Abstract
Acetaminophen (APAP) overdose is the most frequent cause of drug-induced liver injury in humans and a common chemical model to investigate genetic determinants of susceptibility to drug-induced liver injury (DILI). Previous studies performed in our laboratory identified the efflux transporter multidrug resistance-associated protein 4 (Mrp4) as an inducible gene in the liver following toxic APAP exposure in both humans and rodents. In mice, blockade of hepatic Mrp4 induction following APAP administration increases susceptibility towards APAP hepatotoxicity. Collectively, these findings suggest that Mrp4 plays an important role in tolerance to APAP-induced liver injury. To further study the role of Mrp4 in APAP-induced hepatotoxicity, we treated 10–12 weeks old male wild type (WT, C57BL/6J) and Mrp4 knockout (Mrp4−/−) mice with APAP (400 mg/Kg in saline, i.p.) or vehicle. Liver injury endpoints and hepatic gene expression were analyzed at 12, 24 and 48 h post-APAP injections. Unexpectedly, the kinetics of histologically measured liver damage and plasma ALT revealed that Mrp4−/ mice had decreased ALT levels and hepatic necrosis compared to WT mice only at 12 h. Notably, hepatic non-protein sulfhydryl (NPSH) levels were increased in the APAP treated Mrp4−/− mice at intervals less than 24 h, consistent with the capability of Mrp4 to export glutathione. Further gene expression analysis revealed that hepatic drug metabolism genes were downregulated in Mrp4−/− mice at earlier time points post-APAP administration. However, despite significant decreases in endpoints of liver injury detected at an early time point after APAP treatment, these changes were not sustained at later time points as Mrp4−/− mice ultimately had hepatic toxicity at levels comparable to WT mice. In conclusion, our data indicate that lack of Mrp4 by itself in mice does not alter susceptibility to APAP toxicity.
Keywords: Acetaminophen, Mrp4, Drug transporters, Drug-induced liver injury
1. Introduction
Acetaminophen (APAP) is one of the most common medications found in households and the most common drug ingredient in the USA. APAP is generally safe at therapeutic doses but can cause severe acute liver injury at supratherapeutic doses. APAP overdose is among the common causes of drug-induced liver injury (DILI) in the United States and worldwide [1,2]. In rodents, particularly mice, APAP overdose is a common chemical model to study mechanisms of hepatotoxicity and for identifying genetic determinants of susceptibility to DILI [3]. APAP is primarily metabolized in the liver by Phase II drug-metabolizing enzymes to APAP-glucuronide and APAP-sulfate, and excreted through urine and feces viahepatobiliary drug transporters [4]. A minute portion of the administered APAP is converted to N-acetyl-p-benzoquinone imine (NAPQI), a reactive toxic metabolite, by Cytochrome P450 (Cyp) enzymes [4]. In the liver, NAPQI is detoxified by glutathione conjugation and eliminated from hepatocytes by efflux transporters [4]. Previous studies in our laboratory have identified several factors and signaling molecules that can regulate these detoxification processes, which includes drug-metabolizing enzymes, drug transporters, and hepatic resident macrophages [[5], [6], [7]].
Multidrug resistance-associated protein 4 (Mrp4, Abcc4) belongs to the ATP-binding cassette (ABC) class of plasma membrane proteins, which is involved in efflux of several antiviral, anticancer, non-steroid anti-inflammatory drugs (NSAIDs), glutathione conjugates, and endogenous compounds such as bile acids and prostaglandins [8]. Mrp4 is expressed in several tissues, with highest expression in kidneys and choroid plexus [9,10]. In the liver, Mrp4 is expressed on the basolateral membrane of hepatocytes. Mrp4 is involved in excretion of xenobiotics and endogenous compounds from hepatocytes into sinusoidal blood [11]. At basal conditions, Mrp4 has minimal expression level in the liver [12], with substantial increments in expression under several hepatopathological conditions [10,11,13,14]. In mice, Mrp4 deficiency is linked to increased risk of liver injury, altered gut epithelial function and altered drug disposition [9,15,16]. Specifically, the lack of Mrp4 in mouse liver increases susceptibility towards cholestatic liver injury due to decreased hepatic clearance of bile acids [15]. Our laboratory has shown that hepatic Mrp4 expression increases with APAP-induced liver injury in both rodents and humans [6,17,18]. Increased hepatic Mrp4 expression with APAP intoxication is observed in proliferating hepatocytes and confined to centrilobular regions of the liver [5]. We also showed that Kupffer cells play an important role in the upregulation of Mrp4 expression and that blockage of hepatic compensatory cell proliferation and Mrp4 induction by antimitotic agent treatment worsens APAP liver injury [5,6]. Collectively, these studies suggest that upregulation of hepatic Mrp4 expression after APAP administration is critical for the development of tolerance to APAP-induced liver injury and tissue repair. However, the specific role of Mrp4 in the development of tolerance to drug-induced hepatotoxicity is still unknown.
