Abstract
Background:
Surveillance recommendations for patients with low-risk non-muscle-invasive bladder cancer (NMIBC) are based on limited evidence. Our objective was to add to the evidence by assessing outcomes after frequent versus recommended cystoscopic surveillance.
Methods:
This is a retrospective cohort study of patients diagnosed with low-risk (low grade Ta) NMIBC from 2005 – 2011 with follow-up through 2014 from the Department of Veterans Affairs. Patients were classified as having undergone frequent versus recommended cystoscopic surveillance (>3 versus 1–3 cystoscopies in the first 2 years after diagnosis). Using propensity score adjusted models, we estimated the impact of frequent cystoscopy on the number of transurethral resections, the number of resections without cancer in the specimen, and on risk of progression to muscle-invasive cancer or bladder cancer death.
Results:
Among 1,042 patients, 798 (77%) had more frequent cystoscopy than recommended. In adjusted analyses, the frequent cystoscopy group had twice as many transurethral resections (55 versus 26 per 100 person years; p<0.001) and more than 3 times as many resections without cancer in the specimen (5.7 versus 1.6 per 100 person years; p<0.001). Frequent cystoscopy was not associated with time to progression or bladder cancer death (3% at 5.0 years in both groups; p=0.990).
Conclusions:
Frequent cystoscopy among patients with low-risk NMIBC was associated with twice as many transurethral resections and did not decrease the risk for bladder cancer progression or death, supporting current guidelines.
Precis:
Frequent cystoscopic surveillance among patients with low-risk non-muscle-invasive bladder cancer was associated with twice as many transurethral resections and did not decrease the risk for bladder cancer progression or death. This supports current guideline recommendations for less frequent surveillance.
Keywords: bladder cancer, cancer surveillance, cancer progression, cancer mortality, cystoscopy
Introduction
Bladder cancer is a very common cancer, ranking fourth in prevalence in Europe and sixth in Northern America.1 Three quarters of patients are diagnosed with early stage non-muscle-invasive bladder cancer (NMIBC).2 These patients are typically treated by transurethral resection, followed by periodic cystoscopic surveillance to evaluate their bladder for cancer recurrence. Since 2005, multiple international guidelines and panels have recommended low-intensity surveillance for patients at low risk for progression of disease, specifically those diagnosed with low-grade Ta urothelial carcinoma.3 After initial diagnosis and treatment, a surveillance cystoscopy is recommended at 3 months, 12 months, and annually thereafter.3
However, these recommendations are based on scant evidence. For example, the joint American Urological Association / Society of Urologic Oncology guideline from 2016 states that “the data comparing different surveillance regimens for NMIBC are very limited”4. Recommendations are based on a single randomized trial and several observational studies.5–9 The randomized trial included 97 patients with low-risk NMIBC which were assigned to more frequent (every 3 months for the first 2 years) versus less frequent surveillance (every six months for one year, then annually). This study found no significant difference in the risk of recurrence (62% versus 50%, p=0.32) or progression (7% versus 2%, p=0.49),5 but it was substantially underpowered including only 97 patients. The observational studies did not specifically compare more versus less frequent cystoscopic surveillance, but rather indicated that about a quarter of all patients with low-risk NMIBC may experience a recurrence at 5 years and that cancer progression is rare (<15% at 5 years).6–9
Therefore, we set out to add to the evidence-base on surveillance of low-risk NMIBC. Using real-world data from the Department of Veterans Affairs (VA), we assessed associations between surveillance frequency (guideline-recommended versus more frequent surveillance) and outcomes. Our main outcomes were the number of transurethral resections performed, the number of transurethral resections without cancer in the specimen, risk of progression to muscle-invasive disease, and risk of bladder cancer death.
Patients and Methods
Overview
This was a retrospective cohort study of patients newly diagnosed with low-risk NMIBC within the VA. We followed patients during the first two years after diagnosis to assess the frequency with which they underwent cystoscopic surveillance. Finally, we evaluated the association of frequency of cystoscopic surveillance – dichotomized into recommended surveillance versus frequent surveillance – with outcomes, including number of transurethral resections, number of resections without cancer in the specimen, progression to muscle-invasive disease, and bladder cancer death.
