization, hypotension, and neurological impairment appeared; the laboratory analysis showed leukocytosis and the alteration of renal, hepatic and coagulative parameters. Microbiological blood analysis resulted positive for a P mirabilis infection.
Diagnosis:
Multiorgan failure (MOF) with disseminated intravascular coagulation (DIC) due to sepsis was diagnosed.
Interventions:
The practitioners administered intensive support, antibiotic therapy, antithrombin III, vitamin K, and plasma.
Outcomes:
After 3 days the subject died. The autopsy and the microscopic investigation were performed revealing, also, the adrenal diffuse micronodular hyperplasia associated with a cortico-medullary hemorrhagic apoplexy.
Conclusion:
To our knowledge, this is the first case of MOF with WFS due to P mirabilis infection. This case report suggests that P mirabilis should be added to the list of unusual bacteria causing WFS. Furthermore, it supports the theory that any bacterium which causes DIC may cause adrenal hemorrhage and should suggest to clinicians the importance to consider a potential adrenal involvement in every patient with sepsis and DIC.
Keywords: bilateral massive adrenal hemorrhage, forensic sciences, Proteus mirabilis infection, sepsis, Waterhouse-Friderichsen syndrome
1. Introduction
The Waterhouse–Friderichsen syndrome (WFS), also known as purpura fulminans, is a very uncommon event but with high morbidity and mortality, that can occur in association with a common infection. It is described as acute hemorrhagic necrosis of the adrenal glands, often caused by meningococcal infection and is more frequently observed in the pediatric than the adult population.[1]
Adrenal hemorrhage is an infrequent condition that can be associated both to noninfection (including postoperative state, thromboembolic diseases, anticoagulant treatment, burns, trauma, tumor metastasis, and cardiovascular catastrophes) and a variety of infectious diseases. Classically, Neisseria meningitides represents the main microorganism related to WFS but is reported that also other infectious agents can be the possible etiologic agent of this disease, including bacteria (gram-negative and gram-positive) and viruses. Although N meningitidis accounts for more than 80% of WFS cases, other microorganisms implicated include other bacteria as Haemophilus influenzae, Streptococcus pneumoniae, Staphylococcus aureus, group A Streptococcus, Neisseria gonorrhoeae.[2]
The authors report a case of bilateral massive adrenal hemorrhage due to bacteremia of Proteus mirabilis that, according to the literature, is the first case of WFS occurring in the setting of this bacterial infection. Also, the authors report an overview of the syndrome and the main uncommon organisms that are related to this disease.
2. Case report
In winter 2017, a 40 years-old Caucasian man was conducted to the emergency department of the local hospital in Sicily (Italy) after a facial trauma that caused a wound on the nose. On physical examination, no abnormal findings were found, and cranium computed tomography (CT) was negative. The wound was sutured and the patient was discharged. After 2 days, the subject returned to the hospital for a state of unspecific general malaise, and then was transferred to the emergency department where the clinicians observed a neurological impairment (Glasgow Coma Scale: 10) state. Laboratory investigations showed severe hypoglycemia (18 mg/dL), a high anion gap metabolic acidosis; and a glucose-bicarbonate solution was infused. After a few hours, the clinical picture worsened: the man was appearing confused, with the blood pressure of 80/40 mm Hg, heart rate of 96 b/min, oxygen saturation of 96% (with oxygen administration via Ventimask). The other laboratory analyses have shown renal and hepatic impairment, severe thrombocytopenia (5 × 103/mL platelets), partial thromboplastin time 42.2 seconds, prothrombin time 17.2 seconds, prothrombin activity 56%, international normalized ratio 1.46, leukocytosis (29 × 103/mL white blood cells). These data were indicative for severe multiorgan failure (MOF) with disseminated intravascular coagulation (DIC). Abdomen-chest CT revealed a diffuse swelling of both adrenal glands and inflammatory changes in the peri-adrenal and renal fat. The patient was transferred to the intensive care unit and then was intubated; the administration of antibiotic therapy (meropenem, 1 g per day iv), antithrombin III, vitamin K, dobutamine, noradrenalin, and plasma was started. During the following days, the patient remained in critical conditions and the blood microbiological analysis showed positivity for P mirabilis (meropenem sensible <0.25 μg/mL). In the night of the sixth day of hospitalization, the patient was suddenly nonstable hemodynamically and, despite the administration of noradrenaline and adrenaline over the range, he developed a status of severe shock and died. The Judicial Authority ordered the autopsy. The autopsy showed brain congestion and pulmonary hemorrhagic oedema. Splenomegaly, hyperplasia of mesenteric lymph nodes, erosive and hemorrhagic lesions in the mucosa of stomach and intestine were observed. The adrenal lodges showed a fibrino-hemorrhagic infiltration (Fig. 1A and B).