In this study, we used Mrp4 knock out (Mrp4−/−) mice as our animal model to identify the role of Mrp4 in susceptibility to APAP-induced liver injury. We administered both wildtype (WT) and Mrp4−/− male mice a hepatotoxic dose of APAP and evaluated liver injury markers in plasma, histology findings and performed hepatic gene expression analysis of various drug metabolizing enzymes and transporters at different time points post-APAP administration. Our results show that overall, APAP administration resulted in a similar magnitude of liver injury in both genotypes, but the kinetics of hepatic damage were different. In particular, Mrp4−/− mice have a higher hepatic glutathione content and decreased markers of liver injury at an early time point post-APAP administration, but these differences are not maintained throughout all time points. Together our results indicate that the lack of Mrp4 does not alter susceptibility towards APAP-induced hepatotoxicity.
2. Material and methods
2.1. Animals and APAP treatment
Wildtype (WT, C57BL/6J) mice were purchased from Jackson Laboratories (Bar Harbor, ME). Mrp4 knockout (Mrp4−/−) mice were generated by the Schuetz laboratory on a C57BL6/129-SVJ background [9], and subsequently backcrossed for greater than 10 generations into a C57Bl6 genetic background [19]. Mrp4−/− mice were bred at the University of Connecticut. In this study, 10–12-week-old male WT and Mrp4−/− mice were fasted overnight. Following fasting, mice received either vehicle (saline) or APAP (400 mg/Kg, i.p.) [20]. Plasma and liver samples were collected at 12, 24 and 48 h post-injection. The vehicle-treated mice served as the 0 h time point group. One of the APAP-treated WT mice in the 48 h group died, most likely due to severe hepatoxicity, before this time point.
2.2. Plasma alanine aminotransferase (ALT) analysis
Plasma ALT levels were analyzed, as a biochemical index of liver injury, using Infinity ALT/GPT Liquid Reagent (Catalog: TR71121; Thermo Fisher Scientific™, Waltham, MA).
2.3. Hematoxylin and Eosin staining
Liver samples were fixed in 10% neutral buffered zinc formalin and processed for sectioning. Liver sections (5 μm) were stained with hematoxylin and eosin (H&E). Sections were scored for incidence and severity of necrosis by a board-certified veterinary pathologist. The H&E staining liver sections were scored in a blinded fashion and scores were tabulated from 0 to 5, with 0 being no injury and 5 being severe hepatic injury [21]. Scores are presented in Table 1.
Table 1.
Histopathological score of mice livers. Liver sections were incidence and severity scored for degenerative and necrotic changes and scored on a scale of 0–5; 5 being most severe injury and 0 indicative of no injury. Data were tabulated to indicate the number of mice in each group with a given score. Additionally, mice survival rates for each group were also included in the table. Data were ranked prior to statistical analysis. An asterisk “*” denotes significant difference between WT and Mrp4−/− mice.
