Abstract
Stress-related neuropsychiatric disorders such as major depressive disorder and posttraumatic stress disorder exact enormous socio-economic and individual consequences. Resilience, the process of adaptation in the face of adversity, is an important concept that is enabling the field to understand individual differences in stress responses, with the hope of harnessing this information for the development of novel therapeutics that mimic the body’s natural resilience mechanisms. This review provides an update on the current state of research of the neurobiological mechanisms of stress-resilience. We focus on physiological and transcriptional adaptations of specific brain circuits, the role of cellular and humoral factors of the immune system, the gut microbiota and changes at the interface between the brain and the periphery, the blood-brain barrier. We propose to view resilience as a process that requires the integration of multiple central and peripheral systems and that elucidating the underlying neurobiological mechanisms will ultimately lead to novel therapeutic options.
Keywords: stress, resilience, major depressive disorder (MDD), posttraumatic stress disorder (PTSD), mesolimbic reward circuit, inflammation, gut microbiota, blood-brain barrier
Introduction
Psychosocial stress is part of our everyday life – e.g., being bullied at school or work or the recent loss of a close relative– and many people experience physical or sexual abuse. It is however also intuitive that the individual reactions to similar traumatic events can be very different. These range from lifelong disabling mental disorders to relatively moderate acute stress reactions or even a strengthening effect that protects one from future traumas. The topic of the current review is stress-resilience, defined here based on the American Psychological Association as “the process of adapting well in the face of adversity, trauma, tragedy, threats or significant sources of stress” (1).
Our aim is to provide an update on the current state of research of the neurobiological mechanisms of stress-resilience, focusing on literature that has specifically investigated resilience in pre-clinical rodent models relevant to major depressive disorder (MDD) and posttraumatic stress disorder (PTSD).
Stress, resilience and the HPA axis:
An adequate reaction of the body to acute threats is a crucial mechanism to adapt to environmental changes that occur in different developmental stages throughout life. The autonomic nervous system and the hypothalamic-pituitary-adrenal (HPA) axis play a key role in orchestrating the body’s reaction to threats (2). Upon danger, the hypothalamus secretes corticotropin-releasing hormone, which via the pituitary hormone adrenocorticotropin induces production of cortisol from the adrenal cortex. Parallel activation of the sympathetic nervous system leads to effects on several peripheral organs, including release of epinephrine from the adrenal medulla. These responses mediate the necessary acute “fight-or-flight” reaction (3) (4). On the other hand, stress of extreme nature or prolonged duration is among the most important risk factor for many diseases, including neuropsychiatric disorders such as MDD and PTSD (5) (6). An informative conceptualization of this continuum between adaptive and maladaptive stress was introduced with the terms allostasis and allostatic load. While allostasis refers to the adaptive processes that maintain homeostasis, the term allostatic load describes the cumulative burden of adaptations that result when the involved systems fail to shut off after the stressor has subsided or when these systems do not respond adequately (7) (8).
Responses to similar stressors are strikingly distinct across individuals, and the first scientific attention drawn to resilience as a phenomenon of adaptation in the context of risk or adversity took place in the 1970s (9). It was soon established that resilience is a common phenomenon rather than an extraordinary process (10). Over the years, several factors have been linked to resilience, including a strong social support network as well as intrinsic behavioral traits such as optimism (11) (12). Individual coping strategies (13) are particularly relevant to resilience and can be classified into two categories. 1) Active coping responses are intentional efforts of the subject aimed at minimizing the physical, psychological, or social harm of a stressor and are associated with actual or perceived control over the stressor (14). Such coping is considered to lead to changes facilitating an adaptive, resilient response (14). 2) Passive coping, in contrast, includes mechanisms such as avoidance or helplessness and is associated with increased vulnerability (15) (16).
Rodent models of susceptibility and resilience:
The neurobiological mechanisms underlying resilience have long been difficult to uncover, primarily because creating significant adversity in humans in controlled experimental settings is impossible and exploration of molecular and circuit brain mechanisms in humans remains limited. Over the past decade, advances in pre-clinical animal stress models that uncover individual differences in stress-reactivity have allowed detailed neurobiological characterization of the precise mechanisms of stress-vulnerability vs. resilience (14) (17). One of the first animal models demonstrated to separate susceptible and resilient phenotypes is learned helplessness (LH) (18) (19). However, there are considerable shortcomings in the validity of the LH procedure to depression-like behaviors, including that the depression-like behaviors only last a few days and in some strains acute administration of an antidepressant is sufficient to reverse LH behavior (19). Additionally, Nasca et al. showed individual differences in response to both chronic unpredictable stress and restraint stress with some mice exhibiting resilience to development of depression- and anxiety-related behaviors (20).
Another widely used rodent stress model, which distinguishes susceptible and resilient phenotypes with greater etiological validity, is repeated social defeat stress (RSDS) (21) (17). Over a period of usually ten days, a rat or mouse is repeatedly subordinated by a dominant animal; for example, a C57BL6 mouse is defeated by a larger, more aggressive CD1 mouse (22) (23). Importantly, despite undergoing the same stress, individual mice and rats (even from inbred strains) display different behaviors. While susceptible mice are characterized by alterations in behaviors with high face validity to MDD, such as social avoidance and anhedonia (measured by the preferences of a sweet tasting solution over water), resilient mice do no show these changes and display behaviors similar to control mice (24). Until recently, one major limitation of this model was that, mainly because innate aggression of male towards female mice is limited, it could only be applied in male C57BL6 mice (25), although female social defeat has been validated in a different mouse species (26). From a translational perspective, male and female patients differ not only in the prevalence of stress-related neuropsychiatric disorders, but also in their clinical presentations (27) (28). Therefore, it is a major recent advance that two female mouse models, both based on the defeat stress paradigm, have been developed for C57BL6 mice: Harris et al. proposed a model, in which male urine is applied to females to induce CD1 males to attack them (29). Another paradigm uses a DREADD (designer receptors exclusively activated by designer drugs) approach, where induction of aggression in male CD1 mice towards female C57BL6 mice is achieved by activation of the ventromedial hypothalamus (30). Similar to social defeat in males, both stress models lead to different stress responses with some female mice being susceptible and others resilient (29) (30). These models will provide important tools to further elucidate neurobiological mechanisms underlying sex specific differences and commonalities in stress-responses relevant to affective disorders (see (31) for review).
Given that adversity experienced in childhood and during adolescence can profoundly impact individual trajectories, early life stress animal models are of great importance (32). Several established rodent early-life stress paradigms exist, with maternal separation and reduced bedding material being the most commonly used (33). Interestingly, the relation between the extent of stress-exposure and stress response is not linear. While no/low and high levels of stress have a negative effect on performance, moderate exposure to stress can promote active coping responses and therefore have pro-resilient effects. If pups experience maternal-deprivation for a long period, they show higher susceptibility to subsequent stressors in adult life, HPA axis hyperactivity and altered glucocorticoid responses (34) (35). However, if stress exposure is less severe, it can have pro-resilient effects, a process termed stress-inoculation. Rat pups that are exposed to postnatal handling, a moderate early life stress, display lower plasma levels of corticotropin-releasing hormone and an attenuated stress-induced increase in plasma corticosterone compared to both rats that were left undisturbed and those that were severely stressed as pups (36). Additionally, certain behavioral traits that manifest in early life are associated with outcomes in later life. Rats that showed less exploratory behavior of a novel environment in early life had a shorter life span than their more exploratory conspecifics (37).
Decades of research have investigated the importance of the HPA axis in psychosocial stress and indeed led to potential clinically applicable biomarkers, e.g., neuropeptide Y (NPY) or dehydroepiandrosterone (DHEA) (38) (39). Nevertheless, progress in the development of novel therapeutics, including generation of resilience-promoting drugs that directly target the HPA axis, stands in no relation to the vast number of pre-clinical findings that exist, making more effective translational research a high priority.
