Abstract
Background
The pathways and mechanism by which associations between the gut microbiome and the brain, termed the microbiome-gut-brain axis (MGBA), are manifest but remain to be fully elucidated. This study aims to use bibliometric analysis to estimate the global activity within this rapidly developing field and to identify particular areas of focus that are of current relevance to the MGBA during the last decade (2009–2018).
Methods
The current study uses the Scopus for data collection. We used the key terms “microbiome-gut-brain axis” and its synonyms because we are concerned with MGBA per se as a new concept in research rather than related topics. A VOSviewer version 1.6.11 was used to visualize collaboration pattern between countries and authors, and evolving research topics by analysis of the term co-occurrence in the title and abstract of publications.
Results
Between 2009 and 2018, there were 51,504 published documents related to the microbiome, including 1713 articles related to the MGBA: 829 (48.4%) original articles, 658(38.4%) reviews, and 226 (13.2%) other articles such as notes, editorials or letters. The USA took the first place with 385 appearances, followed by Ireland (n = 161), China (n = 155), and Canada (n = 144).The overall citation h-index was 106, and the countries with the highest h-index values were the USA (69), Ireland (58), and Canada (43). The cluster analysis demonstrated that the dominant fields of the MGBA include four clusters with four research directions: “modeling MGBA in animal systems”, “interplay between the gut microbiota and the immune system”, “irritable bowel syndrome related to gut microbiota”, and “neurodegenerative diseases related to gut microbiota”.
Conclusions
This study demonstrates that the research on the MGBA has been becoming progressively more extensive at global level over the past 10 years. Overall, our study found that a large amount of work on MGBA focused on immunomodulation, irritable bowel syndrome, and neurodevelopmental disorders. Despite considerable progress illustrating the communication between the gut microbiome and the brain over the past 10 years, many issues remain about their relevance for therapeutic intervention of many diseases.
Keywords: Gut microbiota, Gut microbiome-brain axis, Microbiome, Bibliometric, Scopus
Background
The interaction between gut and brain has been acknowledged by physicians since antiquity [1]. As far back as the sixteenth century, the association between depression and altered bowel function was recognized and in 1978 Manning and his colleagues described the “irritable bowel syndrome (IBS)” as a gastrointestinal condition which is strongly associated with psychological stress, some authors reporting 50% of sufferers have comorbid depression or anxiety [2]. The pathways and mechanism by which these associations are manifest remain to be fully elucidated. However, recent developments in genome sequencing, metabolomics, functional imaging and computational biology have increased our understanding considerably [3–6].
The rapid development of 16S ribosomal RNA and whole genome sequencing analysis has enabled us to understand the diverse nature of the microbial symbionts that inhabit our gastrointestinal tract [7–9]. Metabolomics is beginning to explain how those microbes produce a range of molecules that impact our behaviors and perceptions. The changes in our microbial diversity, manifest as changes in their metabolic output appear to alter the development of multiple facets of the enteric and central nervous systems including astrocytes, microglial cells and neurons [10, 11]. Functional imaging, functional magnetic resonance imaging and magneto encephalography, have enabled us to identify real time changes in neurological activity and correlate these with changes in behavior or perception [12–14]. Advances in computational biology are beginning to explain how these multifaceted and complex systems interact with each other [15, 16].
The microbiota interacts with the host through their effect on immune, neuro-hormonal and neural pathways. They have been shown to impact a broad range of disease, including neurodegenerative disorders, such as multiple sclerosis and Parkinson’s disease, auto-immune disease and obesity [17, 18]. The gastrointestinal microbiome has also been shown to influence behavior in mammals and man [19, 20]. Transfer of feces from depressed humans to microbiota depleted rats led the recipient rats to display behaviors analogous to depression in the human (anhedonia and anxiety like behaviors) [21, 22]. A strain of bifidobacteria has been demonstrated to increase resilience in people with anxiety [23]. These findings were not observed when healthy people consumed a strain of Lactobacillus [24]. Short chain fatty acids, propionate, butyrate and acetate, are important products of the microbiome and changes in the proportion and quantities of these products alter insulin resistance, ghrelin production and presumably appetite and risk of obesity and diabetes [25, 26].
Bibliometric analyses have been used in various fields to highlight the most influential countries, authors, journals, publications, and institutions [27–42]. These include research related to microbiota [43, 44]. Worldwide, there are more than 330 clinical studies recorded on clinical trials.gov with a specific focus on the microbiome. This is a growing area of importance in order to better understand the impact of specific strains on individuals, and the interaction with pre-existing microbial symbionts. Currently, there is a lack of research concerning assessment of the current status, hot spots, and future outlook on the theme of the microbiome-gut-brain axis (MGBA). This study aims to use bibliometric methods to identify particular areas of research activity in this field and to allow researchers to identify new areas for future development.
Methods
Although a large number of databases are used for evaluation research at global level [45–47], the current study uses the Scopus database which is widely accepted among researchers for the purposes of high quality bibliometric analyses [44, 48–53]. Scopus is the world’s largest abstract and citation database of peer-reviewed research literature, and is an established resource for identifying biomedical research including MEDLINE documents, and includes a higher level of detail than PubMed including the country of origin and citations per document [47, 54].
We used the key terms “microbiome-gut-brain axis” and its synonyms because we are concerned with microbiome-gut-brain axis per se as a new concept in research rather than related topics. Data mining was conducted on July 12, 2019. The central theme in this study was research articles containing “microbiome or microbiota and brain-gut or gut-brain” to identify items based on their search in the fields title, abstract and keyword simultaneously and the time was 10 years between 2009 and 2018.
Data analysis
VOSviewer software (www.vosviewer.com, Van Eck & Waltman version 1.6.11) was used to create a visual representation of collaborations between countries and authors using network maps [55]. Creating a term co-occurrence map in VOSviewer involved only terms that occurred in the title and abstract at least 50 times under binary counting [55]. Terms with the highest relevance score were used to create a term map for network visualization. The algorithm was designed to ensure that terms that co-occurred more frequently had larger bubbles and terms that have a high similarity are located close to each other [55].
Statistical analysis was carried out for the retrieved data by the Statistical Package for the Social Sciences (version 16.0, SPSS Inc., Chicago, IL, USA). Pearson correlation Coefficient was used to test the correlation between some variables (e.g. h-index and number of publications for each country, number of publications and years, and the number of publications related to MGBA and the number of publications related to microbiome in all fields). The analyses carried out in the current study focused largely on the frequencies and percentages of publications for types of documents, countries, journals, and institutes.
