Abstract
Objective:
To estimate the incidence, prevalence and mortality of antiphospholipid syndrome (APS).
Methods:
An inception cohort of patients with incident APS in 2000-2015 in a geographically well-defined population was identified based on comprehensive individual medical record review. All cases met the 2006 Sydney APS criteria (primary definition) or had APS by physician consensus (secondary definition). Lupus anticoagulant, IgM and IgG aCL and anti-β2 glycoprotein-1 antibodies were tested in a centralized lab. Incidence rates were age- and sex-adjusted to the US white 2010 population. Prevalence estimates were obtained from the incidence rates assuming no increased mortality associated with APS and assuming migration in/out of the area was independent of disease status.
Results:
In 2000-2015, 33 cases of incident APS by the Sydney criteria were identified (mean age 54.2 years, 55% female; 97% Caucasian). The annual incidence of APS was 2.1 (95% confidence interval [CI]: 1.4-2.8) per 100,000 population aged ≥ 18 years. Incidence rates were similar in both sexes. The estimated prevalence of APS was 50 per 100,000 (95% CI: 42-58) and was similar in both sexes. Six (18%) patients had a concurrent diagnosis of systemic lupus erythematosus. The most frequent clinical manifestation was deep venous thrombosis. The overall mortality of patients with APS was not significantly different from the general population (standardized mortality ratio: 1.61; 95% CI: 0.74-3.05).
Conclusion:
APS occurred in about 2 persons per 100,000 per year. The estimated prevalence is 50 per 100,000. Overall mortality was not different from the general population.
Introduction
Antiphospholipid Syndrome (APS) is an autoimmune disease characterized by vascular (arterial and/or venous) thrombosis and/or pregnancy morbidity in the presence of antiphospholipid antibodies.(1) APS occurs alone or in association with other autoimmune diseases, particularly systemic lupus erythematosus (SLE).
APS is defined based on the Sydney 2006 international classification criteria consensus.(2) It requires a clinical criterion, either a vascular (venous or arterial) thrombosis or pregnancy morbidity and a laboratory criterion based on persistent antiphospholipid antibodies present on two or more occasions, at least 12 weeks apart. The antiphospholipid antibodies accepted in the laboratory criteria include the lupus anticoagulant (LAC), anticardiolipin (aCL), and anti-β2-glycoprotein I (anti-β2GPI) IgG and IgM antibodies.
It has been speculated that APS is a leading cause of thrombosis and pregnancy morbidity particularly in the young. However, the incidence and prevalence of APS is unknown. Epidemiologic characteristics of the disease have been described in specific disease cohorts such as SLE or stroke, but the burden of the disease in the general population remains unknown.(3)
Estimating the frequency of APS in the general population has been identified as an urgent need in order to understand the magnitude of the disease burden.(4) In a systematic review, the authors concluded that there was a lack of robust scientific data to estimate the frequency of APS in the population. The most common methodological barriers identified were that patients were included with a single positive antiphospholipid result with no confirmation at least 12 weeks later, as well as inclusion of low-titer aCL or anti-β2GPI antibodies by ELISA when the recommended cutoff is 40 units. The available studies are mostly retrospective in nature, done at major referral medical centers, and none were population-based.(5)
The aim of this study was to characterize the annual incidence, prevalence, and mortality of APS in 2000-2015 in a population-based cohort of patients with APS from Olmsted County, Minnesota.
Patients and Methods
Study design
Through the resources of the Rochester Epidemiology Project (REP), a record linkage system, the population of Olmsted County, Minnesota, is well suited for investigation of the epidemiology of APS because comprehensive medical records for all residents seeking medical care are available. The REP allows ready access to the medical records from all health care providers for the local population, including the Mayo Clinic, the Olmsted Medical Center and their affiliated hospitals, local nursing homes, and the few private practitioners. Data about dates and causes of death is routinely tracked and readily available. This system ensures virtually complete ascertainment of all clinically recognized cases of APS among the residents of Olmsted County, Minnesota.(6) The demographics, distribution of morbidity, and death rates in Olmsted County are similar to the state of Minnesota and the upper Midwest. The characteristics and strengths of the REP, as well as its generalizability have been described elsewhere.(7, 8) The population of Olmsted County was 144,248 in 2010, with 74.7% in 2010 being ≥18 years of age. The ethnic distribution was 85.7% white, 4.2% Hispanic, 4.8% African American, 5.5% Asian/Native Hawaiian/Pacific Islander, and 0.2% American Indian/Alaska Native. The study was approved by the institutional review boards of the Mayo Clinic and Olmsted Medical Center.
