Abstract
Red blood cells (RBCs) release ATP in response to haemoglobin deoxygenation, which binds to endothelial purinergic receptors and stimulates vasodilatation. This ATP release is impaired in RBCs from older vs. young adults, but the underlying mechanisms are unknown. Using isolated RBCs from young (24±1) and older (65±2) adults, we tested the hypothesis that age-related changes in RBC deformability (Study 1) and cAMP signalling (Study 2) contribute to the impairment. RBC ATP release during normoxia (PO2 ~112 mmHg) and hypoxia (PO2 ~20 mmHg) was quantified via luciferin-luciferase technique following RBC incubation with either Y-27632 (Rho-kinase inhibitor to increase deformability), diamide (cell-stiffening agent), cilostazol (phosphodiesterase 3 inhibitor), or vehicle control. The mean change in RBC ATP release from normoxia to hypoxia in control conditions was significantly impaired in older vs. young (~50% vs ~120%; P<0.05). RBC deformability was also lower in older vs. young as indicated by a higher RBC transit time (RCTT) measured by blood filtrometry (RCTT: 8.541±0.050 vs. 8.234±0.098 a.u., respectively; P<0.05). Y-27632 improved RBC deformability (RCTT: 8.228±0.083) and ATP release (111.7±17.2%) in older and diamide decreased RBC deformability (RCTT: 8.955±0.114) and ATP release (67.4±11.8%) in young (P<0.05), abolishing the age group differences (P>0.05). Cilostazol did not change ATP release in either age group (P>0.05), and RBC cAMP and ATP release to pharmacological Gi protein activation was similar in both groups (P>0.05). We conclude that decreased RBC deformability is a primary contributor to age-related impairments in RBC ATP release, which may have implications for impaired vascular control with advancing age.
Keywords: red blood cells, hypoxia, deoxygenation, ATP release
Introduction
The local control and regulation of blood flow involves the integration of multiple substances, signalling pathways, and vascular responses, the end goal of which is the matching of oxygen supply to tissue metabolic demand (Clifford & Hellsten, 2004; Mortensen & Saltin, 2014; Joyner & Casey, 2015). Of these substances, circulating adenosine triphosphate (ATP) is among the most unique in that it can stimulate both local and conducted vasodilatation via binding to purinergic P2Y receptors on the endothelium (Collins et al., 1998; Winter & Dora, 2007; Dora, 2017), and it is the only molecule demonstrated to have the intrinsic ability to blunt sympathetically-meditated vasoconstriction when administered exogenously (Rosenmeier et al., 2004; Kirby et al., 2008; Hearon Jr. et al., 2017). Importantly, circulating concentrations of ATP in healthy young adults increase in response to physiological stimuli such as hypoxia and exercise, and are closely correlated with skeletal muscle blood flow during exercise (Mortensen et al., 2011; Kirby et al., 2012). Advancing age in humans is associated with impairments in vasodilatation and regulation of blood flow to the skeletal muscle during exercise, which can contribute to increases in cardiovascular disease morbidity and mortality as well as declines in functional capacity, exercise tolerance, functional independence, and overall quality of life (WHO, 1993; Go et al., 2014; Hearon Jr. & Dinenno, 2016; Mozaffarian et al., 2016). Advancing age is also accompanied by an attenuation in circulating ATP during hypoxia and exercise (Kirby et al., 2012), but not an impaired responsiveness to ATP as determined by measuring vasodilatation in the forearm to brachial artery infusion of ATP (Kirby et al., 2010). Although there is evidence that the vasodilatory responsiveness to ATP may differ with age in the leg vasculature and that this can be modulated by physical activity status (Mortensen et al., 2012), the collective evidence suggests that if ageing adversely affects the contribution of ATP to vascular control and regulation of skeletal muscle blood flow, the impairment is likely related to the source of ATP.
While the exact mechanisms by which local changes in metabolic demand are sensed and vascular responses coordinated to provide the appropriate oxygen supply remain unclear, a growing body of evidence indicates that red blood cells (RBCs) could play a central role in this process (Bergfeld & Forrester, 1992; Ellsworth et al., 1995; Ellsworth, 2000; Jagger et al., 2001; Jensen, 2009; Ellsworth & Sprague, 2012). Specifically, RBCs can stimulate vasodilatation and increase oxygen delivery to the tissue by releasing ATP in direct proportion to the degree of haemoglobin deoxygenation, thus allowing them to act as both a ‘sensor’ for oxygen demand and an ‘effector’ capable increasing blood flow to facilitate the coupling of oxygen delivery to tissue metabolic demand (Dietrich et al., 2000; Jagger et al., 2001; González-Alonso et al., 2002; Sprague et al., 2009). Cell deformation, which occurs as RBCs traverse the microcirculation and is augmented by stimuli associated with the local milieu of contracting skeletal muscle such as elevated shear stress and mechanical compression of blood vessels, also stimulates ATP release and more deformable cells release more ATP in response to a given stimulus (Sprague et al., 1998; Faris & Spence, 2008; Sridharan et al., 2010b; Mortensen et al., 2011; Thuet et al., 2011; Crecelius et al., 2013). Both of these stimuli have been linked to ATP release through activation of heterotrimeric inhibitory G (Gi) proteins (Olearczyk et al., 2004a, 2004b). Downstream of Gi activation, it has been demonstrated that stimulation of adenylyl cyclase (AC) and increases in intracellular cyclic AMP (cAMP), the overall level of which is regulated by the balance between AC-mediated synthesis and hydrolysis by phosphodiesterase 3 (PDE3), are involved in the signalling cascade for RBC ATP release (Sprague et al., 2001a, 2006, 2011; Conti & Beavo, 2007; Adderley & Sprague, 2010; Lomas & Zaccolo, 2014; Brescia & Zaccolo, 2016). In vivo, the aforementioned increase in circulating ATP during exercise has been shown to be dependent on skeletal muscle perfusion, suggesting that intravascular sources such as RBCs play an essential role in this response (Kirby et al., 2013). Consistent with the evidence that RBCs are a primary source of circulating ATP and that circulating ATP responses to hypoxia and exercise are impaired with age, our laboratory was the first to demonstrate that deoxygenation-induced ATP release is impaired in RBCs isolated from healthy older compared with young adults (Kirby et al., 2012).
Although the underlying mechanisms of this impairment in RBC ATP release are unknown, one possibility is the age-associated decrease in RBC membrane fluidity and deformability (Reid et al., 1976; Hegner et al., 1979; Gelmini et al., 1987, 1989). In this context, it has been demonstrated that acute, pharmacologically-induced increases or decreases in RBC deformability produce parallel changes in deoxygenation-induced ATP release from RBCs of young healthy donors (Thuet et al., 2011). Another potential mechanism is altered intracellular cAMP signalling given that RBCs from type 2 diabetics, in addition to exhibiting impaired ATP release responses to cell deformation and deoxygenation (Subasinghe & Spence, 2008; Sprague et al., 2010, 2011; Richards et al., 2014, 2015; Dergunov et al., 2015), also exhibit blunted increases in intracellular cAMP and ATP release following direct pharmacological Gi protein stimulation relative to RBCs from healthy controls (Sprague et al., 2006, 2011). Importantly, treating RBCs from type 2 diabetics with the PDE3 inhibitor cilostazol significantly improves these intracellular cAMP and ATP release responses (Hanson et al., 2010; Sprague et al., 2011; Dergunov et al., 2015). To date, whether changes in red blood cell deformability and/or cAMP signalling are mechanistically-linked with impaired RBC ATP release in older adults has never been experimentally tested.
Accordingly, the primary purpose of the present investigation was to determine if age-related declines in RBC deformability and/or potential alterations in cAMP signalling contribute to impaired deoxygenation-induced ATP release from RBCs of healthy older adults. Specifically, we tested the hypothesis that increasing RBC deformability would improve deoxygenation-induced ATP release in RBCs from older adults and, conversely, that decreasing RBC deformability would attenuate deoxygenation-induced ATP release in RBCs from young adults (Study 1). Further, we hypothesized that treating RBCs with the PDE3 inhibitor cilostazol would improve deoxygenation-induced ATP release from RBCs of healthy older adults, and sought to determine if cellular responses downstream of Gi activation (i.e., increased intracellular cAMP and ATP release) are impaired with advancing age (Study 2).
Methods
Ethical approval and subjects
With approval from the Institutional Review Board at Colorado State University (protocol 16-6361H) and after written informed consent, a total of 17 young and 15 older healthy adults participated in the first series of experiments (Study 1: RBC deformability and ATP release), of which 12 young and 10 older subjects participated in multiple experiments. Similarly, 14 young and 13 older healthy adults participated in the second series of experiments (Study 2: RBC cAMP and ATP release), with 6 young and 6 older subjects participating in multiple experiments. All subjects were free from overt cardiovascular disease as assessed from a medical history, free of cardiovascular medications, non-smokers, non-obese, normotensive, and sedentary to moderately active. Young female subjects were studied during the early follicular phase of their menstrual cycle to minimize any potential cardiovascular effects of sex-specific hormones, whereas older female subjects were post-menopausal and not taking hormone replacement therapy. Additionally, older subjects were further evaluated for clinical evidence of cardiopulmonary disease with a physical examination and resting and exercise (Balke protocol) electrocardiograms. Body composition was determined by whole-body dual-energy X-ray absorptiometry scans (QDR series software, Hologic, Inc., USA). Whole blood lipid panels were run using a Piccolo Xpress chemistry analyser (Abaxis, USA). All studies were performed according to the Declaration of Helsinki.
Isolation of RBCs
Blood was obtained by either venipuncture of the antecubital vein or catheterization of the brachial artery (if the subject was participating in another study in the laboratory) and collected into Vacutainer tubes containing sodium heparin (158 USP units) after a 4 hour fast and 12 hour abstention from caffeine, alcohol, and exercise. RBCs were isolated by centrifugation of the collected whole blood (500g, 4°C, 10 min) followed by removal of the plasma and buffy coat by aspiration. Packed RBCs were resuspended and washed three times in a cell wash buffer (CWB) containing (in mM) 4.7 KCl, 2.0 CaCl2, 1.2 MgSO4, 140.5 NaCl, 21.0 Tris-base, 5.5 glucose, and 0.5% BSA, with pH adjusted to 7.4 at room temperature (Sridharan et al., 2010b; Thuet et al., 2011; Kirby et al., 2012; Richards et al., 2014, 2015). All studies were performed immediately after blood collection and RBC isolation.
RBC drug treatment and deoxygenation
As described previously by our laboratory (Kirby et al., 2012), washed RBCs were diluted to 20% haematocrit with a warmed (37°C) bicarbonate-based buffer containing (in mM) 4.7 KCl, 2.0 CaCl2, 1.2 MgSO4, 140.5 NaCl, 11.1 glucose, 23.8 NaHCO3, and 0.5% BSA and placed in a rotating bulb tonometer (Eschweiler GmbH & Co. KG, Germany). Drug treatments were performed on separate days (Study 1: 1 μM Y-27632 or 500 μM diamide, references below; Study 2: 100 μM cilostazol, Sprague et al., 2011; all drugs from Sigma) and were always paired with a vehicle control in a second tonometer bulb (Study 1: saline; Study 2: dimethylformamide, DMF, Sigma). This concentration of the Rho-kinase inhibitor Y-27632 has been shown to increase deformability and hypoxia-induced ATP release in RBCs from healthy humans (Thuet et al., 2011), whereas the thiol cross-linking agent diamide has been shown to decrease RBC deformability and hypoxia-induced ATP release (Sridharan et al., 2010b; Thuet et al., 2011) without significantly altering haemoglobin or normal cell function (Kosower et al., 1969; Maeda et al., 1983). This concentration of cilostazol has been shown to enhance pharmacologically-stimulated elevations in intracellular cAMP in isolated RBCs (Hanson et al., 2010; Sprague et al., 2011; Leal Denis et al., 2013; Knebel et al., 2013).