| Histological Grade (WT mice) | ||||||||
|---|---|---|---|---|---|---|---|---|
| ID | 0 | 1 | 2 | 3 | 4 | 5 | Percent ≥ 2 | % Survival |
| Veh | 5 | 0 | 100 (5/5) | |||||
| 12 hr | 5 | 100 | 100 (5/5) | |||||
| 24 hr | 3 | 2 | 100 | 100 (5/5) | ||||
| 48 hr | 2 | 2 | 50 | 80 (4/5) | ||||
| Histological Grade (Mrp4−/− mice) | ||||||||
| ID | 0 | 1 | 2 | 3 | 4 | 5 | Percent ≥ 2 | % Survival |
| Veh | 5 | 0 | 100 (5/5) | |||||
| 12 hr | 1 | 4 | 0* | 100 (5/5) | ||||
| 24 hr | 2 | 3 | 60* | 100 (5/5) | ||||
| 48 hr | 3 | 2 | 40 | 100 (5/5) | ||||
2.4. RNA isolation and RT-qPCR analysis
Total RNA was isolated using a phenol-chloroform isolation method. cDNA was synthesized according to manufacturer protocol using iScript™ cDNA synthesis kit (Catalog: 170–8891, Bio-Rad Laboratories Inc., Hercules, CA). Gene expression analysis was performed with qPCR analysis using iTaq™ Universal SYBR® Green Supermix (Catalog: 172–5121, Bio-Rad Laboratories Inc.). Ribosomal protein 36B4 used as housekeeping gene for analysis.
2.5. Protein isolation and quantification
Liver homogenates and plasma membrane fractions were prepared using sucrose-Tris (ST) buffer (0.25 M sucrose, 10 mM Tris−HCl, pH 7.4) [22]. For western blot analysis, cytosolic (40 μg protein/lane) and membrane proteins (75 μg protein/lane) were electrophoretically resolved using 8–10% polyacrylamide gels and trans-blotted onto PVDF membrane [22]. Immunochemical detection of proteins was performed with anti-Mrp4 (M4I-10, Abcam, Cambridge, MA), glutamate-cysteine ligase catalytic (Gclc), glutamate-cysteine ligase modifier (Gclm) and -β-actin (ab8227, Abcam) primary antibodies [5]. Protein-antibody complexes were detected using an Immobilon™ Western chemiluminescent kit (Millipore, Billerica, MA) and exposed to CL-Xposure™ X-ray film (ThermoScientific, Rockford, IL).
2.6. Hepatic non-protein sulfhydryl (NPSH) levels
Liver NPSH values were determined as an indicator of reduced glutathione content using the procedure described by Ellman et al. [23].
2.7. Statistical analysis
