Abstract
In preclinical studies, selenite had single agent activity and radiosensitized tumors in vivo. Here we report results from a Phase 1 trial in 15 patients with metastatic cancer treated with selenite (5.5 to 49.5 mg) orally as a single dose 2 hours before each radiation therapy (RT) treatment. Patients received RT regimens that were standard of care. The primary objective of the study was to assess the safety of this combination therapy. Secondary objectives included measurement of pharmacokinetics (PK) and evaluation of efficacy. Endpoints included assessment of PK, toxicity, tumor response, and pain before and after treatment. The half-life of selenite was 18.5 hours. There were no adverse events attributable to selenite until the 33 mg dose level, at which the primary toxicities were grade 1 GI side effects. One patient treated with 49.5 mg had grade 2 GI toxicity. Although this was not a DLT, it was felt that the highest acceptable dose in this patient population was 33 mg. Most patients had stabilization of disease within the RT fields, with some demonstrating objective evidence of tumor regression. Most patients had a marked improvement in pain and seven out of nine patients with prostate cancer had a decrease in PSA ranging from 11–78%. Doses up to 33 mg selenite were well tolerated in combination with RT. A randomized, well controlled study is needed at the 33 mg dose level to determine if selenite results in clinically meaningful improvements in the response to palliative RT.
Introduction
Patients with metastatic cancer frequently receive palliative radiation therapy to treat painful and symptomatic sites of disease. Despite recent advances in both systemic and local treatment of metastases, many patients have persistent pain or symptoms following treatment. New and improved therapies are needed to increase the efficacy and duration of response to palliative radiation therapy.
Although a large body of data exists from studies of the potential utility of selenium supplementation (using an organic form of selenium) as a chemopreventive strategy, little is known regarding the use of selenium, as inorganic sodium selenite, as a cancer therapy. Our results [1], [2], [3], [4], [5], as well as those of other groups [6], support the novel idea that selenium in the form of selenite can be used to treat prostate as well as other types of cancer. Importantly, selenite is metabolized differently from organic forms of selenium, with the key difference being that the metabolism of selenite depletes cells of an important antioxidant, glutathione (GSH), and results in the generation of superoxide, a highly reactive and toxic radical that results in the generation of reactive oxygen species (ROS).
Our work initially focused on prostate cancer. The rationale for using selenite to treat prostate cancer came from our preclinical studies showing that 1) prostate cancer cells are more sensitive to selenium (sodium selenite)-induced apoptosis than normal prostate epithelial cells, 2) Selenite induces significant growth inhibition of well-established prostate cancer tumors in mice at doses that have no detectable toxicity when administered both ip and po, and 3) Selenite disrupts androgen receptor (AR) signaling, with inhibition of AR expression and activity by selenite occurring via a redox mechanism involving GSH, superoxide, and transcription factor Sp1. Altogether, these findings suggest that selenite may be useful in a variety of potential indications in the natural history of prostate cancer, including both hormone sensitive and hormone refractory prostate cancer, as a single agent, or in combination with radiation, chemotherapy or conventional androgen deprivation therapy (ADT).
Given that depletion of GSH is known to have radiosensitizing effects [7], and generation of superoxide should enhance the efficacy of radiation-induced ROS, selenite has the potential to sensitize a wide range of tumor types. Our data suggests that selenite-mediated tumor-selective radiosensitization in prostate cancer is due, in part, to differences between MnSOD and Bcl-2 family member expression in tumor vs. normal tissue [3]. Similar differences in other tumor types, as well as overexpression of Nrf2 and its downstream target genes in cancer [8], may also contribute to the differential sensitizing effects of selenite.
In the Phase 1 trial described here, sodium selenite (given orally at daily doses of 5.5, 11, 16.5, 33 and 49.5 mg) was given concurrently with palliative radiation therapy in patients with metastatic cancer. The primary objective of the study was to assess the safety and tolerability of this combination therapy. Secondary objectives included measurement of pharmacokinetics and evaluation of efficacy. The underlying hypothesis of this study was that the combination of selenite and radiation therapy would be safe and tolerable, and might have the potential to improve PSA responses in the subset of patients with castration-resistant prostate cancer (CRPC) and local response to radiation therapy in patients with metastatic cancer.