Paired drug- and vehicle-treated RBC suspensions were incubated in tonometer bulbs for 30 min in normoxia (16% O2, 6% CO2, balanced nitrogen; Study 1: PO2 = 111.8 ± 0.7 mmHg and FO2Hb = 94.9 ± 0.1%; Study 2: PO2 = 114.3 ± 0.7 mmHg and FO2Hb = 95.0 ± 0.1%; mean of all age groups and conditions), after which a sample was removed from each tonometer bulb for measurement of extracellular and intracellular ATP (details below). RBCs were then deoxygenated by exposure to hypoxia for 15 min (Study 1: 1% O2, 6% CO2, balanced nitrogen; PO2 = 18.4 ± 0.5 mmHg and FO2Hb = 21.7 ± 1.0%; Study 2: 2.25% O2, 6% CO2, balanced nitrogen; PO2 = 24.1 ± 0.4 mmHg and FO2Hb = 34.8 ± 1.2%; mean of all age groups and conditions) and RBC samples were taken for measurement of ATP as in normoxia. Normoxic and hypoxic gases were blended via gas blender (MCQ Gas Blender Series 100, Italy) and humidified before introduction into the tonometer bulbs. Our goal was to reduce PO2 and FO2Hb to levels within the range observed in vivo during conditions such as exercise (Kirby et al., 2012), and the O2 percentage was adjusted in Study 2 to approach that goal more closely. Blood gases were confirmed by blood gas analysis (Siemens Rapid Point 405 Series Automatic Blood Gas System, Los Angeles, CA) (Kirby et al., 2012).
Measurements of extracellular ATP and RBC total intracellular ATP
ATP was measured via the luciferin-luciferase technique as described previously (Sprague et al., 2001a, 2011; Sridharan et al., 2010b, 2010a; Thuet et al., 2011; Kirby et al., 2012; Richards et al., 2013, 2014, 2015), with light emission during the reaction detected by a luminometer (TD 20/20, Turner Designs). For measurement of extracellular ATP (i.e. ATP release), a 10 μL sample of the 20% haematocrit suspension was taken from each tonometer bulb and diluted 500-fold (0.04% haematocrit), from which 200 μL samples were taken and injected into cuvettes containing 100 μL of firefly tail extract (10 mg/mL deionized water; Sigma) and 100 μL of D-luciferin (0.5 mg/mL deionized water; Research Products International). Peak light output was measured at least in triplicate for each experimental condition and the mean was used for determination of ATP levels by comparison to a standard curve for ATP (Calbiochem) generated on the day of the experiment. Cell counts were obtained from each 0.04% RBC suspension and extracellular ATP was normalized to 4 × 108 cells. To confirm that ATP release was not due to haemolysis, the 0.04% RBC suspensions from which samples for ATP analysis and cell counting were taken were analysed for free haemoglobin by measuring absorbance at 405 nm and samples with significant lysis were excluded, similar to previous reports (Sprague et al., 1998, 2011; Sridharan et al., 2010a; Thuet et al., 2011; Kirby et al., 2012, 2014; Richards et al., 2013, 2014, 2015).
To confirm that the effects of donor age and pharmacological agents on RBC ATP release were not due to differences in total intracellular ATP or the increase in RBC glycolytic activity during hypoxia (Messana et al., 1996; Campanella et al., 2005; Lewis et al., 2009; Kirby et al., 2014), 50 μL samples of drug- and saline-treated RBCs (20% haematocrit) were taken from the tonometer bulbs in normoxia and hypoxia following measurement of extracellular ATP and lysed in deionized water at room temperature (a 20-fold dilution). This lysate was diluted an additional 400-fold (8000-fold total) and ATP was measured by the same approach used for determination of extracellular ATP (Sridharan et al., 2010b, 2010a; Sprague et al., 2011; Thuet et al., 2011; Kirby et al., 2012, 2014). Values were normalized to ATP concentration per RBC.
Measurement of RBC deformability (Study 1)
RBC deformability was measured using the St. George’s blood filtrometer (Carri-Med, Dorking, UK) (Sprague, 1996; Sprague et al., 1998, 2001b, 2001a; Olearczyk et al., 2004a; Sridharan et al., 2010b; Thuet et al., 2011; Clapp et al., 2013; Richards et al., 2014). This device develops a calibrated 3 cm H2O pressure gradient across a vertically mounted, 13 mm diameter polycarbonate filter (Nucleopore) with 9.53 mm exposed surface diameter and average pore size of 5 μm compared to the average RBC size of ~6-8 μM (thus, RBCs must deform to pass through the filter). Distal to the filter, the outflow channel was filled with CWB and flow was prevented by a stopcock. Proximal to the filter, the chamber and an open-ended capillary tube were filled with either CWB (as described above for RBC isolation, but with pH adjusted to 7.4 at 37°C) alone or a 10% haematocrit solution of RBCs and CWB, both warmed to 37°C. For calibration, the time required for CWB alone to pass through the filter was measured by four fibre optic detectors and recorded digitally, with this process repeated until the coefficient of variance between runs was 1% or less; two to four total runs were typically needed throughout the duration of each experiment to achieve this variance criterion. The RBC suspension was then passed through the calibrated filter and red (blood) cell transit time (RCTT) was calculated based on the rate at which the RBC suspension traversed the filter relative to the rate of CWB alone as described previously (Sprague, 1996). If the average filter pore size and haematocrit are kept constant, then RCTT is a unitless index of RBC deformability, with lower RCTT indicating greater RBC deformability.
Measurements of RBC deformability were made after a 30-min incubation with either saline (vehicle control), Y-27632 (1 μM; Sigma), or diamide (500 μM; Sigma). RBC deformability was measured on the same day and in triplicate for each condition, with the treatment order randomized and counterbalanced between subjects. RBC deformability was measured on a different day than the measurements of deoxygenation-induced ATP release, using fresh blood samples on each day in order to ensure that RBCs were studied within ~4 hours of isolation.
Measurement of RBC intracellular cAMP (Study 2)
As described previously (Olearczyk et al., 2004a; Sprague et al., 2005, 2006, 2011; Hanson et al., 2010; Sridharan et al., 2010b), washed RBCs were diluted to a 50% haematocrit in a CWB (as described for isolation of RBCs) and three 1 mL aliquots of this RBC suspension were incubated at room temperature with either DMF for 45 min (vehicle and time control) or the Gi activator mastoparan 7 (Mas 7; 10 μM; Sigma) for 15 min. The reaction was halted by the addition of 4 mL of ice cold acidified ethanol (1.3 μL of 11.6 M HCl in 15 mL of 200 proof ethanol), followed by vortexing and centrifugation (14,000g, 4°C, 10 min). The supernatant was removed and stored overnight at −20°C to precipitate the remaining proteins and centrifuged (3,700g, 4°C, 10 min) the next day. The final supernatant was removed, dried under vacuum centrifugation, and stored at −80°C until enough samples were collected to run the assay. The dried sample was reconstituted in an assay buffer and the concentration of cAMP (fmol) was determined using a commercially available enzyme immunoassay (GE Healthcare; non-acetylation protocol kit). RBCs from 6 young and 6 older subjects were used for this experiment. Treatment of RBCs and measurement of intracellular cAMP was performed in duplicate and averaged for each subject, and the mean was used to determine the relative (%) change in intracellular cAMP compared to the DMF vehicle control (Hanson et al., 2010).
RBC Gi activation (Mas 7) and measurement of extracellular ATP (Study 2)
Washed RBCs were diluted to a 10% haematocrit with a bicarbonate-based buffer (as described above for RBC deoxygenation), placed in a rotating bulb tonometer, and warmed to 37°C in normoxia (15% O2, 6% CO2, balanced nitrogen; PO2 = 118.1 ± 1.1 mmHg and FO2Hb = 93.7 ± 0.2% across both age groups and conditions). After a 15 min equilibration period, RBC samples were taken from each tonometer bulb for baseline measurement of extracellular ATP followed by incubation with saline (vehicle control) or 10 μM Mas 7 (Sprague et al., 2005, 2006; Hanson et al., 2009, 2010; Sridharan et al., 2010b; Thuet et al., 2011). Saline-treated RBCs were sampled for measurement of extracellular ATP at 15 min after the addition of saline, and RBCs incubated with Mas 7 were sampled for extracellular ATP at 5, 10, and 15 min after the addition of Mas 7 and the peak value was used for calculating the relative (%) change in extracellular ATP from baseline (Thuet et al., 2011). Extracellular ATP was measured as described above for the deoxygenation experiments, with the 0.04% haematocrit RBC suspensions obtained by taking a 10 μL sample of the 10% haematocrit suspension from each tonometer bulb and diluting it 250-fold. To confirm that ATP release was not due to haemolysis, free haemoglobin was measured as described for Study 1, but with addition of measures at 570 nm with the background absorbance at 700 nm subtracted as described by (Keller et al., 2017), which was published between the completion of Study 1 and the initiation of Study 2.
Statistics
All values are reported as mean ± SEM. Statistical analyses of absolute ATP values (intracellular and extracellular) were performed using R (R Core Team 2016, R Foundation for Statistical Computing, Vienna, Austria). Absolute ATP values were tested using a 3-way repeated measures ANOVA, with age as the between subjects factor (young vs. older) and drug/gas conditions as the within subject factors (control vs. drug and normoxia vs. hypoxia, respectively). When an interaction or main effect was found, appropriate pairwise comparisons were made. SigmaPlot (Systat Software, San Jose, CA, USA) was used to perform 2-way repeated measures ANOVAs for statistical analyses of the relative (%) change in ATP release from normoxia to hypoxia, RBC deformability (RCTT), the relative change in intracellular cAMP, the relative change in ATP release in response to incubation with Mas 7, and blood gases. In the event of a main effect of age or drug condition or an interaction between the two, post hoc comparisons were made with Tukey’s HSD test. Less than 1% of samples required exclusion due to significant lysis. The relative increase in intracellular cAMP compared to zero was tested using a one-tailed t-test. Significance was set at P < 0.05.
Results
Subjects and blood gases
Subject characteristics are reported in Table 1. Compared to the young adults, older adults had either trending or significant elevations in body mass index (BMI), body fat percentage, and blood lipids; however, all values were still within the normal healthy range. Blood gases for isolated RBCs are reported in Table 2. Most importantly, there were no significant differences in the fraction of oxygenated haemoglobin (FO2Hb) between age groups or pharmacological treatments in normoxia or hypoxia.
Table 1.
