Abstract
Cognitive dysfunction (CD) is an insidious and under diagnosed manifestation of SLE that has a considerable impact on quality of life, which can be devastating. Given inconsistencies in modes of assessment and difficulties in attribution to SLE, the reported prevalence of CD ranges from 5–80%. While clinical studies of SLE-related CD have been hampered by heterogeneous subject populations and a lack of sensitive and standardized cognitive batteries or other validated objective biomarkers for CD, there are nonetheless strong data from mouse models and from the clinical arena that CD is related to known disease mechanisms. Several cytokines, inflammatory molecules and antibodies have been associated with CD. Proposed mechanisms for antibody and cytokine-mediated neuronal injury include abrogation of blood-brain barrier integrity with direct access of soluble molecules in the circulation to the brain and ensuing neurotoxicity and microglial activation. No treatments for SLE-mediated CD exist, but potential candidates include agents that inhibit microglial activation such as angiotensin converting enzyme inhibitors or protect blood brain barrier integrity such as C5a receptor blockers. Structural and functional neuroimaging data have shown a range of regional abnormalities in metabolism and white matter microstructural integrity in SLE patients that correlate with CD and could in the future become diagnostic tools, as well as outcome measures in clinical trials aimed at preserving cognitive function in SLE.
A. Introduction
Neuropsychiatric lupus (NPSLE) encompasses a range of neurologic, psychiatric and cognitive disorders that collectively affect up to 40% of SLE patients at the time of diagnosis and a majority of SLE patients throughout the course of their disease (1). NPSLE is associated with worse quality of life independent of SLE activity and medications (1), high unemployment and disability rates (2), high damage accrual (1), and a three- to nine-fold increase in mortality (3). The American College of Rheumatology (ACR) nomenclature from 1999 organized the heterogeneous NPSLE conditions into 19 standardized “case definitions” (4). These can also be classified as central, peripheral and vascular manifestations, or alternatively, as diffuse and focal manifestations. We will focus this review on cognitive dysfunction, a common diffuse central nervous system (CNS) manifestation of NPSLE.
Cognitive dysfunction (CD) can be slowly progressive, and its presence or progression does not necessarily correlate with disease activity. Because the assessments are not standardized and the attribution to SLE is difficult, the prevalence of CD is highly variable, from 6% to 81% (5). SLE patients identify CD as one of their most distressing symptoms (6) that detracts from quality of life; however, CD, with poor screening and diagnostic metrics, is still grossly under-recognized by rheumatologists. Its pathogenesis is poorly understood and no treatments are available.
The ACR nomenclature defines CD as a significant deficit in any or all of the following cognitive domains: simple or complex attention, reasoning, executive skills, memory, visual-spatial processing, language, and psychomotor speed (4). Previous studies have revealed attention, memory and language to be among the most commonly affected domains in SLE (7). Two major obstacles in our understanding of the contribution of SLE to CD are potential confounders in diagnosis and a lack of understanding of pathogenesis. Neurotoxic medications such as glucocorticoids and cyclophosphamide, infection, metabolic disorder, and hypertension can all cause symptoms that overlap with CD (1). Furthermore, it is important to recognize that other CNS manifestations of SLE, such as seizures, stroke and mood disorders, may also contribute to CD. Another prevalent problem with the current approach to studying CD in SLE is that studies often include patients with focal manifestations, such as ischemic stroke, and diffuse manifestations within a single cohort, although pathogenesis is likely to differ in these two groups.
An additional obstacle in studying CD is that a wide variety of cognitive batteries have been used across cohorts; some of these may not be sensitive to a particular cognitive deficit (7). Studies that explore pathogenic mechanisms and the contribution of autoantibodies, cytokines or other mediators to CD may require specifically designed cognitive assessments and patient selection, as CD in different domains may result from different pathogenic mechanisms.
B. Detection of CD
The ascertainment of CD involves both clinical history, i.e. impaired functioning supported by patient-reported outcomes, and neuropsychological testing.
Neurocognitive testing is the gold standard in the diagnosis of CD in NPSLE. The most frequently used testing batteries to assess cognition in SLE, according to a recent review and meta-analysis (7), are comprehensive traditional batteries that are often administered by a psychologist or trained psychometrist (for example, the Rey Complex Figure Test or Trail Making Test) or the Automated Neuropsychological Assessment Metric (ANAM). Other less frequently used tests include the Modified Mini-Mental State Exam (MMSE), the Montreal Cognitive Assessment (MoCA), the Controlled Oral Word Association Test, the Hopkins Verbal Learning Test-Revised, as well as various additional instruments. Importantly, the meta-analysis reported a wide prevalence of CD ranging between 3% and 81%. Several factors may have contributed to this, including patient heterogeneity (SLE subjects with and without predetermined NPSLE), the use of different assessments including those that may not be sensitive to a particular cognitive deficit, and the lack of a standardized definition for CD despite the ACR guideline. Despite these complexities, the overwhelming evidence supports an increased frequency of CD in SLE compared to the general population.
While many studies have compared cognitive tests in SLE, many have included patients with neurologic or psychiatric disease (7), which makes interpretation difficult. Several studies have compared tests in those without known neuropsychiatric disease. One study compared the ANAM to a set of traditional batteries, including those recommended by the ACR, and found that ANAM subtests, particularly those testing learning and memory, correlated with tests in the traditional batteries (8). In regards to the search for an appropriate screening for CD in SLE, one study compared the MMSE, MoCA and Cognitive Symptom Inventory, and found the MoCA to be the most sensitive/specific and highly correlated with the ACR recommended battery (9).
C. Neuroimaging in CD
Neuroimaging has the potential to be a valuable tool for understanding the pathogenesis of CD in SLE and for monitoring treatment response (Fig 1). We focus here on neuroimaging studies of SLE subjects who lacked confounding CNS manifestations to summarize associations of structural or functional lesions with CD.
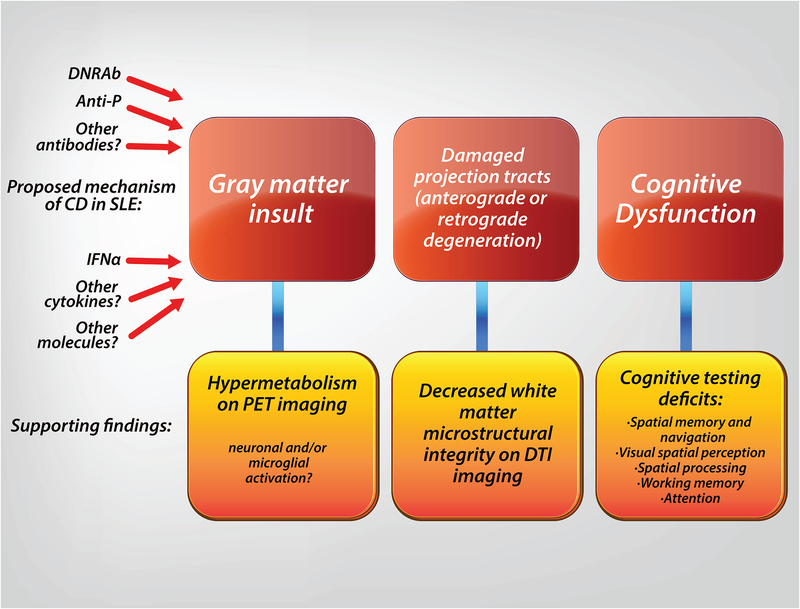
Figure 1: Proposed mechanism of SLE mediated CD.
Neuroimaging studies support a mechanism of CD beginning with hippocampal injury and altered microstructural integrity in the parahippocampus leading to decreased integrity of white matter outflow tracts and resulting in impaired cognition.
