Abstract
Several trials and reviews have outlined the potential role of larviciding for malaria control in sub-Saharan Africa (SSA) to supplement the core indoor insecticide-based interventions. It has been argued that widespread use of long-lasting insecticide-treated nets (LLINs) and indoor residual spraying (IRS) interventions in many parts of Africa result in many new areas with low and focal malaria transmission that can be targeted with larvicides. As some countries in SSA are making good progress in malaria control, larval source management, particularly with bacterial larvicides, could be included in the list of viable options to maintain the gains achieved while paving the way to malaria elimination. We conducted a review of published literature that investigated the application of bacterial larvicides, Bacillus thuringiensis var. israelensis (Bti) and/or Bacillus sphaericus (Bs) for malaria vector control in SSA. Data for the review were identified through PubMed, the extensive files of the authors and reference lists of relevant articles retrieved. A total of 56 relevant studies were identified and included in the review. The findings indicated that, at low application rates, bacterial larvicide products based on Bti and/or Bs were effective in controlling malaria vectors. The larvicide interventions were found to be feasible, accepted by the general community, safe to the non-target organisms and the costs compared fairly well with those of other vector control measures practiced in SSA. Our review suggests that larviciding should gain more ground as a tool for integrated malaria vector control due to the decline in malaria which creates more appropriate conditions for the intervention and to the recognition of limitations of insecticide-based vector control tools. The advancement of new technology for mapping landscapes and environments could moreover facilitate identification and targeting of the numerous larval habitats preferred by the African malaria vectors. To build sustainable anti-larval measures in SSA, there is a great need to build capacity in relevant specialties and develop organizational structures for governance and management of larval source management programmes.
Keywords: Bacterial larvicides, Bacillus thuringiensis var. israelensis, Bacillus sphaericus, Anopheles gambiae (sensu lato), Anopheles gambiae (sensu stricto), Anopheles arabiensis, Anopheles funestus, Sub-Saharan Africa
Background
Malaria mosquito vector control in sub-Saharan Africa (SSA) relies on the use of insecticide-treated bednets and/or indoor residual spraying with insecticide. These interventions have been shown to be effective and the recent decline in malaria prevalence in many parts of Africa has been attributed in part to their wide-scale use for mosquito control [1]. However, emerging and widespread insecticide resistance threatens the success made with these tools [2–4]. In addition, insecticide-based interventions have been reported to be the major drive towards the observed behavioral adaptation by malaria vectors [5, 6]. To maintain the gains achieved in malaria control over the last decade, it is crucial to implement measures that will mitigate insecticide resistance and behavioral adaptation by malaria vectors [7].
Mosquito larval control interventions have proven records of lowering malaria transmission and even eradication of malaria mosquitoes [8]. It has been shown that unlike adult mosquitoes, larvae cannot change their behavior to avoid a control intervention targeted at larval habitats [9]. Moreover, a larval control strategy also serves to extend the useful life of insecticides against adult mosquitoes by reducing the size of the population being selected for resistance and the strategy is equally effective in controlling both indoor and outdoor biting mosquitoes. Integrating larval source reduction with adult mosquito control interventions like insecticide-treated bednets has therefore been considered to be a highly effective strategy to control malaria [10]. Larviciding with chemical agents was historically an important component of malaria vector control [8, 11]. However, due to significant adverse effects to other non-target species, chemical larvicides have received less attention in the past decades. Instead, preference has been shifted to the use of microbial larvicides Bacillus thuringiensis var. israelensis (Bti) and Bacillus sphaericus (Bs), which selectively kill mosquito larvae with negligible effect to non-target organisms [12].
Despite the proven role of larval control and the historical success of such interventions in malaria control, larviciding has remained largely neglected for malaria control in SSA [13]. The World Health Organization (WHO) recommends larviciding to be used in moderate to low transmission settings as a supplement to core interventions (long-lasting insecticide-treated nets [LLINs] and indoor residual spraying [IRS]) in settings where larval habitats are few, fixed and findable, such as urban areas, desert fringes, high altitudes and rural areas with high population densities [14]. Of particular relevance to this recommendation, it has been argued that intensification of LLINs and IRS interventions in many parts of Africa will result in many new areas with low and focal malaria transmission [14]. Moreover, the WHO calls for a search for viable supplementary strategies for managing vector-borne diseases and reducing reliance on chemical insecticides [15]. Likewise, it has been argued that the current malaria control interventions constitute a necessary but insufficient set of measures to ensure a sustainable control and thus larviciding could play an important role when other vector control interventions have achieved their maximum practical impact [14].
In the past decades, there has been growing evidence suggesting that larval source management by applying bacterial larvicides Bti and Bs has the potential to lower the density of mosquito vectors, as previously summarized [12, 16]. The efficacy and safety of Bti and Bs have been reported to be high making them ideal for inclusion in the integrated vector management (IVM) programmes for mosquito-borne disease control [12]. However, control efficacy of Bti and Bs has also been reported to vary greatly, mainly due to factors related to target mosquitoes (species of mosquito, their respective feeding strategies, age and density of larvae), larval habitat conditions (temperature, solar radiation, depth of water, turbidity, organic contents and presence of vegetation) and larvicide properties (application rates, toxin contents, type of carrier, how effective the material reach the target, settling rate, means of application and frequent of treatment) [17]. Due to this heterogeneity of their activity, the general consensus suggests that a larviciding strategy can be appropriate and useful for malaria control in some specific settings, whereas in other settings such efforts are unlikely to be cost-effective [14]. To be effective, application of Bti and Bs, like any other larviciding intervention should be guided by adequate knowledge of the prevailing mosquito vectors, their ecology and the properties of the bacterial larvicide used [12, 16].
As some countries in sub-Saharan Africa are making good progress in malaria control, larviciding, particularly with bacterial larvicides, needs to be included in the list of viable options to intensify elimination campaigns. Thus, information on the effectiveness and feasibility of applying bacterial larvicides for mosquito control is important for designing and implementing larvae control operations to supplement interventions targeting adult mosquitoes. Here, we reviewed the available literature on the use of bacterial larvicides for malaria vector control in SSA in order to provide an informed background for designing and implementing larvae control using bacterial larvicides. The present review was designed to complement available literature on larval source management by reviewing only studies on bacterial larvicides used for malaria vector control and limited its scope to studies conducted in SSA.
Methods
Search strategy and article selection
Articles for this review were identified through PubMed, as well as from the extensive files of the authors and the reference lists of relevant articles. The PubMed search was conducted by using the following search terms: “microbial larvicide” or “Bacillus thuringiensis israelensis” or “Bacillus sphaericus”. The PubMed search resulted in 1112 articles, of which 1077 were excluded (after screening titles and abstracts) because they did not address the effect of bacterial larvicides on malaria vectors or malaria transmission, or the studies were not from the SSA region. Moreover, a total of 21 relevant articles were obtained from the files of the authors and the reference lists of identified relevant articles. Thus, a total of 56 articles were considered for full-text reading and used for this review. Operational studies with more than one published article that reported different outcome measure of interest were all included. For articles that reported the impact of bacterial larvicides on combined malaria and non-malaria vectors or of bacterial larvicides combined with other control methods, only data on malaria vectors and on control effects attributable to microbial larvicides were considered. Data from the selected articles were extracted onto a data extraction form created in Microsoft Excel to easily assess and compare information on key study aspects such as bacterial larvicide products, experimental designs, surveyed larval habitats, the feasibility of the application, the impact of larvicides, effect size, intervention costs, safety and acceptability. Due to the wide range and heterogeneity of the study designs, larvicide products tested, application rates and effect size reported, data for the laboratory, semi-field and field studies are presented separately in the results section. Studies conducted in laboratory settings using laboratory colonized malaria vectors were classified as “laboratory studies” whereas those conducted in simulated field conditions (artificial larval habitats set in open fields) with field collected or laboratory reared mosquitoes were categorized as “semi-field studies”. “Field studies” included trials against natural vector populations in natural breeding habitats of malaria vectors.
Results
Description of the reviewed studies
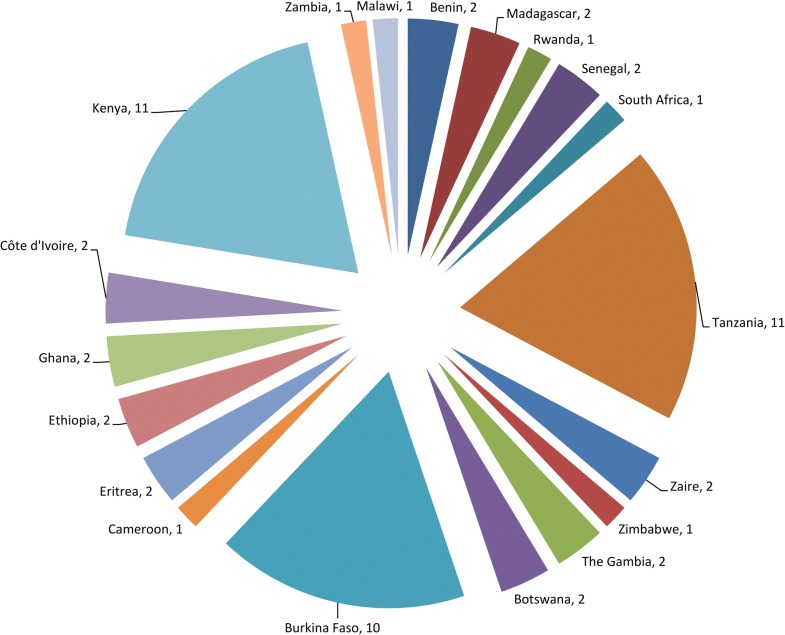
A total of 56 studies were reviewed. More than half (n = 32, 57.1%) were conducted in three countries, namely Kenya (n = 11, 19.6%), Tanzania (n = 11, 19.6%) and Burkina Faso (n = 10, 17.9%) (Fig. 1). The articles were published from 1987 to 2019 and represented over 3 decades of testing of bacterial larvicides in SSA. Of the 56 reviewed articles, 8 (14.3%) had non-interventional components, dealing mainly with community acceptability and/or cost analysis of larvicide interventions. Of the remaining 48 articles, 3, 3 and 32 reported studies that evaluated the activity of larvicides in laboratory, semi-field and field settings only, respectively, whereas 10 articles reported a mixture of these types of studies (Table 1).
Fig. 1.
Number of reviewed publications by country. For publications involving multi-country studies, each country was counted towards the total, e.g. Kenya and Tanzania [94] and Botswana and Zimbabwe [79]. Note: Zaire: now The Democratic Republic of the Congo
Table 1.
Overview of the reviewed articles reporting on bacterial larvicides tested in sub-Saharan Africa, and on the type of studies they describe
| Study typea | No. of articles | References |
|---|---|---|
| Laboratory only | 3 | [21, 64, 65] |
| Laboratory + semi-field | 3 | [66–68] |
| Laboratory + field | 1 | [26] |
| Laboratory + semi-field + field | 3 | [20, 69, 70] |
| Semi-field only | 3 | [71–73] |
| Semi-field + field | 3 | [47, 74, 75] |
| Field only | 32 | [10, 18, 19, 22–25, 27, 30, 32, 48–51, 60, 61, 76–91] |
| Non-interventional | 8 | [28, 29, 31, 52, 92–95] |
| Total | 56 |
aA total of 3 laboratory studies, 3 semi-field studies, 32 field studies, 10 mixed study types and 8 non-interventional studies were reported in the 56 reviewed articles
Of the 39 field studies that involved larvicide application, 24 (61.5%) were conducted in rural settings, whereas 15 (38.5%) were conducted in urban or peri-urban settings (Table 2). Four of these (10.3%) reported on two large-scale larvae control operations conducted in western Kenya and Dar es Salaam, Tanzania. A variety of Anopheles larval habitats were reported in the reviewed articles and these differed considerably within the trial sites. Application of bacterial larvicides in the field studies targeted An. gambiae (s.l.) and An. funestus, the major malaria vectors in SSA (Table 2).
Table 2.