Case finding and ascertainment
Potential cases of APS were identified based on laboratory reports. The case finding strategy was designed to be highly sensitive and comprehensive. We queried the REP patient database for any individuals who were tested for antiphospholipid antibodies (aPL), anti-β2GPI IgG or IgM antibodies or LAC (DRVVT [dilute Russell viper venom time], DRVVT mix, DRVVT confirmation or STACLOT) for whom the test result was reported as out of range or abnormal. Those with at least two abnormal APS tests at any point in time between January 1, 2000 and December 31, 2015 were selected for extensive chart review. All the APS related laboratory studies in Olmsted County, regardless of provider, were performed at the Special Coagulation Laboratory, Mayo Clinic, Rochester, MN, and were interpreted and standardized based on the international consensus criteria.
Identification of the Sydney criteria cohort was performed by rigorous application of its classification criteria, including: 1) all cases had to have a venous or arterial thrombotic event or pregnancy morbidity; 2) at least 12 weeks time interval between initial and repeat laboratory testing; 3) aCL and anti-β2GPI antibodies were considered positive only if they had a value of ≥40 GPL/MPL units. Although in the APS Sydney criteria there is no clear threshold for the anti-β2GPI antibodies, we used this approach since is easier to replicate and apply elsewhere. For those cases where the laboratory test was performed before the implementation of GLP or MPL units, an elevated titer was considered positive, if reported as such by the laboratory.
The Sydney criteria are used in clinical research as classification criteria and commonly used in clinical practice as a framework for diagnosis. However, they do not include all the manifestations of the disease, such as thrombocytopenia or heart valvulopathies. Furthermore, patients that had their confirmatory lab testing done less than 12 weeks apart will not fulfill the classification criteria. Thus, we used two case definitions, one based on the Sydney criteria and one augmented by physician diagnosis. Physician diagnosed cases of APS were those not meeting the Sydney criteria. Patients not classified as APS by the Sydney criteria but who had one of the following characteristics were considered as probable cases: 1) clinical features of APS not included in the updated Sydney criteria, such as thrombocytopenia or Libman-Sacks endocarditis, chorea, etc.(2) 2) diagnosed as having APS by a physician or 3) meeting clinical criteria but not laboratory criteria based on borderline antibodies (i.e. <40 GPL/MPL) or timing of the laboratory criterion confirmation. These potential cases were independently evaluated by two rheumatologists (KM, MP) and one hematologist (RKP), and patients with at least 2/3 physician consensus were included under the physician definition.
The cases identified by the Sydney criteria definition were used for the primary analysis. The expanded cohort, including those who met Sydney criteria and physician diagnosis definition, was used for secondary analysis. The APS incidence date was defined as the earliest date of criteria fulfilment (i.e., date of the laboratory confirmation) or the date of the laboratory tests closest to 12 weeks for the additional patients who did not fulfill the criteria. Patients needed to be Olmsted County residents on the APS incidence date to be included in the inception cohort.
The review of all medical records and data extraction was performed using a standardized data extraction form by two investigators (AD, MP) and verified by the first author. Data regarding age, sex, self-reported race and ethnicity, date of diagnosis and date of last follow up, vital status, clinical characteristics and laboratory findings was recorded.
Antiphospholipid antibody testing.
All reagents were from USA sources unless stated otherwise. Briefly reagents for PT included Dade Innovin (Siemens, Malvern, PA) and HemosIL RecombiPlasTin2G (R2G), (Instrumentation Laboratory, Bedford, MA) and for aPTT Platelin (BioMerieux, Cambridge, MA) and HemosIL SynthASil (Instrumentation Laboratory, Bedford, MA). Source of normal pooled plasma (NPP), and factor deficient plasma was PrecisioBioLogic, Inc, (Nova Scotia, Canada). Over the study period, assays were performed on the MDA-180 (Organon Teknika, Durham, NC), then Sta-R (Stago, Parsipanny, NJ) and more recently ACL TOP 700 (IL, Bedford, MA). All assays were performed according to manufactures’ instructions. LAC testing was performed using, dilute Russell viper venom time (DRVVT) (CRYOcheck, LA CHECK, LA SURE, PrecisionBioLogic, Inc, Nova Scotia, Canada), and STACLOT-LA (Diagnostica Stago, Parsippany, NJ) on the ACL TOP 700.
aCL and anti-β2GPI were performed using the VarelisA kit (Uppsala, Sweden) performed on the Alisei platform (Quebec, Canada). Information about the kits and reagent used for antibody testing before 2005 was not available.