Methods
Patients
This study was approved by the Food and Drug Administration (IND 122151), the Stanford University Internal Review Board, and the Scientific Review Committee for the Stanford Cancer Institute. Fifteen patients with a variety of malignancies were treated on this study. The study was initially open only to prostate cancer patients, but then expanded to include a variety of tumor types. Patient characteristics are summarized in Table 1, including tumor histology, race, sex, age, BSA and history of prior therapy. Patients ranged in age from 37 to 92 years of age, with 13 men and two women. Before study entry, patients had to meet a number of eligibility criteria. Inclusion criteria included a) histologically-confirmed solid tumor malignancy with confirmation of metastasis, multiple myeloma, or plasmacytoma, b) need for palliative radiation therapy, c) for prostate cancer patients, PSA at least 2 ng/mL, except for patients who had recently started androgen deprivation therapy with PSA less than 2 ng/mL, d) age ≥18 years, e) life expectancy greater than 3 months, f) Eastern Cooperative Oncology Group (ECOG) performance status of zero or one or Karnofsky performance status ≥80%, and g) QT interval corrected using Fridericia's method (QTcF) <460 msec. Exclusion criteria included a) Absolute neutrophil count <1500/μL, platelet count ≤100 × 109/L, serum creatinine >2.0 mg/dL, total bilirubin >1.5 × upper limit of normal (ULN), AST, and/or ALT >2 × ULN, hemoglobin <9 g/dL, b) history of other malignancies within 5 years prior to Day 1 except for tumors that in the opinion of the investigators have a negligible risk for metastasis or death, such as adequately controlled basal cell carcinoma, squamous-cell carcinoma of the skin, or early-stage bladder cancer, c) current, or recent (within 4 weeks of the first treatment of this study) cytotoxic chemotherapy (eg, cisplatin, Taxol) or experimental drug therapy, d) uncontrolled inter-current illness, or psychiatric illness/social situations that would limit compliance with study requirements, e) history of myocardial infarction or unstable angina within 6 months prior to study enrollment, f) history of stroke or transient ischemic attack within 6 months prior to study enrollment, g) known human immunodeficiency virus (HIV) positivity while receiving antiretroviral therapies, and i) pregnant or breastfeeding women.
Table 1.
Patient characteristics
| Patient no. | Dose cohort (mg) | Tumor histology | Race | Sex | Age (y) | BSA(m2) | Prior therapy |
|---|---|---|---|---|---|---|---|
| 1 | 5.5 | Prostate | White | M | 92 | 1.8 | N/A |
| 2 | Prostate | African American | M | 76 | 2.2 | RT | |
| 3 | Prostate | White | M | 79 | 2 | RT and ADT | |
| 4 | 11 | Prostate | White | M | 75 | 2.3 | ADT |
| 5 | Prostate | African American | M | 71 | - | N/A | |
| 6 | Prostate | White | M | 82 | 1.9 | N/A | |
| 7 | 16.5 | Prostate | White | M | 68 | - | N/A |
| 8 | Prostate | White | M | 68 | 1.7 | RT | |
| 9 | Prostate | White | M | 65 | 1.7 | RT and ADT | |
| 10 | 33 | Prostate | White | M | 91 | 1.8 | N/A |
| 11 | Multiple myeloma | White | M | 57 | 2.4 | RT, RVD, and CT | |
| 12 | MPNST | Asian | M | 37 | 1.8 | RT and CT | |
| 13 | NSCLC | White | M | 67 | 1.9 | RT and CT | |
| 14 | Multiple myeloma | White | F | 37 | 1.6 | RVD | |
| 15 | 49.5 | NSCLC | African American | F | 56 | 1.7 | RT and CT |
Abbreviations: ADT = androgen deprivation therapy, BSA = body surface area calculated with Du Bois formula, CT = chemotherapy, MPNST = malignant peripheral nerve sheath tumor, NSCLC = non-small cell lung cancer, RT = radiation therapy, RVD = lenalidomide, bortezomib, and dexamethasone.
Study Design
This was a Phase 1 study, with the “3 + 3” rule used for dose escalation of sodium selenite. Patients were treated in groups of three with each receiving the same dose. Sodium selenite (Biosyn, Germany) was given orally 2 hours prior to scheduled daily radiation therapy treatments for the duration of the radiation therapy course. The initial dose escalation schema was 5.5, 11, 16.5, 33, 49.5, 66, 99, and 121 mg daily. Dose escalation was to proceed as follows: a) if none of the three patients experienced a dose limiting toxicity (DLT), dose escalation to the next dose level would occur, b) if one of three patients treated at that dose level experienced a DLT, that dose level would be expanded to six subjects; if no additional patient in that cohort experienced a DLT, dose escalation to the next dose level would occur, c) if two patients in a cohort experienced a DLT, dose escalation would stop and the prior dose would be considered the maximum tolerated dose (MTD). At that point the MTD was to be expanded to a total of six patients.
Baseline evaluations included EKG, PSA for prostate cancer patients, CBC with differential, CMP, LDH, bone scan (BS), or CT, PET/CT or MRI as clinically indicated to monitor response to therapy. Palliative radiation therapy utilized standard of care palliative dose/fractionation regimens. A summary of radiation therapy parameters and concurrent therapy are summarized in Table 2. On Week 1, Day 1 patients underwent physical exam (PE), laboratory studies as above and EKG. All patients completed a pain inventory, the Brief Pain Inventory [9], [10], prior to therapy. Sodium selenite was begun 2 hours prior to the scheduled radiation therapy appointment time. Weekly, during radiation therapy, patients had vital signs performed, with assessment of adverse events (AEs), labs and EKG if clinically indicated. The pain inventory was completed again on the last day of radiation therapy. Following completion of therapy, the first follow-up visit was within 2–3 months +/− 2 weeks, with subsequent follow-up visits optional until progression of disease at the site of radiation. At these visits patients had a PE, labs and imaging. They also completed another pain inventory.
Table 2.
