Abstract
Purpose
In our work we sought to define the prevalence rates of cutaneous events during dasatinib therapy in chronic myeloid leukemia (CML) patients and to investigate the clinical and pathological characteristics of these reactions.
Patients and methods
In our institution, 67 CML patients were treated with dasatinib. it was given as first line treatment in 26 (39%) and subsequent treatment in 41 (61%) CML patients. Flow cytometry analysis of peripheral blood and cutaneous biopsy was done on all CML patients with dermatological lesions appearing during dasatinib treatment.
Results
Among 67 CML patients, 4 (5.9%) showed skin lesions during dasatinib treatment. The cutaneous manifestations were not generalized but mainly located on the back, abdomen, thorax or leg regions. The patients did not show peripheral lymphocytosis at the time when skin lesions appeared. Overall, histological analysis showed that the skin lesions were characterized by a mild perivascular small CD8+ T lymphocytes infiltrate with minimal epidermotropism.
Conclusion
The unusual T cytotoxic cutaneous infiltrate demonstrated in our CML cases could be the expression of a dasatinib-promoted lymphocyte expansion. However, the heterogeneity of the dermatologic manifestations reported in our CML patients could also be related to unknown factors specific to each CML patient. Our work highlights the finding that skin lesions may be associated with dasatinib treatment and that they should not be confused with viral or bacterial infections but rather interpreted as the clinical expression of lymphocytosis promoted by this TKI.
Keywords: inhibitor tyrosine kinase, skin lesions, chronic myeloid leukemia, CD8+ lymphocytes, dasatinib
Introduction
Chronic myeloid leukemia (CML) is a hematological malignancy characterized by the t(9;22)(q34;q11) rearrangement, generating the BCR–ABL1 fusion gene that codes for a chimeric protein with high tyrosine kinase activity. Since the introduction of tyrosine kinase inhibitors (TKIs), targeting the BCR-ABL1 oncoprotein, CML patients can now achieve long-term survival. However, for most CML patients TKIs treatment needs to be taken lifelong,1,2 although multiple studies have shown that TKI discontinuation is feasible and safe in patients with deep and durable molecular responses on-therapy.3 CML outcome does not depend solely on treatment efficacy but also on how well the therapy is tolerated. TKIs have an overall favorable safety profile in clinical practice; adverse events usually occur in early treatment, feature a mild to moderate intensity, and resolve spontaneously.4 Whenever TKI treatment interruption is necessary, re-exposure to the same drug or switch to an alternative TKI yields a response in the majority of CML cases.5 Main safety concerns regarding each TKI are pretty well known;6 the cutaneous reactions are common with TKIs treatment. On the other hand, there is little evidence of cutaneous side effects associated with dasatinib, and even less regarding an underlying immune activation over associated skin lesions/rashes. Anecdotal dermatologic side effects of dasatinib include perifollicular hyperkeratotic eruptions, an acneiform rash, hair depigmentation, vitiligo-like lesions, and panniculitis.7–9 A 23.3% incidence of rashes associated with dasatinib has been estimated. Most rashes were graded as mild, only 1.1% being high grade.10 Several authors have reported immune activation and peripheral T lymphocytosis associated with dasatinib treatment.11–13 In our work we sought to define the prevalence rates of cutaneous events during dasatinib therapy and to investigate the clinical and pathological characteristics of these reactions.
Materials and methods
We retrospectively analyzed 67 consecutive CML patients who were treated with dasatinib in our Institution between 2007 and 2018. The median follow up time was 34 months (min. 5 – max. 144 months). In all patients the CML diagnosis was made on the basis of cytogenetic, reverse transcription polymerase chain reaction, and fluorescence in situ hybridyzation, as previously reported.14–17 Molecular monitoring of the response to dasatinib treatment was performed by real-time PCR, taken as the BCR-ABL1 to control gene (ABL1) transcript ratio, and expressed on the International Scale (IS).18,19 Dasatinib was given as first line treatment in 26 (39%) and subsequent treatment in 41 (61%) CML patients. Flow cytometry analysis of peripheral blood and cutaneous biopsy was done in all CML patients with dermatological lesions appearing during dasatinib treatment. In detail, the skin biopsies were fixed overnight in 10% neutral-buffered formalin, sampled and embedded in paraffin blocks, sectioned at 4 mm thickness and stained with haematoxylin-eosin (H&E). Additional sections, collected on poly-l-lysine-coated slides, were used for immunohistochemical staining. Immunohistochemistry for CD3 (Novocastra; clone: LN10; dilution 1:100), CD4 (Dako; clone: RPA-T4; dilution 1:50), CD20 (Dako; clone: L26; dilution 1:400), CD8 (Dako; clone: 1A5; dilution 1:50), CD56 (Novocastra; clone: 1B6; dilution 1:100) was performed automatically using a Dako Autostainer. The sections were then incubated overnight at 4C with the primary antibodies listed above. Appropriate negative controls, obtained by substituting the primary antibodies with pre-immune serum, as well as positive controls, were included in the procedure. Data on CML patients were retrospectively collected in accordance with the approval of the local ethics committee “Azienda Ospedaliero Universitaria Policlinico di Bari” and the guidelines of the Declaration of Helsinki. Written informed consent was obtained from each CML patient with skin lesions.