Results
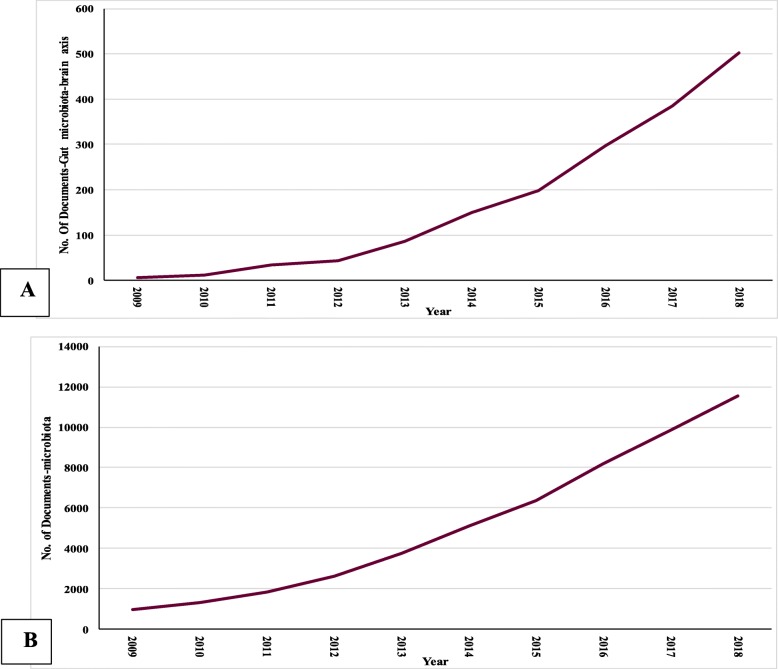
Between 2009 and 2018, there were 51,504 published documents related to the microbiome, including 1713 articles related to the MGBA: 829 (48.4%) original articles, 658(38.4%) reviews, and 226 (13.2%) other articles such as notes, editorials or letters. English was the most frequently used language (n = 1648), followed by French (n = 16), and Chinese (n = 19), with these accounting for 98.2% of publications related to MGBA. Publications related to MGBA and the microbiome are represented in Fig. 1a and b, respectively. Time trend analyses show rising numbers of publications related to MGBA between 2009 and 2018 (r = 0.950; P value< 0.001), and a correlation between overall numbers of microbiome and MGBA publications (r = 0.991, p < 0.001) during the study period.
Fig. 1.
Quantitative growth process of the publications concerning microbiome-gut-brain axis (a) and microbiome in all fields (b) in the period of 10 years
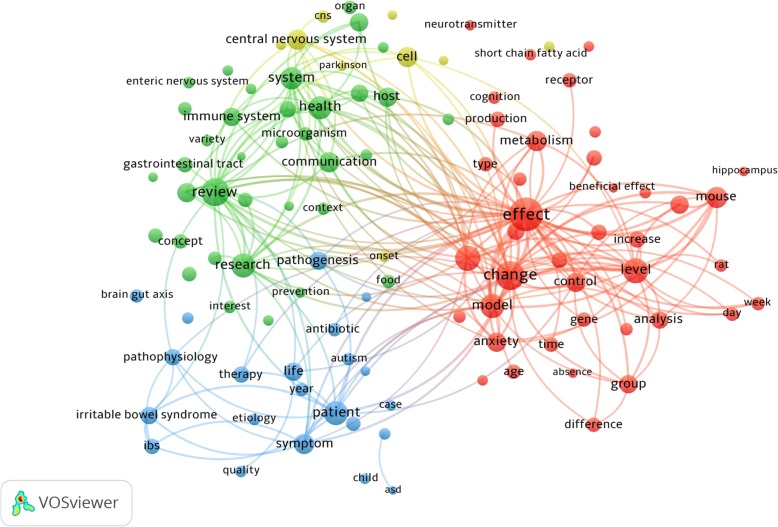
The term analyses maps are presented in Fig. 2: the larger circles representing frequently occurring abstract and title terms. Colors used to differentiate between 4 main topic clusters: 1. “modeling MGBA in animal systems (red cluster)”, 2. “interplay between the gut microbiota and the immune system (green cluster)”, 3. “irritable bowel syndrome related to gut microbiota (blue cluster)”, and 4. “neurodegenerative diseases related to gut microbiota (yellow cluster)”.
Fig. 2.
Research topics clustered by mapping of co-occurrences of terms in title/abstract for publications related to microbiome-gut-brain axis (MGBA). Of the 30,250 terms, 179 terms occurred at least 50 times. For each of the 179 terms, a relevance score was calculated and used to select the 60% most relevant terms. In Fig. 2, the size of the circles represents the occurrences of terms in title/abstract. The largest set of connected terms consists of 107 terms in four clusters. The four clusters can be broadly interpreted as “modeling MGBA in animal systems (red cluster)”, “interplay between the gut microbiota and the immune system (green cluster)”, “irritable bowel syndrome related to gut microbiota (blue cluster)”, and “neurodegenerative diseases related to gut microbiota (yellow cluster)”
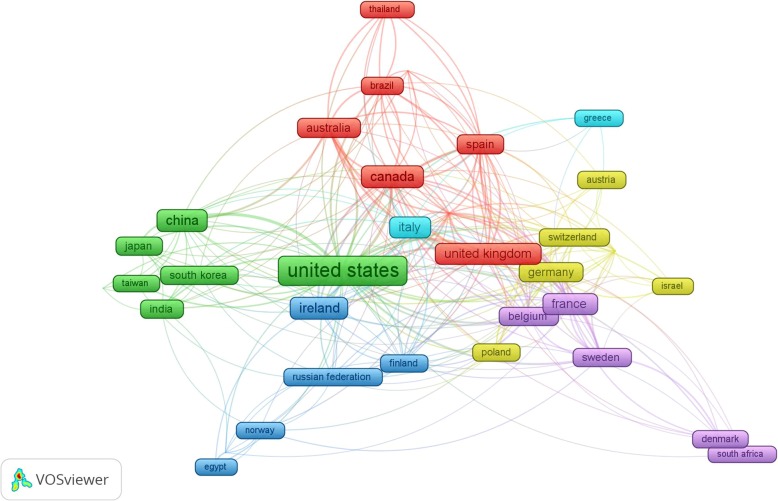
Table 1 presents the 10 most prolific countries related to MGBA publications, with the top 4 being the USA (n = 385), Ireland (n = 161), China (n = 155), and Canada (n = 144). The overall citation h-index was 106, and the countries with the highest h-index values were the USA (69), Ireland (58), and Canada (43). There is a positive modest correlation between h-index and number of published articles (r = 0.817, P-value = 0.004). Figure 3 shows the network visualization map for country collaborations, showing 35 out of a total 86 countries that had more than ten publications; the size of frame represents the number of publications, the thickness of lines signifies the extent of collaboration between the countries.
Table 1.