Identification of a cohort of patients newly diagnosed with low-risk NMIBC
As previously described,10 we used validated algorithms to identify 1,279 patients newly diagnosed with low-risk (low grade Ta) urothelial carcinoma of the bladder between 2005 and 2011 from national VA data (see supplementary methods). We then excluded 99 because of missing covariates (see supplementary methods) and 138 because they died or left VA care during the first 2 years after diagnosis, during which we assessed the frequency of cystoscopic surveillance. This left 1,042 patients for analysis.
Assessing the frequency of cystoscopic surveillance – the exposure
As previously described,10 we used procedure codes (supplementary Table 1) to enumerate the frequency of cystoscopic surveillance during the first 2 years after diagnosis.11,12 The time period during which we assessed cystoscopic surveillance (the surveillance window) started with the diagnosis date (ascertained using validated algorithms11) and ended 2 years after diagnosis, or at the time of cystectomy, radiotherapy, or cancer recurrence, whichever occurred first. We enumerated cystoscopic surveillance procedures only until patients had a cancer recurrence, because a recurrence increases risk for further recurrences. Thus, patients who experience a recurrence are no longer deemed low-risk and warrant more frequent surveillance.13 Recurrences were ascertained from full-text pathology reports using validated natural language processing algorithms.14 Next, we categorized patients into those that received recommended surveillance versus those that received frequent surveillance, based on current consensus guideline recommendations3 and the length of the surveillance window (Figure 1). The surveillance window was categorized into intervals of up to 10.5 months, 10.5 to up to 22.5 months, and over 22.5 months. The rationale for these time intervals was that no more than 1, 2, or 3 surveillance cystoscopies are recommended for patients who are followed for up to 12 months, for 12 months to up to 24 months, or for 24 months, respectively. We then allotted a 1.5-month grace period to allow for surveillance cystoscopies that were done slightly earlier than the 12- and 24-month time points, thus leading to time intervals of up to 10.5 months, 10.5 to up to 22.5 months, and over 22.5 months (Figure 1).
Figure 1. Categorizing patients into those that received recommended versus frequent surveillance.
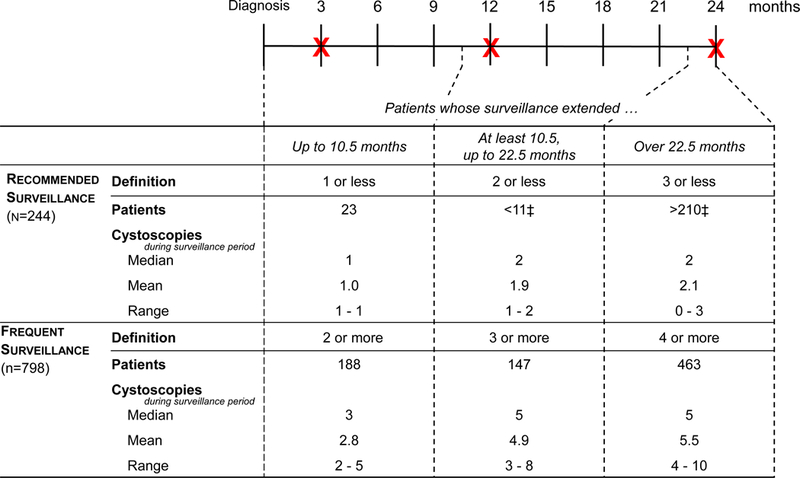
Patients were categorized based on consensus guideline recommendations3 and length of the surveillance window. At the top of the figure, the timeline of the surveillance window is depicted in months. X denotes the recommended time of cystoscopy. We allotted a 1.5-month grace period to allow for surveillance cystoscopies that were done slightly earlier than the 12- and 24-month time points. The lower half of the Figure depicts the number of patients that were categorized into recommended versus frequent surveillance, overall and stratified by the length of the surveillance window, along with the number of cystoscopies they underwent.
‡ Exact numbers not reported to protect confidentiality.