Figure 1.
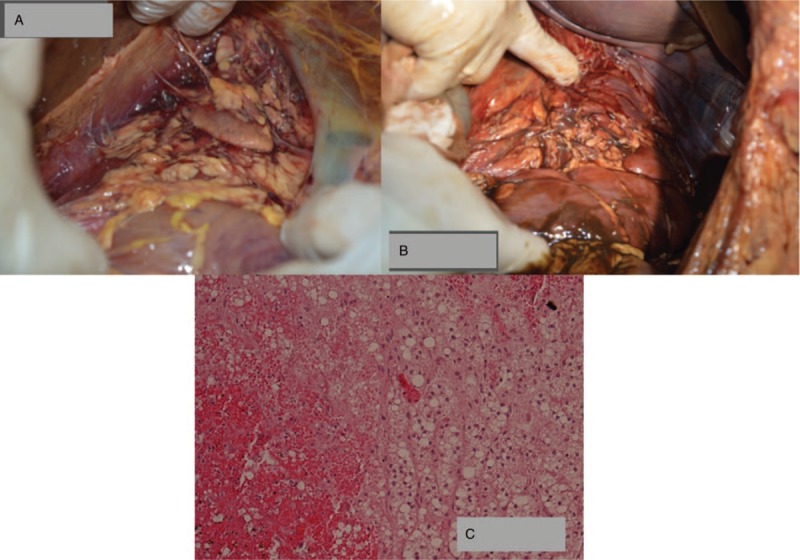
(A and B) Gross examination of adrenal lodges showing the presence of bilateral (respectively A: right; B: left) fibrino-hemorrhagic deposition; (C) Microscopic examination of adrenal gland showing diffuse micronodular hyperplasia associated with cortico-medullary hemorrhagic apoplexy.
The histological analysis showed endobronchial-endoalveolar haemorrhages and arteriolar lung embolus. Spleen had hemorrhagic congestion of the red pulp and hyperplasia of the white pulp. Intestine showed erosive lymphomonocytic inflammation, cytosteatonecrosis lesions of the submucosa and lymphomonocytic inflammation of the myenteric plexus; in the large intestine were also observed submucosal foci of micro-thromboembolism. Both adrenal glands showed diffuse micronodular hyperplasia associated with a cortico-medullary hemorrhagic apoplexy (Fig. 1C).