Subject characteristics
| Study 1 |
Study 2 |
|||||||
|---|---|---|---|---|---|---|---|---|
| Deformability | Y-27632 | Diamide | cAMP and ATP | |||||
| Young | Older | Young | Older | Young | Older | Young | Older | |
| Male:Female | 6:3 | 4:5 | 7:6 | 6:8 | 8:5 | 4:6 | 7:7 | 8:5 |
| Age (years) | 24±1 | 64±2† | 22±1 | 65±2† | 23±1 | 65±3† | 25±1 | 65±2† |
| Body mass index (kg/m2) | 22.7±0.8 | 25.6±1.1 | 23.0±0.8 | 24.9±0.7† | 23.2±0.8 | 25.1±0.9 | 22.4±0.6 | 25.2±0.8† |
| Body fat (%) | 24.3±2.2 | 34.2±3.0† | 24.7±1.8 | 34.9±2.1† | 25.6±2.4 | 34.0±2.9† | 23.7±1.9 | 31.3±2.0† |
| Total cholesterol (mg/dL) | 158±12 | 193±9† | 159±9 | 191±9† | 161±9 | 195±11† | 156±6 | 197±10† |
| LDL cholesterol (mg/dL) | 94±10 | 115±7 | 94±7 | 117±7† | 97±7 | 118±10 | 80±6 | 115±8† |
| HDL cholesterol (mg/dL) | 49±3 | 58±6 | 50±3 | 54±5 | 50±3 | 60±6 | 60±3 | 60±4 |
| LDL:HDL | 2.0±0.2 | 2.2±0.3 | 1.9±0.2 | 2.5±0.3 | 2.0±0.2 | 2.1±0.3 | 1.4±0.1 | 2.0±0.2† |
| Triglycerides (mg/dL) | 80±8 | 103±19 | 75±12 | 100±11 | 77±10 | 87±9 | 80±6 | 107±9† |
P < 0.05 vs. young (within condition)
Table 2.
Isolated red blood cell gases
| pH | PO2 (mmHg) | PCO2 (mmHg) | tHb (g/dL) | FO2Hb (%) | FHHb (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Study 1 | Normoxia | Young | Saline | 7.327±0.010 | 112.1±1.9 | 35.4±1.0 | 7.1±0.2 | 95.1±0.2 | 3.4±0.1 |
| Y-27632 | 7.327±0.009 | 112.5±2.5 | 34.4±0.7 | 7.1±0.2 | 95.1±0.2 | 3.3±0.1 | |||
| Older | Saline | 7.344±0.009 | 111.8±2.7 | 35.0±0.7 | 7.7±0.3 | 95.0±0.2 | 3.6±0.2 | ||
| Y-27632 | 7.360±0.006*† | 114.0±2.7* | 34.1±0.4 | 7.4±0.2 | 95.2±0.2 | 3.4±0.1* | |||
| Hypoxia | Young | Saline | 7.340±0.010 | 19.3±1.2 | 36.5±1.1 | 7.1±0.2 | 24.1±2.8 | 72.1±2.6 | |
| Y-27632 | 7.340±0.010 | 19.6±1.4 | 36.6±0.8 | 7.1±0.2 | 24.1±2.8 | 72.2±2.7 | |||
| Older | Saline | 7.367±0.010 | 18.7±1.7 | 35.8±1.0 | 7.6±0.3 | 23.1±3.4 | 73.1±3.2 | ||
| Y-27632 | 7.375±0.008† | 18.2±1.4 | 35.2±0.4 | 7.3±0.2 | 21.5±3.0 | 74.4±2.9 | |||
| Normoxia | Young | Saline | 7.324±0.007 | 109.6±1.1 | 34.8±0.6 | 6.7±0.1 | 94.6±0.2 | 3.6±0.1 | |
| Diamide | 7.319±0.007 | 110.0±0.8 | 35.1±0.6 | 6.7±0.1 | 94.6±0.2 | 3.5±0.1 | |||
| Older | Saline | 7.314±0.008 | 111.8±1.0 | 33.9±0.9 | 6.5±0.2 | 94.5±0.1 | 3.7±0.2 | ||
| Diamide | 7.306±0.009 | 111.9±1.2 | 34.5±0.6 | 6.5±0.2 | 94.5±0.3 | 3.5±0.2 | |||
| Hypoxia | Young | Saline | 7.348±0.007 | 16.8±0.8 | 34.6±0.6 | 6.5±0.1 | 17.4±1.2 | 78.1±1.2 | |
| Diamide | 7.347±0.007 | 17.5±1.0 | 35.8±0.7 | 6.6±0.2 | 19.6±2.1 | 76.1±1.9 | |||
| Older | Saline | 7.332±0.010 | 18.1±1.1 | 35.0±0.5 | 6.4±0.2 | 17.9±2.0 | 77.7±1.9 | ||
| Diamide | 7.339±0.016 | 21.1±1.7† | 34.5±1.2 | 6.6±0.2 | 28.8±3.9 | 67.3±3.6 | |||
| Study 2 | Normoxia | Young | DMF | 7.330±0.010 | 113.1±1.2 | 34.9±0.6 | 6.6±0.1 | 94.9±0.2 | 3.8±0.1 |
| Cilostazol | 7.325±0.012 | 114.1±1.7 | 34.7±0.7 | 6.5±0.2 | 94.9±0.2 | 3.7±0.1 | |||
| Older | DMF | 7.362±0.013 | 115.7±1.3 | 35.4±0.8 | 6.8±0.1 | 95.1±0.1 | 3.3±0.2 | ||
| Cilostazol | 7.350±0.013* | 114.0±1.1 | 37.0±0.6 | 6.8±0.2 | 95.2±0.1 | 3.5±0.1* | |||
| Hypoxia | Young | DMF | 7.352±0.010 | 23.5±1.1 | 34.8±0.5 | 6.9±0.2 | 33.3±3.2 | 63.2±3.0 | |
| Cilostazol | 7.339±0.009 | 24.5±1.2 | 36.4±0.8* | 6.8±0.2 | 35.7±3.2 | 60.8±2.9 | |||
| Older | DMF | 7.375±0.012 | 24.3±0.6 | 36.2±0.5 | 7.0±0.1 | 34.8±1.7 | 61.9±1.6 | ||
| Cilostazol | 7.361±0.013 | 24.1±0.6 | 36.0±0.7 | 7.0±0.2 | 35.5±1.8 | 61.1±1.7 |
PO2: partial pressure of oxygen, PCO2: partial pressure of carbon dioxide, tHb: total haemoglobin, FO2Hb: fraction of oxygenated haemoglobin, FHHb: fraction of deoxygenated haemoglobin.
P < 0.05 vs. vehicle control (within age)
P < 0.05 vs. young (within condition)
Effect of donor age, Y-27632, and diamide on RBC deformability (Study 1)
RBC deformability (n = 9 young, 9 older) was lower in RBCs from older adults as indicated by the significantly higher RCTT in the saline condition compared to young adults (8.541 ± 0.050 vs. 8.234 ± 0.098, respectively; P < 0.05; Fig. 1). Incubation with the Rho-kinase inhibitor Y-27632 improved RBC deformability significantly relative to the saline condition only in the older adults (RCTT: 8.228 ± 0.083; P < 0.05), such that there was no longer a difference between the age groups (Fig. 1). In contrast, incubation with diamide significantly decreased RBC deformability compared to saline in both young and older adults (RCTT: 8.955 ± 0.114 and 9.242 ± 0.154, respectively; P < 0.05; Fig. 1).
Figure 1. Effect of donor age, Y-27632, and diamide on red blood cell deformability.
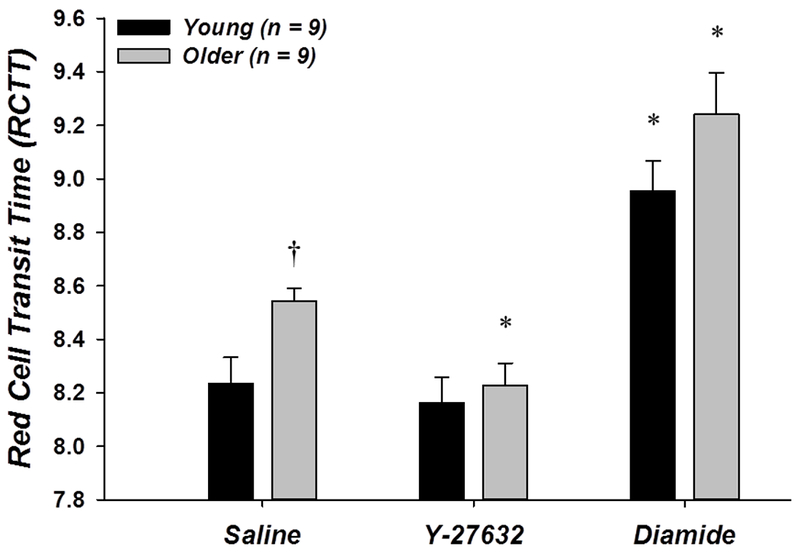
RBCs from older adults were less deformable than RBCs from young adults in control (saline) conditions, as indicated by a greater RCTT. Incubation of RBCs with 1 μM Y-27632 restored deformability in older adults, whereas 500 μM diamide decreased RBC deformability similarly in young and older adults. *P < 0.05 vs. saline (within age); †P < 0.05 vs. young (within condition)
Effect of donor age, Y-27632, and diamide on deoxygenation-induced ATP release from RBCs (Study 1)
In the Y-27632 experiment (n = 13 young, 14 older), extracellular ATP in normoxia was not different between age groups or drug condition (Fig. 2A). With saline, extracellular ATP from RBCs of older adults in hypoxia was significantly lower compared to young adults (19.7 ± 3.1 vs. 29.7 ± 4.3 nmol/4 × 108 RBCs, respectively; P < 0.05; Fig. 2A) and the mean percent increase from normoxia to hypoxia was significantly impaired in the older vs. young adults (35.8 ± 11.1% vs. 114.7 ± 11.0%, respectively; P < 0.05; Fig. 2B). Incubation of RBCs with Y-27632 significantly increased extracellular ATP during hypoxia in older adults (27.4 ± 3.3 nmol/4 × 108 RBCs; P < 0.0001; Fig. 2A) and significantly increased the mean percent increase in extracellular ATP from normoxia to hypoxia in both older and young adults compared to saline (111.7 ± 17.2% and 159.7 ± 22.5%, respectively; P < 0.05), thus reversing the age-related impairment in ATP release compared to control conditions in the young (Fig. 2B).
Figure 2. Effect of donor age and Y-27632 on red blood cell ATP release in normoxia and hypoxia.