Results are expressed as means + S.E.M. of 4–5 mice per group. Data were analyzed by two-way ANOVA followed by Bonferroni post-hoc test using Graph pad Prism5 software (GraphPad Software, Inc., La Jolla, CA). Differences were considered significant at p ≤ 0.05.
3. Results
3.1. Lack of Mrp4 did not alter APAP-induced hepatotoxicity in mice
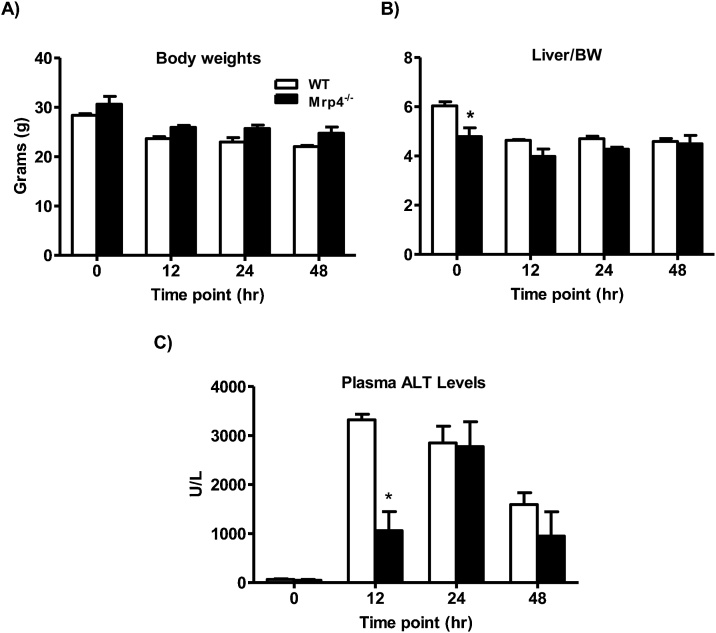
In mice, lack of Mrp4 did not cause detectable changes in body weight, but the liver to body weight ratios were significantly lower in Mrp4−/− mice at basal conditions (vehicle treatment only) (Fig. 1A & B). These differences in the liver to body weight ratios were not observed at any time point post-APAP injection (Fig. 1B). APAP administration (400 mg/Kg, i.p.) induced severe liver injury in both WT and Mrp4−/− mice, as evidenced by increased plasma ALT levels, a marker for liver injury [24], at all time points, compared to 0 h time point. (Fig. 1C). In WT mice, peak plasma ALT levels were observed at 12 h time point, whereas in peak plasma ALT levels in Mrp4−/− mice were observed at 24 h post-APAP injection. Interestingly, Mrp4−/− mice have significantly lower plasma ALT values at 12 h (2,858.8 ± 223.3 U/L) compared to WT mice (1,406 ± 291 U/L). This difference in plasma ALT between genotypes was not observed at the later time points of 24 and 48 h (Fig. 1C). Changes in plasma ALT levels after APAP treatment are in agreement with the H&E staining and histopathology scoring of tissue injury severity (Fig. 2 and Table 1), except for the 24 h time point where the Mrp4−/− mice histology scores are still lesser in magnitude than wild-types despite nearly equal ALT values in both groups.
Fig. 1.
Phenotypic changes in WT and Mrp4−/− mice treated with either vehicle or APAP (400 mg/Kg, i.p.). A) Body weights; B) Liver to body weight ratios; C) Plasma ALT levels. Data are presented as mean ± S.E.M. (p ≤ 0.05). An asterisk “*” denotes significant difference between WT and Mrp4−/− mice.
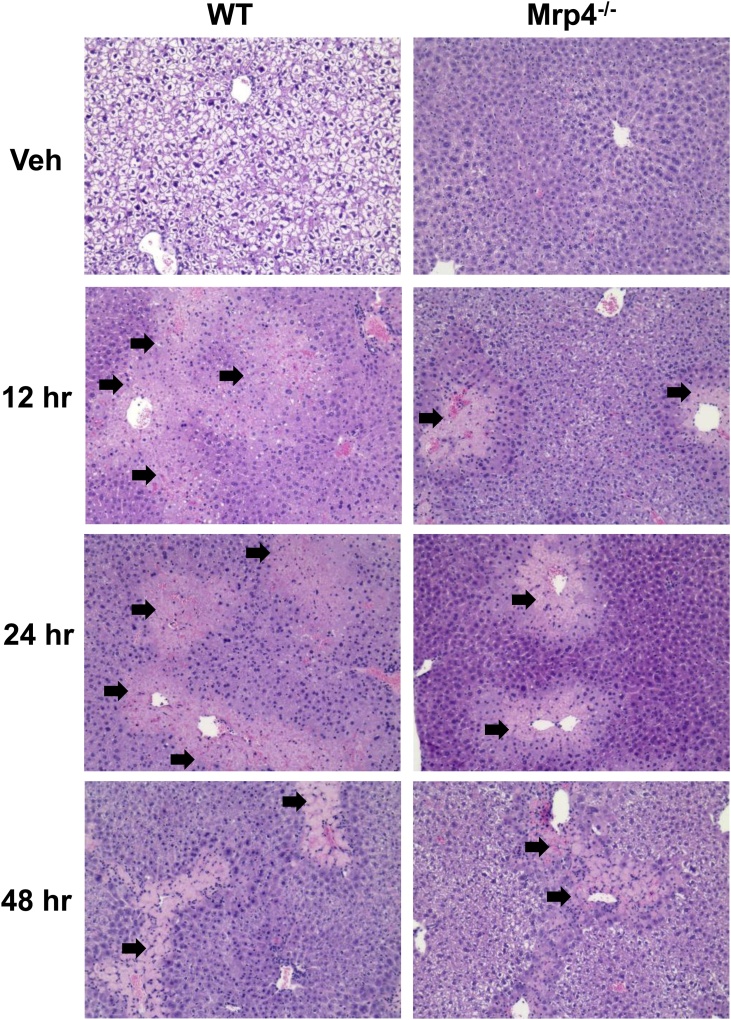
Fig. 2.