MRI studies, both conventional and functional, demonstrate abnormalities in SLE and in SLE patients with CD (10). Conventional MRI studies reveal decreased hippocampal volumes in SLE patients with CD compared to those without CD (10). One study (11) revealed decreased activation of the hippocampus/parahippocampal gyrus on functional MRI during a spatial working memory task in SLE patients, and another found abnormal regional activity in the parahippocampal gyrus on functional MRI during the resting state (12). This is of interest as multiple lines of evidence in rodents reveal that hippocampal integrity is critical for spatial memory (13). Similarly, diffusion tensor imaging (DTI), an advanced MRI technique that assesses white matter integrity, demonstrates SLE-related abnormalities (14). White matter integrity in DTI is often measured by fractional anisotropy (FA), which describes the directionality of water diffusion in tissue. A low FA indicates isotropic diffusion (directionless or random) and represents damaged white matter, which may be due to decreased axonal density, number, diameter, or myelination. Although studies in SLE subjects reveal white matter abnormalities throughout the brain, two independent studies demonstrate an association of decreased FA in the external capsule in SLE patients with CD (15, 16). CD is also correlated with abnormalities in the choline:creatine (Ch/Cr) ratio on magnetic resonance spectroscopy (17, 18). This ratio is used as an index of white matter integrity; choline is essential to neuronal membranes and myelin, while creatinine is a stored phosphate used as a reference. An elevated Ch/Cr is interpreted as increased membrane turnover due to demyelination, ischemia and/or gliosis. Additionally, single photon emission CT displayed a focal area of hypoperfusion in the right precuneus (parietal lobe) in SLE patients with memory impairment compared to those without (19). Hypoperfusion in the parietal lobe is reported in 2 other studies (20, 21).
In vitro studies reveal microglial activation following exposure to SLE serum (22), and studies of murine models of SLE revealed that type I interferon (IFN) mediated microglial activation contributes to CNS damage and possibly to CD (23). Recent advances enhance our ability to assess microglial activity in humans through neuroimaging; the most utilized positron emission tomography (PET) target is the translocator protein 18 kDa (TSPO). PET tracers targeting TSPO have shown that it is expressed on the outer mitochondrial membrane of microglia and is markedly upregulated in response to brain injury and inflammation. In several neurodegenerative diseases, including Alzheimer’s Disease (AD), compelling evidence for TSPO over-expression in disease-specific brain regions exists that associates with poor cognitive performance (24). In SLE, only one neuroimaging study using the TSPO ligand has been performed revealing higher TPSO expression in the cerebellum and hippocampus in those with CD compared to those without (25). Of note, TSPO overexpression in the hippocampus is found in AD and Parkinson’s, and has been found in the cerebellum in AD (24).
Overall, these studies suggest that CD can be assessed through neuroimaging modalities but better definition of cohorts will be needed to correlate specific abnormalities with impairment in specific cognitive domains.
D. Potential molecular mediators of SLE-CD
We highlight several potential mediators of SLE-CD below, although a variety of mechanisms have been proposed (Table 1).
Table 1.
Mechanisms of SLE-CD
| Mechanisms | Antibodies | Cells |
| Disruption of blood brain barrier (5, 60, 62–64, 83) Mononuclear cell infiltration (51, 55, 56) Antibody-mediated injury (43) Cytokine-mediated injury (43) Microglial synaptic pruning (23) |
DNRAb (59, 60, 66) Anti-P/NSPA (45, 84) APL (48) Anti-alpha internexin (41) |
Neuronal injury (13, 23) Microglial activation and pruning (23, 42, 66) |
| Cytokines | Chemokines | Excitotoxic mediators |
| IFNα (23) TWEAK (36) IL6 (35) IL8 (35) |
MCP1/CCL2 (37) | HMGB1(42) Angiotensin II (42) Quinolinic acid (34) MMP-9 (39) Myelin-associated neurite outgrowth inhibitor (38) Lipocalin (40) |
IFNα, interferon alpha; TWEAK, TNF-like weak inducer of apoptosis; IL6, interleukin 6; IL8, interleukin 8; DNRAb, DNA-reactive antibodies (formerly known as anti-NR2 antibodies); Anti-P, anti-ribosomal protein P antibody; NSPA, anti-neuronal-surface P antigen; APL, anti-phospholipid antibodies; MCP1/CCL2, monocyte chemotactic protein 1; HMGB1, high mobility group box 1 protein; MMP9, matrix metalloproteinase 9
D.1. Cytokines and Chemokines
A variety of cytokines, chemokines and other proteins are associated with NPSLE. These associations have usually been studied in subjects with a variety of NPSLE manifestations (diffuse and focal), which limits the ability to link a particular protein to a specific manifestation.
IFNα is the cytokine with the best-described relationship to CD. In a recent study, mice with IFNα-mediated autoimmunity displayed CD that was diminished by an anti-IFNα receptor antibody (23). Moreover, wild type mice injected with IFNα peripherally demonstrated CNS microglial activation with increased engulfment of neuronal synapses (synaptic pruning) and reduced synaptic density in the frontal cortex. The potential importance of IFNα in CD is corroborated by the observation of IFNα gene transcription in activated microglia in SLE brain tissue. These results are in line with clinical observations in patients with hepatitis C and liver cancer receiving exogenous IFNα therapy, which is associated with CD including a spatial memory deficit independent of depression (26) and a lupus-like illness (27).
The source of IFNα in NPSLE can be systemic or central. Intrathecal immune complexes may play a key role in interferon production in the brain in NPSLE, and act as powerful amplifiers of brain inflammation (28). Santer et al showed that CSF of NPSLE patients contains high levels of immune complexes that form as a result of autoantibodies that traverse the BBB or are locally produced by infiltrating B cells. These antibodies bind to cellular antigens that are released by damaged neurons. They further demonstrated ex vivo that these immune complexes bind Fc receptors on microglia and lead to the production of high levels of IFNα as well as other pro-inflammatory mediators, including IFNγ-inducible protein-10, IL-8 and MCP-1.
One candidate mechanism for IFNα-induced CD relates to its activation of indoleamine 2,3-dioxygenase (IDO) in the kynurenine/tryptophan metabolic pathway. IFNα stimulates IDO, catalyzing the breakdown of tryptophan (TRP) into kynurenine (KYN), which is further metabolized to quinolinic acid (QA) or kynurenic acid (KA). QA is an N-methyl D-aspartate receptor (NMDAR) agonist, and can cause excessive glutamate excitotoxicity to neurons (29). QA is synthesized by microglia (29), and neurons cultured in supernatant from IFNγ-stimulated microglia exhibit reduced neurite outgrowth and complexity, which can be prevented by pretreatment of microglia with an IDO inhibitor or an NMDAR inhibitor (30). Notably, KA is a NMDAR antagonist(29), which can protect neurons from excitotoxic damage. An imbalance between QA and KA contributes to spatial memory deficits and brain functional and structural changes in animal models of neuroinflammation (31). In humans with SLE, an increased KYN/TRP ratio in blood has been reported (32) and correlates with IFNα gene expression (33). Additionally, CSF levels of QA are higher in SLE patients with NPSLE syndromes (not limited to CD) than in those with CNS dysfunction not related to SLE or in healthy controls (34).
Among the multiple other cytokines associated with NPSLE, IL-6 and IL-8 have the most plausible association with neuronal damage, given their presence in CSF in association with proteins that are indicative of neuronal and astrocytic damage (35). Other inflammatory mediators, such as TNF-like weak inducer of apoptosis (TWEAK) (36), CCL2 (37), myelin-associated neurite outgrowth inhibitor (38), matrix metalloproteinase-9 (39), lipocalin-2 (40), anti-alpha-internexin (41) and the renin-angiotensin (42) system are associated with CD in SLE. TWEAK, a cytokine in the TNF family, may be implicated in CD as memory impairment is ameliorated in TWEAK deficient MRL/lpr mice (36).
The contribution of cytokines, chemokines and other proteins and molecules to specific molecular mechanisms in NPSLE is for the most part unclear. Cytokines and chemokines are known to recruit immune cells to the CNS, promote intrathecal antibody production by infiltrating B cells, and modulate neurotransmitter release (43). In CD, however, the effects may largely result from direct stimulation of neurons and microglia as evidence for cellular infiltration into the brain is limited.
