Abstract
BACKGROUND:
Clinical studies suggest that heightened peripheral inflammation contributes to the pathogenesis of stress-related disorders including major depressive disorder. However, the molecular mechanisms within peripheral immune cells that mediate enhanced stress vulnerability are not well known. As microRNAs (miRs) are important regulators of immune response, we sought to examine their role in mediating inflammatory and behavioral responses to repeated social defeat stress (RSDS), a mouse model of stress vulnerability that produces susceptible and resilient phenotypes.
METHODS:
We isolated Ly6chigh monocytes via fluorescence-activated cell sorting in the blood of susceptible and resilient mice following RSDS and profiled miR expression via quantitative real-time PCR. Bone marrow chimeric mice were generated to confirm a causal role of the miR 106b~25 cluster in bone marrow–derived leukocytes in mediating stress resilience versus susceptibility.
RESULTS:
We found that RSDS produces an increase in circulating Ly6chigh inflammatory monocytes in both susceptible and resilient mice. We next investigated whether intrinsic leukocyte post-transcriptional mechanisms contribute to individual differences in stress response and the resilient phenotype. Of the miRs profiled in our panel, 8 were significantly regulated by RSDS within Ly6chigh monocytes, including miR-25-3p, a member of the miR-106b~25 cluster. Selective knockout of the miR-106b~25 cluster in peripheral leukocytes promoted behavioral resilience to RSDS.
CONCLUSIONS:
Our results identify the miR-106b~25 cluster as a key regulator of stress-induced inflammation and depression that may represent a novel therapeutic target for drug development.
Keywords: stress, resilience, major depressive disorder, immune system, leukocyte, microRNA
Introduction
While one in five Americans will suffer from Major Depressive Disorder (MDD) in their lifetime (1), approved treatments are ineffective in an estimated 30-50% of MDD patients (2). This high rate of treatment resistance suggests prominent pathophysiological mechanisms that are unresolved by current antidepressant medications (2). Increasing evidence suggests that one such mechanism is heightened peripheral and central inflammation (2, 3). Strikingly, MDD is often comorbid with chronic inflammatory illness, and patients with pre-existing diseases such as multiple sclerosis (4, 5), type 2 diabetes (6, 7), and cardiovascular diseases (8) are at increased risk of developing MDD. can be several times more likely to develop depression than healthy individuals (3). Even in the absence of chronic physical disease, subsets of MDD patients display a dysregulation of inflammatory status (2). Some of the most consistently reported alterations of the immune system in MDD patients include leukocytosis, or an increase in circulating white blood cells, that is specific to inflammatory monocytes and neutrophils (9), and elevations in serum proinflammatory cytokines including tumor necrosis factor (TNF) α (10), interleukin (IL) 1β (11), IL-6 (10, 12), and interferon (IFN) γ (11).
Recent studies have replicated these symptoms in preclinical models and yielded important insights on the role of inflammation in stress-related disorders. Mice exposed to repeated social defeat (RSD), a chronic psychosocial stress paradigm that produces anxiety-like behavior, display a β-adrenergic signaling dependent increase in Ly6chigh inflammatory monocytes and neutrophils in blood, bone marrow and spleen (13). Ly6chigh monocytes are immature, classic inflammatory monocytes that are recruited to sites of inflammation, where they can further differentiate into macrophages or complement the activities of macrophages and dendritic cells (14). A similar pattern of stress-induced myelopoiesis has been reported in mice exposed to chronic variable stress (15). We recently demonstrated that psychosocial stress in the form of repeated social defeat stress (RSDS) elevates peripheral levels of the cytokine Interleukin-6 (IL-6) (16). In contrast to the RSD paradigm, RSDS allows for the separation of resilient mice that behave similarly to unstressed controls from susceptible mice that display depression-like behaviors following RSDS (17). Our previous studies indicate that susceptible mice exhibit elevated serum IL-6 levels compared to both control and resilient mice 48 hrs after RSDS (16, 18, 19). Interestingly, prior to behavioral exposure to RSDS, isolated leukocytes from mice that will go on to become susceptible release more IL-6 in response to in vitro stimulation with lipopolysaccharide (LPS), a bacterial endotoxin and Toll-Like Receptor 4 agonist, than do those of mice that will go on to become resilient (16). This finding suggests that intrinsic mechanisms at the level of the leukocyte likely contribute to increased inflammatory potential in susceptible mice and possible protection from inflammation in resilient mice.
In the present study, we aimed to characterize the effect of RSDS on the circulating leukocyte pool and to identify intrinsic leukocyte post-transcriptional mechanisms contributing to individual differences in stress-induced inflammation and resilience. We focused specifically on epigenetic regulation by microRNAs (miRs) as a potential mediator of the individual differences we observe in C57BL/6J mice—an inbred, highly genetically similar mouse strain. miRs are important regulators of immune processes, including immune cell differentiation and proliferation, and transcription factor, chemokine and cytokine signaling (20). Recent studies in humans have reported dysregulated miR expression in whole blood (21, 22) and peripheral blood mononuclear cells (PBMCs) (23, 24) of depressed patients compared to healthy controls. Human findings also indicate regulation of several blood miRs by antidepressant medication (23, 25, 26) and cognitive behavioral therapy (22) in treatment-responsive patients.
Materials and Methods
Animals
Male CD45.2+ C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME) were shipped to the Icahn School of Medicine at Mount Sinai (ISMMS) animal facility at 6 - 9 weeks of age and allowed at least 1 week of acclimation to the facility prior to the start of experiments. Male CD45.1+ C57BL/6J bone marrow transplant hosts (The Jackson Laboratory, Bar Harbor, ME) were shipped to the ISMMS facility at 3 weeks of age and allowed one week of acclimation prior to irradiation. Homozygous male Mirc3tm1.1Tyj/J mice were bred on a CD45.2+ C57BL/6J background at ISMMS. Male CD-1 mice (Charles River Laboratories, Raleigh, NC) used as aggressors were retired breeders greater than 4 months of age. C57BL/6J mice were group housed prior to social defeat and singly housed after social defeat until sacrifice. CD-1 mice were singly housed before and after social defeat. Animals were maintained on a 12 h light/dark cycle with ad libitum access to food and water. Mouse procedures were performed in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals and the ISMMS Institutional Animal Care and Use Committee.