Radiation treatment parameters
| Patient no. | Radiation field(s) | Dose/Fx† (cGy) | Number of Fx† | Total dose (cGy) | Concurrent therapy |
|---|---|---|---|---|---|
| 1 | Bilateral pelvic bones | 800 | 1 | 800 | Abiraterone |
| 2 | Left shoulder and left hip | 400 | 5 | 2000 | Bicalutamide, leuprolide acetate |
| 3 | Bilateral sacroiliac joints | 400 | 5 | 2000 | Abiraterone, leuprolide acetate |
| 4 | Bilateral sacroiliac joints | 400 | 5 | 2000 | Bicalutamide, leuprolide acetate |
| 5 | Left pelvis and proximal femur | 300 | 10 | 3000 | Leuprolide acetate |
| 6 | T3-T6 | 400 | 5 | 2000 | N/A |
| 7 | C7-T4 & right humerus | 400 | 5 | 2000 | N/A |
| 8 | L2 | 800 | 3 | 2400 | Enzalutamide |
| 9 | T11 | 2000 | 1 | 2000 | Bicalutamide, leuprolide acetate |
| 10 | L1-L4 | 400 | 5 | 2000 | Enzalutamide, leuprolide acetate |
| 11 | Left arm, left and right femur, left leg | 300 | 10 | 3000 | N/A |
| 12 | Right lung | 500 | 10 | 5000 | Olaratumab |
| 13 | Left hip | 300 | 10 | 3000 | Pembrolizumab |
| 14 | Right sacroiliac and sternum | 400 | 5 | 2000 | N/A |
| 15 | Sacrum and skull/dura | 400 | 5 | 2000 | N/A |
Fx = Fraction.
Pharmacokinetic Analysis
Pharmacokinetic Studies
Pharmacokinetic blood sampling was performed on Day 1 pre-dose, 15 minutes +/− 2 minutes, 1 hour +/− 5 minutes, 2 hours +/− 10 minutes, 4 hours +/− 15 minutes, and 24 hours +/− 1 hour. During week 2, on Day 1 pre-dose, 1 hour +/− 5 minutes, and other optional time points were obtained when feasible.
Pharmacokinetic analysis and model simulations
The PK profile of selenite was characterized using nonlinear mixed effects (NLME) modeling. Using NONMEM software (version 7.4; ICON PLC, Dublin, Ireland), a 1-compartment model with oral absorption was fit to the data. PK parameter estimates obtained from the model included bioavailability (F), clearance (CL/F), and volume of distribution (V/F). These estimates were used to determine the half-life (t1/2) using the relationship t1/2 = 0.693/kelimination (kelimination = CL/V) and area under the curve (AUC) using the relationship AUC = (F*dose)/CL. A full description of the structural and statistical model development will be the subject of a separate paper.
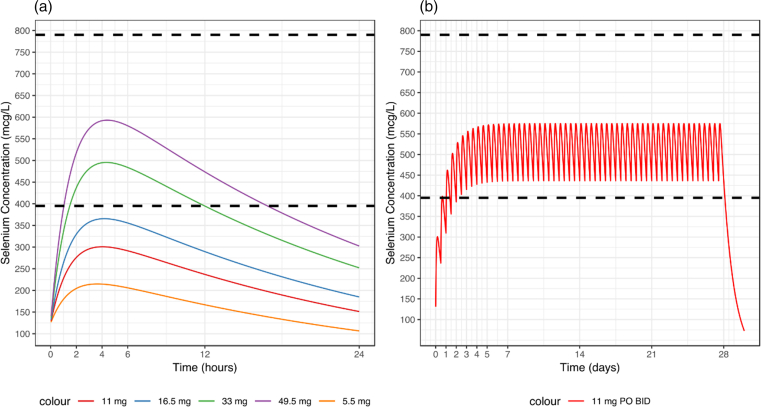
Using the final model and its parameter estimates, a simulation was performed for each dose level (5.5 mg, 11 mg, 16.5 mg, 33 mg, 49.5 mg) assuming a single dose administration. Additionally, using the dose levels 11 mg, 16.5 mg and 33 mg, a dosing regimen (dose, frequency) was proposed to achieve a target selenite concentration of 5–10 μM (395–790 mcg/L). This therapeutic range was determined by concentrations of selenite/selenium that had activity in vitro, as well as PK studies in mice given 2 mg/kg sodium selenite iv, which was a dose that had significant activity in vivo (unpublished data). The PKPDsim package in R was used to perform simulations.
Results
Toxicity Profile
Adverse events are summarized in Table 3 by dose level, in terms of classification, type, grade, number of subjects affected and relationship to selenite. At the 5.5, 11 and 16.5 mg dose levels, there were no AEs attributable to the selenite (data not shown). At the 33 mg dose level the majority of patients had a variety of grade 1 GI toxicities that ranged in attribution from possibly to probable/definitely related. This was the first dose level at which ondansetron and loperamide-Hcl were prescribed prn, and were highly effective in most of the patients. One patient, with a reported low threshold for nausea, had grade 2 nausea and vomiting that was not well controlled with ondansetron and stopped selenite after 4 of 10 planned treatments, with complete resolution of symptoms within 48 hours. Non-GI side effects included one patient with grade 1 fatigue, one patient with grade 1 dizziness, and one patient had grade 2 fatigue, that were possibly related to the selenite treatment. In addition, two patients had grade 1 ECG QTcF prolongations, initially scored as probably related to the selenite. One patient was taken off the study after the first dose of selenite when this occurred given his age of 91 years and relatively frail condition. Pre and post selenite treatment ECGs were subsequently reviewed by Dr. Philip Sager (Department of Medicine, Stanford University; Executive Committee, Cardiac Safety Research Consortium; personal communication). The observed grade 1 ECG changes were determined to be within the range of expected intra-subject variability, but it was not possible to exclude a potential QTc effect of the selenite. The one patient treated at the 49.5 mg dose level had grade 2 diarrhea, nausea and vomiting that was probably related to selenite, as well as grade 1 fatigue that was possibly related to the treatment. This patient required ondansetron every 8 hours as well as loperamide-Hcl, which improved the symptomatology, but did not completely control it. At that point, although this level of toxicity did not meet the strict definition of a DLT, it was felt that this toxicity profile in this patient population was not acceptable, and the highest dose level that was reasonably well tolerated with ondansetron prn was 33 mg.