Reviewed field studies on bacterial larvicides tested in sub-Saharan Africa, including information on study areas, survey periods, larval habitats and their associated malaria vectors
| Study site | Settings | Survey period | Malaria vector | Habitat types | References |
|---|---|---|---|---|---|
| Highland, Madagascar | Rural | Jan 1991–Mar 1992; Jan 1993–Mar 1994 | An. arabiensis | Pools, ditches, rice fields | [25, 82] |
| Cove, Benin | Rural | Nov 2011–Dec 2011 | An. gambiae | Rice fields | [75] |
| Mbita, Kenya | Rural | Jun 2002–Sep 2004 | An. gambiae (s.l.); An. funestus | Rock pools, ditches, puddles, swamps, cement lined pits, burrow pits, footprints, tyre tracks | [49]a |
| Vihiga and Kericho, Kenya | Rural | Apr 2008–Mar 2009 | An. gambiae (s.l.) | Pools, ditches, hoof prints, erosion pits | [76] |
| Floodplains of the River Gambia, Gambia | Rural | Aug 2005–Nov 2005; May 2006–Nov 2007 | An. gambiae (s.l.) | Rice fields, pools, flood water areas, swamps | [50, 69] |
| Muheza, Tanzania | Rural | Oct 1990–Dec 1990 | An. funestus | Pools in blocked streams | [26] |
| Mvomero, Tanzania | Rural | May 2006–Jul 2008; Mar 2012–May 2013 | An. gambiae (s.l.); An. funestus | Ditches, rice fields, puddles, road side canals, swamps, river/stream bed pools, ponds, wetlands, cement lined tanks, streams, wells | [23, 87] |
| Kotiokh, Senegal | Rural | Not stated | An. gambiae (s.l.) | Ponds | [83] |
| Vihiga and Kakamega, Kenya | Rural | Jul 2005–Jan 2007; May 2010–Oct 2010; Feb 2011–Apr 2011; Jan 2016–May 2016 | An. gambiae (s.l.); An. funestus | Drainage canals, abandoned gold mines, ponds, fish ponds, road side canals | [10, 24, 47, 48, 86] |
| Tiémélékro, Côte d’Ivoire | Rural | Dec 2005–Jul 2006 | An. gambiae (s.l.); An. funestus | Not specified | [85] |
| Bobirwa, Botswana; Buhera, Zimbabwe | Rural | Jun 2015–Sep 2015; Aug 2012–Oct 2013 | An. arabiensis | Riverbed pools/drains, hoof prints | [79, 81] |
| Anseba, Gash-Barka, Debub and North Red Sea zones, Eritrea | Rural | Not stated | An. arabiensis | Pools, canals, swamps, dams, stream bed pools, wells, ponds | [88, 89] |
| Chikhwawa, Malawi | Rural | May 2016–April 2018 | Not stated | Not stated | [90] |
| Nouna, Burkina Faso | Rural | 2013–2015 | An. gambiae (s.l.); An. funestus | Rice fields, ponds, brickworks | [61] |
| Maroua, Cameroon | Urban | Feb 1992–Nov 1993 | An. gambiae (s.l.) | Ditches, puddles | [18] |
| Cotonou, Benin | Urban | May 2007–Jul 2008 | Anopheles spp. | Pools | [22] |
| Dar es Salaam, Tanzania | Urban | Mar 2006–Apr 2014 | An. gambiae (s.l.) | Ditches, rice paddies, puddles, swamps, water storage containers, construction pits, ponds, riverbeds, habitats associated with urban agriculture, drains, mangrove swamps | [32, 60, 91]a |
| Kinshasa, Zaireb | Urban | Oct 1989–Jan 1990; May 1991–Sep 1991 | An. gambiae (s.l.) | Irrigation ponds, rice fields, swamps | [51, 78] |
| Bobo-Dioulasso, Burkina Faso | Urban | Jul 1985–Sep 1985; | An. gambiae (s.l.) | Ponds | [20] |
| Ouagadougou, Burkina Faso | Urban | Jul 1984–Aug 1984; 1996–1997 | An. gambiae (s.l.) | Pools, rain puddles | [27, 70] |
| Ouagadougou and Bobo-Dioulasso, Burkina Faso | Urban | Jul 1999–Oct 2000 | An. gambiae (s.l.) | Rain puddles | [84] |
| Dakar, Senegal | Urban | Jan 2012–Dec 2012 | An. gambiae (s.l.) | Rain puddles | [74] |
| Malindi, Kenya | Urban | Jun 2006–Dec 2007 | An. gambiae (s.l.) | Unused swimming pools, drainage, puddles, swamps, water tank/trough, wells, ponds, manholes, car tracks, cesspits | [77, 80] |
| Lusaka, Zambia | Urban | June 2011–Aug 2011 | An. gambiae (s.l.); An. funestus | Dams, marshes, ponds, streams | [19] |
| Kilosa, Tanzania | Rural | Feb 2016–Mar 2016 | – | Rice fields | [30] |
Note: All studies were operational research in design except studies marked with “a” were large-scale control programmes
bZaire: now The Democratic Republic of the Congo
Bacterial larvicide products evaluated
An overview of the evaluated bacterial larvicide products in the reviewed studies is shown in Table 3. Five studies did not specify Bti and/or Bs products evaluated [18–22]. Of the reviewed field studies that reported larvicide application, 13 (33.3%) tested Bti and Bs concurrently (in alternating fashion or in separate larval habitats in the same study site) whereas 13 (33.3%) and 7 (17.9%), respectively, tested only Bti or Bs. Six (15.4%) studies tested larvicide products that were formulated with a combination of both Bti and Bs toxins, namely FourStar® briquettes, VectoMax® corn granules (CG) and Culinexcombi® tablets. Water-dispersible granules (WDG) and CG formulations of the commercial strains of Bti were the majority of larvicide products tested in the reviewed studies, reported by 19 and 11 studies, respectively. In the reviewed studies, application rates for the bacterial larvicide products varied considerably with a strain of the bacterium (Bti or Bs), product formulation and inherent potency of the product as measured by their toxicity in international toxic units per milligram (ITU/mg).
Table 3.
Commercial bacterial larvicide products based on Bacillus thuringiensis var. israelensis and Bacillus sphaericus applied in the reviewed studies from sub-Saharan Africa
| Product (formulation) | Bacterial strain (potency) | Manufacturer | Application rate | References |
|---|---|---|---|---|
| VectoBac® 12 (AS) | Bti (200–1200 ITU/mg) | Abbott Laboratories, North Chicago, IL, USA; Bayer AG, Leverkusen, Germany | 0.3–6.0 l/ha | [78, 81, 82] |
| VectoBac® (G) | Bti (200 ITU/mg) | Abbott Laboratories, North Chicago, IL, USA; Bayer AG, Leverkusen, Germany; Valent Biosciences, Corp, IL, USA | 2.0–20.0 kg/ha | [10, 74, 75, 78, 81, 82, 88, 89] |
| VectoBac® (WDG) | Bti (2700–3000 ITU/mg) | Valent Biosciences, Corp, IL, USA | 0.2–2.0 kg/ha | [10, 29, 32, 49, 50, 52, 60, 61, 67–69, 71, 72, 74, 76, 79, 85, 90, 94] |
| VectoBac® (CG) | Bti (200 ITU/mg) | Valent Biosciences, Corp, IL, USA | 4.0–10.0 kg/ha | [28, 30, 32, 49, 50, 60, 69, 77, 87, 91–94] |
| VectoBac DT (Tab) | Bti (1.3 × 106 ITU Bti) | Valent Biosciences, Corp, IL, USA | 1 tablet/2000 l | [77] |
| Teknar HP-D (LC) | Bti (1500 ITU/mg) | Sandoz, USA | 1.25 l/ha | [25, 70] |
| Bactimos® (WP) | Bti (3500 ITU/mg) | Biochem Products, Moutchanin, USA | 0.25–0.5 kg/ha | [70] |
| VectoBac® (WP) | Bti (200 ITU/mg) | Abbott Laboratories, North Chicago, IL, USA | 0.5–1.0 kg/ha | [70] |
| Bactivec® (WBS) | Bti (Not specified) | Labiofam AS, Havana, Cuba | 5 ml/l | [23, 91] |
| Bactimos® (PP) | Bti (10000 ITU/mg) | Valent Biosciences, Corp, IL, USA | 0.09 kg/ha | [67] |
| ABG6138G | Bti (200 ITU/mg) | Abbott Laboratories, North Chicago, IL, USA | 2.8–5.6 kg/ha | [70] |
| IPS-82 | Bti (not specified) | Institute Pasteur, Paris, France | na | [65] |
| HD-522 | Bti (not specified) | Bacillus Genetic Stock Center, Ohio State University, USA | na | [64] |
| VectoLex® (WDG) | Bs (650 ITU/mg) | Valent Biosciences, Corp, IL, USA | 1.0–10.0 kg/ha | [10, 49, 60, 66, 67, 69, 84, 85] |
| VectoLex® (CG) | Bs (50–670 ITU/mg) | Valent Biosciences, Corp, IL, USA | 11.2–30.0 kg/ha | [49, 60, 69, 88, 89] |
| Spherimos (Briquets) | Bs (Not specified) | Summitt Chemical Co. Baltimore, MD, USA | 1 briquet/m2 | [26] |
| Spherimos (G) | Bs (Not specified) | Abbott Laboratories, North Chicago, IL, USA | 30.0 kg/ha | [83] |
| VectoLex® (G) | Bs (20–200 ITU/mg) | Abbott Laboratories, North Chicago, IL, USA | 10.0–30.0 kg/ha | [10, 51, 78] |
| Spherimos (FC) | Bs (Not specified) | Abbott Laboratories, North Chicago, IL, USA; Duphar BV, Weesp, Netherlands | 30.0–60.0 kg/ha | [26, 83] |
| Griselesf® (WBS) | Bs (Not specified) | Labiofam AS, Havana, Cuba | 10ml/l | [23] |
| ABG6185G | Bs (5 × 1010 spores/g) | Abbott Laboratories, North Chicago, IL, USA | 2.0–8.0 kg/ha | [82] |
| SPH-88 | Bs (not specified) | Institute Pasteur, Paris, France | na | [65] |
| Bs TP | Bs (1600 Bs ITU/mg) | Valent Biosciences, Corp, IL, USA | na | [67] |
| VectoMax® (CG) | Bti + Bs (52 ITU/mg Bti and 50 ITU/mg Bs) | Valent Biosciences, Corp, IL, USA | 7.5 kg/ha | [74, 86] |
| Culinexcombi (Tab) | Bti + Bs (1.0 × 106 ITU Bti + 2.5 × 104 ITU Bs) | Culinex GmbH, Germany | 1 tablet/2000 l | [77, 80] |
| FourStar® (Briquettes) | Bti + Bs (Not specified) | Adapco Inc. Sanford, FL, USA; Central Life Sciences, Sag Harbor, NY, USA | 1 briquette/100 ft2 | [24, 47, 48] |
| BTBSWAX (Wax) | Bti + Bs (Not specified) | ISCA Technologies, Riverside, CA, USA | 1–2 g/m2 | [73] |
Notes: Non-commercial formulations tested included: locally made slow release granular formulation of Bti/Bs [27, 95]. Bs isolate 1593 and 2362 [70] and LL3 [24, 48]. Reported bacterial strains: Bti: AM-6552, IPS-82, HD-522 and BMP 144. Bs: 2362, ABG 6185 and SPH-88. Serotypes: Bti; H-14; Bs; H5a5b
Abbreviations: Bti, Bacillus thuringiensis var. israelensis; Bs, Bacillus sphaericus; AS, aqueous suspension; G, granules; WDG, water-dispersible granules; CG, corn granules; Tab, tablets; LC, liquid concentrate; WP, wettable powder; WBS, water-based suspension; PP, primary powder; TP, technical powder; FC, flowable concentrate; ITU, international toxic units; na, not applicable
The activity of Bti and Bs in laboratory settings
Ten (17.9%) of the reviewed articles presented the findings of the efficacy of Bti and Bs on An. gambiae (s.l.) and An. funestus in laboratory settings (Table 4). The bio-potency of Bti and Bs based products varied between 1500–10,000 Bti ITU/mg and 650–1600 Bs ITU/mg, respectively. In most cases, laboratory experiments were conducted for 24 and 48 h for Bti and Bs, respectively. For Bti, the lethal concentration value that caused 50 and 90/95% mortality of An. gambiae (s.l.) larvae (LC50 and LC90/95) ranged between 0.006–0.662 mg/l and 0.132–1.743 mg/l, respectively. For Bs, the LC50 and LC90/95 values for the same mosquito species ranged between 0.002–0.342 mg/l and 0.018–1.807 mg/l, respectively. For An. funestus, LC50 and LC95 values after 48 h of exposure to Bs were 1.0 mg/l and 6.0 mg/l, respectively. For studies that reported the potency in spores per milliliter, LC50 and LC90 values after 24 h of exposure to Bti in different malaria vectors are presented in Table 4.