D.2. Serology
To date, some antibody specificities have been associated with CD in mice and in patients. Undoubtedly, more await discovery.
Anti-ribosomal protein P antibody (anti-P) or anti-neuronal surface P antigen (NSPA)
Anti-P antibodies associate with psychosis and CD in SLE patients, and studies in mice reveal a plausible pathogenic mechanism behind this association (44). Neuronal surface P antigen (NPSA) is an integral plasma membrane protein that is bound by anti-P. NSPA engagement by anti-P antibodies induces calcium influx and glutamatergic transmission in neurons (45). By activating both a-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and NMDA receptors, anti-P-induced glutamatergic over activation leads to suppression of long-term potentiation (LTP), which provides a mechanism for anti-P mediated pathogenic alterations in the brain. In addition, glutamatergic dysfunction also mediates psychotic symptoms, as in NMDAR encephalitis (46). Although an association of anti-P with depression has not been confirmed in humans, mice injected intracerebroventricularly with anti-P displayed depression-like behavior (47). Anti-NSPA antibodies, induced in rabbits by immunization with NSPA, trigger calcium influx, enhance glutamatergic transmission, and induce memory impairment, mimicking the effect of anti-P antibodies (45).
Antiphospholipid antibodies
Antiphospholipid antibodies (APL) in serum and CSF, specifically anticardiolipin antibodies (aCL IgG) and lupus anticoagulant (LAC), correlate with CD in several studies (48), with up to a three-fold increase of CD in SLE patients with positive APL. In a recent meta-analysis, Ho et al demonstrated a statistically significant association between serum APL (aCL and LAC specifically) and CD (OR 2.01). While APL are well-established in mediating a pro-thrombotic vasculopathy leading to stroke and multi-infarct dementia (48), experimental models of antiphospholipid syndrome suggest that non-thrombotic mechanisms may also be responsible for APL-mediated CD, such as direct toxic effects of APL on neurons and glia (48, 49). For APL to directly bind cells in the brain, they need to traverse the BBB; APL may affect BBB permeability through endothelial cell dysfunction (50).
DNRAb
These antibodies represent a subset of anti-DNA antibodies that cross-react with the NMDAR. They will be discussed in detail below.
E. Pathogenesis
E.1. Mouse models of cognitive dysfunction
Limitations in studying pathogenic mechanisms of CD in humans with SLE, such as the paucity of brain tissue samples, their procurement post-mortem, and the heterogeneity of neuropsychiatric manifestations, have made experimental mouse models fundamental.
The most common and best studied is the MRL/lpr strain. MRL/lpr mice display depression, anxiety and CD by 8 weeks of age that is positively correlated with serum anti-dsDNA antibody titers and pro-inflammatory cytokines and can precede the onset of renal disease (51). These mice exhibit a notable decrease in midbrain and limbic brain volumes by 5–8 weeks of age. Several mechanisms are likely involved in the neuropsychiatric manifestations, including autoantibodies, cytokines, mononuclear cell infiltration and disruption of the blood-brain barrier (BBB) (51). In addition to anti-dsDNA antibodies, other autoantibodies such as anti-P, anti-cardiolipin and DNRAb antibodies are often present in MRL/lpr mice and can lead to CNS disease. An early onset of neuropsychiatric disease may be explained by intrauterine exposure of the fetal brain to maternal autoantibodies or to high cytokine levels (52) and a dysfunctional Fas/Fas receptor signaling pathway, leading to abnormal hippocampal neurogenesis and postnatal brain development (51, 53). Bialas et al (23) demonstrated that Type 1 IFN-mediated microglial activation leads to dendritic pruning in MRL/lpr mice, likely related to the CD observed in this strain.
Several other mouse models have been used to study CD. NZB/NZW F1 mice, another commonly studied lupus strain, display learning difficulties and mood-related disorders that occur later in the course of disease (54), but these findings can be confounded by the high prevalence of brain anomalies in the non-lupus prone NZB parental strain. The mechanisms of disease include mononuclear cell infiltration of different brain regions, most notably the hippocampus and cortex, as well as disturbances in neuropeptides in the affected areas (55, 56). BXSB male mice demonstrate impaired spatial and non-spatial learning. Like NZB mice, they demonstrate congenital structural abnormalities (51). Genetically engineered lupus-prone mouse strains such as the 564Igi strain, a B-cell receptor knock-in model with IFNα receptor 1-dependent pathogenesis (23), and the bicongenic strain, Sle1/Sle 3 (40), also exhibit spatial and object memory impairment, and other behavioral abnormalities.
E.2. DNRAb as a mechanism for CD in SLE
We have been studying DNRAb and their contribution to CD in SLE. Some time ago we identified a subset of anti-dsDNA antibodies, DNRAb, also known as anti-NR2 antibodies, which bind DNA and cross-react with the GluN2A and GluN2B subunits of the NMDAR, the brain’s main excitatory receptor (57). NMDAR are found in the highest numbers in the hippocampus and are integral to learning and memory. DNRAb enhance the excitatory activation of NMDAR, and excessive activation leads to excitotoxic cell death. DNRAb isolated from the serum and CSF of a SLE patient with progressive cognitive decline and injected directly into a mouse brain caused neuronal cell death (57), confirming their ability to mediate brain pathology once present in brain tissue. Serum DNRAb are found in 30–50% SLE patients (58) and pooled data from a recent meta-analysis reveals that SLE patients with NPSLE were more likely to have elevated serum/plasma DNRAb (mean serum levels of 0.4mg/ml in NPSLE patients compared to 0.2mg/ml in non-NPSLE patients) (59). Though some studies have not found an association between serum DNRAb positivity and CD, the presence of DNRAb in CSF associates with diffuse NPSLE, including CD in several studies (mean CSF levels in NPSLE 0.61U/mL while in non-NPSLE SLE 0.31U/mL) (59, 60). Additionally, as a BBB breach is needed to result in CNS disease, serum titers may not accurately reflect CNS disease.
DNRAb should not be confused with the anti-NMDAR antibodies found in autoimmune encephalitis, which bind to the GluN1 subunit of the NMDAR. These antibodies result in internalization of the receptor and subsequently lead to a reversible decrease in NMDAR surface density and thus synaptic dysfunction, without significant neuronal cell death or loss of dendritic tree or spine complexity (61). Clinical manifestations, which include severe neurologic, psychiatric and behavioral symptoms, tend to be transient and positively correlated to CSF antibody titers, as opposed to the manifestations associated with DNRAb, which are persistent.
It is believed that, in SLE, abrogation of BBB integrity and direct access of antibodies to the CNS is needed for antibody-mediated damage, since existing evidence suggests autoantibody is not produced within the CNS in SLE (60). This is based on findings of elevated albumin (normally only found in serum) in CSF of lupus patients with NPSLE compared to those without (60). Several conditions compromise BBB integrity, including viral and bacterial infection, systemic inflammation, stress (epinephrine), ischemia, aging, hypertension, nicotine, alcohol and certain inflammatory cytokines, such as TNFα, IL1β, IL6 and IL8 (62). DNRAb also directly affect BBB integrity by activating endothelial cells and leading to the production of pro-inflammatory cytokines such as TNFα, IL6 and IL8 (63). The complement activation product C5a, present in SLE patients with active disease, alters BBB integrity in MRL/lpr mice through endothelial cell apoptosis (64). In SLE, it is likely that complement, peripheral cytokines and autoantibodies as well as non-disease related mechanisms all compromise BBB integrity.
The mechanism of BBB insult determines the anatomic site of the breach, which dictates the location of antibody-mediated damage. In this way, the same antibody can cause more than one neuropsychiatric manifestation depending on the affected brain region. For example, administration of lipopolysaccharide (LPS) to DNRAb-positive mice leads to hippocampal damage, whereas epinephrine administration causes damage to the amygdala (65). Of note, other mechanisms for antibody and leukocyte entry into the brain have been proposed, including through the choroid plexus, meningeal-arachnoid barrier and glymphatic system (5).