Histology of WT and Mrp4−/− mice livers mice treated with either vehicle or APAP (400 mg/Kg). Representative images of liver sections stained with hematoxylin and eosin for each genotype and time point (magnification 200X). Black arrows indicate necrotic areas.
In both WT and Mrp4−/− mice, APAP caused severe centrilobular necrosis (Fig. 2). Necrosis was most evident at 12 and 24 h in WT mice. Correlating with plasma ALT levels, Mrp4−/− mice had less hepatocellular necrosis at 12 h post-APAP exposure compared to their WT counterparts at the same time point. At 24 and 48 h, Mrp4−/− mice had similar liver injury incidence and severity as WT mice. Semiquantitative incidence and severity scores for necrosis correlated with plasma ALT findings. Collectively, the results of the biochemical and histopathological assessment of hepatocellular necrosis indicate that the absence of Mrp4 does not alter the overall susceptibility of mice toward APAP-induced hepatotoxicity, and that deficiency in Mrp4 function delayed the early appearance of toxicity.
3.2. Lack of Mrp4 alters the expression of hepatic genes expression that are involved in APAP metabolism
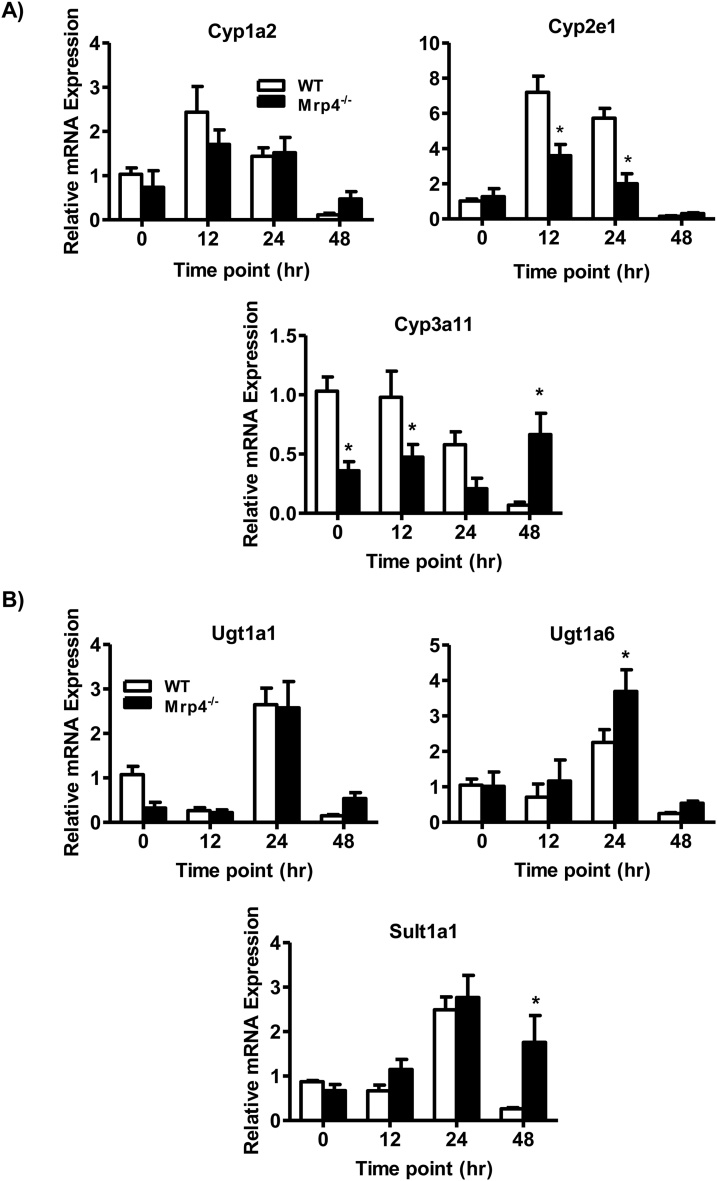
In the liver, APAP is extensively metabolized by Phase I and II drug-metabolizing enzymes. Among the Phase I enzymes, Cyp1a2, 2e1, and 3a11 play a major role in APAP metabolism and are known to be involved in the bioactivation of APAP into its reactive toxic metabolite NAPQI [4]. In mice, lack of Mrp4 does not result in alterations in basal hepatic Cyp1a2 or 2e1 mRNA expression levels. However, the basal mRNA expression of Cyp3a11 is significantly decreased in Mrp4−/− mice (Fig. 3A). This decrease in hepatic Cyp3a11 mRNA in Mrp4−/− mice is similar to decreasing trends reported previously [25]. Following APAP treatment, both Cyp2e1 and 3a11 gene expression is significantly downregulated in Mrp4−/− mice at 12 and 24 h compared to WT mice. Notably, at 48 h post-APAP administration, Cyp3a11 gene expression was