Abstract
Background:
Botulinum toxin type A (BoNTA) is known to prevent fibroblast proliferation and expression of transforming growth factor beta 1 (TGF-β1). It also induces temporary muscle paralysis and decreases tension vectors. Fibroblasts induce scar contracture and hypertrophy by producing collagen fibers in wound healing processes. The aim of this study is to identify the effect of BoNTA on the scar formation.
Methods:
Forty-five patients with forehead laceration were enrolled in this study and randomized into 2 groups with or without injection of BoNTA. When the patients presented to the clinic to remove the stitches, BoNTA was injected to the BoNTA group with 24 patients and saline was injected to the control group with 21 patients. The BoNTA was injected on dermal layer with 5 IU/cm. After that, follow-up was done in 1, 3, and 6 months. The scars were analyzed with the patient and observer scar assessment scale, Stony Brook scar evaluation scales (SBSESs), and visual analog scale (VAS) and analyzed with independent T-test, along with clinical photographs, cutometer, and biopsies.
Results:
In all scar scales, the scores changed into favorable direction in both groups and the changes were larger in BoNTA group compared with the control group. On SBSES and VAS, better improvements on BoNTA group showed statistical significance. Skin biopsy showed less collagen deposition on dermal layer in BoNTA group.
Conclusion:
Improvement of aesthetic, functional, and emotional aspect of the scar formation in the groups treated with BoNTA was illustrated. The application of BoNTA may be expanded to prevent hypertrophic scar after trauma, burns, or operations.
Keywords: botulinum toxins, cicatrix, cosmetics, lacerations, type A
1. Introduction
Skin damage by either trauma or surgical intervention inevitably results in scar formation. In some patients, facial scars can be cosmetically disfiguring and may cause functional impairment and psychosocial withdrawal.[1] Cutaneous scars are generally distinguished from surrounding normal skin by differences in color, thickness, contour, compliance, overall cosmetic, and functional damages such as contracture formation. Not only the disfigurement contributes to the undesirable appearance, but also to prolonged contracture, itching, or tingling which intervenes in the daily-living of patients. Young and Hutchison found that patients were usually dissatisfied with their surgical scars, irrespective of sex, age, ethnicity, or geographical location and that 91% of them would value even a small improvement in their scars.[2] Although surgeons make every effort to prevent widening, hypertrophy, hypo- or hyperpigmentation of scars, in some situations (massive trauma or burn) the situations is out of their hands, resulting in horrible sequalae. Despite numerous methods, such as excision, steroid administration, radiation, laser, and pressure therapy, having been introduced until now, scar management has always been a troublesome and challenging task for surgeons.
Since its 1st introduction in 1973 by Alan Scott by injecting botulinum toxin A on the lateral rectus muscle of a monkey,[3] botulinum toxin has been widely used in various medical conditions, and more importantly for plastic surgeons, in the treatment of facial paralysis and cosmetic procedures. It has been utilized in reducing wrinkles, with its main mechanism being blockade of the neuromuscular junction. Botulinum toxin has also been proved safe and reliable, with reversible complications. One of the major factors that contribute to the appearance of a scar is tension. Wound tension is produced by elastic recoil of the dermal layer and movement of the underlying musculature. In theory, botulinum toxin can play a vital role in scar prevention by reducing contracture and relaxing the adjacent muscles. Furthermore, botulinum toxin seems to be involved in cell signaling during the process of scar formation. In a study conducted in our institution in 2015, it was revealed that botulinum toxin directly inhibits fibroblast-to-myofibroblast differentiation in vitro in hypertrophic scars.[4]
Several studies have suggested the possibility of injecting botulinum toxin into nearby musculature around the traumatic or incisional wounds.[5–7] However, sound clinical evidence has been missing. The aim of this study is to investigate the subjective and objective evidence of the effect that botulinum toxin has on scar formation in human.
2. Patients and methods
This prospective, split-scar, double-blinded, randomized controlled study was approved by the Department of Plastic and Reconstructive Surgery, Kangnam Sacred Heart Hospital, College of Medicine, Hallym University. This study obtained approval of the institutional review board (Kangnam Sacred Heart Hospital Institutional Review Board, IRB number 2012-02-17). The local ethics committee of our hospital approved this study, which conformed to the provisions of the declaration of Helsinki.
2.1. Patient selection
From February 2012 to December 2015, patients who presented forehead lacerations were recruited from the emergency room. All patients came to the emergency room within 2 hours after the forehead relocation caused by trauma. Patients of age below 20 years or above 65 years were excluded from the study. The exclusion criteria were as follows: a known allergy to botulinum toxin; previous injection of botulinum toxin within 6 months before enrollment; underlying neuromuscular disorder; and pregnancy or breast feeding. The subjects involved in the study were assigned the medication for each clinical trial according to the assigned nonexecutive assignment number. To maintain the blinded nature of the study, the generated assignment table and medication for each assignment is disclosed after the clinical test is completed.