The fact that DNRAb are commonly present in SLE patients and can mediate neurotoxicity led us to develop a non-spontaneously autoimmune mouse model to study the effects of DNRAb, and eliminate confounding variables like cytokines and other brain-reactive autoantibodies, which are present in spontaneously autoimmune lupus mouse strains (13). In this model, mice are injected with a consensus sequence contained within the GluN2A and GluN2B subunits that is bound by DNRAb, leading to the production of DNRAb. In order to provide DNRAb with access to brain parenchyma, the BBB is breached with systemic LPS administration. Within one week, in the absence of an inflammatory infiltrate, a 20–25% hippocampal neuronal loss is observed (13), followed by loss of dendritic complexity and spine density with an associated spatial memory impairment that occurs after DNRAb is no longer detectable in the brain (66) (Fig 2).
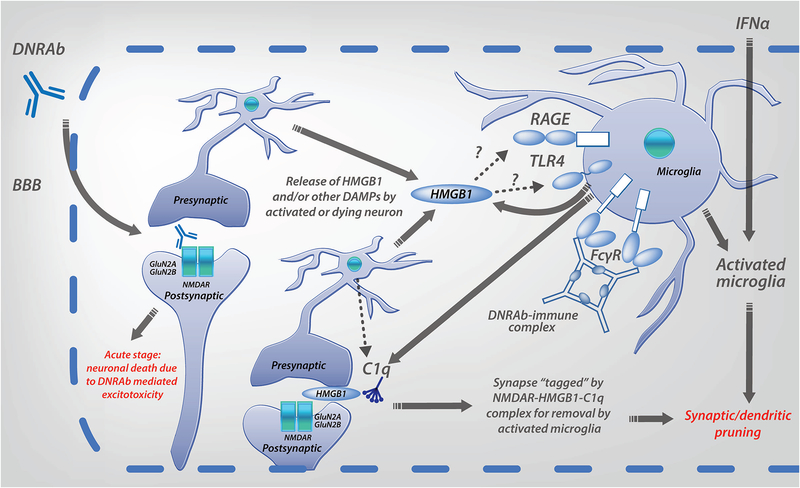
Figure 2: Proposed two stage model for DNRAb mediated neurotoxicity, and the contribution of IFNα to neurotoxicity.
Exposure to DNRAb mediates immediate excitotoxic death of some neurons (acute stage). The surviving neurons experience strong NMDAR stimulation that induces HMGB1 secretion. Microglia are activated following DNRAb penetration of the BBB. There are at least three possible mechanisms for microglial activation in the DNRAb model: binding of secreted HMGB1 to receptor for advanced glycation end products (RAGE) or toll-like receptor 4 (TLR4), engagement of activating Fc receptors (FcR) by DNRAb-immune complexes, and/or exposure to damage-associated molecular patterns (DAMPs) from apoptotic neurons. Activated microglia contribute to the loss of dendrites and synapses, which are “tagged” for destruction by a NMDAR-HMGB1-C1q complex (chronic stage). Interferon-alpha (IFNα) penetrates the BBB, or is produced centrally, and activates microglia, resulting in the loss of neuronal dendrites and synapses. Another mechanism of IFNα-induced neurotoxicity may be through stimulation of the KYN/TRP metabolic pathway in microglia, causing excessive production of QA that results in neuronal excitotoxicity.
Microglia have emerged as central players in human neuropathologies and are increasingly being associated with neuropsychiatric symptoms in murine lupus models as well (23, 42). In DNRAb-mediated CD, microglia may be activated via several mechanisms (Fig 2). This activation also is only detected after DNRAb is no longer detectable in the brain. Activated microglia can phagocytose (or prune) dendritic synapses with a resultant loss in dendritic complexity and spine density. This mechanism is associated with CD in several murine lupus models, including DNRAb+ mice, MRL/lpr mice and NZB/NZW mice (23, 66). DNRAb+ mice develop a selective spatial memory impairment. Microglial depletion in DNRAb+ mice given LPS to allow antibody to penetrate brain parenchyma results in preserved neuronal dendritic architecture (42). The role of complement, notably C1q, in microglial-mediated synaptic pruning is critical. C1q is produced by both neurons and microglia and can “tag” synapses for removal. In DNRAb+ mice, a NMDAR–HMGB1–C1q complex forms at synapses on neuronal dendrites targeting them for destruction. C1q knockout, DNRAb+ mice maintain normal dendritic complexity and spine density following LPS administration (42), confirming a critical contribution of C1q in DNRAb-mediated in pathology.
We have shown that ACE inhibitors, through their centrally-acting effects, reduce microglial activation, prevent loss of dendritic arborization and prevent spatial memory impairment in DNRAb+ mice (42). Treatment with captopril after onset of microglial activation also restored dendritic arborization and spine density, suggesting that surviving neurons in this model do not experience irreversible damage (Fig 3).
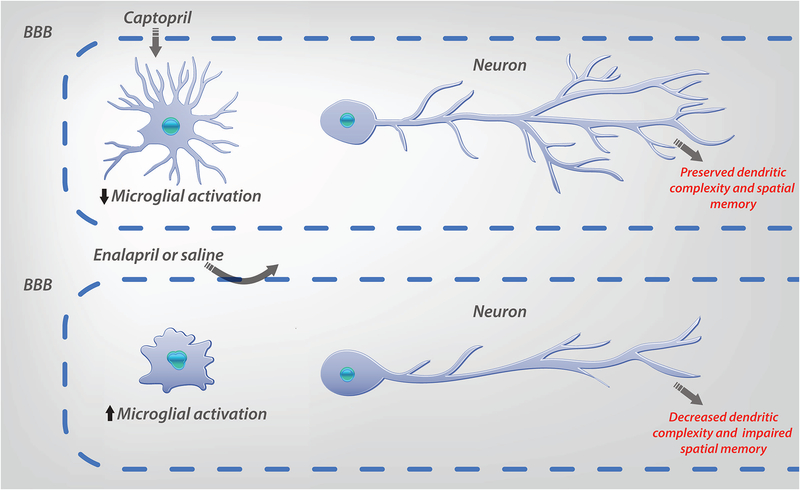
Figure 3: Proposed mechanism of ACE-inhibitor (ACE-I) treatment of SLE mediated CD.
Treatment with a BBB permeable ACE-I (captopril), but not with a BBB impermeable ACE-I (enalapril) or saline, suppresses microglial activation and preserves dendritic complexity and spatial memory in DNRAb+ mice. Importantly, captopril treatment after the onset of microglial activation can restore dendritic complexity, suggesting damaged neurons can recover following treatment.
Multiple behavioral studies have revealed the impact of DNRAb on spatial memory in the mouse model (Table 2). Although it may be difficult to extrapolate results from mice to humans, the mouse model informed our choice of applying tasks related to spatial memory in humans with SLE. Using a 2×2 array of objects that assessed both object recognition and memory for spatial relations, we found that DNRAb is associated with a spatial memory deficit in humans with SLE (66, 67). Additionally, we used a desktop, 3-dimensional spatial navigation task that may be more clinically relevant than the 2×2 array, and found that DNRAb+ SLE patients performed poorly compared to DNRAb- SLE patients, who performed similarly to healthy controls (68). This work demonstrates that mouse models provide structural information related to pathogenic mechanisms in the brain, so that appropriate cognitive tasks may be applied.
Table 2.
