Abstract
BACKGROUND:
Prior research demonstrates a protective role for oxytocin in ovarian cancer based on its anti-proliferative, anti-migratory, and anti-invasive effects in vitro and in vivo. However, the role of endogenous oxytocin has not been examined in ovarian cancer patients. Oxytocin also has anti-inflammatory properties that have not been examined in cancer. The purpose of this investigation was to examine relationships between endogenous oxytocin, tumor-associated inflammation (interleukin-6), and survival in advanced epithelial ovarian cancer patients.
METHODS:
Tumor microenvironment (ascites) and plasma oxytocin levels were analyzed via ELISA on extracted samples obtained from 79 patients. In vitro models were used to characterize oxytocin and oxytocin receptor expression in four ovarian cancer cell lines and to investigate direct anti-inflammatory effects of oxytocin on tumor cell secretion of interleukin-6.
RESULTS:
High and variable levels of oxytocin were observed in ascites, up to 200 times greater than in plasma. Higher levels of ascites oxytocin were associated with lower levels of systemic and tumor-associated interleukin-6, an inflammatory cytokine implicated in ovarian tumor progression. Oxytocin also attenuated interleukin-6 secretion from multiple ovarian tumor cell lines in vitro. Higher levels of ascites oxytocin were associated with a significant survival advantage and statistical mediation analyses suggested this effect was partially mediated by interleukin-6.
CONCLUSIONS:
These data identify a previously unacknowledged hormone in the ovarian tumor microenvironment and provide initial evidence that oxytocin has protective effects in ovarian cancer via anti-inflammatory mechanisms. Future studies should examine the therapeutic utility of oxytocin.
Keywords: ovarian neoplasms, oxytocin, tumor microenvironment, interleukin-6, ascites
Precis for use in table of contents:
This work identifies endogenous oxytocin in the ovarian tumor microenvironment and provides initial evidence that oxytocin may have protective effects in ovarian cancer via its anti-inflammatory action.
1. Introduction
The central and peripheral hormone oxytocin has a well-characterized role in female reproductive function (Gimpl and Fahrenholz, 2001) but has been minimally investigated in gynecologic cancers. Some evidence suggests that oxytocin may also play a broader role in health by dampening the stress response (Neumann et al., 2000; Taylor et al., 2000) and promoting the cellular immune response (Li et al., 2017). While oxytocin is typically conceptualized as a neurohypophyseal hormone, it is also synthesized and released at a number of sites peripherally, including the ovaries (Fuchs, 1988; Gimpl and Fahrenholz, 2001).
Oxytocin has been demonstrated to have protective effects in ovarian cancer based on its anti-proliferative, anti-migratory, and anti-invasive effects on ovarian carcinoma cells in vitro and in vivo (Ji et al., 2018; Mankarious et al., 2016; Morita et al., 2004). In vitro, oxytocin has been shown to inhibit ovarian tumor cell proliferation, invasion, and migration (Ji et al., 2018; Morita et al., 2004) and to reduce the tumor-promoting effects of the stress-hormone cortisol (Mankarious et al., 2016). In a xenograft murine model of ovarian cancer, mice treated with intraperitoneal oxytocin exhibited lower tumor burden, less accumulation of ascites, and less peritoneal dissemination of tumor cells than control mice (Morita et al., 2004). In humans, the oxytocin receptor has been identified on several histologic subtypes of ovarian tumors, including serous, mucinous and endometriod (Morita et al., 2004); and is shown to be significantly upregulated by over five-fold on ovarian tumors compared with healthy ovarian tissue (Mankarious et al., 2016). The presence and relative upregulation of oxytocin receptors on human ovarian tumors suggest that human tumors may be responsive to oxytocin and that conditions unique to the ovarian tumor microenvironment may be modulating the receptor. However, the potential role of endogenous oxytocin in the tumor microenvironment has not been examined in ovarian cancer patients.
In addition to its anti-proliferative and anti-metastatic properties, oxytocin has been shown to reduce inflammation in multiple pre-clinical models of disease (Jankowski et al., 2010; Nation et al., 2010; Yeniel et al., 2014). Inflammation plays a key role in ovarian cancer progression, contributing to tumor angiogenesis, peritoneal dissemination and poorer prognosis (Macciò and Madeddu, 2012). However, the anti-inflammatory properties of oxytocin have not been examined in cancer patients or in vitro, despite evidence that oxytocin impacts cancer-relevant inflammatory pathways (e.g. inhibition of interleukin-6) (Jankowski et al., 2010; Nation et al., 2010; Szeto et al., 2008).
To address these gaps in knowledge, this investigation characterized endogenous levels of tumor microenvironment (ascites) and plasma oxytocin in advanced-stage epithelial ovarian cancer patients and examined relationships between oxytocin and interleukin-6 at the time of surgery. We also examined relationships between ascites oxytocin and ovarian cancer survival. In vitro models were used to characterize oxytocin and oxytocin receptor expression in four ovarian cancer cell lines and to investigate potential anti-inflammatory effects of oxytocin on interleukin-6 secretion from tumor cells under basal and stimulated conditions.
2. Method
2.1. Participants
Women with suspected ovarian cancer were recruited from two large Midwestern university hospitals during a pre-surgical clinic visit as part of a larger IRB-approved study on biobehavioral factors and tumor progression. Eligibility for the study was restricted to patients with primary epithelial ovarian, peritoneal or fallopian tube carcinoma. Histological diagnosis was confirmed by pathology. Exclusion criteria included: age under 18 years, history of previous cancer, comorbid condition with known immune system effects, current pregnancy and inability to accurately answer questions (dementia). Inclusion in the current study was restricted to patients with advanced-stage disease and high grade, serous tumors. Of 243 patients meeting these conditions, 79 had ascites samples available for oxytocin and interleukin-6 analysis, and plasma oxytocin was assayed on a subset of 49 of these patients. The final sample included 79 patients with advanced-stage ovarian cancer.