Results
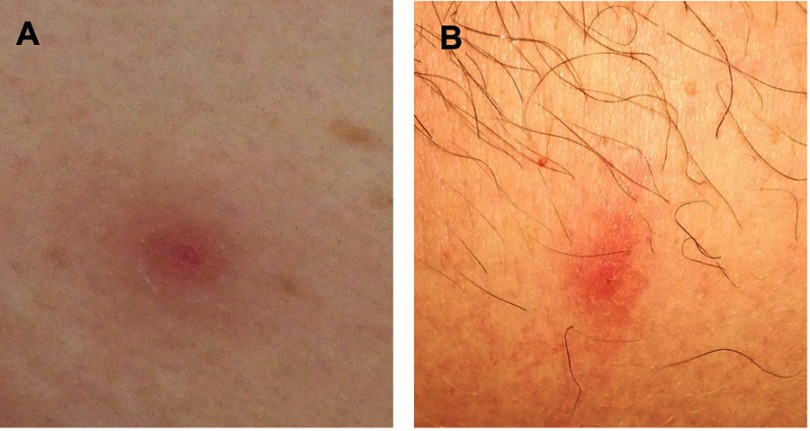
Among 67 CML patients, 4 (5.9%) showed skin lesions during dasatinib treatment; all of these cases except one (Case #2) showed cutaneous events during dasatinib as first line treatment. The median time to onset of skin lesions from the beginning of dasatinib treatment was 47.5 months (min 1 – max 61 months). Moreover, at the time of the appearance of the skin lesions all CML patients except one (Case #3) had achieved a deep molecular response; Case #3 showed early dermatologic lesions after only one month from starting dasatinib (Table 1). The cutaneous manifestations were not generalized but mainly located on the back, abdomen, thorax or leg regions (Table 1). The skin lesions appeared as pink to violaceous erythematous papular lesions, 1 or 2 centimeters in diameter, with oval-shaped edges (Figure 1). The patients did not show peripheral lymphocytosis at the time when skin lesions appeared; moreover, peripheral blood immunophenotypic analysis revealed a normal CD4+/CD8+ ratio, as well as a slightly increased natural killer (NK) cell fraction in Case #4.
Table 1.
Main characteristics of thechronic myeloid leukemia patients showing skin lesions during dasatinib treatment
| Patient | Sex/Age | First line TKI | Skin lesions appearance time after starting dasatinib (months) | Skin lesion site | BCR-ABL1 molecular response | Skin lesions treatment | Dasatinib discontinuation after the skin lesions appearance |
|---|---|---|---|---|---|---|---|
| Case#1 | M/58 | Dasatinib | 61 | Abdomen, back | MR4.5 | Prednisone | Yes |
| Case#2 | F/43 | Nilotinib | 51 | Chest | MR3 | None | No |
| Case#3 | F/34 | Dasatinib | 1 | Leg | Not assessed | Prednisone | No |
| Case#4 | M/46 | Dasatinib | 44 | Leg | MR3 | None | No |
Abbreviation: TKI, tyrosine kinase inhibitors.
Figure 1.
Skin lesions of the abdomen in Case #1 (A) and leg in Case #4 (B). See text for the description.