Ten leading countries in the publications concerning microbiome-gut-brain axis
| SCR | Country | Number of documents (%) | h-index | No. of collaborated countries | No. of articles from collaboration |
|---|---|---|---|---|---|
| 1st | United States | 585 (34.2) | 69 | 48 | 189 |
| 2nd | Ireland | 161 (9.4) | 58 | 21 | 58 |
| 3rd | China | 155 (9.1) | 28 | 22 | 56 |
| 4th | Canada | 144 (8.4) | 43 | 30 | 67 |
| 5th | United Kingdom | 127 (7.4) | 37 | 31 | 83 |
| 6th | Italy | 121 (7.1) | 26 | 28 | 42 |
| 7th | France | 102 (6.0) | 29 | 28 | 48 |
| 8th | Australia | 82 (4.8) | 25 | 19 | 43 |
| 9th | Germany | 81 (4.7) | 24 | 24 | 45 |
| 10th | Spain | 65 (3.8) | 21 | 29 | 34 |
SCR Standard competition ranking
Fig. 3.
Network visualization map for country collaboration. Of the 86 countries, 35 had at least ten publications; the largest set of connected countries consists of 34 countries. The size of frame represents the number of publications of the country and the thickness of lines signifies the size of collaboration between the countries, while 6 different colors seen in this figure represent the collaboration cluster of the countries
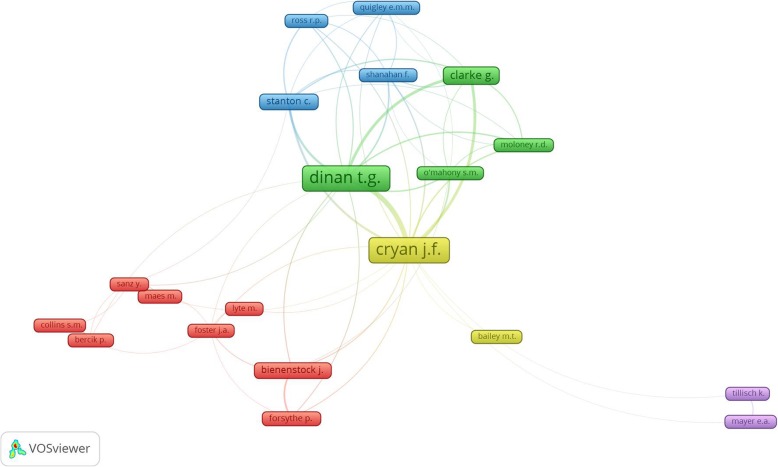
Co-authorship in the field of MGBA is shown in Fig. 4, with 5 clusters identified; the size of frame represents the number of publications by an author, and the thickness of lines signifies the extent of collaboration between authors. Of the 6054 authors, 25 had at least ten publications including the most active author Cryan, J.F. with 120 (7.0%) publications.
Fig. 4.
Network visualization map for author collaboration. Of the 6054 authors, 25 had at least ten publications; the largest set of connected authors consists of 20 authors. The size of frame represents the number of publications of the author and the thickness of lines signifies the size of collaboration between the authors, while 5 different colors seen in this figure represent the collaboration cluster of the authors
The 10 most influential journals covering the MGBA research with their IFs are shown in Table 2. The three most influential journals from the top 10 influential journals are Brain Behavior and Immunity (49 articles), Plos One (34 articles), and Scientific Reports (33 articles). Table 3 shows the list of top 20 most-cited articles [56–75] on MGBA. The most prolific institutions were University College Cork (152 articles), McMaster University (67 articles), and INSERM (Institut National de la Santé et de la Recherche Médicale, French National Institute of Health and Medical Research, 43 articles) (Table 4).
Table 2.
The most productive journals in the microbiome-gut-brain axis research
| SCRa | Journal | Frequency (%) | IFb |
|---|---|---|---|
| 1st | Brain Behavior and Immunity | 49 (2.86) | 6.170 |
| 2nd | Scientific Reports | 34 (1.98) | 4.011 |
| 3rd | Plos One | 33 (1.93) | 2.776 |
| 4th | Gut Microbes | 23 (1.34) | 7.823 |
| 4th | World Journal of Gastroenterology | 23 (1.34) | 3.411 |
| 6th | Neurogastroenterology and Motility | 22 (1.28) | 3.803 |
| 7th | Frontiers in Microbiology | 20 (1.17) | 4.259 |
| 8th | Nutrients | 19 (1.11) | 4.171 |
| 9th | Advances in Experimental Medicine and Biology | 15 (0.88) | 2.126 |
| 10th | Nature Reviews Gastroenterology and Hepatology | 14 (0.82) | 23.57 |
SCR Standard competition ranking, IF Impact factor
aEqual journals have the same ranking number, and then a gap is left in the ranking numbers
bImpact factors (IF) based on Journal Citation Reports (JCR) 2018 from Clarivate Analytics
Table 3.
The 20 most influential articles in the microbiome-gut-brain axis research
| SCRa | Authors | Title | Year of publication | Source title | Cited by |
|---|---|---|---|---|---|
| 1st | Nicholson et al. [56] | “Host-gut microbiota metabolic interactions” | 2012 | Science | 1490 |
| 2nd | Cryan and Dinan [57] | “Mind-altering microorganisms: The impact of the gut microbiota on brain and behavior” | 2012 | Nature Reviews Neuroscience | 1204 |
| 3rd | Heijtz et al. [58] | “Normal gut microbiota modulates brain development and behavior” | 2011 | Proceedings of the National Academy of Sciences of the United States of America | 1116 |
| 4th | Hsiao et al. [59] | “Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders” | 2013 | Cell | 1041 |
| 5th | Bravo et al. [60] | “Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve” | 2011 | Proceedings of the National Academy of Sciences of the United States of America | 1028 |
| 6th | Foster and McVey Neufeld [61] | “Gut-brain axis: How the microbiome influences anxiety and depression” | 2013 | Trends in Neurosciences | 612 |
| 7th | Bercik et al. [62] | “The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice” | 2011 | Gastroenterology | 602 |
| 8th | Collins et al. [63] | “The interplay between the intestinal microbiota and the brain” | 2012 | Nature Reviews Microbiology | 566 |
| 8th | Berer et al. [64] | “Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination” | 2011 | Nature | 566 |
| 10th | De Vadder et al. [65] | “Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits” | 2014 | Cell | 525 |
| 11th | Neufeld et al. [66] | “Reduced anxiety-like behavior and central neurochemical change in germ-free mice” | 2011 | Neurogastroenterology and Motility | 522 |
| 12th | O’Mahony et al. [67] | “Early Life Stress Alters Behavior, Immunity, and Microbiota in Rats: Implications for Irritable Bowel Syndrome and Psychiatric Illnesses” | 2009 | Biological Psychiatry | 521 |
| 13th | Clarke et al. [68] | “The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner” | 2013 | Molecular Psychiatry | 476 |
| 14th | Sampson et al. [69] | “Gut microbiota regulate motor deficits and neuroinflammation in a model of parkinson’s disease” | 2016 | Cell | 455 |
| 15th | Tillisch et al. [70] | “Consumption of fermented milk product with probiotic modulates brain activity” | 2013 | Gastroenterology | 445 |
| 16th | Rhee et al. [71] | “Principles and clinical implications of the brain-gut-enteric microbiota axis” | 2009 | Nature Reviews Gastroenterology and Hepatology | 444 |
| 17th | Braniste et al. [72] | “The gut microbiota influences blood-brain barrier permeability in mice” | 2014 | Science Translational Medicine | 378 |
| 18th | Scheperjans et al. [73] | “Gut microbiota are related to Parkinson’s disease and clinical phenotype” | 2015 | Movement Disorders | 361 |
| 19th | O’Mahony et al. [74] | “Serotonin, tryptophan metabolism and the brain-gut-microbiome axis” | 2015 | Behavioural Brain Research | 356 |
| 20th | Cryan and O’Mahony [75] | “The microbiome-gut-brain axis: From bowel to behavior” | 2011 | Neurogastroenterology and Motility | 347 |
SCR Standard competition ranking
aEqual citations have the same ranking number, and then a gap is left in the ranking numbers
Table 4.
