Abstract
Diverticulitis is a prevalent gastrointestinal disorder that is associated with significant morbidity and health care costs. Approximately 20% of patients with incident diverticulitis have at least 1 recurrence. Complications of diverticulitis, such as abdominal sepsis, are less likely to occur with subsequent events. Several risk factors, many of which are modifiable, have been identified including obesity, diet, and physical inactivity. Diet and lifestyle factors could affect risk of diverticulitis through their effects on the intestinal microbiome and inflammation. Preliminary studies have found that the composition and function of the gut microbiome differ between individuals with vs without diverticulitis. Genetic factors, as well as alterations in colonic neuromusculature, can also contribute to the development of diverticulitis. Less-aggressive and more-nuanced treatment strategies have been developed. Two multicenter, randomized trials of patients with uncomplicated diverticulitis found that antibiotics did not speed recovery or prevent subsequent complications, and guidelines now recommend antibiotics for only specific patients. Elective surgical resection is no longer recommended solely based on number of recurrent events or young patient age and might not be necessary for some patients with diverticulitis complicated by abscess. Randomized trials of hemodynamically stable patients who require more emergent surgery for acute, complicated diverticulitis that has not improved with antibiotics provide evidence to support primary anastomosis vs sigmoid colectomy with end colostomy. Despite these advances, more research is needed to increase our understanding of the pathogenesis of diverticulitis and to clarify treatment algorithms.
Keywords: chronic manifestations, smoldering diverticulitis, functional symptoms, laparoscopic lavage, immunosuppression
Lay Summary
Diverticulitis is a prevalent condition of the colon. New evidence indicates that diet and lifestyle may interact with the gut microbiota to initiate inflammation. Less aggressive treatment paradigms are under active investigation.
Diverticular disease, once a rarely diagnosed medical curiosity, is now one of the most common gastrointestinal disorders among inpatients and outpatients.1–3 Painter and Burkitt first documented a large increase in the prevalence of diverticular disease beginning at the time of the industrial revolution and differences in prevalence between Western and Eastern countries.4 These observations led to the hypothesis that diverticular disease resulted from dietary fiber deficiency. According to this theory, a diet low in fiber resulted in small-caliber stools, increased intracolonic pressures, and herniation of the colonic mucosa through the muscular layers adjacent to the vasa recta. Diverticulitis was thought to ensue when a diverticulum became obstructed with stool, resulting in fecal stasis, mucosal trauma, and ischemia. Diverticular disease was readily attributed to fiber deficiency, surgery and antibiotics became the primary treatments for diverticulitis, and research in the field stagnated.
In the past 2 decades, there has been a resurgent interest in diverticular disease. Modern imaging techniques and the widespread use of flexible endoscopy have enabled more accurate diagnosis of diverticulitis and asymptomatic diverticulosis, increasing our understanding of their epidemiology. The discovery that diverticulitis is not a progressive led to new and less-aggressive surgical and medical approaches.5, 6 Recognition that diverticulitis shares many risk factors with other inflammatory diseases has prompted new theories of pathogenesis and opportunities for research and treatment. We review the latest data and concepts regarding the epidemiology, pathophysiology, and treatment of diverticulitis of the colon.
Terminology
“Diverticular disease” is used to describe asymptomatic diverticulosis and the spectrum of complications of colonic diverticulosis. However, because the term indicates the presence of symptoms or complications, diverticular disease is more appropriate for diverticulitis and other complications of diverticulosis such as bleeding, rather than for asymptomatic diverticulosis. In addition, diverticula can occur in the small bowel and other areas of the intestinal tract. This review focuses on diverticulitis and complicated diverticulitis (diverticulitis with abscess, perforation, stricture, obstruction and/or fistula) of the colon. Other complications of diverticular disease not covered in this review include segmental colitis associated with diverticular disease (SCAD), an uncommon manifestation that shares many clinical and histologic features with inflammatory bowel diseases (IBD),7 and symptomatic uncomplicated diverticular disease (SUDD). SUDD is most commonly defined as gastrointestinal symptoms in the setting of diverticulosis without evidence of overt inflammation or diverticulitis.8 However, definitions vary, there is significant overlap of symptoms with irritable bowel syndrome (IBS), and the diagnosis is controversial. Some patients develop chronic abdominal symptoms following an episode of diverticulitis, a syndrome sometimes referred to as post-diverticulitis functional bowel disease.9
Epidemiology
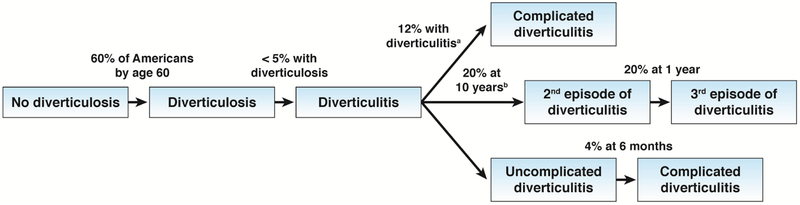
The lifetime risk of diverticulitis in a person with diverticulosis was reported to range from 10% to 25%.10 However, these estimates predate the routine use of flexible endoscopy, and therefore, an accurate assessment of prevalence. In addition, the diagnosis of diverticulitis was made on clinical grounds. Modern estimates based on colonoscopy and computed tomography (CT) indicate that fewer than 5% of individuals with diverticulosis develop diverticulitis (Figure 1).11 Nonetheless, because more than 50% of Americans older than 60 years of age have diverticulosis, diverticulitis is highly prevalent.12 Annually, in the United States (US), there are more than 2.7 million outpatient visits and 200,000 inpatient admissions for diverticulitis at a cost of more than $2 billion.1, 3 The incidence of diverticulitis has increased over time and increases with patient age.13, 14 However, the relative increase in diverticulitis in recent decades has been greatest in young patients. For example, from 1980 through 2007, the incidence of diverticulitis in individuals 40–49 years old increased by 132%.13
Figure 1.
Natural history of diverticulosis and diverticulitis. aThe majority of complications occur during the first or second episode of diverticulitis.29, 30 bEstimates of recurrence are for all patients with diverticulitis regardless of the presence of complications and in the absence of prophylactic surgery. Of note, patients with complicated diverticulitis treated medically do not appear to be at increased risk of recurrent diverticulitis compared with uncomplicated diverticulitis. However, the risk of recurrence is significantly lower in those who undergo surgery for complicated diverticulitis.14
Diverticulitis is more common in men than women until the 6th decade, when it becomes more common in women. 13, 14 The prevalence of hospitalization for diverticulitis in the US is greatest in whites (62/100,000), similar in African Americans and Hispanics (approximately 30/100,000), and lowest in Asians (10/100,00).14 Individuals living in urban areas are more likely to be hospitalized for diverticulitis than those in rural areas,15 as are those with lower income and educational levels.16 Diverticulitis is more common in developed countries, but could be increasing in other parts of the world.17 After immigration, non-Western individuals have a lower risk of hospitalization for diverticular disease than Western natives, although risk increases with time since immigration.18
Progression of diverticulosis and diverticulitis
Complications of diverticulitis occur in approximately 12% of patients. The most common complication is phlegmon or abscess (approximately 70% of patients with complications) followed by peritonitis, obstruction, and fistula.13 Mortality following complicated diverticulitis (diverticulitis with phlegmon, abscess, perforation or fistula) is increased compared to uncomplicated diverticulitis, and is highest among individuals with perforation or abscess.13, 19 In a population-based cohort study in the United Kingdom, mortality at 1 year was 20% in patients with perforated diverticulitis compared to 4% in age- and sex-matched controls.19
A small percentage of patients (4%–10%) have ongoing or smoldering diverticulitis, defined as ongoing diverticulitis (pain with increased white blood cell counts or markers of inflammation, fever, or CT evidence of inflammation) despite antibiotic treatment or re-exacerbation after cessation of treatment.20, 21 An estimated 8% of patients with incident disease have recurrences within the first year after complete recovery from the incident episode, and 20% have recurrences within 10 years. The risk of recurrence increases with subsequent episodes. After a second episode, the risk is 18% at 1 year and 55% at 10 years, and after a third episode it is 40% at 3 years.13 These estimates are derived from a population-based cohort of all patients with diverticulitis. Most other estimates of recurrence are derived from selected populations, such as those who were hospitalized for treatment. In addition to the number of recurrences, risk factors for recurrence include young age at onset,22, 23 severity of the incident event,24–26 extent of colon involvement, family history of diverticulitis,25 smoking, male sex, and obesity.27
Diverticulitis was once thought to be a progressive disease with an increasing risk of complications as the number of episodes increased. This belief informed guidelines for aggressive surgical intervention. However, complications, with the exception of fistula formation, occur more commonly during the first episode of diverticulitis than after subsequent episodes.28 For example, in a prospective study of 900 patients, the risk of free perforation was 25% at the first episode and decreased to zero after the 4th episode.29 In another prospective study, only 4% of patients developed complicated diverticulitis within 2 years of presentation with uncomplicated diverticulitis.30 The reduced risk of subsequent perforation is believed to be due to occlusion of microperforations by nearby tissues or organs during the initial inflammatory episode. Population-based data also indicate that the risk of recurrence after complicated diverticulitis treated medically is similar to the risk following uncomplicated diverticulitis.13 Nonetheless, retrospective studies reporting a high rate of recurrence following medical treatment of complicated diverticulitis continue to dictate surgical treatment.24, 31, 32
Chronic manifestations
Patients with a history of diverticulitis can develop chronic manifestations aside from recurrent or smoldering diverticulitis, stricture, and fistula. In a randomized controlled trial (RCT) of antibiotics vs no antibiotics for treatment of uncomplicated diverticulitis, approximately 40% of patients with CT- confirmed diverticulitis had mild to moderate abdominal pain and/or changes in bowel habits at 1 year of follow up.33 In a large retrospective study of US Veterans, the risk of IBS and functional bowel diseases were 5- and 2.5-fold higher, respectively, in patients with a history of diverticulitis than without.9 Patients with diverticulitis were also more than twice as likely to develop mood disorders.9 A study that evaluated quality of life in patients with a history of diverticulitis found that negative psychological, social as well as gastrointestinal, symptoms are common after resolution of the acute episode, and that patients attribute these symptoms specifically to prior diverticulitis.34 Although functional symptoms without overt inflammation appear to be common following a diagnosis of diverticulitis, it is less clear whether patients with diverticulosis without a history of diverticulitis can develop chronic symptoms (such as SUDD). Many studies of SUDD included patients with prior diverticulitis and it is difficult to distinguish SUDD from IBS.8 In a large, prospective colonoscopy study, no association was found between diverticulosis and IBS.35
There may be long-term, extra-intestinal implications of diverticulitis. In a nationwide study in Sweden, individuals with diverticular disease had a modest increase in risk of thromboembolic events compared with matched controls.36 It should be a priority to increase our understanding of the long-term, global effects of diverticulitis on health and quality of life.