Informed consent was obtained during the patients’ pre-surgical visit and blood was collected in the morning prior to surgery. Samples of ascites fluid were obtained during surgery and taken immediately to the lab for processing. Blood samples were collected in heparinized tubes (BD Biosciences, San Jose, CA.). All samples were centrifuged at 2200 rpm at 20°C for 10 minutes. Ascites supernatant and blood plasma were stored at −80°C until analyzed. Survival time was determined by the number of days between primary surgery and date of death. Survival information was censored on December 30, 2016 or on the date of last known contact. By this date, 59 patients were deceased and 20 were censored.
2.2. Biochemical Assays
Oxytocin immunoreactivity in plasma, ascites, and cell culture supernatant was analyzed by ELISA (Arbor Assays, Ann Arbor, MI) following extraction of samples using C18 Sep-Pak columns (Waters; Milford, MA) as described (Szeto et al., 2011). Oxytocin immunoassay was performed following manufacturer’s instructions; the lower limit of detection was approximately 1 pg/well and the intra and inter-assay coefficient of variance (CV) were < 8% and < 12%, respectively.
Interleukin-6 in plasma and ascites was analyzed by ELISA (R&D Systems, Minneapolis, MN) and in cell culture supernatants using an ELISA from BD Biosciences (Cat # 555220; Franklin Lakes, NJ). The minimum detectable level was less than 7 pg/mL and inter-assay CV ranged from 3.3% to 6.4%. Samples with interleukin-6 levels below the sensitivity of the regular assay were quantified using the R&D high sensitivity ELISA; intra-assay CV was less than 10%.
2.3. Cell cultures
Human ovarian cancer cell lines, OVCAR-8, HEYA8, and OV432 were kindly provided by Dr. Anil Sood (University of Texas MD Anderson Cancer Center) and the SKOV-3 cell line was obtained directly from ATCC. Cell lines were routinely screened for mycoplasma species (GenProbe detection kit; Fisher, Itasca, IL). Ovarian cancer cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, 2 mM glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin at 37°C in a humidified 95% air-5% CO2 incubator. For experiments, cells were seeded in 24-well plates, 60mm or 100-mm dishes at 100,000, 1×106 or 5×106 cells/dish, respectively, and used when cells reached ~80% confluence.
2.4. mRNA Expression
Total RNA (optical density ratio of 260/280 nm between 1.8 – 2.1) was isolated from approximately 106 cells using the RNAeasy kit (Cat no 74186, Qiagen, Valencia, CA) and treated with DNAse I (Cat no. 79254, Qiagen, Valencia, CA) and stored at −80°C. First-strand cDNA synthesis was performed using High Capacity cDNA reverse transcription kit following the manufacturer’s protocol (Applied Biosystems by Thermo Fisher. Cat no: 4374967, Waltham, MA). The reaction was carried using 50 ng RNA in the presence of master mix (RT buffer, dNTP mix, random primers, RNase inhibitor, and reverse transcriptase) incubated at 25°C for 10 min, then at 37°C for 120 min, denatured at 85°C for 5 min and then cooled to 4°C.s Quantitative gene expression by real-time PCR was performed with the TaqMan gene expression assay (Cat no. 4352042, Applied Biosystems by Thermo Fisher). Inventoried primers for polymerase chain reactions (PCR) were from Applied Biosystems; human OXTR (Hs00168573_m1), oxytocin (Hs00792417_g1), human IL-6 (Hs00985639_m1), 18S (Hs03928985_g1). cDNA was amplified with TaqMan Universal PCR Master Mix in 10 μl reactions and run using universal cycling conditions on an Applied Biosystems Step-One Plus RT-PCR System. Non-template controls were also performed to ensure that there was no amplification of genomic DNA. Samples were analyzed in triplicate and were normalized to the reference gene, 18S. The ΔΔCT (threshold cycle; (Pfaffl, 2001)) method was used to analyze changes in gene expression. Relative quantification was expressed as the fold change compared with the appropriate control condition (Schmittgen and Livak, 2008).
2.5. Western Blotting for oxytocin receptor
OXTR protein expression was examined in total cell homogenates as previously described (Szeto et al., 2017). PBS-washed cells were collected by scraping and solubilized in SDS lysis buffer (50 mM Tris, pH 8.6, 1% SDS). Protein was measured with BCA Protein Assay (Pierce, Rockford, IL). For immunoblotting, 10 μg of protein was separated by denaturing and reducing electrophoresis on 10–20% Tris-glycine polyacrylamide gradient gels (Lonza, Walkersville, MD). After separation, proteins were transferred to nitrocellulose and membranes were blocked in Tris-buffered saline containing 5% nonfat powdered milk and 0.1% Tween-20 for 1 h. Samples were probed with polyclonal anti-rabbit OXTR (cat. no. ab181077; Abcam, Cambridge, MA) diluted 1:1,000 in blocking buffer at 4°C overnight. Immunoreactive bands were detected with peroxidase conjugated donkey-anti-rabbit IgG secondary antibody (Jackson ImmunoResearch; West Grove, PA) at a 1:1000 dilution for 1 h then visualized using a chemiluminescent substrate (cat no 1705060S; BioRad, Hercules, CA). Immunoreactive bands were imaged with a FluorChem E analyzer (ProteinSimple, San Jose CA) and bands quantified by densitometry using FluorChem analysis software. To normalize for protein loading, membranes were probed with chicken anti-actin antibody (cat. no. SAB3500350; Sigma, St. Louis, MO) diluted 1:500 and detected using peroxidase conjugated donkey anti-chicken IgY secondary antibody (cat no 703-035-155; Jackson ImmunoResearch; 1:2000 dilution).