Central Nervous System Mechanisms of Resilience
Hippocampal neurogenesis:
The hippocampus is important in mediating responses to stress. Both mineralocorticoid and glucocorticoid receptors are vastly expressed in the hippocampus, making it a region highly responsive to activation of the HPA axis (40) (41). The dentate gyrus of the hippocampus is capable of generating functional neurons from adult neural precursors, a process termed adult neurogenesis (42). Stress and glucocorticoid release decrease adult hippocampal neurogenesis, a process that is reversed by treatment with some but not all antidepressants (43) (44). The findings regarding the role of adult hippocampal neurogenesis in mediating RSDS-induced susceptibility vs. resilience, however, are inconsistent. Lagace et al. showed that, compared with resilient and control mice, susceptible mice display enhanced survival of dentate gyrus neurons four weeks after the defeat that were born 24 hours after but not before defeat stress. Irradiation-induced ablation of neurogenesis led to pro-resilient behaviors. The authors suggested that this compensatory enhancement in hippocampal neurogenesis is related to the maladaptive stress response (45). In contrast, a recent study reported that increasing hippocampal neurogenesis promotes resilience to social defeat stress (46) (Fig. 1B). The authors used a gain-of-function model, where deletion of the pro-apoptotic gene Bax from adult neural stem cells was sufficient to increase hippocampal neurogenesis, and showed that this manipulation protects from social defeat-induced social avoidance and anxiety-like behaviors (46). The authors additionally describe a population of stress-responsive cells that are inhibited by adult-born neurons and suggested that direct silencing of these cells confers resilience to stress (46).
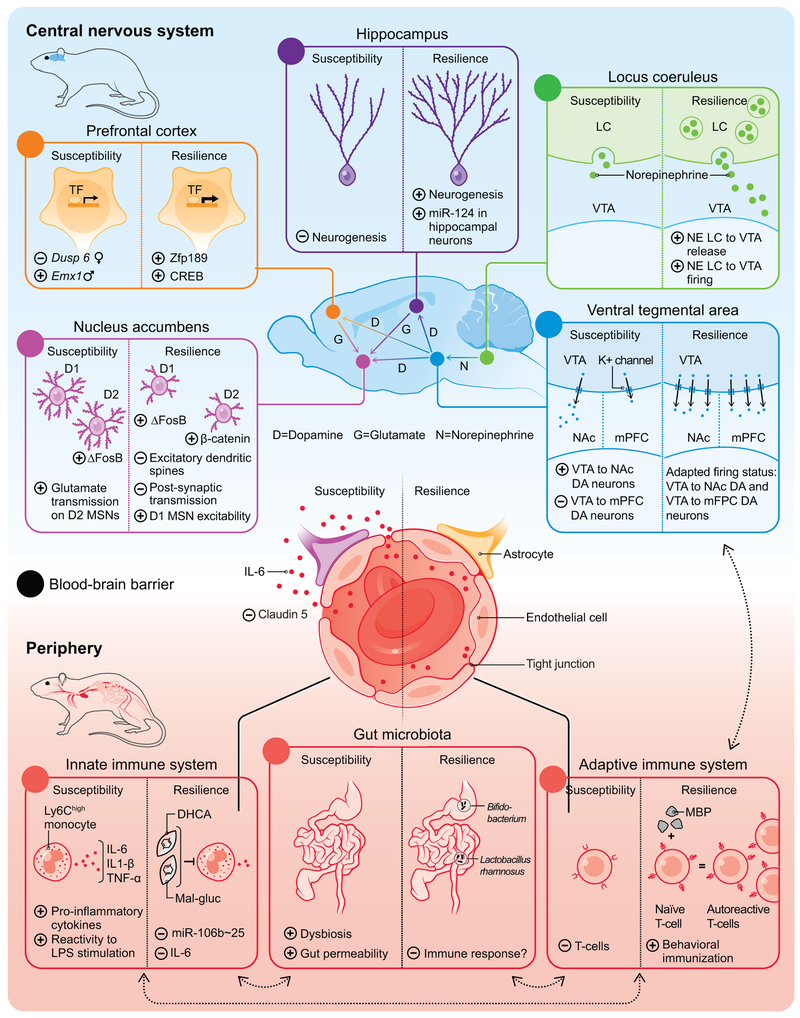
Fig. 1.
Stress-resilience, the process of positive adaptation in the face of psychosocial stress, is a complex process that involves both central and peripheral mechanisms. In the central nervous system, several specific brain regions and circuits are crucial in mediating responses to stress (Fig. A-E). In the periphery, cellular and humoral factors of the immune system (Fig. G, H) and changes in gut microbiota composition (Fig. I) contribute to the development of resilience. Recently, the blood-brain barrier has been implicated as an additional factor (Fig. F). Importantly, these compartments interact extensively with each other.
Abbreviations: CREB: cAMP response element-binding protein; DA: Dopamine; DHCA: Dihydrocaffeic acid; Mal-gluc: Malvidin-3′-O-glucoside; Dusp6: Dual Specificity Phosphatase 6; Emx1: Empty spiracles homeobox; IL: Interleukin; K+: Potassium; LC: Locus coeruleus; LPS: Lipopolysaccharides; MBP: Myelin basic protein; miR: microRNA; mPFC: Medial prefrontal cortex; MSN: Medium spiny neurons; NE: Norepinephrine; NAc: Nucleus accumbens; TNF: Tumor necrosis factor: VTA: Ventral tegmental area; Zfp189: Zinc finger protein 189.
Ventral tegmental area (VTA) dopamine (DA) neurons:
The mesolimbic dopamine pathway is a key reward circuit, in which DA neurons project from the VTA to the nucleus accumbens (NAc), hippocampus, prefrontal cortex (PFC), and other forebrain regions (47). Neurons of the VTA release dopamine in response to both rewarding and aversive stimuli and different stressors can differentially influence the dopaminergic neurons of the VTA (48) (49) (50). One of the key findings of this circuit is that stress-susceptible mice show increased firing of DA neurons projecting from the VTA to the NAc, whereas projections from the VTA to the mPFC display the opposite (Figure 1D) (49). Interestingly, resilient mice display control-level firing activity in both of the aforementioned circuits (51) (52). Further supporting the role of the VTA in actively mediating resilience are data from gene expression studies, where both in the VTA and NAc significantly more genes were regulated in resilient compared to susceptible mice (24). Particularly interesting, the microarray data revealed upregulation of four distinct potassium (K+) channel subunits in the VTA of resilient mice only (Fig. 1D) (24). These findings suggest that K+ channels may play an active functional role in driving the higher firing of VTA DA neurons back to normal levels in resilient mice, indicating that resilience represents a physiological state distinct from control mediated by a host of resilience-promoting mechanisms. It is known that RSDS increases the excitatory Ih current in VTA DA neurons of susceptible mice, and induces even greater increases in Ih in these neurons of resilient mice (51) (53) (54). Further studies showed that local infusion of HCN (Hyperpolarization-activated cyclic nucleotide–gated) channel inhibitors into the VTA rapidly normalized social avoidance in susceptible mice (53) (51). This finding suggests that the force that drives the pathological higher firing exists in resilient mice, but that additional compensative ionic mechanisms such as K+ channel induction could drive the higher firing back to normal levels in resilient mice as stated above. Further, measuring K+ currents revealed a selective increase in resilient mice (51). Among these voltagegated K+ currents, KCNQ subtype of K+ channels plays a key role in regulating the firing activity of VTA DA neurons and pharmacologically enhancing KCNQ channels showed significant antidepressant-like effects in the RSDS model (51). Informed by these pre-clinical results, a recent study reported that a 10-week treatment with a non-selective KCNQ channel opener, ezogabine, decreases depressive symptoms in MDD patients, an effect associated with changes of ventral striatal connectivity as a function of clinical improvement (55).