Risk factors
Modern studies have confirmed and expanded the role of diet and other modifiable lifestyle factors in the natural history of diverticulitis (Table 1). Lifestyle factors associated with increased risk include Western dietary patterns (high in red meat, fat, and refined grains) and red meat consumption alone.37, 38 Obesity, and central obesity in particular, increases the risk of diverticulitis.39–42 Smoking is also associated with an increased risk of diverticulitis—particularly complicated diverticulitis.43–46 On the other hand, dietary fiber intake and prudent diets (high in fruits, vegetables, and whole grains) reduce the risk of diverticulitis.38, 47, 48 Nuts and seeds do not appear to increase the risk, and in a large, prospective cohort nuts and popcorn were associated with reduced risk of diverticulitis.49. Physical activity (particularly vigorous activity, such as running) is associated with decreased risk.39, 50, 51 It is not clear whether alcohol use affects risk of diverticulitis.43, 52
Table 1.
Risk Factors for Incident Diverticulitis
| Risk Factor | Category | RR/ORa | References |
|---|---|---|---|
| Diet | |||
| Fiber | Highest quintile | 0.57–0.75 | 48, 49 |
| Nuts | > 2 times/week | 0.80 | 50 |
| Popcorn | > 2 times/week | 0.72 | 50 |
| Vegetarian diet | Yes/no | 0.69 | 49 |
| Prudent dietary patternb | Highest quintile | 0.74 | 39 |
| Western dietary patternc |
Highest quintile | 1.55 | 39 |
| Red meat | Highest quintile | 1.58 | 38 |
| Lifestyle | |||
| Physical activity | Highest quintile | 0.63–0.75 | 40, 51, 52 |
| Body mass index (BMI) | BMI ≥ 30 | 1.33–4.4 | 40–42 |
| Waist-to-hip ratio | Highest quintile | 1.62 | 42 |
| Smoking | Current or ≥ 15 cigarettes/day | 1.23–1.89 | 41, 45, 46 |
| Medications | |||
| Non-aspirin NSAIDs | ≥ 2 times/week | 1.72 | 58 |
| Aspirin | Ever or ≥ 2 times/week | 1.25–1.32 | 57, 58 |
| All NSAIDs | ≥ 2 times/week | 1.62 | 58 |
| Corticosteroids | Current use | 2.74 | 57 |
| Opiate analgesics | Current use | 2.16 | 57 |
| Statins | Current use | 0.44 | 57 |
| Vitamin D | Highest quintile | 0.49 | 61 |
| Sibling with diverticular disease | Yes/no | 2.92 | 97 |
Effect estimates are from select, large, population-based cohort or case-controls studies with adjustment for confounding variables. The study outcomes include hospitalization for diverticulitis, symptomatic diverticular disease and diverticulitis managed in the inpatient or outpatient setting depending on the study.
Prudent dietary pattern is high in fruits, vegetables and whole grains
Western dietary pattern is high in red meat, high-fat dairy, and refined grains
A study examining the joint contribution of multiple lifestyle risk factors on risk of incident diverticulitis found that adherence to a low-risk lifestyle decreased the risk of diverticulitis by nearly 75%. A low-risk lifestyle was defined as fewer than 4 servings of red meat per week, at least 23g of fiber per day, 2 hrs of vigorous activity per week, body mass index 18.5–24.9, and no history of smoking. Assuming causal associations, it was estimated that a low-risk lifestyle could prevent half of diverticulitis cases.53 These findings highlight the importance of diet and lifestyle modification in the prevention of diverticulitis.
Several medications have been associated with increased risk of diverticulitis. Prospective cohort and case-control studies have found a consistent positive association between nonsteroidal anti-inflammatory drug (NSAIDs) use and diverticulitis.54–57 The association appears to be stronger for non-aspirin NSAIDs than for aspirin.57 Non-aspirin NSAIDs also appear to be more strongly associated with complicated or perforated diverticulitis than with uncomplicated disease.57 Opiate analgesics as well as corticosteroids are also associated with diverticulitis and perforated diverticulitis.56 Several medications have been associated with a decreased risk of diverticulitis including statins, calcium channel blockers, and metformin, although these studies were at risk of bias and confounding—further studies are needed.56, 58, 59 A case–control study comparing 25(OH)D levels and risk of diverticulitis, as well as a study of ultraviolet light exposure, identified low vitamin D levels as a risk factor for diverticulitis.15, 60, 61 Medication use, particularly NSAID use, is generally one of the easiest targets for risk factor modification.
Immunosuppression is thought to increase the risk of diverticulitis. The risk associated with corticosteroids is well-defined. However, studies of other forms of immunosuppression such as chemotherapy, organ transplant, and renal failure have been small, retrospective, and heterogeneous with respect to the type and degree of immunosuppression and associated risk for diverticulitis. Although the mechanism of increased risk is unclear, it is plausible that impaired healing contributes to perforation, and the lack of an immune response might mask signs and symptoms. Although their symptoms may be less pronounced, immunocompromised patients appear to be more likely to present with complicated disease, and are therefore more likely to require surgical intervention than their non-immunocompromised counterparts.62 It is unclear whether these patients are more likely to develop recurrent disease, but poor outcomes during recurrent episodes, including death, appear more likely.62, 63
Pathophysiology
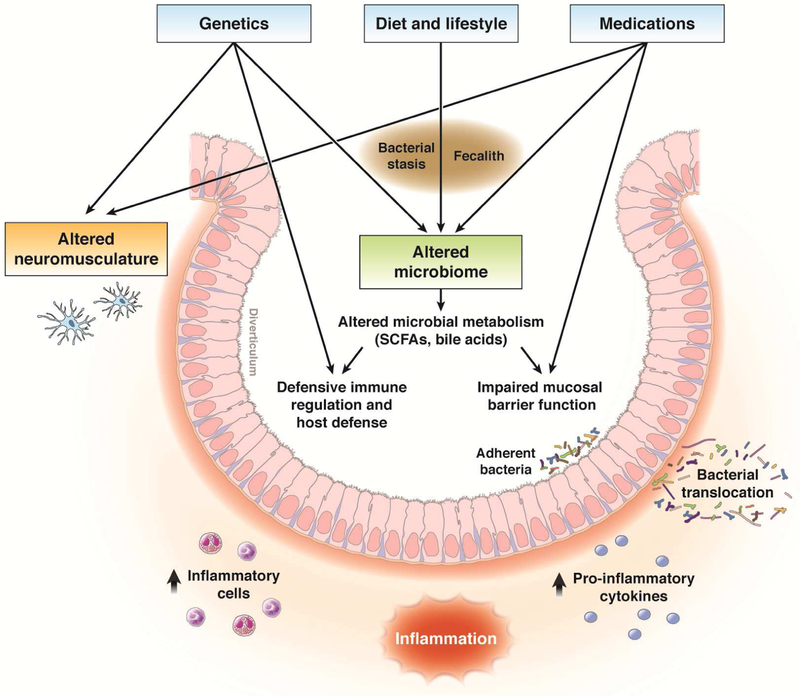
The pathophysiology of diverticulitis is incompletely understood. Long-standing but unproven theories suggest that diverticulitis results from obstruction and trauma to a diverticulum with subsequent ischemia, microperforation, and infection.64 This theory led to the wide-spread belief that patients with diverticulosis should avoid eating nuts and seeds, and the widespread use of antibiotics for diverticulitis treatment. However, more recent studies indicated that nut and seed consumption do not increase risk of diverticulitis,49 and that antibiotics may not hasten recovery or improve outcomes.21, 33 These findings have led to models of diverticulitis pathogenesis that involve chronic inflammation and alterations in the gut microbiome (see Figure 2).
Figure 2.
Proposed pathophysiology of acute colonic diverticulitis. Diverticulitis is hypothesized to arise from the complex interaction of diet and lifestyle factors, medications, genetics, and the gut microbiome. Alterations in the gut microbiome composition (eg, ↓short chain fatty acid, SCFA, producers, ↑invasive pathogens) and function (↓SCFAs, altered bile acids) result in defects in the mucosal barrier and immune function leading to an inflammatory cascade and mucosal inflammation.
Chronic inflammation
Several studies have associated diverticulitis with a chronic inflammatory state. The most convincing evidence, although indirect, is that many risk factors for diverticulitis are associated with chronic, systemic inflammation. For example, obesity, physical inactivity, and a Western diet are risk factors for other diseases believed to be caused by chronic inflammation, including cardiovascular disease and diabetes—these risk factors increase levels of biomarkers of inflammation.65–67 Increased expression of matrix metalloproteases and histamine, which are associated with intestinal inflammation, have also been linked to diverticulitis.68, 69 Intestinal mucosal inflammation observed in patients with SCAD has morphological and clinical overlap with IBD,7. Findings from a case series study indicated that patients with diverticulosis, particularly those with abdominal symptoms or SUDD, have microscopic inflammation.70, 71 However, SCAD and SUDD are separate manifestations of diverticular disease, and, in a study of more than 600 individuals undergoing screening colonoscopies, after adjustment for potential confounders, there was no association between diverticulosis and levels of immune markers or cytokines implicated in IBD and IBS. In addition, there was no association between diverticulosis and IBS and no difference in mucosal inflammatory markers between diverticulosis patients with and without abdominal symptoms, although the subset with symptoms was small.35 Therefore, diverticulosis itself may not be associated with mucosal inflammation, and functional symptoms in patients with diverticulosis might not be related to inflammation or even to diverticulosis itself. However, these studies did not investigate whether patients who later developed diverticulitis or had recurrent diverticulitis had subclinical mucosal or systemic inflammation.