Table 4.
Laboratory trials using Bacillus thuringiensis var. israelensis and Bacillus sphaericus against malaria vectors in sub-Saharan Africa
| Site (Country) | Bacterial strain (potency) | Product (formulations) | Species tested (larval instar) |
Exposure time (h) | LC50 | LC90/95 | References |
|---|---|---|---|---|---|---|---|
| ICIPE (Kenya) | Bti (2700 ITU/mg) | VectoBac® (WDG) | An. gambiae (s.s.) (3rd) | 24 | 0.021 | 0.201 | [67] |
| Bs (650 BsITU/mg) | VectoLex® (WDG) | An. gambiae (s.s.) (3rd) | 24 | 0.004 | 0.038 | ||
| Bti (10000 ITU/mg) | Bactimos® (PP) | An. gambiae (s.s.) (3rd) | 24 | 0.006 | 0.090 | ||
| Bs (1600 ITU/mg) | (TP) | An. gambiae (s.s.) (3rd) | 24 | 0.002 | 0.018 | ||
| Ouagadougou (Burkina Faso) | Bti (3500 ITU/mg) | Bactimos® (WP) | An. gambiae (s.l.) (3rd and 4th) | 24 | 0.081 | 0.231a | [70] |
| Bti (2000 ITU/mg) | VectoBac® (WP) | An. gambiae (s.l.) (3rd and 4th) | 24 | 0.110 | 0.375a | ||
| Bti (1500 ITU/mg) | Teknar (FC) | An. gambiae (s.l.) (3rd and 4th) | 24 | 0.662 | 1.743a | ||
| Bs (not stated) | 1593 IF-119 (SD) | An. gambiae (s.l.) (3rd and 4th) | 48 | 0.043 | 0.107a | ||
| Bs (not stated) | 2362 IF-118 (SD) | An. gambiae (s.l.) (3rd and 4th) | 48 | 0.022 | 0.130a | ||
| MRC, Farafenni (Gambia) | Bs (650 Bs ITU/mg) | VectoLex® (WDG) | An. gambiae (s.s.) (3rd) | 24 | 0.004 | 0.023 | [69] |
| Bti (3000 ITU/mg) | VectoBac® (WDG) | An. gambiae (s.s.) (3rd) | 24 | 0.039 | 0.132 | ||
| KEMRI, Kisumu (Kenya) | Bti (7000 ITU/mg) | Not stated | An. gambiae (s.l.) (3rd) | 24 | 0.062 | 0.797 | [21] |
| Bs (1000 ITU/mg) | Not stated | An. gambiae (s.l.) (3rd) | 48 | 0.058 | 0.451 | ||
| KCCR, Kumasi (Ghana) | Bti (3000 ITU/mg) | VectoBac® (WDG) | An. gambiae (3rd) | 24 | 0.026 | 0.136 | [68] |
| Bobo-Dioulasso (Burkina Faso) | Bs (not stated) | (FC) | An. gambiae (s.s.) (3rd) | 48 | 0.342 | 1.807 | [20] |
| UFS, Muheza (Tanzania) | Bs (not stated) | Spherimos (FC) | An. funestus (4th) | 48 | 1.0 | 6.0 | [26] |
| KCCR, Kumasi (Ghana) | Bs (not stated) | VectoLex® (WDG) | An. gambiae (s.l.) (3rd and 4th) | 24 | 0.0027 | 0.0086 | [66] |
| NOCMVD, Nazareth (Ethiopia) | Bti (not stated) | IPS-82 | An. arabiensis (3rd) | 48 | 0.0018b | – | [65] |
| Bs (not stated) | SPH-88 | An. arabiensis (3rd) | 48 | 0.0076b | – | ||
| NICD, Johannesburg (South Africa) | Bti (not stated) | Not stated | An. quadriannulatus (3rd) | 24 | 2.97 | 5.02 | [64] |
| – | An. arabiensis (3rd) | 24 | 3.72 | 10.10a | |||
| – | An. gambiae (3rd) | 24 | 3.76 | 7.70a | |||
| – | An. merus (3rd) | 24 | 3.82 | 6.65a | |||
| – | An. funestus (3rd) | 24 | 4.44 | 13.50a |
aLC90/95: a dose marked with a represents LC90; unmarked doses represent LC95
bDoses provided in µg/l were converted to mg/l for a uniform presentation. Concentrations reported in mg/l except for study [64] are given in 104 spores/ml
Abbreviations: Bti, Bacillus thuringiensis var. israelensis; Bs, Bacillus sphaericus; ITU, international toxic units; WDG, water-dispersible granules; PP, primary powder; TP, technical powder; FC, flowable concentrate; SD, spray dried; ICIPE, International Centre of Insect Physiology and Ecology; MRC, Medical Research Council; KCCR, Kumasi Centre for Collaborative Research; UFS, Ubwari Field Station; NOCMVD, National Organization for the Control of Malaria and other Vector-borne Diseases; NICD, National Institute of Communicable Diseases; LC, lethal concentration (concentration that kills 50/95% of the test subjects)
The activity of Bti and Bs in semi-field conditions
A total of 12 (21.4%) studies reported experiments with Bti and Bs conducted in semi-field conditions to establish their effectiveness and duration of control (Table 5). The larval habitats treated contained Anopheles gambiae (s.l.), a mixture of Anopheles and culicine species, An. arabiensis and An. gambiae (s.s.) in 5, 4, 2 and 1 of these studies, respectively. Larvicide application rates varied considerably among the studies with products based on Bti having relatively lower application rates compared to Bs. With respect to larvicide formulation, application rates for water-dispersible granules (WDG) were lower than corn granules (CG) or granules (G) due to their inherently high potency. The studies reported appreciable larval reductions in the treated larval habitats for 2 to 14 days post-treatment. Of the tested products, the highest larval reductions and the most prolonged effect was seen in studies that tested VectoMax® CG, with 98–100% reduction in late larval instars for 2 weeks. On the other hand, the pupal reductions in treated larval habitats varied between 64–100%, with residual effects ranging from 7 days to 3 months. A very high residual effect in pupal control was observed in a study that tested a slow release formulation of bacterial larvicide (FourStar®) that combined both Bti and Bs (Table 5).
Table 5.
Semi-field trials using commercial products of Bacillus thuringiensis var. israelensis and Bacillus sphaericus against malaria vectors in sub-Saharan Africa
| Test site (Country) | Larvicide type (potency) | Product (formulation) | Species tested | Application rate | % larval reductione (Residual) | % pupae reduction (Residual) | References |
|---|---|---|---|---|---|---|---|
| CRSN, Nouna (Burkina Faso) | Bti (3000 ITU/mg) | VectoBac® (WDG) | An. gambiae (s.l.) | 0.2–1.0 mg/l | 90–100 (4 days) | 98.5 (21 days) | [71] |
| CREC (Benin) | Bti (200 ITU/mg) | VectoBac® (GR) | An. gambiae | 0.6–1.2 g/m2 | – | > 80 (19 days) | [75] |
| MRC, Farafenni (Gambia) | Bti (3000 ITU/mg) | VectoBac® (WDG) | Anopheles and Culicinaea | 0.2 kg/ha | 81–100 (5 days) | 64–94 (7 days) | [69] |
| Bs (650 ITU/mg) | VectoLex® (WDG) | Anopheles and Culicinaea | 0.5–5.0 kg/ha | 96–100 (1–2 days) | > 95 (7 days) | ||
| KCCR, Kumasi (Ghana) | Bti (not stated) | VectoBac® (WDG) | Anopheles and Culicinaeb | 0.2–0.4 mg/l | 51–100 (4 days) | – | [68] |
| Bobo-Dioulasso (Burkina Faso) | Bs (not stated) | 2362 (FC) | An. gambiae (s.l.) | 0.1–10.0 g/m2 | 100 (7–10 days) | 92 (3–10 days) | [20] |
| KCCR,Kumasi (Ghana) | Bs (not stated) | VectoLex® (WDG) | Anopheles and Culicinaec | 0.5–1.0 mg/l | 70–100 (10 days) | 100 (12 days) | [66] |
| ICIPE, Suba (Kenya) | Bti (2700 ITU/mg) | VectoBac® (WDG) | Anopheles and Culicinaed | 0.2–1.6 mg/l | 88–100 (4 days) | 95 | [67] |
| Bs (650 Bs ITU/mg) | VectoLex® (WDG) | Anopheles and Culicinaed | 1 and 5 mg/l | 100 (11 days) | 100 (14 days) | ||
| Kisian, Kisumu (Kenya) | Bti + Bs (not stated) | FourStar® (Briquettes) | An. gambiae (s.l.) | 1 briquette per 9.3 m2 | – | 85.5 (180 days) | [47] |
| Ouagadougou (Burkina Faso) | Bs (not stated) | 1593 IF-119 (Spray-dried) | An. gambiae (s.l.) | 0.12–0.24 kg/ha | 95.8–100 (2 days) | – | [70] |
| Bs (not stated) | 2362 IF-118 (spray dried) | An. gambiae (s.l.) | 0.12–0.24 kg/ha | 100 (2 days) | – | ||
| Tolay (Ethiopia) | Bti (3000 ITU/mg) | VectoBac® (WDG) | An. arabiensis | 0.05–0.2 g/m2 | 50–100 (13 days) | – | [72] |
| DP, Dakar (Senegal) | Bti (3000 ITU/mg) | VectoBac® (WDG) | An. arabiensis | 0.03 g/m2 | 94–100 (14 days) | – | [74] |
| Bti (200 ITU/mg) | VectoBac® (GR) | An. arabiensis | 0.5 g/m2 | 92–100 (14 days) | – | ||
| Bti/Bs (52/50 ITU/mg) | VectoMax® (CG) | An. arabiensis | 0.75 g/m2 | 98–100 (14 days) | – | ||
| Bouake (Côte d’Ivoire) | Bti + Bs (Not stated) | BTBSWAX® (Wax) | An. gambiae (s.s.) | 1–2 g/m2 | – | < 10– > 80 (10 days) | [73] |
Note: Proportional of tested Anopheles: aAnopheles population accounted for 40%
bAnopheles population accounted for 87%
cAnopheles population accounted for 89%
dAnopheles population accounted for 85%
eLarval reductions: based on late instars except study [66] in which reductions were based on all larval instars
Abbreviations: CRSN, Centre de Recherche en Sante de Nouna; ITU, international toxic units; CREC, Centre de Recherche Entomologique de Cotonou; WDG, water-dispersible granules; GR, granules; FC, flowable concentrate; Bti, Bacillus thuringiensis var. israelensis; Bs, Bacillus sphaericus; MRC, Medical Research Council; KCCR, Kumasi Centre for Collaborative Research; ICIPE, International Centre of Insect Physiology and Ecology; DP, Department of Pikine
The activity of Bti and Bs on immature and adult mosquitoes in field conditions
A total of 39 (69.6%) reviewed studies evaluated the activity of Bti and/or Bs in field conditions (Table 2). Of these, seven commenced the evaluations of Bti and/or Bs from the laboratory and/or semi-field conditions. Five bacterial larvicide products evaluated in the semi-field trials were also tested in the field conditions (Table 6). WDG and CG formulations of Bti and Bs were the majority of the evaluated products, with VectoBac® WDG tested in 14 of the field studies. Reported larval reductions varied considerably with the test site, larvicide product applied and application rate. Overall, larval reductions ranging from 47 to 100% were recorded, with the residual effect lasting for 2 to 28 days following single or repeated applications of the larvicide. Substantial pupal reductions were also reported, with the most marked impact observed with FourStar®, a slow release bacterial larvicide formulation (Table 6). The least larval reductions were recorded with Bactimos® and VectoBac® wettable powder (WP) when applied once in rain pools. In the reviewed articles, it was not possible to analyze the difference in susceptibility between An. gambiae (s.l.) and An. funestus or variation in different ecological settings due to heterogeneity in testing conditions, products used and pooling of mosquito species data in some studies. However, the reviewed laboratory studies indicated that An. gambiae (s.l.) were more sensitive to Bti and Bs than An. funestus (Table 4). On the other hand, a total of 14 (35.9%) reviewed field studies reported the activity of Bti and/or Bs in adult mosquitoes and/or malaria transmission. Different levels of reductions in adult mosquitoes and/or malaria transmission were reported with single or repeated applications of Bti and/or Bs (Table 7).