3. Discussion and conclusions
Infections and septic shock are among the most common causes of death that, not infrequently, are related to sudden unexpected death and medical malpractice.[3–5] The present case, in fact, initially come to authors attention to evaluate if there were any professional liability profiles, that, on the basis of the cause of death and management of the patient, that were subsequentially excluded. The clinical data and, in particular, the autopsy and histological findings, suggested that the subject died for MOF associated with DIC and bilateral adrenal apoplexy (WFS) due to sepsis sustained by P mirabilis. The clinical danger entailed in bilateral adrenal hemorrhage is acute adrenal insufficiency in the setting of severe illness, which can lead to rapid cardiovascular collapse and death.[6] The acute hemorrhagic necrosis of the adrenal glands is considered a subtype of severe infection or endotoxemia and a lot of microorganisms that can induce DIC can lead to intravascular changes of the adrenal vessels (forming a unique blood supply, where an arterial subcapsular plexus drains on relatively few venules) and to apoplexy adrenal glands. Thus, endotoxin-mediated septic shock is the most frequent clinical condition seen in patients with infectious adrenal hemorrhage. These endotoxins (lipopolysaccharide in the case of Gram-negative bacteria and peptidoglycan for Gram-positive bacteria), bind the toll-like receptors of endothelial and inflammatory cells, inducing a number of signaling pathways that lead to transcription of coagulation, fibrinolysis, and pro-inflammatory cytokine genes activation (as tissue factor, interleukin [IL]-1, IL-6, and tumor necrosis factor-alpha).[7]
It has been proposed that uni-visceral (or single organ type) Shwartzman mechanism may play an important role in the pathogenesis of adrenal haemorrhage and necrosis in humans. Since stress induces adrenal over-function through adrenocorticotropic hormone (ACTH) secretion, it can be claimed that stress is capable to injure the adrenal tissue directly, or that the shock state, caused by successive stresses, may provoke peripheral circulatory disturbance which would influence the adrenal gland.[8] The ACTH stimulates cortisol synthesis and secretion by regulating multiple steps in the steroidogenetic pathway. Activation of the melanocortin receptor 2 by ACTH in the adrenals also induces the adrenal production of factors affecting adrenal growth and its blood flow. Furthermore, ACTH stimulates the intra-adrenal production of vascular endothelial growth factor and the vaso-relaxant epoxy-eicosatrienoic acids.[9,10]
However, it is also described that diffuse haemorrhage and necrosis of the adrenal do not occur easily either after ACTH administration or under stress conditions in humans. Additionally, stronger stimulation may be necessary to provoke a real lesion of the adrenal, and this could be given by an infection or by endotoxemia.[8]
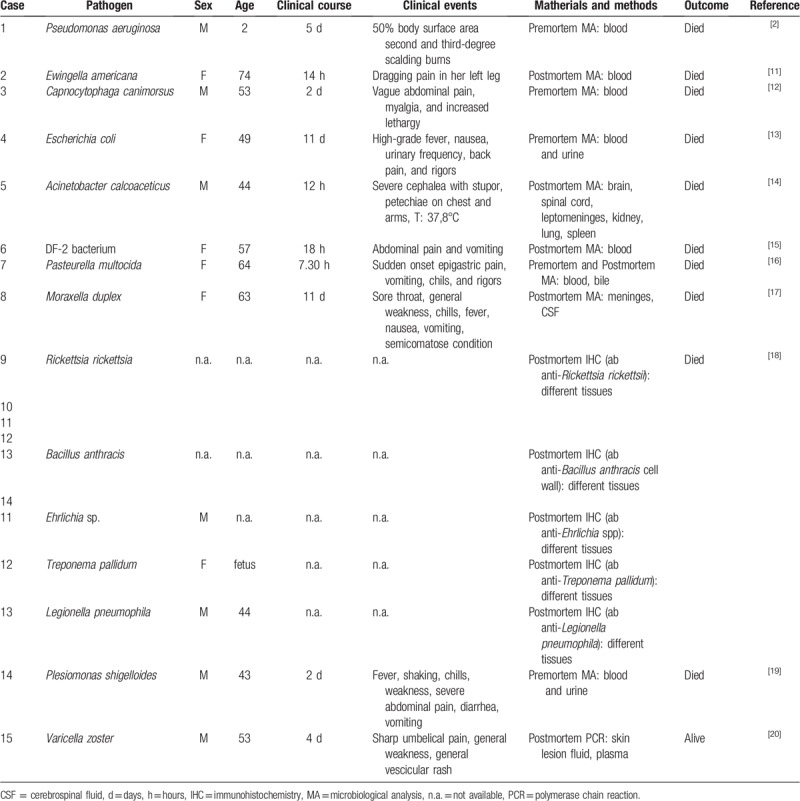
Concerning the triggering factor represented by endotoxemia, even if the N meningitidis is the most common pathogens associated with WFS, other microorganisms were identified as the possible cause of this adrenal disease. These microorganisms are H influenzae, S pneumoniae, S. aureus, group A Streptococcus, and N gonorrhoeae. The literature shows, also, unique or rare cases in which the WFS was related to infection caused by Pseudomonas aeruginosa, Klebsiella oxytoca, Ewingella americana, Capnocytophaga canimorsus, Escherichia coli, Acinetobacter calcoaceticus, DF-2 (Dysgonic fermenter 2) bacterium, Pasteurella multocida, Moraxella duplex, Rickettsia rickettsia, Bacillus anthracis, Ehrlichia sp., Treponema pallidum, Legionella pneumophila, Plesiomonas shigelloides and also by viruses as Varicella zoster.[2,11–20] The main information about these cases is summarized in Table 1.