The top ten most productive institutes
| SCRa | Institute | Country | n (%) |
|---|---|---|---|
| 1st | University College Cork | Ireland | 152 (8.87) |
| 2nd | McMaster University | Canada | 67 (3.91) |
| 3rd | INSERM (Institut national de la santé et de la recherche médicale) | France | 43 (2.51) |
| 4th | INRA (Institut National de La Recherche Agronomique) | France | 41 (2.39) |
| 5th | University of California, Los Angeles | USA | 29 (1.69) |
| 6th | Teagasc - Irish Agriculture and Food Development Authority | Ireland | 28 (1.63) |
| 7th | St. Joseph’s Healthcare Hamilton | Canada | 26 (1.52) |
| 8th | David Geffen School of Medicine at UCLA | USA | 23 (1.34) |
| 9th | The University of North Carolina at Chapel Hill | USA | 22 (1.28) |
| 10th | Universite Catholique de Louvain | Belgium | 19 (1.11) |
| 10th | University of California, San Diego | USA | 19 (1.11) |
| 10th | Københavns Universitet | Denmark | 19 (1.11) |
aEqual institutions have the same ranking number, and then a gap is left in the ranking numbers
Discussion
This is the first application of bibliometric quantitatively and qualitatively methods regarding the MGBA involving 1713 papers retrieved from Scopus. The results of this bibliometric analysis present a comprehensive overview of the development of the scientific literature in the MGBA field over the past 10 years.
The number of articles concerning MGBA research increased rapidly between 2009 and 2018. This increase is likely related to the many experts in psychiatry, neurology and gastroenterology fields (e.g. Cryan J.F., Dinan T.G., Clarke G., Bienenstock J., Forsythe P., Stanton C., Quigley E.M.M., Bercik P., O’Mahony S.M., Shanahan F., Foster J.A., Moloney R.D., and others) developing their interest in the physiological role of the guts’ microbiota on brain and behavior as an emerging platform for therapeutic intervention of many diseases. Furthermore, the increased number of publications may relate to several hot topics [56–68, 70–72, 74–77] which were published during this period, revealing novel findings that open the door for new areas of investigation. These studies propose novel concepts for treating several conditions such as IBS, autism, depression, multiple sclerosis, auto-immune disease, Parkinson’s disease, and obesity [78–85].
Since 2012, there has been growing research output in the field of MGBA, which is consistent with increasing research activity related to the microbiome in general. Similar findings have been reported in other bibliometric studies [43, 44, 86–89]. A possible underlying explanation for the rising publication numbers is that in 2013 the National Institutes of Health (NIH) launched the second phase of Integrative Human Microbiome Project (iHMP) [90].
Research output related to MGBA most often originated from the United States, as reported in other bibliometric studies regarding microbiome research [43, 44, 86–89]. Our study clearly reveals that the United States is at the forefront of studies on MGBA. The research output from the USA may be associated with the wide range of researchers with an interest within this field and a substantial amount of financial support to researchers. In 2013 the USA launched a special research project on gut microbiota-brain axis [91]. Since then, there has been increasing neuroscience interest in the role of gut microbiota on animal and human brain behavior and cognitive development [92, 93]. Ireland featured as the second most prolific nation and this might be related to Professor John F Cryan and Professor Ted Dinan, with their team who are the most active authors in this field, and principal investigators at the Alimentary Pharmabiotic Centre (APC) in University College Cork. [94] The APC is funded by Science Foundation Ireland (SFI) [75], and has conducted studies in collaboration with several companies including GlaxoSmithKline, Cremo, Suntory, Pfizer, Wyeth and Mead Johnson which consequently provided more funding for conducting research in the field of psychobiotics [75], thus may contribute to increasing number of publications regarding gut microbiota-brain axis.
The number of citations for the top 20 articles in the current study varied from 1490 to 347, which is higher range of citations than in other medical fields such as mobile-health [95], toxicology [28], social media in psychology [96], parasitic diseases [51, 97], and viral diseases [98–100]. Additionally, it also reveals that researchers paid great attention on the MGBA mostly in recent years, and published several outstanding articles on top-ranking journals in the medical field such as Science [56] and Nature [64]. The most cited article is “Host-gut microbiota metabolic interactions” a review by Nicholson et al., 2012 [56], published in Science, where the authors suggest that the manipulation of the gut microbiota to optimize new therapeutic strategies could control many diseases and improve health. The second most cited article “Mind-altering microorganisms: The impact of the gut microbiota on brain and behavior” was published in the Nature Reviews Neuroscience in 2012 by Cryan and Dinan [57], where the authors suggest that the concept of a microbiota-gut-brain axis may lead to the development of novel therapeutics for management of several neurological and psychiatric disorders.
Finally, there are some limitations for our study findings. First, the search was limited to publications listed in Scopus, which is the largest biomedical database and the most frequently used database for bibliometric analyses, although it might not contain all publications relevant to MGBA research. MGBA publications that do not include this term or its synonyms in the title, abstract or key words might not be taken into account for our analysis. Secondly, a general limitation of the bibliometric approach is that there is no weighting to take account of the quality or scientific rigor of any individual publication. Despite these limitations, we still consider that the findings offer a valid representation of MGBA research output at a global level.