Altered microbiomes
Alterations in the gut microbiota are implicated in the pathogenesis of many intestinal disorders. Diet and lifestyle factors may induce alterations in the gut microbiome that lead to mucosal inflammation and diverticulitis.72, 73 Several lines of evidence support a role for gut microbiota in diverticulitis. Acute diverticulitis involves micro- or macro-perforation with translocation of commensal bacteria across the colon mucosal barrier, sometimes resulting in frank infections, including abscess formation and peritonitis. The standard treatment for diverticulitis is antibiotics.74 Worldwide differences in the prevalence of diverticular disease mirror geographical differences in gut microbial composition.4, 75–77 Diet and lifestyle risk factors for diverticulitis affect the gut microbiome. For example, obesity and a Western dietary pattern are linked to decreased microbial diversity in the intestine and changes in the microbiome composition and its functions.78–81 On the other hand, diets high in fiber increase gut microbiome diversity and richness.82–84 Dietary fiber is an important source of energy for intestinal microbes, which metabolize complex carbohydrates into short-chain fatty acids (SCFAs).85 SCFAs increase the production of mucus and antimicrobial peptides and mediate immune homeostasis, intestinal barrier function, and proper levels of cell proliferation.86
Preliminary studies indicate that the composition of the intestinal microbiome differs in patients with vs without diverticular disease. Most studies have focused on the microbiota of patients with SUDD. In general, these studies have found decreases in bacteria that produce SCFAs and in Akkermansia, a mucin-degrading bacteria that promotes epithelial barrier integrity and suppresses inflammation.70, 87, 88 However, it is difficult to extrapolate findings from studies of SUDD to diverticulitis given the controversy surrounding the diagnosis of SUDD and the lack of similarities in pathophysiology. A large study of the mucosal adherent bacteria in incidental diverticulosis (n=226) vs no diverticulosis (n=309) found only weak associations with the phylum Proteobacteria and the family Comamonadaceae. It concluded that mucosal adherent bacteria are unlikely to contribute to development of diverticulosis.89
Several studies have examined the intestinal microbiomes of patients with diverticulitis. A PCR analysis of surgical resection specimens from patients with diverticulitis found higher levels of Bifidobacterium than in patients with colon cancer or IBD.90 Daniels et al studied the microbiomes of patients with acute diverticulitis who underwent colonoscopies vs controls. The authors performed PCR amplification of the 16S–23S rDNA interspace region of specific phyla and found the diversity of Proteobacteria to be higher in patients with diverticulitis. The Enterobacteriaceae family, which includes Escherichia coli, had the highest degree of discrimination—also observed in studies of patients with IBD.91 Lower levels of Clostridiales (which produce SCFAs) such as Lachnospiraceae and of Erysipelotrichaceae (also decreased in patients with IBD)92, 93 were found in cases with a history of diverticulitis compared to controls with asymptomatic diverticulosis. PICRUSt analysis predicted that microbes involved in carbohydrate metabolism and biosynthesis of secondary metabolites were significantly decreased in cases.94 Overall, changes in the intestinal microbiome associate with development of diverticulitis and symptoms associated with diverticulosis (such as SUDD). Bacteria involved in SCFA metabolism, mucosal barrier function, and potentially invasive bacteria appear to be particularly important. However, it is difficult to determine whether changes in the fecal microbiome are causally associated with diverticulitis. In small, cross-sectional studies, diet and lifestyle changes following diagnosis and treatment with antibiotics may have altered gut microbiota profiles.
Genetics
Two large, Scandinavian twin or family studies provided evidence that genetic factors affect risk for diverticular disease. In these studies, the odds of developing diverticular disease if a co-twin had the disease was significantly higher among monozygotic twins than dizygotic twins and higher in non-twin siblings than the general population. Statistical modeling estimated that genetic factors accounted for 40%–50% of risk for diverticular disease.95, 96 These studies could not clearly distinguish diverticulitis from diverticulosis. However, results were similar the analysis was restricted to complicated diverticulitis.96
A genome-wide association study in Iceland and Denmark identified variants in genes (ARHGAP15, COLQ, and FAM155A) that associated with diverticular disease. Variants in FAM155A associated specifically with diverticulitis.97 A variant in ARHGAP15 is associated with phagocyte function and inflammation. Maguire et al used the UK Biobank to perform a retrospective genome-wide association study of 28,000 patients admitted to the hospital with diverticular disease. The authors identified 42 risk loci, 8 of which were replicated in a separate cohort. Candidate risk loci contained genes that regulate immunity, the extracellular matrix, cell adhesion, membrane transport, and intestinal motility. The previously identified associations with ARGAP15, COLQ, and FAM155A were confirmed in this study. 98 Two small studies of patients with diverticulitis who required surgery found an association with several single-nucleotide polymorphisms in the TNFSF15 gene.99, 100 Variants in this gene, which encodes for a cytokine in the tumor necrosis family, have been associated with severe forms of IBD. A study of 5 members of a family with early-onset diverticulitis identified a rare single-nucleotide polymorphisms in the laminin subunit beta 4 (LAMB4).101 Laminins are part of the extracellular matrix and involved in development of the enteric nervous system.
Alterations in colonic neuromusculature
Localized high-pressure zones in the colon were originally believed to lead to formation of diverticula at weak spots in the colonic musculature. This might account for the observation that diverticulosis occurs most frequently in the sigmoid, where the colon is less distensible, than the proximal colon and rectum. Furthermore, diverticula are not found in the rectum, which functions as a reservoir and is highly distensible, and where the tenia coli flare to create an additional, circumferential layer to the bowel wall. Alterations in the enteric nervous system, connective tissue, smooth muscle, and colonic motility are believed to contribute to the development of diverticulosis and functional symptoms in the setting of diverticulosis.102 It is less clear if these neuromotor abnormalities are involved in the development of diverticulitis.
Early-onset diverticulosis occurs in patients with connective tissue disorders, supporting a role for connective tissue defects in the formation of diverticulosis103–105. Alternations in collagen composition and content as well as connective tissue metabolism have been identified in colons of patients with diverticulosis.106 Reduced numbers of ganglionic and neuronal cells107, as well as imbalances in neurotrophic factors and neuropeptides such as serotonin and acetyl choline, are also found in patients with diverticular disease.108–110 These alternations might cause symptoms in patients with diverticulosis.111 Motility studies of patients with diverticulosis have, in general, found increased motility indices and propulsive activity.112 It is not clear whether these neuromuscular alterations predate the formation of diverticulosis or diverticulitis, and therefore contribute to disease development, or are a result of processes that occur after diverticulosis formation.
Treatment
Antibiotics, dietary modification, and pain control have been the mainstays of treatment for patients with uncomplicated diverticulitis; surgical resection has been the cornerstone for treatment of complicated diverticulitis and recurrence. However, these interventions are based largely on dogma and expert opinion rather than data. Traditional surgical recommendations were comfortingly definitive but ultimately have not been supported by evidence. Although more recent data and technology advances have reduced risk through less-invasive treatment, clinical guidelines have become more difficult to define due to the need for individualized treatment.74 The concept that diverticulitis is an inflammatory as well as an infection-associated disease have sparked interest in new medical and surgical treatment paradigms. In general, there has been a shift towards less-aggressive medical and surgical management.
Pharmacologic agents
Two RCTs and several observational studies have challenged the need for antibiotic treatment for patients with uncomplicated diverticulitis (Table 2). The antibotika vid okomplicerad divertikulit study group randomly assigned 623 patients with CT-proven uncomplicated diverticulitis from 10 centers in Sweden and Iceland to groups given intravenous followed by oral antibiotics (carbapenem or piperacillin and tazobactam followed by oral metronidazole combined with either ciprofloxacin or cefadroxil) or intravenous fluids alone. Patients were followed for 12 months.33 In the diverticulitis: antibiotics or close observation (DIABLO) study, 570 patients with CT-proven uncomplicated diverticulitis (Hinchey 1a or 1b; supplementary table 1) from 22 centers in the Netherlands were randomly assigned to groups given amoxicillin-clavulanic acid (intravenously, for least 48 hrs, and then oral) or placebo and followed for 6 months.21 Each study found that antibiotics did not hasten recovery or prevent subsequent surgery or complications.
Table 2.
Selected Multi-center Randomized Controlled Trials of Treatment for Acute Diverticulitis
| Study name | Author, year | Inclusion criteria | Intervention | Primary Outcome | Main findings |
|---|---|---|---|---|---|
| Medical Treatment | |||||
| AVOD | Chabok, 2012 | CT-based uncomplicated left-sided diverticulitis; Hinchey 1a | IV carbapenem or piperacillin/tazobactam then oral metronidazole plus ciprofloxacin or cefadroxil (n=314) vs. IV fluids (n=309) | Complication rate | No difference in time to recovery, complications, recurrent diverticulitis |
| DIABLO | Daniels, 2016 | CT-based left-sided uncomplicated diverticulitis; Hinchey 1a-1b | IV then PO amoxicillin- clavulanic acid (n=266) vs. observation alone (n=262) | Time to recovery | No difference in time to recovery, complications, recurrent diverticulitis |
| Mora Lopez, 2017 | Mild, CT-based uncomplicated diverticulitis; Neff grade 0 | Oral amoxicillin, ibuprofen, acetaminophen (n=230) vs. ibuprofen and acetaminophen(n=230) | Readmission | Anticipated recruitment until July 2020 (NCT02785549) | |
| Surgical Treatment | |||||
| ColonPerfRCT | Oberkofler, 2012 | Hinchey III-IV | Sigmoidectomy with anastomosis and diverting ostomy (n=32) vs. Hartmann procedure (n=30) | Postoperative complication rate | No differences in postoperative complications. Discontinued due to significant difference favoring primary anastomosis with diverting ostomy and declining enrollment. |
| SCANDIV | Schultz, 2017 | CT-based Hinchey grades I-IV | Laparoscopic lavage (n=101) vs. sigmoidectomy (n=98) | Severe postoperative complication within 90 days | Lavage did not reduce severe postoperative complications. The stoma rate was lower in the lavage group (14% vs. 42%; P<0.001). 4 cancers missed with lavage. |
| DILALA | Kohl, 2018 | Laparoscopy-based Hinchey grade III | Laparoscopic lavage (n=43) vs. Hartmann procedure (n=40) | Reoperation within 24 months | Fewer patients in the lavage group than in the Hartmann arm had at least 1 reoperation within 12 months (42% vs. 67.5%, P = 0.012). Mortality and severe adverse events did not differ between groups. |