Table 3.
Adverse events
| Cohort-selenite dose (mg) | Classification | Type | Grade attribution | Number of occurrences | Number of subjects affected | Relation to selenite |
|---|---|---|---|---|---|---|
| 33 | Blood and lymphatic system | Anemia | 3 | 1 | 1 | Unrelated |
| Gastrointestinal | Abdominal pain | 1 | 3 | 3 | 1-Possible, 2-Probably | |
| Diarrhea | 1 | 5 | 4 | 2-Possible, 3-Probable | ||
| Dysphagia | 1 | 1 | 1 | Unrelated | ||
| Nausea | 1 | 8 | 5 | 7-Probable, 1-Unrelated | ||
| Nausea | 2 | 1 | 1 | Probable/ Definite | ||
| Vomiting | 1 | 6 | 2 | 4-Probable, 2-Unlikely | ||
| Vomiting | 2 | 1 | 1 | Probable | ||
| General | Facial pain | 1 | 1 | 1 | Unrelated | |
| Fatigue | 1 | 2 | 2 | Possible | ||
| 2 | 2 | 1 | Unrelated, Possible | |||
| Flu-like symptoms | 1 | 2 | 1 | Unrelated | ||
| Infection and Infestation | Upper respiratory infection | 1 | 1 | 1 | Unrelated | |
| Injury | Fall (Mechanical) | 2 | 2 | 1 | Unrelated | |
| Investigations | ECG QTcF prolonged | 1 | 2 | 2 | Probable | |
| Musculoskeletal and Connective Tissue | Bone pain | 1 | 1 | 1 | Unrelated | |
| Back pain | 1 | 1 | 1 | Unrelated | ||
| Nervous System | Dizziness | 1 | 1 | 1 | Probable | |
| Paresthesia | 1 | 1 | 1 | Unrelated | ||
| Respiratory, Thoracic and Mediastinal | Dyspnea | 1 | 1 | 1 | Unrelated | |
| Dyspnea | 2 | 1 | 1 | Unrelated | ||
| Sore throat | 1 | 1 | 1 | Unrelated | ||
| 49.5 | Gastrointestinal | Diarrhea | 2 | 1 | 1 | Possible |
| Nausea | 1 | 1 | 1 | Probable | ||
| 2 | 1 | 1 | Probable | |||
| Vomiting | 1 | 1 | 1 | Probable | ||
| 2 | 1 | 1 | Probable | |||
| General | Fatigue | 1 | 1 | 1 | Possible | |
| Musculoskeletal and Connective Tissue | Back pain | 2 | 1 | 1 | Unrelated | |
| Pain in extremity | 2 | 1 | 1 | Unrelated |
Pharmacokinetics
Pharmacokinetic analysis and model simulations
Using the parameter estimates from the population PK model, the half-life was calculated to be approximately 18.5 hours. Table 4 provides the area under the curve, the maximum concentration (Cmax), and the time to maximum concentration (tmax) for each dose level. Figure 1A provides a simulation using the final parameter estimates from the PK model for each dose level after a single dose administration, which is in alignment with the data that were collected from the study and modeled. This simulation reveals that only the higher dose levels (33 mg and 49.5 mg) reach the desired therapeutic range after a single dose. Figure 1B provides a simulation for a new proposed dosing regimen of 11 mg dosed twice daily to achieve and remain within the desired concentration range.
Table 4.
PK parameters; parameters derived from the final population PK model per dose level
| Dose (mcg) |
AUC (mcg • hour/L) |
Cmax (mcg/L) |
tmax |
|---|---|---|---|
| (hour) | |||
| 5500 | 3629 | 214.9 | 3.65 |
| 1100 | 6160 | 300.8 | 4.05 |
| 16,500 | 8277 | 365.6 | 4.15 |
| 33,000 | 13,259 | 495.5 | 4.35 |
| 49,500 | 16,997 | 593.0 | 4.40 |
Fig. 1.
Dosing simulations. (a) Single dose simulation and (b) proposed dose simulation using the final population PK model; desired systemic sodium selenite range demarcated by 395 and 790 mcg/L (lower and upper black dotted lines, respectively).