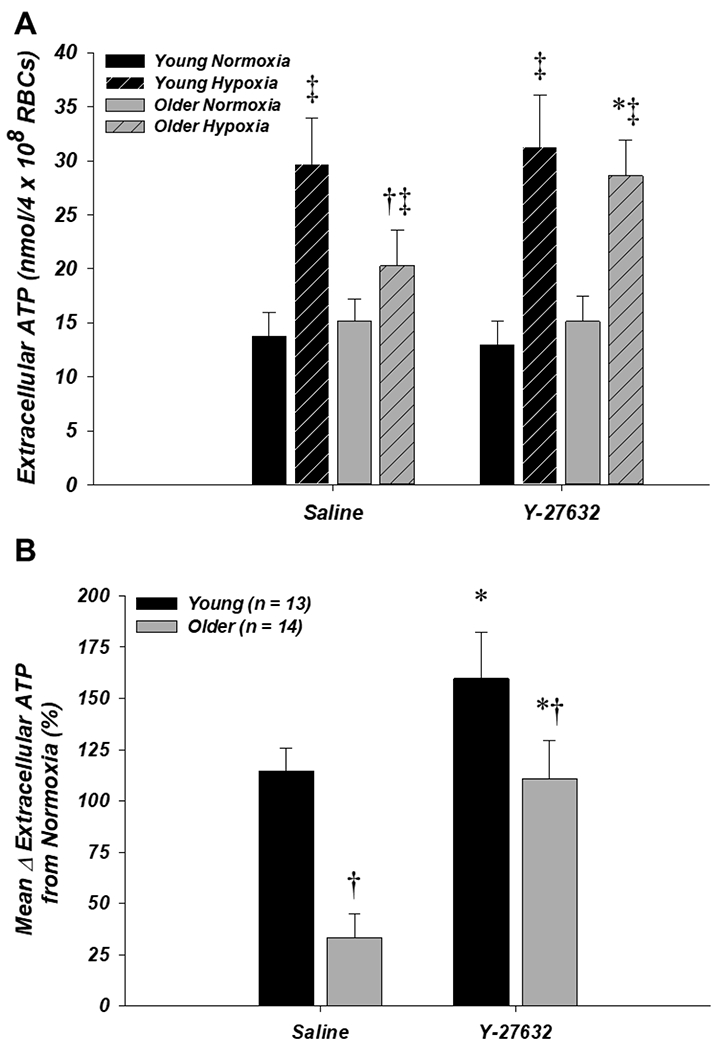
A: extracellular ATP (i.e., ATP release) from RBCs of older adults was significantly lower compared to young adults during hypoxia with saline (control) but not Y-27632, as Y-27632 significantly increased extracellular ATP during hypoxia from RBCs of older adults. B: the mean percent increase in extracellular ATP from normoxia to hypoxia was impaired from RBCs of older adults in the saline condition. Incubation with Y-27632 normalized this response from RBCs of older adults relative to the young saline control. *P < 0.05 vs. saline (within age); †P < 0.05 vs. young (within condition); ‡P < 0.05 vs. normoxia (within condition)
In the diamide experiment (n = 13 young, 10 older), extracellular ATP in normoxia was not different between age groups or drug condition (Fig. 3A). As in the Y-27632 experiment, extracellular ATP from RBCs of older adults in the saline hypoxia condition was significantly lower compared to young adults (18.0 ± 3.0 vs. 25.8 ± 2.5 nmol/4 × 108 RBCs, respectively; P < 0.05; Fig. 3A) and the mean percent increase from normoxia to hypoxia was significantly impaired vs. young adults (57.7 ± 14.2% vs. 137.9 ± 25.3%, respectively; P < 0.05; Fig. 3B). Relative to the saline condition, incubation of RBCs with diamide resulted in significantly lower extracellular ATP during hypoxia in older and young adults (14.1 ± 2.9 and 15.7 ± 2.6 nmol/4 × 108 RBCs, respectively; P < 0.05) (Fig. 2A) and significantly blunted the mean percent increase in RBC ATP release from normoxia to hypoxia in young adults (67.4 ± 11.8%; P < 0.05) such that it was not different from older adults in either condition (Fig. 3B).
Figure 3. Effect of donor age and diamide on red blood cell ATP release in normoxia and hypoxia.
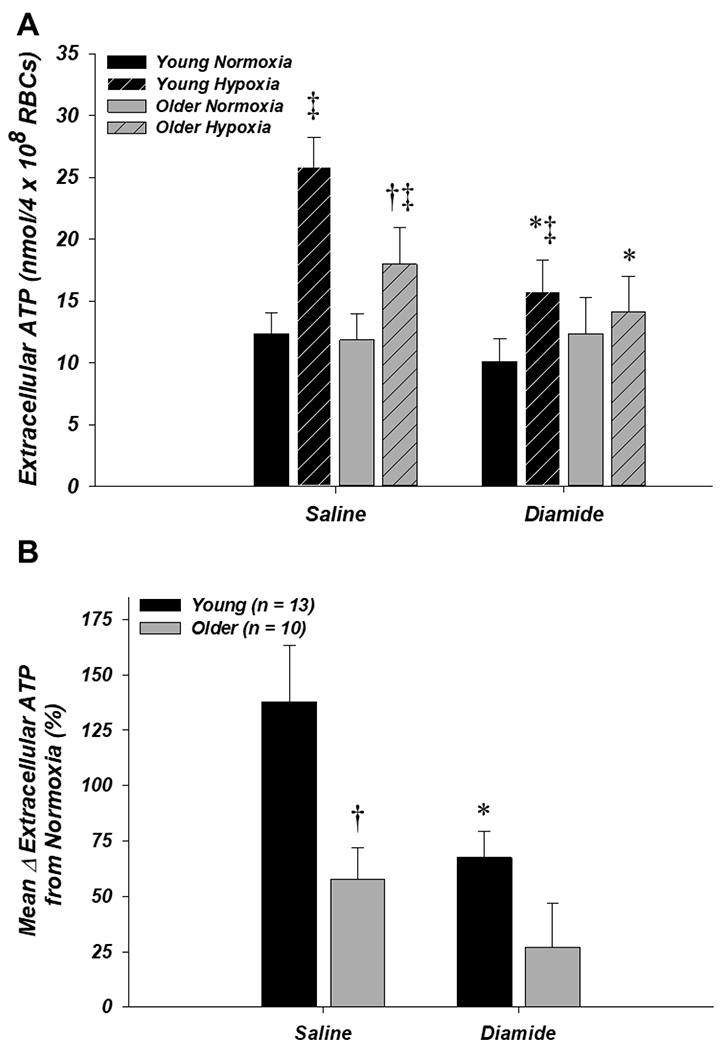
A: extracellular ATP (i.e., ATP release) from RBCs of older adults was significantly lower compared to young adults during hypoxia with saline (control) but not diamide, as diamide significantly decreased extracellular ATP during hypoxia from RBCs of both age groups. B: the mean percent increase in extracellular ATP from normoxia to hypoxia was impaired from RBCs of older adults in the saline condition. Incubation with diamide significantly decreased ATP release from RBCs of young adults such that it was no longer different from older adults. *P < 0.05 vs. saline (within age); †P < 0.05 vs. young (within condition); ‡P < 0.05 vs. normoxia (within condition)
Effect of donor age and cilostazol on deoxygenation-induced ATP release from RBCs (Study 2)
Extracellular ATP (n = 10 young, 12 older) in normoxia was not different between age groups or drug conditions (Fig. 4A). In the DMF vehicle control condition, extracellular ATP from RBCs of older adults in hypoxia trended towards being lower compared to young adults (10.7 ± 1.4 vs. 15.0 ± 2.5 nmol/4 × 108 RBCs, respectively; P = 0.07; Fig. 4A) and the mean percent increase from normoxia to hypoxia was significantly impaired in the older vs. young adults (46.7 ± 8.0% vs. 117.6 ± 13.3%, respectively; P < 0.05; Fig. 4B). This age-related impairment in ATP release during hypoxia was unaffected by incubation of RBCs with cilostazol, as both extracellular ATP and the percent increase from normoxia to hypoxia remained significantly lower in older vs. young adults (10.7 ± 1.2 vs. 16.7 ± 3.4 nmol/4 × 108 RBCs and 64.2 ± 11.4% vs. 141.0 ± 25.6%, respectively; both P < 0.05; Figs. 4A and 4B).
Figure 4. Effect of donor age and cilostazol on red blood cell ATP release in normoxia and hypoxia.
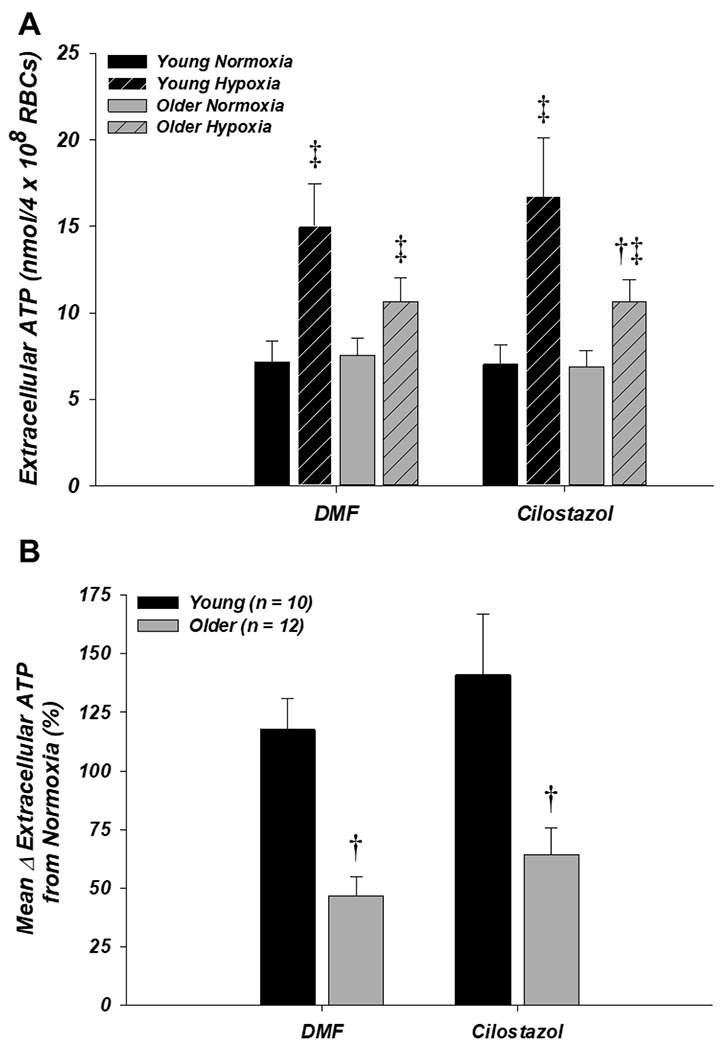
A: extracellular ATP (i.e., ATP release) from RBCs of older adults trended towards being lower compared to young adults during hypoxia with DMF (control; P = 0.07) and was significantly lower with cilostazol. Cilostazol had no effect on extracellular ATP from RBCs of young or older adults during normoxia or hypoxia. B: the mean percent increase in extracellular ATP from normoxia to hypoxia was impaired from RBCs of older adults in both the DMF (control) and cilostazol conditions. †P < 0.05 vs. young (within condition); ‡P < 0.05 vs. normoxia (within condition)
Effect of donor age on RBC intracellular cAMP and ATP release responses to Mas 7 (Study 2)
For measurements of the RBC intracellular cAMP response to incubation with the Gi activator Mas 7 (n = 6 young, 6 older), the concentration of intracellular cAMP in unstimulated (DMF vehicle control) RBCs was not significantly different between young and older adults (233.2 ± 41.0 fmol vs. 191.4 ± 38.0 fmol, respectively; P = 0.47). Incubation of RBCs with Mas 7 significantly increased intracellular cAMP relative to the DMF condition in both young and older adults (52.3 ± 15.2% and 60.8 ± 22.8%, respectively; P < 0.05 vs. zero; Fig. 5A). For measurements of the RBC ATP release response to incubation with Mas 7 (n = 4 young, 4 older), baseline extracellular ATP prior to the addition of saline or Mas 7 was not different in RBCs from young (10.8 ± 5.6 and 12.5 ± 6.1 nmol/4 × 108 RBCs, respectively; P > 0.05) or older (7.5 ± 1.2 and 6.4 ± 1.9 nmol/4 × 108 RBCs, respectively; P > 0.05) adults. ATP release from RBCs of both young and older adults did not change significantly following incubation with saline for 15 min (Fig. 5B). Following incubation with Mas 7, the mean percent increase in ATP release from baseline to peak was significantly greater than saline in RBCs from both young and older adults (231.8 ± 91.4% and 259.8 ± 43.8%, respectively; P < 0.05) and was not different between age groups (P > 0.05; Fig. 5B).