The NAc and its inputs:
The NAc integrates dopaminergic projections from the VTA and glutamatergic inputs from the hippocampus, PFC, amygdala and thalamus and is comprised largely of two subtypes of GABAergic medium spiny neurons (MSNs) that express predominantly either dopamine 1 (D1) or D2 receptors and play important roles in a host of reward-related behaviors (56) (57) (58). Distinct glutamatergic inputs to the NAc allow this region to bidirectionally regulate reward and aversion (59) (60) (61) (62), leading to the hypothesis that depression—and by extension resilience—may be due in part to alterations in glutamatergic function within the NAc (63). For example, it was observed that susceptible mice have more excitatory dendritic spines and increased postsynaptic transmission onto NAc MSNs compared to resilient mice (Fig. 1E) (64) (65). Francis and colleagues (66) expanded upon these initial findings to show increased glutamate transmission specifically on D2 MSNs of susceptible mice relative to resilient mice (Fig. 1E). While there were no changes in excitatory currents in D1 MSNs following RSDS, the authors found an increase in excitability of D1 MSNs in resilient mice relative to susceptible mice (Fig. 1E). Subsequent studies by Khibnik et al. suggested that the upregulated amplitude of unitary excitatory postsynaptic currents specifically on mushroom spines of D1-MSNs could represent an active adaptation enabling the mice to better cope with the effects of social stress (67). Together, this work points to cell- and possibly circuit-specific glutamatergic signaling within the NAc that promotes stress resilience. Given what we know about NAc glutamate signaling in susceptibility vs. resilience, it has been suggested that distinct inputs to NAc may control positive vs. negative mood states following chronic stress contributing to either susceptible or resilient phenotypes. To test this hypothesis, research has employed in vivo optogenetic approaches to stimulate or inhibit PFC-NAc, thalamus-NAc, BLA-NAc or ventral subiculum (vSub)-NAc glutamate pathways in mediating stress responses during social defeat stress (62) (61). It was found that stimulation of glutamate inputs from either the thalamus or vSub both potentiate social avoidance following subthreshold social defeat stress. By contrast, stimulation of PFC-NAc glutamate inputs promotes resilience, but only under very specific conditions, and studies to silence these inputs using halorhodopsin have no effect (62) (61). This suggests that PFC neurons either promote resilience via collateral pathways or that different stimulation parameters evoke different postsynaptic effects on NAc MSNs. While these findings clearly define the importance of input specificity to NAc neurons in encoding susceptibility vs. resilience, we still have a limited understanding of how these inputs differentially activate the NAc. One possibility is that specific inputs are differentially connected to D1 vs. D2 MSNs or perhaps to GABAergic vs. cholinergic interneurons. In vivo tracing studies support this possibility (68), however, functional studies in mouse models of resilience are required to confirm this hypothesis.
The locus coeruleus (LC) and its outputs:
There is increasing evidence that the LC, a norepinephrine (NE) producing brainstem nucleus, plays a role in stress susceptibility and resilience (69) (70). The LC provides virtually all of the NE input throughout the forebrain and also innervates the VTA. Isingrini et al. showed that resilient mice display increased NE release from LC neurons that project to the VTA (Fig. 1C) (71). In a recent study, Zhang et al. reported that resilient but not susceptible mice show increased firing of LC neurons that project to the VTA (Fig. 1C) and that mimicking this adaptive change by optogenetic stimulation in stress-susceptible mice promotes resilience (72). Molecular profiling and pharmacological studies identify α1- and β3-adrenergic receptors expressed by VTA DA neurons as being sufficient and necessary to induce resilience, providing an additional potential pharmacological target which now warrants clinical investigation (72).
Transcriptional and epigenetic mechanisms:
Transcription factors have been implicated as important mechanisms in mediating environmental influences on the brain (73). As alluded to above, several brain-region specific gene expression studies have indicated that resilience is an active process with the involvement of greater transcriptional activity than stress susceptibility (74) (75). Several forms of stress induce ΔFosB, a truncated product of the FosB immediate early gene, in specific brain regions, including the NAc (76) (77). Interestingly, ΔFosB induction in the NAc after RSDS is cell-type specific. The modest induction of ΔFosB in susceptible mice takes place in D2 MSNs, whereas the more robust induction in resilient mice is specific to D1 MSNs (Fig. 1E) (78) (79). Viral overexpression of ΔFosB in D1 MSNs promotes a resilient behavioral phenotype and is necessary for the antidepressant action of fluoxetine (78) (79) (80) (81). Further supporting its importance, ΔFosB is reduced in post-mortem NAc tissue of patients with MDD (78). Additionally, β-catenin, a downstream factor of WNT (wingless) signaling, is highly regulated in the NAc of resilient mice (82). Again, this effect is cell-type specific, since overexpression of β-catenin in D2- but not D1-type MSNs induces a pro-resilient phenotype, mediated in part through activation of Dicer1 and downstream generation of microRNAs (miRNAs) (Fig. 1E) (83). In a recent study, Lorsch et al. identified in the PFC the zinc-finger transcription factor Zfp189 as a key hub gene in a resilient-specific gene module (84). The authors reported that cAMP response element-binding protein (CREB) was the strongest predicted upstream regulator of genes within this module and showed that overexpression of Zfp189 in the PFC promoted resilience (Fig. 1A).
The initial findings linking epigenetic alterations and MDD were that broad inhibition of histone deacetylases (HDACs) in several brain regions, including NAc, hippocampus and PFC, led to antidepressant-like effects in stressed rodents (85). Additional evidence came from studies suggesting that antidepressant effects of fluoxetine were in part mediated by histone acetylation (86). However, alternate studies have begun to unravel the complex mechanisms of histone modifications and have revealed opposing effects of certain HDACs. For example, RSDS decreased expression of Hdac5 in the NAc of susceptible mice and chronic imipramine administration increased its expression, therefore suggesting a potential pro-resilient effect (87). AAV-mediated Hdac2 overexpression in the same brain region protected mice from chronic ultra-mild stress induced social avoidance (88). These findings suggest that different HDACs regulate different genes to promote susceptibility versus resilience.
DNA methylation is a process during which a methyl group is covalently attached to cytosine (and rarely other nucleotides) and leads generally via hyper-methylation of gene promotors to inactivation of gene expression (89). One interesting target relevant to stress susceptibility and resilience is the DNA methyltransferase, DNMT3a: DNMT3a expression is elevated both in the NAc of human MDD patients and in stress-susceptible mice. Interestingly, Dnmt3a manipulation seems to have sex-specific effects: overexpression of Dnmt3a in the NAc makes both female and male mice susceptible to subthreshold variable stress, while knockout of Dnmt3a in the NAc promotes resilience selectively in females (90) (91).
Another mechanism of transcriptional regulation takes place through non-protein coding RNAs (92). Recent transcriptional studies have shown that stress leads to brain region specific changes in miRNA expression (93) (94). As an example of the function of miRNAs in promoting resilience, Higuchi and colleagues showed that overexpression of miR-124, an endogenous small, noncoding RNA that represses gene expression post-transcriptionally, in hippocampal neurons confers stress-resilience (Fig. 1B) (95). Recent advances in molecular methods and gene-editing technologies will enable precise cell-type specific manipulation of transcription factors or epigenetic modifications hopefully further increasing our understanding of the transcriptional and chromatin-based mechanisms of resilience (96) (97) (81).
Peripheral Mechanisms of Resilience
The innate immune system:
Both pre-clinical animal models and human studies show that repeated psychosocial stress leads to profound peripheral immunological changes (98) (99). Evidence from human studies linking stress-vulnerability and resilience to immune-alterations exists at multiple levels: a subset of patients with MDD show elevated levels of several pro-inflammatory cytokines (100) (101), MDD has high comorbidity with chronic inflammatory illnesses such as autoimmune disorders, cardiovascular disorders or cancer (102) (103) (104) and certain anti-inflammatory therapies potentially elicit antidepressant effects (105). Whether traditional antidepressants reduce peripheral cytokine levels, remains controversial, with a recent meta-analysis indicating a reduction of levels of interleukin (IL)-1β and possibly IL-6 (106). Interestingly, there is evidence that the rapid acting antidepressant ketamine reduces levels of pro-inflammatory cytokines (107) (108). However, whether these inflammatory changes are causally linked to antidepressant effects remains unclear.