Measuring outcomes
We assessed the number of transurethral resections, number of resections without cancer in the specimen, progression to muscle-invasive disease, and bladder cancer death. We calculated the number of transurethral resections each patient underwent during the follow-up period (including cystoscopy with biopsy; referred to as transurethral resections hereafter) using Current Procedural Terminology (52204, 52224, 52234, 52235, 52240) and International Classification of Diseases, Ninth Revision, procedural codes (57.33, 57.49). The follow-up period started with the date of diagnosis and ended with the date of cystectomy, radiotherapy, cancer recurrence, death, or last contact with the VA system, or at the end of study (12/31/2014), whichever came first. We only counted resections that were at least 30 days apart to avoid over-counting of re-resections and of resections double-coded in different subfiles.
We a priori specified to examine the number of transurethral resections without cancer in the specimen, as we hypothesized that more frequent surveillance may lead to more incidental findings and subsequent resections without cancer in the specimen. For this, we used validated natural language processing algorithms14 to enumerate the number of transurethral resections without cancer in the specimen for each patient during the follow-up period defined above (see also supplementary methods).
We ascertained progression to muscle-invasive disease using validated natural language processing algorithms14 followed by chart review of full-text pathology reports (see supplementary methods). To determine bladder cancer death and death from any cause, we obtained the date of death for each patient from the VA Corporate Data Warehouse Vital Status file. Cause of death data was retrieved from the National Death Index.15 Bladder cancer death was defined based on International Classification of Diseases, Tenth Revision, codes from the cause of death field within the National Death Index (C67.x, C68.8, D09.0, D09.10, D09.19, D41.4, D41.8, D41.9, D49.4).
Statistical analyses
Data management was performed from December 2015 through April 2018. Data analyses were performed from April 2018 to February 2019. We compared patient characteristics between patients undergoing frequent versus recommended surveillance with the Chi-square and Wilcoxon tests for categorical and continuous variables, respectively. We then performed propensity score adjusted analyses to assess differences in outcomes between patients undergoing frequent versus recommended surveillance. We calculated the propensity score for each patient to undergo frequent surveillance using logistic regression and all covariates listed in Table 1 (see supplementary methods for details on covariates).16–18 We then adjusted all subsequent analyses using this propensity score as a covariate.
Table 1.
Baseline characteristics of the 1,042 low-risk patients included, stratified by recommended (3 or less cystoscopies in the first 2 years) versus frequent cystoscopic surveillance (> cystoscopies in the first 2 years).
| Cystoscopic Surveillance Frequency | |||
|---|---|---|---|
| Characteristic | Frequent (n=798) |
Recommended (n=244) |
p-value † |
| Age (median, range) | 75 (66–95) | 76 (66–93) | 0.42 |
| Age ≥80 | 233 (29%) | 78 (32%) | 0.41 |
| Male Sex | 788 (99%) | >241 (99%) | 0.98 |
| Race | 0.38 | ||
| White‡ | >689 (>86%) | >201 (>83%) | |
| Black | 50 (6%) | 17 (7%) | |
| Asian‡ | <11 (<2%) | <11 (<5%) | |
| Hispanic‡ | 12 (2%) | <11 (<5%) | |
| Native American‡ | <11 (<2%) | <11 (<5%) | |
| Unknown | 34(4%) | 15 (6%) | |
| Urban (v. Rural) Residence | 444 (56%) | 141 (58%) | 0.55 |
| Proportion living in ZIP code with ≥25% college graduates | 298 (37%) | 99 (41%) | 0.36 |
| Comorbidity16 | 0.05 | ||
| 0 | 115 (14%) | 46 (19%) | |
| 1 | 199 (25%) | 47 (19%) | |
| 2 | 206 (26%) | 53 (22%) | |
| ≥3 | 278 (35%) | 98 (40%) | |
| Nosos-p score § (median, range)17, 18 | 1.6 (0.4–7.2) | 1.5 (0.4–7.1) | 0.56 |
| Year of diagnosis | 0.14 | ||
| 2005‡ | 15 (2%) | <11 (<5%) | |
| 2006 | 95 (12%) | 16 (7%) | |
| 2007 | 111 (14%) | 31 (13%) | |
| 2008 | 134 (17%) | 40 (16%) | |
| 2009 | 145 (18%) | 46 (19%) | |
| 2010 | 145 (18%) | 60 (25%) | |
| 2011‡ | 153 (19%) | >40 (>16%) | |
From Chi-square test for categorical and Wilcoxon test for continuous variables.
Exact numbers not reported to protect confidentiality.