Figure 5. Effect of donor age on RBC responses to Mas 7.
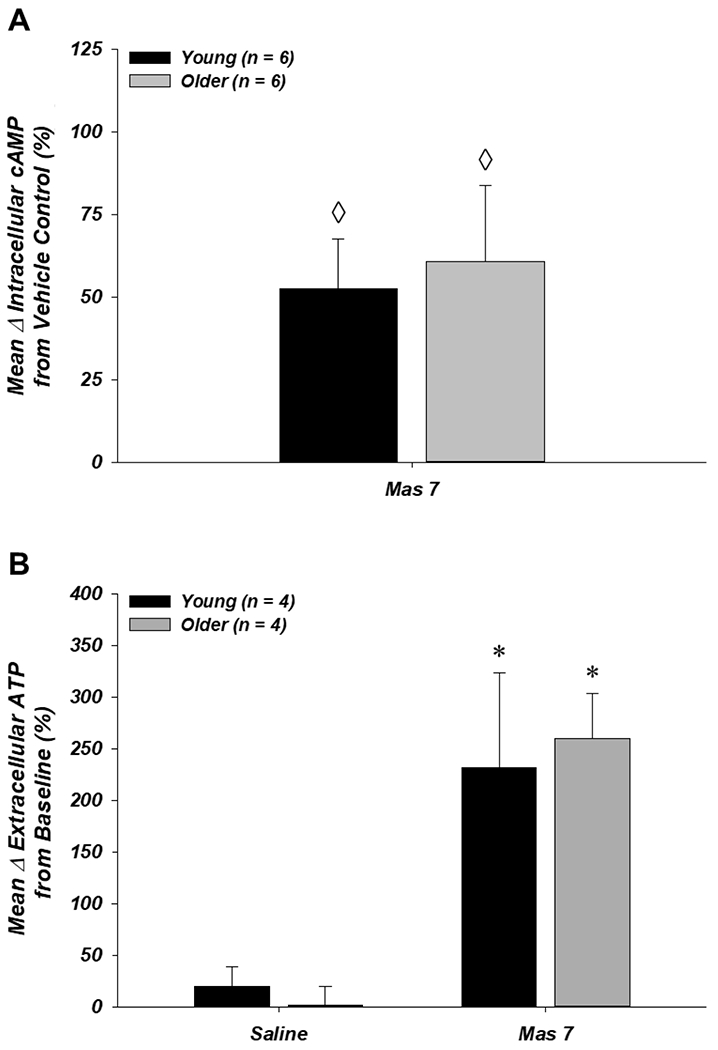
A: the Gi activator Mas 7 increased intracellular cAMP similarly from RBCs of young and older adults. B: extracellular ATP (i.e. ATP release) from RBCs of young and older adults was unaffected by incubation with saline (vehicle and time control) and increased significantly following incubation with Mas 7. ◊P < 0.05 vs. zero; *P < 0.05 vs. saline
Effect of donor age and drug treatments on RBC intracellular ATP (Studies 1 and 2)
RBC intracellular ATP increased significantly from normoxia to hypoxia during control conditions in both age groups (P < 0.05) and this was unaffected by incubation of RBCs with Y-27632 (Fig. 6A), diamide (Fig. 6B), or cilostazol (Fig. 6C). Intracellular ATP was significantly lower in RBCs from older vs. young adults during hypoxia with Y-27632 (1.88 ± 0.07 vs. 2.12 ± 0.13 mM/RBC, respectively; P < 0.05; Fig. 6A), although the mean change from normoxia to hypoxia was unaffected by age (0.24 ± 0.05 vs. 0.28 ± 0.07 mM/RBC for older vs. young, respectively; P > 0.05). Similarly, diamide significantly decreased intracellular ATP compared to saline during normoxia and hypoxia in both age groups (P < 0.05; Fig. 6B), but the mean change from normoxia to hypoxia with diamide vs. saline was not significantly different in young (0.24 ± 0.03 vs. 0.26 ± 0.04 mM/RBC, respectively) or older (0.17 ± 0.03 vs. 0.13 ± 0.05 mM/RBC, respectively) adults. Intracellular ATP was unaffected by age group or drug condition in the experiment with cilostazol (Fig. 6C).
Figure 6. Effect of donor age and pharmacology on red blood cell intracellular ATP in normoxia and hypoxia.
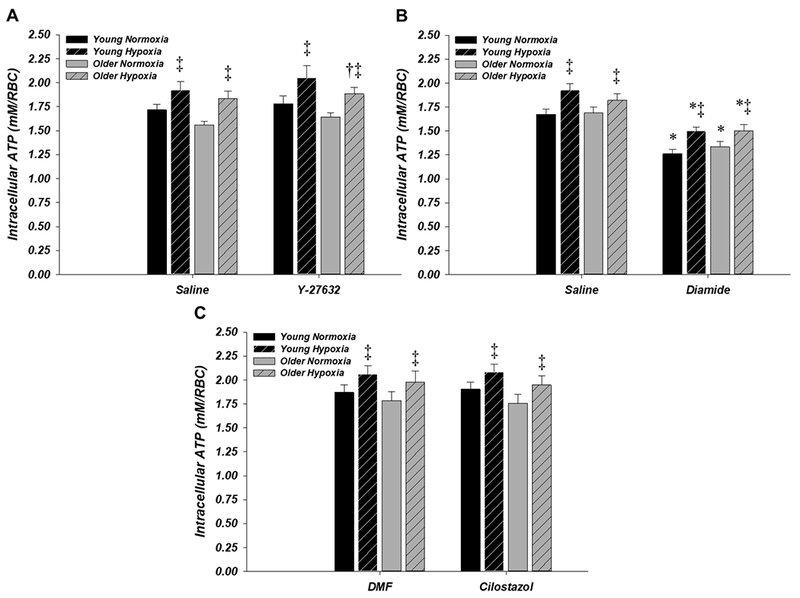
RBC intracellular ATP increased significantly during hypoxia in all age groups and drug conditions. A: intracellular ATP was significantly lower in older (n = 10) compared to young (n = 9) adults during hypoxia with Y-27632, although the mean change from normoxia to hypoxia was unaffected by age. B: diamide significantly decreased intracellular ATP vs. saline during normoxia and hypoxia in young (n = 11) and older (n = 10) adults. C: intracellular ATP in young (n = 10) and older (n = 12) adults was unaffected by age group or drug condition. *P < 0.05 vs. control (within age); ‡P < 0.05 vs. normoxia (within condition)
Discussion
The primary new findings from the present investigation are as follows. First, Rho-kinase inhibition completely reversed age-related declines in RBC deformability and in doing so, abolished the impairment in deoxygenation-induced ATP release from RBCs of older adults (Figs. 1 and 2). Second, decreasing RBC deformability in young adults impaired deoxygenation-induced ATP release to the same degree as occurs with advancing donor age (Figs. 1 and 3). Third, treatment of RBCs from older adults with the PDE3 inhibitor cilostazol did not improve the age-related impairment in deoxygenation-induced ATP release, which may be related to the lack of an impairment in RBC intracellular cAMP and ATP release responses to direct pharmacological Gi activation in older compared to younger adults (Figs. 4 and 5). Finally, and to the best of our knowledge, this is the first study to demonstrate that the age-related impairment in deoxygenation-induced ATP release was not due to changes in RBC metabolism (i.e., glycolysis), as intracellular ATP in normoxia and its increase during hypoxia were unaffected by donor age (Fig. 6). These collective findings provide the first insight into the underlying mechanisms of impaired deoxygenation-induced ATP release from RBCs of healthy older adults, indicating that the age-related reduction in RBC deformability is a key mechanism (Fig. 7).
Figure 7. Experimental targets and working hypothesis for the mechanism of impaired deoxygenation-induced from RBCs of older adults.
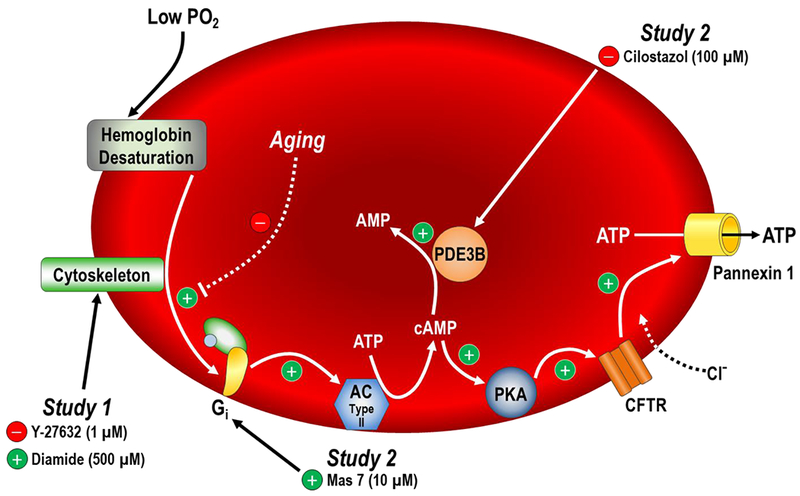
Pharmacological manipulations in Study 1 targeted the RBC cytoskeleton and in Study 2 targeted cAMP-related components of the proposed signalling cascade for deoxygenation-induced ATP release (adapted from Ellsworth & Sprague, 2012). Our findings indicate that reductions in RBC deformability with advancing donor age may limit activation of the signalling cascade downstream of haemoglobin desaturation, ultimately leading to impaired deoxygenation-induced RBC ATP release in older adults.
Mechanisms of impaired deoxygenation-induced ATP release from RBCs of older adults
The findings from Study 1 of the present study are the first experimental evidence that the age-related decline in RBC deformability is a primary mechanism of impaired deoxygenation-induced ATP release from RBCs of healthy older adult humans. In this context, we demonstrate that increasing deformability of RBCs from older adults and decreasing deformability of RBCs from young adults (Fig. 1) abolished the difference in deoxygenation-induced ATP release between the age groups (Figs. 2 and 3). This is aligned with earlier studies using RBCs from young donors, which demonstrated that acute, pharmacologically-induced increases or decreases in RBC deformability produce corresponding increases or decreases in deoxygenation-induced ATP release (Sridharan et al., 2010b; Thuet et al., 2011). Collectively, this suggests that these two properties of RBCs are linked. Although the precise pathway for RBC ATP release in response to deoxygenation and the mechanism(s) by which RBC deformability alters this process remain to be fully elucidated, the physiological events associated with haemoglobin’s conformational change from the oxygenated (oxyHb) to deoxygenated (deoxyHb) state provide valuable insight. Specifically, the reversible association of deoxyHb with band 3 in the RBC membrane, increased intracellular glycolysis, and Gi protein activation are all linked to this conformational change and have been shown to be required for deoxygenation-induced ATP release (Jagger et al., 2001; Olearczyk et al., 2004b; Chu et al., 2016). In addition, the association of deoxyHb with band 3 increases RBC deformability by displacing band 3 from the cytoskeletal protein ankyrin (Stefanovic et al., 2013; Chu et al., 2016; Zhou et al., 2019), which is closely associated with deoxygenation-induced increases in RBC capillary velocity (Zhou et al., 2019), and also facilitates an increase in glycolysis by displacing a complex of glycolytic enzymes from band 3 (Campanella et al., 2005, 2008; Chu & Low, 2006; Lewis et al., 2009; Puchulu-Campanella et al., 2013; Chu et al., 2016). Increases in intracellular ATP also produce fluctuations, or “flickering”, of the RBC membrane, (Park et al., 2010), which could activate mechanosensitive proteins such as Gi proteins or Piezo1 channels (Gudi et al., 1998; Cinar et al., 2015) and facilitate the subsequent release of ATP.