Table 1.
Summary of the literature reports on WFS caused by unexpected/unusual infective agents.
Patients with the WFS usually show a sudden onset of vague and nonspecific symptoms as cough, dizziness, headache, sore throat, chills, rigors, weakness, malaise, restlessness, apprehension, myalgias, arthralgias, and fever. More than 75% of patients develop a generalized rash and, if no treatment is administered, patients may present vomiting, seizures, mental status changes, violent chills, circulatory collapse, and shock.[1]
Concerning the laboratory examinations, leukocytosis is often seen; the chemistry profile may have an elevated anion gap metabolic acidosis and, if there is bilateral adrenal hemorrhage, hyponatremia, hyperkalemia, mild azotemia, and occasionally hypoglycemia may be found.[21] Coagulation abnormalities are consistent with DIC and consumption of clotting factors.[22] Thus, the clinical findings could be considered very unspecific, making a clinical diagnosis of WFS extremely challenging. Other useful information could be obtained from Gram's stains and cultures from blood, cerebrospinal fluid, urine, and sputum to have.
If clinically suspected, the CT scan of the abdomen is strongly suggested as “gold standard” in the diagnosis of adrenal hemorrhage.[23] The therapy should be started as soon as the diagnosis is suspected, due to the lethal and fulminant course of this syndrome. It consists of antibiotic administration and, in particular, Penicillin G is the most suggested in N meningitidis infection, while third-generation cephalosporin can be used when the organism is unknown.[24] The need for corticosteroidal administration was a belief fervently held by Friderichsen in his later writings.[25] Some authors hold corticosteroid therapy as not recommended, maintaining that adrenal insufficiency is very rare and that serum cortisol levels are usually elevated in patients with adrenal hemorrhage; nevertheless, documentation of dramatic clinical improvement and survival following treatment with cortisone exists.[26] Obviously, the administration of therapy aimed to manage the clinical and laboratory tests alterations and the clinical manifestations directly related to the disease (ie, anticoagulant therapy for DIC treatment; supportive therapy in circulatory collapse and shock) is also very important.[27]
In light of this brief overview on WFS, the presented case confirms that the collection of clinical, laboratory tests and microbiological data, together with imaging, could be useful in practical diagnosis, even if, nowadays, the assessment of WFS is often given by postmortem findings.
The main evidence of the analyzed case was that WFS occurred in the setting of P mirabilis bacteremia. P mirabilis is described as a urease positive, highly motile bacterium, member of the Enterobacteriaceae family. It is normally found in soil, water, and sewage, and is part of the normal fecal flora. It has been related to community-acquired and nosocomial illnesses, including urinary tract infection (cystitis, pyelonephritis, and prostatitis), septicemia, and wound infections, but also in neonatal meningoencephalitis, empyema, and osteomyelitis.[28,29]
This is the first case, reported in the literature, in which the P mirabilis infection was complicated by WFS. Therefore, this case report demonstrates the difficulty in diagnosing and treating the WFS and supports the theory that any bacterium which causes DIC may cause adrenal hemorrhage. Finally, the evidence of WFS related to several uncommon microorganisms should suggest to clinicians the importance to consider a potential adrenal involvement in every patient with sepsis and DIC.
Author contributions
Conceptualization: Elvira Ventura Spagnolo, Cristina Mondello, Cataldo Raffino.
Data curation: Elvira Ventura Spagnolo, Cristina Mondello, Salvatore Roccuzzo, Chiara Stassi, Luigi Cardia, Angela Grieco, Cataldo Raffino.
Formal analysis: Elvira Ventura Spagnolo, Salvatore Roccuzzo, Cataldo Raffino.
Investigation: Cataldo Raffino.