The Nosos-p score is a risk-adjustment score based on diagnosis codes, biographic information (including gender, date of birth, insurance coverage, race, marital status, VA priority (priority 1–9), and inclusion in a VA registry), drug prescription data and utilization costs. The “-p” indicates it is a prospective score, using data from one fiscal year to predict future health care utilization in the next fiscal year. For example, a Nosos-p score of 1.5 denotes that a patient has an expected cost in the next fiscal year that is 1.5 times higher than the average VA patient.17, 18
We used Poisson regression models offset by the natural log of the follow-up period to assess the association of surveillance frequency with number of transurethral resections and with number of transurethral resections without cancer in the specimen (over-dispersion was addressed with the deviance method19). We used Fine-and-Gray competing risks regression20 to estimate the association of surveillance frequency with progression to muscle-invasive disease or death from bladder cancer (for details on these models see supplementary methods).
To illustrate the magnitude of effects, we back calculated the number of resections (overall and without cancer in the specimen) per 100 person-years, the risk of progression to muscle-invasive disease or death, and the risk of bladder cancer death from these adjusted models. We used an alpha level of less than 0.05 to indicate statistical significance. The study was approved by the Veteran’s Institutional Review Board of Northern New England (#897920) and the University of Utah Institutional Review Board (#00079402). All analyses were performed using SAS Enterprise Guide 7.15.
Sensitivity analyses
Recognizing that some patients in our cohort may have had intermediate risk disease based on factors that could not be extracted using our validated natural language processing algorithms (e.g., multifocality, tumor size >3cm), we performed sensitivity analyses assuming all patients had intermediate risk disease. For these analyses, we categorized patients as having undergone recommended surveillance if they received no more than five surveillance cystoscopy procedures in the first two years after diagnosis.21 We then recalculated the propensity score using this alternative definition as well as all adjusted models described above.
Results
Frequent versus recommended surveillance
Among the 1,042 patients with low-risk NMIBC, 798 (77%) underwent frequent surveillance and 244 (23%) underwent recommended surveillance. There were no significant differences in baseline characteristics (Table 1). Figure 1 describes the number of cystoscopy procedures for patients who underwent recommended versus frequent surveillance according to the length of the surveillance window. The surveillance window was up to 10.5 months among 23 of the 244 patients (9.4%) who underwent recommended surveillance compared to 188 of the 798 patients (23.6%) who underwent frequent surveillance (p<0.001, Figure 1). The surveillance window tended to be shorter among patients who underwent frequent surveillance, because a higher proportion of them experienced a recurrence within the first 2 years (42.0% versus 12.3%; 335 of 798 versus 30 of 244 patients, p<0.001). Recurrences are associated with an increased risk for further recurrences and were thus reason for ending their surveillance window earlier.
Associations between surveillance frequency and outcomes
In propensity score adjusted analyses, patients who underwent frequent versus recommended surveillance had twice as many transurethral resections (55 [95% Confidence Interval (CI) 52–60] versus 26 [95% CI 22–30] per 100 person years; p<0.001) and more than 3 times as many resections without cancer in the specimen (5.7 [95% CI 5.0–6.5] versus 1.6 [95% CI 1.1–2.3] per 100 person years; p<0.001; Figure 2). Progression to muscle-invasive disease was rare with only 15 of the 1,042 patients progressing (1.4%) during a median follow-up time of 4.8 years. Frequent cystoscopy was not associated with time to progression or death in adjusted competing risks analyses (3% at 5.0 years for both groups; p=0.990, HR 1.01 [95% CI 0.46–2.22], Figure 3A). Bladder cancer death occurred infrequently and was not associated with frequent versus recommended cystoscopy (3% at 5.4 years versus 3% at 5.1 years, p=0.817, HR 0.90 [95% CI 0.39–2.13], Figure 3B).
Figure 2. Number of transurethral resections and of transurethral resections without cancer in the specimen among patients who underwent frequent versus recommended surveillance.
