To the editor:
We thank Dr. Feng and colleagues for their interest in our article (1) and for sharing their data demonstrating the association of several autoantibodies with systemic lupus erythematosus (SLE) disease activity in a Chinese cohort of patients with SLE (2). As the authors know, autoantibodies in SLE patients arise years prior to the onset of clinical detection (3), drive the formation of immune complexes which activate complement to induce target organ damage, and may serve as biomarkers of disease activity (4).
Tang et al. used a multiplexed bead platform to examine the relationship between the titers of 15 autoantibodies with SLE disease activity. In cross-sectional analyses, a correlation between anti-nucleosome, anti-dsDNA, anti-Smith, anti-ribosomal P, and anti-histone titers with SLEDAI scores (treated as a continuous variable) was observed in hospitalized patients. The correlation coefficients were modest (~0.3). This may be due to a substantial number of subjects in their cohort with absent or low titers of autoantibodies, but they were included in the correlation analysis. In a subset of these patients (21), a longitudinal post-hospitalization sample was examined. It revealed a stronger association between these same autoantibodies with a change in SLEDAI scores except curiously for anti-dsDNA (which may be due to detection of low-affinity antibodies as the authors correctly noted (5)).
Two limitations exist for utilizing autoantibody titers as a surrogate of SLE disease activity. As mentioned, not all patients may have autoreactivity to any given autoantigen. The other issue is the variance observed between the assays used for autoantibody assessment. This is best described for dsDNA (reviewed in (5)), but this is also true for ANA testing where discordances using similar immunofluorescence assays have been reported (6). A “gold standard platform” for autoantibody detection has not yet been resolved, which currently limit the biomarker potential of autoantibodies.
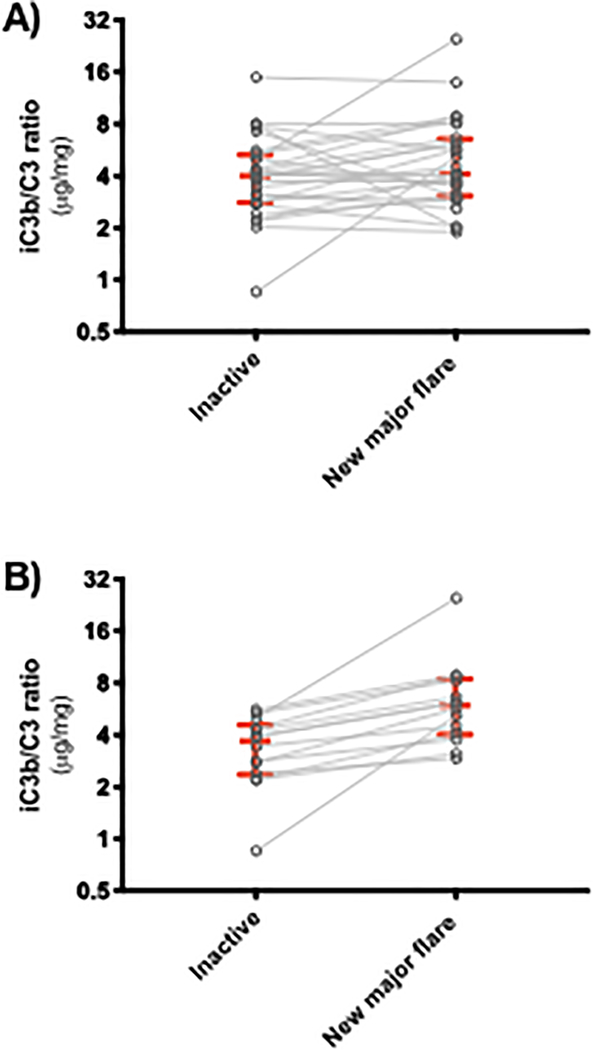
A single biomarker is likely insufficient to assess SLE disease activity. Indeed, while we have demonstrated the independent association of iC3b:C3 ratios with SLE disease activity (1), not all subjects possessed this association. For example, we have observed that only 14 out of 27 subjects in our cohort had a rise in iC3b:C3 ratios which correlated with a new major flare using the Fortin definition (7) (Figure 1). The assessment of additional complement species such as hydrolyzed C3 (8) or other complement activation products (9, 10) may be needed to fully categorize the complement activation signatures in patients with SLE. Furthermore, using intraindividual changes of complement levels over time will likely have more clinical value than comparisons to the lower limit of normal cutoff defined in healthy controls. This is due to known alterations in complement metabolism in SLE. For example, C3 tickover is ongoing at low levels even in patients with inactive disease (11). This dramatically limits the appropriate interpretation of complement levels if compared to healthy control reference values.
Figure 1. iC3b/C3 ratios associate with new major flares in a proportion of subjects with SLE.