Conclusions
The characteristics of the MGBA related publications from 2009 to 2018 are investigated through the bibliometrics analysis based on the Scopus database. This study demonstrates that the research on the MGBA has been becoming progressively more extensive at global level over the past 10 years. Overall, our study found a large amount of work on MGBA, focused on immunomodulation, irritable bowel syndrome, and neurodevelopmental disorders. Despite considerable progress illustrating the communication between the gut microbiome and the brain over the past 10 years, many issues remain to fully realize their relevance for therapeutic intervention of many diseases.
Acknowledgements
The authors would like to thank An-Najah National University for all administrative support throughout the implementation of this project.
Abbreviations
- IBS
Irritable bowel syndrome
- IFs
Impact factors
- JCR
Journal citation reports
- SCR
Standard competition ranking
- SPSS
Statistical package for social sciences
Authors’ contributions
SHZ designed the study, collected the data, analysed the data, and drafting the manuscript, SWA, WMS participated in the study design, involved in interpretation of the data, and made revisions to the initial draft, and SS and WSW contributed towards the conception, wrote part of the article, involved in interpretation of the data, and made revisions to the initial draft. All authors approved this final manuscript and also presented critical review before the final submission.
Funding
No funding was received for writing this study.
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sa’ed H. Zyoud, Email: saedzyoud@yahoo.com, Email: saedzyoud@najah.edu
Simon Smale, Email: simonsmale1969@gmail.com.
W. Stephen Waring, Email: stephen.waring@york.nhs.uk.
Waleed M. Sweileh, Email: waleedsweileh@yahoo.com
Samah W. Al-Jabi, Email: samahjabi@yahoo.com
References
- 1.Miller I. The gut-brain axis: historical reflections. Microb Ecol Health Dis. 2018;29(1):1542921. doi: 10.1080/16512235.2018.1542921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Manning AP, Thompson WG, Heaton KW, Morris AF. Towards positive diagnosis of the irritable bowel. Br Med J. 1978;2(6138):653–654. doi: 10.1136/bmj.2.6138.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Enaud R, Vandenborght LE, Coron N, Bazin T, Prevel R, Schaeverbeke T, Berger P, Fayon M, Lamireau T, Delhaes L. The mycobiome: a neglected component in the microbiota-gut-brain axis. Microorganisms. 2018;6(1):22. doi: 10.3390/microorganisms6010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cussotto S, Clarke G, Dinan TG, Cryan JF. Psychotropics and the microbiome: a chamber of secrets. Psychopharmacology. 2019;236(5):1411–1432. doi: 10.1007/s00213-019-5185-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cussotto S, Sandhu KV, Dinan TG, Cryan JF. The neuroendocrinology of the microbiota-gut-brain axis: a behavioural perspective. Front Neuroendocrinol. 2018;51:80–101. doi: 10.1016/j.yfrne.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Cussotto S, Strain CR, Fouhy F, Strain RG, Peterson VL, Clarke G, Stanton C, Dinan TG, Cryan JF. Differential effects of psychotropic drugs on microbiome composition and gastrointestinal function. Psychopharmacology. 2019;236(5):1671–1685. doi: 10.1007/s00213-018-5006-5. [DOI] [PubMed] [Google Scholar]
- 7.Human Microbiome Project Consortium Structure, function and diversity of the healthy human microbiome. Nature. 2012;486(7402):207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Human Microbiome Jumpstart Reference Strains Consortium A catalog of reference genomes from the human microbiome. Science. 2010;328(5981):994–999. doi: 10.1126/science.1183605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Human Microbiome Project Consortium A framework for human microbiome research. Nature. 2012;486(7402):215–221. doi: 10.1038/nature11209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang J. Microbial metabolomics. Curr Genomics. 2011;12(6):391–403. doi: 10.2174/138920211797248619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sekirov I, Russell SL, Antunes LC, Finlay BB. Gut microbiota in health and disease. Physiol Rev. 2010;90(3):859–904. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- 12.Wang H, Lee IS, Braun C, Enck P. Effect of probiotics on central nervous system functions in animals and humans: a systematic review. J Neurogastroenterol Motil. 2016;22(4):589–605. doi: 10.5056/jnm16018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lener MS, Niciu MJ, Ballard ED, Park M, Park LT, Nugent AC, Zarate CA., Jr Glutamate and gamma-aminobutyric acid systems in the pathophysiology of major depression and antidepressant response to ketamine. Biol Psychiatry. 2017;81(10):886–897. doi: 10.1016/j.biopsych.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al Omran Y, Aziz Q. Functional brain imaging in gastroenterology: to new beginnings. Nat Rev Gastroenterol Hepatol. 2014;11(9):565–576. doi: 10.1038/nrgastro.2014.89. [DOI] [PubMed] [Google Scholar]
- 15.Malan-Muller S, Valles-Colomer M, Raes J, Lowry CA, Seedat S, Hemmings SMJ. The gut microbiome and mental health: implications for anxiety- and trauma-related disorders. OMICS. 2018;22(2):90–107. doi: 10.1089/omi.2017.0077. [DOI] [PubMed] [Google Scholar]
- 16.Cong X, Henderson WA, Graf J, McGrath JM. Early life experience and gut microbiome: the brain-gut-microbiota signaling system. Adv Neonatal Care. 2015;15(5):314–323. doi: 10.1097/ANC.0000000000000191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tlaskalova-Hogenova H, Stepankova R, Kozakova H, Hudcovic T, Vannucci L, Tuckova L, Rossmann P, Hrncir T, Kverka M, Zakostelska Z, et al. The role of gut microbiota (commensal bacteria) and the mucosal barrier in the pathogenesis of inflammatory and autoimmune diseases and cancer: contribution of germ-free and gnotobiotic animal models of human diseases. Cell Mol Immunol. 2011;8(2):110–120. doi: 10.1038/cmi.2010.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014;157(1):121–141. doi: 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng P, Zeng B, Zhou C, Liu M, Fang Z, Xu X, Zeng L, Chen J, Fan S, Du X, et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host's metabolism. Mol Psychiatry. 2016;21(6):786–796. doi: 10.1038/mp.2016.44. [DOI] [PubMed] [Google Scholar]