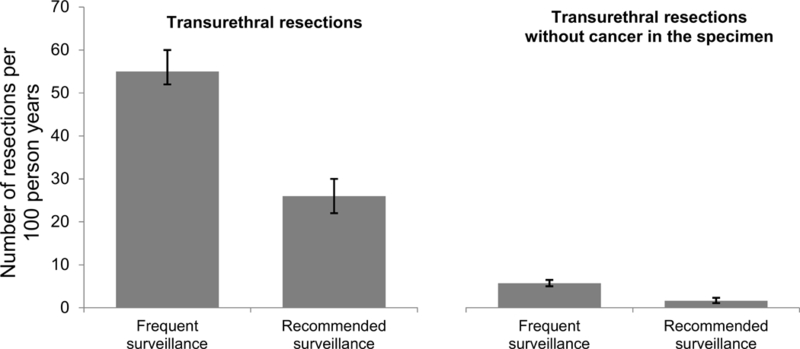
Patients who underwent frequent versus recommended surveillance underwent twice as many transurethral resections and more than 3 times as many transurethral resections without cancer in the specimen (p<0.001 for both).
Figure 3. Cumulative incidence plots showing the probability of progression to muscle-invasive bladder cancer (MIBC) or bladder cancer death (panel A) and of bladder cancer death (panel B).
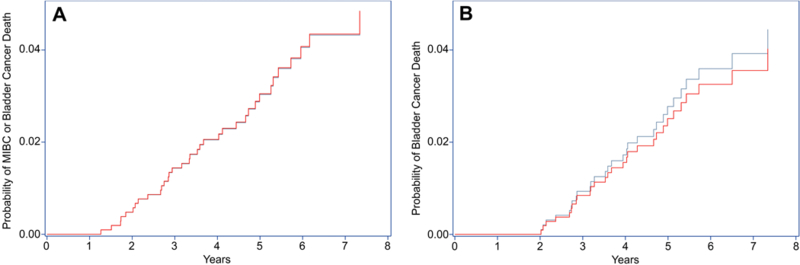
Data are from propensity score adjusted Fine and Gray competing risk models with death from other causes modeled as a competing risk. Median length of follow-up was 4.8 years.
Blue line: recommended surveillance; red line: frequent surveillance.
Note: In panel A, blue and red lines almost completely overlap.
Sensitivity analyses
In sensitivity analyses assuming all patients had intermediate risk disease, 469 patients (45.0%) underwent frequent surveillance and 573 patients (55.0%) underwent recommended surveillance. Patients who underwent frequent versus recommended surveillance had again more than twice as many transurethral resections (74 [95% CI 68–81] versus 32 [95% CI 29–35] per 100 person years; p<0.001) and approximately 3 times as many resections without cancer in the specimen (7.7 [95% CI 6.6–9.0] versus 2.8 [95% CI 2.3–3.4] per 100 person years; p<0.001). There was a shorter time to progression to muscle-invasive disease or death among those with frequent versus recommended surveillance (3% at 4.0 years versus 3% at 6.2 years; p=0.03, HR 2.13 [95% CI 1.06–4.27], Supplementary Figure 1A). There was no statistically significant difference in bladder cancer death (3% at 4.7 years versus 3% at 7.3 years, p=0.07, HR 2.01 [95% CI 0.95–4.25], Supplementary Figure 1B).
Discussion
We found that patients with low-risk NMIBC who underwent frequent surveillance had twice as many transurethral resections as those who underwent recommended surveillance (55 versus 26 per 100 patients followed for one year) and more than three times as many resections without cancer in the specimen (5.7 versus 1.6 per 100 patients followed for one year). Thus, frequent surveillance exposes patients to more resections and the associated additional peri-operative risks.22–24 There were no apparent benefits of frequent cystoscopy: It was not associated with a longer time to progression to muscle-invasive disease or bladder cancer death.