| DIVERTI | Bridoux, 2017 | CT-based Hinchey grade III-IV | Primary anastomosis with diverting stoma (n=50) vs. Hartmann procedure (n=52) | Mortality within 18 months | Mortality did not differ Hartmann and primary anastomosis (7.7% vs. 4%; p = 0.4233). Morbidity and stoma reversal were comparable (39 vs 44%; p = 0.4233). At 18 months, 96% of PA patients and 65% of HP patients had a stoma reversal (p = 0.0001). |
| DIRECT | van de Wall, 2017 | CT-, US-, or endoscopy confirmed diverticulitis with persistent pain > 3 months and/or 3+ recurrences/2 years | Elective laparoscopic or open sigmoidectomy (n=53) vs. conservative management (n=56) | Health-related quality of life | Prematurely terminated due to increasing difficulty with enrollment. Elective sigmoidectomy resulted in better quality of life than conservative management (Gastrointestinal Quality of Life Index mean difference 14.2, 95% CI 7.2–21.1, p<0.0001). |
| LADIES-LOLA arm | Vennix, 2015 | Laparoscopy-based Hinchey grade III | Laparoscopic lavage (n=45) vs. sigmoidectomy (n=42) | Composite morbidity* and mortality | Prematurely terminated due to safety concerns in lavage arm. |
| LADIES-DIVA arm | 2010-present | Laparoscopy-based Hinchey grade III-IV | Sigmoidectomy with anastomosis (n=118, anticipated) vs. Hartmann procedure (n=118, anticipated) | Stoma-free survival at 12 months | Anticipated recruitment until March 2017 (NCT01317485) |
| STELLA | Tartaglia, 2015-present | CT-based Hinchey grade II unresponsive to conservative therapy and grade III | Laparoscopic lavage (n=50 anticipated) vs. Laparoscopic sigmoidectomy (n=50 anticipated) | 30-day morbidity, 30-day mortality, 30-day sepsis control 15-day re-intervention | Anticipated recruitment until December 2018 (NCT03008707) |
| DEBUT | Flum, 2016-present | CT-based Hinchey grades I-IV | No intervention prospective cohort) (n=750) | Patient-reported symptom burden and quality of life | Anticipated recruitment until December 2019 (NCT02776787) |
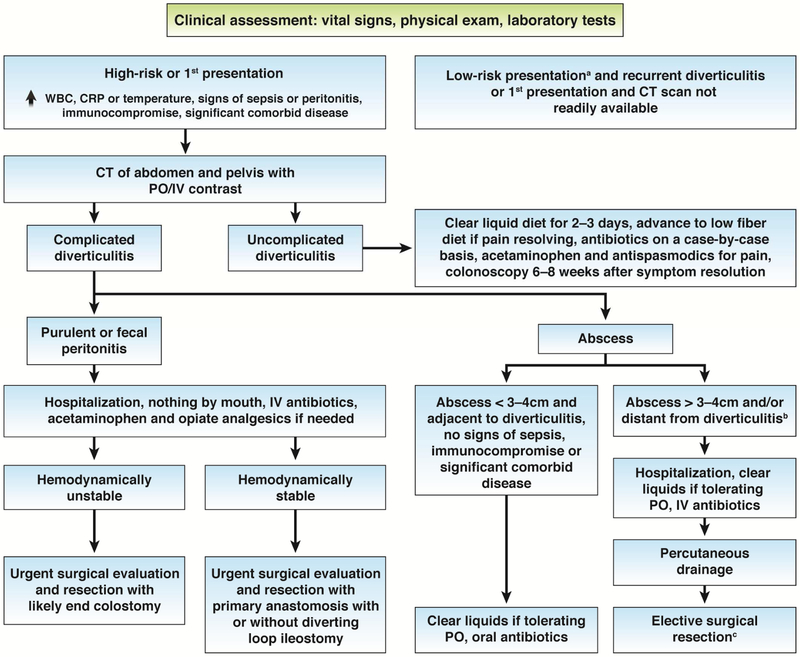
Based on these findings and growing concerns about antibiotic-related complications and resistance, several European guidelines have stopped recommending antibiotics for uncomplicated diverticulitis.113–115 The American Gastroenterological Association’s 2015 guideline gave a conditional recommendation for selective rather than routine use of antibiotics in uncomplicated diverticulitis in the absence of severe disease, immunocompromise, pregnancy or significant comorbidity,116 noting the low quality of evidence including inadequate power to assess risk of serious complications.117 In a subsequent analysis of 2-year follow-up of the DIABLO trial, rates of progression to complicated or recurrent diverticulitis were similar.118 However, higher proportions of patients in the placebo group underwent elective surgery (7.7%) than in the antibiotic group (4.2%; P=.09), and the indications for surgery in the placebo group were more frequently obstruction and recurrent diverticulitis. Furthermore, the risk of elective surgery in the placebo group might have been underestimated. The proportion would increase to 10% if patients who were censored after enrolling in another trial of elective surgery vs conservative management were included in the analysis.119 Therefore, the long-term safety of withholding antibiotics from patients with uncomplicated diverticulitis is uncertain; larger long-term follow-up studies are needed before well-supported consensus treatment guidelines can be developed. Nonetheless, data indicate that the risk of serious complications after acute uncomplicated diverticulitis is low (4%) whether or not patients receive antibiotics.118 The decision to withhold antibiotics from stable, immunocompetent, adherent patients with uncomplicated diverticulitis involves shared decision making and close follow up (see Figure 3). This option may be particularly appealing to patients with recurrent diverticulitis who have had mild episodes and repeated exposure to antibiotics.
Figure 3.
Management algorithm for acute diverticulitis. Evaluation and treatment approach depends on the severity of presentation, presence of complications (peritonitis, abscess), and comorbid conditions. aLow-risk presentation includes no markedly elevated WBC, CRP, or temperature, no signs of sepsis or peritonitis, no immunocompromise or significant comorbid disease. bSuch as a pelvic abscess. cRecommended by current guidelines, but some evidence to suggest good outcomes without resection in selected patients. CRP, C-reactive protein; IV, intravenous; PO, per os; WBC, white blood cell count.
Other approaches that have been studied for uncomplicated diverticulitis, although with less rigor, include shorter treatment courses, oral vs intravenous antibiotics, and outpatient vs inpatient management. A small RCT indicated that the treatment failure rate was similar in patients receiving 4 days vs 7 days of antibiotics.120 Small studies have also demonstrated that outpatient treatment (with antibiotics) is safe in select patients with uncomplicated diverticulitis who can tolerate oral intake and do not have significant comorbid disease121, 122. A trial is underway to compare outpatient treatment with vs without antibiotics.123
Unfortunately, there are few data to guide dietary recommendations and pain management for patients with acute diverticulitis. Patients have been instructed to consume a clear-liquid or low-residue diet. A prospective, uncontrolled study of an unrestricted diet in 86 patients with uncomplicated diverticulitis concluded that this was well-tolerated, although 8% had serious adverse events and 20% had ongoing symptoms.124 A multicenter trial is underway to evaluate an unrestricted vs a progressive diet in uncomplicated diverticulitis. Most guidelines do not address pain control. NSAIDs and opiates are associated with increased risk of incident and complicated diverticulitis but have not been studied in patients with acute disease.
Selection is key to management of outpatients with diverticulitis. The diagnostic accuracy of diverticulitis based on clinical grounds alone has been reported to be low (diagnoses of diverticulitis are incorrect for as many as 60% of cases), and clinical decision tools are imperfect.125 CT or ultrasound imaging is required for identification of complicated diverticulitis (Figure 3). A multi-slice CT with intravenous contrast identifies patients with diverticulitis with 95% sensitivity and 96% specificity.126 In Europe, ultrasound is frequently used and identifies diverticulitis with 94% accuracy, but is operator dependent.127 Ultrasound-based strategies would reduce radiation exposure—particularly in patients with recurrent disease, but further studies are needed.
Pharmacologic agents might be used to prevent recurrence of diverticulitis. Patients with incident diverticulitis have an approximately 10% risk of recurrence within 1 year—risk increases substantially with each subsequent episode.13 Even after a segmental colectomy, as many as 15% of patients have recurrences; 10%–20% of patients have serious post-operative complications and as many as 20% report no relief in symptoms of pain and bloating.117, 128–130 A medical means to prevent recurrence would be transformative. Several medications have been tested under the premise that diverticulitis is an acute inflammatory disorder. Six trials have evaluated the efficacy of mesalamine in the prevention of recurrent diverticulitis in more than 2000 patients.131–134 Unfortunately, none of these trials found a significant difference in the rate of recurrent diverticulitis. However, there may be other indications for mesalamine in diverticulitis. A small retrospective study of patients with acute uncomplicated diverticulitis found that mesalamine led to faster recovery, and uncontrolled studies indicate benefits to patients with SUDD.135, 136 Other pharmaceutical agents that have been tested for prevention of recurrent diverticulitis include rifaximin and probiotics.137, 138 However, these studies have been small and of low quality.117 Pharmacologic agents are needed to reduce risk of recurrent diverticulitis.
Surgery
Patients with a phlegmon or small abscess (Hinchey grade I or II) can be managed with bowel rest, antibiotics, and if appropriate with percutaneous drainage (Figure 3). Surgical treatment is rarely warranted due to the minimal opportunity to prevent recurrence or perforation. Specifically, after an incident diagnosis of uncomplicated diverticulitis in an otherwise healthy patient, the risk of recurrence is only 13%–23%, and risk of perforation or other complications in patients with disease recurrence is less than 6%.5, 13 In a RCT of surgery vs conservative management of patients with ongoing and recurrent disease, fewer than 42% of patients actually had recurrence.139 Surgical guidelines therefore urge caution with respect to surgical resection after uncomplicated diverticulitis.5, 74 Moreover, among patients who underwent surgical resection, as many as 15% had a recurrence and as many as 25% have continued chronic pain without imaging evidence of inflammation.20, 140, 141 The decision to move forward with surgery, therefore, should include a discussion with patients about the possibility of persistent or recurrent pain as well as the 10%–20% risk of surgical complications.74 When surgeons and patients together decide for surgery, we recommend complete resection of the apparently affected portion of the colon with primary anastomosis to the rectum and avoidance of a diverting stoma unless the patient is immunocompromised or nutritionally depleted, specifically requests a stoma, or has unexpected surgical findings.
In patients with complicated diverticulitis, the need for operative intervention and type of operation are areas of active investigation. Patients with diverticula-associated fistula, by definition, have chronic disease and can almost always undergo an elective operation with a primary anastomosis. Increasingly, only patients who are hemodynamically unstable with severe sepsis and/or peritonitis require an urgent Hartmann procedure (a sigmoid colectomy with end colostomy). The rationale for colostomy in this case is that infection, inflammation, and shock will compromise healing of a descending colon–rectum anastomosis. However, this rationale has been based on tradition and expert opinion, rather than data. Even among patients with purulent or fecal peritonitis (Hinchey grade III–IV), the need for an urgent colostomy is increasingly questioned.
Patients with acute complicated diverticulitis who are hemodynamically stable but are not improving clinically after several days of conservative management will likely require an operative intervention. Several recent surgical RCTs from Europe have compared the Hartmann procedure to anastomosis with diverting loop ileostomy, anastomosis without stoma, and laparoscopic lavage among patients with CT- or laparoscopically-diagnosed Hinchey III-IV diverticulitis (Table 2).142, 143 These findings indicate the difficulty of applying RCT principles to studies of acute disorders that require surgery—recruitment was hampered, blinding was impossible, and rigorous technical standardization was not realistic. Several studies closed prematurely due to slow or inadequate recruitment.139, 143 Each study used a different primary outcome, making it difficult to summarize or compare results. In general, however, post-operative complications, mortality, and stoma-free survival after a Hartmann procedure were equivalent or inferior to colectomy with a primary anastomosis with or without a diverting loop ileostomy. Investigators also found that diverting loop ileostomies were more likely to be closed within 12 months of the incident diagnosis than colostomies, supporting primary anastomosis over the Hartmann procedure.