Statistical analysis
Age and sex-specific incidence rates were calculated for both the Sydney criteria and the expanded cohorts by using the number of incident cases as the numerator and population estimates based on decennial census counts as the denominator, with linear interpolation used to estimate population size for intercensal years. Overall incidence rates were age- and/or sex-adjusted to the estimated 2010 white population of the US. In order to compute 95% confidence intervals (95% CI) for incidence rates, it was assumed that the number of incident cases followed a Poisson distribution. Trends in incidence rates were examined using Poisson regression methods with smoothing splines for age and calendar year. Survival rates following the diagnosis of APS were estimated using Kaplan-Meier methods and were compared to expected survival of the Minnesota white population.
Prevalence was calculated using a cohort method. Prevalence can be easily estimated from incidence when the following 3 conditions are met: (1) the disease is not associated with any excess mortality; (2) there are no important calendar time trends; and (3) migration in or out of the census population is independent of disease status. The method involves applying age, sex, and calendar year-specific incidence rates of disease and mortality rates from life tables to a hypothetical population to yield estimates of prevalence.(9) Confidence intervals for the prevalence estimates were obtained using bootstrap methods. The estimated number of persons in the US with APS on January 1, 2015 was estimated by applying the age- and sex-specific US population counts from the US Census Bureau to the estimated prevalence rates. Analyses were performed using SAS software version 9.4 (SAS Institute) and R version 3.4.2 (R Foundation for Statistical Computing).
Results
Demographic, clinical and laboratory characteristics of the APS incident cohort and time of diagnosis
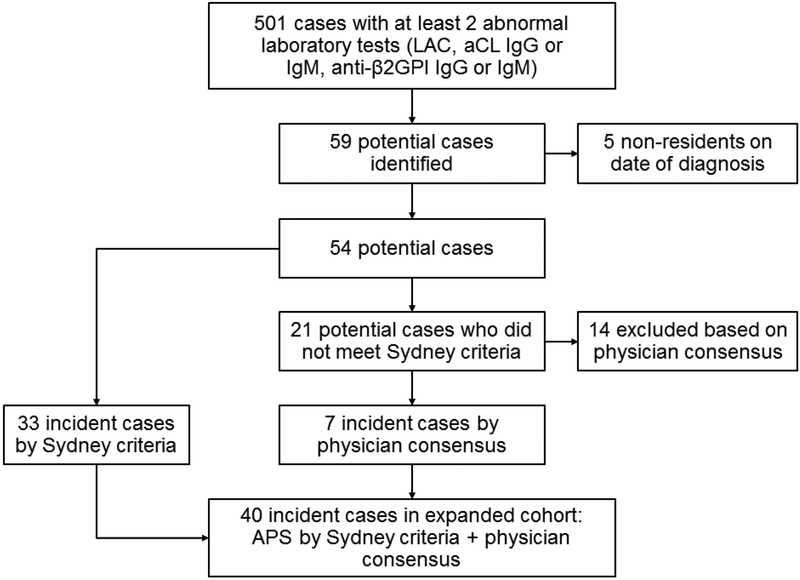
As illustrated in the flow diagram in Figure 1, 501 cases with at least two abnormal laboratory results reported by the laboratory were screened. A total of 59 cases were identified as meeting the eligibility criteria. Five cases were excluded since they were not residents of Olmsted County at the time of diagnosis. The primary analysis included 33 cases who met the Sydney APS criteria. An additional 21 cases were reviewed by three physician experts and seven cases were identified, the rest (14 cases) were excluded. A total of 40 incident cases of APS were identified by the Sydney criteria and physician diagnosis consensus for the secondary analysis.
Figure 1:
Flowchart describing the screening process for the identification of patients with antiphospholipid Syndrome (APS) diagnosed in Olmsted County, Minnesota from 2000-2015. aCL; anticadiolipin, anti-β2GPI; anti-β2-glycoprotein I.