2.6. Experiments evaluating the anti-inflammatory effects of oxytocin on ovarian cancer cell lines
To evaluate the effects of oxytocin on interleukin-6 secretion, ovarian tumor cells were incubated with oxytocin (Bachem, Torrance, CA) for six hours under basal or norepinephrine-stimulated conditions. Norepinephrine stimulation was used to enhance an inflammatory challenge as norepinephrine has been demonstrated to enhance interleukin-6 secretion by ovarian tumor cells (Nilsson et al., 2007). In the norepinephrine-stimulated conditions, ovarian tumor cells were concurrently incubated with 10 μM norepinephrine (Sigma, St. Louis, MO) with or without 1 nM oxytocin for 6 hours. The oxytocin dose of 1 nM was chosen because it is near the Kd of the ligand binding to the oxytocin receptor (Gimpl and Fahrenholz, 2001) and it was also in the upper range of oxytocin detected in clinical samples of patients’ ascites fluid. To evaluate the specificity of the oxytocin response, HEYA8 and SKOV-3 cells were incubated with 10 nM of Atosiban (cat. No. A3480, Sigma-Millipore, St. Louis, MO), an oxytocin receptor antagonist, during a six hour incubation in the presence of 10 μM norepinephrine and with or without 1 nM oxytocin.
2.7. Statistical analyses
The Statistical Package for the Social Sciences (V.23, Armonk, NY) was used for analysis of relationships between oxytocin, interleukin-6 and clinical/demographic variables. Distributions were examined for violations of normality and potential outliers. Oxytocin and interleukin-6 values were log10 transformed to normalize their distribution. Pearson correlations were used to examine associations between oxytocin, interleukin-6, and patient age. In vitro results were compared by paired independent t-tests with post hoc Bonferroni correction.
Survival analyses were conducted in MPLUS version 8.0 using Cox proportional hazards models and maximum likelihood estimation. Because there are no existing studies to guide classification of levels of ascites oxytocin in ovarian cancer, patients were divided into high and low oxytocin groups using the distribution of data. Ascites oxytocin was divided into three tertiles; the top tertile was taken as representative of ‘high oxytocin ascites’ (n=26, range 147 −2383 pg/mL) and compared to the bottom two tertiles (n=53, range = 1.7–107 pg/mL). Ancillary analyses were conducted using the log-transformed level of ascites oxytocin as a continuous variable in survival analyses to determine if the results were impacted by the use of tertiles. Assumptions of proportional hazards were tested using scaled Schoenfeld residuals. Each model controlled for patient age and volume of residual disease (optimal/suboptimal debulking). In the first model, only ascites oxytocin was added as a predictor of survival. In the second model, ascites interleukin-6 was also added. In the final model, both variables were included, and a potential mediation effect was tested via bias-corrected bootstrapped confidence intervals (20,000 resamples) around the indirect path between high ascites oxytocin and survival through ascites interleukin-6. This model was designed to determine if any survival advantage associated with ascites oxytocin was linked to lower levels of ascites interleukin-6.
3. Results
The clinical characteristics of participants are shown in Table 1. The mean age of the ovarian cancer patients (N=79) was 59.9 years (±11.9 years). All patients had advanced-stage disease (89.9% Stage III) and high-grade, serous tumors. Average survival time was 3.03 (±2.11) years.
Table 1.
Participant Characteristics
| Characteristica | Ovarian Cancer Patients (N, %) |
|---|---|
| Age, Years, Mean (S.D.; range) | 59.86 (±11.9; 27–83) |
| Race | |
| Caucasian | 74 (93.7%) |
| Black/African American | 3 (3.8%) |
| American Indian/Alaskan Native | 1 (1.3%) |
| Chose not to answer | 1 (1.3%) |
| Ethnicity | |
| Non-Hispanic | 77 (97.5%) |
| Chose not to answer | 2 (2.5%) |
| Stage | |
| III | 71 (89.9%) |
| IV | 8 (10.1%) |
| Grade | |
| High | 79 (100%) |
| Histology | |
| Serous | 79 (100%) |
| Oxytocin pg/mL, Mean (S.D; range) | |
| Plasma (N=49) | 8.85 (±5.0; 1.6–22.6) |
| Ascites | 193.57 (±360.1; 1.7–2383.0) |
| Interleukin-6 pg/mL, Mean (S.D.; range) | |
| Plasma (N=49) | 37.53 (±48.0; 1.7–250.0) |
| Ascites | 7703.50 (±8724.2; 87.9–37687.2) |
| Total Ascites Fluid in mL, Mean (S.D.; range) | 2518.90 (±2044.7; 15.0–8000.0) |
N is 79 for each characteristic unless otherwise noted
3.1. Characterization of endogenous oxytocin levels and associations with interleukin-6
High and variable levels of oxytocin were identified in ascites (mean=193.57±360.1 pg/mL; range=1.7–2383.0 pg/mL), over 20 times the average level of plasma oxytocin in this sample (mean=8.85±5.0 pg/mL; range=1.6–22.6 pg/mL). Plasma oxytocin levels were relatively high compared to population norms of peripheral blood extracted and analyzed with similar methods from healthy, non-pregnant, non-lactating women, values which typically range from 1–5pg/mL (Leng and Sabatier, 2016; McCullough et al., 2013). Levels of oxytocin in plasma and ascites were not related (r=.100, p=.493). Neither plasma nor ascites oxytocin levels were related to patient age (plasma: r=.222, p=.125; ascites: r= −.092, p=.419).
Higher levels of ascites oxytocin were associated with lower ascites interleukin-6 (r=−.323, p=.004) and lower plasma interleukin-6 (r=−.251, p=.036). Plasma oxytocin was not related to interleukin-6 in plasma (r=.092, p=.527) or in ascites (r=−.001, p=.993).