Methodology: Cristina Mondello, Luigi Cardia.
Project administration: Elvira Ventura Spagnolo.
Resources: Angela Grieco, Cataldo Raffino.
Supervision: Elvira Ventura Spagnolo, Cristina Mondello.
Validation: Elvira Ventura Spagnolo, Cristina Mondello, Cataldo Raffino.
Visualization: Chiara Stassi.
Writing – original draft: Elvira Ventura Spagnolo, Cristina Mondello.
Writing – review and editing: Luigi Cardia.
Footnotes
Abbreviations: ACTH = adrenocorticotropic hormone, CT = computed tomography, DIC = disseminated intravascular dissemination, MOF = multiorgan failure, WFS = Waterhouse–Friderichsen syndrome.
In the reported case there are no personal-specific medical information that allow to identify the subject because the described clinical picture, demonstrating septic shock, is common in many patients and the data concerning the patient have been sufficiently anonymized (eg, no name or photographs of the patient are included in the paper). Moreover, even if the patient is dead, we sought permission from a relative as a matter of courtesy and medical ethics. Unfortunately, no relatives were contactable. Finally, the authors got the permission to publish the case by the Public Prosecutor who performed the forensic investigations. Informed consent to publish the case report was obtained from the public prosecutor who performed the forensic investigations.
We asked for ethical approval but it was not necessary because the present work is a descriptive case report with no ethical implications.
The autopsy was performed for legal reasons because it was necessary to exclude the possibility that death was related to a crime.
The authors have no conflicts of interest to disclose.
References
- [1].Varon J, Chen K, Sternbach George L. Rupert Waterhouse and Carl Friderichsen: adrenal apoplexy. J Emerg Med 1998;16:643–7. [DOI] [PubMed] [Google Scholar]
- [2].Tormos LM, Schandl CA. The significance of adrenal hemorrhage undiagnosed Waterhouse-Friderichsen syndrome. A case series. J Forensic Sci 2013;58:1071–4. [DOI] [PubMed] [Google Scholar]
- [3].Laganà P, Dattilo G, Mondello C, et al. A case of infective endocarditis due to Salmonella enterica phagetype 35. First report. Clin Ter 2017;168:e397–400. [DOI] [PubMed] [Google Scholar]
- [4].Ventura Spagnolo E, Mondello C, Roccuzzo S, et al. A lethal tick-borne encephalitis (TBE) due to TBE virus in Sicily (Italy): a case of IgG+/IgM- response? Clin Ter 2018;169:e145–8. [DOI] [PubMed] [Google Scholar]
- [5].Ventura Spagnolo E, Mondello C, Cardia L, et al. Odontogenic abscess complicated by descending necrotizing mediastinitis: evidence of medical and dental malpractice. Minerva Stomatol 2016;65:412–5. [PubMed] [Google Scholar]
- [6].Simon DR, Palese MA. Clinical update on the management of adrenal hemorrhage. Curr Urol Rep 2009;10:78–83. [DOI] [PubMed] [Google Scholar]
- [7].Levi M, Keller T, van Grop E, et al. Infection and inflammation and the coagulation system. Cardiovasc Res 2003;60:26–39. [DOI] [PubMed] [Google Scholar]
- [8].Aoyama H, Kikuchi F, Mori W. Acute, massive, haemorrhagic adrenal necrosis experimentally produced by the Shwartzman mechanism in rabbits. Virchows Arch A Pathol Anat Histopathol 1987;412:11–6. [DOI] [PubMed] [Google Scholar]
- [9].Lefebvre H, Thomas M, Duparc C, et al. Role of ACTH in the interactive/paracrine regulation of adrenal steroid secretion in physiological and pathophysiological conditions. Front Endocrinol (Lausanne) 2016;20:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ansurudeen I, Kopf PG, Gauthier KM, et al. Aldosterone secretagogues increase adrenal blood flow in male rats. Endocrinology 2014;155:127–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Tsokos M. Fatal Waterhouse-Friderichsen syndrome due to Ewingella americana infection. Am J Forensic Med Pathol 2003;24:41–4. [DOI] [PubMed] [Google Scholar]