Repeated Social Defeat Stress
RSDS was performed as described previously (16, 17). Briefly, CD-1 mice were selected as RSDS aggressors following repeated screening for intruder-elicited aggressive responses in the home cage. Twenty-four hours prior to the start of RSDS, CD-1 aggressor mice were singly housed in static hamster cages with woodchip bedding on one side of a clear, perforated Plexiglas partition. Each day for 10 consecutive days, experimental mice were exposed to a novel CD-1 aggressor mouse in its home cage for a 5-10-min bout of physical aggression. After the defeat bout, experimental mice were moved to the opposite side of the perforated partition for 24 hours, allowing for physical separation but continuous sensory contact. Control mice were housed in mouse static cages with woodchip bedding, 2 mice to a cage, on opposite sides of a perforated Plexiglas divider. Controls were rotated daily to simulate the rotation of the defeat group but were never exposed to CD-1 aggressors. Twenty-four hours after the final defeat bout, experimental mice were singly housed in static mouse cages with woodchip bedding. Animals were visually inspected for wounding during the course of RSDS. Mice with excessive wounding and moribund mice were immediately sacrificed. To quantify sickness behavior, food and water consumption were assessed by measuring the difference between the weight of the food pellets or water bottles immediately after the defeat and 16 hours later.
Social Avoidance Testing (Social Interaction Test)
Social Interaction (SI) testing was performed as described previously (16, 27). Under red light conditions, experimental mice were placed in a novel, Plexiglas open-field arena with a small, wire enclosure placed at one end. The SI test comprised two phases. In the first phase, mouse baseline exploratory behavior was tracked for 2.5 minutes in the absence of a novel CD-1 mouse using Ethovision 3.0 software (Noldus Information Technology, Leesburg, VA). In the second phase, a novel CD-1 mouse was placed in the wire enclosure, and exploratory behavior of the experimental mouse was recorded for 2.5 minutes. See supplemental methods for calculation of SI ratio.
Accelerated Defeat
To measure susceptibility and resilience to RSDS within the five-day treatment constraints of the MC-21 antibody, we performed an adapted, accelerated RSDS protocol as described previously (28). Experimental mice received two, five-minute defeats per day for four days, totaling 8 defeats. Defeats were administered in the morning and evening. Accelerated defeat is not a subthreshold stressor as this protocol is sufficient to induce social avoidance behavior under control conditions (29). Data shown for accelerated defeat are combined from two separate cohorts. Animals were visually inspected for wounding during the course of accelerated RSDS. Mice with excessive wounding and moribund mice were immediately sacrificed.
MC-21 / MC-67 Antibody Treatment
Male CD45.2+C57BL/6J mice were given daily injections (i.p.). of saline, rat anti- mouse CCR2 mAb MC-21 (lot no. AK1480/01) or IgG2b kappa isotype control MC-67 (lot no. AK1481/02). Antibodies were administered at a dose of 4 ug per mouse per day in 100 uL sterile saline. Antibodies were administered at least 5 minutes prior to defeat or social interaction testing. Animals showing poor depletion in subsequent flow cytometry analysis were excluded.
Generation of Bone Marrow Chimeras
As described in Hodes et al. (16), four week old, CD45.1+C57BL/6J host mice were irradiated with 1,200 rad delivered in two doses of 600 rad, 10-11 hours apart, to ablate the peripheral immune system. During irradiation, animals were anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg) and placed in lead tubes (Nuclead, West Bridgewater, MA) such that only the head was shielded. Donor bone marrow progenitor cells were lavaged from femurs of four male Mirc3tm1.1Tyj/J−/− and four male Mirc3tm1.1Tyj/J+/+ mice with fluorescence-activated cell sorting (FACS) buffer (2% heat-inactivated FBS, 5 mM EDTA in 1X Dulbecco’s PBS, Thermofisher, Waltham, MA). Progenitor cells were next incubated with 1X red blood cell (RBC) lysis solution (BioLegend, San Diego, CA), washed in FACS buffer, and counted with a hemocytometer. Shortly following the second bout of host irradiation, approximately 2×106 donor bone marrow progenitor cells were transplanted through retro-orbital injection. Host mice received three weeks of antibiotic (Sulfatrim) treatment, followed by 5 weeks of rest, for a total of 8 weeks recovery and donor repopulation. At sacrifice, the level of chimerism was assayed via flow cytometry by comparing populations of CD45.1+ leukocytes (host) and CD45.2+ leukocytes (donor). One statistical outlier mouse was excluded due to low chimerism.
Blood Sample Collection, Processing and Antibody Staining
Trunk blood was collected following rapid decapitation. For FACS, 500 uL to 1 mL of blood was collected from each mouse into 3 mL FACS buffer. For flow cytometry, 100 uL of blood was collected into 2-3 mL FACS buffer. Cells were depleted of RBCs with 1X RBC lysis solution (BioLegend), filtered and washed in FACS buffer, and surface stained in 50 uL FACS buffer for 20-30 minutes on ice. See supplemental methods for antibodies used for FACS.
Flow Cytometry and FACS
Multiparameter flow cytometry analysis was performed on an LSRII Fortessa cytometer (BD Biosciences, San Jose, CA). Flow data was analyzed with FlowJo software (Tree Star, Ashland, OR). FACS was performed on a FacsAria II sorter (BD Biosciences) followed by analysis with FlowJo software (Tree Star). DAPI+ dead cells and doublets were excluded from analysis and collection. Cells were sorted directly into 500 uL Trizol LS reagent (Ambion, Waltham, MA), lysed, and flash frozen on dry ice.