- 20.Dill-McFarland KA, Tang ZZ, Kemis JH, Kerby RL, Chen G, Palloni A, Sorenson T, Rey FE, Herd P. Close social relationships correlate with human gut microbiota composition. Sci Rep. 2019;9(1):703. doi: 10.1038/s41598-018-37298-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelly JR, Borre Y, O’Brien C, Patterson E, El Aidy S, Deane J, Kennedy PJ, Beers S, Scott K, Moloney G, et al. Transferring the blues: depression-associated gut microbiota induces neurobehavioural changes in the rat. J Psychiatr Res. 2016;82:109–118. doi: 10.1016/j.jpsychires.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 22.Wrzosek L, Ciocan D, Borentain P, Spatz M, Puchois V, Hugot C, Ferrere G, Mayeur C, Perlemuter G, Cassard AM. Transplantation of human microbiota into conventional mice durably reshapes the gut microbiota. Sci Rep. 2018;8(1):6854. doi: 10.1038/s41598-018-25300-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okubo R, Koga M, Katsumata N, Odamaki T, Matsuyama S, Oka M, Narita H, Hashimoto N, Kusumi I, Xiao J, et al. Effect of bifidobacterium breve A−1 on anxiety and depressive symptoms in schizophrenia: a proof-of-concept study. J Affect Disord. 2019;245:377–385. doi: 10.1016/j.jad.2018.11.011. [DOI] [PubMed] [Google Scholar]
- 24.Kelly JR, Allen AP, Temko A, Hutch W, Kennedy PJ, Farid N, Murphy E, Boylan G, Bienenstock J, Cryan JF, et al. Lost in translation? The potential psychobiotic Lactobacillus rhamnosus (JB-1) fails to modulate stress or cognitive performance in healthy male subjects. Brain Behav Immun. 2017;61:50–59. doi: 10.1016/j.bbi.2016.11.018. [DOI] [PubMed] [Google Scholar]
- 25.den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud DJ, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. 2013;54(9):2325–2340. doi: 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kasubuchi M, Hasegawa S, Hiramatsu T, Ichimura A, Kimura I. Dietary gut microbial metabolites, short-chain fatty acids, and host metabolic regulation. Nutrients. 2015;7(4):2839–2849. doi: 10.3390/nu7042839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zyoud SH, Al-Jabi SW, Sweileh WM, Awang R, Waring WS. Global research productivity of N-acetylcysteine use in paracetamol overdose: a bibliometric analysis (1976-2012) Hum Exp Toxicol. 2015;34(10):1006–1016. doi: 10.1177/0960327114565494. [DOI] [PubMed] [Google Scholar]
- 28.Zyoud SH, Al-Jabi SW, Sweileh WM, Awang R, Waring WS. Bibliometric profile of the global scientific research on methanol poisoning (1902-2012) J Occup Med Toxicol. 2015;10:17. doi: 10.1186/s12995-015-0062-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zyoud SH, Al-Jabi SW, Sweileh WM, Waring WS. Scientific research related to calcium channel blockers poisoning: bibliometric analysis in Scopus, 1968-2012. Hum Exp Toxicol. 2015;34(11):1162–1170. doi: 10.1177/0960327115571768. [DOI] [PubMed] [Google Scholar]
- 30.Zyoud SH, Waring WS, Al-Jabi SW, Sweileh WM. Global cocaine intoxication research trends during 1975-2015: a bibliometric analysis of web of science publications. Subst Abuse Treat Prev Policy. 2017;12(1):6. doi: 10.1186/s13011-017-0090-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zyoud SH, Waring WS, Al-Jabi SW, Sweileh WM. Global research production in glyphosate intoxication from 1978 to 2015: a bibliometric analysis. Hum Exp Toxicol. 2017;36(10):997–1006. doi: 10.1177/0960327116678299. [DOI] [PubMed] [Google Scholar]
- 32.Zyoud SH, Waring WS, Al-Jabi SW, Sweileh WM, Awang R. The 100 most influential publications in paracetamol poisoning treatment: a bibliometric analysis of human studies. Springerplus. 2016;5(1):1534. doi: 10.1186/s40064-016-3240-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zyoud SH, Waring WS, Al-Jabi SW, Sweileh WM, Rahhal B, Awang R. Intravenous lipid emulsion as an antidote for the treatment of acute poisoning: a bibliometric analysis of human and animal studies. Basic Clin Pharmacol Toxicol. 2016;119(5):512–519. doi: 10.1111/bcpt.12609. [DOI] [PubMed] [Google Scholar]
- 34.Zyoud SH, Waring WS, Sweileh WM, Al-Jabi SW. Global research trends in lithium toxicity from 1913 to 2015: a bibliometric analysis. Basic Clin Pharmacol Toxicol. 2017;121(1):67–73. doi: 10.1111/bcpt.12755. [DOI] [PubMed] [Google Scholar]
- 35.Albuquerque PC, Castro MJ, Santos-Gandelman J, Oliveira AC, Peralta JM, Rodrigues ML. Bibliometric indicators of the zika outbreak. PLoS Negl Trop Dis. 2017;11(1):e0005132. doi: 10.1371/journal.pntd.0005132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sweileh WM, Al-Jabi SW, Sawalha AF, AbuTaha AS, Zyoud SH. Bibliometric analysis of publications on campylobacter: (2000-2015) PLoS Negl Trop Dis. 2016;35(1):39. doi: 10.1186/s41043-016-0076-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sweileh WM, Al-Jabi SW, Zyoud SH, Sawalha AF, Abu-Taha AS. Global research output in antimicrobial resistance among uropathogens: a bibliometric analysis (2002-2016) J Glob Antimicrob Resist. 2018;13:104–114. doi: 10.1016/j.jgar.2017.11.017. [DOI] [PubMed] [Google Scholar]
- 38.Sweileh WM, Shraim NY, Al-Jabi SW, Sawalha AF, AbuTaha AS, Zyoud SH. Bibliometric analysis of global scientific research on carbapenem resistance (1986-2015) Ann Clin Microbiol Antimicrob. 2016;15(1):56. doi: 10.1186/s12941-016-0169-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sweileh WM, Shraim NY, Al-Jabi SW, Sawalha AF, Rahhal B, Khayyat RA, Zyoud SH. Assessing worldwide research activity on probiotics in pediatrics using Scopus database: 1994-2014. World Allergy Organ J. 2016;9:25. doi: 10.1186/s40413-016-0116-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang M, Gao M, Yue S, Zheng T, Gao Z, Ma X, Wang Q. Global trends and future prospects of food waste research: a bibliometric analysis. Environ Sci Pollut Res Int. 2018;25(25):24600–24610. doi: 10.1007/s11356-018-2598-6. [DOI] [PubMed] [Google Scholar]
- 41.Zheng M, Fu HZ, Ho YS. Research trends and hotspots related to ammonia oxidation based on bibliometric analysis. Environ Sci Pollut Res Int. 2017;24(25):20409–20421. doi: 10.1007/s11356-017-9711-0. [DOI] [PubMed] [Google Scholar]