This is the first study to quantify downstream consequences associated with frequent surveillance cystoscopy among patients with low-risk NMIBC. These patients underwent substantially more surveillance cystoscopy procedures over the surveillance period (Figure 1) – procedures that are associated with additional anxiety, discomfort, and opportunity cost, such as lost wages for travel time and time spent in the clinic.25,26 Frequent cystoscopic surveillance also substantially increases healthcare costs with only relatively small gains in quality adjusted life years based on a recent cost-effectiveness analysis.27 In our current study, frequent cystoscopic surveillance was associated with double the number of transurethral resections. More frequent cystoscopic surveillance likely also led to more frequent subtle lesions detected in the bladder, which then led to additional transurethral resections without cancer in the specimen. These additional resections subject patients to peri-operative risks, including a peri-operative complication rate of 4%22 to 8%23 – most commonly urinary tract infections and hematuria22,24 – and an up to 3% 90-day peri-operative mortality rate.22
While current guidelines advise against frequent surveillance, some may argue that frequent surveillance could have benefits in terms of cancer control. Earlier detection of recurrence may lead to earlier treatment and thus may prevent further adverse cancer outcomes such as progression to muscle-invasive disease or bladder cancer death. However, in our cohort, progression to muscle-invasive disease or bladder cancer death was rare (3% at 5.0 years, Figure 3A), a finding that is consistent with the <5% progression rate reported by others.8,9 Further, frequent versus recommended surveillance was not associated with progression to muscle-invasive disease or bladder cancer death in our main analyses. As such, our findings support current consensus guideline recommendations for infrequent surveillance,3 and even less frequent surveillance (e.g., stopping if a patient has remained recurrence-free at one year as recommended by the United Kingdom’s National Institute for Health and Care Excellence28) might further reduce burden on patients.
Our study has several limitations. First, our data are from the VA and may not be generalizable to a non-Veteran population. However, the majority of incident bladder cancer cases occur among men older than 65 years,29 and our Veteran population represents this important segment of the population. Second, we only had a moderate length of follow-up for progression and bladder cancer death (median 4.8 years), limiting our power to detect any differences. However, these outcomes were quite rare and there was no clinically meaningful separation of the cumulative incidence curves (Figure 3). Thus, it is unlikely that a clinically meaningful difference would emerge even with longer follow-up. Third, while we accounted for observed confounding in propensity score adjusted analyses, our data are subject to unobserved confounding. While all patients had low-grade non-invasive urothelial carcinoma, the pathology reports used for risk-classification did not allow us to assess factors that may put patients at intermediate risk for recurrence, e.g. multifocality or large tumor size. Thus, we performed sensitivity analyses assuming an extreme case in which every patient had intermediate risk disease. While most results remained unchanged in the sensitivity analyses, there was one notable difference: Frequent versus recommended surveillance was associated with a shorter time to muscle-invasive bladder cancer or death, likely due to unobserved confounding. For example, those undergoing frequent surveillance may on average have had larger or more multifocal tumors putting them at a higher risk of progression to muscle-invasive disease and bladder cancer death. However, their absolute risk for bladder cancer progression or death remained quite low at <5% at 5 years (Supplementary Figure 1A). To address unobserved differences, we attempted to perform instrumental variable analyses,30 but were unable to find a suitable instrument.
In spite of these limitations, our study has important strengths. It is the first population-based study that could assess outcomes relevant to both patients and physicians. We had full-text pathology reports available and thus were able to ascertain resections without cancer in the specimen as well as progression to muscle-invasive disease. Thus, we were able to overcome limitations of other commonly used databases, such as the Surveillance Epidemiology and End Results-Medicare database and the National Cancer Database, both of which do not include pathologic details of resections performed for suspected cancer recurrence.31,32
Conclusions
Our findings demonstrate that three out of four patients with low-risk NMIBC underwent too many surveillance cystoscopy procedures, without any apparent benefit in terms of cancer control. These procedures are not harmless; there are risks associated with frequent surveillance, as patients undergoing frequent surveillance had twice as many transurethral resections. Our results support current guideline recommendations for the surveillance of low-risk NMIBC and add to the currently limited evidence-base supporting these recommendations.
Supplementary Material
Acknowledegements:
This study was supported using resources and facilities at the White River Junction Department of Veterans Affairs (VA) Medical Center, the VA Salt Lake City Health Care System, and the VA Informatics and Computing Infrastructure (VINCI), VA HSR RES 13–457. Support for VA and Centers for Medicare & Medicaid Services data is provided with support from the VA Information Resource Center, Project Numbers SDR 02–237 and 98–004. We acknowledge programming assistance by Steven J. Oostema, MS, and Ben Viernes, MPH.