Laparoscopic lavage, which initially appeared promising among small cohort studies, has not performed well and even led to early closure of the RCT LADIES-LOLA (LaparOscopic LAvage) group due to an increased need for unplanned urgent surgery, in spite of fewer stomas in the lavage arm than the surgical resection arm.144 The Scandinavian diverticulitis trial similarly found more reoperations but ultimately a lower stoma rate in the lavage arm.145 The diverticulitis-laparoscopic lavage trial found fewer operations and improved stoma-free survival in the lavage group, and, in contrast to the 2 previous studies, concluded that the use of lavage was feasible and safe.146 Unfortunately, lavage has been studied only as a substitute for resection (definitive treatment) rather than as a bridge to resection (damage control) with a primary anastomosis.147 At this time, the use of laparoscopic lavage is not recommended outside of clinical trials given its association with persistent and recurrent abdominal sepsis.
There are several special case patient profiles that deserve mention: young patients, the elderly, and those who are immunocompromised. Although epidemiologic trends indicate that younger patients comprise an increasingly large proportion of all patients diagnosed and have an increased risk of recurrence,13, 14 there is no evidence that they fare worse than their older counterparts if treated non-operatively.148 Similarly, the elderly are not more likely to experience recurrence. Based on nationally representative longitudinal data, more than 85% of inpatient cases were managed non-operatively and only 3% required a future operation. Odds of recurrence were even lower among those aged 80 years and older.149 On the other hand, elective surgery in elderly patients is associated with higher rates of mortality, intestinal diversion and hospital readmission.150 Therefore, aggressive intervention is not indicated based on young age, and conservative management is strongly recommended for stable elderly patients who are not suffering from obstructive symptoms or fistula.
Elective surgery for stable patients with resolved complicated diverticulitis or multiple recurrent episodes of disease might reduce the risk of further recurrence or ongoing symptoms. It is unclear whether immunocompromised patients are more likely to develop recurrent disease, but delayed diagnosis and poor outcomes during recurrent episodes, including mortality, appear to be more likely.62, 63 Guidelines recommend a lower threshold for prophylactic surgery in the setting of ongoing immunocompromise.74
Although the indications for elective surgery for diverticulitis have become more nuanced and require an assessment of patient values and preferences, the goals of surgical treatment are unchanged. Surgical priorities focus on first controlling the source of infection, second reducing morbidity, and third increasing quality of life.5 Controlling the source of infection requires resection of affected sigmoid colon, and not merely oversewing, or lavaging the area of abscess. The most important adverse outcomes of surgical treatment are persistent or recurrent abdominal sepsis, unplanned reoperation, and long-term colostomy or ileostomy. To minimize such events, we recommend surgical mobilization of the splenic flexure of the colon to achieve a tension-free and well-perfused anastomosis, complete resection of thickened or inflamed colon, ensuring that the distal anastomotic edge is rectum (not residual sigmoid) as indicated by flared tenia and no distal diverticula, and avoidance of the Hartmann procedure when feasible.5 Increasing quality of life usually implies avoidance of a colostomy, although select patients may actually experience an improved quality of life with a colostomy. Concerns about impaired anastomotic healing should prompt consideration of a primary colon to rectum anastomosis with a diverting loop ileostomy given a reasonably clean surgical field.
Post-treatment recommendations
Guidelines recommend that a colonoscopy be performed 4–8 weeks following recovery from an episode of diverticulitis in patients who have not had a recent, high-quality colonoscopy even among those who were diagnosed via CT scanning.116 This recommendation is based on multiple observational studies of patients with imaging-diagnosed diverticulitis that subsequently underwent colonoscopy. A pooled analysis indicated that 1 out of 67 patients initially diagnosed with diverticulitis would have a misdiagnosed colon cancer on follow up colonoscopy.117 These cancers were almost exclusively located in the area of suspected previous diverticulitis. Patients with suspicious imaging or presentations were probably more likely to be referred for colonoscopy than those with a typical course, thereby biasing the results of such observational studies. In a randomized trial of antibiotics vs no antibiotics, 6 patients out of 893 screened for eligibility were initially felt to have a clinical presentation and imaging consistent with diverticulitis but were subsequently found to have colon cancer.21
Future Directions
Diverticulitis has a considerable impact on patients and the health care systems. Large, prospective cohort studies have identified the importance of diet, lifestyle, medication, and genetic factors in development. Increased understanding of the natural history of diverticulosis and diverticulitis has led to less aggressive management approaches, and findings from studies of inflammation and the intestinal microbiome have stimulated new avenues of research and treatment. Diverticular disease research has increased rapidly in the last 2 decades. An informal PubMed search for this article using the terms diverticulosis, diverticulitis, or diverticular disease found that the number of published articles increased 3.5-fold, from approximately 200 in 2000 to nearly 700 in 2017. Nonetheless, our understanding of the pathogenesis of diverticulitis is superficial, and treatment algorithms are still largely based on medical dogma and expert opinion.
RCTs have begun to guide evidence-based care of patients with diverticulitis. However, it is difficult to perform high-quality RCTs due to factors such as inadequate sample sizes to detect differences in infrequent but serious events, and inability to blind treatment allocation. Well-supported, definitive consensus treatment guidelines therefore do not exist, so clinicians must often use their judgment to select treatment. The fact that most major RCTs of medical and surgical treatment have been performed in Europe is a call to action for investigators in the US. The medical community has much to learn, and patients have much to gain from future research of this common, costly, and complicated disease (Table 3).
Table 3.
Areas of Unmet Need in Diverticular Disease
| Epidemiology |
| Delineate factors associated with the rapid rise in diverticulitis incidence among young patients |
| Understand the long-term, global impact of acute diverticulitis on patients |
| Identify markers (clinical and biomarkers) of risk of disease progression/recurrence |
| Examine sex and racial differences in the incidence of diverticulitis |
| Define the natural history of diverticulitis in immunocompromised patients |
| Pathogenesis |
| Determine the role of systemic and mucosal inflammation in the development diverticulitis |
| Examine prospectively the gut microbiota and related metabolites in diverticulitis |
| Clarify risk genes within genetic risk loci associated with diverticulitis and determine mechanisms |
| Delineate the role of connective tissue and enteric nerve abnormalities in diverticulitis |
| Medical Treatment |
| Develop accurate clinical prediction tools to aide in the diagnosis and triage of acute diverticulitis |
| Examine the role of ultrasound in the diagnosis of diverticulitis (in the U.S.) |
| Define the long-term consequences of withholding antibiotics in uncomplicated diverticulitis |
| Compare dietary strategies in the treatment of uncomplicated diverticulitis |
| Study pain control strategies for complicated and uncomplicated diverticulitis |
| Surgical Treatment |
| Compare outcomes in patients undergoing surgery vs no surgery for recurrent diverticulitis |
| Understand long-term outcomes of medical vs surgical treatment of complicated diverticulitis |
| Identify predictors of postoperative complications |
| Identify predictors of resolution of symptoms following surgical resection |
| Determine the optimal timing of surgery among patient with persistent inflammatory symptoms |
| Evaluate the modern prognostic value of the Hinchey classification system |
| Prevention |
| Define the role of diet and lifestyle modification in the prevention of recurrent diverticulitis |
| Develop and test new pharmaceutical agents in the prevention of recurrent diverticulitis |
| Compare medical vs surgical approach to prevention of recurrent diverticulitis |
Supplementary Material
What You Need to Know.
BACKGROUND AND CONTEXT
Diverticulitis of the colon is one of the most common gastrointestinal disorders in the outpatient and inpatient setting. Research on the epidemiology, pathophysiology and treatment of this disorder has accelerated over the past decade.
NEW FINDINGS
Sigmoid diverticulitis is increasingly prevalent, partly associated with the aging population, but also due to increased incidence among younger Americans. Recurrence occurs in approximately 20% of patients, and disease complicated by peritonitis is more common in the first episode. Diet, lifestyle and certain medications are risk factors for incident diverticulitis. The etiopathogenesis of diverticulitis is thought to involve diet and lifestyle factors, microbiota alterations, neuromuscular abnormalities and genetic predisposition. Randomized control trials indicate that antibiotics may not be helpful for uncomplicated diverticulitis. The decision for elective and even acute surgery should be individualized and primary anastomosis with or without a diverting loop ileostomy is safe in most situations.
LIMITATIONS
Observational studies have been limited by potential selection bias and surgical randomized trials have been hampered by difficulty with recruitment.
IMPACT
Data regarding risk for and treatment of diagnosis have illuminated previous misconceptions about diverticulitis. Much work remains, however, to better understand the pathophysiology of diverticulitis and the best means to prevent incident and recurrent disease as well as more definitive recommendations for management.