- 42.Zongyi Y, Dongying C, Baifeng L. Global regulatory T-cell research from 2000 to 2015: a bibliometric analysis. PLoS One. 2016;11(9):e0162099. doi: 10.1371/journal.pone.0162099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tian J, Li M, Lian F, Tong X. The hundred most-cited publications in microbiota of diabetes research: a bibliometric analysis. Medicine (Baltimore) 2017;96(37):e7338. doi: 10.1097/MD.0000000000007338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yao H, Wan JY, Wang CZ, Li L, Wang J, Li Y, Huang WH, Zeng J, Wang Q, Yuan CS. Bibliometric analysis of research on the role of intestinal microbiota in obesity. PeerJ. 2018;6:e5091. doi: 10.7717/peerj.5091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bakkalbasi N, Bauer K, Glover J, Wang L. Three options for citation tracking: google scholar, scopus and web of science. Biomed Digit Libr. 2006;3:7. doi: 10.1186/1742-5581-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Falagas ME, Pitsouni EI, Malietzis GA, Pappas G. Comparison of PubMed, scopus, web of science, and google scholar: strengths and weaknesses. FASEB J. 2008;22(2):338–342. doi: 10.1096/fj.07-9492LSF. [DOI] [PubMed] [Google Scholar]
- 47.Kulkarni AV, Aziz B, Shams I, Busse JW. Comparisons of citations in web of science, scopus, and google scholar for articles published in general medical journals. Jama. 2009;302(10):1092–1096. doi: 10.1001/jama.2009.1307. [DOI] [PubMed] [Google Scholar]
- 48.Hernandez-Vasquez A, Alarcon-Ruiz CA, Bendezu-Quispe G, Comande D, Rosselli D. A bibliometric analysis of the global research on biosimilars. J Pharm Policy Pract. 2018;11:6. doi: 10.1186/s40545-018-0133-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khalili M, Rahimi-Movaghar A, Shadloo B, Mojtabai R, Mann K, Amin-Esmaeili M. Global scientific production on illicit drug addiction: a two-decade analysis. Eur Addict Res. 2018;24(2):60–70. doi: 10.1159/000487590. [DOI] [PubMed] [Google Scholar]
- 50.Lee RP, Xu R, Dave P, Ajmera S, Lillard JC, Wallace D, Broussard A, Motiwala M, Norrdahl S, Howie C, et al. Taking the next step in publication productivity analysis in pediatric neurosurgery. J Neurosurg Pediatr. 2018;21(6):655–665. doi: 10.3171/2018.1.PEDS17535. [DOI] [PubMed] [Google Scholar]
- 51.Sweileh WM. Global output of research on epidermal parasitic skin diseases from 1967 to 2017. Infect Dis Poverty. 2018;7(1):74. doi: 10.1186/s40249-018-0456-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Teles RHG, Moralles HF, Cominetti MR. Global trends in nanomedicine research on triple negative breast cancer: a bibliometric analysis. Int J Nanomedicine. 2018;13:2321–2336. doi: 10.2147/IJN.S164355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zyoud SH. Investigating global trends in paraquat intoxication research from 1962 to 2015 using bibliometric analysis. Am J Ind Med. 2018;61(6):462–470. doi: 10.1002/ajim.22835. [DOI] [PubMed] [Google Scholar]
- 54.Agarwal A, Durairajanayagam D, Tatagari S, Esteves SC, Harlev A, Henkel R, Roychoudhury S, Homa S, Puchalt NG, Ramasamy R, et al. Bibliometrics: tracking research impact by selecting the appropriate metrics. Asian J Androl. 2016;18(2):296–309. doi: 10.4103/1008-682X.171582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van Eck N, Waltman L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics. 2009;84(2):523–538. doi: 10.1007/s11192-009-0146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, Pettersson S. Host-gut microbiota metabolic interactions. Science. 2012;336(6086):1262–1267. doi: 10.1126/science.1223813. [DOI] [PubMed] [Google Scholar]
- 57.Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13(10):701–712. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- 58.Diaz Heijtz R, Wang S, Anuar F, Qian Y, Bjorkholm B, Samuelsson A, Hibberd ML, Forssberg H, Pettersson S. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci U S A. 2011;108(7):3047–3052. doi: 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, Codelli JA, Chow J, Reisman SE, Petrosino JF, et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155(7):1451–1463. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Bienenstock J, Cryan JF. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A. 2011;108(38):16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Foster JA, McVey Neufeld KA. Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 2013;36(5):305–312. doi: 10.1016/j.tins.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 62.Bercik P, Denou E, Collins J, Jackson W, Lu J, Jury J, Deng Y, Blennerhassett P, Macri J, McCoy KD, et al. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology. 2011;141(2):599–609. doi: 10.1053/j.gastro.2011.04.052. [DOI] [PubMed] [Google Scholar]
- 63.Collins SM, Surette M, Bercik P. The interplay between the intestinal microbiota and the brain. Nat Rev Microbiol. 2012;10(11):735–742. doi: 10.1038/nrmicro2876. [DOI] [PubMed] [Google Scholar]
- 64.Berer K, Mues M, Koutrolos M, Rasbi ZA, Boziki M, Johner C, Wekerle H, Krishnamoorthy G. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature. 2011;479(7374):538–541. doi: 10.1038/nature10554. [DOI] [PubMed] [Google Scholar]
- 65.De Vadder F, Kovatcheva-Datchary P, Goncalves D, Vinera J, Zitoun C, Duchampt A, Backhed F, Mithieux G. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell. 2014;156(1–2):84–96. doi: 10.1016/j.cell.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 66.Neufeld KM, Kang N, Bienenstock J, Foster JA. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol Motil. 2011;23(3):255–264. doi: 10.1111/j.1365-2982.2010.01620.x. [DOI] [PubMed] [Google Scholar]
- 67.O'Mahony SM, Marchesi JR, Scully P, Codling C, Ceolho AM, Quigley EM, Cryan JF, Dinan TG. Early life stress alters behavior, immunity, and microbiota in rats: implications for irritable bowel syndrome and psychiatric illnesses. Biol Psychiatry. 2009;65(3):263–267. doi: 10.1016/j.biopsych.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 68.Clarke G, Grenham S, Scully P, Fitzgerald P, Moloney RD, Shanahan F, Dinan TG, Cryan JF. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatry. 2013;18(6):666–673. doi: 10.1038/mp.2012.77. [DOI] [PubMed] [Google Scholar]
- 69.Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE, Challis C, Schretter CE, Rocha S, Gradinaru V, et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of parkinson’s disease. Cell. 2016;167(6):1469–1480.e1412. doi: 10.1016/j.cell.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tillisch K, Labus J, Kilpatrick L, Jiang Z, Stains J, Ebrat B, Guyonnet D, Legrain-Raspaud S, Trotin B, Naliboff B, et al. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology. 2013;144(7):1394–1401. doi: 10.1053/j.gastro.2013.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rhee SH, Pothoulakis C, Mayer EA. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat Rev Gastroenterol Hepatol. 2009;6(5):306–314. doi: 10.1038/nrgastro.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Toth M, Korecka A, Bakocevic N, Ng LG, Kundu P, et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med. 2014;6(263):263ra158. doi: 10.1126/scitranslmed.3009759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Scheperjans F, Aho V, Pereira PA, Koskinen K, Paulin L, Pekkonen E, Haapaniemi E, Kaakkola S, Eerola-Rautio J, Pohja M, et al. Gut microbiota are related to Parkinson's disease and clinical phenotype. Mov Disord. 2015;30(3):350–358. doi: 10.1002/mds.26069. [DOI] [PubMed] [Google Scholar]