RNA Isolation and Quantitative Real-Time PCR (qPCR)
Total RNA was isolated using the DirectZol RNA MiniPrep and RNA Clean & Concentrator-5 kits (Zymo Research, Irvine, CA) according to the manufacturer’s instructions. miR expression was assayed using the miRCURY LNA™ Universal RT microRNA PCR protocol with custom Pick-&-Mix panels (Exiqon, Woburn, MA) according to the manufacturer’s instructions, with modifications for low input. 1.35 ng of total RNA was reverse transcribed to cDNA for each sample (Universal cDNA Synthesis Kit II, Exiqon). The cDNA product was diluted 50X in nuclease-free water, combined 1:1 with PCR Master Mix (ExiLENT SYBR® Green master mix, Exiqon), and dispensed into custom 384 well plates. Samples were run in duplicate, and two no-template controls (NTC) were included. Real-time PCR amplification followed by melting curve analysis was performed on an Applied Biosystems 7900HT machine (ThermoFisher). Samples were heated to 95°C for 10 min followed by 40 amplification cycles of 95°C for 10 seconds and 60°C for 1 min at a ramp-rate 1.6°C/ s. See supplemental methods for detailed description of pcr analysis.
Statistical Analysis
Comparisons of three or more means were analyzed by one-way analysis of variance (ANOVA) with Tukey’s post hoc tests. Comparisons of multiple factors were analyzed by two-way ANOVA with Bonferroni post hoc tests. Data were log transformed prior to ANOVA when variances were unequal. Statistical analysis was performed using Prism 5.0 software (GraphPad, La Jolla, CA) and statistical significance was set at p<0.05. Outliers for behavioral testing were identified as being greater than two standard deviations from the mean and were excluded from statistical analysis.
Results
Chronic social stress promotes a robust induction of Ly6chigh inflammatory monocytes and neutrophils in peripheral blood
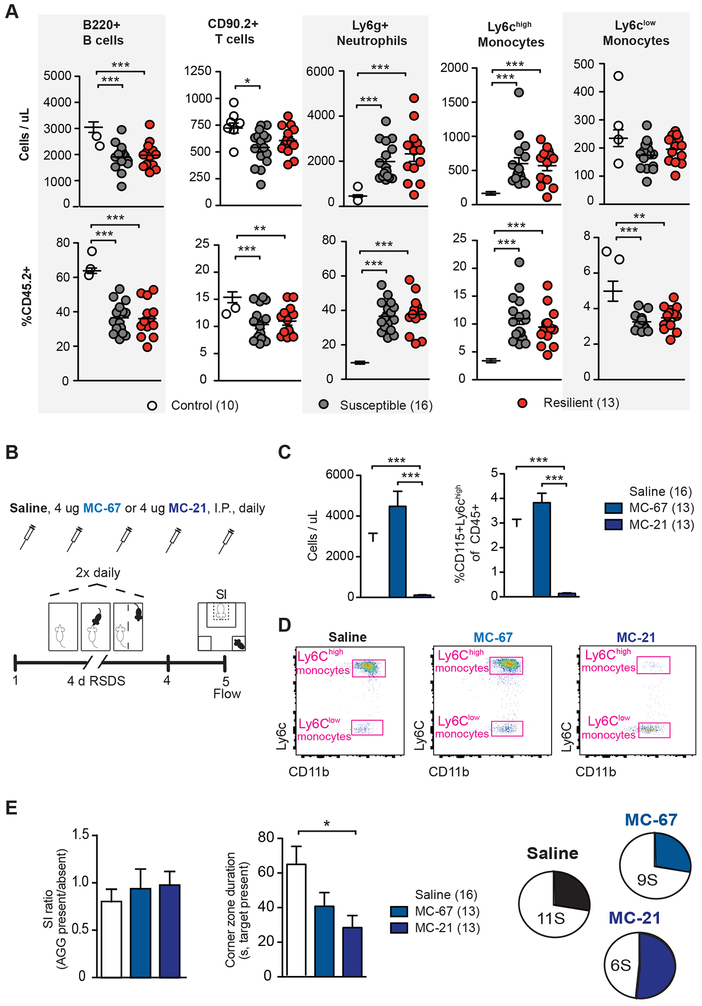
In order to assess the effect of RSDS on the circulating leukocyte pool, we exposed mice to 10 days of RSDS and performed flow cytometry on blood collected 48 hours after the last defeat bout (Supplementary Figure 1A and B) Lymphocytes, monocytes and neutrophils have been implicated in response to (30, 31) or regulated by (13, 15) other stress paradigms. Flow cytometry analysis revealed stress-induced changes in the absolute counts and frequencies of several leukocyte subtype populations 48 hours after defeat (Figure 1A). In both susceptible and resilient mice compared to controls, RSDS exposure induced an increase in absolute count and frequency of circulating neutrophils (F2,36=37.55, p<0.0001 [count]; F2,36=86.90, p<0.0001 [frequency]) and Ly6chigh monocytes (F2,36=21.04, p<0.0001 [count]; F2,36=37.91, p<0.0001 [frequency]). RSDS exposure induced a robust decrease in absolute count (F2,36=15.97, p<0.0001) and frequency (F2,36=38.69, p<0.0001) of circulating B cells in susceptible and resilient groups compared to controls. RSDS led to a decrease in absolute count of T cells (F2,36=4.51, p<0.05) only in susceptible mice. While we observed no group differences in absolute count of Ly6clow monocytes, total frequency was decreases (F2,36=9.026, p<0.001) in both susceptible and resilient mice compared to control mice. These differences in leukocyte cell populations were not related to wounding (Supplementary Figure 1C) or to changes in the total number of circulating leukocytes (Supplementary Figure 1D). In order to characterize longer term changes in the leukocyte pool, we next performed flow cytometry nine days after defeat in a second cohort of mice. We observed sustained changes in leukocytes following RSDS only in susceptible mice at this time point. For example, Ly6chigh monocytes remained increased both in absolute count and frequency (F2,36=3.45, p<0.05 [count]; F2,36=4.80, p<0.05 [frequency]), neutrophil frequencies were increased (F2,36=3.33, p<0.05) and B-cell frequencies decreased (F2,36=5.42, p<0.01) (Supplementary Figure 2).
Figure 1. Role of Ly6chigh monocytes in behavioral response to repeated social defeat stress.