Abbreviations:
- IBD
inflammatory bowel disease
- SCAD
segmental colitis associated with diverticular disease
- SUDD
symptomatic uncomplicated diverticular disease
- IBS
irritable bowel syndrome
- NSAID
nonsteroidal anti-inflammatory drug
- RCT
randomized controlled trial
- SCFAs
short chain fatty acids
- RR
relative risk
- OR
odds ratio
Footnotes
Conflict of interest: The authors have no relevant conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Peery AF, Crockett SD, Barritt AS, et al. Burden of Gastrointestinal, Liver, and Pancreatic Diseases in the United States. Gastroenterology 2015;149:1731–1741 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Etzioni DA, Mack TM, Beart RW Jr., et al. Diverticulitis in the United States: 1998–2005: changing patterns of disease and treatment. Ann Surg 2009;249:210–7. [DOI] [PubMed] [Google Scholar]
- 3.Peery AF, Dellon ES, Lund J, et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology 2012;143:1179–1187 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Painter NS, Burkitt DP. Diverticular disease of the colon: a deficiency disease of Western civilization. Br Med J 1971;2:450–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Regenbogen SE, Hardiman KM, Hendren S, et al. Surgery for diverticulitis in the 21st century: a systematic review. JAMA Surg 2014;149:292–303. [DOI] [PubMed] [Google Scholar]
- 6.Morris AM, Regenbogen SE, Hardiman KM, et al. Sigmoid diverticulitis: a systematic review. JAMA 2014;311:287–97. [DOI] [PubMed] [Google Scholar]
- 7.Lamps LW, Knapple WL. Diverticular disease-associated segmental colitis. Clin Gastroenterol Hepatol 2007;5:27–31. [DOI] [PubMed] [Google Scholar]
- 8.Spiller R Is it diverticular disease or is it irritable bowel syndrome? Dig Dis 2012;30:64–9. [DOI] [PubMed] [Google Scholar]
- 9.Cohen E, Fuller G, Bolus R, et al. Increased risk for irritable bowel syndrome after acute diverticulitis. Clin Gastroenterol Hepatol 2013;11:1614–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hughes LE. Postmortem survey of diverticular disease of the colon. II. The muscular abnormality of the sigmoid colon. Gut 1969;10:344–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shahedi K, Fuller G, Bolus R, et al. Long-term risk of acute diverticulitis among patients with incidental diverticulosis found during colonoscopy. Clin Gastroenterol Hepatol 2013;11:1609–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peery AF, Keku TO, Martin CF, et al. Distribution and Characteristics of Colonic Diverticula in a United States Screening Population. Clin Gastroenterol Hepatol 2016;14:980–985 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bharucha AE, Parthasarathy G, Ditah I, et al. Temporal Trends in the Incidence and Natural History of Diverticulitis: A Population-Based Study. Am J Gastroenterol 2015;110:1589–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wheat CL, Strate LL. Trends in Hospitalization for Diverticulitis and Diverticular Bleeding in the United States From 2000 to 2010. Clin Gastroenterol Hepatol 2016;14:96–103 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maguire LH, Song M, Strate LL, et al. Association of geographic and seasonal variation with diverticulitis admissions. JAMA Surg 2015;150:74–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nikberg M, Ji J, Leppert J, et al. Socioeconomic characteristics and comorbidities of diverticular disease in Sweden 1997–2012. Int J Colorectal Dis 2017;32:1591–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fong SS, Tan EY, Foo A, et al. The changing trend of diverticular disease in a developing nation. Colorectal Dis 2011;13:312–6. [DOI] [PubMed] [Google Scholar]
- 18.Hjern F, Johansson C, Mellgren A, et al. Diverticular disease and migration--the influence of acculturation to a Western lifestyle on diverticular disease. Aliment Pharmacol Ther 2006;23:797–805. [DOI] [PubMed] [Google Scholar]
- 19.Humes DJ, Solaymani-Dodaran M, Fleming KM, et al. A population-based study of perforated diverticular disease incidence and associated mortality. Gastroenterology 2009;136:1198–205. [DOI] [PubMed] [Google Scholar]
- 20.Boostrom SY, Wolff BG, Cima RR, et al. Uncomplicated diverticulitis, more complicated than we thought. J Gastrointest Surg 2012;16:1744–9. [DOI] [PubMed] [Google Scholar]
- 21.Daniels L, Unlu C, de Korte N, et al. Randomized clinical trial of observational versus antibiotic treatment for a first episode of CT-proven uncomplicated acute diverticulitis. Br J Surg 2017;104:52–61. [DOI] [PubMed] [Google Scholar]
- 22.Ambrosetti P, Robert JH, Witzig JA, et al. Acute left colonic diverticulitis in young patients. J Am Coll Surg 1994;179:156–60. [PubMed] [Google Scholar]
- 23.Anaya DA, Flum DR. Risk of emergency colectomy and colostomy in patients with diverticular disease. Arch Surg 2005;140:681–5. [DOI] [PubMed] [Google Scholar]
- 24.Kaiser AM, Jiang JK, Lake JP, et al. The management of complicated diverticulitis and the role of computed tomography. Am J Gastroenterol 2005;100:910–7. [DOI] [PubMed] [Google Scholar]
- 25.Hall JF, Roberts PL, Ricciardi R, et al. Long-term follow-up after an initial episode of diverticulitis: what are the predictors of recurrence? Dis Colon Rectum 2011;54:283–8. [DOI] [PubMed] [Google Scholar]
- 26.Dickerson EC, Chong ST, Ellis JH, et al. Recurrence of Colonic Diverticulitis: Identifying Predictive CT Findings-Retrospective Cohort Study. Radiology 2017;285:850–858. [DOI] [PubMed] [Google Scholar]
- 27.El-Sayed C, Radley S, Mytton J, et al. Risk of Recurrent Disease and Surgery Following an Admission for Acute Diverticulitis. Dis Colon Rectum 2018;61:382–389. [DOI] [PubMed] [Google Scholar]
- 28.Humes DJ, West J. Role of acute diverticulitis in the development of complicated colonic diverticular disease and 1-year mortality after diagnosis in the UK: population-based cohort study. Gut 2011. [DOI] [PubMed] [Google Scholar]
- 29.Ritz JP, Lehmann KS, Frericks B, et al. Outcome of patients with acute sigmoid diverticulitis: multivariate analysis of risk factors for free perforation. Surgery 2011;149:606–13. [DOI] [PubMed] [Google Scholar]
- 30.van Dijk ST, Daniels L, Nio CY, et al. Predictive factors on CT imaging for progression of uncomplicated into complicated acute diverticulitis. Int J Colorectal Dis 2017;32:1693–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ambrosetti P, Becker C, Terrier F. Colonic diverticulitis: impact of imaging on surgical management -- a prospective study of 542 patients. Eur Radiol 2002;12:1145–9. [DOI] [PubMed] [Google Scholar]
- 32.Gregersen R, Andresen K, Burcharth J, et al. Long-term mortality and recurrence in patients treated for colonic diverticulitis with abscess formation: a nationwide register-based cohort study. Int J Colorectal Dis 2018;33:431–440. [DOI] [PubMed] [Google Scholar]
- 33.Chabok A, Pahlman L, Hjern F, et al. Randomized clinical trial of antibiotics in acute uncomplicated diverticulitis. Br J Surg 2012;99:532–9. [DOI] [PubMed] [Google Scholar]
- 34.Spiegel BM, Reid MW, Bolus R, et al. Development and validation of a disease-targeted quality of life instrument for chronic diverticular disease: the DV-QOL. Qual Life Res 2015;24:163–79. [DOI] [PubMed] [Google Scholar]
- 35.Peery AF, Keku TO, Addamo C, et al. Colonic Diverticula Are Not Associated With Mucosal Inflammation or Chronic Gastrointestinal Symptoms. Clin Gastroenterol Hepatol 2018;16:884–891 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Strate LL, Erichsen R, Horvath-Puho E, et al. Diverticular disease is associated with increased risk of subsequent arterial and venous thromboembolic events. Clin Gastroenterol Hepatol 2014;12:1695–701 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cao Y, Strate LL, Keeley BR, et al. Meat intake and risk of diverticulitis among men. Gut 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strate LL, Keeley BR, Cao Y, et al. Western Dietary Pattern Increases, and Prudent Dietary Pattern Decreases, Risk of Incident Diverticulitis in a Prospective Cohort Study. Gastroenterology 2017;152:1023–1030 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hjern F, Wolk A, Hakansson N. Obesity, physical inactivity, and colonic diverticular disease requiring hospitalization in women: a prospective cohort study. Am J Gastroenterol 2012;107:296–302. [DOI] [PubMed] [Google Scholar]
- 40.Rosemar A, Angeras U, Rosengren A. Body Mass Index and Diverticular Disease: A 28-Year Follow-Up Study in Men. Dis Colon Rectum 2007. [DOI] [PubMed] [Google Scholar]
- 41.Strate LL, Liu YL, Aldoori WH, et al. Obesity increases the risks of diverticulitis and diverticular bleeding. Gastroenterology 2009;136:115–122 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ma W, Jovani M, Liu PH, et al. Association Between Obesity and Weight Change and Risk of Diverticulitis in Women. Gastroenterology 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aldoori WH, Giovannucci EL, Rimm EB, et al. A prospective study of alcohol, smoking, caffeine, and the risk of symptomatic diverticular disease in men. Ann Epidemiol 1995;5:221–8. [DOI] [PubMed] [Google Scholar]
- 44.Hjern F, Wolk A, Hakansson N. Smoking and the risk of diverticular disease in women. Br J Surg 2011;98:997–1002. [DOI] [PubMed] [Google Scholar]
- 45.Humes DJ, Ludvigsson JF, Jarvholm B. Smoking and the Risk of Hospitalization for Symptomatic Diverticular Disease: A Population-Based Cohort Study from Sweden. Dis Colon Rectum 2016;59:110–4. [DOI] [PubMed] [Google Scholar]
- 46.Aune D, Sen A, Leitzmann MF, et al. Tobacco smoking and the risk of diverticular disease - a systematic review and meta-analysis of prospective studies. Colorectal Dis 2017;19:621–633. [DOI] [PubMed] [Google Scholar]
- 47.Aldoori WH, Giovannucci EL, Rimm EB, et al. A prospective study of diet and the risk of symptomatic diverticular disease in men. Am J Clin Nutr 1994;60:757–64. [DOI] [PubMed] [Google Scholar]
- 48.Crowe FL, Appleby PN, Allen NE, et al. Diet and risk of diverticular disease in Oxford cohort of European Prospective Investigation into Cancer and Nutrition (EPIC): prospective study of British vegetarians and non-vegetarians. BMJ 2011;343:d4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Strate LL, Liu YL, Syngal S, et al. Nut, corn, and popcorn consumption and the incidence of diverticular disease. JAMA 2008;300:907–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aldoori WH, Giovannucci EL, Rimm EB, et al. Prospective study of physical activity and the risk of symptomatic diverticular disease in men. Gut 1995;36:276–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Strate LL, Liu YL, Aldoori WH, et al. Physical activity decreases diverticular complications. Am J Gastroenterol 2009;104:1221–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tonnesen H, Engholm G, Moller H. Association between alcoholism and diverticulitis. Br J Surg 1999;86:1067–8. [DOI] [PubMed] [Google Scholar]