Clinical Responses
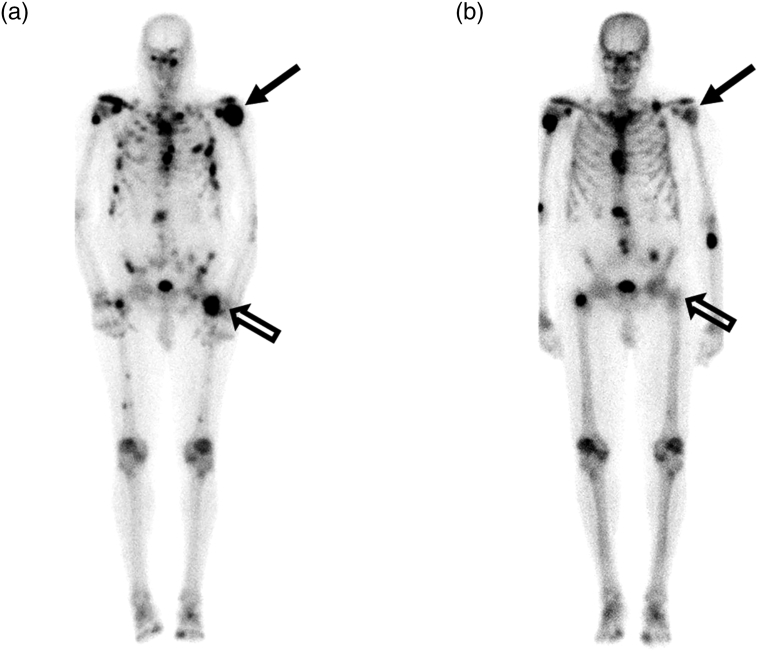
It is important to note that patient numbers were small, and the patient population heterogeneous, so it is not possible to draw any definitive conclusions about efficacy. Nevertheless, data were gathered for PSA in the subset of patients with prostate cancer (Table 5). All patients completed a pain inventory prior to treatment, on the last day of radiation therapy and at their first follow up visit (Table 6). Tumor response in the irradiated field was assessed as well (Table 7). An example of the response observed in a patient with CRPC is shown in Fig. 2. A comparison of pre and post treatment bone scans demonstrates a near complete response in the irradiated left hip in Patient 2.
Table 5.
PSA data
| Cohort-selenite dose (mg) | Patient number | PSA value Day 1 (ng/ml) | PSA value FU (ng/ml) | Time to FU visit (M) | % Change | Change |
|---|---|---|---|---|---|---|
| 5.5 | 2 | 39.83 | 8.79 | 2 | −77.9 | ↓ |
| 3 | 225 | 239 | 2.25 | 6.2 | ↑ | |
| 11 | 4 | 131.83 | 35.77 | 3.25 | −72.9 | ↓ |
| 5 | 40.61 | 19.25 | 4 | −52.6 | ↓ | |
| 6 | 0.98 | 0.32 | 4 | −67.3 | ↓ | |
| 16.5 | 7 | 1.74 | 21.85 | 2.25 | 1155.7 | ↑↑ |
| 8 | 3.3 | 1.34 | 2.75 | −59.4 | ↓ | |
| 9 | 1 | 0.89 | 3.25 | −11 | ↓ | |
| 33 | 10 | 196 | 64.46 | 3 | −67.1 | ↓ |
Table 6.
Average pain reduction
| Average change in numerical value from baseline |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dose cohort (mg) | Total (N = 15) | Assessment timepoint | Worst pain last 24 h mean ± SD | Least pain last 24 h mean ± SD | Average pain mean ± SD | Pain right now mean ± SD | ||||
| 5.5 mg | 3 | End of RT | −4 | ± 1.4 | −1.5 | ± 2.1 | −2.5 | ± 0.7 | −2 | ± 0 |
| First follow-up | −2.5 | ± 0.7 | −1.5 | ± 1.4 | −1.5 | ± 0.7 | −2 | ± 0 | ||
| 11 mg | 3 | End of RT | −1.7 | ± 1.5 | −2.7 | ± 3.8 | −2.3 | ± 4.0 | 1.7 | ± 2.9 |
| First follow-up | −0.7 | ± 5.0 | −1.3 | ± 4.9 | −0.7 | ± 4.6 | 1.7 | ± 1.2 | ||
| 16.5 mg | 3 | End of RT | −1 | ± 1.4 | −1 | ± 2.8 | −1.5 | ± 3.5 | −0.5 | ± 2.1 |
| First follow-up | −0.7 | ± 0.6 | −2.3 | ± 2.1 | −2 | ± 2 | −2.7 | ± 3.8 | ||
| 33 mg | 5 | End of RT | −1 | ± 2 | −0.2 | ± 0.5 | −1.2 | ± 1.3 | −0.6 | ± 1.1 |
| First follow-up | −2 | ± 2 | 0 | ± 1 | −1 | ± 1 | −0.8 | ± 1.3 | ||
| 49.5 mg | 1 | End of RT | −6 | ± 0 | −5 | ± 0 | −4.5 | ± 0 | −4 | ± 0 |
| First follow-up | −10 | ± 0 | −9 | ± 0 | −8.5 | ± 0 | −8 | ± 0 | ||
Abbreviation: RT = radiation therapy.
Table 7.