2.2. Wound management
Wound closure was conducted by 2 plastic surgeons (JWL and SJL) in the emergency room under local anesthesia. The entire subcutaneous layer was sutured with 6-0 Vicryl (6-0 coated VICRYL, Livingston, UK) at every 3 to 4 mm and the skin was sutured with simple interrupted 6-0 nylon sutures (6-0 ETHILON; ETHICON, Livingston, UK) at every 1 to 2 mm. The patients were then asked to visit the outpatient clinic for wound management and assessment every other day for a week. All sutures were removed on postoperative day 5 and all patients were asked to wear sunblock. After obtaining written consent, basic demographic data including age, sex, and wound length were collected, and the patients were randomized into control and botulinum toxin type A (BoNTA) groups. Placebo drug was prepared as a vial containing 0.9% saline which is similar to BoNTA. BoNTA Inj. Botulinum toxin type A (Hugel, Chuncheon, South Korea) was prepared by mixing 4 mL of 0.9% saline solution with 100 units of BoNTA (25 IU/mL). After the wound closure, removal of stitches was done in 5 days, and the BoNTA (BoNTA group) or saline solution (control group) was injected at multiple points around the sutured site within a 0.5 cm distance. The 5 IU/cm amount was injected into multiple sites that are symmetrical in the bottom side, centered on the suture line, and all injections were performed by a single plastic surgeon (ISS) in the intralesional and intradermal layer. To prevent eyebrow ptosis, the drugs were not injected around the supraorbital rim. The BoNTA remaining after treatment was discarded.
The subjects involved in the study were assigned medication for each clinical trial according to the assigned nonexecutive assignment number.
2.3. Wound assessment
Follow-up was conducted at 1, 3, and 6 months postoperatively, and scar evaluation was conducted in each visit. Digital photographs (Canon 700D, Tokyo, Japan) of the scar were taken under the same light source and illumination conditions using a standard light source box. Scar evaluation was done in 2 ways: subjective and objective. As for subjective evaluation, clinical photographs were taken, and various scar scale evaluations were done. Patient and observer scar assessment scale,[8–10] stony brook scar evaluation scales (SBSESs)[11] and visual analog scale (VAS)[12,13] were used as scar evaluation scales. Changes in value of these scales from 1 to 6 months postoperatively were calculated to measure the true improvement or worsening of the scar.
As for objective evaluation, we performed a scar removal procedure for patients who wanted it after 6 months, and we performed pathologic examination by obtaining biopsy specimen. Traditional hematoxylin and eosin staining and Masson trichrome stain were used to reveal the density of the collagen fiber between 2 groups. Also, Cutometer MPA 580 (Courage & Khazaka Electronic GmbH, Cologne, Germany) was used to evaluate skin elasticity, and Mexameter X18 (Courage & Khazaka Electronic GmbH) was used to measure pigmentation and erythema. The skin properties were measured from scar and contralateral normal skin of the forehead to adjust the differences between individuals, and the subtracted value of normal skin and scar tissue was used to measure relative difference between the 2 groups.