- 53.Liu PH, Cao Y, Keeley BR, et al. Adherence to a Healthy Lifestyle is Associated With a Lower Risk of Diverticulitis among Men. Am J Gastroenterol 2017;112:1868–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kvasnovsky CL, Papagrigoriadis S, Bjarnason I. Increased diverticular complications with nonsteriodal anti-inflammatory drugs and other medications: a systematic review and meta-analysis. Colorectal Dis 2014;16:O189–96. [DOI] [PubMed] [Google Scholar]
- 55.Aldoori WH, Giovannucci EL, Rimm EB, et al. Use of acetaminophen and nonsteroidal anti-inflammatory drugs: a prospective study and the risk of symptomatic diverticular disease in men. Arch Fam Med 1998;7:255–60. [DOI] [PubMed] [Google Scholar]
- 56.Humes DJ, Fleming KM, Spiller RC, et al. Concurrent drug use and the risk of perforated colonic diverticular disease: a population-based case-control study. Gut 2011;60:219–24. [DOI] [PubMed] [Google Scholar]
- 57.Strate LL, Liu YL, Huang ES, et al. Use of Aspirin or Nonsteroidal Anti-inflammatory Drugs Increases Risk for Diverticulitis and Diverticular Bleeding. Gastroenterology 2011;140:1427–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Freckelton J, Evans JA, Croagh D, et al. Metformin use in diabetics with diverticular disease is associated with reduced incidence of diverticulitis. Scand J Gastroenterol 2017;52:969–972. [DOI] [PubMed] [Google Scholar]
- 59.Morris CR, Harvey IM, Stebbings WS, et al. Do calcium channel blockers and antimuscarinics protect against perforated colonic diverticular disease? A case control study. Gut 2003;52:1734–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maguire LH, Song M, Strate LE, et al. Higher Serum Levels of Vitamin D Are Associated With a Reduced Risk of Diverticulitis. Clin Gastroenterol Hepatol 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McGuire HH Jr. Bleeding colonic diverticula. A reappraisal of natural history and management. Ann Surg 1994;220:653–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Biondo S, Borao JL, Kreisler E, et al. Recurrence and virulence of colonic diverticulitis in immunocompromised patients. Am J Surg 2012;204:172–9. [DOI] [PubMed] [Google Scholar]
- 63.Samdani T, Pieracci FM, Eachempati SR, et al. Colonic diverticulitis in chemotherapy patients: should operative indications change? A retrospective cohort study. Int J Surg 2014;12:1489–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Humes DJ, Spiller RC. Review article: The pathogenesis and management of acute colonic diverticulitis. Aliment Pharmacol Ther 2014;39:359–70. [DOI] [PubMed] [Google Scholar]
- 65.Pai JK, Mukamal KJ, Rexrode KM, et al. C-reactive protein (CRP) gene polymorphisms, CRP levels, and risk of incident coronary heart disease in two nested case-control studies. PLoS One 2008;3:e1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pradhan AD, Manson JE, Rifai N, et al. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 2001;286:327–34. [DOI] [PubMed] [Google Scholar]
- 67.Ridker PM, Buring JE, Cook NR, et al. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: an 8-year follow-up of 14 719 initially healthy American women. Circulation 2003;107:391–7. [DOI] [PubMed] [Google Scholar]
- 68.Altadill A, Eiro N, Gonzalez LO, et al. Comparative analysis of the expression of metalloproteases and their inhibitors in resected crohn’s disease and complicated diverticular disease. Inflamm Bowel Dis 2012;18:120–30. [DOI] [PubMed] [Google Scholar]
- 69.von Rahden BH, Jurowich C, Kircher S, et al. Allergic predisposition, histamine and histamine receptor expression (H1R, H2R) are associated with complicated courses of sigmoid diverticulitis. J Gastrointest Surg 2012;16:173–82; discussion 182. [DOI] [PubMed] [Google Scholar]
- 70.Barbara G, Scaioli E, Barbaro MR, et al. Gut microbiota, metabolome and immune signatures in patients with uncomplicated diverticular disease. Gut 2017;66:1252–1261. [DOI] [PubMed] [Google Scholar]
- 71.Tursi A, Brandimarte G, Elisei W, et al. Faecal calprotectin in colonic diverticular disease: a case-control study. Int J Colorectal Dis 2009;24:49–55. [DOI] [PubMed] [Google Scholar]
- 72.Floch MH, White J. Diverticulitis: new concepts and new therapies. J Clin Gastroenterol 2005;39:355–6. [DOI] [PubMed] [Google Scholar]
- 73.Simpson J, Scholefield JH, Spiller RC. Pathogenesis of colonic diverticula. Br J Surg 2002;89:546–54. [DOI] [PubMed] [Google Scholar]
- 74.Feingold D, Steele SR, Lee S, et al. Practice parameters for the treatment of sigmoid diverticulitis. Dis Colon Rectum 2014;57:284–94. [DOI] [PubMed] [Google Scholar]
- 75.De Filippo C, Cavalieri D, Di Paola M, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A 2010;107:14691–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tomkins AM, Bradley AK, Oswald S, et al. Diet and the faecal microflora of infants, children and adults in rural Nigeria and urban U.K. J Hyg (Lond) 1981;86:285–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kang JY, Dhar A, Pollok R, et al. Diverticular disease of the colon: ethnic differences in frequency. Aliment Pharmacol Ther 2004;19:765–9. [DOI] [PubMed] [Google Scholar]
- 78.Lopez-Garcia E, Schulze MB, Fung TT, et al. Major dietary patterns are related to plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr 2004;80:1029–35. [DOI] [PubMed] [Google Scholar]
- 79.Turnbaugh PJ, Backhed F, Fulton L, et al. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe 2008;3:213–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Turnbaugh PJ, Gordon JI. The core gut microbiome, energy balance, and obesity. J Physiol 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Martinez-Medina M, Denizot J, Dreux N, et al. Western diet induces dysbiosis with increased E coli in CEABAC10 mice, alters host barrier function favouring AIEC colonisation. Gut 2014;63:116–24. [DOI] [PubMed] [Google Scholar]
- 82.Claesson MJ, Jeffery IB, Conde S, et al. Gut microbiota composition correlates with diet and health in the elderly. Nature 2012;488:178–84. [DOI] [PubMed] [Google Scholar]
- 83.Cotillard A, Kennedy SP, Kong LC, et al. Dietary intervention impact on gut microbial gene richness. Nature 2013;500:585–8. [DOI] [PubMed] [Google Scholar]
- 84.De Filippis F, Pellegrini N, Vannini L, et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut 2016;65:1812–1821. [DOI] [PubMed] [Google Scholar]
- 85.Makki K, Deehan EC, Walter J, et al. The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe 2018;23:705–715. [DOI] [PubMed] [Google Scholar]
- 86.Koh A, De Vadder F, Kovatcheva-Datchary P, et al. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016;165:1332–1345. [DOI] [PubMed] [Google Scholar]
- 87.Kvasnovsky CL, Leong LEX, Choo JM, et al. Clinical and symptom scores are significantly correlated with fecal microbiota features in patients with symptomatic uncomplicated diverticular disease: a pilot study. Eur J Gastroenterol Hepatol 2018;30:107–112. [DOI] [PubMed] [Google Scholar]
- 88.Tursi A, Mastromarino P, Capobianco D, et al. Assessment of Fecal Microbiota and Fecal Metabolome in Symptomatic Uncomplicated Diverticular Disease of the Colon. J Clin Gastroenterol 2016;50 Suppl 1:S9–S12. [DOI] [PubMed] [Google Scholar]
- 89.Jones RB, Fodor AA, Peery AF, et al. An Aberrant Microbiota is not Strongly Associated with Incidental Colonic Diverticulosis. Sci Rep 2018;8:4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gueimonde M, Ouwehand A, Huhtinen H, et al. Qualitative and quantitative analyses of the bifidobacterial microbiota in the colonic mucosa of patients with colorectal cancer, diverticulitis and inflammatory bowel disease. World J Gastroenterol 2007;13:3985–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Daniels L, Budding AE, de Korte N, et al. Fecal microbiome analysis as a diagnostic test for diverticulitis. Eur J Clin Microbiol Infect Dis 2014;33:1927–36. [DOI] [PubMed] [Google Scholar]
- 92.Gevers D, Kugathasan S, Denson LA, et al. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe 2014;15:382–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Frank DN, St Amand AL, Feldman RA, et al. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A 2007;104:13780–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hullar MA, Sandstrom R, Lampe JW, et al. The fecal microbiome differentiates patients with a history of diverticulitis vs those with uncomplicated diverticulosis. Gastroenterology 2017;152:S624. [Google Scholar]
- 95.Granlund J, Svensson T, Olen O, et al. The genetic influence on diverticular disease - a twin study. Aliment Pharmacol Ther 2012. [DOI] [PubMed] [Google Scholar]
- 96.Strate LL, Erichsen R, Baron JA, et al. Heritability and familial aggregation of diverticular disease: a population-based study of twins and siblings. Gastroenterology 2013;144:736–742 e1. [DOI] [PubMed] [Google Scholar]
- 97.Sigurdsson S, Alexandersson KF, Sulem P, et al. Sequence variants in ARHGAP15, COLQ and FAM155A associate with diverticular disease and diverticulitis. Nat Commun 2017;8:15789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Maguire LH, Handelman SK, Du X, et al. Genome-wide association analyses identify 39 new susceptibility loci for diverticular disease. Nat Genet 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Connelly TM, Berg AS, Hegarty JP, et al. The TNFSF15 gene single nucleotide polymorphism rs7848647 is associated with surgical diverticulitis. Ann Surg 2014;259:1132–7. [DOI] [PubMed] [Google Scholar]
- 100.Connelly TM, Choi CS, Berg AS, et al. Diverticulitis and Crohn’s disease have distinct but overlapping tumor necrosis superfamily 15 haplotypes. J Surg Res 2017;214:262–269. [DOI] [PubMed] [Google Scholar]
- 101.Coble JL, Sheldon KE, Yue F, et al. Identification of a rare LAMB4 variant associated with familial diverticulitis through exome sequencing. Hum Mol Genet 2017;26:3212–3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bassotti G, Villanacci V, Bernardini N, et al. Diverticular Disease of the Colon: Neuromuscular Function Abnormalities. J Clin Gastroenterol 2016;50 Suppl 1:S6–8. [DOI] [PubMed] [Google Scholar]