3.2. Oxytocin and survival
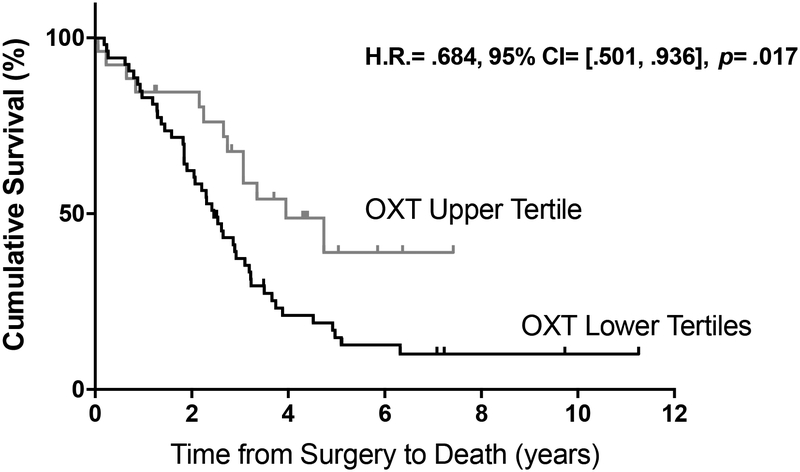
In a multivariate Cox model controlling a priori for volume of residual disease and patient age, higher levels of oxytocin in ascites were independently associated with a decreased risk of death from disease (Figure 1; top tertile vs. bottom 2 tertiles; b=−.379, H.R.=.684, 95% CI=[.501, .936], p=.017). Since all patients had advanced-stage, high-grade serous ovarian carcinomas (Table 1), the survival differences were not confounded by these variables. Similar effects were observed when ascites oxytocin was treated as a continuous predictor: (b=−.286, H.R.=.751, 95% CI=[.576, .979], p=.034). When interleukin-6 was entered as a covariate in the model, there was a significant indirect (i.e., mediated) effect of high ascites oxytocin on survival, through lower ascites interleukin-6 (b=−.127, H.R.=.881, bootstrapped 95% CI =[.702, .985]). These findings suggest that the effect of oxytocin on survival may be mediated in part by lower levels of tumor-associated inflammation in the presence of high oxytocin.
Figure 1.
Survival curves for high ascites oxytocin (top tertile, n = 26, grey) and low ascites oxytocin (bottom tertiles, n = 53, black). Patients in the high ascites oxytocin group have a decreased risk of death from disease; b= −.379, H.R.= .684, 95% CI= [.501, .936], p= .017.
3.3. In vitro expression of oxytocin, oxytocin receptor and interleukin-6 by ovarian cancer cell lines
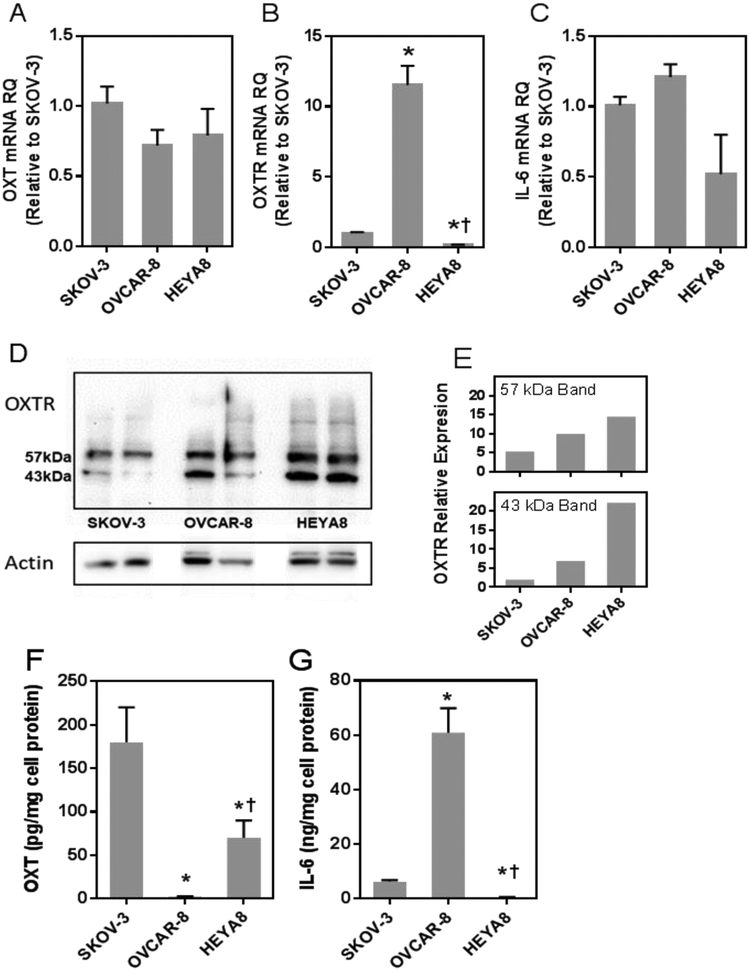
mRNA and protein expression of oxytocin, oxytocin receptor and interleukin-6 were measured to evaluate the expression phenotype of four ovarian cancer cell lines: SKOV-3, OVCAR-8, HEYA8 and OVCA432. OVCA432 cells had no detectable mRNA or protein expression for any of the target genes (data not shown) and were not studied further. The other cell lines expressed mRNA and protein of the three target genes (Figure 2 panels A-G). Oxytocin peptide mRNA levels were not significantly different between the SKOV-3, OVCAR-8 and HEYA8 cells (Figure 2A). However, constitutive secretion of oxytocin protein expression differed between cell lines, ranging from 2.2 pg/mg cell protein for the OVCAR-8 cells to 180 pg/mg cell protein for SKOV-3 cells (Figure 2F).
Figure 2.
Expression of oxytocin, oxytocin receptor and interleukin-6 in SKOV-3, OVCAR-8 and HEYA8 human ovarian cancer cell lines. Panels A-C, mRNA expression of oxytocin, oxytocin receptor and interleukin −6 for each of the cell lines studied; mRNA levels are expressed as relative quantitation (RQ) and were arbitrarily normalized to levels of SKOV-3 cells. Panel D, oxytocin receptor protein expression in replicate samples of SKOV-3, OVCAR-8 and HEYA8 cells evaluated by Western blotting, demonstrating two immunoreactive bands representing a lower unglycoslyated 43 kDa OTR and a 57 kDa glycosylated receptor. Panel E, mean expression level (in arbitrary units) of each band normalized for actin. Panel F and G, supernatant interleukin-6 and oxytocin protein levels after a six-hour incubation (ng of I interleukin-6 per mg of cell protein or pg of oxytocin/mg of cell protein). * indicates p <0.001 comparing expression levels of HEYA8 or OVCAR-8 to SKOV-3 cells and † indicates p <0.02 comparing HEYA8 to OVCAR-8.