Table 6.
Field trials of commercial products of Bacillus thuringiensis var. israelensis and Bacillus sphaericus against immature stages of malaria vectors in sub-Saharan Africa
| Country | Vector targeted | Product (Potency) | Application rate | Application cycle | % Larval reduction | Residual effect | References |
|---|---|---|---|---|---|---|---|
| Mahitsy, Madagascar | An. arabiensis | VectoBac® 12 AS (1200 ITU/mg) | 0.3–1.0 l/ha | Once | 89–100a | 2 days | [82] |
| VectoBac® G (200 ITU/mg) | 2.0–10.0 kg/ha | Once | 67–100a | 2 days | |||
| ABG6185 (G) | 2.0–18.0 kg/ha | Once | 37–100a | 2 days | |||
| Mbita, Kenya | An. gambiae (s.l.); An. funestus | VectoBac® WDG (3000 ITU/mg) / VectoBac® CG (200 ITU/mg) | 0.2 kg/ha / 5.0 kg/ha | Variableb | 99 | 7 days | [49] |
| VectoLex® WDG (650 ITU/mg) / VectoLex® CG (50 ITU/mg) | 1.0 kg/ha / 15.0 kg/ha | 2 weeks | 99 | 23 days | |||
| Dar es Salaam, Tanzania | An. gambiae (s.l.) | VectoBac® WDG (3000 ITU/mg)/ VectoBac® CG (200 ITU/mg) | 0.4 kg/ha / 10.0 kg/ha | 1 week | 96a | 7 days | [32, 60] |
| VectoLex® WDG (650 ITU/mg)/ VectoLex® CG (50 ITU/mg) | 2.0 kg/ha / 30.0 kg/ha | 1 week | 96a | 7 days | |||
| Kakamega and Vihiga, Kenya | An. gambiae (s.l.); An. funestus | VectoBac® WDG/G; VectoLex® WDG/G | – | 1 week | 91.1 | 7 days | [10] |
| Malindi, Kenya | An. gambiae (s.l.) | VectoBac DT | 1 tab/2000 l | Once | 89–99 | 8 days | [77] |
| Culinexcombi Tab (1.0 × 106 ITU Bti + 2.5 × 104 ITU Bs) | 1 tab/2000 l | Once | 77–100 | 8 days | |||
| Kinshasa, Zaireg | An. gambiae (s.l.) | VectoBac® G (200 ITU/mg) | 10.0–0.0 l/ha | Once | 86–100a | 2 days | [78] |
| VectoLex® G (200 ITU/mg) | 10.0–30.0 l/ha | Once | 95–100a | 2 days | |||
| Floodplains of the River Gambia, Gambia | Anopheles sp. | VectoBac® WDG 3000 ITU/mg)/ VectoBac® CG (200 ITU/mg) | 0.2 kg/ha / 4.0 kg/ha | 1 week | 100 | 7 days | [69] |
| VectoLex® WDG (650 ITU/mg) | 1.0 kg/ha | 1 week | 100 | 2 days | |||
| Floodplains of the River Gambia, Gambia | An. gambiae (s.l.) | VectoBac® WDG (3000 ITU/mg)/ VectoBac® CG (200 ITU/mg) | 0.2 kg/ha / 5.0 kg/ha | 1 week | 88a | 7 days | [50] |
| Bobirwa, Botswana; Buhera, Zimbabwe | Anopheles sp. | VectoBac® WDG | 0.3 kg/ha | 2 weeks | 47–95a | 14 days | [79] |
| Bobirwa, Botswana | An. arabiensis | VectoBac® 12 AS (1200 ITU/mg)/ VectoBac® G (200 ITU/mg) | 2.0 l/ha / 2 g/m2 | Once | 81–97 | 2 days | [81] |
| Anseba, Gash-Barka, Debub and North Red Sea zones, Eritrea | An. arabiensis | VectoBac® G (200 ITU/mg) | 5.6–11.2 kg/ha | Once | 54–100a | 14–21 days | [88, 89] |
| VectoLex® CG (670 ITU/mg) | 11.2–22.4 kg/ha | Once | 73.8–100a | 14–21 days | |||
| Anjiro, Madagascar | An. gambiae (s.s.) | Teknar HP–D LC | 1.25 l/ha | Once | 95.3–100a | 3 days | [25] |
| Dakar, Senegal | An. gambiae (s.l.) | VectoBac® WDG (3000 ITU/mg)/ VectoBac® G (200 ITU/mg)/ VectoMax® CG (Bti 52ITU/mg and 50 Bs ITU/mg) | 0.05 g/m2 / 0.5 g/m2 / 0.75 g/m2 | Once | 100a | 3 days | [74] |
| Ouagadougou, Burkina Faso | An. gambiae (s.l.) | Bactimos® WP (3500 ITU/mg) | 0.25–0.5 kg/ha | Once | 82–95 a | 1 day | [70] |
| An. gambiae (s.l.) | VectoBac® WP (2000 ITU/mg) | 0.5–1.0 kg/ha | Once | 86–95 a | 1 day | ||
| Mvomero, Tanzania | An. gambiae (s.l.); An. funestus | Bactivec® WBS | 5 ml/l | Variablec | 79.3–98a | 14 days | [23] |
| Griselesf® WBS | 10 ml/l | Variablec | 47–76.6a | 14 days | |||
| Kinshasa, Zaireg | An. gambiae (s.l.) | VectoLex® G (200 ITU/mg) | 10.0 kg/ha | 15 days | 98a | 2 days | [51] |
| Bobo-Dioulasso, Burkina Faso | An. gambiae | Spherimos FC | 0.1–10.0 g/m2 | Once | 100 | 1–10 days | [20] |
| Muheza, Tanzania | An. funestus | Spherimos FC | 60 mg/l | Once | 100 a | 28 days | [26] |
| Kotiokh, Senegal | An. gambiae (s.l.) | Spherimos FC | 3 g/m2 | Once | 95–100a | 5 days | [83] |
| Spherimos G | 3 g/m2 | Once | 100a | 15 days | |||
| Western, Kenya | An. gambiae (s.l.); An. funestus | VectoMax® CG | – | 4 weeks | ~100a | 10 days | [86] |
| Cove, Benind | An. gambiae (s.l.) | VectoBac® G (200 ITU/mg) | 1.2 g/m2 | Once | 73–95 | 3 days | [75] |
| Malindi, Kenya | An. gambiae (s.l.) | Culinexcombi Tablets (1.0 x 106 ITU Bti + 2.5 × 104 ITU Bs) | 1 tab/2000 l | 2 weeks | 99–100 | 10 days | [80] |
| Western Kenya, Kenyae | An. gambiae (s.l.); An. funestus | FourStar® Briquettes (not stated) | 1 briq/100ft2 | Once | – | 5 months | [47] |
| Vihiga and Kakamega, Kenya | An. gambiae s.l.; An. funestus | FourStar®Briquettes (not stated) | 1 briq/100ft2 | Once | 80a | 4 weeks | [48] |
| Chikhwawa, Malawi | Not stated | VectoBac®WDG (not stated) | Not stated | Not stated | Not stated | Not stated | [90] |
| Lusaka, Zambia | An. gambiae (s.l.); An. funestus | Not stated | 5 ml/m2 | Not stated | Not stated | Not stated | [19] |
| Ouagadougou, Burkina Faso | An. gambiae (s.l.) | Granular Bs (1520 ITU/mg) / Spherimos FC (300 ITU/mg) | 3.0 ml / 3.0 g/m2 | Once | 60–97 | 10 days | [27] |
| Ouagadougou and Bobo-Dioulasso, Burkina Faso | An. gambiae (s.l.) | Spherimos FC (80 ITU/mg) / VectoLex WSM (100% TP) | 30 g/m2 / 0.5 g/m2 | 1 week | Not stated | Not stated | [84] |
| Maroua, Cameroon | An. gambiae (s.l.); An. funestus; An. pharoensis | Bs strain 2362 (not specified) | 10 g/m2 | 3 roundsf | Not stated | Not stated | [18] |
| Tiémélékro, Côte d’Ivoire | An. gambiae (s.l.); An. funestus (s.l.) | VectoBac® WDG (3000 ITU/mg) / VectoLex® WDG (650 ITU/mg) | 0.8 mg/l / 10 mg/l | ~3 weeks | Reduced to zero | 21 days | [85] |
| Vihiga and Kericho, Kenya | An. gambiae (s.l.); An. funestus | VectoBac® WDG (not stated) | 200 g/ha | 1 week | 91 | 7 days | [76] |
| Cotonou, Benin | Anopheles | Bti (not stated) | 50 mg/l | 2 weeks | Significantly reduced | 9 days | [22] |
| Mvomero, Tanzania | An. gambiae (s.l.); An. funestus | VectoBac® CG (ITU/mg)/ | 10 kg/ha | 1 week | Not stated | Not stated | [87] |
| Nouna, Burkina Faso | An. gambiae (s.l.); An. funestus | VectoBac® WDG (not stated) | Not stated | 10 days | Not stated | Not stated | [61] |
| Kilosa, Tanzania | Not stated | VectoBac® CG | Not stated | Not stated | Not stated | Not stated | [30] |
aLarval reductions based on all larval instars; unmarked are based on late instars only
bWeekly application cycles for the first four rounds after which re-treatment was conducted when late instars were noted
cLarval habitats were re-treated when late instars were detected during weekly monitoring
dPupal reduction reported 100% for up to 3 days [75]
ePupal reduction reported 87.4–95.4% for up to 5 months [47]
fThree rounds of applications between March 1992–Nov 1993
gZaire: now The Democratic Republic of the Congo
Abbreviations: ITU, international toxic units; AS, aqueous suspension; G, granules; WDG, water-dispersible granules; CG, corn granules; LC, liquid concentrate; WP, wettable powder, WBS, water-based suspension; FC, flowable concentrate; WSM, water-suspendable micro-granule; Bti, Bacillus thuringiensis var. israelensis; Bs, Bacillus sphaericus; tab, tablets; briq, briquettes
Table 7.