For statistical analysis, independent T-test was used to compare the median values of the changes of scar scales from 1 to 6 months postoperatively. P-value below .05 indicates that one group showed more favorable changes regarding scar scales. The results were analyzed with IBM SPSS Statistics for Windows (Version 22.0; IBM, Armonk, NY). Statistical significance was defined as P <.05.
3. Results
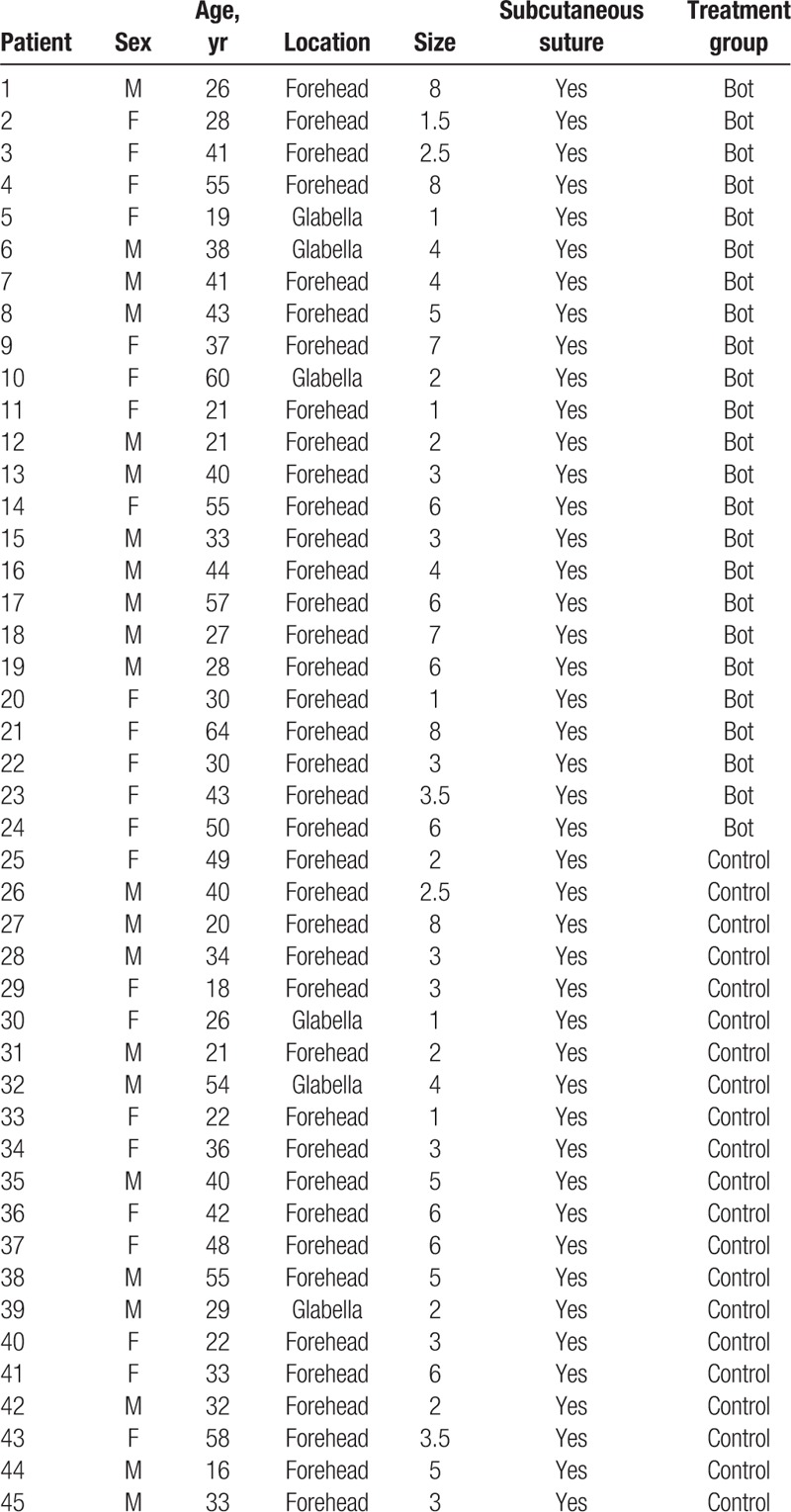
Sixty forehead laceration patients were recruited. Among them, 15 patients were lost by the time of follow-up because they did not attend the 6-month visit. Forty-five patients completed the follow-up. After the randomization process, there were 21 patients in the control group and 24 patients in the BoNTA group. No complications or significant adverse effects were noted during the follow-up period. There were no significant adverse events in all patients. We performed perilesional and intradermal injection and did not induce muscle contracture, so frontalis muscle paralysis did not occur. Basic patient demographics including age, gender, and photo type according to the Fitzpatrick scale are listed in Table 1. More detailed patient data with location, wound length and treatment group are listed in Table 2.
Table 1.
Patient demographics.
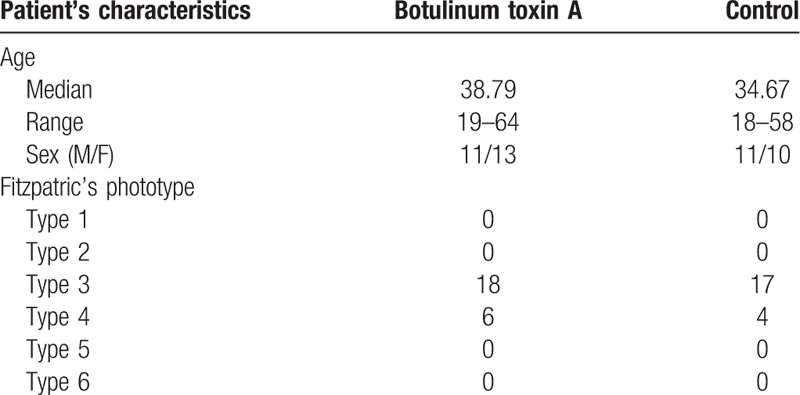
Table 2.
Patient data with location and size.
All scar assessment scales showed favorable changes in both control and BoNTA groups (Fig. 1). However, when the amount of changes of the scar scales was investigated, there were more favorable changes in BoNTA group, which was proved statistically in Stony Brook scar evaluation scales (SBSESs) (P = .047) and VAS (P = .046). Even without statistical significance, there were more favorable changes in BoNTA group in patient scar assessment scale (PSAS) (P = .110) and observer scar assessment scale (OSAS) (P = .169) (Fig. 2). At the 6-month visit, the overall median OSAS score was 14.83 in the BoNTA group (minimum 12, maximum 18) compared with 15.95 in the control group (minimum 13, maximum 18), with no significant different improvement grade between the 2 groups on the independent T-test. At the 6-month visit, the overall median PSAS score was 14.54 in the BoNTA group (minimum 12, maximum 18) compared with 14.81 in the control group (minimum 13, maximum 16), with no significant different improvement grade between the 2 groups, based on the independent T-test. The overall median VAS score was 7.00 in the BoNTA group (minimum 6, maximum 8) compared with 6.05 in the control group (minimum 5, maximum 7), with a significantly more acceptable result in the toxin group based on the independent T-test. The overall median SBSES score was 4.08 in the BoNTA group (minimum 3, maximum 5) compared with 3.19 in the control group (minimum 2, maximum 4), with a significantly more positive result in the toxin group based on the independent T-test (Table 3). The scar pigmentation, width, height, and pliability showed favorable changes in both the groups, but improvements of these properties during the time from the 1st month to the 6th were not revealed clearly in the scores.