The innate immune system, which represents the first line of host defense during infection, plays an important role in the early recognition and subsequent triggering of a pro-inflammatory response to invading pathogens (109) (110). Similar to the response to pathogens, chronic stress leads to an increase of inflammatory cells and pro-inflammatory mediators, e.g., Ly6chigh monocytes and neutrophils or IL-1β, IL-6 and tumor necrosis factor (TNF)-α, respectively (111) (112). Hodes et al. was one of the first to investigate differences between stress-susceptible vs. -resilient phenotypes. After RSDS, resilient mice displayed lower blood levels of IL-6 than susceptible mice (Fig. 1G) and both neutralizing IL-6 with a systemically-administered antibody and depleting IL-6 from bone-derived leukocytes using chimeric mice promoted resilience (113). Additionally, pre-defeat inflammatory markers predicted how mice will respond to RSDS: mice susceptible after RSDS displayed more pre-existing circulating leukocytes than resilient mice and IL-6 release upon stimulation with the bacterial endotoxin lipopolysaccharide (LPS) correlated negatively with social interaction scores (113). Additional work by Pfau et al. investigated the potential role of stress-induced epigenetic regulation of leukocytes by miRNAs (114). The authors reported that, within Ly6Chigh monocytes of mice exposed to RSDS, several miRNAs were regulated by RSDS, including miR-25-3p, a member of the miR-106b~25 cluster (114). Selective knockout of the miR-106b~25 cluster in peripheral leukocytes promoted behavioral resilience to RSDS (114). Given that Ly6Chigh monocytes tend to be more inflammatory in nature, it is thought that these cells may be a prominent source of inflammatory molecules following stress and therapeutic strategies targeting Ly6Chigh may promote resilience by reducing inflammation. Indeed, systemic administration of the phytochemicals, dihydrocaffeic acid (DHCA) and malvidin-3′-O-glucoside (Mal-gluc), promoted stress-resilience in mice by decreasing IL-6 release from leukocytes (115) (Fig. 1G).
The adaptive immune system:
The adaptive immune system is involved in the later phase of an infection, where it fights invading pathogens with an immune response characterized by clonal gene rearrangement of antigen-specific receptors on lymphocytes and the formation of an immunological memory (116). Far fewer studies have investigated its main cellular components, B and T lymphocytes (117), in stress responses. One meta-analysis concludes that MDD patients show reduced T-cell proportions and a moderate increase in the ratio of CD4/CD8 T-cells in blood (118). Rodent studies have indicated a potential neuroprotective or pro-resilient effect of T cells (119). Immunization of rats with modified myelin basic protein (MBP), which leads to the induction of autoreactive T-cells, prior to chronic mild stress (CMS), reduced depressive-like behaviors such as anhedonia endpoints and immobility in the forced swim test (Fig. 1H) (120). These changes went along with the rescue of CMS-induced BDNF decrease in the hippocampus (120). Interestingly, recruitment of T-cells to the CNS correlated positively with stress-resilience (121). The authors showed that T-cells infiltrated the choroid plexus, which displayed an increase in the intracellular adhesion molecule ICAM-1 (121). Additionally, mice depleted of lymphocytes (Rag2−/−) receiving lymphocytes from defeated donors displayed less anxiety-like behaviors, reduced pro-inflammatory cytokine levels and microglia shifting towards an anti-inflammatory phenotype, compared with those receiving no cells or cells from unstressed donors (122). This work suggests that psychosocial stress imprints onto the adaptive immune system which then influences the outcome of stress exposure. It can be speculated that resilience to psychosocial stress may be promoted via behavioral immunization, where in analogy to traditional vaccination strategies, exposure to an attenuated antigen can protect against successive stressful events (Fig. 1H) (123) (124).
Gut microbiota:
Microbiota refers to the collection of microorganisms in a particular habitat, e.g., the skin or gut (125). The gut microbiota has been implicated in a wide range of physiological processes including interactions with the host immune system and direct effects on the brain, for example, by production of neuroactive metabolites (126). These pathways, subsumed in the term “microbiota-gut-brain axis”, are an important modulator of the body’s response to stress (127) (126). Several studies reported disturbances in gut microbiota composition in MDD patients compared to healthy controls (128) (129). In a seminal mouse study, germ-free mice (animals that lack bacterial colonization) displayed increased motor activity and reduced anxiety-like behavior coincident with elevated NE, DA and serotonin turnover in the striatum (130). Interestingly, it was possible to transfer an “anxious” behavioral phenotype between two mouse strains (BALB/c vs. NIH Swiss) via fecal microbiota transfer (131). Additionally, fecal microbiota transplantation of germ-free mice with microbiota derived from MDD patients resulted in increased depression-like behaviors, compared to mice colonized with microbiota from healthy controls (132). Regarding resilience, a small study reported that oral intake of Bifidobacterium significantly increases the number of resilient mice after RSDS compared with vehicle-treated mice (133) (Fig. 1I). Treatment with Lactobacillus rhamnosus led to decreased RSDS-induced anxiety-like behaviors, prevented deficits in social interaction with conspecifics and attenuated stress-related activation of dendritic cells while increasing IL-10+ regulatory T cells, suggesting a potential resilience-promoting interaction with the immune system (134). However, the mechanisms that link gut dysbiosis to stress-susceptibility and resilience associated immune-disturbances, remain to be elucidated.
The blood-brain barrier:
The blood-brain barrier, comprised of brain microvascular endothelial cells, astrocytes and pericytes, is an important interface between the brain and the systemic circulation (135). Under homeostatic conditions, the BBB tightly controls the communication between these two compartments: cytokines for example do not passively diffuse into the brain but are, in a saturable manner, transported actively from the blood to the brain (136). Under stress conditions however, studies in humans and rodents have implicated neurovascular impairment in stress responses (137) (138). One study revealed that RSDS in mice decreases the endothelial tight junction protein Claudin-5, resulting in a higher permeability to the peripheral cytokine IL-6 (139) (Fig. 1F). In this study, viral-mediated downregulation of Claudin-5 promoted greater susceptibility to RSDS. Importantly, Claudin-5 was found to be downregulated in postmortem NAc tissue from MDD patients (139). Another study conducted with rats found that passive coping animals display greater vascular remodeling than active coping animals, with active coping seen as a pro-resilience phenotype (140). Using a murine LH model, Cheng et al. showed that BBB permeability increases in the hippocampus of mice after LH induction, and this was maintained in mice with prolonged LH, whereas the BBB permeability had normalized in mice that recovered from LH (141).
Neuro-immune interactions.
A wealth of evidence indicates that stress influences the peripheral immune system resulting in depression-associated behavioral changes. However, the specific mechanisms are still not well understood. It has been suggested that because RSDS leads to brain region specific BBB disruption (i.e. increased permeability in the NAc but not in other brain regions), infiltrating cytokines may act directly on these brain regions to affect neuronal function (139). In accord with this hypothesis, it was recently shown that peripheral IL-6 is necessary for maladaptive synaptic plasticity in NAc of susceptible mice following RSDS (115). Another interesting possibility is that the central nervous system itself might attract peripheral immune cells, in a region-specific way, to impact brain circuits. A recent study by McKim et al. (142) reported that the development of anxiety-like behaviors during stress was dependent on microglial recruitment of IL-1β-producing monocytes, which stimulated brain endothelial IL-1R1 (142). This study adds to the increasing evidence that glial cells, the non-neuronal cells of the nervous system constitute an important interface between the periphery and neuronal dysfunction (143). While an in-depth discussion of the role of glial cells is beyond the scope of this review, it is important to take into account the regulatory and immune surveillance functions of microglia, the brain-resident macrophages (see (144) for review). Different lines of evidence indicate a role of microglia in stress-associated neuropsychiatric disorders. Social defeat stress leads to morphological and functional changes in microglia (145). Post-mortem brain analysis in patients that committed suicide showed significant microgliosis (146) and brain translocator protein density, a marker of increased microglial activation, was elevated in MDD patients (147). With regard to resilience, the antibiotic minocycline prevented chronic unpredictable stress induced anhedonia in rats, indicating that manipulations of microglia could be pro-resilient (148). Given that metabolites produced by the gut microbiota can not only influence the immune system but also directly affect glial cells (149) and the BBB (150), the concept of providing resilience via specific modulation of the gut microbiota could provide a promising avenue for novel treatments.
Conclusion and Outlook
The societal and individual burden entailed by stress-related neuropsychiatric disorders is immense. Past efforts in developing treatments for such disorders have focused on preventing or reversing the damaging effects of stress. Understanding the neurobiological mechanisms that promote resilience to stress in some individuals, but lacking in those who are inherently more susceptible, constitutes a novel, additional important approach in stress biology. Indeed, early clinical studies suggest that inducing mechanisms of natural resilience in depressed humans might be an effective route for antidepressant drug discovery. As shown in this review, the neurobiology of resilience is complex, involving many convergent systems that ultimately affect brain function and behavior. One of the main challenges will be to gain a holistic model of resilience that encompasses both peripheral systems and key circuits in the brain to answer central questions in the field. To address the many open questions in the field, with the ultimate goal of developing much needed therapeutic options, a multi-disciplinary, translational approach, incorporating multiple levels of analysis of the brain as well as studies of several peripheral organs, will be critical.