A) Spaghetti plots of 27 subjects with inactive disease in which a new major flare was identified in consecutive visits in our Complement Activation Signatures in Systemic Lupus Erythematosus (CASTLE) cohort. iC3b :C3 ratios in the inactive [median = 4.012, interquartile range (IQR) = 2.803 – 3.068] were only marginally lower than the new major flare (median = 4.099, IQR = 3.068 – 6.544) group (p-value = 0.147). B) Fourteen of these subjects had a rise in iC3b:C3 levels (inactive median = 3.532, IQR = 2.359 – 4.565; new major flare median = 5.938, IQR = 4.017 – 8.430; p-value = 0.001).
We envision that complement activation products could be combined with autoantibody profiles, clinical manifestations, and additional investigational biomarkers to define clusters of SLE patients, each with a defined set of biomarkers that best associate with disease activity.
Acknowledgments
Funding
This research was funded/supported by Kypha, Inc. and National Institutes of Health (NIH)/National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) under Award Number R21AR069833. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Competing Interests
AHJK and JPA received funding related to this work from Kypha, Inc. AHJK participated in consulting, advisory board, and speaker’s bureaus for Exagen Diagnostics, Inc. VS is a consultant to Kypha, Inc., Abbvie, Inc., Amgen, Inc., AstraZeneca plc, Bristol Myers Squibb, Celgene Corporation, EMD Serono, Inc., Genentech/Roche Holding AG, GlaxoSmithKline plc, Janssen Pharmaceutica, Eli Lilly and Company, Novartis International AG, Pfizer, Inc. and Union Chimique Belge (UCB). JPA is a consultant or serves on scientific advisory boards for Kypha, Inc., Compliment Corporation, Gemini Therapeutics, Inc., Celldex Therapeutics, Clinical Pharmacy Services, CDMI, Omeros Corporation, Achillion Pharmaceuticals, Inc., True North Therapeutics, BioMarin Pharmaceutical, Inc., Annexon Biosciences, Inc., and AdMiRx, Inc.
References
- 1.Kim AHJ, Strand V, Sen DP, Fu Q, Mathis NL, Schmidt MJ, et al. Association of Blood Concentrations of Complement Split Product iC3b and Serum C3 With Systemic Lupus Erythematosus Disease Activity. Arthritis Rheumatol. 2019;71(3):420–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang X, Ning M, Zhu Y, Zhu Y, Xu Y, Wu S, et al. Association of autoantibody quantification with SLE disease activity: Comment on the Article by Kim et al. Arthritis Rheumatol. 2019. [DOI] [PubMed] [Google Scholar]
- 3.Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, James JA, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. The New England journal of medicine. 2003;349(16):1526–33. [DOI] [PubMed] [Google Scholar]
- 4.Rahman A, Isenberg DA. Systemic lupus erythematosus. The New England journal of medicine. 2008;358(9):929–39. [DOI] [PubMed] [Google Scholar]
- 5.Pisetsky DS. Anti-DNA antibodies--quintessential biomarkers of SLE. Nat Rev Rheumatol. 2016;12(2):102–10. [DOI] [PubMed] [Google Scholar]
- 6.Pisetsky DS, Spencer DM, Lipsky PE, Rovin BH. Assay variation in the detection of antinuclear antibodies in the sera of patients with established SLE. Annals of the rheumatic diseases. 2018;77(6):911–3. [DOI] [PubMed] [Google Scholar]
- 7.Fortin PR, Ferland D, Moore AD, Belisle P, Joseph L, Clarke AE. Rates and predictors of lupus flares. Arthritis Rheum-Us. 1998;41(9):S218–S. [Google Scholar]
- 8.Kulkarni HS, Elvington ML, Perng YC, Liszewski MK, Byers DE, Farkouh C, et al. Intracellular C3 Protects Human Airway Epithelial Cells from Stress-associated Cell Death. Am J Respir Cell Mol Biol. 2019;60(2):144–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buyon J, Furie R, Putterman C, Ramsey-Goldman R, Kalunian K, Barken D, et al. Reduction in erythrocyte-bound complement activation products and titres of anti-C1q antibodies associate with clinical improvement in systemic lupus erythematosus. Lupus Sci Med. 2016;3(1):e000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kao AH, Navratil JS, Ruffing MJ, Liu CC, Hawkins D, McKinnon KM, et al. Erythrocyte C3d and C4d for monitoring disease activity in systemic lupus erythematosus. Arthritis Rheum. 2010;62(3):837–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swaak AJ, van Rooyen A, Vogelaar C, Pillay M, Hack E. Complement (C3) metabolism in systemic lupus erythematosus in relation to the disease course. Rheumatol Int. 1986;6(5):221–6. [DOI] [PubMed] [Google Scholar]