If the initial stimulus for RBC ATP release following haemoglobin deoxygenation is mechanical in nature, as the aforementioned evidence suggests, then the parallel effects of increasing or decreasing RBC deformability on deoxygenation-induced ATP release are likely due to respective increases or decreases in the activation of mechanosensitive pathways that facilitate RBC ATP release. Thus, we propose that impaired deoxygenation-induced RBC ATP release with advancing age is primarily due to reductions in RBC deformability limiting the mechanosensitive stimulation of ATP release rather than an impairment in other components of the signalling cascade (Fig. 7). The findings from Study 2 suggest that this may be the case given that treatment with cilostazol to target PDE3, which is multiple steps downstream of the initial haemoglobin deoxygenation stimulus in the proposed RBC signalling cascade for deoxygenation-induced ATP release (Ellsworth & Sprague, 2012), had no effect on impaired ATP release from RBCs of older adults during hypoxia (Fig. 4), although effective PDE3 inhibition was not confirmed as addressed in the “experimental considerations and limitations” section below. Further, no age-related impairments were observed in RBC intracellular cAMP responses or ATP release following direct pharmacological activation of Gi using a single concentration of Mas 7 (Fig. 5), providing the first experimental evidence that elements of the proposed intracellular signalling cascade downstream of deoxygenation may remain intact with age. Finally, RBCs from older adults retained the ability to increase glycolysis during hypoxia, which has been shown to be required for deoxygenation-induced ATP release despite the presence of a large intracellular pool of ATP (Jagger et al., 2001; Kirby et al., 2014; Chu et al., 2016).
Potential causes of decreased RBC deformability with advancing donor age
Advancing donor age is associated with multiple deleterious changes in RBC properties, including increased fragility (Detraglia et al., 1974; Bowdler et al., 1981), morphological changes (Bowdler et al., 1981), and decreases in membrane fluidity and deformability (Reid et al., 1976; Hegner et al., 1979; Gelmini et al., 1987, 1989). Among these, the age-associated decline in RBC antioxidant capacity (Glass & Gershon, 1984; Gershon & Gershon, 1988; Gil et al., 2006; Rizvi & Maurya, 2007; Chaleckis et al., 2016) is likely one of the most detrimental given that RBCs can generate substantial amounts of reactive oxygen/nitrogen species (Johnson et al., 2005; Cimen, 2008; Rifkind & Nagababu, 2013; Kuhn et al., 2017), which cause oxidative damage that has been clearly linked to decreased RBC deformability (Haest et al., 1977; Wang et al., 1999; Tsantes et al., 2006; Rifkind & Nagababu, 2013; Mohanty et al., 2014; Xiong et al., 2017). Further, this would likely be exacerbated given the increased susceptibility of RBCs from older adults to oxidative damage (Glass & Gershon, 1984; Gershon & Gershon, 1988; Gil et al., 2006; Rizvi & Maurya, 2007). Although studies testing the effects of increasing antioxidant capacity (exogenously or endogenously) on age-associated decrements in RBC properties are limited (Nelson et al., 2006; Cazzola et al., 2012; Xiong et al., 2017), reduced RBC deformability in aged rats can be improved following single or combined administration of vitamins C and E (Xiong et al., 2017).
Experimental considerations and limitations
In Study 1, incubation of RBCs with 500 μM diamide significantly decreased intracellular ATP in normoxia and hypoxia compared to the saline control condition (Fig. 6B). However, this effect of diamide was the same in RBCs from both age groups and it also did not affect the upregulation of glycolytic activity and increase in intracellular ATP in hypoxia (Fig. 6B), which is required for deoxygenation-induced ATP release (Jagger et al., 2001). In both age groups in the present study, diamide did not alter RBC characteristics such as haemoglobin concentration or oxygen saturation relative to saline in normoxia or hypoxia (Table 2). Further, other functional properties of RBCs including survival, osmotic fragility, density distribution, and haemoglobin polymerization have been shown to be unaffected by treatment with diamide at similar concentrations (Kosower et al., 1969; Schmid-Schönbein & Gaehtgens, 1981; Maeda et al., 1983). Diamide-mediated decreases in RBC deformability and deoxygenation-induced ATP release have also been shown to be abolished by subsequent incubation of RBCs with Y-27632 (Thuet et al., 2011). Thus, the decrease in deoxygenation-induced ATP release from RBCs following incubation with diamide in Study 1 is likely to be due primarily to the decrease in RBC deformability.
It has been suggested recently that RBC ATP release occurs primarily through haemolysis rather than a regulated export process (Sikora et al., 2014; Grygorczyk & Orlov, 2017). However, while haemolysis can certainly contribute to ATP release and is an important methodological challenge that must be controlled for in studies such as these (Keller et al., 2017), the collective experimental evidence does not support the hypothesis that haemolysis is a primary mechanism for ATP release from human RBCs (Kirby et al., 2015). Accordingly, there were no significant differences in haemolysis between age groups or drug treatments during normoxia or hypoxia in the present investigation, and no significant correlations between haemolysis and ATP release in young or older adults during any of the experimental conditions (adjusted r2 ranged from −0.042-0.056). Thus, RBC ATP release in the present study was primarily due to a regulated export process that was dependent on the oxygenation state of haemoglobin and influenced by donor age and pharmacological manipulations of deformability.
Cilostazol did not have an effect on deoxygenation-induced ATP release in Study 2 of the present investigation, and it cannot be ruled out that this was due to ineffective PDE3 inhibition as we were unable to measure intracellular cAMP in the presence of cilostazol due to unexpected technical issues. While this limitation prevents definitive conclusions regarding the contribution of cAMP-related signalling to impaired RBC ATP release with advancing age from being made, the 100μM concentration of cilostazol used in the present study has been shown to be an effective inhibitor of PDE3 in a variety of other isolated RBC studies (Hanson et al., 2010; Sprague et al., 2011; Leal Denis et al., 2013; Knebel et al., 2013). The intact cAMP and ATP release responses to pharmacological Gi activation of RBCs from older compared to young adults provides additional supporting evidence that intracellular signalling downstream of deoxygenation may not be impaired with age, but this finding is limited by the use of a single concentration of Mas 7 that resulted in an approximately two-fold greater increase in ATP release relative to that observed during hypoxia from RBCs of young adults. As such, it is possible that more subtle differences with age might be observed with a lower concentration of Mas 7, although other isolated RBC studies have demonstrated impaired ATP release responses to the same 10μM concentration of Mas 7 used in the present study (Sprague et al., 2006; Hanson et al., 2010).
An alternative explanation for the lack of effect of cilostazol on ATP release in Study 2 is that cAMP may not actually be involved in the signalling cascade for RBC ATP release, as suggested by Keller et al. (2017) based on their recent experimental findings. This proposal is based on the specific findings that incubation of RBCs with the active cAMP analogue 8Br-cAMP did not induce any ATP release independent of significant increases in RBC lysis and that treatment of RBCs with various compounds that increased intracellular cAMP had no effect of ATP release (Keller et al., 2017). These findings are in direct contrast to work performed by Sprague et al. (2001) demonstrating that incubation of RBCs with the active cAMP analogue Sp-cAMP stimulates ATP release, whereas pretreatment with Rp-cAMP, an inactive cAMP analogue and inhibitor of protein kinase A, blocks deformation-induced RBC ATP release. Studies demonstrating the efficacy of cilostazol for improving intracellular cAMP responses and ATP release from RBCs patients with type 2 diabetes or RBCs co-incubated with insulin also support a role for cAMP in the signalling cascade for ATP release (Hanson et al., 2010; Sprague et al., 2011; Dergunov et al., 2015). The reasons for the discrepant results with pharmacological stimuli are unclear, but this is an issue that warrants further investigation, perhaps using comparable physiological stimuli such as hypoxia or deformation in order to define more clearly the intracellular factors that regulate the release of ATP from RBCs.
Conclusions
ATP is a unique vasoactive molecule that can stimulate vasodilatation and blunt sympathetically-mediated vasoconstriction to help facilitate appropriate regulation of blood flow to active skeletal muscle. RBCs are a key source of circulating ATP, and the ability to release ATP in response to haemoglobin deoxygenation allows RBCs to both detect and initiate a vascular response to imbalances between local oxygen supply and demand. Deoxygenation-induced ATP release is impaired from RBCs of healthy older adults, and the present findings indicate that reduced RBC deformability with age is a primary mechanism. In contrast, these data suggest that healthy ageing may not be associated with changes in intracellular signalling downstream of Gi activation. The mechanistic contribution of impaired RBC ATP release to declines in vascular function and blood flow regulation in healthy older adults, and whether enhancing RBC ATP release to physiological stimuli in vivo can improve vascular function with age, remains to be determined.
Key Points.
Red blood cells (RBCs) release ATP in response to deoxygenation, which can increase blood flow to help match oxygen supply with tissue metabolic demand.
This release of ATP is impaired in RBCs from older adults, but the underlying mechanisms are unknown.
In this study, improving RBC deformability in older adults restored deoxygenation-induced ATP release, whereas decreasing RBC deformability in young adults reduced ATP release to that of older adults.
In contrast, treating RBCs with a phosphodiesterase 3 inhibitor did not affect ATP release in either age group, possibly due to intact intracellular signalling downstream of deoxygenation as indicated by preserved cAMP and ATP release responses to pharmacological Gi protein activation in RBCs from older adults.
These findings are the first to demonstrate that the age-related decrease in RBC deformability is a primary mechanism of impaired deoxygenation-induced ATP release, which may have implications for treating impaired vascular control with advancing age.
Acknowledgements
We thank the subjects who volunteered to participate, Dr. Randy Sprague for the generous donation of the St. George’s blood filtrometer and methodological guidance, and Brett Kirby, Jennifer Richards, Christopher Hearon Jr., Anne Crecelius, and Janée Terwoord for their assistance in conducting these studies.
Funding
This research was supported by the National Institutes of Health awards HL119337 (F.A.D., M.J.J.) and F31HL126377 (M.L.R., F.A.D.).