(A) Absolute counts (top) and relative frequencies (bottom) of B cells, T cells, Neutrophils, Ly6chigh monocytes, and Ly6clow monocytes in peripheral blood 48 hours post repeated social defeat stress (RSDS). (B) Experimental timeline of 4-day accelerated RSDS, daily injection of saline, MC-21 anti-CCR2 antibody or MC-67 isotype control, social interaction (SI) testing, and flow cytometry analysis (flow). (C) Depletion of Ly6chigh monocytes in peripheral blood by daily injection of MC-21, shown as a decrease in absolute count (left) and frequency (right). (D) Representative flow scatter plots of blood from Saline (left), MC-67 (middle), and MC-21 (right) treated mice. (E) MC-21 treated mice showed a nonsignificant increase in social interaction ratio compared to the saline treated group (left). MC-21 mice spent less time in the corner zones than saline treated mice when the target was present (middle). Whereas saline and MC-67 groups showed an approximately 1:2 ratio of resilience to susceptibility, MC-21 treated mice showed a roughly 1:1 ratio (right). For bar graphs, data represent mean + SEM. For dot plots, data represent mean ± SEM. The number of animals (n) within each group is indicated below graphs. *p<0.05, **p<0.01, ***p<0.0001 (one-way ANOVA with Tukey’s post hoc tests [A, E].
Depletion of Ly6chigh monocytes is mildly antidepressant
Because we observed an RSDS-induced increase in Ly6chigh monocytes in susceptible and resilient mice, and due to prior publications attributing the leukocyte transcriptional response to stress to the monocyte compartment (13), we next sought to determine the functional role of Ly6chigh monocytes in behavioral responses to RSDS. We administered daily systemic injections of the rat anti-mouse CCR2 antibody MC-21, its IgG2b isotype control MC-67, or saline and performed an accelerated RSDS protocol (Figure 1B). CCR2, a receptor for the chemokine CCL2 necessary for Ly6chigh monocyte recruitment from bone marrow, is expressed by Ly6chigh but not Ly6clow monocytes (32). Daily injection of MC-21 has been shown to deplete Ly6chigh monocytes in blood for 5 days, after which the antibody becomes ineffective due to humoral immune response and the generation of mouse anti-rat antibodies (32), necessitating our use of the accelerated defeat protocol (Figure 1B). We observed a robust depletion of Ly6chigh monocytes in blood, both in relative frequency (F2,39=192.0, p<0.0001) and absolute count (F2,39=129.4, p<0.0001) in mice treated with MC-21 antibody injections (Figure 1C and D). Compared to saline treated mice, MC-21 treated mice showed a higher SI ratio, however this trend did not reach statistical significance (Figure 1E). When the aggressor was present, MC-21 treated mice spent significantly less time in the corners than saline treated mice (Figure 1E, F2,39=4.345, p<0.05). Overall, more mice were classified as resilient in the MC-21-treated group as indicated by the pie charts in Figure 1E, suggesting that Ly6chigh depletion has a mild antidepressant effect. There were no differences between stressed mice receiving MC-21 or MC-67 in measures of sickness behavior (Supplementary Figure 3). Lastly, in unstressed mice, there was no difference between mice reciveing MC-21 or MC-67 on social interaction behavior (Supplementary Figure 4).
RSDS regulates miR expression in Ly6chigh monocytes
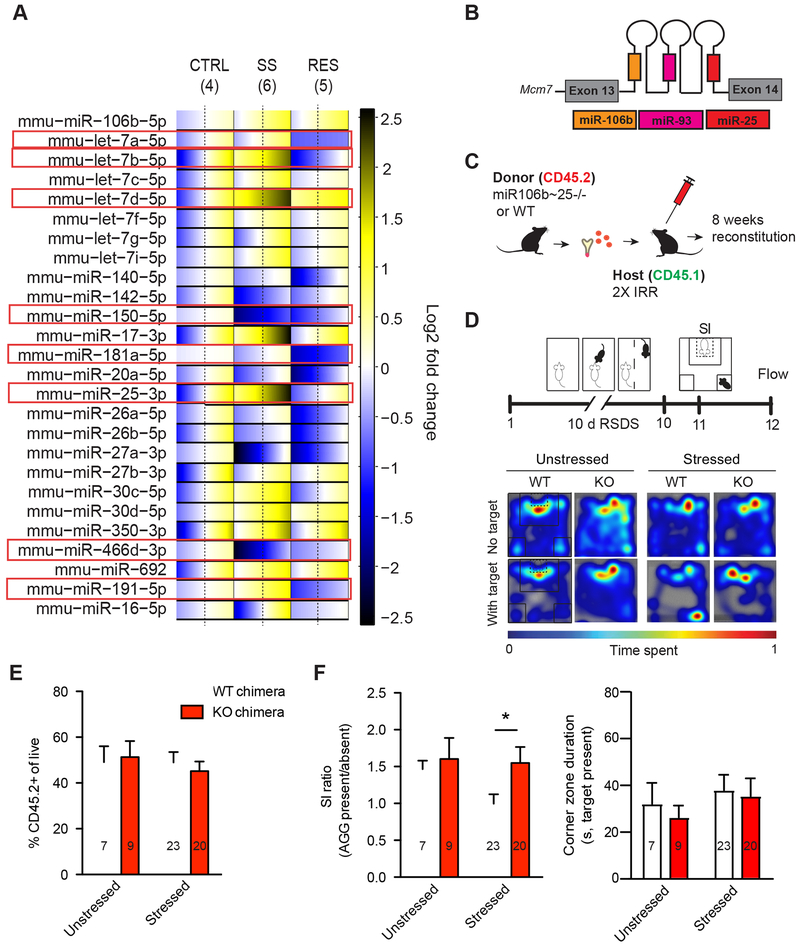
Given that RSDS similarly increased Ly6chigh monocytes in susceptible and resilient mice and that Ly6chigh monocyte depletion had only mild effects on social avoidance, we next sought to profile molecular signatures within Ly6chigh monocytes that may contribute to individual differences in behavioral and inflammatory responses to RSDS. We collected Ly6chigh monocytes via FACS 48-hours post-RSDS and isolated total RNA (Supplementary Figures, 5 and 6A, B). We profiled 44 miRs using a custom-designed qPCR panel (Supplementary Table 1). miRs were chosen based on fulfillment of at least three of the following criteria: 1) Predicted to target cytokines (IL-6, IL-10 or IL-1β) dysregulated in social defeat (16), 2) highly expressed in mouse Ly6chigh monocytes or human PBMCs (33, 34), 3) regulated in human depression or antidepressant response (24-26, 35), 4) regulated by LPS (36-38), or 5) dysregulated in chronic inflammatory disorders. Thirty of the 44 miRs assayed were detectable in our samples (see Figure 2A for an expression heat map of assays not used for reference normalization). We identified 8 miRs that were significantly regulated by RSDS by univariate ANOVA, with significant or strongly trending post hoc differences between groups (Figure 2A, highlighted in red boxes, Supplementary Table 2).