Acknowledgments and disclosure
This review was supported by Mental Health grants RO1 MH090264, P50 MH096890 (SJR), P50 AT008661-01 (SJR), RO1 MH104559 (SJR), R56 MH115409 (MHH), P50 MH096890 (EJN), an Early Postdoc Mobility Fellowship of the Swiss National Science Foundation (FC) and a Walter and Gertrud Siegenthaler Postdoctoral Fellowship (FC).
In the past 5 years, Dr. Russo has provided consultation services to Danone and Sunovion Pharmaceuticals and has received research support from Janssen Pharmaceuticals. Dr. Murrough has provided consultation services to Boehreinger Ingelheim, Sage Therapeutics, Novartis, Allergan, Fortress Biotech, Janssen Research and Development, Medavante-Prophase, and Global Medical Education (GME) and has received research support from Avanir Pharmaceuticals, Inc. Dr. Murrough is named on a patent pending for neuropeptide Y as a treatment for mood and anxiety disorders. Dr. Nestler has provided consultation services to PsychoGenics, Merck, and Janssen and is a Director of Berg. All other authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Association AP (2018): The road to resilience. http://wwwapaorg/helpcenter/road-resilienceaspx.
- 2.Herman JP, McKlveen JM, Ghosal S, Kopp B, Wulsin A, Makinson R, et al. (2016): Regulation of the Hypothalamic-Pituitary-Adrenocortical Stress Response. Comprehensive Physiology. 6:603–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith SM, Vale WW (2006): The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues in clinical neuroscience. 8:383–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ulrich-Lai YM, Herman JP (2009): Neural regulation of endocrine and autonomic stress responses. Nature reviews Neuroscience. 10:397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hammen C (2005): Stress and Depression. Annual review of clinical psychology. 1:293–319. [DOI] [PubMed] [Google Scholar]
- 6.Yehuda R, LeDoux J (2007): Response variation following trauma: a translational neuroscience approach to understanding PTSD. Neuron. 56:19–32. [DOI] [PubMed] [Google Scholar]
- 7.McEwen BS (1998): Stress, adaptation, and disease. Allostasis and allostatic load. Annals of the New York Academy of Sciences. 840:33–44. [DOI] [PubMed] [Google Scholar]
- 8.Charney DS (2004): Psychobiological mechanisms of resilience and vulnerability: implications for successful adaptation to extreme stress. The American journal of psychiatry. 161:195–216. [DOI] [PubMed] [Google Scholar]
- 9.Garmezy N (1971): Vulnerability research and the issue of primary prevention. The American journal of orthopsychiatry. 41:101–116. [DOI] [PubMed] [Google Scholar]
- 10.Masten AS (2001): Ordinary magic. Resilience processes in development. The American psychologist. 56:227–238. [DOI] [PubMed] [Google Scholar]
- 11.Ozbay F, Fitterling H, Charney D, Southwick S (2008): Social support and resilience to stress across the life span: a neurobiologic framework. Current psychiatry reports. 10:304–310. [DOI] [PubMed] [Google Scholar]
- 12.Feder A, Nestler EJ, Charney DS (2009): Psychobiology and molecular genetics of resilience. Nature reviews Neuroscience. 10:446–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koolhaas JM, Korte SM, De Boer SF, Van Der Vegt BJ, Van Reenen CG, Hopster H, et al. (1999): Coping styles in animals: current status in behavior and stress-physiology. Neuroscience and biobehavioral reviews. 23:925–935. [DOI] [PubMed] [Google Scholar]
- 14.Russo SJ, Murrough JW, Han MH, Charney DS, Nestler EJ (2012): Neurobiology of resilience. Nature neuroscience. 15:1475–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wood SK, Bhatnagar S (2014): Resilience to the effects of social stress: evidence from clinical and preclinical studies on the role of coping strategies. Neurobiology of stress. 1: 164–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Southwick SM, Vythilingam M, Charney DS (2005): The psychobiology of depression and resilience to stress: implications for prevention and treatment. Annual review of clinical psychology. 1:255–291. [DOI] [PubMed] [Google Scholar]
- 17.Hammels C, Pishva E, De Vry J, van den Hove DL, Prickaerts J, van Winkel R, et al. (2015): Defeat stress in rodents: From behavior to molecules. Neuroscience and biobehavioral reviews. 59:111–140. [DOI] [PubMed] [Google Scholar]
- 18.Seligman ME, Beagley G (1975): Learned helplessness in the rat. Journal of comparative and physiological psychology. 88:534–541. [DOI] [PubMed] [Google Scholar]
- 19.Cryan JF, Mombereau C (2004): In search of a depressed mouse: utility of models for studying depression-related behavior in genetically modified mice. Mol Psychiatry. 9:326–357. [DOI] [PubMed] [Google Scholar]
- 20.Nasca C, Bigio B, Zelli D, Nicoletti F, McEwen BS (2015): Mind the gap: glucocorticoids modulate hippocampal glutamate tone underlying individual differences in stress susceptibility. Mol Psychiatry. 20:755–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, et al. (2006): Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science (New York, NY). 311:864–868. [DOI] [PubMed] [Google Scholar]
- 22.Golden SA, Covington HE, Berton O, Russo SJ (2011): A standardized protocol for repeated social defeat stress in mice. Nature protocols. 6:1183–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wood SK, Walker HE, Valentino RJ, Bhatnagar S (2010): Individual differences in reactivity to social stress predict susceptibility and resilience to a depressive phenotype: role of corticotropin-releasing factor. Endocrinology. 151:1795–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, et al. (2007): Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 131:391–404. [DOI] [PubMed] [Google Scholar]
- 25.Beery AK, Zucker I (2011): Sex bias in neuroscience and biomedical research. Neuroscience and biobehavioral reviews. 35:565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steinman MQ, Trainor BC (2017): Sex differences in the effects of social defeat on brain and behavior in the California mouse: Insights from a monogamous rodent. Seminars in cell & developmental biology. 61:92–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kessler RC (2003): Epidemiology of women and depression. Journal of affective disorders. 74:5–13. [DOI] [PubMed] [Google Scholar]
- 28.Martin LA, Neighbors HW, Griffith DM (2013): The experience of symptoms of depression in men vs women: analysis of the National Comorbidity Survey Replication. JAMA psychiatry. 70:1100–1106. [DOI] [PubMed] [Google Scholar]
- 29.Harris AZ, Atsak P, Bretton ZH, Holt ES, Alam R, Morton MP, et al. (2017): A Novel Method for Chronic Social Defeat Stress in Female Mice. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 43:1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takahashi A, Chung JR, Zhang S, Zhang H, Grossman Y, Aleyasin H, et al. (2017): Establishment of a repeated social defeat stress model in female mice. Scientific reports. 7:12838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bale TL, Epperson CN (2015): Sex differences and stress across the lifespan. Nature neuroscience. 18:1413–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Danese A, Lewis SJ (2016): Psychoneuroimmunology of Early-Life Stress: The Hidden Wounds of Childhood Trauma? Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 42:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murthy S, Gould E (2018): Early Life Stress in Rodents: Animal Models of Illness or Resilience? Frontiers in Behavioral Neuroscience. 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lupien SJ, McEwen BS, Gunnar MR, Heim C (2009): Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature reviews Neuroscience. 10:434–445. [DOI] [PubMed] [Google Scholar]
- 35.Tractenberg SG, Levandowski ML, de Azeredo LA, Orso R, Roithmann LG, Hoffmann ES, et al. (2016): An overview of maternal separation effects on behavioural outcomes in mice: Evidence from a four-stage methodological systematic review. Neuroscience and biobehavioral reviews. 68:489–503. [DOI] [PubMed] [Google Scholar]
- 36.Plotsky PM, Meaney MJ (1993): Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Brain research Molecular brain research. 18:195–200. [DOI] [PubMed] [Google Scholar]
- 37.Cavigelli SA, McClintock MK (2003): Fear of novelty in infant rats predicts adult corticosterone dynamics and an early death. Proceedings of the National Academy of Sciences of the United States of America. 100:16131–16136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kautz M, Charney DS, Murrough JW (2017): Neuropeptide Y, resilience, and PTSD therapeutics. Neuroscience letters. 649:164–169. [DOI] [PubMed] [Google Scholar]