A summary of the demographic and clinical characteristics at diagnosis time of the APS cohort by criteria and the expanded cohort with physician diagnosis is available in Table 1. The mean age of the cohorts was 54.2 years and 55% were female. The majority of the patients were white and one patient was black. Around one fifth of the cohort had a diagnosis of SLE by ACR criteria. The demographic characteristics of the expanded cohort were similar. Thrombotic events were significantly more frequent than obstetric events and non-criteria manifestations of the disease. In this population-based cohort, deep vein thrombosis (DVT) (42%) followed closely by pulmonary embolism (PE) (39%) were the most frequent thrombotic manifestations of APS, while the most frequent arterial manifestations were ischemic strokes and peripheral arterial thrombosis. No myocardial infarctions were recorded at time of diagnosis. Pregnancy morbidity was identified in 3/18 (17%) of the female patients identified. These three patients had three or more embryonic losses before week 10 of pregnancy, two of these patients also experienced at least one fetal death after week 10, and one gave birth to a premature baby before week 34th. Forty percent of the patients had at least one non-criteria manifestation: thrombocytopenia was the most frequently observed (15%) while chronic cutaneous ulcers and cardiac valve disease were observed in two (6%) and one (3%) patient, respectively.
Table 1.
Demographic and clinical characteristics of patients with incident antiphospholipid syndrome diagnosed in Olmsted County, Minnesota from 2000-2015 who met Sydney criteria and an expanded cohort including 7 patients based on physician diagnosis. SD; standard deviation, SLE; systemic lupus erythematosus, DVT; deep vein thrombosis, PE; pulmonary embolism, TIA; transient ischemic attack, MI; myocardial infarction, aPL; antiphospholipid, LAC; lupus anticoagulant, aCL; anticardiolipin, anti-β2GPI; anti -β2 glycoprotein I.
| Characteristic | Sydney Criteria cohort (n= 33) |
Expanded cohort (n= 40) |
|---|---|---|
| Age, Mean ± SD years | 54.2 (18.5) | 55.7 (19.0) |
| Female sex | 18 (55%) | 20 (50%) |
| White race | 32 (97%) | 39 (98%) |
| Length of follow-up (y), mean ± SD | 8.3 (4.9) | 8.2 (4.7) |
| Smoker (Current or former) | 13 (39%) | 16 (40%) |
| SLE diagnosis | 6 (18%) | 7 (18%) |
| Thrombosis | 33 (100%) | 40 (100%) |
| DVT | 14 (42%) | 19 (48%) |
| PE | 13 (39%) | 15 (38%) |
| TIA | 4 (12%) | 4 (10%) |
| Stroke | 11 (33%) | 14 (35%) |
| MI | 0 | 0 |
| Peripheral arterial thrombosis | 2 (6%) | 3 (8%) |
| Biopsy-proven microvascular thrombosis | 2 (6%) | 2(5%) |
| Pregnancy morbidity | 3/18 (17%) | 3/20 (15%) |
| Non-criteria manifestations | 13 (39%) | 16 (40%) |
| Livedo | 3 (9%) | 3 (8%) |
| Superficial thrombophlebitis | 1 (3%) | 3 (8%) |
| Chronic cutaneous ulcers | 2 (6%) | 2 (5%) |
| Cardiac valve disease | 1 (3%) | 1 (3%) |
| Pulmonary hypertension | 4 (12%) | 5 (13%) |
| aPL nephropathy | 0 | 0 |
| Thrombocytopenia (<100,000) | 5 (15%) | 5 (13%) |
| Other neurologic manifestations1 | 2 (6%) | 2 (5%) |
| APS profile | ||
| Positive LAC | 24/32 (75%) | 27/39 (69%) |
| Positive aCL | 23/33 (70%) | 24/40 (60%) |
| IgG | 13/33 (39%) | 13/40 (33%) |
| IgM | 14/33 (42%) | 15/38 (38%) |
| Positive anti-β2GPI | 5/9 (56%) | 5/12 (42%) |
| IgG | 2/9 (22%) | 2/12 (17%) |
| IgM | 1/9 (11%) | 2/12 (8%) |
Seizures, cognitive dysfunction.
LAC and aCL IgG and IgM antibodies were assessed in the majority of patients, but only 9/33 had anti-β2GPI IgG and IgM tested. Overall three fourths of the cases were positive for LAC, and either IgG or IgM aCL, while 20% or less of those tested for anti-β2GPI were positive.
The expanded cohort had similar proportions of clinical manifestations except in the antibody profile, where the proportion of patients with positive antibodies (GPL, MPL ≥ 40) in the expanded cohort was lower, as expected. The characteristics of the seven patients in the expanded cohort are detailed in Supplementary Table 1. Three patients did not meet criteria because the laboratory confirmation was performed in less than 12 weeks, while four of them did not meet criteria because of titers lower than the cut off.