Assessments of spatial cognition in the DNRAb+ mouse model
| Approach | Cognitive feature tested | Description of test | Behavior in mice with hippocampal dysfunction |
|---|---|---|---|
| Object place memory (OPM) task in tandem with the Novel Object Recognition (NOR) task |
|
The mouse is placed in a chamber and allowed to explore 2 objects. Then, either of one of the 2 procedures below is followed:
|
Behavior in the DNRAb mouse model, in which DNRAb+ mice display enlarged place field size on recordings from CA1 hippocampal neurons, indicating a spatial map with less resolution: |
| T or Y maze | Spatial working memory | The mouse is placed in a T- or Y-shaped chamber with one arm blocked off. Afterwards, the barrier is removed and the mouse is again allowed to explore. Because mice have an innate preference for novelty, the mouse will spend more of its time in the unexplored arm. This requires that mice recognize which arm of the maze they had previously explored. | DNRAb+ mice spent less time alternating between the two arms of a T maze than DNRAb- mice (13, 65) |
| Morris water maze | Spatial reference memory | The mouse is placed in a pool of water with a hidden platform located just below the surface. Mice learn to escape from water by swimming to the platform over repeated sessions. This is followed by a trial in which the platform is removed. Mice that memorize the position of the platform preferentially swim in that area (trained sector). Since mice may find swimming stressful leading to difficulties in interpreting results, the paddling pool maze was designed to overcome this problem (see below). | DNRAb+ mice display reduced exploration of the trained sector compared to DNRAb- mice (13) |
| Training to criterion task | Spatial flexibility | Mice are required to find 5 consecutive locations of the platform in the Morris water maze. | DNRAb+ mice have a poor ability to learn a given location compared to DNRAb- mice (13, 65) |
| Shallow water paddling pool maze | Spatial memory; spatial flexibility | Mice are placed in a large, bright, circular arena with transparent sides containing shallow water. Around the perimeter are 12 potential exits, one of which is connected to an escape tunnel (target). The target is in a fixed position until the mouse finds it, then a new target position is selected. The mice are trained to find sequential targets in the maze. | DNRAb+ mice need more trials to reach a moved target relative to DNRAb- mice (85) |
Our group is also investigating the impact of DNRAb on brain structure and function in patients with SLE with stable disease activity and without CNS disease. We found that these SLE subjects demonstrate hypermetabolism on FDG-PET in the hippocampus, among other brain regions, and hippocampal hypermetabolism correlates with poor working memory (69), demonstrating that SLE patients with no other NPSLE symptoms may exhibit CD that correlates with clear abnormalities in brain function. Further, DNRAb antibody positivity was shown to correlate with hippocampal hypermetabolism (69) and decreased white matter microstructural integrity in the parahippocampal gyrus on DTI (67). The decreased microstructural integrity in the parahippocampal gyrus on DTI correlated with increased serum DNRAb and poor spatial memory performance. DTI findings did not correlate with deficits in other cognitive domains. FDG-PET studies performed concurrently with DTI revealed hypermetabolism in gray matter areas, such as the hippocampus, adjacent to areas with decreased white matter microstructural integrity, suggesting that changes in regional metabolism may indicate a pathophysiological process leading to structural changes (Fig 1). Hypermetabolism and reduced white matter microstructural integrity were stable over a mean of 15 months. These findings suggest that metabolic activity in these regions may be a marker for SLE that is potentially responsive to targeted therapies, and possibly useful as an outcome measure in clinical trials.
F. Implications for therapy of SLE-CD
There are no treatments for CD in SLE and there is limited/scant data on the use of immunosuppressive therapy in CD. The insidious nature of CD and its occurrence independent of systemic disease activity have shifted the risk-benefit assessment in favor of using less aggressive, less immunosuppressive options, despite emerging evidence of immune-mediated mechanisms. In one small prospective double-blind, placebo-controlled study, a trial of glucocorticoid therapy (0.5mg/kg prednisone) led to clinical improvement in 5/8 patients with mild SLE and CD who completed the trial (70). The duration of therapy varied from 2–19 months, and relapse of CD after taper was not reported.
Given its moderate success in slowing cognitive decline in AD (71), memantine, a NMDA receptor antagonist, was tested in SLE patients with mild self-reported baseline CD, but did not exhibit significant improvement in cognitive performance in SLE patients compared to placebo (72). The study was not powered to test for an effect in DNRAb positive patients (of which there were only five), though DNRAb positive mouse treated with memantine prior to breaching the BBB demonstrated no evidence of antibody-mediated neuronal death (65). That said, long-term attenuation of the NMDAR could have deleterious impacts on brain function and it is therefore an unfavorable therapeutic option (73).
Although no studies have assessed the benefit of anticoagulation or anti-platelet therapy in SLE patients with CD without thromboembolic phenomena, anti-platelet therapy such as low dose aspirin or anti-malarials may be considered in SLE patients with positive antiphospholipid antibodies and CD. In a 3-year prospective observational study assessing predictors of CD in SLE patients, regular use of low dose aspirin improved cognitive function in SLE patients with or without APL compared to those not taking aspirin (74).
One potential therapeutic strategy is to protect and enhance BBB integrity. C5a receptor (C5aR) blockade ameliorates BBB disruption and attenuates behavioral abnormalities in MRL/lpr mice (75), revealing a potential therapeutic target for CD. While sphingosine-1-phosphate (S1P) receptor modulation with FTY720 stabilizes the BBB in MRL/lpr mice and mitigates CD (76), its use in SLE will be limited by its known toxicity.
Another therapeutic strategy is to block microglial activation. The renin-angiotensin system, best known for maintaining hemodynamic and mineralocorticoid homeostasis, consists of multiple neuroactive peptides that when disbalanced play a significant role in the neuroinflammatory processes central to CD (77). The most potent component of this complex system is angiotensin II, which activates microglia to assume a pro-inflammatory phenotype, and when overexpressed, is directly neurotoxic, resulting in neuronal injury and cell death. Another pro-inflammatory mechanism of the renin-angiotensin system is the ACE-mediated inactivation of bradykinin, which has anti-inflammatory effects, suppresses microglial activation and diminishes Type 1 IFN responses in normal and lupus prone mice (78). GWA studies identify an ACE allele as a risk factor in SLE with the risk allele leading to increased serum ACE. In a randomized trial (79) and several observational studies (80), ACE inhibition retarded cognitive decline in Alzheimer’s Disease. These data, together with murine studies, support the potential use of ACE inhibitors as novel neuroprotective therapeutics for CD in SLE. Angiotensin receptor blockers may also be a useful therapeutic alternative.
Minocycline has also emerged as a potent inhibitor of microglial activation with benefits in several neurological conditions (81); however, its toxicity profile and potential risk of drug-induced lupus may limit its usefulness.
The current approach of broad immunosuppression or no treatment for CD in SLE, with the inherent dangers of immunosuppression or increasing impairment, respectively, illustrates that clinical trials are greatly needed. Several potential treatment strategies appear promising; however, much work is required to confirm suitable biomarkers and endpoints for use in clinical trials. When trials begin, they need to be performed in well-defined SLE populations.
G. Considerations for potential trials of neuroprotection in SLE-CD
A key question that arises related to potential clinical trials in SLE-CD is whether or not CD may be fixed versus decelerated or reversed, with or without therapy. Several longitudinal studies have examined SLE-CD (7); however, only two studies included patients without any history of neuropsychiatric disease, and in one of these studies CD was not detected, which limits interpretation of results. Our study examining ACE inhibitors and microglia supports the idea that cognition may be “retrieved” rather than only prevented in decline, as ACE inhibitors given after the onset of microglia activation restored dendritic arborization and spine density (unpublished data).
Another key question related to potential trials is: what design and outcome measures should be used? Clinical trials in neurodegenerative diseases that affect cognition, such as in AD, lend some insight into potential trials in SLE-CD, but mostly with respect to therapeutic targets rather than cognitive testing as an outcome measure. This is because the broad cognitive tests that are utilized in AD, such as the MMSE, lack sensitivity to mild CD (82), which is often encountered in SLE. Given this problem, we have chosen to use more sensitive tests such as the ANAM, and specific tests related to a known pathogenic mechanism (e.g. DNRAb and a spatial memory deficit). However, the use of cognitive testing as an outcome measure has several limitations in potential trials. Even with a relatively sensitive test such as the ANAM, many patients are needed to ensure adequate power to detect a significant change in performance over the limited time of a trial. Furthermore, the clinically meaningful change in cognitive tests is unclear. Therefore, clinical trials that employ imaging as an outcome measure based on a plausible pathogenic mechanism, such as DNRAb mediated neurotoxicity and microglia activation, may be advantageous before moving onto larger clinical trials with cognitive testing. Several longitudinal studies of TSPO-PET in AD reveal increased microglia activation over time, and in one study it correlated with worsening CD (24). These results suggest that TSPO-PET imaging may apply as a biomarker of SLE-CD.