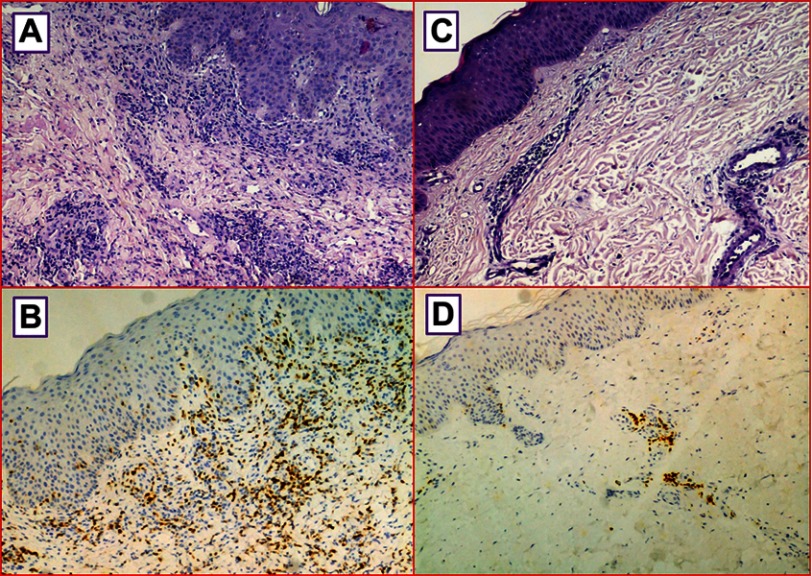
In cases #2 and #4 the cutaneous lesions were self-regressing while in cases #1 and #3 they regressed after brief oral steroids treatment. Dasatinib was discontinued only in Case #1; in this patient the discontinuation was permanent because pleural effusion relapse followed to the disappearance of the skin lesions. Overall, histological analysis showed that the skin lesions were characterized by a mild perivascular small T cell lymphocytes dermal infiltrate with minimal epidermotropism (Figure 2A and C), and in Case #1 a focal band-like infiltrate and a proliferation of small dilatated vessels were also evident (Figure 2A). The lymphocytes were CD8+ cytotoxic T-cells (Figure 2B and D). In all skin biopsies, immunohistochemistry staining for CD56 was negative. There were only sporadic eosinophils. The epidermis showed a normal basal layer, mild hyperkeratosis and absence of spongiosis. There was no papillary dermal fibrosis, characteristically consistent with a chronic process.
Figure 2.
The cutis is infiltrated by perivascular small lymphocytes (A and C) (H&E; original magnification, 100×); the lymphocytes show CD8 positivity (B and D) (immunoperoxidase staining; original magnification, 100×).
Discussion
Dermatologic events have been reported in CML patients treated with TKIs. During imatinib therapy any grade and grade 3–4 rashes within 12 months were reported in 33.9% and 2% of patients, respectively.20 Cutaneous lesions induced by imatinib are usually maculopapular eruptions, although other patterns have been reported, such as pigmentary changes, photosensitization, lichenoid reactions, psoriasiform eruptions, pseudoporphyria, exanthematous pustulosis, neutrophilic dermatosis, panniculitis, and Stevens-Johnson syndrome.8,21 In CML patients treated with nilotinib the frequency by 12 months of any grade and grade 3–4 rashes was 31% and <1%, respectively;22 other frequent dermatologic events include mild to moderate pruritus, skin xerosis, alopecia, and body hair loss. During bosutinib treatment following imatinib failure in patients with chronic phase-CML, rashes, all of grades 1–2, were very frequent, occurring in 44% of patients.23 Ponatinib treatment was associated with the onset of erythematous and papular rashes; moreover, skin xerosis was very frequent.24 The above cutaneous events described during TKIs treatment can be explained by direct toxic pharmacological effects rather than immunogenic or allergic mechanisms. On the other hand, this may not be entirely true for dasatinib. In the DASISION trial, all grades rashes were observed in 17% of CML patients but grades 3–4 in none.7 The dominant clinical characteristic of the rash was a pruritic keratosis pilaris-like eruption. In addition, a large variety of rashes have been reported with dasatinib: erythema, erythema multiforme, urticaria, photosensitivity, nail disorders, neutrophilic dermatosis, palmoplantar erythrodysesthesia syndrome, asteatosis, leukocytoclastic vasculitis, skin ulcer, skin fibrosis, and Stevens-Johnson syndrome.8 Dasatinib has a well known spectrum of side effects (mostly pleural effusions, pulmonary hypertension and myelosuppression),6 particularly due to its multiple kinase targets. There is very little evidence about its cutaneous effects, and probably many of these are missed during treatment for two reasons: firstly, they are not specific and therefore could easily be evaluated as viral, bacterial or transient skin lesions; secondly, CML patients on dasatinib may tend to underestimate the skin lesions, that are mainly transient and localized, and therefore they may find it unnecessary to report them to the hematologist if the lesions appear outside of already scheduled visits. In our series of CML patients treated with dasatinib the cutaneous events occurred predominantly in molecular remission (MR3 or MR4.5). The cutaneous lesions described in our four CML cases had various macroscopic aspects (basically featuring a vesicular lesion of less than one centimeter), were clinically relevant (painful, itching) or mild, and tended to regress when treated with steroids and/or temporary dasatinib discontinuation, if clinically advisable. It is noteworthy that in the CML patients we describe there was a contrast between the clinical heterogeneity of the skin lesions and the uniformity of the histological pattern emerging from cutaneous biopsies. In this regard it could be a relevant point that during dasatinib treatment, a significant expansion of large granular lymphocytes (LGLs) has previously been reported.25,26 The etiology of LGL expansions during dasatinib seems to be multifactorial and a clear mechanism has not yet been identified. One hypothesis is that dasatinib could directly activate or modulate the proliferation and function of NK cells. The pre-existing clonal T or NK cells are probably expanded by dasatinib through the inhibition of tyrosine kinases, as well as several subtypes of Src family kinases such as Fyn or Lck, which are known to be involved in the regulation or activation of NK cells.25,26 The LGL-increase phenomenon has not been reported in patients treated with imatinib or nilotinib, which cannot inhibit Src family kinases. The unusual T cytotoxic cutaneous infiltrate demonstrated in our CML cases could be the expression of a dasatinib-promoted lymphocyte expansion. However, the heterogeneity of the dermatologic manifestations reported in our CML patients could also be related to unknown factors specific to each CML patient. Another aspect that will need to be clarified is whether the presence of skin lesions during treatment with dasatinib may have a prognostic significance given that most of them appear when the CML patient has achieved a molecular response.