Compared to SKOV-3 cells, OVCAR-8 cells had the highest oxytocin receptor mRNA levels (11 times greater) and the HEYA8 cells showed the lowest expression (5-fold lower; Figure 2B, all p <0.01). In contrast, oxytocin receptor protein levels, measured by Western Blot, were highest in HEYA8 cells, lower in OVCAR-8 and lowest in SKOV-3 (Figure 2D). Note that the Western blot showed two immunoreactive bands representing the 43 kDa unglycosylated receptor and a 57 kDa glycosylated receptor (Szeto et al., 2017), and the relative expression pattern among the cell lines was similar for both bands (Figure 2E). There were no significant differences in interleukin-6 mRNA expression among the cell lines (Figure 2C); however, interleukin-6 protein secretion was significantly greater in OVCAR-8 compared to the other cell lines (Figure 2G). Taken together, these results demonstrate that these cell lines express oxytocin, oxytocin receptor and interleukin-6 mRNA and protein. Oxytocin mRNA expression does not parallel protein expression in these cell lines, reflecting a fundamental difference in transcription of mRNA and translation of protein in these cells.
3.4. Anti-inflammatory effects of oxytocin in ovarian cancer cell lines
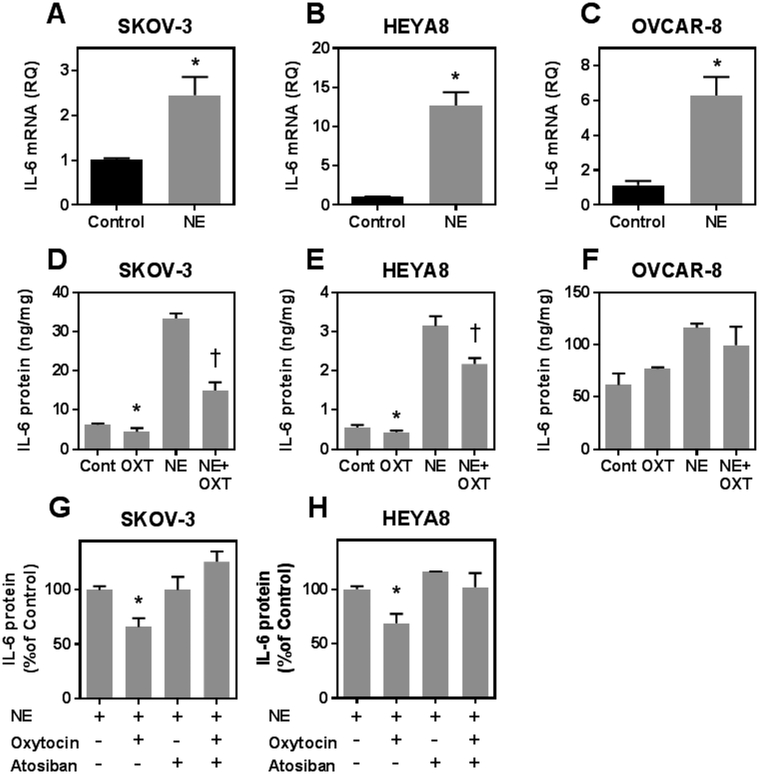
We evaluated the ability of the oxytocin/oxytocin receptor system to attenuate interleukin-6 secretion from human ovarian cancer cell lines under basal and norepinephrine-stimulated conditions. When tumor cells were incubated with oxytocin alone, supernatant interleukin-6 was significantly reduced in SKOV-3 and HEYA8 cells, but not in OVCAR-8 cells (Figure 3D–F). Incubation with norepinephrine significantly increased supernatant interleukin-6 levels in all three cell lines (Figure 3D–F). This increase was attenuated by co-incubation with oxytocin in SKOV-3 and HEYA8 cells (Figure 3D and E), but, again, not in the OVCAR-8 cell line (Figure 3F). This suggests that the OVCAR-8 cell line, which exhibited the highest levels of basal inflammation (Figures 2G and 3D–F), was oxytocin resistant, even though these cells expressed oxytocin receptors within the range of the other cell lines (Figure 2D). To show that the effects of oxytocin on reducing norepinephrine -induced interleukin-6 secretion was mediated by activation of the oxytocin receptor, SKOV-3 and HEYA8 cells (i.e., those that responded to oxytocin) were treated with the oxytocin receptor antagonist, Atosiban, during incubations with norepinephrine and with or without oxytocin. No change was observed when cells were incubated with norepinephrine and Atosiban compared with norepinephrine alone. However, when Atosiban was added with oxytocin and norepinephrine, Atosiban effectively abolished the anti-inflammatory action of oxytocin, and the reductive effect of oxytocin on interleukin-6 was no longer observed (Figure 3G and 3H).
Figure 3.
Effect of oxytocin on ovarian tumor cell interleukin-6 secretion under basal and stimulated conditions. Panels A-C show interleukin-6 mRNA expression after exposing cells to control medium or medium with 10 μM norepinephrine (NE) for 6 hours. Panels D-F show interleukin-6 (IL-6) secretion from different cell lines following incubation without (Control) or with 1 nM oxytocin (OXT) under basal or NE stimulated conditions. All data are expressed as the mean ± SD of 3 or 4 dishes and representative of at least two similar experiments. * and † indicate p< 0.05 by Students t-test compared to control or NE stimulated conditions, respectively. Panels G and H show IL-6 secretion from SKOV-3 and HEYA8 cells treated with 10 μM NE for 6 hours alone or in the presence of 1 nM OXT, 10 nM Atosiban or OXT and Atosiban simultaneously as indicated. Data the mean ± SD of 3 dishes expressed as % IL-6 secretion relative to cells treated with NE (Control condition) and * indicates p< 0.05 by Students t-test compared to control.