Figure 2. Peripheral knockdown of miR-106b~25 cluster promotes behavioral resilience to repeated social defeat stress (RSDS).
(A) microRNA profiling in Ly6chigh monocytes 48 hours after RSDS. Heatmap illustrating normalized expression pattern of detectable microRNAs (miRs) in resilient (right) and susceptible mice (middle) relative to unstressed controls (left). The range of colors indicates individual differences within each group (standard error of the mean, SEM) from the average, which is represented by a dashed line. Blue indicates lower expression, yellow indicates increased expression, and white indicates no change. (B) Schematic of the polycistronic miR-106b~25 cluster. (C) Schematic illustrating the generation of miR-106b~25−/− bone marrow chimeras. (D) Experimental timeline of 10-day RSDS, social interaction (SI) testing, and flow cytometry. (E) % CD45.2+ cells in WT and miR-106b~25−/− chimeras showing no differences in level of chimerism. (F) Social interaction ratios in stressed and unstressed WT and miR-106b~25−/− chimeras. 106b~25−/− chimeras exhibited significantly higher social interaction ratios than stressed WT chimeras and no differences in corner zone duration. Representative heat maps of social interaction tests are shown on the top right. For bar graphs, data represent mean + SEM. The number of animals (n) within each group is indicated on the graphs. *p<0.05 (two-way ANOVA with Bonferroni post hoc test).
Downregulation of the miR-106b~25 cluster promotes behavioral resilience to RSDS
Of the 8 miRs we identified to be regulated by RSDS, we chose to focus on miR-25-3p as its expression was oppositely regulated in stressed mice and because it was negatively correlated with social interaction (p<0.05) (Supplementary Figure 6C). miR-25-3p is a member of the miR-106b~25 polycistronic cluster encoded within the thirteenth intron of the minichromosome maintenance complex component 7 (Mcm7) DNA replication gene (Figure 2B) (39). The cluster is widely expressed and has been primarily studied for its oncogenic properties (40). In order to functionally interrogate the role of the miR-106b~25 cluster in behavioral susceptibility and resilience, we generated bone marrow chimeric mice, using either homozygous miR-106b~25 cluster KO mice (Mirc3tm1.1Tyj/J+/−, referred to henceforth as miR-106b~25−/−) or littermate, wild type controls (Mirc3tm1.1Tyj/J+/+, referred to henceforth as WT) as bone marrow donors (Figure 2C). Based on our miR profiling results, we hypothesized that miR-106b~25−/− chimeras would display enhanced behavioral resilience. We exposed chimeric mice to RSDS followed by behavioral analysis and blood flow cytometry to confirm chimerism (Figure 2D). In both groups, we observed ~50% chimerism within leukocytes, a partial chimerism likely attributable to our use of lead head shielding to prevent radiation-induced changes in hippocampal neurogenesis and blood-brain barrier permeability (Figure 2E). We observed no group differences in chimerism within leukocyte subtypes (Supplementary Figure 7). Consistent with our hypothesis, our behavioral results indicated that downregulation of the cluster promotes resilience to RSDS. miR-106b~25−/− chimeras exposed to RSDS displayed higher social interaction ratios than stressed WT chimeras (Figure 2F, chimerism effect F1,57=2.70, Bonferroni post hoc test p<0.05 for stressed mice) but no effect on corner time.
Discussion
We report that RSDS induces changes in leukocyte subtype frequency in peripheral blood, including a robust induction of Ly6chigh monocytes in both suscpetible and resilient mice. Depletion of peripheral Ly6chigh monocytes is mildly antidepressant, leading us to explore whether intrinsic post-transcriptional mechanisms are involved in resilience. Within Ly6chigh monocytes of mice exposed to RSDS, we identify several miRs regulated by RSDS 48 hours after defeat, including miR-25-3p, a member of the miR-106b~25 cluster. Peripheral downregulation of this cluster through generation of bone marrow chimeras promotes behavioral resilience to RSDS.
Our findings of stress induction of monocytes and neutrophils in blood support previous findings in mice (13, 15, 41) and humans (13, 15) exposed to chronic stress. Interestingly, we also observed a decrease in absolute counts of circulating T cells in susceptible animals vs. controls. Recent studies have highlighted a potential role for T cells in stress resilience. Cohen et al. (30) report that mice deficient in mature T cells are more susceptible to predator odor stress than wild type mice. This phenomenon may be related to behavioral “immunization” to stress that is mediated by T cells (42). Indeed, Brachman et al. (31) find that infusion of lymphocytes (which include both T and B cells) from mice exposed to RSDS into mice deficient in mature lymphocytes confers resilience to developing depression and anxiety-like behaviors. Further study of T cells in the RSDS model, as well as of potential miR-mediated mechanisms for establishing immunological memory of stress, may yield additional insights into the biology of resilience.
We observe that peripheral antibody depletion of CCR2+Ly6chigh monocytes is mildly antidepressant, supporting and expanding upon recent studies indicating anxiolytic effects of whole body CCR2 knock out (41) and antidepressant effects of Ly6c mAb treatment (43). We did not, however, find that depleting blood monocytes prevents susceptibility to RSDS. Zheng et al. (43) have used similar monocyte-depletion approaches, reporting that peripheral depletion of Ly6chigh monocytes with a Ly6c mAb prevents LPS-induced depression-like behavior in the sucrose preference and forced swim tests and attenuates LPS-induced proinflammatory transcription in brain. They also report results from enriching for Ly6chigh monocytes, in which they took advantage of the recovery of peripheral monocytes heavily enriched for the Ly6chigh subtype that follows clodronate liposome depletion. Mice with enriched Ly6chigh monocyte populations in blood displayed enhanced LPS-induced depression-like behavior in the sucrose preference and forced swim tests. While this group reports antidepressant findings in the RSDS behavioral model, for these studies they admininister ginsenoside Rg1, which does not deplete Ly6chigh monocytes in blood, but rather reduces Ly6chigh monocyte recruitment to the CNS through its inhibition of cerebral CCL2. Collectively, these results indicate that the antidepressant effects of monocyte depletion may require both reduced peripheral circulation and reduced signaling with the CNS.