- 39.Yehuda R, Brand SR, Golier JA, Yang RK (2006): Clinical correlates of DHEA associated with post-traumatic stress disorder. Acta psychiatrica Scandinavica. 114:187–193. [DOI] [PubMed] [Google Scholar]
- 40.Levone BR, Cryan JF, O'Leary OF (2015): Role of adult hippocampal neurogenesis in stress resilience. Neurobiology of stress. 1: 147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jankord R, Herman JP (2008): Limbic regulation of hypothalamo-pituitary-adrenocortical function during acute and chronic stress. Annals of the New York Academy of Sciences. 1148:64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ming GL, Song H (2011): Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 70:687–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, et al. (2003): Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science (New York, NY). 301:805–809. [DOI] [PubMed] [Google Scholar]
- 44.Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA (2011): Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature. 476:458–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lagace DC, Donovan MH, DeCarolis NA, Farnbauch LA, Malhotra S, Berton O, et al. (2010): Adult hippocampal neurogenesis is functionally important for stress-induced social avoidance. Proceedings of the National Academy of Sciences of the United States of America. 107:4436–4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anacker C, Luna VM, Stevens GS, Millette A, Shores R, Jimenez JC, et al. (2018): Hippocampal neurogenesis confers stress resilience by inhibiting the ventral dentate gyrus. Nature. 559:98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nestler EJ, Carlezon WA Jr. (2006): The mesolimbic dopamine reward circuit in depression. Biological psychiatry. 59:1151–1159. [DOI] [PubMed] [Google Scholar]
- 48.Lammel S, Lim BK, Malenka RC (2014): Reward and aversion in a heterogeneous midbrain dopamine system. Neuropharmacology. 76:351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chaudhury D, Walsh JJ, Friedman AK, Juarez B, Ku SM, Koo JW, et al. (2013): Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature. 493:532–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tye KM, Mirzabekov JJ, Warden MR, Ferenczi EA, Tsai HC, Finkelstein J, et al. (2013): Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature. 493:537–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Friedman AK, Walsh JJ, Juarez B, Ku SM, Chaudhury D, Wang J, et al. (2014): Enhancing depression mechanisms in midbrain dopamine neurons achieves homeostatic resilience. Science (New York, NY). 344:313–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Han MH, Nestler EJ (2017): Neural Substrates of Depression and Resilience. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 14:677–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cao JL, Covington HE 3rd, Friedman AK, Wilkinson MB, Walsh JJ, Cooper DC, et al. (2010): Mesolimbic dopamine neurons in the brain reward circuit mediate susceptibility to social defeat and antidepressant action. The Journal of neuroscience : the official journal of the Society for Neuroscience. 30:16453–16458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ku SM, Han MH (2017): HCN Channel Targets for Novel Antidepressant Treatment. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 14:698–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tan A, Costi S, Morris LS, Van Dam NT, Kautz M, Whitton AE, et al. (2018): Effects of the KCNQ channel opener ezogabine on functional connectivity of the ventral striatum and clinical symptoms in patients with major depressive disorder. Mol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Russo SJ, Nestler EJ (2013): The brain reward circuitry in mood disorders. Nature reviews Neuroscience. 14:609–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lobo MK, Covington HE 3rd, Chaudhury D, Friedman AK, Sun H, Damez-Werno D, et al. (2010): Cell type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science (New York, NY). 330:385–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aleyasin H, Flanigan ME, Golden SA, Takahashi A, Menard C, Pfau ML, et al. (2018): Cell-Type-Specific Role of DeltaFosB in Nucleus Accumbens In Modulating Intermale Aggression. The Journal of neuroscience : the official journal of the Society for Neuroscience. 38:5913–5924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stuber GD, Sparta DR, Stamatakis AM, van Leeuwen WA, Hardjoprajitno JE, Cho S, et al. (2011): Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature. 475:377–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Britt JP, Benaliouad F, McDevitt RA, Stuber GD, Wise RA, Bonci A (2012): Synaptic and behavioral profile of multiple glutamatergic inputs to the nucleus accumbens. Neuron. 76:790–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bagot RC, Parise EM, Pena CJ, Zhang HX, Maze I, Chaudhury D, et al. (2015): Ventral hippocampal afferents to the nucleus accumbens regulate susceptibility to depression. Nature communications. 6:7062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Christoffel DJ, Golden SA, Walsh JJ, Guise KG, Heshmati M, Friedman AK, et al. (2015): Excitatory transmission at thalamo-striatal synapses mediates susceptibility to social stress. Nature neuroscience. 18:962–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Christoffel DJ, Golden SA, Russo SJ (2011): Structural and synaptic plasticity in stress-related disorders. Reviews in the neurosciences. 22:535–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Christoffel DJ, Golden SA, Dumitriu D, Robison AJ, Janssen WG, Ahn HF, et al. (2011): IkappaB kinase regulates social defeat stress-induced synaptic and behavioral plasticity. The Journal of neuroscience : the official journal of the Society for Neuroscience. 31:314–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Golden SA, Christoffel DJ, Heshmati M, Hodes GE, Magida J, Davis K, et al. (2013): Epigenetic regulation of RAC1 induces synaptic remodeling in stress disorders and depression. Nat Med. 19:337–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Francis TC, Chandra R, Friend DM, Finkel E, Dayrit G, Miranda J, et al. (2015): Nucleus accumbens medium spiny neuron subtypes mediate depression-related outcomes to social defeat stress. Biological psychiatry. 77:212–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Khibnik LA, Beaumont M, Doyle M, Heshmati M, Slesinger PA, Nestler EJ, et al. (2016): Stress and Cocaine Trigger Divergent and Cell Type-Specific Regulation of Synaptic Transmission at Single Spines in Nucleus Accumbens. Biological psychiatry. 79:898–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wall NR, De La Parra M, Callaway EM, Kreitzer AC (2013): Differential innervation of direct- and indirect-pathway striatal projection neurons. Neuron. 79:347–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Krystal JH, Neumeister A (2009): Noradrenergic and serotonergic mechanisms in the neurobiology of posttraumatic stress disorder and resilience. Brain research. 1293:13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hermans EJ, van Marie HJ, Ossewaarde L, Henckens MJ, Qin S, van Kesteren MT, et al. (2011): Stress-related noradrenergic activity prompts large-scale neural network reconfiguration. Science (New York, NY). 334:1151–1153. [DOI] [PubMed] [Google Scholar]
- 71.Isingrini E, Perret L, Rainer Q, Amilhon B, Guma E, Tanti A, et al. (2016): Resilience to chronic stress is mediated by noradrenergic regulation of dopamine neurons. Nature neuroscience. 19:560–563. [DOI] [PubMed] [Google Scholar]