4. Discussion
This study identifies endogenous oxytocin in the human ovarian tumor microenvironment and provides initial clinical and pre-clinical evidence that oxytocin may be protective against ovarian tumor-related inflammation. In a cohort of patients with advanced-stage epithelial ovarian cancer, high and variable levels of oxytocin were observed in ascites, with concentrations up to 200 times greater than the plasma levels observed in this study. Higher levels of ascites oxytocin were associated with lower levels of plasma and ascites interleukin-6, an inflammatory cytokine implicated in ovarian tumor growth and metastasis (Obata et al., 1997). Higher ascites oxytocin was also associated with a survival advantage among advanced-stage epithelial ovarian cancer patients. Patients in the highest tertile of ascites oxytocin lived an average of 1.5 years longer and had a 34% decreased risk of death from disease than patients in the lower tertiles of oxytocin. Statistical mediation analyses were consistent with the hypothesis that these effects were mediated by associated differences in ascites interleukin-6 levels.
We evaluated the ability of the oxytocin/oxytocin receptor system to reduce interleukin-6 secretion in several human ovarian cancer cell lines. We found that oxytocin significantly reduced interleukin-6 secretion in two out of the three cell lines that expressed oxytocin and oxytocin receptors, under both basal and norepinephrine-stimulated conditions. This effect was blocked the oxytocin receptor antagonist, Atosiban, suggesting that the observed effects were mediated by specific receptor activation. We noted that oxytocin was less effective at reducing interleukin-6 in the OVCAR8 cells. These cells expressed the highest levels of interleukin-6, indicative of a higher inflammatory state under basal culture conditions, suggesting that some ovarian cancer cells may have unique inflammatory properties that make them resistant to the anti-inflammatory effects of oxytocin. Notwithstanding, the clinical and in vitro data presented in this study suggest that oxytocin may protect against inflammation-mediated tumor progression.
This interpretation is consistent with studies showing that exogenous oxytocin has anti-ovarian tumor effects in vitro and in vivo (Cassoni et al., 2004; Ji et al., 2018; Morita et al., 2004) and broad anti-inflammatory effects in other diseases (Jankowski et al., 2010; Nation et al., 2010; Yeniel et al., 2014), including an attenuation of interleukin-6 secretion from endotoxin-stimulated human macrophages (Szeto et al., 2017). The attenuation of macrophage-derived cytokine secretion by oxytocin could be relevant to ovarian cancer, as tumor-associated macrophages play an integral role in tumor growth and spread of disease (Solinas et al., 2009).
This study provides initial evidence that endogenous oxytocin may be a protective factor in human ovarian cancer. As a relatively new line of inquiry, there are notable study limitations and questions open to further investigation. First, these clinical data come from a correlational study and the underlying mechanisms remain to be determined. That said, the in vitro analyses reported here suggest it is plausible that endogenous oxytocin could inhibit tumor progression by reducing tumor production of pro-inflammatory cytokines such as interleukin-6. Second, the source of oxytocin in the ovarian tumor microenvironment is unclear. In this study, ovarian tumor cells were shown to express oxytocin. Thus, it is possible that some of the oxytocin identified in the tumor microenvironment is tumor-derived. Healthy ovarian cells secrete oxytocin as a part of their normal function (Fuchs, 1988; Gimpl and Fahrenholz, 2001) and tumor cells derived from these tissues may continue to express oxytocin as a retained function of the original cells. However, it is currently unknown whether healthy ovarian cells secrete oxytocin in response to inflammation. It is possible that some of the oxytocin in the tumor microenvironment is secreted from healthy ovarian or reproductive tissue. This question should be answered in future research using healthy ovarian tissue or in vivo models. Oxytocin in plasma primarily originates from the posterior pituitary. As ascites oxytocin levels were not associated with plasma levels and were considerably higher, it is highly likely that oxytocin in the tumor microenvironment does not originate from the posterior pituitary and is locally produced. Pathways underlying this local production remain to be elucidated. Finally, levels of oxytocin in ascites were highly variable and it is unclear what contributes to these individual differences. As oxytocin has been demonstrated to interact with other hormones such as cortisol (Mankarious et al., 2016), norepinephrine (Kotwica et al., 1991), and estrogen (Vasudevan et al., 2001), it is possible that interactions between oxytocin and other hormones in the tumor microenvironment contribute to oxytocin variability between patients. For example, norepinephrine has been demonstrated to stimulate the release of ovarian oxytocin in animal studies examining reproductive function (Kotwica et al., 1991). Oxytocin is also linked to psychosocial factors such as stress (Amico et al., 2004; Neumann, 2002; Taylor et al., 2000) and social support (Heinrichs et al., 2003; Neumann, 2008; Taylor et al., 2000). While these studies are typically examining centrally released oxytocin, relationships between tumor microenvironment oxytocin and psychosocial factors should be investigated as both social isolation and stress have been linked to disease processes in ovarian cancer (Cole and Sood, 2012; Lutgendorf et al., 2012; Lutgendorf et al., 2011; Thaker et al., 2006). Moreover, it would be helpful to compare ascites oxytocin levels in ovarian cancer patients to patients with other cancers characterized by ascites fluid. It would be particularly interesting to see ascites oxytocin levels of another female reproductive system cancer (e.g. breast cancer) versus a non-reproductive system cancer (e.g. lung cancer). To our knowledge, this study is the first to identify oxytocin in cancer-related ascites fluid. Characterizing the source and moderators of oxytocin in the ovarian tumor microenvironment, along with the molecular basis of its effects in ovarian cancer, are critical next steps in determining how, and for whom, oxytocin may be protective.