Field trials of commercial products of Bacillus thuringiensis var. israelensis and Bacillus sphaericus against adult malaria vectors and the effect on malaria transmission in sub-Saharan Africa
| Country | Vectors targeted | Product (application rate) | Application cycle | Percentage reduction | References | ||
|---|---|---|---|---|---|---|---|
| Adult density/biting | Transmission/EIR | Malaria prevalence | |||||
| Mbita, Kenya | An. gambiae (s.l.); An. funestus | VectoBac® WDG (0.2 kg/ha); VectoBac® CG (5.0 kg/ha); VectoLex® WDG (1.0 kg/ha) and VectoLex® CG (15.0 kg/ha) | 1/2 weeke | 92.0a | – | – | [49] |
| Dar es Salaam, Tanzania | An. gambiae (s.l.) | VectoBac® WDG (0.4 kg/ha); VectoBac® CG (10.0 kg/ha); VectoLex® WDG (2.0 kg/ha); VectoLex® CG (30.0 kg/ha) | 1 week | 31.3a | 71c | 40.0 | [60] |
| VectoBac® (Not stated); Bactivec® (not stated) | – | Reducedb | – | Reduced | [91] | ||
| Kakamega and Vihiga, Kenya | An. gambiae (s.l.); An. funestus | VectoBac® WDG; VectoBac® CG; VectoLex® WDG; VectoLex® CG | 1 week | 85.9b | 73.1d | – | [10] |
| Dar es Salaam, Tanzania | An. gambiae (s.l.); An. funestus; An. coustani | VectoBac® WDG (0.4 kg/ha); VectoBac® CG (10.0 kg/ha) | 1 week | 72.0b | 32.0d | Reduced | [32] |
| Kinshasa, Zaireg | An. gambiae (s.l.) | VectoLex® G (10.0 kg/ha) | 2 weeks | 13.6a | 39.9d,f | – | [51] |
| Western, Kenya | An. gambiae (s.l.); An. funestus | FourStar® Briquettes (1 briq/100 ft2) | Once | 60.0–85.0b | – | – | [47] |
| Nouna, Burkina Faso | An. gambiae (s.l.); An. funestus | VectoBac® WDG (not stated) | 10 days | 72–80b | – | – | [61] |
| Bobirwa, Botswana | An. arabiensis | VectoBac® 12 AS (2.0 l/ha); VectoBac® G (2.0 g/m2) | Once | Reducedb | Reducedc | Decreased | [81] |
| Eritrea | An. arabiensis | VectoBac® G (11.2 kg/ha); VectoLex® CG (22.4 kg/ha) | 1 week | Significantly reducedb | – | – | [89] |
| Tiémélékro, Côte d’Ivoire | An. gambiae (s.l.); An. funestus (s.l.) | VectoBac® (0.8 mg/l); VectoLex® (10 mg/l) | 3 weeks | Significantly reduceda | Significantly reducedd | – | [85] |
| Maroua, Cameroon | An. gambiae (s.l.); An. funestus; | Bs strain 2362 (10 g/m2) | 3 rounds | Reduceda | Reducedc | – | [18] |
| Cotonou, Benin | Anopheles | Bti (50 mg/l) | 2 weeks | Reduceda | – | Reduced | [22] |
| Gambia | An. gambiae (s.l.) | VectoBac® WDG (0.2 kg/ha); VectoBac® CG (5.0 kg/ha) | 1 week | Limitedb | Unsatisfactoryc | No effect | [50] |
Reported percentage reductions are based on: ahuman biting; badult density; cmalaria transmission; and dEIR
e1 week for Vectobac® and 2 weeks for Vectolex®
fEstimated from reduction in EIR from 0.238 to 0.143
gZaire: now The Democratic Republic of the Congo
Notes: Tested product (potency): VectoBac® WDG (3000 ITU/mg); Vectobac® CG (200 ITU/mg); VectoLex® WDG (650 ITU/mg); Vectolex® CG (50 ITU/mg); VectoLex® G (200 ITU/mg)
Abbreviations: briq, briquettes; CG, corn granules; G, granules; ITU, international toxic units; WDG, water-dispersible granules; EIR, entomological inoculation rate
Safety, cost effectiveness and acceptability
Five of the reviewed studies evaluated the safety of Bti and/or Bs to non-target organism co-habiting with mosquito larvae in natural larval habitats. Of these, 4 [23–26] reported that the products were fairly safe to the non-target organisms whereas the fifth [27] indicated that Bti caused mortalities in Psychodidae larvae. Six studies evaluated the economic costs of implementing bacterial larvicides interventions in the tropical conditions of SSA (Table 8). The costs varied greatly depending on the ecology of the vectors, the larvicide product deployed and the size of the human population covered by the intervention. The cost per person protected per year (PPPY) varied from USD 0.44 in Ouagadougou, Burkina Faso to USD 2.50 in Mbita, Kenya. The cost PPPY was relatively higher in the rural (range of USD 0.77–2.50) than in the urban settings (range of USD 0.44–0.94). Five of the reviewed studies monitored the acceptability of microbial larvicide interventions to the community members and concluded that they were highly accepted by the general community [23, 28–31]. However, challenges related to accessing larval habitats in people’s compounds were reported from the large-scale larviciding intervention conducted in Dar es Salaam, Tanzania [32]. In general, Bti and/or Bs larvicide products were perceived as relatively easy to use and suitable to apply with hand and/or conventional sprayers.
Table 8.
Cost (in USD) of bacterial larvicide interventions for malaria control in sub-Saharan Africa
| Country (costing year) |
Location (settings) | Population protected | Larvicide (potency) | Product (formulation) | Targeting strategy | Average annual costs | Cost PPPY | References |
|---|---|---|---|---|---|---|---|---|
| Tanzania (2006) | Dar es Salaam (urban) | 592338 | Bti (200 ITU/mg) | VectoBac® (CG) | None | 559476.3d | 0.94 | [94] |
| Kenya (2006) | Vihiga (rural) | 609324 | Bti (200 ITU/mg) | VectoBac® (CG) | Spatial and temporala | 916908d | 1.50 | [94] |
| Kenya (2006) | Vihiga (rural) | 609324 | Bti (3000 ITU/mg) | VectoBac® (WDG) | Spatial and temporala | 480735d | 0.79 | [94] |
| Kenya (2006) | Mbita (rural) | 55558 | Bti (200 ITU/mg) | VectoBac® (CG) | Spatialb | 138866d | 2.50 | [94] |
| Kenya (2006) | Mbita (rural) | 55558 | Bti (3000 ITU/mg) | VectoBac® (WDG) | Spatialb | 107669d | 1.94 | [94] |
| Burkina Faso (2013) | Nouna (rural) | 156000 | Bti (3000 ITU/mg) | VectoBac® (WDG) | None | 163038 | 1.05 | [52] |
| Burkina Faso (2013) | Nouna (rural) | 156000 | Bti (3000 ITU/mg) | VectoBac® (WDG) | Spatialc | 120239 | 0.77 | [52] |
| Tanzania (2014) | Mvomero (rural) | 37083 | Bti (not stated) | VectoBac® (CG) | None | 53782.53 | 1.44 | [93] |
| Tanzania (2012) | Dar es Salaam (urban) | 6875784 | Bti (200 ITU/mg) | VectoBac® (CG) | None | 5111234 | 0.87 | [92] |
| Kenya (2005) | Mbita (rural) | 8000 | Bti (200 and3000) + Bs (50 and 650 ITU/mg) | VectoBac® (WDG & CG) + VectoLex® (WDG & CG) | Spatialb | 6773–7026 | 0.85–0.89 | [49] |
| Burkina Faso (1999) | Bobo-Dioulasso (urban) | 19245 | Bs (not stated) | VectoLex® (WDG); Spherimos (FC); Locally made (SRG) | None | 8400e | 0.44 | [95] |
| Burkina Faso (1999) | Ouagadougou (urban) | 17776 | Bs (not stated) | VectoLex® (WDG), Spherimos (FC), Locally made (SRG) | None | 8400e | 0.47 | [95] |
aIntervention in valley bottoms during the main rainy season
bIntervention covering two third of the populated lowlands
cTargeted application of 50% of the most productive habitats
dMid-point published price of larvicide
eCosting included interventions to control both Anopheles gambiae and Culex quinquefasciatus (1€ was approximately 1 USD in the costing year)
Abbreviations: PPPY, per person protected per year; Bti, Bacillus thuringiensis var. israelensis; Bs, Bacillus sphaericus; ITU, international toxic units; CG, custom granules; WDG, water-dispersible granules; SRG, sustained-release granular; FC, fluid concentrate
Discussion
After several years of encouraging reports on global malaria decline [33], the 2018 world malaria report indicated that no further significant progress in reducing global malaria cases was made during the 2015–2017 time frame [34]. The persisting malaria transmission occurs despite implementation in time and space of widely effective malaria control interventions, mainly anti-malarial drugs and insecticide-based vector control methods [34]. With the observed resilience in malaria transmission, the current control interventions need to be complemented with other novel methods in an integrated manner to further reduce the malaria burden. Larval source management is an important strategy in malaria control and its potential to lower malaria transmission has been well documented [8, 11, 35–37]. When integrated with adult mosquito control interventions, such as LLINs or IRS, the strategy has been found to have a complementary role in lowering malaria transmission [10]. The present article reviews the available literature on implementation of bacterial larvicides for malaria vector control in sub-Saharan Africa (SSA) to provide an informed background for designing larval source management using these agents.
Reduction in malaria burden and intensity of parasite transmission [expressed as entomological inoculation rate (EIR), a measure of infectious bite per person per unit time] recorded over the past two decades is an important epidemiological juncture to intensify malaria control measures. It has been shown that once EIRs fall below one infectious bite per person per year, malaria burden becomes much more responsive to further reductions in transmission [38]. Thus, as malaria continues to decline, larviciding interventions may have a much greater epidemiological impact as a supplementary intervention, secondary to primary front-line options like LLINs/IRS. Moreover, larviciding becomes a more feasible intervention for IVM once malaria transmission has been reduced to low and moderate levels by LLINs/IRS or once these tools have reached their maximum practical effect [14]. Recognizing the limitations of the primary front-line vector control tools and reduced progress of malaria control recorded in recent years, call for accelerated development and adoption of diverse options available for malaria vector control [39].
The reviewed studies on bacterial larviciding conducted in field conditions were carried out in typical Anopheles breeding habitats found in SSA and targeted the main malaria vectors An. gambiae (s.l.) and An. funestus. The laboratory and semi-field studies targeted the same vector species. In this region, An. gambiae (s.l.) is known to breed in clear, temporary water bodies exposed to direct sunlight, whereas An. funestus prefers semi-permanent to permanent water bodies with some degree of shading [40]. To be effective, bacterial larvicide interventions require that the habitats that produce malaria vectors are targeted continuously and for indefinite basis. Due to the ephemeral nature of Anopheles larval habitats, identifying and targeting these numerous larval habitats have been considered important challenges of larviciding interventions [13]. However, the advancements in geographical information system (GIS) technology, satellite imagery and drone-based multispectral imagery, have made mapping and generation of high-resolution geo-referenced landscape images possible [41–43]. With these new technologies, larval habitats can be relatively easily identified, mapped and targeted for larviciding, thereby overcoming the constraints of the traditional laborious methods of identifying and mapping the habitats.
Various bacterial larvicide products were tested in the reviewed studies, including most of the products available in the market or developed for mosquito control [12, 17]. WDG and CG formulations were the most preferred and these were also used in the large-scale control programmes. While the CG were easily applied by hand and were suitable in larval habitats with dense vegetation, WDG had a lower application rate due to their high toxin content (measured in international toxic units per milligram, ITU/mg). These properties also had implications for transport and storage costs. The newly formulated bacterial larvicides based on granules, tablets and briquettes were designed to offer flexibility in the application in different larval habitat types which vary in their physical characteristics and larvae productivity [12]. The reviewed articles indicated that products based on Bti and/or Bs were fairly easy to apply by hand or with conventional sprayers depending on the formulation and habitat characteristics. Although the larvicidal activity of used products is known to vary with mosquito species, the reviewed studies indicated that they were generally effective in controlling An. gambiae (s.l.), An. funestus (s.l.) and culicine mosquitoes.
The findings of studies implemented in diverse ecological conditions across SSA indicated that at low application rates, bacterial larvicides based on Bti and/or Bs were effective in controlling malaria vectors. The reported effectiveness of these agents in mosquito control corroborates well with findings of other studies conducted elsewhere outside SSA [44–46]. It was found that Bti and/or Bs caused a reduction in larval density, vector density, vector biting and malaria transmission in most of the tested areas. However, due to their short duration of activity, repeated applications at weekly or bi-weekly intervals were required to sustain control. On the other hand, products based on sustained slow release formulations showed relatively high residual activity ranging from 3–6 months in selected larval habitats [47, 48]. Moreover, the reviewed studies showed that the efficacy and residual activity of Bti and/or Bs on malaria vectors varied considerably with the prevailing ecological settings of the study site, the test products, as well as the study design. The activity of Bti and Bs is known to be influenced by factors related to the target mosquito, larval habitat conditions and larvicide properties as reported elsewhere [17]. The inherent variation in their activity in different ecological settings needs to be taken into account when designing and scaling-up larvicide interventions.