Conclusion
In conclusion, our work highlights the finding that skin lesions may be associated with dasatinib treatment and that they should not be confused with viral or bacterial infections but rather interpreted as the clinical expression of lymphocytosis promoted by this TKI. It remains to be seen whether such skin lesions are part of the presumed immunomodulatory activity characterizing dasatinib, apart from its tyrosine kinase inhibition activity.
Acknowledgment
The authors would like to thank Ms. MVC Pragnell, B.A. for language revision of the manuscript. This work was supported by the Associazione Italiana contro le Leucemie (AIL) - BARI.
Disclosure
Professor Eugenio Maiorano reports personal fees from Roche, personal fees from Genomic Health and personal fees from Genactis, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
- 1.Huang X, Cortes J, Kantarjian H. Estimations of the increasing prevalence and plateau prevalence of chronic myeloid leukemia in the era of tyrosine kinase inhibitor therapy. Cancer. 2012;118:3123–3127. doi: 10.1002/cncr.26523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baccarani M, Deininger MW, Rosti G, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122:872–884. doi: 10.1182/blood-2012-12-471029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rea D, Cayuela JM. Treatment-free remission in patients with chronic myeloid leukemia. Int J Hematol. 2018;108:355–364. doi: 10.1007/s12185-017-2295-0 [DOI] [PubMed] [Google Scholar]
- 4.Rea D. Management of adverse events associated with tyrosine kinase inhibitors in chronic myeloid leukemia. Ann Hematol. 2015;94(Suppl 2):S149–S158. doi: 10.1007/s00277-015-2318-y [DOI] [PubMed] [Google Scholar]
- 5.Russo Rossi A, Breccia M, Abruzzese E, et al. Outcome of 82 chronic myeloid leukemia patients treated with nilotinib or dasatinib after failure of two prior tyrosine kinase inhibitors. Haematologica. 2013;98:399–403. doi: 10.3324/haematol.2012.064337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steegmann JL, Baccarani M, Breccia M, et al. European LeukemiaNet recommendations for the management and avoidance of adverse events of treatment in chronic myeloid leukaemia. Leukemia. 2016;30:1648–1671. doi: 10.1038/leu.2016.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kantarjian H, Shah NP, Hochhaus A, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2010;362:2260–2270. doi: 10.1056/NEJMoa1002315 [DOI] [PubMed] [Google Scholar]
- 8.Brazzelli V, Grasso V, Borroni G. Imatinib, dasatinib and nilotinib: a review of adverse cutaneous reactions with emphasis on our clinical experience. J Eur Acad Dermatol Venereol. 2013;27:1471–1480. doi: 10.1111/jdv.12172 [DOI] [PubMed] [Google Scholar]
- 9.Amitay-Laish I, Stemmer SM, Lacouture ME. Adverse cutaneous reactions secondary to tyrosine kinase inhibitors including imatinib mesylate, nilotinib, and dasatinib. Dermatol Ther. 2013;24:386–395. doi: 10.1111/j.1529-8019.2011.01431.x [DOI] [PubMed] [Google Scholar]
- 10.Bergman JC, Ly TY, Keating MM, et al. Recurrent and fixed neutrophilic dermatosis associated with dasatinib. J Cutan Med Surg. 2018;22:621–623. doi: 10.1177/1203475418775663 [DOI] [PubMed] [Google Scholar]
- 11.Kreutzman A, Ilander M, Porkka K, et al. Dasatinib promotes Th1-type responses in granzyme B expressing T-cells. OncoImmunology. 2014;3:e28925. doi: 10.4161/onci.28925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iriyama N, Fujisawa S, Yoshida C, et al. Early cytotoxic lymphocyte expansion contributes to a deep molecular response to dasatinib in patients with newly diagnosed chronic myeloid leukemia in the chronic phase: results of the D-first study. Am J Hematol. 2015;90:819–824. doi: 10.1002/ajh.24096 [DOI] [PubMed] [Google Scholar]