We generated bone marrow chimeras using miR-106b~25−/− mice in order to investigate the functional importance of the miR-106b~25 cluster in behavioral response to stress. miR-25-3p, a member of the cluster, was regulated by RSDS in monocytes 48 hours after defeat, and its expression was signficantly correlated with individual social interaction ratios of stressed mice. We also included miR-106b-5p in our panel, observing nonsignificant increased expression in both susceptible and resilient mice. The miR-106b~25 cluster has been shown to mediate TGF-β signaling (39), which can potentiate inflammatory signaling in monocytes (44), but this is the first demonstration linking this specifc cluster to depression or mood disorders. One study reported elevated miR-106b-5p expression in the cerebro spinal fluid of MDD patients vs. control subjects (45). While it is still unclear what inflammatory targets miR106b~25 may regulate to promote resilience, recent studies have shown IL-10 to be post-transcriptionally regulated by miR106b~25 (46) (47). Thus, it’s possible that depletion of miR106b~25 results in increased levels of the anti-inflammatory cytokine IL-10. Future studies are required to investigate the potential downstream mechanisms of miR-106b and their roles in promoting resilience vs. susceptibility.
miR profiling in monocytes revealed several additional targets of interest. For instance, we observed a reduction of miR-150-5p in both susceptible and resilient mice. miR-150-5p is enriched in monocytes (48, 49) and has been shown to regulate monocytosis in acute myocardial infarction (MI), a condition associated with depressive symptoms in 65% of patients (50). MI results in an inflammatory response that triggers the recruitment of neutrophils and monocytes to the heart (49). miR-150-5p is downregulated in human and mouse monocytes after MI, and in vitro overexpression of miR-150-5p in THP-1 (human monocytic leukemia) cells inhibits migration and proinflammatory cytokine release, whereas blockade produces the opposite effect (49). miR-150-5p−/− bone marrow chimeras exhibit a prominent post-MI infltration of Ly6chigh monocytes in blood and spleen (49). Unexpectedly, we observed a pattern of downregulation of monocyte-dervived let-7 miRs in resilient mice and upregulation in susceptible mice. This was surprising as the ubiquitous let-7 family has anti-inflammatory activities (51) and is a known regulator of IL-6 (52, 53). However, these results may be related to our post-defeat time point, and may indicate that the let-7 family serves as a homeostatic mechanism to control excessive inflammation in susceptible mice. miR-181a-5p, which was selectively downregulated in monocytes of resilient mice, has been shown to be an important regulator of T cell receptor (TCR) sensitivity and age-related changes in adaptive immunity (54, 55). miR-191-5p showed a similar expression pattern to miR-25-3p and, like miR-25-3p, is a member of an oncogenic miR cluster (56). It has also been shown to be dysregulated in plasma of patients with chronic conditions including Type II diabetes (57) and Alzheimer’s disease (58).
In conclusion, we find that RSDS induces changes in circulating leukocyte subpopulations and monocyte miR expression profiles. Inflammatory monocyte depletion is mildly antidepressant, whereas downregulation of the miR-106b~25 cluster promotes behavioral resilience. These findings shed light on the role of peripheral inflammation in depression and identify novel targets for further research and potential therapeutic intervention.
Supplementary Material
Acknowledgements
This research was supported by Mental Health grants RO1 MH090264, P50 MH096890 (SJR), P50 AT008661-01 (S.J.R.), RO1 MH104559 (to S.J.R. and M.M.), T32 MH087004 (M.L.P. and M.F.), T32 MH096678 (M.L.P.), F31 MH105217 (M.L.P.), a Janssen/IMHRO Rising Star Translational Research Award (SJR), a Irma T. Hirschl/Monique Weill-Caulier Trust Research Awards, a Swiss National Science Foundation Early / Advanced Postdoc Mobility fellowship (to V.K. and F.C.), a Walter and Gertrud Siegenthaler Postdoctoral Fellowship (FC) and Brain and Behavior Research Foundation NARSAD young investigator awards (C.M. and G.E.H.). The authors would like to thank Andrea Ventura (Sloan Kettering) for generously providing the miR106b−/− mice as well as the Center for Comparative Medicine and Surgery housing facilities staff for their work and support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures section:
The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE (2005): Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of general psychiatry. 62:593–602. [DOI] [PubMed] [Google Scholar]
- 2.Hodes GE, Kana V, Menard C, Merad M, Russo SJ (2015): Neuroimmune mechanisms of depression. Nat Neurosci. 18:1386–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Menard C, Pfau ML, Hodes GE, Russo SJ (2016): Immune and Neuroendocrine Mechanisms of Stress Vulnerability and Resilience. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marrie RA, Reingold S, Cohen J, Stuve O, Trojano M, Sorensen PS, et al. (2015): The incidence and prevalence of psychiatric disorders in multiple sclerosis: a systematic review. Mult Scler. 21:305–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siegert RJ, Abernethy DA (2005): Depression in multiple sclerosis: a review. J Neurol Neurosurg Psychiatry. 76:469–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ali S, Stone MA, Peters JL, Davies MJ, Khunti K (2006): The prevalence of comorbid depression in adults with Type 2 diabetes: a systematic review and meta-analysis. Diabet Med. 23:1165–1173. [DOI] [PubMed] [Google Scholar]
- 7.Anderson RJ, Freedland KE, Clouse RE, Lustman PJ (2001): The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care. 24:1069–1078. [DOI] [PubMed] [Google Scholar]