Abbreviations:
- AC
adenylyl cyclase
- ATP
adenosine triphosphate
- BMI
body mass index
- cAMP
cyclic AMP
- CWB
cell wash buffer
- deoxyHb
deoxygenated haemoglobin
- DMF
dimethylformamide
- FHHb
fraction of deoxygenated haemoglobin
- FO2Hb
fraction of oxygenated haemoglobin
- Gi
inhibitory G (proteins)
- Mas 7
mastoparan 7
- oxyHb
oxygenated haemoglobin
- PCO2
partial pressure of carbon dioxide
- PDE3
phosphodiesterase 3
- PO2
partial pressure of oxygen
- RBCs
red blood cells
- RCTT
red (blood) cell transit time
- tHb
total haemoglobin
Footnotes
Competing interests
None
References
- Adderley SP & Sprague RS (2010). Regulation of cAMP by phosphodiesterases in erythrocytes. Pharmacol Reports 62, 475–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergfeld G & Forrester T (1992). Release of ATP from human erythrocytes in response to a brief period of hypoxia and hypercapnia. Cardiovasc Res 26, 40–47. [DOI] [PubMed] [Google Scholar]
- Bowdler A, Dougherty R & Bowdler N (1981). Age as a factor affecting erythrocyte osmotic fragility in males. Gerontology 27, 224–231. [DOI] [PubMed] [Google Scholar]
- Brescia M & Zaccolo M (2016). Modulation of compartmentalised cyclic nucleotide signalling via local inhibition of phosphodiesterase activity. Int J Mol Sci 17, 1672–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campanella ME, Chu H & Low PS (2005). Assembly and regulation of a glycolytic enzyme complex on the human erythrocyte membrane. PNAS 102, 2402–2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campanella ME, Chu H, Wandersee NJ, Peters LL, Mohandas N, Gilligan DM & Low PS (2008). Characterization of glycolytic enzyme interactions with murine erythrocyte membranes in wild-type and membrane protein knockout mice. Blood 112, 3900–3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazzola R, Rondanelli M, Faliva M & Cestaro B (2012). Effects of DHA-phospholipids, melatonin and tryptophan supplementation on erythrocyte membrane physico-chemical properties in elderly patients suffering from mild cognitive impairment. Exp Gerontol 47, 974–978. [DOI] [PubMed] [Google Scholar]
- Chaleckis R, Murakami I, Takada J, Kondoh H & Yanagida M (2016). Individual variability in human blood metabolites identifies age-related differences. PNAS 113, 4252–4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu H & Low PS (2006). Mapping of glycolytic enzyme-binding sites on human erythrocyte band 3. Biochem J 400, 143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu H, McKenna MM, Krump NA, Zheng S, Mendelsohn L, Thein SL, Garrett LJ, Bodine DM & Low PS (2016). Reversible binding of hemoglobin to band 3 constitutes the molecular switch that mediates O2 regulation of erythrocyte properties. Blood 128, 2708–2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimen MYB (2008). Free radical metabolism in human erythrocytes. Clin Chim Acta 390, 1–11. [DOI] [PubMed] [Google Scholar]
- Cinar E, Zhou S, DeCourcey J, Wang Y, Waugh RE & Wan J (2015). Piezo1 regulates mechanotransductive release of ATP from human RBCs. PNAS 112, 11783–11788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp KM, Ellsworth ML, Sprague RS & Stephenson AH (2013). Simvastatin and GGTI-2133, a geranylgeranyl transferase inhibitor, increase erythrocyte deformability but reduce low O(2) tension-induced ATP release. Am J Physiol - Heart Circ Physiol 304, H660–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford PS & Hellsten Y (2004). Vasodilatory mechanisms in contracting skeletal muscle. J Appl Physiol 97, 393–403. [DOI] [PubMed] [Google Scholar]
- Collins DM, McCullough WT & Ellsworth ML (1998). Conducted vascular responses: communication across the capillary bed. Microvasc Res 56, 43–53. [DOI] [PubMed] [Google Scholar]
- Conti M & Beavo J (2007). Biochemistry and physiology of cyclic nucleotide phosphodiesterases: essential components in cyclic nucleotide signaling. Annu Rev Biochem 76, 481–511. [DOI] [PubMed] [Google Scholar]
- Crecelius AR, Kirby BS, Richards JC & Dinenno FA (2013). Mechanical effects of muscle contraction increase intravascular ATP draining quiescent and active skeletal muscle in humans. J Appl Physiol 114, 1085–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dergunov SA, Bowles EA, Gordon W, Green M, Bierman A, Ellsworth ML, Pinkhassik E & Sprague RS (2015). Liposomal delivery of a phosphodiesterase 3 inhibitor rescues low oxygen-induced ATP release from erythrocytes of humans with type 2 diabetes. Biochem Biophys Reports 2, 137–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detraglia M, Cook F, Stasiw D & Cerny L (1974). Erythrocyte fragility in aging. Biochim Biophys Acta 345, 213–219. [DOI] [PubMed] [Google Scholar]
- Dietrich HH, Ellsworth ML, Sprague RS & Dacey RG (2000). Red blood cell regulation of microvascular tone through adenosine triphosphate. Am J Physiol - Heart Circ Physiol 278, H1294–8. [DOI] [PubMed] [Google Scholar]
- Dora KA (2017). Conducted dilatation to ATP and K+ and in rat skeletal muscle arterioles. Acta Physiol 219, 202–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellsworth ML (2000). The red blood cell as an oxygen sensor: what is the evidence? Acta Physiol Scand 168, 551–559. [DOI] [PubMed] [Google Scholar]
- Ellsworth ML, Forrester T, Ellis CG & Dietrich HH (1995). The erythrocyte as a regulator of vascular tone. Am J Physiol 269, H2155–61. [DOI] [PubMed] [Google Scholar]
- Ellsworth ML & Sprague RS (2012). Regulation of blood flow distribution in skeletal muscle: role of erythrocyte-released ATP. J Physiol 590, 4985–4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faris A & Spence DM (2008). Measuring the simultaneous effects of hypoxia and deformation on ATP release from erythrocytes. Analyst 133, 678–682. [DOI] [PubMed] [Google Scholar]
- Gelmini G, Coiro V, Ferretti P, Baroni M & Delsignore R (1989). Evaluation of whole blood filterability with increasing age in healthy men and women. Haematologica 74, 15–18. [PubMed] [Google Scholar]
- Gelmini G, Delsignore R & Coiro V (1987). Evaluation of erythrocyte deformability in pre-menopausal and post-menopausal women. Maturitas 9, 275–281. [DOI] [PubMed] [Google Scholar]
- Gershon H & Gershon D (1988). Altered enzyme function and premature sequestration of erythrocytes in aged individuals. Blood Cells 14, 93–101. [PubMed] [Google Scholar]
- Gil L, Siems W, Mazurek B, Gross J, Schroeder P, Voss P & Grune T (2006). Age-associated analysis of oxidative stress parameters in human plasma and erythrocytes. Free Radic Res 40, 495–505. [DOI] [PubMed] [Google Scholar]
- Glass GA & Gershon D (1984). Decreased enzymic protection and increased sensitivity to oxidative damage in erythrocytes as a function of cell and donor aging. Biochem J 218, 531–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go AS et al. (2014). Heart disease and stroke statistics - 2014 update: a report from the American Heart Association. Circulation 129, e28–e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Alonso J, Olsen DB & Saltin B (2002). Erythrocyte and the regulation of human skeletal muscle blood flow and oxygen delivery: role of circulating ATP. Circ Res 91, 1046–1055. [DOI] [PubMed] [Google Scholar]
- Grygorczyk R & Orlov SN (2017). Effects of hypoxia on erythrocyte membrane properties - Implications for intravascular hemolysis and purinergic control of blood flow. Front Physiol 8, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudi S, Nolan J & Frangos J (1998). Modulation of GTPase activity of G proteins by fluid shear stress and phospholipid composition. PNAS 95, 2515–2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haest CW, Kamp D, Plasa G & Deuticke B (1977). Intra- and intermolecular cross-linking of membrane proteins in intact erythrocytes and ghosts by SH-oxidizing agents. Biochim Biophys Acta 469, 226–230. [DOI] [PubMed] [Google Scholar]
- Hanson MS, Ellsworth ML, Achilleus D, Stephenson AH, Bowles EA, Sridharan M, Adderley S & Sprague RS (2009). Insulin inhibits low oxygen-induced ATP release from human erythrocytes: implication for vascular control. Microcirculation 16, 424–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson MS, Stephenson AH, Bowles EA & Sprague RS (2010). Insulin inhibits human erythrocyte cAMP accumulation and ATP release: role of PDE3 and PI3K. Exp Biol Med 235, 256–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearon C Jr. & Dinenno F (2016). Regulation of skeletal muscle blood flow during exercise in ageing humans. J Physiol 594, 2261–2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearon CM Jr., Richards JC, Racine ML, Luckasen GJ, Larson DG, Joyner MJ & Dinenno FA (2017). Sympatholytic effect of intravascular ATP is independent of nitric oxide, prostaglandins, Na+/K+-ATPase and KIR channels in humans. J Physiol 15, 5175–5190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegner D, Platt D, Heckers H, Schloeder U & Breuninger V (1979). Age-dependent physiochemical and biochemical studies of human red cell membranes. Mech Ageing Dev 10, 117–130. [DOI] [PubMed] [Google Scholar]
- Jagger JE, Bateman RM, Ellsworth ML & Ellis CG (2001). Role of erythrocyte in regulating local O2 delivery mediated by hemoglobin oxygenation. Am J Physiol Heart Circ Physiol 280, H2833–9. [DOI] [PubMed] [Google Scholar]
- Jensen FB (2009). The dual roles of red blood cells in tissue oxygen delivery: oxygen carriers and regulators of local blood flow. J Exp Biol 212, 3387–3393. [DOI] [PubMed] [Google Scholar]
- Johnson RM, Goyette G, Ravindranath Y & Ho Y-S (2005). Hemoglobin autoxidation and regulation of endogenous H2O2 levels in erythrocytes. Free Radic Biol Med 39, 1407–1417. [DOI] [PubMed] [Google Scholar]
- Joyner MJ & Casey DP (2015). Regulation of increased blood flow (hyperemia) to muscles during exercise: a hierarchy of competing physiological needs. Physiol Rev 95, 549–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller AS, Diederich L, Panknin C, DeLalio LJ, Drake JC, Sherman R, Jackson EK, Yan Z, Kelm M, Cortese-Krott MM & Isakson BE (2017). Possible roles for ATP release from RBCs exclude the cAMP-mediated Panx1 pathway. Am J Physiol - Cell Physiol 313, C593–C603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby B, Schwarzbaum P, Lazarowski E, Dinenno F & McMahon T (2015). Liberation of ATP secondary to hemolysis is not mutually exclusive of regulated export. Blood 125, 1844–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby BS, Crecelius AR, Richards JC & Dinenno FA (2013). Sources of intravascular ATP during exercise in humans: critical role for skeletal muscle perfusion. Exp Physiol 98, 988–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby BS, Crecelius AR, Voyles WF & Dinenno FA (2010). Vasodilatory responsiveness to adenosine triphosphate in ageing humans. J Physiol 588, 4017–4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby BS, Crecelius AR, Voyles WF & Dinenno FA (2012). Impaired skeletal muscle blood flow control with advancing age in humans: attenuated ATP release and local vasodilation during erythrocyte deoxygenation. Circ Res 111, 220–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby BS, Hanna G, Hendargo HC & McMahon TJ (2014). Restoration of intracellular ATP production in banked red blood cells improves inducible ATP export and suppresses RBC-endothelial adhesion. Am J Physiol - Heart Circ Physiol 307, H1737–H1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby BS, Voyles WF, Carlson RE & Dinenno FA (2008). Graded sympatholytic effect of exogenous ATP on postjunctional alpha-adrenergic vasoconstriction in the human forearm: implications for vascular control in contracting muscle. J Physiol 586, 4305–4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knebel SM, Elrick MM, Bowles EA, Zdanovec AK, Stephenson AH, Ellsworth ML & Sprague RS (2013). Synergistic effects of prostacyclin analogs and phosphodiesterase inhibitors on cyclic adenosine 3’,5’ monophosphate accumulation and adenosine 3’5’ triphosphate release from human erythrocytes. Exp Biol Med 238, 1069–1074. [DOI] [PubMed] [Google Scholar]