Many typical larval habitats for malaria vector mosquitoes, particularly the all-important An. gambiae complex in SSA, are in nature transient and shifting [40]. Most of these habitats originate from a wide range of economic important human activities such as agriculture, construction and mining [49]. Although some of the habitats are relatively more permanent and may contain some water for the most of the year, their size and, more importantly, the location of water margin where most of mosquito breeding activities take place fluctuates from week to week depending on weather conditions (mainly rain and sun). In addition to natural forces of the weather, man-made habitats are constantly modified to serve the purpose of which were created for, during cultivation and resumption of construction or mining activities. These activities may end up creating more new habitats or eliminate some altogether. Thus, many active larval habitats for malaria mosquitoes are not always static, but sometimes dynamic and a moving target. For this reason, irrespective of the residuality of the product applied, treated sites must be visited on a regular basis to identify and treat new active larval habitats that may have arisen or to re-treat the existing ones which have been affected by human activities. For this case, residuality is less valuable than it would otherwise be because of the duration and nature of the habitats themselves. Although long-lasting, slow release formulations of larvicides are desirable, less persistent conventional products have wide application in tropical weather conditions and more appropriate for the transient An. gambiae complex larval habitats.
Although the reviewed studies demonstrated the effectiveness of the bacterial larvicides based on Bti and Bs in malaria vector control, the products were found to be less effective in riverine areas with extensive flooding in The Gambia [50], in rice fields and swamps in Zaire (now The Democratic Republic of the Congo) [51], in transient rain puddles in Burkina Faso [27] and in overgrown wetlands in Tanzania [23]. These findings support the view that manual application of bacterial larvicides Bti and/or Bs by ground teams is not a strategy for all larval habitat types [14]. However, as malaria prevalence continues to decline, high transmission areas are attaining low to focal transmission, creating more conducive conditions appropriate for larviciding intervention. If sustained, the decline in malaria parasite transmission intensity creates an important opportunity for adoption of larviciding as a supplementary measure, though the strategy may not be suitable as a stand-alone intervention in many transmission settings. Moreover, it was evident from the reviewed studies that effective control of mosquito larvae can be achieved with repeated treatment of breeding sites and that malaria vector control with bacterial larvicides demands much greater ecological information with regard to water quality and the nature of the mosquito breeding habitats. It was also evident that larvicide intervention was more cost-effective in urban than in rural areas. To be effective, larviciding intervention with Bti and Bs needs to be well adapted to the prevailing local malaria vectors and their ecology.
High implementation costs have been considered as the main factor that prevents broader use of larvicide interventions particularly in SSA [13, 52]. Despite variation in the cost of these interventions reported in different areas of SSA, the cost compared favorably with those for LLINs and IRS interventions [53–55]. Some of the reviewed studies indicated that the interventions based on Bti and/or Bs were readily accepted by the general community in the intervention areas [23, 28–31]. In addition to participation in the larvicide applications, some community members indicated willingness to pay for the intervention [28–31]. Moreover, the safety of the tested products was found to be high [23–26], targeting only mosquito larvae and with no deleterious effect to non-target organisms, as also reported in other studies conducted outside SSA [56–58]. However, one of the reviewed articles reported that Bti treatment caused mortalities in Psychodidae larvae when applied at the recommended rate [27]. Species of the Psychodidae are among the dipterans that have been shown to be affected by Bti treatment as summarized elsewhere [59].
Larviciding intervention, particularly for control of mosquito with diverse breeding habitats like the major malaria vector in SSA, is labor-intensive undertaking. To be effective, larviciding teams must cover a large number of Anopheles larval habitats, some of which appear and disappear frequently in space and time with high-cost implications. In SSA, financial resources (for equipment, supplies and personnel costs) required to manage larviciding programmes remain intermittent and unreliable [60, 61]. Thus, mobilizing reliable sources of funding is crucial for the success of larvicide interventions. It was also found out that larvicides application by ground teams in rural areas with extensive larval habitats was inappropriate [50]. In areas with large flood plains, extensive wetland and rice cultivation which are largely inaccessible on foot, aerial application of larvicides is more appropriate. Further analysis of the reviewed field studies has shown that a wide range of larvicide products including non-WHOPES-recommended products [14] was evaluated in SSA (Tables 6, 7). This variation in product types tested coupled with heterogeneity in test conditions, did not permit unambiguous analysis of the efficacy of the interventions by geographical settings and/or malaria vector species. While more countries in SSA are adopting (larval source management, LSM) for control of malaria, it is crucial that a package of products for larviciding is streamlined based on WHOPES recommendations to allow for appropriate scale-up of the intervention in the future.
Apart from financial constraints, other major obstacles to delivering larviciding interventions to local communities in SSA include scarcity of trained personnel in the field of vector ecology/biology and lack of organizational structures for governance and management of vertical, decentralized LSM programmes [39, 62]. While it is crucial that these rare specialties are developed, a partnership between academic/research institutions and communities have been applied effectively to fill this technical gap in the implementation of larviciding or other vector control programmes in SSA [60, 62]. With the renewed interest in LSM in SSA, there is an urgent need to develop operational teams and robust organization structures for governance of these programmes in the near future [39, 60, 63]. Beside the outlined shortfalls, SSA is better positioned with adequate human resources to manage labor-intensive larval source management programmes. The availability of effective and yet safe microbial larvicides is making larviciding interventions feasible with such community-based staff with a minimal level of training.
Based on the effectiveness of larvicide products reported in the reviewed studies (Tables 6, 7), the historical success of larvicide interventions for malaria vector control [8, 11] and expert opinions [13, 37], larviciding remains a feasible option that can be included in the IVM programmes to supplement other vector control tools. Following decades of neglect of this strategy in SSA, research is still needed to improve quality of evidence and build skills especially in areas of malaria vector ecology, designing, monitoring and evaluation of larval source management programmes.
Conclusions
The findings from the reviewed studies indicated that, at low application rates, bacterial larvicide products based on Bti and Bs were effective against malaria vectors in SSA. Furthermore, the larvicide intervention was found to be feasible and safe to non-target organisms and its cost compared fairly well with those of other vector control measures practiced in SSA. However, interventions based on these agents require substantial knowledge of larval ecology due to the effect of environment and larval habitat characteristics on these agents. As malaria continues to decline in SSA, larviciding should gain more ground due to shrinking of transmission areas and creation of more appropriate conditions for the intervention, and in order to delay the evolution of insecticide resistance and behavioral adaptations by the vectors. Moreover, the advancement of technology for mapping landscapes could facilitate the identification and targeting the numerous larval habitats preferred by the African malaria vectors. To build sustainable anti-larval measures in SSA, there is a great need to build capacity in relevant specialties in vector control and develop organizational structures for governance and management of larval source management programmes.
Acknowledgements
We are grateful to Dr Paul Erik Simonsen from the University of Copenhagen, Denmark for constructive comments to the manuscript.
Abbreviations
- Bti
Bacillus thuringiensis var. israelensis
- Bs
Bacillus sphaericus
- ITU
international toxic units
- WDG
water-dispersible granules
- CG
corn granules
- G
granules
- FC
flowable concentrate
- WP
wettable powder
- SRG
sustained-release granular
- WBS
water-based suspension
- PP
primary powder
- TP
technical powder
- LC
liquid concentrate
- AS
aqueous suspension
- LLINs
long-lasting insecticide-treated nets
- IRS
indoor residual spraying
- SSA
sub-Saharan Africa
- IVM
integrated vector management
- GIS
geographical information system
- EIR
entomological inoculation rate
- PPPY
person protected per year
- WHOPES
World Health Organization Pesticides Evaluation Scheme
- LSM
larval source management
Authors’ contributions
YAD, FWM conceived and designed the review. YAD conducted literature search and drafted the manuscript with inputs from EJK, FWM, AKG and WNK. All authors read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
All relevant data supporting the conclusions of this article are included within the article.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yahya A. Derua, Email: yaderua@gmail.com
Eliningaya J. Kweka, Email: eliningaya.kweka@tpri.go.tz
William N. Kisinza, Email: wkisinza@nimr.or.tz
Andrew K. Githeko, Email: githeko@yahoo.com
Franklin W. Mosha, Email: fwmosha@gmail.com
References
- 1.Bhatt S, Weiss DJ, Cameron E, Bisanzio D, Mappin B, Dalrymple U, et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature. 2015;526:207–211. doi: 10.1038/nature15535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kleinschmidt I, Bradley J, Knox TB, Mnzava AP, Kafy HT, Mbogo C, et al. Implications of insecticide resistance for malaria vector control with long-lasting insecticidal nets: a WHO-coordinated, prospective, international, observational cohort study. Lancet Infect Dis. 2018;18:640–649. doi: 10.1016/S1473-3099(18)30172-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hancock PA, Wiebe A, Gleave KA, Bhatt S, Cameron E, Trett A, et al. Associated patterns of insecticide resistance in field populations of malaria vectors across Africa. Proc Natl Acad Sci USA. 2018;115:5938–5943. doi: 10.1073/pnas.1801826115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Protopopoff N, Mosha JF, Lukole E, Charlwood JD, Wright A, Mwalimu CD, et al. Effectiveness of a long-lasting piperonyl butoxide-treated insecticidal net and indoor residual spray interventions, separately and together, against malaria transmitted by pyrethroid-resistant mosquitoes: a cluster, randomised controlled, two-by-two factorial design trial. Lancet. 2018;391:1577–1588. doi: 10.1016/S0140-6736(18)30427-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sougoufara S, Doucouré S, Sembéne PMB, Harry M, Sokhna C. Challenges for malaria vector control in sub-Saharan Africa: resistance and behavioral adaptations in Anopheles populations. J Vector Borne Dis. 2017;54:4–15. [PubMed] [Google Scholar]
- 6.Kamdem C, Fouet C, Gamez S, White BJ. Pollutants and insecticides drive local adaptation in African malaria mosquitoes. Mol Biol Evol. 2017;34:1261–1275. doi: 10.1093/molbev/msx087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Killeen GF, Ranson H. Insecticide-resistant malaria vectors must be tackled. Lancet. 2018;391:1551–1552. doi: 10.1016/S0140-6736(18)30844-4. [DOI] [PubMed] [Google Scholar]
- 8.Killeen GF, Fillinger U, Kiche I, Gouagna LC, Knols BGJ. Eradication of Anopheles gambiae from Brazil: lessons for malaria control in Africa? Lancet Infect Dis. 2002;2:618–627. doi: 10.1016/S1473-3099(02)00397-3. [DOI] [PubMed] [Google Scholar]
- 9.Killeen GF, Fillinger U, Knols BGJ. Advantages of larval control for African malaria vectors: low mobility and behavioural responsiveness of immature mosquito stages allow high effective coverage. Malar J. 2002;1:8. doi: 10.1186/1475-2875-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fillinger U, Ndenga B, Githeko A, Lindsay SW. Integrated malaria vector control with microbial larvicides and insecticide-treated nets in western Kenya: a controlled trial. Bull World Health Organ. 2009;87:655–665. doi: 10.2471/BLT.08.055632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shousha AT. Species-eradication. The eradication of Anopheles gambiae from Upper Egypt 1942–1945. Bull World Health Organ. 1948;1:309–342. [PMC free article] [PubMed] [Google Scholar]
- 12.Walker K, Lynch M. Contributions of Anopheles larval control to malaria suppression in tropical Africa: review of achievements and potential. Med Vet Entomol. 2007;21:2–21. doi: 10.1111/j.1365-2915.2007.00674.x. [DOI] [PubMed] [Google Scholar]
- 13.Fillinger U, Lindsay SW. Larval source management for malaria control in Africa: myths and reality. Malar J. 2011;10:353. doi: 10.1186/1475-2875-10-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.WHO . a supplementary measure for malaria control. An operational manual. Geneva: World Health Organization; 2013. [Google Scholar]
- 15.WHO. World Health Assembly Resolution 50.13: Promotion of chemical safety, with special attention to persistent organic pollutants. Geneva: World Health Organization; 1997. https://www.who.int/ipcs/publications/wha/whares_53_13/en/. Accessed 1 Mar 2019.