- 8.Rudisch B, Nemeroff CB (2003): Epidemiology of comorbid coronary artery disease and depression. Biol Psychiatry. 54:227–240. [DOI] [PubMed] [Google Scholar]
- 9.Maes M, Van der Planken M, Stevens WJ, Peeters D, DeClerck LS, Bridts CH, et al. (1992): Leukocytosis, monocytosis and neutrophilia: hallmarks of severe depression. J Psychiatr Res. 26:125–134. [DOI] [PubMed] [Google Scholar]
- 10.Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, et al. (2010): A meta-analysis of cytokines in major depression. Biol Psychiatry. 67:446–457. [DOI] [PubMed] [Google Scholar]
- 11.Schiepers Oj, Wichers MC, Maes M (2005): Cytokines and major depression. Prog Neuropsychopharmacol Biol Psychiatry. 29:201–217. [DOI] [PubMed] [Google Scholar]
- 12.Maes M, Bosmans E, De Jongh R, Kenis G, Vandoolaeghe E, Neels H (1997): Increased serum IL-6 and IL-1 receptor antagonist concentrations in major depression and treatment resistant depression. Cytokine. 9:853–858. [DOI] [PubMed] [Google Scholar]
- 13.Powell ND, Sloan EK, Bailey MT, Arevalo JM, Miller GE, Chen E, et al. (2013): Social stress up-regulates inflammatory gene expression in the leukocyte transcriptome via beta-adrenergic induction of myelopoiesis. Proceedings of the National Academy of Sciences of the United States of America. 110:16574–16579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ginhoux F, Jung S (2014): Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat Rev Immunol. 14:392–404. [DOI] [PubMed] [Google Scholar]
- 15.Heidt T, Sager HB, Courties G, Dutta P, Iwamoto Y, Zaltsman A, et al. (2014): Chronic variable stress activates hematopoietic stem cells. Nature medicine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hodes GE, Pfau ML, Leboeuf M, Golden SA, Christoffel DJ, Bregman D, et al. (2014): Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proceedings of the National Academy of Sciences of the United States of America. 111:16136–16141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Golden SA, Covington HE 3rd, Berton O, Russo SJ (2011): A standardized protocol for repeated social defeat stress in mice. Nature protocols. 6:1183–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Menard C, Pfau ML, Hodes GE, Kana V, Wang VX, Bouchard S, et al. (2017): Social stress induces neurovascular pathology promoting depression. Nat Neurosci. 20:1752–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang J, Hodes GE, Zhang H, Zhang S, Zhao W, Golden SA, et al. (2018): Epigenetic modulation of inflammation and synaptic plasticity promotes resilience against stress in mice. Nat Commun. 9:477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee HM, Nguyen DT, Lu LF (2014): Progress and challenge of microRNA research in immunity. Front Genet. 5:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maffioletti E, Cattaneo A, Rosso G, Maina G, Maj C, Gennarelli M, et al. (2016): Peripheral whole blood microRNA alterations in major depression and bipolar disorder. J Affect Disord. 200:250–258. [DOI] [PubMed] [Google Scholar]
- 22.Issler O, Haramati S, Paul ED, Maeno H, Navon I, Zwang R, et al. (2014): MicroRNA 135 is essential for chronic stress resiliency, antidepressant efficacy, and intact serotonergic activity. Neuron. 83:344–360. [DOI] [PubMed] [Google Scholar]
- 23.He S, Liu X, Jiang K, Peng D, Hong W, Fang Y, et al. (2016): Alterations of microRNA-124 expression in peripheral blood mononuclear cells in pre- and post-treatment patients with major depressive disorder. J Psychiatr Res. 78:65–71. [DOI] [PubMed] [Google Scholar]
- 24.Fan HM, Sun XY, Guo W, Zhong AF, Niu W, Zhao L, et al. (2014): Differential expression of microRNA in peripheral blood mononuclear cells as specific biomarker for major depressive disorder patients. J Psychiatr Res. 59:45–52. [DOI] [PubMed] [Google Scholar]
- 25.Bocchio-Chiavetto L, Maffioletti E, Bettinsoli P, Giovannini C, Bignotti S, Tardito D, et al. (2012): Blood microRNA changes in depressed patients during antidepressant treatment. Eur Neuropsychopharmacol. [DOI] [PubMed] [Google Scholar]
- 26.Belzeaux R, Bergon A, Jeanjean V, Loriod B, Formisano-Treziny C, Verrier L, et al. (2012): Responder and nonresponder patients exhibit different peripheral transcriptional signatures during major depressive episode. Transl Psychiatry. 2:e185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, et al. (2006): Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 311:864–868. [DOI] [PubMed] [Google Scholar]
- 28.Wilkinson MB, Dias C, Magida J, Mazei-Robison M, Lobo M, Kennedy P, et al. (2011): A Novel Role of the WNT-Dishevelled-GSK3 beta Signaling Cascade in the Mouse Nucleus Accumbens in a Social Defeat Model of Depression. J Neurosci. 31:9084–9092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, et al. (2007): Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 131:391–404. [DOI] [PubMed] [Google Scholar]
- 30.Cohen H, Ziv Y, Cardon M, Kaplan Z, Matar MA, Gidron Y, et al. (2006): Maladaptation to mental stress mitigated by the adaptive immune system via depletion of naturally occurring regulatory CD4+CD25+ cells. J Neurobiol. 66:552–563. [DOI] [PubMed] [Google Scholar]
- 31.Brachman RA, Lehmann ML, Maric D, Herkenham M (2015): Lymphocytes from chronically stressed mice confer antidepressant-like effects to naive mice. J Neurosci. 35:1530–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bruhl H, Cihak J, Plachy J, Kunz-Schughart L, Niedermeier M, Denzel A, et al. (2007): Targeting of Gr-1+,CCR2+ monocytes in collagen-induced arthritis. Arthritis Rheum. 56:2975–2985. [DOI] [PubMed] [Google Scholar]