Incidence and Prevalence of APS in the study population
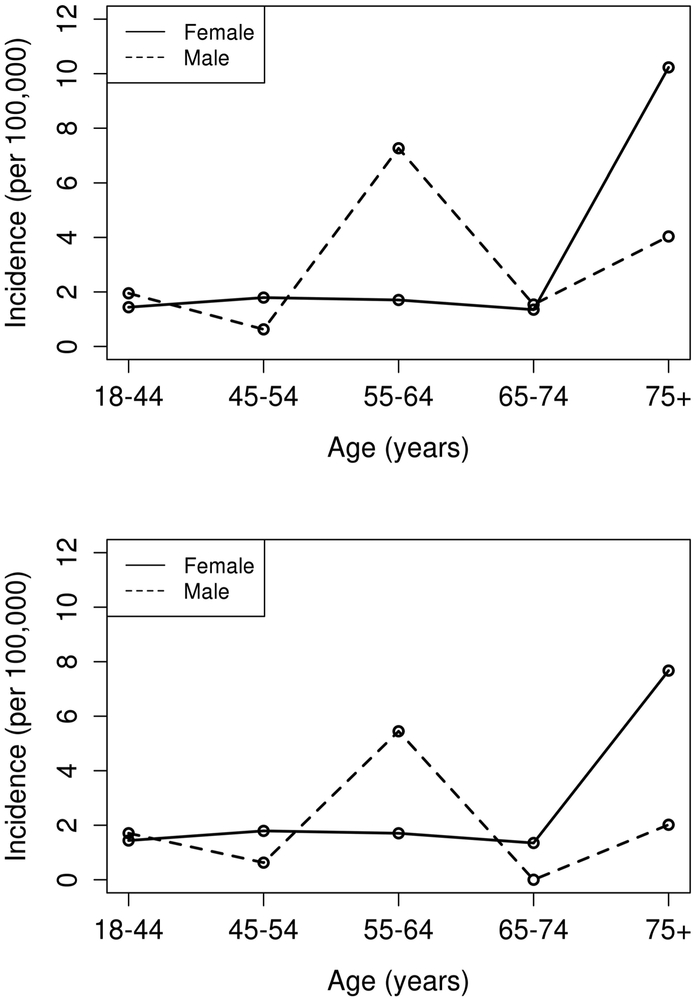
Using the Sydney criteria definition, 33 cases of APS were diagnosed during 2000-2015. Annual incidence rates stratified by age and sex are detailed in Table 2. The overall annual incidence rate of adults aged ≥ 18 years with APS age- and sex-adjusted to the 2010 US white population was 2.1 (95% confidence interval [CI]: 1.4-2.8)per 100,000 population. The age-adjusted incidence rates was 2.1 (95% CI 1.1-3.1) for the female population and 2.0 (95% 1.0-3.0) for the male population. Age-specific rates (Figure 2) peaked in those who were 75 years or older, the incidence of APS increases significantly with age (p=0.007). There was no evidence of a difference in APS rates by sex and no evidence of a differential age effect in males vs females. The incidence rates were slightly higher in the expanded cohort. We did not observe trends in the incidence rates over the time period.
Table 2.
Annual incidence and prevalence rates of antiphospholipid syndrome in Olmsted County, 2000-2015, according to Sydney criteria or expanded case definition. CI; Confidence interval.
| Sydney Criteria cohort | Expanded cohort | |||
|---|---|---|---|---|
| Incidence | No. of Cases | Incidence rate (95% CI) |
No of Cases | Incidence rate (95% CI) |
| Females by age group | ||||
| 18-44 | 6 | 1.4 | 6 | 1.4 |
| 45-54 | 3 | 1.8 | 3 | 1.8 |
| 55-64 | 2 | 1.7 | 2 | 1.7 |
| 65-74 | 1 | 1.3 | 1 | 1.3 |
| >75 | 6 | 7.7 | 8 | 10.2 |
| Totala | 18 | 2.1 (1.1 – 3.1) | 20 | 2.4 (1.3 – 3.4) |
| Males by age group | ||||
| 18-44 | 7 | 1.7 | 8 | 2.0 |
| 45-54 | 1 | 0.6 | 1 | 0.6 |
| 55-64 | 6 | 5.4 | 8 | 7.3 |
| 65-74 | 0 | 0.0 | 1 | 1.5 |
| >75 | 1 | 2.0 | 2 | 4.0 |
| Totala | 15 | 2.0 (1.0 - 3.0) | 20 | 2.7 (1.5 – 3.9) |
| Overall by age group | ||||
| 18-44 | 13 | 1.6 | 14 | 1.7 |
| 45-54 | 4 | 1.2 | 4 | 1.2 |
| 55-64 | 8 | 3.5 | 10 | 4.4 |
| 65-74 | 1 | 0.7 | 2 | 1.4 |
| >75 | 7 | 5.5 | 10 | 7.8 |
| Totalb | 33 | 2.1 (1.4 – 2.8) | 40 | 2.6 (1.8 – 3.4) |
Age-adjusted to the 2010 US white population
Age- and sex-adjusted to the 2010 US white population.