- 13.Kreutzman A, Juvonen V, Kairisto V, et al. Mono/oligoclonal T and NK cells are common in chronic myeloid leukemia patients at diagnosis and expand during dasatinib therapy. Blood. 2010;116:772–782. doi: 10.1182/blood-2009-12-256800 [DOI] [PubMed] [Google Scholar]
- 14.Zagaria A, Anelli L, Albano F, et al. Molecular cytogenetic characterization of deletions on der(9) in chronic myelocytic leukemia. Cancer Genet Cytogenet. 2006;167:97–102. doi: 10.1016/j.cancergencyto.2006.01.011 [DOI] [PubMed] [Google Scholar]
- 15.Albano F, Anelli L, Zagaria A, et al. “Home-brew” FISH assay shows higher efficiency than BCR-ABL dual color, dual fusion probe in detecting microdeletions and complex rearrangements associated with t(9;22) in chronic myeloid leukemia. Cancer Genet Cytogenet. 2007;174:121–126. doi: 10.1016/j.cancergencyto.2006.09.025 [DOI] [PubMed] [Google Scholar]
- 16.Albano F, Anelli L, Zagaria A, et al. Non random distribution of genomic features in breakpoint regions involved in chronic myeloid leukemia cases with variant t(9;22) or additional chromosomal rearrangements. Mol Cancer. 2010;9:120. doi: 10.1186/1476-4598-9-120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Albano F, Anelli L, Zagaria A, et al. Decreased TET2 gene expression during chronic myeloid leukemia progression. Leuk Res. 2011;35:e220–e222. doi: 10.1016/j.leukres.2011.07.013 [DOI] [PubMed] [Google Scholar]
- 18.Cross NC, White HE, Colomer D, et al. Laboratory recommendations for scoring deep molecular responses following treatment for chronic myeloid leukemia. Leukemia. 2015;29:999–1003. doi: 10.1038/leu.2015.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cross NC, White HE, Ernst T, et al. Development and evaluation of a secondary reference panel for BCR-ABL1 quantification on the International Scale. Leukemia. 2016;30:1844–1852. doi: 10.1038/leu.2016.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348:994–1004. doi: 10.1056/NEJMicm020037 [DOI] [PubMed] [Google Scholar]
- 21.Amitay-Laish I, Stemmer SM, Lacouture ME. Adverse cutaneous reactions secondary to tyrosine kinase inhibitors including imatinib mesylate, nilotinib, and dasatinib. Dermatol Ther. 2011;24:386–395. doi: 10.1111/j.1529-8019.2011.01431.x [DOI] [PubMed] [Google Scholar]
- 22.Saglio G, Kim DW, Issaragrisil S, et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med. 2010;362:2251–2259. doi: 10.1056/NEJMoa0912614 [DOI] [PubMed] [Google Scholar]
- 23.Cortes JE, Kantarjian HM, Brümmendorf TH, et al. Safety and efficacy of bosutinib (SKI-606) in chronic phase Philadelphia chromosome-positive chronic myeloid leukemia patients with resistance or intolerance to imatinib. Blood. 2011;118:4567–4576. doi: 10.1182/blood-2011-02-334870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cortes JE, Kim DW, Pinilla-Ibarz J, et al. A phase 2 trial of ponatinib in Philadelphia chromosome-positive leukemias. N Engl J Med. 2013;369:1783–1796. doi: 10.1056/NEJMoa1306494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schiffer CA, Cortes JE, Hochhaus A, et al. Lymphocytosis after treatment with dasatinib in chronic myeloid leukemia: effects on response and toxicity. Cancer. 2016;122:1398–1407. doi: 10.1002/cncr.29933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qiu Z-Y, Xu W, Li. J-Y. Large granular lymphocytosis during dasatinib therapy. Cancer Biology & Therapy. 2014;15:247–255. doi: 10.4161/cbt.27310 [DOI] [PMC free article] [PubMed] [Google Scholar]