If experimental administration of oxytocin reliably downregulates tumor progression in vivo, as shown in (Ji et al., 2018; Morita et al., 2004), it may emerge as a valuable therapeutic adjunct in advanced-stage ovarian cancer. In addition having anti-tumor effects (Ji et al., 2018; Mankarious et al., 2016; Morita et al., 2004), oxytocin has been shown to protect against cisplatin-induced ovarian damage (Erbaş et al., 2014), renal damage (Erbas et al., 2014) and neurotoxicity (Akman et al., 2015) in mice treated with oxytocin while receiving chemotherapy. Thus, it is possible that oxytocin could protect against adverse chemotoxic effects, in addition to reducing ovarian tumor-related inflammation. If proven useful, oxytocin has the potential to be readily translated into clinical use, as a viable synthetic compound (Pitocin) is widely used in medical practice.
4.1. Conclusion
Oxytocin is a naturally-occurring hormone which is demonstrated to have anti-ovarian tumor effects in vitro and in vivo (Ji et al., 2018; Mankarious et al., 2016; Morita et al., 2004). The current investigation extends these pre-clinical findings by showing associations between endogenous oxytocin, reduced systemic and tumor-associated interleukin-6, and longer survival in a clinical sample of advanced epithelial ovarian cancer patients. The in vitro data presented in this study provides further evidence that oxytocin may be protective against ovarian tumor-associated inflammation. Continued investigation of the role of oxytocin in ovarian cancer may uncover novel mechanisms of ovarian cancer pathophysiology. Future studies should examine the therapeutic utility of oxytocin as well as relationships between psychosocial factors and oxytocin in the tumor microenvironment.
Highlights.
Oxytocin is present in the ascites fluid of advanced stage ovarian cancer patients
Higher ascites oxytocin is associated with lower tumor interleukin-6 and longer survival
In vitro, oxytocin reduced interleukin-6 secretion from ovarian tumor cells
Oxytocin may have protective effects in ovarian cancer via anti-inflammatory mechanisms
Acknowledgments:
We would like to acknowledge Desire Christensen and Sarah Strack for their helpful support during this project.
Funding: This project was supported in part by NIH grants CA193249 (SL), CA140933 (SL), T32GM108540 (SL), CA109298 (AKS), CA209904 (AKS), AG017265 (SC), AG043404 (SC), HL116387 (PMM), P30CA086862 (PI George Weiner) and American Cancer Society grant RP-16-240-01-TBG (AKS).
Conflict of interest disclosure statement: Dr. Thaker has done consulting and/or speaking for Stryker, Iovance Biotherapeutics, Abbvie/Stemcentrx, Clovis Oncology, Unleash Immunolytics, Celsion, Merck, and Tesaro, has research funding from Merck, and is a Celsion shareholder; Dr. Sood is on the Scientific Advisory Board of Kiyatec, has research funding from M-Trap, and is a Biopath shareholder; other authors declare no potential conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akman T, Akman L, Erbas O, Terek MC, Taskiran D, Ozsaran A, 2015. The preventive effect of oxytocin to cisplatin-induced neurotoxicity: an experimental rat model. BioMed research international. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amico J, Mantella R, Vollmer R, Li X, 2004. Anxiety and stress responses in female oxytocin deficient mice. Journal of neuroendocrinology 16, 319–324. [DOI] [PubMed] [Google Scholar]
- Cassoni P, Sapino A, Marrocco T, Chini B, Bussolati G, 2004. Oxytocin and oxytocin receptors in cancer cells and proliferation. Journal of neuroendocrinology 16, 362–364. [DOI] [PubMed] [Google Scholar]
- Cole SW, Sood AK, 2012. Molecular pathways: beta-adrenergic signaling in cancer. Clinical cancer research 18, 1201–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erbaş O, Akman L, Yavaşoğlu A, Terek MC, Akman T, Taskiran D, 2014. Oxytocin improves follicular reserve in a cisplatin-induced gonadotoxicity model in rats. BioMed research international. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erbas O, Korkmaz HA, Oltulu F, Aktug H, Yavasoglu A, Akman L, Solmaz V, Taskiran D, 2014. Oxytocin alleviates cisplatin-induced renal damage in rats. Iranian journal of basic medical sciences 17, 747. [PMC free article] [PubMed] [Google Scholar]
- Fuchs A, 1988. Oxytocin and ovarian function. Journal of reproduction and fertility. Supplement 36, 39–47. [PubMed] [Google Scholar]
- Gimpl G, Fahrenholz F, 2001. The oxytocin receptor system: structure, function, and regulation. Physiological reviews 81, 629–683. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U, 2003. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biological psychiatry 54, 1389–1398. [DOI] [PubMed] [Google Scholar]
- Jankowski M, Bissonauth V, Gao L, Gangal M, Wang D, Danalache B, Wang Y, Stoyanova E, Cloutier G, Blaise G, Gutkowska J, 2010. Anti-inflammatory effect of oxytocin in rat myocardial infarction. Basic Reseach in Cardiology 105, 205–218. [DOI] [PubMed] [Google Scholar]
- Ji H, Liu N, Yin Y, Wang X, Chen X, Li J, Li J, 2018. Oxytocin inhibits ovarian cancer metastasis by repressing the expression of MMP-2 and VEGF. Journal of Cancer 9, 1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotwica J, Skarynski D, Jaroszewski J, Kotwica G, 1991. Effect of norepinephrine on the release of progesterone and ovarian oxytocin in cattle. Animal Reproduction Science 26, 179–191. [Google Scholar]