- 16.Mittal PK. Biolarvicides in vector control: challenges and prospects. J Vector Borne Dis. 2003;40:20–32. [PubMed] [Google Scholar]
- 17.Lacey LA. Bacillus thuringiensis serovariety israelensis and Bacillus sphaericus for mosquito control. Am Mosq Control Assoc. 2007;23:133–163. doi: 10.2987/8756-971X(2007)23[133:BTSIAB]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 18.Barbazan P, Baldet T, Darrieit E, Escaffre H, Haman DD, Hougard J-M. Impact of treatments with Bacillus sphaericus on Anopheles populations and the transmission of malaria in Maroua, a large city in a Savannah Region of Cameroon. J Am Mosq Control Assoc. 1998;14:33–39. [PubMed] [Google Scholar]
- 19.Kandyata A, Mbata KJ, Shinondo CJ, Katongo C, Kamuliwo RM, Nyirenda F, et al. Impacts of Bacillus thuringiensis var. israelensis and Bacillus sphaericus insect larvicides on mosquito larval densities in Lusaka, Zambia. Med J Zambia. 2012;39:33–38. [Google Scholar]
- 20.Nicolas L, Darriet F, Hougard JM. Efficacy of Bacillus sphaericus 2362 against larvae of Anopheles gambiae under laboratory and field conditions in West Africa. Med Vet Entomol. 1987;1:157–162. doi: 10.1111/j.1365-2915.1987.tb00337.x. [DOI] [PubMed] [Google Scholar]
- 21.Derua YA, Kahindi SC, Mosha FW, Kweka EJ, Atieli HE, Zhou G, et al. Susceptibility of Anopheles gambiae complex mosquitoes to microbial larvicides in diverse ecological settings in western Kenya. Med Vet Entomol. 2019;33:220–227. doi: 10.1111/mve.12353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kinde-Gazard D, Baglo T. Assessment of microbial larvicide spraying with Bacillus thuringiensis israelensis, for the prevention of malaria. Med Mal Infect. 2012;42:114–118. doi: 10.1016/j.medmal.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 23.Magesa SM, Athumani Y, Barongo V, Sambu EZ, Senkoro KP, Mboera LEG, et al. Efficacy of Bacillus thuringiensis var. israelensis (Bactivec®) and Bacillus sphaericus (Griselesf®) for control of mosquito larvae: a field trial in Mvomero and Bagamoyo districts. Dar es Salaam: National Institute for Medical Research; 2009. [Google Scholar]
- 24.Derua YA, Kahindi SC, Mosha FW, Kweka EJ, Atieli HE, Wang X, et al. Microbial larvicides for mosquito control: impact of long lasting formulations of Bacillus thuringiensis var. israelensis and Bacillus sphaericus on non-target organisms in western Kenya highlands. Ecol Evol. 2018;8:7563–7573. doi: 10.1002/ece3.4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ravoahangimalala O, Thiery I, Sinegre G. Rice field efficacy of deltamethrin and Bacillus thuringiensis israelensis formulations on Anopheles gambiae s.s. in the Anjiro Region of Madagascar. Bull Soc Vector Ecol. 1994;19:169–174. [Google Scholar]
- 26.Ragoonanansingh RN, Njunwa KJ, Curtis CF, Becker N. A field study of Bacillus sphaericus for the control of culicine and anopheline mosquito larvae in Tanzania. Bull Soc Vector Ecol. 1992;17:45–50. [Google Scholar]
- 27.Skovmand O, Sanogo E. Experimental formulations of Bacillus sphaericus and B. thuringiensis israelensis against Culex quinquefasciatus and Anopheles gambiae (Diptera: Culicidae) in Burkina Faso. J Med Entomol. 1999;36:62–67. doi: 10.1093/jmedent/36.1.62. [DOI] [PubMed] [Google Scholar]
- 28.Mboera LEG, Kramer RA, Miranda ML, Kilima SP, Shayo EH, Lesser A. Community knowledge and acceptance of larviciding for malaria control in a rural district of east-central Tanzania. Int J Environ Res Public Health. 2014;11:5137–5154. doi: 10.3390/ijerph110505137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ingabire CM, Hakizimana E, Rulisa A, Kateera F, Van Den Borne B, Muvunyi CM, et al. Community-based biological control of malaria mosquitoes using Bacillus thuringiensis var. israelensis (Bti) in Rwanda: community awareness, acceptance and participation. Malar J. 2017;16:399. doi: 10.1186/s12936-017-2046-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mazigo HD, Massawe IS, Rumisha SF, Kweka EJ, Mboera LEG. Rice farmers’ perceptions and acceptability in the use of a combination of biolarvicide (Bacillus thuringiensis var. israeliensis) and fertilizers application for malaria control and increase rice productivity in a rural district of central Tanzania. Malar J. 2019;18:71. doi: 10.1186/s12936-019-2697-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dambach P, Jorge MM, Traoré I, Phalkey R, Sawadogo H, Zabré P, et al. A qualitative study of community perception and acceptance of biological larviciding for malaria mosquito control in rural Burkina Faso. BMC Public Health. 2018;18:399. doi: 10.1186/s12889-018-5299-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Geissbühler Y, Kannady K, Chaki PP, Emidi B, Govella NJ, Mayagaya V, et al. Microbial larvicide application by a large-scale, community-based program reduces malaria infection prevalence in urban Dar es Salaam, Tanzania. PLoS ONE. 2009;4:e5107. doi: 10.1371/journal.pone.0005107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.WHO . World malaria report 2017. Geneva: World Health Organization; 2017. [Google Scholar]
- 34.WHO . World malaria report 2018. Geneva: World Health Organization; 2018. [Google Scholar]
- 35.Utzinger J, Tozan Y, Singer BH. Efficacy and cost-effectiveness of environmental management for malaria control. Trop Med Int Health. 2001;6:677–687. doi: 10.1046/j.1365-3156.2001.00769.x. [DOI] [PubMed] [Google Scholar]
- 36.Utzinger J, Tozan Y, Doumani F, Singer BH. The economic payoffs of integrated malaria control in the Zambian Copperbelt between 1930 and 1950. Trop Med Int Health. 2002;7:657–677. doi: 10.1046/j.1365-3156.2002.00916.x. [DOI] [PubMed] [Google Scholar]
- 37.Tusting LS, Thwing J, Sinclair D, Fillinger U, Gimnig J, Bonner KE, et al. Mosquito larval source management for controlling malaria. Cochrane Database Syst Rev. 2015;8:CD008923. doi: 10.1002/14651858.CD008923.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beier JC, Killeen GF, Githure JI. Short report: entomologic inoculation rates and Plasmodium falciparum malaria prevalence in Africa. Am J Trop Med Hyg. 1999;61:109–113. doi: 10.4269/ajtmh.1999.61.109. [DOI] [PubMed] [Google Scholar]
- 39.Killeen GF, Tatarsky A, Diabate A, Chaccour CJ, Marshall JM, Okumu FO, et al. Developing an expanded vector control toolbox for malaria elimination. BMJ Glob Health. 2017;2:e000211. doi: 10.1136/bmjgh-2016-000211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gillies MT, Coetzee M. A supplement to the Anophelinae of Africa south of the Sahara (Afrotropical region) Johannesburg: South Africa Institute for Medical Research; 1987. [Google Scholar]
- 41.Mushinzimana E, Munga S, Minakawa N, Li L, Feng C-C, Bian L, et al. Landscape determinants and remote sensing of anopheline mosquito larval habitats in the western Kenya highlands. Malar J. 2006;5:13. doi: 10.1186/1475-2875-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carrasco-Escobar G, Manrique E, Ruiz-Cabrejos J, Saavedra M, Alava F, Bickersmith S, et al. High-accuracy detection of malaria vector larval habitats using drone-based multispectral imagery. PLoS Negl Trop Dis. 2019;13:e0007105. doi: 10.1371/journal.pntd.0007105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hardy A, Makame M, Cross D, Majambere S, Msellem M. Using low-cost drones to map malaria vector habitats. Parasit Vectors. 2017;10:29. doi: 10.1186/s13071-017-1973-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kumar A, Sharma VP, Sumodan PK, Thavaselvam D. Field trials of biolarvicide Bacillus thuringiensis var. israelensis strain 164 and the larvivorous fish Aplocheilus blocki against Anopheles stephensi for malaria control in Goa, India. J Am Mosq Control Assoc. 1998;14:457–462. [PubMed] [Google Scholar]
- 45.Kroeger A, Horstick O, Riedl C, Kaiser A, Becker N. The potential for malaria control with the biological larvicide Bacillus thuringiensis israelensis (Bti) in Peru and Ecuador. Acta Trop. 1995;60:47–57. doi: 10.1016/0001-706X(95)00101-J. [DOI] [PubMed] [Google Scholar]
- 46.Becker N. Microbial control of mosquitoes: management of the Upper Rhine mosquito population as a model programme. Parasitol Today. 1997;13:485–487. doi: 10.1016/S0169-4758(97)01154-X. [DOI] [PubMed] [Google Scholar]
- 47.Afrane YA, Mweresa NG, Wanjala CL, Gilbreath-III TM, Zhou G, Lee M-C, et al. Evaluation of long-lasting microbial larvicide for malaria vector control in Kenya. Malar J. 2016;15:577. doi: 10.1186/s12936-016-1626-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kahindi SC, Muriu S, Derua YA, Wang X, Zhou G, Lee M-C, et al. Efficacy and persistence of long-lasting microbial larvicides against malaria vectors in western Kenya highlands. Parasit Vectors. 2018;11:438. doi: 10.1186/s13071-018-3009-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fillinger U, Lindsay SW. Suppression of exposure to malaria vectors by an order of magnitude using microbial larvicides in rural Kenya. Trop Med Int Health. 2006;11:1629–1642. doi: 10.1111/j.1365-3156.2006.01733.x. [DOI] [PubMed] [Google Scholar]
- 50.Majambere S, Pinder M, Fillinger U, Ameh D, Conway DJ, Green C, et al. Is mosquito larval source management appropriate for reducing malaria in areas of extensive flooding in the Gambia? A cross-over intervention trial. Am J Trop Med Hyg. 2010;82:176–184. doi: 10.4269/ajtmh.2010.09-0373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Karch S, Asid N, Manzambi M, Salaun JJ. Efficacy of Bacillus sphaericus against the malaria vector Anopheles gambiae and other mosquitoes in swamps and rice fields in Zaire. J Am Mosq Control Assoc. 1992;8:376–380. [PubMed] [Google Scholar]
- 52.Dambach P, Schleicher M, Stahl HC, Traoré I, Becker N, Kaiser A, et al. Routine implementation costs of larviciding with Bacillus thuringiensis israelensis against malaria vectors in a district in rural Burkina Faso. Malar J. 2016;15:380. doi: 10.1186/s12936-016-1438-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yukich J, Tediosi F, Lengeler C. Operations, costs and cost-effectiveness of five insecticide-treated net programs (Eritrea, Malawi, Tanzania, Togo, Senegal) and two indoor residual spray programs (Kwa-Zulu-Natal, Mozambique) Washington: USAID; 2007. [Google Scholar]
- 54.Yukich JO, Lengeler C, Tediosi F, Brown N, Mulligan JA, Chavasse D, et al. Costs and consequences of large-scale vector control for malaria. Malar J. 2008;7:258. doi: 10.1186/1475-2875-7-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Worrall E, Connor S, Thomson M. Improving the cost-effectiveness of IRS with climate informed health surveillance systems. Malar J. 2008;7:263. doi: 10.1186/1475-2875-7-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lacey LA, Merritt RW. The safety of bacterial microbial agents used for black fly and mosquito control in aquatic environments. In: Hokkanen HMT, Hajek AE, editors. Environmental impact of microbial insecticides: need and methods for risk assessment. Dordrecht: Kluwer Academic Publishers; 2003. pp. 151–168. [Google Scholar]