Funding: FRS is supported by a Conquer Cancer Foundation Career Development Award and by the Dow-Crichlow Award of the Department of Surgery at the Dartmouth-Hitchcock Medical Center. PPG is supported by the Department of Veterans Affairs Health Services Research & Development (IIR 15–085, 1I01HX001880–01A2). The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Disclaimer: Opinions expressed in this manuscript are those of the authors and do not constitute official positions of the U.S. Federal Government or the Department of Veterans Affairs.
Conflicts of Interest: none
References:
- 1.International Agency for Research on Cancer. GLOBOCAN 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012. http://globocan.iarc.fr/Pages/pie_pop_prev_sel.aspx. Published July 5, 2018. Accessed July 5, 2018.
- 2.Snyder C, Harlan L, Knopf K, Potosky A, Kaplan R. Patterns of care for the treatment of bladder cancer. J Urol. 2003;169(5):1697–1701. doi: 10.1097/01.ju.0000056727.30546.b7 [DOI] [PubMed] [Google Scholar]
- 3.Schroeck FR, Smith N, Shelton JB. Implementing risk-aligned bladder cancer surveillance care. Urol Oncol Semin Orig Investig. 2018;36(5):257–264. doi: 10.1016/j.urolonc.2017.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang SS, Boorjian SA, Chou R, et al. Non-muscle invasive bladder cancer: American Urological Association / SUO guideline. https://www.auanet.org/education/guidelines/non-muscle-invasive-bladder-cancer.cfm. Published May 2016. Accessed May 3, 2016.
- 5.Olsen LH, Genster HG. Prolonging follow-up intervals for non-invasive bladder tumors: a randomized controlled trial. Scand J Urol Nephrol Suppl. 1995;172:33–36. [PubMed] [Google Scholar]
- 6.Leblanc B, Duclos AJ, Bénard F, et al. Long-term followup of initial Ta grade 1 transitional cell carcinoma of the bladder. J Urol. 1999;162(6):1946–1950. [DOI] [PubMed] [Google Scholar]
- 7.Mariappan P, Smith G. A surveillance schedule for G1Ta bladder cancer allowing efficient use of check cystoscopy and safe discharge at 5 years based on a 25-year prospective database. J Urol. 2005;173(4):1108–1111. doi: 10.1097/01.ju.0000149163.08521.69 [DOI] [PubMed] [Google Scholar]
- 8.Rieken M, Shariat SF, Kluth L, et al. Comparison of the EORTC tables and the EAU categories for risk stratification of patients with nonmuscle-invasive bladder cancer. Urol Oncol Semin Orig Investig. 2018;36(1):8.e17–8.e24. doi: 10.1016/j.urolonc.2017.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cambier S, Sylvester RJ, Collette L, et al. EORTC Nomograms and Risk Groups for Predicting Recurrence, Progression, and Disease-specific and Overall Survival in Non–Muscle-invasive Stage Ta–T1 Urothelial Bladder Cancer Patients Treated with 1–3 Years of Maintenance Bacillus Calmette-Guérin. Eur Urol. 2016;69(1):60–69. doi: 10.1016/j.eururo.2015.06.045 [DOI] [PubMed] [Google Scholar]
- 10.Schroeck FR, Lynch KE, Chang J won, et al. Extent of Risk-Aligned Surveillance for Cancer Recurrence Among Patients With Early-Stage Bladder Cancer. JAMA Netw Open. 2018;1(5):e183442. doi: 10.1001/jamanetworkopen.2018.3442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schroeck FR, Sirovich BE, Seigne JD, Robertson DJ, Goodney PP. Assembling and validating data from multiple sources to study care for Veterans with bladder cancer. BMC Urol. 2017;17(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hollenbeck BK, Ye Z, Dunn RL, Montie JE, Birkmeyer JD. Provider treatment intensity and outcomes for patients with early-stage bladder cancer. J Natl Cancer Inst. 2009;101(8):571–580. doi: 10.1093/jnci/djp039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Babjuk M, Boehle A, Burger M, et al. European Association of Urology (EAU) guidelines on non-muscle-invasive bladder cancer: update 2016. Eur Urol. 2017;71(3):447–461. [DOI] [PubMed] [Google Scholar]
- 14.Schroeck FR, Patterson OV, Alba P, et al. Development of a Natural Language Processing engine to generate bladder cancer pathology data for health services research. Urology. 2017;110:84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Center of Excellence for Suicide Prevention. Joint Department of Veterans Affairs (VA) and Department of Defense (DoD) Suicide Data Repository – National Death Index (NDI). 2017. http://vaww.virec.research.va.gov/Mortality/Overview.htm. Accessed August 30, 2017.