Figure 1.
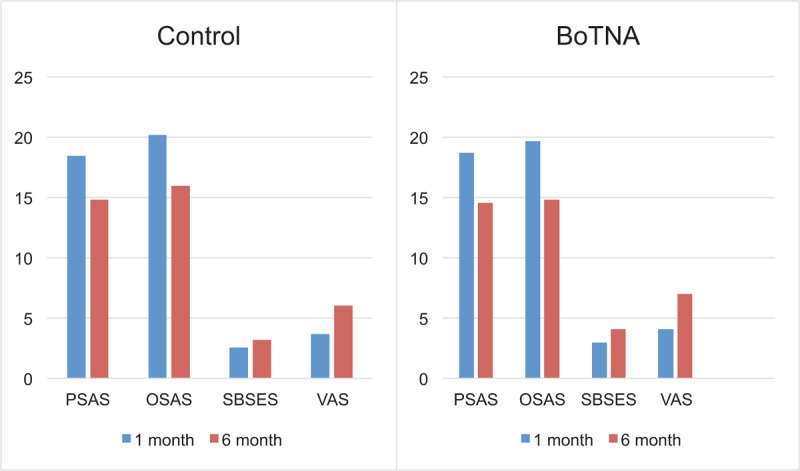
Trend of median value of scar assessment scales of control (left) and BoNTA group (right) from 1 to 6 months after injection. BoTNA = botulinum toxin type A, OSAS = observer scar assessment scale, PSAS = patient scar assessment scale, SBSES = Stony Brook scar evaluation scale, VAS = visual analog scale.
Figure 2.
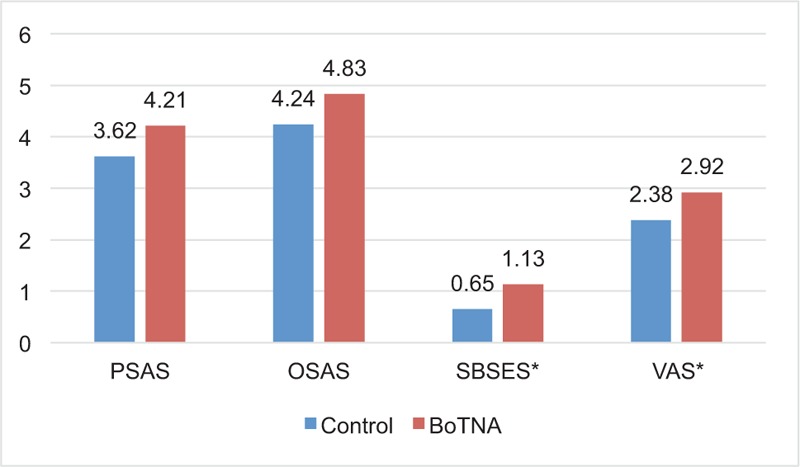
Difference of scar scale between 1 and 6 months after injection in both groups. ∗Statistically significant (P < .05). BoTNA = botulinum toxin type A, OSAS = observer scar assessment scale, PSAS = patient scar assessment scale, SBSES = Stony Brook scar evaluation scale, VAS = visual analog scale.
Table 3.
Statistical results of scar scales on both groups about improvement grade.
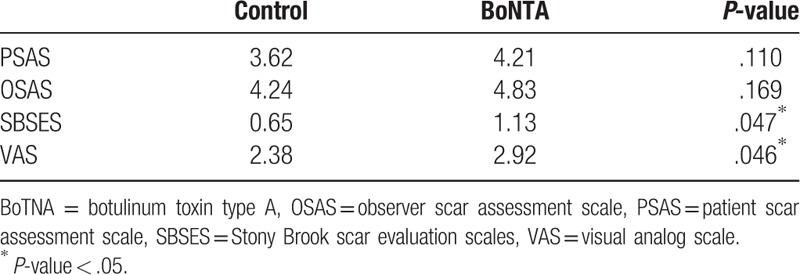
The gross findings followed the normal wound healing path, which exhibited as normalized color, texture, and pliability in both groups. The biopsies obtained from the control and BoNTA group supported these findings. In hematoxylin and eosin stain and Masson trichrome stain, there was a denser deposition of collagen fibers of the specimen belonging to the control group compared to the BoNTA group (Fig. 3). The collagen deposition was shown in blue in Masson trichrome stain and showed clearer differences between the 2 groups. Even the conventional staining which stains the collagen fiber in pink, showed a denser deposition in the control group, indicating more likelihood of scar formation those subjects. The collagen deposition the in BoNTA group was observed to be small compared to the collagen deposition in the control group.