Tumor response in irradiated field
| Patient no. | Tumor response | Field | Imaging modality (months after treatment) |
|---|---|---|---|
| 1 | NE | Bone | BS (N/A) |
| 2 | Field 1: CR, Field 2: PR | Bone | BS (2 mo) |
| 3 | SD | Bone | BS (1 mo) |
| 4 | CR | Bone | BS (1 mo) |
| 5 | SD (↓ Intensity of uptake) | Bone | BS (3 mo) |
| 6 | PR (change from diffuse to patch involvement with ↓ intensity of uptake) | Bone | BS (5 mo) |
| 7 | SD (↓ intensity of uptake) | Bone | BS (1 mo) |
| 8 | SD | Bone | MRI (2 mo) |
| 9 | SD | Bone | CT and MRI (3 mo) |
| 10 | Field 1: PD, Field 2: SD | Bone | CT (4 mo) |
| 11 | PR (almost complete metabolic resolution) | Bone | PET-CT (6 mo) |
| 12 | SD | Lymph node | CT (1 mo) |
| 13 | PD | Bone | CT, MRI and PET-CT (1 mo) |
| 14 | NE (no FU scan because of clinical progression) | Bone | PET-CT (N/A) |
| 15 | SD | Bone | BS (2 mo) |
NE = not evaluated; no follow-up imaging.
For CT or MRI imaging: PR ≥30% ↓ sum of diameters.
PD ≥20% ↑ sum of diameters
For bone scan: CR resolution of uptake.
PR decrease in extent of involvement (# and size of lesion ≥50% observed).
SD same extent of involvement (# and size of lesions).
Fig. 2.
Patient #2, a 76-year-old male with disseminated prostate carcinoma who received sodium selenite in combination with radiotherapy for metastases in the left shoulder and left hip. Tc-99 m MDP bone scans pre (A) and 15 months post (B) treatment demonstrate near complete response in the left hip (open arrows) and partial response in the left shoulder (arrows).
Table 5 shows PSA values on Day 1 prior to the initiation of selenite and radiation therapy and the PSA value at the time of the first follow up, which ranged from 2–4 months following the completion of radiation therapy. Seven out of nine patients had a decrease in PSA with the magnitude of change ranging from an 11% to a 77.9% decrease. There was no evidence of a selenite dose response relationship, but patient numbers were too small and the patients too heterogeneous to draw a definitive conclusion about the presence or absence of a dose response relationship. These results are confounded by the concurrent use of ADT in the majority of the patients with CRPC. Of note, patients were maintained on whatever ADT they were on before study entry, as discontinuation of ADT could have been even more of a confounding variable. Interestingly, two of the three patients that were not receiving ADT had an increase in PSA.
The pain inventory captured four categories of pain as follows: worst pain in last 24 hours, least pain in last 24 hours, average pain and pain right now. Pain was scored using a 10 point scale, on which 0 was no pain and 10 was severe pain. Table 6 shows the average change in numerical value compared to baseline for two time points, baseline compared to the last day of radiation therapy, and baseline compared to the first follow up visit as a function of dose level. A change of at least one point was felt to be clinically meaningful. As can be seen, there was a generalized improvement in pain in all four categories at both time points, with a suggestion of a dose response relationship with some increase in pain (decreased change from baseline) in the lowest dose groups of 5.5 and 11 mg, but sustained or improved pain in the higher dose groups. These results are potentially confounded by a variety of factors including extent of disease, systemic therapy and pain medicine usage.
Lastly, tumor responses in the irradiated field are summarized in Table 7. All but one patient received palliative radiation therapy to symptomatic bone metastases. Quantitation of response in bone is difficult and imprecise. Furthermore, patients were imaged as per standard of care, using a variety of imaging modalities, including bone scan, which is not applicable to RECIST scoring. Acknowledging these limitations, of the evaluable patients (n = 13), eight patients had stable disease (SD) within the irradiated field (includes patient 10 with two sites of disease irradiated, with site dependent SD and progressive disease (PD) in the two sites respectively. Another patient also had PD. The remaining patients had significant improvement with complete resolution of bone scan abnormalities in two irradiated fields (patients two and four). Patient two also had a greater than 50% decrease in the number and size of lesions on bone scan, and patient 11 had almost complete metabolic resolution on PET CT. There was no apparent dose response relationship.
Discussion
Given the promising results with inorganic sodium selenite in preclinical tumor models and some early clinical trials, there is increasing interest in using selenite as a cytotoxic agent, and/or as a sensitizer. For example, in a study of newly diagnosed patients with non-Hodgkin's lymphoma treated with standard chemotherapy with or without adjuvant sodium selenite (0.2 mg/kg per day for 30 days), the patients receiving selenite had down-regulated levels of Bcl-2 and improved clinical outcomes [11]. In another study of selenite (0.2 mg/kg per day for 7 days) in combination with chemotherapy, addition of selenite resulted in a significant increase in the percentage of apoptotic lymphoma cells and clinical response compared to patients treated with chemotherapy alone [12]. Sodium selenite has also been studied in a variety of other tumor types, including colon cancer [13], and head and neck cancer [14]. In addition, patients with multiple tumor types were enrolled in a Phase one trial: the SECAR study, in which 34 patients with different resistant tumor types received i.v. sodium selenite daily for 5 consecutive days either for 2 or 4 weeks [15]. The MTD was defined as 10.2 mg/m2 with a calculated median plasma half-life of 18.25 hours. The most common side effects were fatigue, nausea and cramps in fingers and legs [15].