- 33.Liang Y, Ridzon D, Wong L, Chen C (2007): Characterization of microRNA expression profiles in normal human tissues. BMC Genomics. 8:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vaz C, Ahmad HM, Sharma P, Gupta R, Kumar L, Kulshreshtha R, et al. (2010): Analysis of microRNA transcriptome by deep sequencing of small RNA libraries of peripheral blood. BMC Genomics. 11:288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oved K, Morag A, Pasmanik-Chor M, Oron-Karni V, Shomron N, Rehavi M, et al. (2012): Genome-wide miRNA expression profiling of human lymphoblastoid cell lines identifies tentative SSRI antidepressant response biomarkers. Pharmacogenomics. 13:1129–1139. [DOI] [PubMed] [Google Scholar]
- 36.Liu Y, Chen Q, Song Y, Lai L, Wang J, Yu H, et al. (2011): MicroRNA-98 negatively regulates IL-10 production and endotoxin tolerance in macrophages after LPS stimulation. FEBS Lett. 585:1963–1968. [DOI] [PubMed] [Google Scholar]
- 37.Schulte LN, Eulalio A, Mollenkopf HJ, Reinhardt R, Vogel J (2011): Analysis of the host microRNA response to Salmonella uncovers the control of major cytokines by the let-7 family. The EMBO journal. 30:1977–1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun Y, Cai J, Ma F, Lu P, Huang H, Zhou J (2012): miR-155 mediates suppressive effect of progesterone on TLR3, TLR4-triggered immune response. Immunol Lett. 146:25–30. [DOI] [PubMed] [Google Scholar]
- 39.Smith AL, Iwanaga R, Drasin DJ, Micalizzi DS, Vartuli RL, Tan AC, et al. (2012): The miR-106b-25 cluster targets Smad7, activates TGF-beta signaling, and induces EMT and tumor initiating cell characteristics downstream of Six1 in human breast cancer. Oncogene. 31:5162–5171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tan W, Li Y, Lim SG, Tan TM (2014): miR-106b-25/miR-17–92 clusters: polycistrons with oncogenic roles in hepatocellular carcinoma. World J Gastroenterol. 20:5962–5972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wohleb ES, Powell ND, Godbout JP, Sheridan JF (2013): Stress-induced recruitment of bone marrow-derived monocytes to the brain promotes anxiety-like behavior. J Neurosci. 33:13820–13833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lewitus GM, Schwartz M (2009): Behavioral immunization: immunity to self-antigens contributes to psychological stress resilience. Mol Psychiatry. 14:532–536. [DOI] [PubMed] [Google Scholar]
- 43.Zheng X, Ma S, Kang A, Wu M, Wang L, Wang Q, et al. (2016): Chemical dampening of Ly6C(hi) monocytes in the periphery produces anti-depressant effects in mice. Sci Rep. 6:19406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA (2006): Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol. 24:99–146. [DOI] [PubMed] [Google Scholar]
- 45.Wan Y, Liu Y, Wang X, Wu J, Liu K, Zhou J, et al. (2015): Identification of differential microRNAs in cerebrospinal fluid and serum of patients with major depressive disorder. PLoS One. 10:e0121975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sharma A, Kumar M, Aich J, Hariharan M, Brahmachari SK, Agrawal A, et al. (2009): Posttranscriptional regulation of interleukin-10 expression by hsa-miR-106a. Proceedings of the National Academy of Sciences of the United States of America. 106:5761–5766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Quinn SR, O'Neill LA (2014): The role of microRNAs in the control and mechanism of action of IL-10. Current topics in microbiology and immunology. 380:145–155. [DOI] [PubMed] [Google Scholar]
- 48.Zhang Y, Liu D, Chen X, Li J, Li L, Bian Z, et al. (2010): Secreted monocytic miR-150 enhances targeted endothelial cell migration. Mol Cell. 39:133–144. [DOI] [PubMed] [Google Scholar]
- 49.Liu Z, Ye P, Wang S, Wu J, Sun Y, Zhang A, et al. (2015): MicroRNA-150 protects the heart from injury by inhibiting monocyte accumulation in a mouse model of acute myocardial infarction. Circ Cardiovasc Genet. 8:11–20. [DOI] [PubMed] [Google Scholar]
- 50.Guck TP, Kavan MG, Elsasser GN, Barone EJ (2001): Assessment and treatment of depression following myocardial infarction. Am Fam Physician. 64:641–648. [PubMed] [Google Scholar]
- 51.Kumar M, Ahmad T, Sharma A, Mabalirajan U, Kulshreshtha A, Agrawal A, et al. (2011): Let-7 microRNA-mediated regulation of IL-13 and allergic airway inflammation. The Journal of allergy and clinical immunology. 128:1077–1085 e1071–1010. [DOI] [PubMed] [Google Scholar]
- 52.Sung SY, Liao CH, Wu HP, Hsiao WC, Wu IH, Jinpu, et al. (2013): Loss of let-7 microRNA upregulates IL-6 in bone marrow-derived mesenchymal stem cells triggering a reactive stromal response to prostate cancer. PLoS One. 8:e71637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Iliopoulos D, Hirsch HA, Struhl K (2009): An epigenetic switch involving NF-kappaB, Lin28, Let-7 MicroRNA, and IL6 links inflammation to cell transformation. Cell. 139:693–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mele F, Basso C, Leoni C, Aschenbrenner D, Becattini S, Latorre D, et al. (2015): ERK phosphorylation and miR-181a expression modulate activation of human memory TH17 cells. Nat Commun. 6:6431. [DOI] [PubMed] [Google Scholar]
- 55.Li G, Yu M, Lee WW, Tsang M, Krishnan E, Weyand CM, et al. (2012): Decline in miR-181a expression with age impairs T cell receptor sensitivity by increasing DUSP6 activity. Nature medicine. 18:1518–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nagpal N, Kulshreshtha R (2014): miR-191: an emerging player in disease biology. Front Genet. 5:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zampetaki A, Kiechl S, Drozdov I, Willeit P, Mayr U, Prokopi M, et al. (2010): Plasma microRNA profiling reveals loss of endothelial miR-126 and other microRNAs in type 2 diabetes. Circ Res. 107:810–817. [DOI] [PubMed] [Google Scholar]
- 58.Kumar P, Dezso Z, MacKenzie C, Oestreicher J, Agoulnik S, Byrne M, et al. (2013): Circulating miRNA biomarkers for Alzheimer's disease. PLoS One. 8:e69807. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.