- Leng G, Sabatier N, 2016. Measuring oxytocin and vasopressin: bioassays, immunoassays and random numbers. Journal of neuroendocrinology 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Wang P, Wang SC, Wang Y-F, 2017. Approaches mediating oxytocin regulation of the immune system. Frontiers in immunology 7, 693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutgendorf SK, De Geest K, Bender D, Ahmed A, Goodheart MJ, Dahmoush L, Zimmerman MB, Penedo FJ, Lucci JA III, Ganjei-Azar P, 2012. Social influences on clinical outcomes of patients with ovarian cancer. Journal of Clinical Oncology 30, 2885–2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutgendorf SK, DeGeest K, Dahmoush L, Farley D, Penedo F, Bender D, Goodheart M, Buekers TE, Mendez L, Krueger G, 2011. Social isolation is associated with elevated tumor norepinephrine in ovarian carcinoma patients. Brain, behavior, and immunity 25, 250–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macciò A, Madeddu C, 2012. Inflammation and ovarian cancer. Cytokine 58, 133–147. [DOI] [PubMed] [Google Scholar]
- Mankarious A, Dave F, Pados G, Tsolakidis D, Gidron Y, Pang Y, Thomas P, Hall M, Karteris E, 2016. The pro-social neurohormone oxytocin reverses the actions of the stress hormone cortisol in human ovarian carcinoma cells in vitro. International journal of oncology 48, 1805–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough ME, Churchland PS, Mendez AJ, 2013. Problems with measuring peripheral oxytocin: can the data on oxytocin and human behavior be trusted? Neuroscience & Biobehavioral Reviews 37, 1485–1492. [DOI] [PubMed] [Google Scholar]
- Morita T, Shibata K, Kikkawa F, Kajiyama H, Ino K, Mizutani S, 2004. Oxytocin inhibits the progression of human ovarian carcinoma cells in vitro and in vivo. International journal of cancer 109, 525–532. [DOI] [PubMed] [Google Scholar]
- Nation D, Szeto A, Mendez AJ, Brooks LG, Zaias J, Herderick EE, Gonzales J, Noller CM, Schneiderman N, McCabe PM, 2010. Oxytocin attenuates atherosclerosis and adipose tissue inflammation in socially isolated ApoE−/− mice. Psychosom Med 72, 376–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann ID, 2002. Involvement of the brain oxytocin system in stress coping: interactions with the hypothalamo-pituitary-adrenal axis. Progress in brain research 139, 147–162. [DOI] [PubMed] [Google Scholar]
- Neumann ID, 2008. Brain oxytocin: a key regulator of emotional and social behaviours in both females and males. Journal of neuroendocrinology 20, 858–865. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Wigger A, Torner L, Holsboer F, Landgraf R, 2000. Brain oxytocin inhibits basal and stress-induced activity of the hypothalamo-pituitary-adrenal axis in male and female rats: partial action within the paraventricular nucleus. Journal of neuroendocrinology 12, 235–244. [DOI] [PubMed] [Google Scholar]
- Nilsson MB, Armaiz-Pena G, Takahashi R, Lin YG, Trevino J, Li Y, Jennings N, Arevalo J, Lutgendorf SK, Gallick GE, Sanguino AM, Lopez-Berestein G, Cole SW, Sood AK, 2007. Stress Hormones Regulate Interleukin-6 Expression by Human Ovarian Carcinoma Cells through a Src-dependent Mechanism. Journal of Biological Chemistry 282, 29919–29926. [DOI] [PubMed] [Google Scholar]
- Obata N, Tamakoshi K, Shibata K, Kikkawa F, Tomoda Y, 1997. Effects of interleukin-6 on in vitro cell attachment, migration and invasion of human ovarian carcinoma. Anticancer research 17, 337–342. [PubMed] [Google Scholar]
- Pfaffl MW, 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic acids research 29, e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ, 2008. Analyzing real-time PCR data by the comparative C(T) method. Nature protocols 3, 1101–1108. [DOI] [PubMed] [Google Scholar]
- Solinas G, Germano G, Mantovani A, Allavena P, 2009. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. Journal of leukocyte biology 86, 1065–1073. [DOI] [PubMed] [Google Scholar]
- Szeto A, McCabe PM, Nation D, Tabak BA, Rossetti MA, McCullough ME, Schneiderman N, Mendez AJ, 2011. Evaluation of enzyme immunoassay and radioimmunoassay methods for the measurement of plasma oxytocin. Psychosomatic medicine 73, 393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeto A, Nation D, Mendez AJ, Dominguez-Bendala J, Brooks LG, Schneiderman N, McCabe PM, 2008. Oxytocin attenuates NADPH-dependent superoxide activity and IL-6 secretion in macrophages and vascular cells. American Journal of Physiology-Endocrinology and Metabolism 295, E1495–E1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeto A, Sun-Suslow N, Mendez AJ, Hernandez RI, Wagner KV, McCabe PM, 2017. Regulation of the macrophage oxytocin receptor in response to inflammation. American Journal of Physiology-Endocrinology and Metabolism 312, E183–E189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S, Klein LC, Lewis BP, Gruenewald TL, Gurung RA, Updegraff JA, 2000. Biobehavioral responses to stress in females: tend-and-befriend, not fight-or-flight. Psychological review 107, 411. [DOI] [PubMed] [Google Scholar]
- Thaker PH, Han LY, Kamat AA, Arevalo JM, Takahashi R, Lu C, Jennings NB, Armaiz-Pena G, Bankson JA, Ravoori M, 2006. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nature medicine 12, 939–944. [DOI] [PubMed] [Google Scholar]
- Vasudevan N, Davidkova G, Zhu Y-S, Koibuchi N, Chin WW, Pfaff D, 2001. Differential interaction of estrogen receptor and thyroid hormone receptor isoforms on the rat oxytocin receptor promoter leads to differences in transcriptional regulation. Neuroendocrinology 74, 309–324. [DOI] [PubMed] [Google Scholar]
- Yeniel AO, Erbas O, Ergenoglu AM, Aktug H, Taskiran D, Yildirim N, Ulukus M, 2014. Effect of oxytocin treatment on explant size, plasma and peritoneal levels of MCP-1, VEGF, TNF-alpha and histopathological parameters in a rat endometriosis model. European Journal of Obstetrics & Gynecology and Reproductive Biology 175, 134–139. [DOI] [PubMed] [Google Scholar]