- 103.Beighton PH, Murdoch JL, Votteler T. Gastrointestinal complications of the Ehlers-Danlos syndrome. Gut 1969;10:1004–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Stagi S, Lapi E, Chiarelli F, et al. Incidence of diverticular disease and complicated diverticular disease in young patients with Williams syndrome. Pediatr Surg Int 2010;26:943–4. [DOI] [PubMed] [Google Scholar]
- 105.Suster SM, Ronnen M, Bubis JJ. Diverticulosis coli in association with Marfan’s syndrome. Arch Intern Med 1984;144:203. [PubMed] [Google Scholar]
- 106.Wedel T, Barrenschee M, Lange C, et al. Morphologic Basis for Developing Diverticular Disease, Diverticulitis, and Diverticular Bleeding. Viszeralmedizin 2015;31:76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bassotti G, Battaglia E, Bellone G, et al. Interstitial cells of Cajal, enteric nerves, and glial cells in colonic diverticular disease. J Clin Pathol 2005;58:973–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bottner M, Barrenschee M, Hellwig I, et al. The GDNF System Is Altered in Diverticular Disease - Implications for Pathogenesis. PLoS One 2013;8:e66290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bottner M, Barrenschee M, Hellwig I, et al. The enteric serotonergic system is altered in patients with diverticular disease. Gut 2013;62:1753–62. [DOI] [PubMed] [Google Scholar]
- 110.Banerjee S, Akbar N, Moorhead J, et al. Increased presence of serotonin-producing cells in colons with diverticular disease may indicate involvement in the pathophysiology of the condition. Int J Colorectal Dis 2007;22:643–9. [DOI] [PubMed] [Google Scholar]
- 111.Simpson J, Sundler F, Humes DJ, et al. Post inflammatory damage to the enteric nervous system in diverticular disease and its relationship to symptoms. Neurogastroenterol Motil 2009;21:847–e58. [DOI] [PubMed] [Google Scholar]
- 112.Bassotti G, Battaglia E, Spinozzi F, et al. Twenty-four hour recordings of colonic motility in patients with diverticular disease: evidence for abnormal motility and propulsive activity. Dis Colon Rectum 2001;44:1814–20. [DOI] [PubMed] [Google Scholar]
- 113.Andeweg CS, Mulder IM, Felt-Bersma RJ, et al. Guidelines of diagnostics and treatment of acute left-sided colonic diverticulitis. Dig Surg 2013;30:278–92. [DOI] [PubMed] [Google Scholar]
- 114.Kruis W, Germer CT, Leifeld L, et al. Diverticular disease: guidelines of the german society for gastroenterology, digestive and metabolic diseases and the german society for general and visceral surgery. Digestion 2014;90:190–207. [DOI] [PubMed] [Google Scholar]
- 115.Vennix S, Morton DG, Hahnloser D, et al. Systematic review of evidence and consensus on diverticulitis: an analysis of national and international guidelines. Colorectal Dis 2014;16:866–78. [DOI] [PubMed] [Google Scholar]
- 116.Stollman N, Smalley W, Hirano I, et al. American Gastroenterological Association Institute Guideline on the Management of Acute Diverticulitis. Gastroenterology 2015;149:1944–9. [DOI] [PubMed] [Google Scholar]
- 117.Strate LL, Peery AF, Neumann I. American Gastroenterological Association Institute Technical Review on the Management of Acute Diverticulitis. Gastroenterology 2015;149:1950–1976 e12. [DOI] [PubMed] [Google Scholar]
- 118.van Dijk ST, Daniels L, Unlu C, et al. Long-Term Effects of Omitting Antibiotics in Uncomplicated Acute Diverticulitis. Am J Gastroenterol 2018;113:1045–1052. [DOI] [PubMed] [Google Scholar]
- 119.Peery AF. It’s Actually a Little Complicated: Antibiotics for Uncomplicated Diverticulitis. Am J Gastroenterol 2018;113:949–950. [DOI] [PubMed] [Google Scholar]
- 120.Schug-Pass C, Geers P, Hugel O, et al. Prospective randomized trial comparing short-term antibiotic therapy versus standard therapy for acute uncomplicated sigmoid diverticulitis. Int J Colorectal Dis 2010;25:751–9. [DOI] [PubMed] [Google Scholar]
- 121.Biondo S, Golda T, Kreisler E, et al. Outpatient versus hospitalization management for uncomplicated diverticulitis: a prospective, multicenter randomized clinical trial (DIVER Trial). Ann Surg 2014;259:38–44. [DOI] [PubMed] [Google Scholar]
- 122.Mizuki A, Nagata H, Tatemichi M, et al. The out-patient management of patients with acute mild-to-moderate colonic diverticulitis. Aliment Pharmacol Ther 2005;21:889–97. [DOI] [PubMed] [Google Scholar]
- 123.Mora Lopez L, Ruiz-Edo N, Serra Pla S, et al. Multicentre, controlled, randomized clinical trial to compare the efficacy and safety of ambulatory treatment of mild acute diverticulitis without antibiotics with the standard treatment with antibiotics. Int J Colorectal Dis 2017;32:1509–1516. [DOI] [PubMed] [Google Scholar]
- 124.Stam MA, Draaisma WA, van de Wall BJ, et al. An unrestricted diet for uncomplicated diverticulitis is safe: results of a prospective diverticulitis diet study. Colorectal Dis 2017;19:372–377. [DOI] [PubMed] [Google Scholar]
- 125.Andeweg CS, Knobben L, Hendriks JC, et al. How to diagnose acute left-sided colonic diverticulitis: proposal for a clinical scoring system. Ann Surg 2011;253:940–6. [DOI] [PubMed] [Google Scholar]
- 126.Andeweg CS, Wegdam JA, Groenewoud J, et al. Toward an evidence-based step-up approach in diagnosing diverticulitis. Scand J Gastroenterol 2014;49:775–84. [DOI] [PubMed] [Google Scholar]
- 127.Hollerweger A, Macheiner P, Rettenbacher T, et al. Colonic diverticulitis: diagnostic value and appearance of inflamed diverticula-sonographic evaluation. Eur Radiol 2001;11:1956–63. [DOI] [PubMed] [Google Scholar]
- 128.Andeweg C, Peters J, Bleichrodt R, et al. Incidence and risk factors of recurrence after surgery for pathology-proven diverticular disease. World J Surg 2008;32:1501–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Binda GA, Arezzo A, Serventi A, et al. Multicentre observational study of the natural history of left-sided acute diverticulitis. Br J Surg 2012;99:276–85. [DOI] [PubMed] [Google Scholar]
- 130.Thaler K, Weiss EG, Nogueras JJ, et al. Recurrence rates at minimum 5-year follow-up: laparoscopic versus open sigmoid resection for uncomplicated diverticulitis. Surg Laparosc Endosc Percutan Tech 2003;13:325–7. [DOI] [PubMed] [Google Scholar]
- 131.Raskin JB, Kamm MA, Jamal MM, et al. Mesalamine did not prevent recurrent diverticulitis in phase 3 controlled trials. Gastroenterology 2014;147:793–802. [DOI] [PubMed] [Google Scholar]
- 132.Parente F, Bargiggia S, Prada A, et al. Intermittent treatment with mesalazine in the prevention of diverticulitis recurrence: a randomised multicentre pilot double-blind placebo-controlled study of 24-month duration. Int J Colorectal Dis 2013;28:1423–31. [DOI] [PubMed] [Google Scholar]
- 133.Kruis W, Kardalinos V, Eisenbach T, et al. Randomised clinical trial: mesalazine versus placebo in the prevention of diverticulitis recurrence. Aliment Pharmacol Ther 2017;46:282–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Stollman N, Magowan S, Shanahan F, et al. A randomized controlled study of mesalamine after acute diverticulitis: results of the DIVA trial. J Clin Gastroenterol 2013;47:621–9. [DOI] [PubMed] [Google Scholar]
- 135.Barbara G, Cremon C, Barbaro MR, et al. Treatment of Diverticular Disease With Aminosalicylates: The Evidence. J Clin Gastroenterol 2016;50 Suppl 1:S60–3. [DOI] [PubMed] [Google Scholar]
- 136.Nespoli L, Lo Bianco G, Uggeri F, et al. Effect of oral mesalamine on inflammatory response in acute uncomplicated diverticulitis. World J Gastroenterol 2015;21:8366–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Dughera L, Serra AM, Battaglia E, et al. Acute recurrent diverticulitis is prevented by oral administration of a polybacterial lysate suspension. Minerva Gastroenterol Dietol 2004;50:149–53. [PubMed] [Google Scholar]
- 138.Lanas A, Ponce J, Bignamini A, et al. One year intermittent rifaximin plus fibre supplementation vs. fibre supplementation alone to prevent diverticulitis recurrence: a proof-of-concept study. Dig Liver Dis 2013;45:104–9. [DOI] [PubMed] [Google Scholar]
- 139.van de Wall BJM, Stam MAW, Draaisma WA, et al. Surgery versus conservative management for recurrent and ongoing left-sided diverticulitis (DIRECT trial): an open-label, multicentre, randomised controlled trial. Lancet Gastroenterol Hepatol 2017;2:13–22. [DOI] [PubMed] [Google Scholar]
- 140.Wolff BG, Boostrom SY. Prophylactic resection, uncomplicated diverticulitis, and recurrent diverticulitis. Dig Dis 2012;30:108–13. [DOI] [PubMed] [Google Scholar]
- 141.Horgan AF, McConnell EJ, Wolff BG, et al. Atypical diverticular disease: surgical results. Dis Colon Rectum 2001;44:1315–8. [DOI] [PubMed] [Google Scholar]
- 142.Bridoux V, Regimbeau JM, Ouaissi M, et al. Hartmann’s Procedure or Primary Anastomosis for Generalized Peritonitis due to Perforated Diverticulitis: A Prospective Multicenter Randomized Trial (DIVERTI). J Am Coll Surg 2017;225:798–805. [DOI] [PubMed] [Google Scholar]
- 143.Oberkofler CE, Rickenbacher A, Raptis DA, et al. A multicenter randomized clinical trial of primary anastomosis or Hartmann’s procedure for perforated left colonic diverticulitis with purulent or fecal peritonitis. Ann Surg 2012;256:819–26; discussion 826–7. [DOI] [PubMed] [Google Scholar]
- 144.Vennix S, Musters GD, Mulder IM, et al. Laparoscopic peritoneal lavage or sigmoidectomy for perforated diverticulitis with purulent peritonitis: a multicentre, parallel-group, randomised, open-label trial. Lancet 2015;386:1269–1277. [DOI] [PubMed] [Google Scholar]
- 145.Schultz JK, Wallon C, Blecic L, et al. One-year results of the SCANDIV randomized clinical trial of laparoscopic lavage versus primary resection for acute perforated diverticulitis. Br J Surg 2017;104:1382–1392. [DOI] [PubMed] [Google Scholar]
- 146.Kohl A, Rosenberg J, Bock D, et al. Two-year results of the randomized clinical trial DILALA comparing laparoscopic lavage with resection as treatment for perforated diverticulitis. Br J Surg 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Morris AM. Laparoscopic peritoneal lavage for perforated diverticulitis: in search of evidence. Lancet 2015;386:1219–1221. [DOI] [PubMed] [Google Scholar]
- 148.Etzioni DA, Cannom RR, Ault GT, et al. Diverticulitis in California from 1995 to 2006: increased rates of treatment for younger patients. Am Surg 2009;75:981–5. [PubMed] [Google Scholar]
- 149.Lidor AO, Segal JB, Wu AW, et al. Older patients with diverticulitis have low recurrence rates and rarely need surgery. Surgery 2011;150:146–53. [DOI] [PubMed] [Google Scholar]
- 150.Lidor AO, Schneider E, Segal J, et al. Elective surgery for diverticulitis is associated with high risk of intestinal diversion and hospital readmission in older adults. J Gastrointest Surg 2010;14:1867–73; discussion 1873–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.