Importantly, future clinical trials in SLE-CD will require specifically defined patient samples. Current clinical trials may include SLE patients with neurologic or psychiatric diseases apart from SLE as a cause, which can potentially confound interpretation of results.
H. Conclusion
Cognitive dysfunction in SLE, although insidious and sometimes difficult to diagnose, can be devastating with a considerable impact on quality of life. The pathogenesis of CD in SLE is poorly understood, which translates into a lack of biomarkers that can aid in diagnosis. However, efforts to dismiss CD as merely a confounding symptom in SLE are shortsighted and do a disservice to patients. While clinical studies to date have been hampered by heterogeneous subject populations and a lack of sensitive and standardized cognitive batteries that test for an association of a specific cognitive deficit with a defined pathogenic mechanism, there is strong data from the clinical arena and from mouse models that CD is present in many patients and is related to known disease mechanisms. Future clinical studies that utilize sensitive and specific tests for cognitive deficits related to known pathogenic mechanisms (for example, spatial memory deficits related to DNRAb) are necessary and will provide the information needed to design clinical trials to preserve cognitive function and improve quality of life for patients.
References
- 1.Hanly JG, Kozora E, Beyea SD, Birnbaum J. Review: Nervous System Disease in Systemic Lupus Erythematosus: Current Status and Future Directions. Arthritis Rheumatol. 2019;71(1):33–42. [DOI] [PubMed] [Google Scholar]
- 2.Campbell R, Cooper GS, Gilkeson GS. The impact of systemic lupus erythematosus on employment. J Rheumatol. 2009;36(11):2470–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahn GY, Kim D, Won S, Song ST, Jeong HJ, Sohn IW, et al. Prevalence, risk factors, and impact on mortality of neuropsychiatric lupus: a prospective, single-center study. Lupus. 2018;27(8):1338–47. [DOI] [PubMed] [Google Scholar]
- 4.The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes. Arthritis Rheum. 1999;42(4):599–608. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz N, Stock AD, Putterman C. Neuropsychiatric lupus: new mechanistic insights and future treatment directions. Nature Reviews Rheumatology. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lupus: Patient Voices. 2018.
- 7.Rayes HA, Tani C, Kwan A, Marzouk S, Colosimo K, Medina-Rosas J, et al. What is the prevalence of cognitive impairment in lupus and which instruments are used to measure it? A systematic review and meta-analysis. Semin Arthritis Rheum. 2018. [DOI] [PubMed] [Google Scholar]
- 8.Roebuck-Spencer TM, Yarboro C, Nowak M, Takada K, Jacobs G, Lapteva L, et al. Use of computerized assessment to predict neuropsychological functioning and emotional distress in patients with systemic lupus erythematosus. Arthritis Rheum. 2006;55(3):434–41. [DOI] [PubMed] [Google Scholar]
- 9.Paez-Venegas N, Jordan-Estrada B, Chavarria-Avila E, Perez-Vazquez F, Gómez-Bañuelos E, Medina-Dávalos R, et al. The Montreal Cognitive Assessment Test: A Useful Tool in Screening of Cognitive Impairment in Patients With Systemic Lupus Erythematosus. J Clin Rheumatol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Postal M, Lapa AT, Reis F, Rittner L, Appenzeller S. Magnetic resonance imaging in neuropsychiatric systemic lupus erythematosus: current state of the art and novel approaches. Lupus. 2017;26(5):517–21. [DOI] [PubMed] [Google Scholar]
- 11.Zhu CM, Ma Y, Xie L, Huang JZ, Sun ZB, Duan SX, et al. Spatial Working Memory Impairment in Patients with Non-neuropsychiatric Systemic Lupus Erythematosus: A Blood-oxygen-level Dependent Functional Magnetic Resonance Imaging Study. J Rheumatol. 2017;44(2):201–8. [DOI] [PubMed] [Google Scholar]
- 12.Liu S, Cheng Y, Xie Z, Lai A, Lv Z, Zhao Y, et al. A Conscious Resting State fMRI Study in SLE Patients Without Major Neuropsychiatric Manifestations. Front Psychiatry. 2018;9:677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kowal C, DeGiorgio LA, Nakaoka T, Hetherington H, Huerta PT, Diamond B, et al. Cognition and immunity; antibody impairs memory. Immunity. 2004;21(2):179–88. [DOI] [PubMed] [Google Scholar]
- 14.Costallat BL, Ferreira DM, Lapa AT, Rittner L, Costallat LTL, Appenzeller S. Brain diffusion tensor MRI in systematic lupus erythematosus: A systematic review. Autoimmun Rev. 2018;17(1):36–43. [DOI] [PubMed] [Google Scholar]
- 15.Corrêa DG, Zimmermann N, Borges RS, Pereira DB, Doring TM, Tukamoto G, et al. White-matter integrity in patients with systemic lupus erythematosus and memory deficits. Neuroradiol J. 2018;31(6):587–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jung RE, Chavez RS, Flores RA, Qualls C, Sibbitt WL, Roldan CA. White matter correlates of neuropsychological dysfunction in systemic lupus erythematosus. PLoS One. 2012;7(1):e28373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kozora E, Arciniegas DB, Duggan E, West S, Brown MS, Filley CM. White matter abnormalities and working memory impairment in systemic lupus erythematosus. Cogn Behav Neurol. 2013;26(2):63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Filley CM, Kozora E, Brown MS, Miller DE, West SG, Arciniegas DB, et al. White matter microstructure and cognition in non-neuropsychiatric systemic lupus erythematosus. Cogn Behav Neurol. 2009;22(1):38–44. [DOI] [PubMed] [Google Scholar]
- 19.Oh DH, Kim SH, Jung S, Sung YK, Bang SY, Bae SC, et al. Precuneus hypoperfusion plays an important role in memory impairment of patients with systemic lupus erythematosus. Lupus. 2011;20(8):855–60. [DOI] [PubMed] [Google Scholar]
- 20.Kao CH, Ho YJ, Lan JL, Changlai SP, Liao KK, Chieng PU. Discrepancy between regional cerebral blood flow and glucose metabolism of the brain in systemic lupus erythematosus patients with normal brain magnetic resonance imaging findings. Arthritis Rheum. 1999;42(1):61–8. [DOI] [PubMed] [Google Scholar]
- 21.Castellino G, Padovan M, Bortoluzzi A, Borrelli M, Feggi L, Caniatti ML, et al. Single photon emission computed tomography and magnetic resonance imaging evaluation in SLE patients with and without neuropsychiatric involvement. Rheumatology (Oxford). 2008;47(3):319–23. [DOI] [PubMed] [Google Scholar]
- 22.Wang J, Yang C, Zhao Q, Zhu Z, Li Y, Yang P. Microglia activation induced by serum of SLE patients. J Neuroimmunol. 2017;310:135–42. [DOI] [PubMed] [Google Scholar]
- 23.Bialas AR, Presumey J, Das A, van der Poel CE, Lapchak PH, Mesin L, et al. Microglia-dependent synapse loss in type I interferon-mediated lupus. Nature. 2017;546(7659):539–43. [DOI] [PubMed] [Google Scholar]
- 24.Cerami C, Iaccarino L, Perani D. Molecular Imaging of Neuroinflammation in Neurodegenerative Dementias: The Role of In Vivo PET Imaging. Int J Mol Sci. 2017;18(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, Coughlin JM, Ma S, Endres CJ, Kassiou M, Sawa A, et al. Neuroimaging of translocator protein in patients with systemic lupus erythematosus: a pilot study using [(11)C]DPA-713 positron emission tomography. Lupus. 2017;26(2):170–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lieb K, Engelbrecht MA, Gut O, Fiebich BL, Bauer J, Janssen G, et al. Cognitive impairment in patients with chronic hepatitis treated with interferon alpha (IFNalpha): results from a prospective study. Eur Psychiatry. 2006;21(3):204–10. [DOI] [PubMed] [Google Scholar]