Figure 3.
Representative biopsy specimen with hematoxylin and eosin stain (upper, ×100) and Masson trichrome stain (lower, ×100). Left column, control group. Right column, botulinum toxin A (BoNTA) group. Note the denser deposition of collagen fibers of control group compared with BoNTA group in both stains (arrows).
Furthermore, skin property values further backed up the results. Elasticity, melanin (pigmentation) and erythema values were more similar in the BoNTA group compared to normal skin than the values of the control group (Figs. 4 and 5). The differences of elasticity of scar from the control area were 0.056 (Uf), melanin, 83 and erythema, 76 in control group while the differences of elasticity of scar from the control area was 0.038 (Uf), melanin, 64 and erythema, 45 in BoNTA group.
Figure 4.
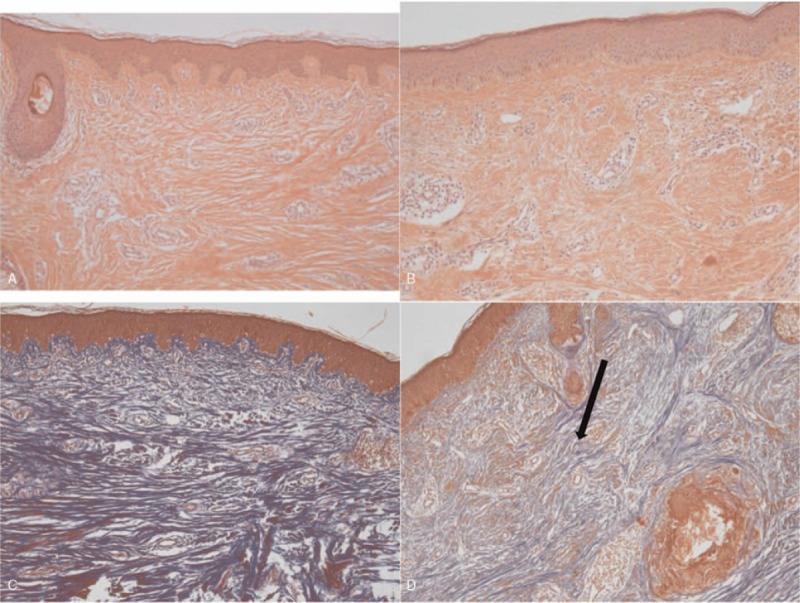
Representative case of the control group which had saline injection after stitch out (S019). Photographs are showing wound healing and scar formation process. Upper left, right after stitch out. Upper right, 1 month after the injection. Lower left, 3 months after the injection. Lower right, 6 months after injection. Changes of scar scale values from 1 to 6 months after the injection: from 15 to 13 (patient scar assessment scale), from 17 to 15 (observer scar assessment scale), from 3 to 4 (Stony Brook scar evaluation scale), from 6 to 7 (visual analog scale). Cutometer value (Uf): 0.006 (scar), 0.062 (normal skin), 0.056 (difference). Melanin: 249 (scar), 332 (normal skin), 83 (difference). Erythema: 426 (scar), 350 (normal skin), 76 (difference).
Figure 5.
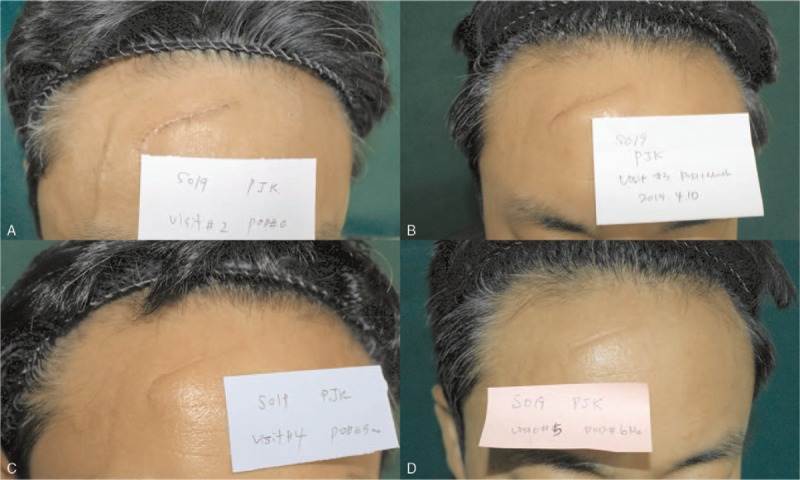
Representative case of BoNTA group which had botulinum toxin A injection after stitch out (S021). Photographs showing wound healing and scar formation process. Upper left, right after stitch out. Upper right, 1 month after the injection. Lower left, 3 months after the injection. Lower right, 6 months after injection. Changes of scar scale value from 1 to 6 months after injection: from 16 to 11 (patient scar assessment scale), from 16 to 12 (observer scar assessment scale), from 2 to 4 (Stony Brook scar evaluation scale), from 5 to 7 (visual analog scale). Cutometer value: 0.074 (scar), 0.036 (normal skin), 0.038 (difference); melanin: 236 (scar), 172 (normal skin), 64 (difference); erythema: 424 (scar), 379 (normal skin), 45 (difference).