CI: Confidence interval
Figure 2.
Age- and sex- specific incidences of antiphospholipid syndrome by the Sydney criteria definition (top panel) and the expanded cohort by physician consensus (bottom panel).
The estimated prevalence of APS adjusted to the US white 2010 population was 50 per 100,000 (95% CI: 42-58). The prevalence among females was 51 per 100,000 (95% CI: 31- 72) and 48 (95% CI: 29 – 68) for males. Based on this and US census data, an estimated 119,300 persons in the US were affected by APS in 2015. The overall and per sex prevalence in the expanded cohort was somewhat higher (Table 3).
Table 3.
Estimated age and sex adjusted prevalence rate (per 100,000) of antiphospholipid syndrome by Sydney criteria and expanded definition in Olmsted County, Minnesota, 200-2015. CI; Confidence interval.
| Sydney Criteria cohort Prevalence Rate (95% CI) |
Expanded cohort Prevalence Rate (95% CI) |
|
|---|---|---|
| Overall | 50 (42 – 58) | 59 (52 – 67) |
| Females | 51 (31 – 72) | 54 (41 – 70) |
| Males | 48 (29 – 68) | 64 (44 – 86) |
Mortality rates among APS cases compared to the general population of the geographic region
During a median follow-up of 8.3 years, there were nine deaths in the incident APS cohort by the Sydney criteria. Based on the Minnesota lifetables, 5.6 deaths were expected. The SMR of APS by Sydney criteria was 1.61 (95% CI: 0.74-3.05). The 10-year survival rate was (80%; 95% CI: 66-100%) after APS incidence. Results were similar in the expanded cohort (Table 4).
Table 4.
Mortality and survival rates of incident antiphospholipid syndrome by the Sydney Criteria and Physician definition among residents of Olmsted County, Minnesota from 2000 – 2015. CI; Confidence interval.
| Measure | Sydney Criteria cohort |
Expanded cohort |
|---|---|---|
| No. of Patients | 33 | 40 |
| Observed no. of deaths | 9 | 10 |
| Expected no. of deaths | 5.6 | 7.6 |
| SMR (95% CI) | 1.61 (0.74 – 3.05) | 1.32 (0.63 - 2.42) |
| Survival rate, % (95% CI) | ||
| 2 years | 97 (91 - 100) | 98 (93 - 100) |
| 5 years | 90 (79 - 100) | 91 (82 - 100) |
| 10 years | 80 (66 - 100) | 83 (70 - 98) |
Discussion
As was described in Erkan and Lockshin’s recently published APS book, “Although there are many speculations, epidemiology of APS is yet to be elucidated”.(10) Previous studies reported the incidence and prevalence in particular disease cohorts (e.g., SLE) and the available APS studies are based on data from referral centers and rheumatology practices.(11, 12) This population-based study of clinically identified APS is the first to describe basic and fundamental epidemiologic data on incidence, prevalence and mortality rates needed to understand the population burden of APS. Overall, the age- and sex- adjusted annual incidence of APS was 2.1, and the prevalence was 50 per 100,000 population. The incidence was similar in women and men and mortality was not different from that of the general population.
In general, the incidence of autoimmune diseases, such as Sjögren syndrome, rheumatoid arthritis and SLE in particular, tend to be more common in females than males.(13-15) In this incident APS cohort, the rates were very similar between men and women. In a large APS European cohort, the female/male ratio was 5. However, after exclusion of the SLE patients, the female to male ratio decreased to 3.5 in patients with primary APS, and decreased to 1.0 after the obstetric APS cases were excluded. This estimate is similar to our findings and those reported by the APS Alliance for Clinical Trials and international Networking (APS ACTION) clinical database.(10)
The frequency of antiphospholipid antibodies reportedly increases with age, but it remains unclear if these antibodies are pathogenic or an epiphenomenon of a malignancy or other underlying process.(16) Furthermore, thrombotic events increase in relation to aging as well. These factors are limitations of the current definition of APS. In accordance with current classification standards, incidence rates observed in the current study peaked late in life, with a notably higher incidence after the age of 55 years of age and especially over the age of 75 years.