- 72.Zhang H, Chaudhury D, Nectow AR, Friedman AK, Zhang S, Juarez B, et al. (2018): alpha1- and beta3-Adrenergic Receptor-Mediated Mesolimbic Homeostatic Plasticity Confers Resilience to Social Stress in Susceptible Mice. Biological psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nestler EJ (2015): Chapter Six - Role of the Brain's Reward Circuitry in Depression: Transcriptional Mechanisms In: De Biasi M, editor. International Review of Neurobiology: Academic Press, pp 151–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bagot RC, Cates HM, Purushothaman I, Lorsch ZS, Walker DM, Wang J, et al. (2016): Circuit-wide Transcriptional Profiling Reveals Brain Region-Specific Gene Networks Regulating Depression Susceptibility. Neuron. 90:969–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bagot RC, Cates HM, Purushothaman I, Vialou V, Heller EA, Yieh L, et al. (2017): Ketamine and Imipramine Reverse Transcriptional Signatures of Susceptibility and Induce Resilience-Specific Gene Expression Profiles. Biological psychiatry. 81:285–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Perrotti LI, Hadeishi Y, Ulery PG, Barrot M, Monteggia L, Duman RS, et al. (2004): Induction of deltaFosB in reward-related brain structures after chronic stress. The Journal of neuroscience : the official journal of the Society for Neuroscience. 24:10594–10602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nestler EJ (2015): FosB: a transcriptional regulator of stress and antidepressant responses. European journal of pharmacology. 753:66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vialou V, Robison AJ, Laplant QC, Covington HE 3rd, Dietz DM, Ohnishi YN, et al. (2010): DeltaFosB in brain reward circuits mediates resilience to stress and antidepressant responses. Nature neuroscience. 13:745–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lobo MK, Zaman S, Damez-Werno DM, Koo JW, Bagot RC, DiNieri JA, et al. (2013): DeltaFosB induction in striatal medium spiny neuron subtypes in response to chronic pharmacological, emotional, and optogenetic stimuli. The Journal of neuroscience : the official journal of the Society for Neuroscience. 33:18381–18395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Donahue RJ, Muschamp JW, Russo SJ, Nestler EJ, Carlezon WA Jr. (2014): Effects of striatal DeltaFosB overexpression and ketamine on social defeat stress-induced anhedonia in mice. Biological psychiatry. 76:550–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hamilton PJ, Burek DJ, Lombroso SI, Neve RL, Robison AJ, Nestler EJ, et al. (2018): Cell-Type-Specific Epigenetic Editing at the Fosb Gene Controls Susceptibility to Social Defeat Stress. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 43:272–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wilkinson MB, Dias C, Magida J, Mazei-Robison M, Lobo M, Kennedy P, et al. (2011): A novel role of the WNT-dishevelled-GSK3beta signaling cascade in the mouse nucleus accumbens in a social defeat model of depression. The Journal of neuroscience : the official journal of the Society for Neuroscience. 31:9084–9092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dias C, Feng J, Sun H, Shao NY, Mazei-Robison MS, Damez-Werno D, et al. (2014): beta-catenin mediates stress resilience through Dicer1/microRNA regulation. Nature. 516:51–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lorsch ZS, Hamilton PJ, Ramakrishnan A, Parise EM, Wright WJ, Salery M, et al. (2018): Zfp189 Mediates Stress Resilience Through a CREB-Regulated Transcriptional Network in Prefrontal Cortex. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Covington HE 3rd, Maze I, LaPlant QC, Vialou VF, Ohnishi YN, Berton O, et al. (2009): Antidepressant actions of histone deacetylase inhibitors. The Journal of neuroscience : the official journal of the Society for Neuroscience. 29:11451–11460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ (2006): Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nature neuroscience. 9:519–525. [DOI] [PubMed] [Google Scholar]
- 87.Renthal W, Maze I, Krishnan V, Covington HE 3rd, Xiao G, Kumar A, et al. (2007): Histone deacetylase 5 epigenetically controls behavioral adaptations to chronic emotional stimuli. Neuron. 56:517–529. [DOI] [PubMed] [Google Scholar]
- 88.Uchida S, Hara K, Kobayashi A, Otsuki K, Yamagata H, Hobara T, et al. (2011): Epigenetic status of Gdnf in the ventral striatum determines susceptibility and adaptation to daily stressful events. Neuron. 69:359–372. [DOI] [PubMed] [Google Scholar]
- 89.Fuks F (2005): DNA methylation and histone modifications: teaming up to silence genes. Current opinion in genetics & development. 15:490–495. [DOI] [PubMed] [Google Scholar]
- 90.Hodes GE, Pfau ML, Purushothaman I, Ahn HF, Golden SA, Christoffel DJ, et al. (2015): Sex Differences in Nucleus Accumbens Transcriptome Profiles Associated with Susceptibility versus Resilience to Subchronic Variable Stress. The Journal of neuroscience : the official journal of the Society for Neuroscience. 35:16362–16376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.LaPlant Q, Vialou V, Covington HE 3rd, Dumitriu D, Feng J, Warren BL, et al. (2010): Dnmt3a regulates emotional behavior and spine plasticity in the nucleus accumbens. Nature neuroscience. 13:1137–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Issler O, Chen A (2015): Determining the role of microRNAs in psychiatric disorders. Nature reviews Neuroscience. 16:201–212. [DOI] [PubMed] [Google Scholar]
- 93.Pfau ML, Purushothaman I, Feng J, Golden SA, Aleyasin H, Lorsch ZS, et al. (2016): Integrative Analysis of Sex-Specific microRNA Networks Following Stress in Mouse Nucleus Accumbens. Frontiers in molecular neuroscience. 9:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Torres-Berrio A, Lopez JP, Bagot RC, Nouel D, Dal Bo G, Cuesta S, et al. (2017): DCC Confers Susceptibility to Depression-like Behaviors in Humans and Mice and Is Regulated by miR-218. Biological psychiatry. 81:306–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Higuchi F, Uchida S, Yamagata H, Abe-Higuchi N, Hobara T, Hara K, et al. (2016): Hippocampal MicroRNA-124 Enhances Chronic Stress Resilience in Mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 36:7253–7267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Heller EA, Cates HM, Pena CJ, Sun H, Shao N, Feng J, et al. (2014): Locus-specific epigenetic remodeling controls addiction- and depression-related behaviors. Nature neuroscience. 17:1720–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lee HB, Sundberg BN, Sigafoos AN, Clark KJ (2016): Genome Engineering with TALE and CRISPR Systems in Neuroscience. Frontiers in genetics. 7:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dantzer R (2018): Neuroimmune Interactions: From the Brain to the Immune System and Vice Versa. Physiological reviews. 98:477–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Steptoe A, Hamer M, Chida Y (2007): The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain Behav Immun. 21:901–912. [DOI] [PubMed] [Google Scholar]
- 100.Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, et al. (2010): A meta-analysis of cytokines in major depression. Biological psychiatry. 67:446–457. [DOI] [PubMed] [Google Scholar]
- 101.Kohler CA, Freitas TH, Maes M, de Andrade NQ, Liu CS, Fernandes BS, et al. (2017): Peripheral cytokine and chemokine alterations in depression: a meta-analysis of 82 studies. Acta psychiatrica Scandinavica. 135:373–387. [DOI] [PubMed] [Google Scholar]
- 102.Chan KL, Cathomas F, Russo SJ (2019): Central and Peripheral Inflammation Link Metabolic Syndrome and Major Depressive Disorder. Physiology (Bethesda, Md). 34:123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Iwata M, Ota KT, Duman RS (2013): The inflammasome: Pathways linking psychological stress, depression, and systemic illnesses. Brain, Behavior, and Immunity. 31:105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dunn JH, Ellis LZ, Fujita M (2012): Inflammasomes as molecular mediators of inflammation and cancer: potential role in melanoma. Cancer letters. 314:24–33. [DOI] [PubMed] [Google Scholar]
- 105.Kohler O, Benros ME, Nordentoft M, Farkouh ME, Iyengar RL, Mors O, et al. (2014): Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: a systematic review and meta-analysis of randomized clinical trials. JAMA psychiatry. 71:1381–1391. [DOI] [PubMed] [Google Scholar]
- 106.Hannestad J, DellaGioia N, Bloch M (2011): The effect of antidepressant medication treatment on serum levels of inflammatory cytokines: a meta-analysis. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 36:2452–2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tan S, Wang Y, Chen K, Long Z, Zou J (2017): Ketamine Alleviates Depressive-Like Behaviors via Down-Regulating Inflammatory Cytokines Induced by Chronic Restraint Stress in Mice. Biological & pharmaceutical bulletin. 40:1260–1267. [DOI] [PubMed] [Google Scholar]
- 108.Chen MH, Li CT, Lin WC, Hong CJ, Tu PC, Bai YM, et al. (2018): Rapid inflammation modulation and antidepressant efficacy of a low-dose ketamine infusion in treatment-resistant depression: A randomized, double-blind control study. Psychiatry research. 269:207–211. [DOI] [PubMed] [Google Scholar]
- 109.Mogensen TH (2009): Pathogen recognition and inflammatory signaling in innate immune defenses. Clinical microbiology reviews. 22:240–273, Table of Contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Medzhitov R, Janeway C Jr. (2000): Innate immunity. The New Englandjournal of medicine. 343:338–344. [DOI] [PubMed] [Google Scholar]