- 57.Merritt RW, Lessard JL, Wessell KJ, Hernandez O, Berg MB, Wallace JR, et al. Lack of effects of Bacillus sphaericus (Vectolex) on nontarget organisms in a mosquito-control program in southeastern Wisconsin: a 3-year study. J Am Mosq Control Assoc. 2005;21:201–212. doi: 10.2987/8756-971X(2005)21[201:LOEOBS]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 58.Lagadic L, Roucaute M, Caquet T. Bti sprays do not adversely affect non-target aquatic invertebrates in French Atlantic coastal wetlands. J Appl Ecol. 2014;51:102–113. doi: 10.1111/1365-2664.12165. [DOI] [Google Scholar]
- 59.Boisvert M, Boisvert J. Effects of Bacillus thuringiensis var. israelensis on target and nontarget organisms: a review of laboratory and field experiments. Biocontrol Sci Technol. 2000;10:517–561. doi: 10.1080/095831500750016361. [DOI] [Google Scholar]
- 60.Fillinger U, Kannady K, William G, Vanek MJ, Dongus S, Nyika D, et al. A tool box for operational mosquito larval control: preliminary results and early lessons from the Urban Malaria Control Programme in Dar es Salaam, Tanzania. Malar J. 2008;7:20. doi: 10.1186/1475-2875-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dambach P, Traoré I, Kaiser A, Sié A, Sauerborn R, Becker N. Challenges of implementing a large scale larviciding campaign against malaria in rural Burkina Faso - lessons learned and recommendations derived from the EMIRA project. BMC Public Health. 2016;16:1023. doi: 10.1186/s12889-016-3587-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mukabana WR, Kannady K, Kiama GM, Ijumba JN, Mathenge EM, Kiche I, et al. Ecologists can enable communities to implement malaria vector control in Africa. Malar J. 2006;5:9. doi: 10.1186/1475-2875-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chaki PP, Kannady K, Mtasiwa D, Tanner M, Mshinda H, Kelly AH, et al. Institutional evolution of a community-based programme for malaria control through larval source management in Dar es Salaam, United Republic of Tanzania. Malar J. 2014;13:245. doi: 10.1186/1475-2875-13-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ketseoglou I, Koekemoer LL, Coetzee M, Bouwer G. The larvicidal efficacy of Bacillus thuringiensis subsp. israelensis against five African Anopheles (Diptera: Culicidae) species. Afr Entomol. 2011;19:146–150. doi: 10.4001/003.019.0110. [DOI] [Google Scholar]
- 65.Seyoum A, Abate D. Larvicidal efficacy of Bacillus thuringiensis var. israelensis and Bacillus sphaericus on Anopheles arabiensis in Ethiopia. World J Microbiol Biotechnol. 1997;13:21–24. doi: 10.1007/BF02770802. [DOI] [Google Scholar]
- 66.Baffour-awuah S, Owusu-Dabo E, Kruppa T, Annan A, Nartey R, Dogbe J, et al. Lysinibacillus sphaericus biolarvicide, an efficacious tool in the control of Anopheles gambiae in Kumasi, Ghana. East Afr J Public Health. 2014;11:851–861. [Google Scholar]
- 67.Fillinger U, Knols BGJ, Becker N. Efficacy and efficiency of new Bacillus thuringiensis var. israelensis and Bacillus sphaericus formulations against Afrotropical anophelines in western Kenya. Trop Med Int Health. 2003;8:37–47. doi: 10.1046/j.1365-3156.2003.00979.x. [DOI] [PubMed] [Google Scholar]
- 68.Nartey R, Owusu-Dabo E, Kruppa T, Baffour-Awuah S, Annan A, Oppong S, et al. Use of Bacillus thuringiensis var. israelensis as a viable option in an integrated malaria vector control programme in the Kumasi Metropolis, Ghana. Parasit Vectors. 2013;6:116. doi: 10.1186/1756-3305-6-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Majambere S, Lindsay SW, Green C, Kandeh B, Fillinger U. Microbial larvicides for malaria control in The Gambia. Malar J. 2007;6:76. doi: 10.1186/1475-2875-6-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Majori G, Ali A, Sabatinelli G. Laboratory and field efficacy of Bacillus thuringiensis var. israelensis and Bacillus sphaericus against Anopheles gambiae s.l. and Culex quinquefasciatus in Ouagadougou, Burkina Faso. J Am Mosq Control Assoc. 1987;3:20–25. [PubMed] [Google Scholar]
- 71.Dambach P, Louis VRV, Kaiser A, Ouedraogo S, Sie A, Sauerborn R, et al. Efficacy of Bacillus thuringiensis var. israelensis against malaria mosquitoes in northwestern Burkina Faso. Parasit Vectors. 2014;7:371. doi: 10.1186/1756-3305-7-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Demissew A, Balkew M, Girma M. Larvicidal activities of chinaberry, neem and Bacillus thuringiensis israelensis (Bti) to an insecticide resistant population of Anopheles arabiensis from Tolay, Southwest Ethiopia. Asian Pac J Trop Biomed. 2016;6:554–561. doi: 10.1016/j.apjtb.2016.03.013. [DOI] [Google Scholar]
- 73.Zogo B, Tchiekoi BNC, Koffi AA, Dahounto A, Ahoua Alou LP, Dabiré RK, et al. Impact of sunlight exposure on the residual efficacy of biolarvicides Bacillus thuringiensis israelensis and Bacillus sphaericus against the main malaria vector, Anopheles gambiae. Malar J. 2019;18:55. doi: 10.1186/s12936-019-2687-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Diédhiou SM, Konaté L, Doucouré S, Samb B, Niang EA, Sy O, et al. Effectiveness of three biological larvicides and of an insect growth regulator against Anopheles arabiensis in Senegal. Bull Soc Pathol Exot. 2016;110:102–115. doi: 10.1007/s13149-016-0531-4. [DOI] [PubMed] [Google Scholar]
- 75.Djènontin A, Pennetier C, Zogo B, Soukou KB, Ole-Sangba M, Akogbéto M, et al. Field efficacy of vectobac GR as a mosquito larvicide for the control of anopheline and culicine mosquitoes in natural habitats in Benin, West Africa. PLoS ONE. 2014;9:e87934. doi: 10.1371/journal.pone.0087934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Imbahale SS, Githeko A, Mukabana WR, Takken W. Integrated mosquito larval source management reduces larval numbers in two highland villages in western Kenya. BMC Public Health. 2012;12:362. doi: 10.1186/1471-2458-12-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kahindi SC, Midega JT, Mwangangi JM, Kibe LW, Nzovu J, Luethy P, et al. Efficacy of vectobac DT and Culinexcombi against mosquito larvae in unused swimming pools in Malindi, Kenya. J Am Mosq Control Assoc. 2008;24:538–542. doi: 10.2987/5734.1. [DOI] [PubMed] [Google Scholar]
- 78.Karch S, Manzambi ZA, Salaun JJ. Field trials with Vectolex (Bacillus sphaericus) and Vectobac (Bacillus thuringiensis (H-14)) against Anopheles gambiae and Culex quinquefasciatus breeding in Zaire. J Am Mosq Control Assoc. 1991;7:176–179. [PubMed] [Google Scholar]
- 79.Mpofu M, Becker P, Mudambo K, De Jager C. Field effectiveness of microbial larvicides on mosquito larvae in malaria areas of Botswana and Zimbabwe. Malar J. 2016;15:586. doi: 10.1186/s12936-016-1642-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mwangangi JM, Kahindi SC, Kibe LW, Nzovu JG, Luethy P, Githure JI, et al. Wide-scale application of Bti/Bs biolarvicide in different aquatic habitat types in urban and peri-urban Malindi, Kenya. Parasitol Res. 2011;108:1355–1363. doi: 10.1007/s00436-010-2029-1. [DOI] [PubMed] [Google Scholar]
- 81.Obopile M, Segoea G, Waniwa K, Ntebela DS, Moakofhi K, Motlaleng M, et al. Did microbial larviciding contribute to a reduction in malaria cases in eastern Botswana in 2012–2013? PHA. 2018;8(Suppl. 1):50–54. doi: 10.5588/pha.17.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Romi R, Ravoniharimelina B, Ramiakajato M, Majori G. Field trials of Bacillus thuringiensis H-14 and Bacillus sphaericus (strain 2362) formulations against Anopheles arabiensis in the central highlands of Madagascar. J Am Mosq Control Assoc. 1993;9:325–329. [PubMed] [Google Scholar]
- 83.Skovmand O, Bauduin S. Efficacy of a granular formulation of Bacillus sphaericus against Culex quinquefasciatus and Anopheles gambiae in West African countries. J Vector Ecol. 1997;22:43–51. [PubMed] [Google Scholar]
- 84.Skovmand O, Ouedraogo TDA, Sanogo E, Samuelsen H, Toé LP, Baldet T. Impact of slow-release Bacillus sphaericus granules on mosquito populations followed in a tropical urban environment. J Med Entomol. 2009;46:67–76. doi: 10.1603/033.046.0109. [DOI] [PubMed] [Google Scholar]
- 85.Tchicaya ES, Koudou BG, Keiser J, Adja AM, Cisse G, Tanner M, et al. Effect of repeated application of microbial larvicides on malaria transmission in central Cote d’Ivoire. J Am Mosq Control Assoc. 2009;25:382–385. doi: 10.2987/08-5809.1. [DOI] [PubMed] [Google Scholar]
- 86.Zhou G, Afrane YA, Dixit A, Atieli HE, Lee MC, Wanjala CL, et al. Modest additive effects of integrated vector control measures on malaria prevalence and transmission in western Kenya. Malar J. 2013;12:256. doi: 10.1186/1475-2875-12-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kramer RA, Mboera LEG, Senkoro K, Lesser A, Shayo EH, Paul CJ, Miranda ML. A randomized longitudinal factorial design to assess malaria vector control and disease management interventions in rural Tanzania. Int J Environ Res Public Health. 2014;11:5317–5332. doi: 10.3390/ijerph110505317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shililu JI, Tewolde GM, Brantly E, Githure JI, Mbogo CM, Beier JC, et al. Efficacy of Bacillus thuringiensis israelensis, Bacillus sphaericus and temephos for managing Anopheles larvae in Eritrea. J Am Mosq Control Assoc. 2003;19:251–258. [PubMed] [Google Scholar]
- 89.Shililu J, Mbogo C, Ghebremeskel T, Githure J, Novak R. Mosquito larval habitats in a semiarid ecosystem in Eritrea: impact of larval habitat management on Anopheles arabiensis population. Am J Trop Med Hyg. 2007;76:103–110. doi: 10.4269/ajtmh.2007.76.103. [DOI] [PubMed] [Google Scholar]
- 90.Van Den Berg H, Van Vugt M, Kabaghe AN, Nkalapa M, Kaotcha R, Truwah Z, et al. Community-based malaria control in southern Malawi: a description of experimental interventions of community workshops, house improvement and larval source management. Malar J. 2018;17:266. doi: 10.1186/s12936-018-2415-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Msellemu D, Namango HI, Mwakalinga VM, Ntamatungiro AJ, Mlacha Y, Mtema ZJ, et al. The epidemiology of residual Plasmodium falciparum malaria transmission and infection burden in an African city with high coverage of multiple vector control measures. Malar J. 2016;15:288. doi: 10.1186/s12936-016-1340-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Maheu-Giroux M, Castro MC. Cost-effectiveness of larviciding for urban malaria control in Tanzania. Malar J. 2014;13:477. doi: 10.1186/1475-2875-13-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rahman R, Lesser A, Mboera L, Kramer R. Cost of microbial larviciding for malaria control in rural Tanzania. Trop Med Int Health. 2016;21:1468–1475. doi: 10.1111/tmi.12767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Worrall E, Fillinger U. Large-scale use of mosquito larval source management for malaria control in Africa: a cost analysis. Malar J. 2011;10:338. doi: 10.1186/1475-2875-10-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Skovmand O, Ouedraogo TDA, Sanogo E, Samuelsen H, Toé LP, Bosselmann R, et al. Cost of integrated vector control with improved sanitation and road infrastructure coupled with the use of slow-release Bacillus sphaericus granules in a tropical urban setting. J Med Entomol. 2011;48:813–821. doi: 10.1603/ME10041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data supporting the conclusions of this article are included within the article.