Although the mortality rate was not statistically different among cases with APS and the general Minnesota population, these findings do not exclude the possibility of increased mortality among those with APS. In the Euro-phospholipid cohort, the unadjusted SMR was 1.8, consistent with our findings. However, the 5 and 10 year survival was lower in our inception cohort, likely due to differences in the age of the cases.(17)
The clinical manifestations of the current cohort were consistent with those reported by others. DVT was the most frequent manifestation occurring in 42% of the cases, while strokes were the most common arterial event (33%) and 17% of the women in the study had pregnancy morbidity. Although the Sydney criteria only includes thrombotic events and pregnancy morbidity, 40% of the cases had at least one non-criteria manifestation of the disease, highlighting the importance of non-criteria manifestations and the systemic nature of the disease. However, other manifestations, such as livedo reticularis and renal disease were not as frequent. Since these data were recorded in routine healthcare encounters, the lack of systematic assessment may explain some of the discrepancies.
This study has several limitations inherent to all retrospective designs. First, the study was based on medical record review of clinically identified cases of APS and not on a serologic survey; thus, case ascertainment depended on the completeness of workup and documentation by the providers. Consequently, the burden of under-studied and undiagnosed cases remains unknown. We rigorously applied the Sydney criteria; the GPL and MPL cut off had to be ≥40 and the aPL testing had to be confirmed 12 weeks later. While almost all the cases were tested for aCL and lupus anticoagulant, only 9/33 were evaluated for anti-β2GPI. This may be due to the wide variety of specialties that cared for these patients and lack of familiarity with current assessment standards. Although the APS literature is published in the fields of rheumatology and hematology, patients in this cohort were assessed by a wide variety of clinicians including neurologists, primary care physicians, cardiologists and others. Second, our results can be generalized to subjects with a demographic profile similar to the upper Midwest. APS is more common in patients with SLE, therefore it is possible that the burden of APS may be higher in those ethnic groups in which SLE is more common. Our results may not be generalizable to more diverse populations with other ethnic or racial compositions. Third, our study population was small to provide stable incidence rates. This limitation is inherent to the population covered by the record-linkage system of the REP, which in this study includes only residents of Olmsted County.
The major strengths of our study are that the case finding relied on laboratory data that was performed by the same laboratory throughout the span of the study. The record linkage system of the REP afforded us a population-based study where all the cases of clinically detected APS in the community were identified and verified by comprehensive medical record review, minimizing referral bias and misclassification.
In conclusion, results from this population-based study revealed that definite APS occurred in about 2 persons per 100,000 per year. The estimated prevalence was 50 per 100,000. Overall mortality was not different from the general population. The incidence and prevalence of APS in the same population was at least as common as SLE.(14)
Supplementary Material
Acknowledgments
Financial support:
This study was made possible using the resources of the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the National Institutes of Health under Award Number R01-AG-034676 and CTSA Grant Number TL1 TR002380 from the National Center for Advancing Translational Science. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Ali Duarte-Garcia is supported by the Mayo Clinic Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery, which receives no industry funding
Footnotes
Disclosures: Nothing to disclose.