- 27.Niewold TB. Interferon alpha-induced lupus: proof of principle. J Clin Rheumatol. 2008;14(3):131–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Santer DM, Yoshio T, Minota S, Möller T, Elkon KB. Potent induction of IFN-alpha and chemokines by autoantibodies in the cerebrospinal fluid of patients with neuropsychiatric lupus. J Immunol. 2009;182(2):1192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwarcz R, Stone TW. The kynurenine pathway and the brain: Challenges, controversies and promises. Neuropharmacology. 2017;112(Pt B):237–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O’Farrell K, Fagan E, Connor TJ, Harkin A. Inhibition of the kynurenine pathway protects against reactive microglial-associated reductions in the complexity of primary cortical neurons. Eur J Pharmacol. 2017;810:163–73. [DOI] [PubMed] [Google Scholar]
- 31.Li Y, Eskelund AR, Zhou H, Budac DP, Sanchez C, Gulinello M. Behavioral Deficits Are Accompanied by Immunological and Neurochemical Changes in a Mouse Model for Neuropsychiatric Lupus (NP-SLE). Int J Mol Sci. 2015;16(7):15150–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiang ZY, Tang AG, Ren YP, Zhou QX, Luo XB. Simultaneous determination of serum tryptophan metabolites in patients with systemic lupus erythematosus by high performance liquid chromatography with fluorescence detection. Clin Chem Lab Med. 2010;48(4):513–7. [DOI] [PubMed] [Google Scholar]
- 33.Lood C, Tyden H, Gullstrand B, Klint C, Wenglen C, Nielsen CT, et al. Type I interferon-mediated skewing of the serotonin synthesis is associated with severe disease in systemic lupus erythematosus. PLoS One. 2015;10(4):e0125109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vogelgesang SA, Heyes MP, West SG, Salazar AM, Sfikakis PP, Lipnick RN, et al. Quinolinic acid in patients with systemic lupus erythematosus and neuropsychiatric manifestations. J Rheumatol. 1996;23(5):850–5. [PubMed] [Google Scholar]
- 35.Kwiecinski J, Klak M, Trysberg E, Blennow K, Tarkowski A, Jin T. Relationship between elevated cerebrospinal fluid levels of plasminogen activator inhibitor 1 and neuronal destruction in patients with neuropsychiatric systemic lupus erythematosus. Arthritis Rheum. 2009;60(7):2094–101. [DOI] [PubMed] [Google Scholar]
- 36.Stock AD, Wen J, Putterman C. Neuropsychiatric Lupus, the Blood Brain Barrier, and the TWEAK/Fn14 Pathway. Front Immunol. 2013;4:484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duarte-Garcia A, Romero-Diaz J, Juarez S, Cicero-Casarrubias A, Fragoso-Loyo H, Nunez-Alvarez C, et al. Disease activity, autoantibodies, and inflammatory molecules in serum and cerebrospinal fluid of patients with Systemic Lupus Erythematosus and Cognitive Dysfunction. PLoS One. 2018;13(5):e0196487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lei HW, Wang JY, Dang QJ, Yang F, Liu X, Zhang JH, et al. Neuropsychiatric involvement in lupus is associated with the Nogo-a/NgR1 pathway. J Neuroimmunol. 2017;311:22–8. [DOI] [PubMed] [Google Scholar]
- 39.Ainiala H, Hietaharju A, Dastidar P, Loukkola J, Lehtimaki T, Peltola J, et al. Increased serum matrix metalloproteinase 9 levels in systemic lupus erythematosus patients with neuropsychiatric manifestations and brain magnetic resonance imaging abnormalities. Arthritis Rheum. 2004;50(3):858–65. [DOI] [PubMed] [Google Scholar]
- 40.Mike EV, Makinde HM, Gulinello M, Vanarsa K, Herlitz L, Gadhvi G, et al. Lipocalin-2 is a pathogenic determinant and biomarker of neuropsychiatric lupus. J Autoimmun. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu X, Chen X, Huang L, Zhu C, Gu Y, Ye S. Anti-α-Internexin Autoantibody from Neuropsychiatric Lupus Induce Cognitive Damage via Inhibiting Axonal Elongation and Promote Neuron Apoptosis. PLoS One. 5 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nestor J, Arinuma Y, Huerta TS, Kowal C, Nasiri E, Kello N, et al. Lupus antibodies induce behavioral changes mediated by microglia and blocked by ACE inhibitors. J Exp Med. 2018;215(10):2554–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jeltsch-David H, Muller S. Neuropsychiatric systemic lupus erythematosus: pathogenesis and biomarkers. Nat Rev Neurol. 2014;10(10):579–96. [DOI] [PubMed] [Google Scholar]
- 44.Gonzalez A, Massardo L. Antibodies and the brain: antiribosomal P protein antibody and the clinical effects in patients with systemic lupus erythematosus. Curr Opin Neurol. 2018;31(3):300–5. [DOI] [PubMed] [Google Scholar]
- 45.Segovia-Miranda F, Serrano F, Dyrda A, Ampuero E, Retamal C, Bravo-Zehnder M, et al. Pathogenicity of lupus anti-ribosomal P antibodies: role of cross-reacting neuronal surface P antigen in glutamatergic transmission and plasticity in a mouse model. Arthritis Rheumatol. 2015;67(6):1598–610. [DOI] [PubMed] [Google Scholar]
- 46.Ellul P, Groc L, Tamouza R, Leboyer M. The Clinical Challenge of Autoimmune Psychosis: Learning from Anti-NMDA Receptor Autoantibodies. Front Psychiatry. 2017;8:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Katzav A, Ben-Ziv T, Blank M, Pick CG, Shoenfeld Y, Chapman J. Antibody-specific behavioral effects: intracerebroventricular injection of antiphospholipid antibodies induces hyperactive behavior while anti-ribosomal-P antibodies induces depression and smell deficits in mice. J Neuroimmunol. 2014;272(1–2):10–5. [DOI] [PubMed] [Google Scholar]
- 48.Yelnik CM, Kozora E, Appenzeller S. Cognitive disorders and antiphospholipid antibodies. Autoimmun Rev. 2016;15(12):1193–8. [DOI] [PubMed] [Google Scholar]
- 49.Shoenfeld Y, Nahum A, Korczyn AD, Dano M, Rabinowitz R, Beilin O, et al. Neuronal-binding antibodies from patients with antiphospholipid syndrome induce cognitive deficits following intrathecal passive transfer. Lupus. 2003;12(6):436–42. [DOI] [PubMed] [Google Scholar]
- 50.Soltesz P, Der H, Veres K, Laczik R, Sipka S, Szegedi G, et al. Immunological features of primary anti-phospholipid syndrome in connection with endothelial dysfunction. Rheumatology (Oxford). 2008;47(11):1628–34. [DOI] [PubMed] [Google Scholar]
- 51.Jeltsch-David H, Muller S. Neuropsychiatric systemic lupus erythematosus and cognitive dysfunction: the MRL-lpr mouse strain as a model. Autoimmun Rev. 2014;13(9):963–73. [DOI] [PubMed] [Google Scholar]
- 52.Lee JY, Huerta PT, Zhang J, Kowal C, Bertini E, Volpe BT, et al. Neurotoxic autoantibodies mediate congenital cortical impairment of offspring in maternal lupus. Nat Med. 2009;15(1):91–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ishimura R, Martin GR, Ackerman SL. Loss of apoptosis-inducing factor results in cell-type-specific neurogenesis defects. J Neurosci. 2008;28(19):4938–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schrott LM, Crnic LS. Anxiety behavior, exploratory behavior, and activity in NZB × NZW F1 hybrid mice: role of genotype and autoimmune disease progression. Brain Behav Immun. 1996;10(3):260–74. [DOI] [PubMed] [Google Scholar]