While there have been no clinical trials to date studying sodium selenite as a potential radiosensitizer, a randomized Phase three trial studied the ability of selenium to function as a radioprotector of normal tissues presumed to be secondary to enhanced antioxidant capacity, as organic selenium is used for the synthesis of antioxidant enzymes. This trial compared selenium supplementation (500 μg po on days of radiation therapy and 300 μg on days without radiation) with observation in patients with gynecologic malignancies treated with radiation therapy [16]. Interestingly, there was a reduction in the number of episodes and severity of radiation-induced diarrhea.
More relevant to our study, is the study by Corcoran et al. [17], using the inorganic form of selenium, sodium selenate (SeO4-, which is not as reactive with thiols as selenite, SeO32-). In this study, patients with castration-resistant prostate cancer received escalating doses of selenate orally. The MTD was 60 mg daily. Dose limiting toxicity (fatigue and diarrhea) occurred at 90 mg daily, with no grade 4 toxicity. One patient treated with 60 mg/day had a PSA response greater than 50% for 11 weeks, and the mean PSA doubling time nearly doubled in patients following treatment.
Given that sodium selenite depletes GSH and generates superoxide radicals when metabolized, it has the potential to radiosensitize multiple tumor types. Since in our preclinical studies, it radiosensitized tumors in vivo and did not sensitize GI epithelium to radiation (in fact it had a slight protective effect), it has the theoretical potential to significantly increase the therapeutic window for radiation therapy. This and other preclinical data to date, as well as early clinical data for other indications, provided a compelling rationale for the study of sodium selenite in combination with radiation therapy. In the study described here, the safety, tolerability and PK of sodium selenite was studied in 15 patients with advanced/metastatic tumors receiving concurrent sodium selenite with palliative radiation therapy. The 33 mg dose level had acceptable tolerability, with the primary toxicity being grade 1 GI side effects. These side effects were well controlled with ondansetron and loperamide-Hcl prn. It was concluded that 33 mg would be a reasonable dose for future studies when given orally, one time per day, with no oral intake for at least 2 hours prior.
The half-life obtained from the parameter estimates of the PK model is in agreement with what is reported in the literature. The SECAR study reported a median half-life of approximately 18 hours in patients with malignant disease receiving IV sodium selenite as a single agent [15]. Corcoran et al. noted significant accumulation of selenite, the active metabolite of selenate, following selenate administration in male patients diagnosed with castrate-resistant prostate cancer [17]. The PK model we have developed for this study captures the accumulation of selenite and appropriately characterizes the sparse data and variability in the patient population.
From the simulations for the PK model, it takes approximately 1 day to enter the desired therapeutic range for the 11 mg dose level when given twice daily. While a few dosing regimens are possible, 11 mg was chosen to minimize nausea.
Efforts were made to assess potential efficacy signals. Given the inherent limitations of small numbers, and the heterogeneous patient population in terms of tumor type, site of irradiation, radiation dose/fractionation, prior and concurrent systemic therapies, and lack of randomization, it is not possible to draw any definitive conclusions. Nevertheless, the majority of patients with prostate cancer did exhibit a decrease in PSA following treatment, and the majority of patients on the study had a decrease in pain indices. Lastly, the majority of patients had stabilization of disease within the radiation therapy field(s), with some demonstrating objective evidence of tumor regression. Similar findings can be observed in patients treated with radiation therapy without selenite, and a randomized, well controlled, study will be needed at the 33 mg dose level to determine if selenite results in clinically meaningful improvements in the response to palliative radiation therapy.
Acknowledgments
Acknowledgments
We thank Dr. Philip Sager for his expert review of ECGs, and the following physicians for facilitating the enrollment of their patients on this study: Drs. Beth Kidd, Susan Hiniker, Wendy Hara, and Steven Hancock. We also thank Rie von Eyben for assisting with the statistical plan for the protocol. SPARK Translational Research Program at the Stanford Cancer Institute provided funding for the clinical trial. The Office of Technology and Licensing at Stanford University provided funding for the manufacturing of the study drug, sodium selenite. Finally, the NIH National Center for Advancing Translational Sciences (Grant ULI-TR001085) provided funding for the Cancer Institute Clinical Trials Research Unit at Stanford University.
Declaration of Interest
Susan J. Knox is the co-founder of RadioRx, Inc. (now EpicentRx) and serves as a consultant to EpicentRX. Stacy L. Stamps-DeAnda is a shareholder with GlaxoSmithKline and AbbVie. Rada Savic serves as a paid consultant to Medimmune, Sanofi, Aventis, and UNITAID; she is also the founder of Insight Rx and scientific advisor to TB Alliance. Lei Shura is employed by Pharmacyclics an AbbVie Company. Authors have no other conflicts to report.
Funding
This work was supported by SPARK at Stanford University as well as the National Institutes of Health [Grant ULITR001085].
Role of the Funding Source
This study was initiated by Dr. Susan J. Knox who served as both PI and sponsor. Dr. Knox was involved with all aspects of the study and had full access to all data as well as full responsibility for the decision to publish. SPARK at Stanford University and the National Institutes of Health provided funding, but had no such involvement.