References
- 1.Corban MT, Duarte-Garcia A, McBane RD, Matteson EL, Lerman LO, Lerman A. Antiphospholipid Syndrome: Role of Vascular Endothelial Cells and Implications for Risk Stratification and Targeted Therapeutics. J Am Coll Cardiol. 2017;69(18):2317–30. doi: 10.1016/j.jacc.2017.02.058. PubMed PMID: 28473138. [DOI] [PubMed] [Google Scholar]
- 2.Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4(2):295–306. doi: 10.1111/j.1538-7836.2006.01753.x. PubMed PMID: 16420554. [DOI] [PubMed] [Google Scholar]
- 3.Petri M Update on anti-phospholipid antibodies in SLE: the Hopkins’ Lupus Cohort. Lupus. 2010;19(4):419–23. doi: 10.1177/0961203309360541. PubMed PMID: 20353980. [DOI] [PubMed] [Google Scholar]
- 4.Erkan D, Derksen R, Levy R, Machin S, Ortel T, Pierangeli S, et al. Antiphospholipid Syndrome Clinical Research Task Force report. Lupus. 2011;20(2):219–24. Epub 2011/02/10. doi: 10.1177/0961203310395053. PubMed PMID: 21303838. [DOI] [PubMed] [Google Scholar]
- 5.Andreoli L, Chighizola CB, Banzato A, Pons-Estel GJ, Ramire de Jesus G, Erkan D. Estimated frequency of antiphospholipid antibodies in patients with pregnancy morbidity, stroke, myocardial infarction, and deep vein thrombosis: a critical review of the literature. Arthritis Care Res (Hoboken). 2013;65(11):1869–73. doi: 10.1002/acr.22066. PubMed PMID: 23861221. [DOI] [PubMed] [Google Scholar]
- 6.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ 3rd, Rocca WA Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester epidemiology project. Am J Epidemiol. 2011;173(9):1059–68. doi: 10.1093/aje/kwq482. PubMed PMID: 21430193; PubMed Central PMCID: PMCPMC3105274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.St Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ 3rd, Rocca WA. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clinic proceedings. 2012;87(2):151–60. Epub 2012/02/07. doi: 10.1016/j.mayocp.2011.11.009. PubMed PMID: 22305027; PubMed Central PMCID: PMCPMC3538404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ 3rd, Pankratz JJ, Brue SM, et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol. 2012;41(6):1614–24. doi: 10.1093/ije/dys195. PubMed PMID: 23159830; PubMed Central PMCID: PMCPMC3535751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lyon JL, Gardner JW. THE RISING FREQUENCY OF HYSTERECTOMY: ITS EFFECT ON UTERINE CANCER RATES1. American Journal of Epidemiology. 1977;105(5):439–43. doi: 10.1093/oxfordjournals.aje.a112402. [DOI] [PubMed] [Google Scholar]
- 10.Erkan D, Lockshin MD. Antiphospholipid Syndrome: Current Research Highlights and Clinical Insights: Springer; 2017. [Google Scholar]
- 11.Cervera R, Boffa MC, Khamashta MA, Hughes GR. The Euro-Phospholipid project: epidemiology of the antiphospholipid syndrome in Europe. Lupus. 2009;18(10):889–93. doi: 10.1177/0961203309106832. PubMed PMID: 19671788. [DOI] [PubMed] [Google Scholar]
- 12.Peschken CA, Katz SJ, Silverman E, Pope JE, Fortin PR, Pineau C, et al. The 1000 Canadian faces of lupus: determinants of disease outcome in a large multiethnic cohort. J Rheumatol. 2009;36(6):1200–8. doi: 10.3899/jrheum.080912. PubMed PMID: 19369456. [DOI] [PubMed] [Google Scholar]
- 13.Maciel G, Crowson CS, Matteson EL, Cornec D. Incidence and Mortality of Physician-Diagnosed Primary Sjogren Syndrome: Time Trends Over a 40-Year Period in a Population-Based US Cohort. Mayo Clinic proceedings. 2017;92(5):734–43. doi: 10.1016/j.mayocp.2017.01.020. PubMed PMID: WOS:000401119800011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jarukitsopa S, Hoganson DD, Crowson CS, Sokumbi O, Davis MD, Michet CJ Jr., et al. Epidemiology of systemic lupus erythematosus and cutaneous lupus erythematosus in a predominantly white population in the United States. Arthritis Care Res (Hoboken). 2015;67(6):817–28. Epub 2014/11/06. doi: 10.1002/acr.22502. PubMed PMID: 25369985; PubMed Central PMCID: PMCPMC4418944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Myasoedova E, Crowson CS, Kremers HM, Therneau TM, Gabriel SE. Is the incidence of rheumatoid arthritis rising?: Results from Olmsted County, Minnesota, 1955–2007. Arthritis & Rheumatism. 2010;62(6):1576–82. doi: doi: 10.1002/art.27425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manukyan D, Rossmann H, Schulz A, Zeller T, Pfeiffer N, Binder H, et al. Distribution of antiphospholipid antibodies in a large population-based German cohort. Clin Chem Lab Med. 2016;54(10):1663–70. doi: 10.1515/cclm-2016-0014. PubMed PMID: 27028736. [DOI] [PubMed] [Google Scholar]
- 17.Cervera R, Serrano R, Pons-Estel GJ, Ceberio-Hualde L, Shoenfeld Y, de Ramon E, et al. Morbidity and mortality in the antiphospholipid syndrome during a 10-year period: a multicentre prospective study of 1000 patients. Ann Rheum Dis. 2015;74(6):1011–8. doi: 10.1136/annrheumdis-2013-204838. PubMed PMID: 24464962. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.