significantly elevated in Mrp4−/− mice. Among the various phase II enzymes, Ugt1a1, 1a6 and Sult1a1 are majorly involved in APAP metabolism [4]. In mice, Mpr4 deficiency did not affect basal hepatic Ugt1a1, 1a6 and Sult1a1 gene expression (vehicle treated, time zero groups) (Fig. 3B). However, in mice receiving APAP, Ugt1a6 and Sult1a1 mRNA expression is significantly upregulated in Mrp4−/− mice at only 24 and 48 h, respectively, compared to WT mice at the same time points (Fig. 3B).
Fig. 3.
Gene expression analysis of hepatic Phase I and II enzymes involved in APAP metabolism. A) Cytochrome p450s: 1a2, 2e1 and 3a11 (Phase I); B) Ugt1a1, 1a6 and Sul1a1 (Phase II). Data are presented as mean ± S.E.M. (p ≤ 0.05). An asterisk “*” denotes significant difference between WT and Mrp4−/− mice.
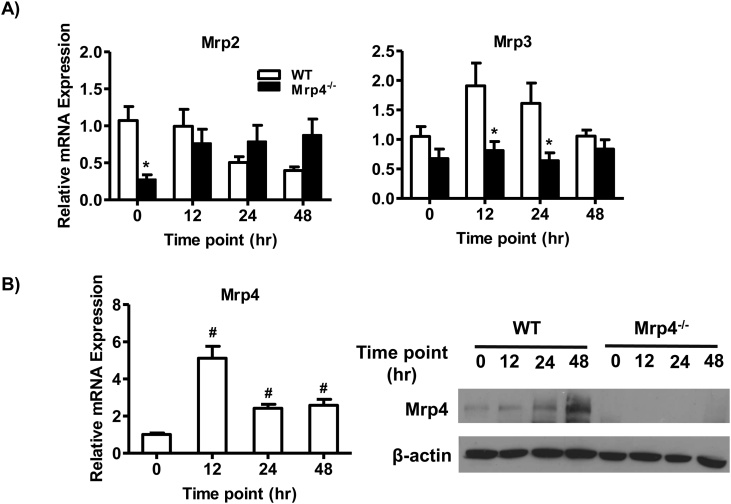
Hepatic efflux transporter such as multidrug resistance-associated protein 2 and 3, 4 are known to play an important role in APAP metabolism and disposition. The various conjugated metabolites of APAP are known substrates of efflux transporters [4]. In both humans and rodents, altered expression of hepatic Mrp proteins is shown to alter APAP disposition [26,27]. Similarly, Mrp transporters knockout mice have been reported to have altered susceptibility to APAP toxicity [[28], [29], [30]]. Under baseline conditions, hepatic Mrp2 mRNA levels were decreased in Mrp4−/− mice compared WT mice while hepatic Mrp3 mRNA expression was slightly lower though not statistically significant (Fig. 4A). These differences in hepatic Mrp2 expression were not observed post-APAP administration. Following APAP administration, Mrp4−/− mice had significantly lower hepatic Mpr3 mRNA values at 12 and 24 than WT groups (Fig. 4A). Our laboratory previously showed that hepatic Mrp4 gene and protein expression increases following toxic APAP exposure in mice [17] and humans [18]. In agreement with our previously published work, hepatic Mrp4 mRNA and protein expression increased in WT mice (Fig. 4B).