- Kosower N, Kosower E & Wertheim B (1969). Diamide, a new reagent for the intracellular oxidation of glutathione to the disulfide. Biochem Biophys Res Commun 37, 1967–1970. [DOI] [PubMed] [Google Scholar]
- Kuhn V, Diederich L, Keller TCS, Kramer CM, Lückstädt W, Panknin C, Suvorava T, Isakson BE, Kelm M & Cortese-Krott MM (2017). Red blood cell function and dysfunction: redox regulation, nitric oxide metabolism, anemia. Antioxid Redox Signal 26, 718–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal Denis MF, Incicco JJ, Espelt MV, Verstraeten SV., Pignataro OP, Lazarowski ER & Schwarzbaum PJ (2013). Kinetics of extracellular ATP in mastoparan 7-activated human erythrocytes. Biochim Biophys Acta 1830, 4692–4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis IA, Campanella ME, Markley JL & Low PS (2009). Role of band 3 in regulating metabolic flux of red blood cells. PNAS 106, 18515–18520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomas O & Zaccolo M (2014). Phosphodiesterases maintain signaling fidelity via compartmentalization of cyclic nucleotides. Physiology 29, 141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda N, Kon K, Imaizumi K, Sekiya M & Shiga T (1983). Alteration of rheological properties of human erythrocytes by crosslinking of membrane proteins. Biochim Biophys Acta 735, 104–112. [DOI] [PubMed] [Google Scholar]
- Messana I, Orlando M, Cassiano L, Pennacchietti L, Zuppi C, Castagnola M & Giardina B (1996). Human erythrocyte metabolism is modulated by the O2-linked transition of hemoglobin. FEBS Lett 390, 25–28. [DOI] [PubMed] [Google Scholar]
- Mohanty JG, Nagababu E & Rifkind JM (2014). Red blood cell oxidative stress impairs oxygen delivery and induces red blood cell aging. Front Physiol 5, 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen S, Nyberg M, Winding K & Saltin B (2012). Lifelong physical activity preserves functional sympatholysis and purinergic signalling in the ageing human leg. J Physiol 590, 6227–6236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen S & Saltin B (2014). Regulation of the skeletal muscle blood flow in humans. Exp Physiol 12, 1552–1558. [DOI] [PubMed] [Google Scholar]
- Mortensen SP, Thaning P, Nyberg M, Saltin B & Hellsten Y (2011). Local release of ATP into the arterial inflow and venous drainage of human skeletal muscle: insight from ATP determination with the intravascular microdialysis technique. J Physiol 589, 1847–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozaffarian D et al. (2016). Heart disease and stroke statistics - 2016 update. Circulation 133, e38–e360. [DOI] [PubMed] [Google Scholar]
- Nelson SK, Bose SK, Grunwald GK, Myhill P & McCord JM (2006). The induction of human superoxide dismutase and catalase in vivo: a fundamentally new approach to antioxidant therapy. Free Radic Biol Med 40, 341–347. [DOI] [PubMed] [Google Scholar]
- Olearczyk JJ, Stephenson AH, Lonigro AJ & Sprague RS (2004a). Heterotrimeric G protein Gi is involved in a signal transduction pathway for ATP release from erythrocytes. Am J Physiol Heart Circ Physiol 286, H940–5. [DOI] [PubMed] [Google Scholar]
- Olearczyk JJ, Stephenson AH, Lonigro AJ & Sprague RS (2004b). NO inhibits signal transduction pathway for ATP release from erythrocytes via its action on heterotrimeric G protein Gi. Am J Physiol - Heart Circ Physiol 287, H748–54. [DOI] [PubMed] [Google Scholar]
- Park Y, Best CA, Auth T, Gov NS, Safran SA, Popescu G, Suresh S & Feld MS (2010). Metabolic remodeling of the human red blood cell membrane. PNAS 107, 1289–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puchulu-Campanella E, Chu H, Anstee DJ, Galan JA, Tao WA & Low PS (2013). Identification of the components of a glycolytic enzyme metabolon on the human red blood cell membrane. J Biol Chem 288, 848–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid H, Barnes A, Lock P, Dormandy A & Dormandy T (1976). A simple method for measuring erythrocyte deformability. J Clin Pathol 29, 855–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JP, Bowles EA, Gordon WR, Ellsworth ML, Stephenson AH & Sprague RS (2015). Mechanisms of C-peptide-mediated rescue of low O2-induced ATP release from erythrocytes of humans with type 2 diabetes. Am J Physiol Regul Integr Comp Physiol 308, R411–8. [DOI] [PubMed] [Google Scholar]
- Richards JP, Stephenson AH, Ellsworth ML & Sprague RS (2013). Synergistic effects of C-peptide and insulin on low O2-induced ATP release from human erythrocytes. Am J Physiol Regul Integr Comp Physiol 305, R1331–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JP, Yosten GLC, Kolar GR, Jones CW, Stephenson AH, Ellsworth ML & Sprague RS (2014). Low O2-induced ATP release from erythrocytes of humans with type 2 diabetes is restored by physiological ratios of C-peptide and insulin. Am J Physiol Regul Integr Comp Physiol 307, R862–8. [DOI] [PubMed] [Google Scholar]
- Rifkind JM & Nagababu E (2013). Hemoglobin redox reactions and red blood cell aging. Antioxid Redox Signal 18, 2274–2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizvi SI & Maurya PK (2007). Markers of oxidative stress in erythrocytes during aging in humans. Ann N Y Acad Sci 1100, 373–382. [DOI] [PubMed] [Google Scholar]
- Rosenmeier JB, Hansen J & González-Alonso J (2004). Circulating ATP-induced vasodilatation overrides sympathetic vasoconstrictor activity in human skeletal muscle. J Physiol 558, 351–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid-Schönbein H & Gaehtgens P (1981). What is red cell deformability? Scand J Clin Lab Investig 156, 13–26. [DOI] [PubMed] [Google Scholar]
- Sikora J, Orlov SN, Furuya K & Grygorczyk R (2014). Hemolysis is a primary ATP-release mechanism in human erythrocytes. Blood 124, 2150–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague R (1996). ATP: the red blood cell link to NO and local control of the pulmonary circulation. Am J Physiol 271, H2717–H2722. [DOI] [PubMed] [Google Scholar]
- Sprague R, Goldman D, Bowles E, Achilleus D, Stephenson A, Ellis C & Ellsworth M (2010). Divergent effects of low-O2 tension and iloprost on ATP release from erythrocytes of humans with type 2 diabetes: implications for O2 supply to skeletal muscle. Am J Physiol - Heart Circ Physiol 299, 566–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague RS, Bowles EA, Achilleus D, Stephenson AH, Ellis CG & Ellsworth ML (2011). A selective phosphodiesterase 3 inhibitor rescues low PO2-induced ATP release from erythrocytes of humans with type 2 diabetes: implication for vascular control. Am J Physiol - Heart Circ Physiol 301, 2466–2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague RS, Bowles EA, Stumpf M, Ricketts G, Freidman A, Hou W-H, Stephenson A & Lonigro A (2005). Rabbit erythrocytes possess adenylyl cyclase type II that is activated by the heterotrimeric G proteins Gs and Gi. Pharmacol Reports 57 Suppl, 222–228. [PubMed] [Google Scholar]
- Sprague RS, Ellsworth ML, Stephenson AH, Kleinhenz ME & Lonigro AJ (1998). Deformation-induced ATP release from red blood cells requires CFTR activity. Am J Physiol - Heart Circ Physiol 275, H1726–H1732. [DOI] [PubMed] [Google Scholar]
- Sprague RS, Ellsworth ML, Stephenson AH & Lonigro AJ (2001a). Participation of cAMP in a signal-transduction pathway relating erythrocyte deformation to ATP release. Am J Physiol Cell Physiol 281, C1158–64. [DOI] [PubMed] [Google Scholar]
- Sprague RS, Hanson MS, Achilleus D, Bowles EA, Stephenson AH, Sridharan M, Adderley S & Ellsworth ML (2009). Rabbit erythrocytes release ATP and dilate skeletal muscle arterioles in the presence of reduced oxygen tension. Pharmacol Reports 61, 183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague RS, Stephenson AH, Bowles EA, Stumpf MS & Lonigro AJ (2006). Reduced expression of G(i) in erythrocytes of humans with type 2 diabetes is associated with impairment of both cAMP generation and ATP release. Diabetes 55, 3588–3593. [DOI] [PubMed] [Google Scholar]
- Sprague RS, Stephenson AH, Ellsworth ML, Keller C & Lonigro AJ (2001b). Impaired release of ATP from red blood cells of humans with primary pulmonary hypertension. Exp Biol Med 226, 434–439. [DOI] [PubMed] [Google Scholar]
- Sridharan M, Adderley SP, Bowles EA, Egan TM, Stephenson AH, Ellsworth ML & Sprague RS (2010a). Pannexin 1 is the conduit for low oxygen tension-induced ATP release from human erythrocytes. Am J Physiol Heart Circ Physiol 299, H1146–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan M, Sprague RS, Adderley SP, Bowles EA, Ellsworth ML & Stephenson AH (2010b). Diamide decreases deformability of rabbit erythrocytes and attenuates low oxygen tension-induced ATP release. Exp Biol Med 235, 1142–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanovic M, Puchulu-Campanella E, Kodippili G & Low PS (2013). Oxygen regulates the band 3-ankyrin bridge in the human erythrocyte membrane. Biochem J 449, 143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subasinghe W & Spence DM (2008). Simultaneous determination of cell aging and ATP release from erythrocytes and its implications in type 2 diabetes. Anal Chim Acta 618, 227–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuet KM, Bowles EA, Ellsworth ML, Sprague RS & Stephenson AH (2011). The Rho kinase inhibitor Y-27632 increases erythrocyte deformability and low oxygen tension-induced ATP release. Am J Physiol - Heart Circ Physiol 301, H1891–H1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsantes AE, Bonovas S, Travlou A & Sitaras NM (2006). Redox imbalance, macrocytosis, and RBC homeostasis. Antioxid Redox Signal 8, 1205–1216. [DOI] [PubMed] [Google Scholar]
- Wang X, Wu Z, Song G, Wang H, Long M & Cai S (1999). Effects of oxidative damage of membrane protein thiol groups on erythrocyte membrane viscoelasticities. Clin Hemorheol Microcirc 21, 137–146. [PubMed] [Google Scholar]
- WHO (1993). Aging and working capacity. World Health Organ Tech Rep Ser 835, 1–56. [PubMed] [Google Scholar]
- Winter P & Dora KA (2007). Spreading dilatation to luminal perfusion of ATP and UTP in rat isolated small mesenteric arteries. J Physiol 582, 335–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Xiong Y, Zhou S, Sun Y, Zhao Y, Ren X, Zhang Y & Zhang N (2017). Vitamin C and E supplements enhance the antioxidant capacity of erythrocytes obtained from aged rats. Rejuvenation Res 20, 85–92. [DOI] [PubMed] [Google Scholar]
- Zhou S, Giannetto M, DeCourcey J, Kang H, Kang N, Li Y, Zheng S, Zhao H, Simmons WR, Wei HS, Bodine DM, Low PS, Nedergaard M & Wan J (2019). Oxygen tension-mediated erythrocyte membrane interactions regulate cerebral capillary hyperemia. Sci Adv 5, eaaw4466. [DOI] [PMC free article] [PubMed] [Google Scholar]