- 16.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. [DOI] [PubMed] [Google Scholar]
- 17.Wagner TH, Upadhyay A, Cowgill E, et al. Risk Adjustment Tools for Learning Health Systems: A Comparison of DxCG and CMS-HCC V21. Health Serv Res. 2016;51(5):2002–2019. doi: 10.1111/1475-6773.12454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Health Economics Resource Center. Risk Adjustment. https://www.herc.research.va.gov/include/page.asp?id=risk-adjustment. Published 2018. Accessed October 1, 2018.
- 19.Pedan A Analysis of Count Data Using the SAS® System. Proc Twenty-Sixth Annu SAS Users Group Int Conf 2001;Paper 247–26. http://www2.sas.com/proceedings/sugi26/p247-26.pdf. Accessed October 18, 2018. [Google Scholar]
- 20.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. J Am Stat Assoc. 1999;94(446):496–509. doi: 10.2307/2670170 [DOI] [Google Scholar]
- 21.The National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: bladder cancer Version 4. https://www.nccn.org/professionals/physician_gls/pdf/bladder.pdf.Published 2018. Accessed July 2, 2018.
- 22.Hollenbeck BK, Miller DC, Taub D, et al. Risk factors for adverse outcomes after transurethral resection of bladder tumors. Cancer. 2006;106(7):1527–1535. doi: 10.1002/cncr.21765 [DOI] [PubMed] [Google Scholar]
- 23.Gregg JR, McCormick B, Wang L, et al. Short term complications from transurethral resection of bladder tumor. Can J Urol. 2016;23(2):8198–8203. [PubMed] [Google Scholar]
- 24.Matulewicz RS, Sharma V, McGuire BB, Oberlin DT, Perry KT, Nadler RB. The effect of surgical duration of transurethral resection of bladder tumors on postoperative complications: An analysis of ACS NSQIP data. Urol Oncol Semin Orig Investig. 2015;33(8):338.e19–338.e24. doi: 10.1016/j.urolonc.2015.05.011 [DOI] [PubMed] [Google Scholar]
- 25.Koo K, Zubkoff L, Sirovich BE, et al. The burden of cystoscopic bladder cancer surveillance: anxiety, discomfort, and patient preferences for decision making. Urology. 2017;108:122–128. doi: 10.1016/j.urology.2017.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ray KN, Chari AV, Engberg J, Bertolet M, Mehrotra A. Opportunity Costs of Ambulatory Medical Care in the United States. Am J Manag Care. 2015;21(8):9. [PMC free article] [PubMed] [Google Scholar]
- 27.Heijnsdijk EAM, Nieboer D, Garg T, Lansdorp‐Vogelaar I, Koning HJ de, Nielsen ME. Cost-effectiveness of surveillance schedules in older adults with non-muscle-invasive bladder cancer. BJU Int. 2019;123(2):307–312. doi: 10.1111/bju.14502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.National Institute for Health and Care Excellence (NICE). Bladder cancer: diagnosis and management. February 2015. https://www.nice.org.uk/guidance/ng2/evidence. Accessed July 24, 2017. [PubMed]
- 29.Noone AM, Howlader N, Krapcho M, et al. Seer Cancer Statistics Review, 1975–2015. Bethesda MD: Natl Cancer Inst; April 2018. http://seer.cancer.gov/csr/1975_2015/. Accessed November 25, 2018. [Google Scholar]
- 30.MacKenzie TA, Tosteson TD, Morden NE, Stukel TA, O’Malley AJ. Using instrumental variables to estimate a Cox’s proportional hazards regression subject to additive confounding. Health Serv Outcomes Res Methodol. 2014;14(1–2):54–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40(8):IV–3. [DOI] [PubMed] [Google Scholar]
- 32.Merkow RP, Rademaker AW, Bilimoria KY. Practical Guide to Surgical Data Sets: National Cancer Database (NCDB). JAMA Surg. 2018;153(9):850–851. doi: 10.1001/jamasurg.2018.0492 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