- [12].Cooper JD, Dorion RP, Smith JL. A rare case of Waterhouse-Friderichsen syndrome caused by Capnocytophaga canimorsus in an immunocompetent patient. Infection 2015;43:599–602. [DOI] [PubMed] [Google Scholar]
- [13].Khwaja J. Bilateral adrenal hemorrhage in the background of Escherichia coli sepsis: a case report. J Med Case Rep 2017;11:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ferrara SD, Bertoloni G, Terribile V. Sudden death due to waterhouse-Friderichsen syndrome and purulent Leptomeningitis caused by Acinetobacter calcoaceticus (Mima polymorpha). Z Rechtsmed 1977;80:73–8. [DOI] [PubMed] [Google Scholar]
- [15].Chaudhuri AK, Hartley RB, Maddocks AC, et al. Waterhouse-Friderichsen syndrome caused by a DF-2 bacterium in a splenectomised patient. J Clin Pathol 1981;34:172–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Morrison U, Taylor M, Sheahan DG, et al. Waterhouse-Friderichsen syndrome complicating primary biliary sepsis due to Pasteurella multocida in a patient with cirrhosis. J Clin Pathol 1995;48:775–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lewis JF, Marshburn ET, Singletary HP, et al. Fatal meningitis due to Moraxella duplex: report of a case with Waterhouse-Friderichsen syndrome. South Med J 1968;61:539–41. [DOI] [PubMed] [Google Scholar]
- [18].Guarner J, Paddock CD, Bartlett J, et al. Adrenal gland hemorrhage in patients with fatal bacterial infections. Mod Pathol 2008;21:1113–20. [DOI] [PubMed] [Google Scholar]
- [19].Curti AJ, Lin JH, Szabo K. Overwhelming post-splenectomy infection with Plesiomonas shigelloides in a patient cured of Hodgkin's disease. A case report. Am J Clin Pathol 1985;83:522–4. [DOI] [PubMed] [Google Scholar]
- [20].Heitz AFN, Hofstee HMA, Gelinck LBS, et al. A rare case of Waterhouse-Friderichsen syndrome during primary Varicella zoster infection. Neth J Med 2017;75:351–3. [PubMed] [Google Scholar]
- [21].Xarli VP, Steel A, Davis PJ, et al. Adrenal hemorrhage in the adult. Medicine 1978;57:211–21. [DOI] [PubMed] [Google Scholar]
- [22].Kunzer W, Schindera F, Schenk W, et al. Waterhouse-Friderichsen-syndrome. Dtsch Med Wochenschr 1972;97:270–3. [DOI] [PubMed] [Google Scholar]
- [23].Munoz Corsini L, Delgado Arnaiz C, Garcõa del Valle S, et al. Postoperative bilateral adrenal hemorrhage: correlation between clinical and radiological signs. J Clin Anesth 2008;20:605–8. [DOI] [PubMed] [Google Scholar]
- [24].Salzman MB, Rubin LG. Meningococcemia. Inf Dis Clin North Am 1996;10:709–25. [DOI] [PubMed] [Google Scholar]
- [25].Friderichsen C. Waterhouse-Friderichsen syndrome. Acta Endocrinol 1955;18:482–92. [DOI] [PubMed] [Google Scholar]
- [26].Jafari HS, McCracken GH. Sepsis and septic shock: a review for clinicians. Pediatr Inf Dis J 1992;11:739–49. [DOI] [PubMed] [Google Scholar]
- [27].Carcillo JA, Davis AL, Zaritzky A. Role of early fluid resuscitation in pediatric septic shock. JAMA 1991;266:1242–5. [PubMed] [Google Scholar]
- [28].Kim BN, Nam NJ, Kim MN, et al. Bacteraemia due to tribe proteeae: a review of 132 cases during a decade. Scand J Infect Dis 2003;35:98–103. [DOI] [PubMed] [Google Scholar]
- [29].Chen CY, Chen YH, Lu PL, et al. Proteus mirabilis urinary tract infection and bacteremia: risk factors, clinical presentation, and outcomes. J Microbiol Immunol Infect 2012;45:228–36. [DOI] [PubMed] [Google Scholar]