4. Discussion
Patients who have a scar on their face are deeply influenced by the presence of it mostly in negative ways. A visible scar disturbs a person's psychologic well-being, and sometimes give negative impressions to others, thereby reducing a person's social role. In minimizing the formation of a conspicuous scar, measures routinely employed to facilitate favorable healing include minimizing reactive suture material, performing a good quality closure, applying occlusive or semi-occlusive dressings, avoiding sun exposure, and applying various types of scar care products.[14] Multiple factors contribute to an undesirable scar, including the patient's ethnic background, the anatomical location of the incision, surgical techniques used, and postoperative infections.[15] The beneficial effect of BoNTA in the treatment of facial wounds are well known. In addition, BoTNA is associated with immobilization and inhibition of myofibroblast differentiation. Fibroblasts induce collagen synthesis, especially type I collagen which constitutes a major proportion of the extracellular matrix, in the scar formation process. Myofibroblasts are cells differentiated from fibroblasts, which induce scar contracture and secrete TGF-β1 which level was proven to increase in abnormal scar formation. Several studies suggested the possibility of regulating scar formation by controlling these factors.[16]
In an in vitro study in our institution, BoTNA directly inhibits fibroblast-to-myofibroblast differentiation in the hypertrophic scar.[4] BoNTA delays fibroblast growth by inhibiting the cell cycle and, thereby, reducing hypertrophic scar development. In addition, BoNTA decreases the expression of connective tissue growth factor, which is a downstream regulator of the TGF-β1, and inhibits the growth of fibroblasts as well as the scar expansion.[17] In fact, BoNTA reduces the concentration of TGF-β1 in fibroblasts; the more BoNTA is given the more the concentration of TGF-β1 is downscaled.[18] Cutaneous injuries lead to an inflammatory response, and many important cellular mediators in this response affect melanocytes and melanogenesis in a variety of ways: nitric oxide, histamine, p53, and TGF-β1, which are released by the inflammatory response, induce melanogenesis.[19] The mechanism behind post inflammatory hyperpigmentation is not fully understood but may imply activation of melanocytes by inflammatory mediators or reactive oxidative species released from the damaged skin.[20] BoNTA decreases the infiltration of inflammatory cells during wound healing, reduces fibrosis, but counterintuitively causes the extension of its length.[21] Furthermore, BoNTA determines muscular paralysis and reduces muscle tension during the healing of a wound, thereby being suitable for cosmetic outcome improvement, for example, in a rabbit postoperative ear scar.[6] BoNTA is absorbed from the neuromuscular junction and blocks the secretion of acetylcholine, inhibiting muscle contraction.[22] Based on this action mechanism, several studies investigated the effect of BoTNA to improve scar by reducing tensile strength of the adjacent musculature. Our results are consistent with those of the previously mentioned studies. Based on this study, we contemplated on clinical applications of BoNTA to prevent scar formation.
Our results show that the PSAS and OSAS failed to get a significant objective difference between the 2 groups, which is a similar result to that of the study by Markam.[23] The overall median PSAS and OSAS were not statistically significantly different between the BoNTA group and the control group. This is due to the fact that the validated assessment scales[9,24–27] routinely used to evaluate the healing outcome of the complex wounds (burns, loss of bulk) are not discriminant enough to compare 2 groups of patients with simple facial wounds without tissue loss and sutured in good conditions.
On the contrary, the VAS and SBSES were significantly favorable to the BoNTA group. The VAS and SBSES appear to be more suitable for the assessment of simple facial wounds because it is ready to use, easy to use, sensitive and its results are reproducible. However, VAS and SBSES scores are methods with limitations, which is highly clinician-dependent, not considering patients’ real discomfort. In this study, the scar scales were investigated at 1 and 6 months after the wound closure, and the preoperative photos were subtracted to show the differences. In this way, the degree of improvement of the scar could be evaluated without the bias of the diverse scar conditions. The changes of the scar scales showed statistically significant improvement from 1 to 6 months after the wound closure. This result suggests that BoTNA has favorable effects on scar formation. In addition, our biopsy result further supports this hypothesis. Collagen fibers were more densely deposited in the specimen from the control group compared with the BoNTA group. Masson trichrome stain showed even clearer differences between 2 groups (Fig. 3).
The measured values of skin properties from cutometer and mexameter also showed favorable scar formation in the BoNTA group compared with the control group. Since skin properties are different in each patient, the scar was compared to the contralateral normal skin on the forehead of the patients. Cutometer has recently emerged as a novel, objective skin evaluation tool by using the suction chamber method, measuring vertical deformation of the skin. By extensive literature review regarding the measuring properties of scar tissue, maximal skin extension (Uf) was recorded to compare skin properties of scar tissue and contralateral normal skin.[28,29] Also, mexameter, which measures skin vascularity and pigmentation by the narrow-wave length light absorption method showed acceptable reliability in literature.[28] Although it has limitations regarding the change of measuring location and pressure application between investigators, the result of this study showed correlations with other evaluation tools.