- 74.O'Mahony SM, Clarke G, Borre YE, Dinan TG, Cryan JF. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav Brain Res. 2015;277:32–48. doi: 10.1016/j.bbr.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 75.Cryan JF, O'Mahony SM. The microbiome-gut-brain axis: from bowel to behavior. Neurogastroenterol Motil. 2011;23(3):187–192. doi: 10.1111/j.1365-2982.2010.01664.x. [DOI] [PubMed] [Google Scholar]
- 76.Desbonnet L, Garrett L, Clarke G, Kiely B, Cryan JF, Dinan TG. Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience. 2010;170(4):1179–1188. doi: 10.1016/j.neuroscience.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 77.Grenham S, Clarke G, Cryan JF, Dinan TG. Brain-gut-microbe communication in health and disease. Front Physiol. 2011;2:94. doi: 10.3389/fphys.2011.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bonaz BL, Bernstein CN. Brain-gut interactions in inflammatory bowel disease. Gastroenterology. 2013;144(1):36–49. doi: 10.1053/j.gastro.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 79.Dinan TG, Cryan JF. The microbiome-gut-brain axis in health and disease. Gastroenterol Clin N Am. 2017;46(1):77–89. doi: 10.1016/j.gtc.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 80.Dinan TG, Stanton C, Cryan JF. Psychobiotics: a novel class of psychotropic. Biol Psychiatry. 2013;74(10):720–726. doi: 10.1016/j.biopsych.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 81.Evrensel A, Ceylan ME. The gut-brain axis: the missing link in depression. Clin Psychopharmacol Neurosci. 2015;13(3):239–244. doi: 10.9758/cpn.2015.13.3.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mayer EA, Tillisch K, Gupta A. Gut/brain axis and the microbiota. J Clin Invest. 2015;125(3):926–938. doi: 10.1172/JCI76304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Misra Snigdha, Mohanty Debapriya. Psychobiotics: A new approach for treating mental illness? Critical Reviews in Food Science and Nutrition. 2017;59(8):1230–1236. doi: 10.1080/10408398.2017.1399860. [DOI] [PubMed] [Google Scholar]
- 84.Petra AI, Panagiotidou S, Hatziagelaki E, Stewart JM, Conti P, Theoharides TC. Gut-microbiota-brain axis and its effect on neuropsychiatric disorders with suspected immune dysregulation. Clin Ther. 2015;37(5):984–995. doi: 10.1016/j.clinthera.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sarkar A, Lehto SM, Harty S, Dinan TG, Cryan JF, Burnet PWJ. Psychobiotics and the manipulation of bacteria-gut-brain signals. Trends Neurosci. 2016;39(11):763–781. doi: 10.1016/j.tins.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Huang X, Fan X, Ying J, Chen S. Emerging trends and research foci in gastrointestinal microbiome. J Transl Med. 2019;17(1):67. doi: 10.1186/s12967-019-1810-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ding-Qi B, Hui-Bo C, Xin-Yang L, Yi Z, Lan-Hua L, Yun-Hai G. Analysis of research status and hotspots of snail intestinal flora based on bibliometrics. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2018;30(5):571–574. doi: 10.16250/j.32.1374.2018189. [DOI] [PubMed] [Google Scholar]
- 88.Ejtahed HS, Tabatabaei-Malazy O, Soroush AR, Hasani-Ranjbar S, Siadat SD, Raes J, Larijani B. Worldwide trends in scientific publications on association of gut microbiota with obesity. Iran J Basic Med Sci. 2019;22(1):65–71. doi: 10.22038/ijbms.2018.30203.7281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li Y, Zou Z, Bian X, Huang Y, Wang Y, Yang C, Zhao J, Xie L. Fecal microbiota transplantation research output from 2004 to 2017: a bibliometric analysis. PeerJ. 2019;7:e6411. doi: 10.7717/peerj.6411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Integrative HMP (iHMP) Research Network Consortium The Integrative human microbiome project: dynamic analysis of microbiome-host omics profiles during periods of human health and disease. Cell Host Microbe. 2014;16(3):276–289. doi: 10.1016/j.chom.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang HX, Wang YP. Gut Microbiota-brain Axis. Chin Med J. 2016;129(19):2373–2380. doi: 10.4103/0366-6999.190667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schmidt C. Mental health: thinking from the gut. Nature. 2015;518(7540):S12–S15. doi: 10.1038/518S13a. [DOI] [PubMed] [Google Scholar]
- 93.Smith PA. The tantalizing links between gut microbes and the brain. Nature. 2015;526(7573):312–314. doi: 10.1038/526312a. [DOI] [PubMed] [Google Scholar]
- 94.O'Connor A. The Psychobiotic revolution. Lancet Gastroenterol Hepatol. 2017;2(12):854. doi: 10.1016/S2468-1253(17)30336-9. [DOI] [Google Scholar]
- 95.Sweileh WM, Al-Jabi SW, AbuTaha AS, Zyoud SH, Anayah FMA, Sawalha AF. Bibliometric analysis of worldwide scientific literature in mobile - health: 2006-2016. BMC Med Inform Decis Mak. 2017;17(1):72. doi: 10.1186/s12911-017-0476-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zyoud SH, Sweileh WM, Awang R, Al-Jabi SW. Global trends in research related to social media in psychology: mapping and bibliometric analysis. Int J Ment Heal Syst. 2018;12:4. doi: 10.1186/s13033-018-0182-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zyoud SH. Global toxocariasis research trends from 1932 to 2015: a bibliometric analysis. Health Res Policy Syst. 2017;15(1):14. doi: 10.1186/s12961-017-0178-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Al-Jabi SW. Global research trends in West Nile virus from 1943 to 2016: a bibliometric analysis. Glob Health. 2017;13(1):55. doi: 10.1186/s12992-017-0284-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sweileh WM. Global research trends of World Health Organization's top eight emerging pathogens. Glob Health. 2017;13(1):9. doi: 10.1186/s12992-017-0233-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zyoud SH. Global research trends of Middle East respiratory syndrome coronavirus: a bibliometric analysis. BMC Infect Dis. 2016;16:255. doi: 10.1186/s12879-016-1600-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.