- 55.Kier AB. Clinical neurology and brain histopathology in NZB/NZW F1 lupus mice. J Comp Pathol. 1990;102(2):165–77. [DOI] [PubMed] [Google Scholar]
- 56.Bracci-Laudiero L, Aloe L, Lundeberg T, Theodorsson E, Stenfors C. Altered levels of neuropeptides characterize the brain of lupus prone mice. Neurosci Lett. 1999;275(1):57–60. [DOI] [PubMed] [Google Scholar]
- 57.DeGiorgio LA, Konstantinov KN, Lee SC, Hardin JA, Volpe BT, Diamond B. A subset of lupus anti-DNA antibodies cross-reacts with the NR2 glutamate receptor in systemic lupus erythematosus. Nat Med. 2001;7(11):1189–93. [DOI] [PubMed] [Google Scholar]
- 58.Hanly JG, McCurdy G, Fougere L, Douglas JA, Thompson K. Neuropsychiatric events in systemic lupus erythematosus: attribution and clinical significance. J Rheumatol. 2004;31(11):2156–62. [PubMed] [Google Scholar]
- 59.Tay SH, Fairhurst AM, Mak A. Clinical utility of circulating anti-N-methyl-d-aspartate receptor subunits NR2A/B antibody for the diagnosis of neuropsychiatric syndromes in systemic lupus erythematosus and Sjogren’s syndrome: An updated meta-analysis. Autoimmun Rev. 2017;16(2):114–22. [DOI] [PubMed] [Google Scholar]
- 60.Hirohata S, Arinuma Y, Yanagida T, Yoshio T. Blood-brain barrier damages and intrathecal synthesis of anti-N-methyl-D-aspartate receptor NR2 antibodies in diffuse psychiatric/neuropsychological syndromes in systemic lupus erythematosus. Arthritis Res Ther. 2014;16(2):R77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hughes EG, Peng X, Gleichman AJ, Lai M, Zhou L, Tsou R, et al. Cellular and synaptic mechanisms of anti-NMDA receptor encephalitis. J Neurosci. 2010;30(17):5866–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Diamond B, Huerta PT, Mina-Osorio P, Kowal C, Volpe BT. Losing your nerves? Maybe it’s the antibodies. Nat Rev Immunol. 2009;9(6):449–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yoshio T, Onda K, Nara H, Minota S. Association of IgG anti-NR2 glutamate receptor antibodies in cerebrospinal fluid with neuropsychiatric systemic lupus erythematosus. Arthritis Rheum. 2006;54(2):675–8. [DOI] [PubMed] [Google Scholar]
- 64.Mahajan SD, Tutino VM, Redae Y, Meng H, Siddiqui A, Woodruff TM, et al. C5a induces caspase-dependent apoptosis in brain vascular endothelial cells in experimental lupus. Immunology. 2016;148(4):407–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huerta PT, Kowal C, DeGiorgio LA, Volpe BT, Diamond B. Immunity and behavior: antibodies alter emotion. Proc Natl Acad Sci U S A. 2006;103(3):678–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chang EH, Volpe BT, Mackay M, Aranow C, Watson P, Kowal C, et al. Selective Impairment of Spatial Cognition Caused by Autoantibodies to the N-Methyl-D-Aspartate Receptor. EBioMedicine. 2015;2(7):755–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mackay M, Vo A, Tang CC, Small M, Anderson EW, Ploran EJ, et al. Metabolic and microstructural alterations in the SLE brain correlate with cognitive impairment. JCI Insight. 2019;4(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Anderson E, Ploran E, Diamond B, Volpe B, Aranow C, Mackay M. Spatial Navigation Impairment Associated with Anti-NMDA Receptor Antibodies in Systemic Lupus Erythematosus. 2017. [Google Scholar]
- 69.Mackay M, Tang CC, Volpe BT, Aranow C, Mattis PJ, Korff RA, et al. Brain metabolism and autoantibody titres predict functional impairment in systemic lupus erythematosus. Lupus Sci Med. 2015;2(1):e000074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Denburg SD, Carbotte RM, Denburg JA. Corticosteroids and neuropsychological functioning in patients with systemic lupus erythematosus. Arthritis Rheum. 1994;37(9):1311–20. [DOI] [PubMed] [Google Scholar]
- 71.Reisberg B, Doody R, Stöffler A, Schmitt F, Ferris S, Möbius HJ, et al. Memantine in moderate-to-severe Alzheimer’s disease. N Engl J Med. 2003;348(14):1333–41. [DOI] [PubMed] [Google Scholar]
- 72.Petri M, Naqibuddin M, Sampedro M, Omdal R, Carson KA. Memantine in systemic lupus erythematosus: a randomized, double-blind placebo-controlled trial. Semin Arthritis Rheum. 2011;41(2):194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Willmore CB, LaVecchia KL, Wiley JL. NMDA antagonists produce site-selective impairment of accuracy in a delayed nonmatch-to-sample task in rats. Neuropharmacology. 2001;41(8):916–27. [DOI] [PubMed] [Google Scholar]
- 74.McLaurin EY, Holliday SL, Williams P, Brey RL. Predictors of cognitive dysfunction in patients with systemic lupus erythematosus. Neurology. 2005;64(2):297–303. [DOI] [PubMed] [Google Scholar]
- 75.Alexander JJ, Jacob A, Vezina P, Sekine H, Gilkeson GS, Quigg RJ. Absence of functional alternative complement pathway alleviates lupus cerebritis. Eur J Immunol. 2007;37(6):1691–701. [DOI] [PubMed] [Google Scholar]
- 76.Shi D, Tian T, Yao S, Cao K, Zhu X, Zhang M, et al. FTY720 attenuates behavioral deficits in a murine model of systemic lupus erythematosus. Brain Behav Immun. 2018;70:293–304. [DOI] [PubMed] [Google Scholar]
- 77.Jackson L, Eldahshan W, Fagan SC, Ergul A. Within the Brain: The Renin Angiotensin System. Int J Mol Sci. 2018;19(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Seliga A, Lee MH, Fernandes NC, Zuluaga-Ramirez V, Didukh M, Persidsky Y, et al. Kallikrein-Kinin System Suppresses Type I Interferon Responses: A Novel Pathway of Interferon Regulation. Front Immunol. 2018;9:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ohrui T, Tomita N, Sato-Nakagawa T, Matsui T, Maruyama M, Niwa K, et al. Effects of brain-penetrating ACE inhibitors on Alzheimer disease progression. Neurology. 2004;63(7):1324–5. [DOI] [PubMed] [Google Scholar]
- 80.Gebre AK, Altaye BM, Atey TM, Tuem KB, Berhe DF. Targeting Renin-Angiotensin System Against Alzheimer’s Disease. Front Pharmacol. 2018;9:440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Garrido-Mesa N, Zarzuelo A, Gálvez J. Minocycline: far beyond an antibiotic. Br J Pharmacol. 2013;169(2):337–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Posner H, Curiel R, Edgar C, Hendrix S, Liu E, Loewenstein DA, et al. Outcomes Assessment in Clinical Trials of Alzheimer’s Disease and its Precursors: Readying for Short-term and Long-term Clinical Trial Needs. Innov Clin Neurosci. 2017;14(1–2):22–9. [PMC free article] [PubMed] [Google Scholar]
- 83.Mahajan SD, Parikh NU, Woodruff TM, Jarvis JN, Lopez M, Hennon T, et al. C5a alters blood-brain barrier integrity in a human in vitro model of systemic lupus erythematosus. Immunology. 2015;146(1):130–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Massardo L, Bravo-Zehnder M, Calderon J, Flores P, Padilla O, Aguirre JM, et al. Anti-N-methyl-D-aspartate receptor and anti-ribosomal-P autoantibodies contribute to cognitive dysfunction in systemic lupus erythematosus. Lupus. 2015;24(6):558–68. [DOI] [PubMed] [Google Scholar]
- 85.Kowal C, Degiorgio LA, Lee JY, Edgar MA, Huerta PT, Volpe BT, et al. Human lupus autoantibodies against NMDA receptors mediate cognitive impairment. Proc Natl Acad Sci U S A. 2006;103(52):19854–9. [DOI] [PMC free article] [PubMed] [Google Scholar]