Although this study showed promising results, there were several limitations to mention.
First, the small sample size could have impaired the results. Some patients dropped out from the study, and this could have deviated the results by leaving patients who were more cooperative in scar management.
Second, because scar revision was not performed in all patients, sufficient biopsy specimen could not be obtained, and quantitative analysis could not be performed.
Third, we would have liked to be able to follow-up the patients for up to 1 year or longer, but we could only do it for 6 months due to the real problem of the patient's appliance. Many studies used 6 months follow-up for evaluating scar improvement.[30–32] However, this effect had been negated by randomization of the patients into control and intervention groups. Also, scar evaluation by scar assessment scales is subjective in nature. To support the study, we used 3 different types of scar scales to evaluate diverse aspects of the scar and adopted analyses of biopsy specimen. Clearly, further evaluation is needed in dosage, injecting methods, and complete action mechanisms through larger clinical trials.
5. Conclusion
Based on the findings, BoNTA can improve scar properties in various aspects, especially in decreasing collagen synthesis. The gross findings also showed favorable changes. Despite several limitations, this study provides useful indication of application of BoNTA in scar prevention with promising results. The result should be backed up with further studies to establish its clinical use.
Author contributions
Conceptualization: Hii Sun Jeong, In Suck Suh.
Data curation: Seong Hwan Kim, Seong Joo Lee, In Suck Suh.
Formal analysis: Seong Joo Lee.
Investigation: Seong Hwan Kim, Seong Joo Lee, Jun Won Lee.
Methodology: Seong Joo Lee.
Resources: Jun Won Lee, Hii Sun Jeong, In Suck Suh.
Software: Seong Joo Lee.
Supervision: In Suck Suh.
Visualization: Jun Won Lee.
Writing – original draft: Seong Hwan Kim.
Writing – review & editing: Seong Hwan Kim, Seong Joo Lee.
Footnotes
Abbreviations: BoTNA = botulinum toxin type A, OSAS = observer scar assessment scale, PSAS = patient scar assessment scale, SBSES = Stony Brook scar evaluation scales, TGF-β1 = transforming growth factor beta 1, VAS = visual analog scale.
Presented at the 74th, 75th Congress of the Korean Society of Plastic and Reconstructive Surgeons (PRS Korea 2016 and 2017), and Aesthetic Plastic Surgery (APS) 2018 in Seoul, Republic of Korea (clinical trial information: Clinical Research Information Service [Republic of Korea], #KCT0002742, March 19, 2018).
The authors have no funding and conflicts of interest to disclose.
References
- [1].Tebble NJ, Thomas DW, Price P. Anxiety and self-consciousness in patients with minor facial lacerations. J Adv Nurs 2004;47:417–26. [DOI] [PubMed] [Google Scholar]
- [2].Young VL, Hutchison J. Insights into patient and clinician concerns about scar appearance: semiquantitative structured surveys. Plast Reconstr Surg 2009;124:256–65. [DOI] [PubMed] [Google Scholar]
- [3].Scott AB, Rosenbaum A, Collins CC. Pharmacologic weakening of extraocular muscles. Invest Ophthalmol 1973;12:924–7. [PubMed] [Google Scholar]
- [4].Jeong HS, Lee BH, Sung HM, et al. Effect of botulinum toxin type A on differentiation of fibroblasts derived from scar tissue. Plast Reconstr Surg 2015;136:171e–8e. [DOI] [PubMed] [Google Scholar]
- [5].Sherris DA, Gassner HG. Botulinum toxin to minimize facial scarring. Facial Plast Surg 2002;18:35–9. [DOI] [PubMed] [Google Scholar]
- [6].Gassner HG, Sherris DA, Otley CC. Treatment of facial wounds with botulinum toxin A improves cosmetic outcome in primates. Plast Reconstr Surg 2000;105:1948–53. [DOI] [PubMed] [Google Scholar]
- [7].Wilson AM. Use of botulinum toxin type A to prevent widening of facial scars. Plast Reconstr Surg 2006;117:1758–66. [DOI] [PubMed] [Google Scholar]
- [8].Draaijers LJ, Tempelman FR, Botman YA, et al. The patient and observer scar assessment scale: a reliable and feasible tool for scar evaluation. Plast Reconstr Surg 2004;113:1960–5. [DOI] [PubMed] [Google Scholar]
- [9].Truong PT, Lee JC, Soer B, et al. Reliability and validity testing of the patient and observer scar assessment scale in evaluating linear scars after breast cancer surgery. Plast Reconstr Surg 2007;119:487–94. [DOI] [PubMed] [Google Scholar]
- [10].van de Kar AL, Corion LU, Smeulders MJ, et al. Reliable and feasible evaluation of linear scars by the patient and observer scar assessment scale. Plast Reconstr Surg 2005;116:514–22. [DOI] [PubMed] [Google Scholar]