Data Sharing
Data beyond what is presented in the manuscript will not be made available to others. This manuscript provides detailed and comprehensive results. The study protocol and informed consent form will be provided upon e-mail request from the corresponding author (sknox@stanford.edu).
References
- 1.Husbeck B, Bhattacharyya RS, Feldman D, Knox SJ. Inhibition of androgen receptor signaling by selenite and methylseleninic acid in prostate cancer cells: two distinct mechanisms of action. Mol Cancer Ther. 2006;5(8):2078–2085. doi: 10.1158/1535-7163.MCT-06-0056. [DOI] [PubMed] [Google Scholar]
- 2.Husbeck B, Peehl DM, Knox SJ. Redox modulation of human prostate carcinoma cells by selenite increases radiation-induced cell killing. Free Radic Biol Med. 2005;38(1):50–57. doi: 10.1016/j.freeradbiomed.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 3.Husbeck B, Nonn L, Peehl DM, Knox SJ. Tumor-selective killing by selenite in patient-matched pairs of normal and malignant prostate cells. Prostate. 2006;66(2):218–225. doi: 10.1002/pros.20337. [DOI] [PubMed] [Google Scholar]
- 4.Bhattacharyya RS, Husbeck B, Feldman D, Knox SJ. Selenite treatment inhibits LAPC-4 tumor growth and prostate-specific antigen secretion in a xenograft model of human prostate cancer. Int J Radiat Oncol Biol Phys. 2008;72(3):935–940. doi: 10.1016/j.ijrobp.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 5.Tian J, Ning S, Knox SJ. Sodium selenite radiosensitizes hormone-refractory prostate cancer xenograft tumors but not intestinal crypt cells in vivo. Int J Radiat Oncol Biol Phys. 2010;78(1):230–236. doi: 10.1016/j.ijrobp.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 6.Corcoran NM, Najdovska M, Costello AJ. Inorganic selenium retards progression of experimental hormone refractory prostate cancer. J Urol. 2004;171(2):907–910. doi: 10.1097/01.ju.0000092859.16817.8e. Pt 1. [DOI] [PubMed] [Google Scholar]
- 7.Bump EA, Brown JM. Role of glutathione in the radiation response of mammalian cells in vitro and in vivo. Pharmacol Ther. 1990;47(1):117–136. doi: 10.1016/0163-7258(90)90048-7. [DOI] [PubMed] [Google Scholar]
- 8.Lau A, Villeneuve NF, Sun Z. Dual roles of Nrf2 in cancer. Pharmacol Res. 2008;58(5–6):262–270. doi: 10.1016/j.phrs.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cleveland CS, Ryan KM. Pain assessment: Global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23(2):129–138. [PubMed] [Google Scholar]
- 10.Wu JS, Beaton D, Smith PM, Hagen HA. Patterns of pain and interference in patients with painful bone metastases: a brief pain inventory validation study. J Pain Symptom Manage. 2010;39(2):230–240. doi: 10.1016/j.jpainsymman.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 11.Asfour IA, Fayek M, Raouf S. The impact of high-dose sodium selenite therapy on bcl-2 expression in adult non-hodgkin's lymphoma patients: correlation with response and survival. Biol Trace Elem Res. 2007;120(1–3):1–10. doi: 10.1007/s12011-007-0029-5. [DOI] [PubMed] [Google Scholar]
- 12.Asfour IA, El-Tehewi MM, Ahmed MH. High-dose sodium selenite can induce apoptosis of lymphoma cells in adult patients with non-Hodgkin's lymphoma. Biol Trace Elem Res. 2009;127(3):200–210. doi: 10.1007/s12011-008-8240-6. [DOI] [PubMed] [Google Scholar]
- 13.Al-Taie OH, Seufert J, Karvar S. Selenium supplementation enhances low selenium levels and stimulates glutathione peroxidase activity in peripheral blood and distal colon mucosa in past and present carriers of colon adenomas. Nutr Cancer. 2003;46(2):125–130. doi: 10.1207/S15327914NC4602_04. [DOI] [PubMed] [Google Scholar]
- 14.Büntzel J, Micke O, Kisters K. Selenium substitution during radiotherapy of solid tumours - laboratory data from two observation studies in gynaecological and head and neck cancer patients. Anticancer Res. 2010;30(5):1783–1786. [PubMed] [Google Scholar]
- 15.Brodin O, Eksborg S, Wallenberg M. Pharmacokinetics and toxicity of sodium selenite in the treatment of patients with carcinoma in a Phase I clinical trial: The SECAR Study. Nutrients. 2015;7(6):4978–4994. doi: 10.3390/nu7064978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muecke R, Schomburg L, Glatzel M. Multicenter phase 3 trial comparing selenium supplementation with observation in gynecologic radiation oncology. Int J Radiation Oncology Biol Phys. 2010;78(3):828–835. doi: 10.1016/j.ijrobp.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 17.Corcoran NM, Hovens CM, Michael M, Rosenthal MA, Costello AJ. Open-label phase I dose escalation study of sodium selenite, a novel activator of PP2A, in patients with castration-resistant prostate cancer. Br J Cancer. 2010;103(4):462–468. doi: 10.1038/sj.bjc.6605798. [DOI] [PMC free article] [PubMed] [Google Scholar]