- 111.Powell ND, Sloan EK, Bailey MT, Arevalo JM, Miller GE, Chen E, et al. (2013): Social stress up-regulates inflammatory gene expression in the leukocyte transcriptome via beta-adrenergic induction of myelopoiesis. Proceedings of the National Academy of Sciences of the United States of America. 110:16574–16579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Heidt T, Sager HB, Courties G, Dutta P, Iwamoto Y, Zaltsman A, et al. (2014): Chronic variable stress activates hematopoietic stem cells. Nature Medicine. 20:754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hodes GE, Pfau ML, Leboeuf M, Golden SA, Christoffel DJ, Bregman D, et al. (2014): Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proceedings of the National Academy of Sciences of the United States of America. 111:16136–16141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pfau M, Hodes GE, Kana V, Menard C, Aleyasin H, Lavin Y, et al. (2016): Role of leukocyte derived microRNAs in stress induced inflammation and depression Abstract, Annual Meeting of the Society for Neuroscience. [Google Scholar]
- 115.Wang J, Hodes GE, Zhang H, Zhang S, Zhao W, Golden SA, et al. (2018): Epigenetic modulation of inflammation and synaptic plasticity promotes resilience against stress in mice. Nature communications. 9:477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bonilla FA, Oettgen HC (2010): Adaptive immunity. The Journal of allergy and clinical immunology. 125:S33–40. [DOI] [PubMed] [Google Scholar]
- 117.Miller AH (2010): Depression and immunity: a role for T cells? Brain Behav Immun. 24:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zorrilla EP, Luborsky L, McKay JR, Rosenthal R, Houldin A, Tax A, et al. (2001): The relationship of depression and stressors to immunological assays: a meta-analytic review. Brain Behav Immun. 15:199–226. [DOI] [PubMed] [Google Scholar]
- 119.Lewitus GM, Schwartz M (2009): Behavioral immunization: immunity to self-antigens contributes to psychological stress resilience. Mol Psychiatry. 14:532–536. [DOI] [PubMed] [Google Scholar]
- 120.Lewitus GM, Wilf-Yarkoni A, Ziv Y, Shabat-Simon M, Gersner R, Zangen A, et al. (2009): Vaccination as a Novel Approach for Treating Depressive Behavior. Biological psychiatry. 65:283–288. [DOI] [PubMed] [Google Scholar]
- 121.Lewitus GM, Cohen H, Schwartz M (2008): Reducing post-traumatic anxiety by immunization. Brain, Behavior, and Immunity. 22:1108–1114. [DOI] [PubMed] [Google Scholar]
- 122.Brachman RA, Lehmann ML, Maric D, Herkenham M (2015): Lymphocytes from chronically stressed mice confer antidepressant-like effects to naive mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 35:1530–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Toben C, Baune BT (2015): An Act of Balance Between Adaptive and Maladaptive Immunity in Depression: a Role for T Lymphocytes. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology. 10:595–609. [DOI] [PubMed] [Google Scholar]
- 124.Dantzer R, Cohen S, Russo SJ, Dinan TG (2018): Resilience and immunity. Brain Behav Immun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Clemente JC, Ursell LK, Parfrey LW, Knight R (2012): The impact of the gut microbiota on human health: an integrative view. Cell. 148:1258–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cryan JF, Dinan TG (2012): Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nature reviews Neuroscience. 13:701–712. [DOI] [PubMed] [Google Scholar]
- 127.Mayer EA, Knight R, Mazmanian SK, Cryan JF, Tillisch K (2014): Gut microbes and the brain: paradigm shift in neuroscience. The Journal of neuroscience : the official journal of the Society for Neuroscience. 34:15490–15496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jiang H, Ling Z, Zhang Y, Mao H, Ma Z, Yin Y, et al. (2015): Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav Immun. 48:186–194. [DOI] [PubMed] [Google Scholar]
- 129.Naseribafrouei A, Hestad K, Avershina E, Sekelja M, Linlokken A, Wilson R, et al. (2014): Correlation between the human fecal microbiota and depression. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 26:1155–1162. [DOI] [PubMed] [Google Scholar]
- 130.Diaz Heijtz R, Wang S, Anuar F, Qian Y, Bjorkholm B, Samuelsson A, et al. (2011): Normal gut microbiota modulates brain development and behavior. Proceedings of the National Academy of Sciences of the United States of America. 108:3047–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bercik P, Denou E, Collins J, Jackson W, Lu J, Jury J, et al. (2011): The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology. 141:599–609, 609.e591-593. [DOI] [PubMed] [Google Scholar]
- 132.Zheng P, Zeng B, Zhou C, Liu M, Fang Z, Xu X, et al. (2016): Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host's metabolism. Mol Psychiatry. 21:786–796. [DOI] [PubMed] [Google Scholar]
- 133.Yang C, Fujita Y, Ren Q, Ma M, Dong C, Hashimoto K (2017): Bifidobacterium in the gut microbiota confer resilience to chronic social defeat stress in mice. Scientific reports. 7:45942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bharwani A, Mian MF, Surette MG, Bienenstock J, Forsythe P (2017): Oral treatment with Lactobacillus rhamnosus attenuates behavioural deficits and immune changes in chronic social stress. BMC Medicine. 15:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Abbott NJ, Patabendige AAK, Dolman DEM, Yusof SR, Begley DJ (2010): Structure and function of the blood–brain barrier. Neurobiology of Disease. 37:13–25. [DOI] [PubMed] [Google Scholar]
- 136.Banks WA (2005): Blood-brain barrier transport of cytokines: a mechanism for neuropathology. Current pharmaceutical design. 11:973–984. [DOI] [PubMed] [Google Scholar]
- 137.Friedman A, Kaufer D, Shemer J, Hendler I, Soreq H, Tur-Kaspa I (1996): Pyridostigmine brain penetration under stress enhances neuronal excitability and induces early immediate transcriptional response. Nat Med. 2:1382–1385. [DOI] [PubMed] [Google Scholar]
- 138.Niklasson F, Agren H (1984): Brain energy metabolism and blood-brain barrier permeability in depressive patients: analyses of creatine, creatinine, urate, and albumin in CSF and blood. Biological psychiatry. 19:1183–1206. [PubMed] [Google Scholar]
- 139.Menard C, Pfau ML, Hodes GE, Kana V, Wang VX, Bouchard S, et al. (2017): Social stress induces neurovascular pathology promoting depression. Nature neuroscience. 20:1752–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pearson-Leary J, Eacret D, Chen R, Takano H, Nicholas B, Bhatnagar S (2017): Inflammation and vascular remodeling in the ventral hippocampus contributes to vulnerability to stress. Translational psychiatry. 7:e1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cheng Y, Desse S, Martinez A, Worthen RJ, Jope RS, Beurel E (2018): TNFalpha disrupts blood brain barrier integrity to maintain prolonged depressive-like behavior in mice. Brain Behav Immun. 69:556–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.McKim DB, Weber MD, Niraula A, Sawicki CM, Liu X, Jarrett BL, et al. (2018): Microglial recruitment of IL-1beta-producing monocytes to brain endothelium causes stress-induced anxiety. Mol Psychiatry. 23:1421–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Rajkowska G, Miguel-Hidalgo JJ (2007): Gliogenesis and glial pathology in depression. CNS & neurological disorders drug targets. 6:219–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wohleb ES, Franklin T, Iwata M, Duman RS (2016): Integrating neuroimmune systems in the neurobiology of depression. Nature reviews Neuroscience. 17:497–511. [DOI] [PubMed] [Google Scholar]
- 145.Wohleb ES, Hanke ML, Corona AW, Powell ND, Stiner LM, Bailey MT, et al. (2011): beta-Adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. The Journal of neuroscience : the official journal of the Society for Neuroscience. 31:6277–6288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Steiner J, Bielau H, Brisch R, Danos P, Ullrich O, Mawrin C, et al. (2008): Immunological aspects in the neurobiology of suicide: elevated microglial density in schizophrenia and depression is associated with suicide. Journal of psychiatric research. 42:151–157. [DOI] [PubMed] [Google Scholar]
- 147.Setiawan E, Wilson AA, Mizrahi R, Rusjan PM, Miler L, Rajkowska G, et al. (2015): Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA psychiatry. 72:268–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kreisel T, Frank MG, Licht T, Reshef R, Ben-Menachem-Zidon O, Baratta MV, et al. (2014): Dynamic microglial alterations underlie stress-induced depressive-like behavior and suppressed neurogenesis. Mol Psychiatry. 19:699–709. [DOI] [PubMed] [Google Scholar]
- 149.Rothhammer V, Borucki DM, Tjon EC, Takenaka MC, Chao CC, Ardura-Fabregat A, et al. (2018): Microglial control of astrocytes in response to microbial metabolites. Nature. 557:724–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Toth M, et al. (2014): The gut microbiota influences blood-brain barrier permeability in mice. Science translational medicine. 6:263ra158. [DOI] [PMC free article] [PubMed] [Google Scholar]