- [11].Fearmonti R, Bond J, Erdmann D, et al. A review of scar scales and scar measuring devices. Eplasty 2010;10:e43. [PMC free article] [PubMed] [Google Scholar]
- [12].Duncan JA, Bond JS, Mason T, et al. Visual analogue scale scoring and ranking: a suitable and sensitive method for assessing scar quality? Plast Reconstr Surg 2006;118:909–18. [DOI] [PubMed] [Google Scholar]
- [13].Durani P, McGrouther DA, Ferguson MW. Current scales for assessing human scarring: a review. J Plast Reconstr Aesthet Surg 2009;62:713–20. [DOI] [PubMed] [Google Scholar]
- [14].Due E, Rossen K, Sorensen LT, et al. Effect of UV irradiation on cutaneous cicatrices: a randomized, controlled trial with clinical, skin reflectance, histological, immunohistochemical and biochemical evaluations. Acta Derm Venereol 2007;87:27–32. [DOI] [PubMed] [Google Scholar]
- [15].Larrabee WF., Jr Treatment of facial wounds with botulinum toxin A improves cosmetic outcome in primates. Plast Reconstr Surg 2000;105:1954–5. [DOI] [PubMed] [Google Scholar]
- [16].Xiao Z, Qu G. Effects of botulinum toxin type A on collagen deposition in hypertrophic scars. Molecules 2012;17:2169–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Xiao Z, Zhang M, Liu Y, et al. Botulinum toxin type a inhibits connective tissue growth factor expression in fibroblasts derived from hypertrophic scar. Aesthetic Plast Surg 2011;35:802–7. [DOI] [PubMed] [Google Scholar]
- [18].Xiao Z, Zhang F, Cui Z. Treatment of hypertrophic scars with intralesional botulinum toxin type A injections: a preliminary report. Aesthetic Plast Surg 2009;33:409–12. [DOI] [PubMed] [Google Scholar]
- [19].Chadwick S, Heath R, Shah M. Abnormal pigmentation within cutaneous scars: a complication of wound healing. Indian J Plast Surg 2012;45:403–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ortonne JP, Bissett DL. Latest insights into skin hyperpigmentation. J Investig Dermatol Symp Proc 2008;13:10–4. [DOI] [PubMed] [Google Scholar]
- [21].Lee BJ, Jeong JH, Wang SG, et al. Effect of botulinum toxin type a on a rat surgical wound model. Clin Exp Otorhinolaryngol 2009;2:20–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wheeler A, Smith HS. Botulinum toxins: mechanisms of action, antinociception and clinical applications. Toxicology 2013;306:124–46. [DOI] [PubMed] [Google Scholar]
- [23].Ziade M, Domergue S, Batifol D, et al. Use of botulinum toxin type A to improve treatment of facial wounds: a prospective randomised study. J Plast Reconstr Aesthet Surg 2013;66:209–14. [DOI] [PubMed] [Google Scholar]
- [24].Nguyen DQ, Potokar T, Price P. A review of current objective and subjective scar assessment tools. J Wound Care 2008;17:101–2. [DOI] [PubMed] [Google Scholar]
- [25].Stavrou D, Haik J, Weissman O, et al. Patient and observer scar assessment scale: how good is it? J Wound Care 2009;18:171–6. [DOI] [PubMed] [Google Scholar]
- [26].Vercelli S, Ferriero G, Sartorio F, et al. How to assess postsurgical scars: a review of outcome measures. Disabil Rehabil 2009;31:2055–63. [DOI] [PubMed] [Google Scholar]
- [27].Roques C, Teot L. A critical analysis of measurements used to assess and manage scars. Int J Low Extrem Wounds 2007;6:249–53. [DOI] [PubMed] [Google Scholar]
- [28].Nedelec B, Correa JA, Rachelska G, et al. Quantitative measurement of hypertrophic scar: interrater reliability and concurrent validity. J Burn Care Res 2008;29:501–11. [DOI] [PubMed] [Google Scholar]
- [29].Draaijers LJ, Botman YA, Tempelman FR, et al. Skin elasticity meter or subjective evaluation in scars: a reliability assessment. Burns 2004;30:109–14. [DOI] [PubMed] [Google Scholar]
- [30].Jang JU, Kim SY, Yoon ES, et al. Comparison of the effectiveness of ablative and non-ablative fractional laser treatments for early stage thyroidectomy scars. Arch Plast Surg 2016;43:575–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Borges J, Cuzzi T, Mandarim-de-Lacerda CA, et al. Fractional Erbium laser in the treatment of photoaging: randomized comparative, clinical and histopathological study of ablative (2940 nm) vs. non-ablative (1540 nm) methods after 3 months. An Bras Dermatol 2014;89:250–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Jared Christophel J, Elm C, Endrizzi BT, et al. A randomized controlled trial of fractional laser therapy and dermabrasion for scar resurfacing. Dermatol Surg 2012;38:595–602. [DOI] [PubMed] [Google Scholar]