Fig. 4.
Gene and protein expression analysis of hepatic efflux transporters involved in APAP biotransformation and disposition. A) mRNA levels of Mrp2, Mrp3, and Mrp4; B) Protein expression of Mrp4 from crude liver membrane fractions. Data are presented as mean ± S.E.M. (p ≤ 0.05). An asterisk “*” denotes significant difference between WT and Mrp4−/− mice. A pound “#” denotes significance between 0 h time point and other time points in WT mice.
3.3. Mrp4 alters hepatic glutathione levels and the expression of glutathione-related genes
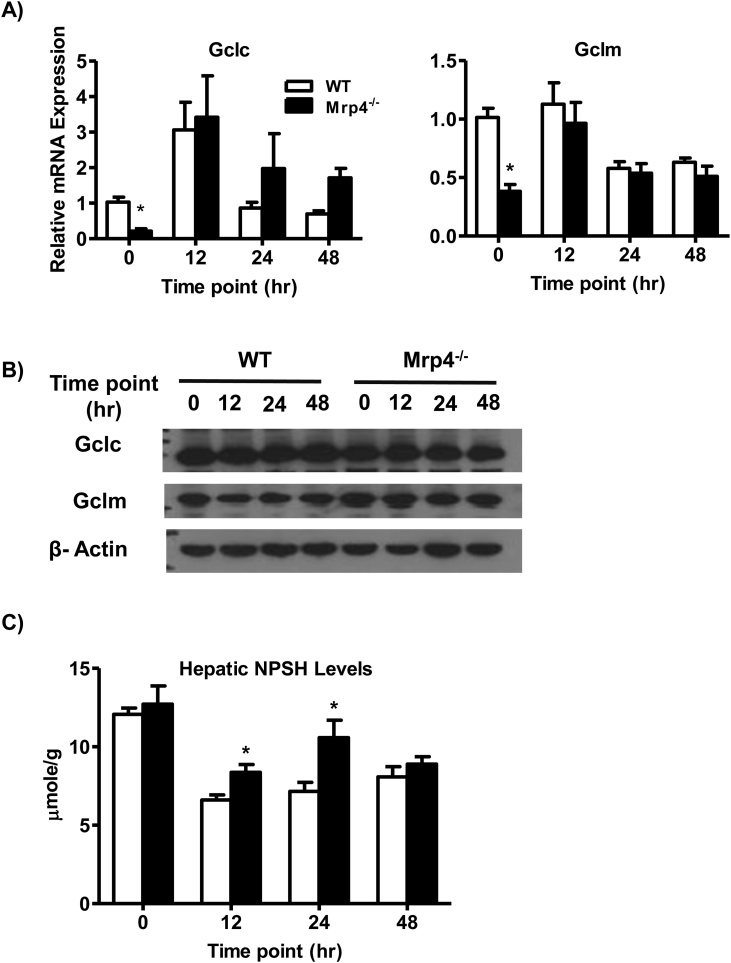
Hepatic glutathione levels play a pivotal role in the detoxification of the APAP toxic metabolite NAPQI [4]. Decreased hepatic glutathione content and gene expression of glutathione-related genes are known to increase susceptibility toward APAP-induced liver injury [31,32]. Fig. 5A shows that Mrp4 deficiency results in decreased expression of the glutathione synthesis genes Gclc and Gclm mRNA under basal condition. These differences in Gclc and Gclm gene expression are not observed post-APAP injection. Although basal Gclc and Gclm mRNA levels are significantly downregulated in Mrp4−/− mice, protein expression for these genes was not different between genotypes also under basal conditions (Fig. 5B). In agreement with the protein expression data for glutathione synthesis enzymes, basal hepatic NPSH levels, a marker for tissue glutathione levels, are similar between WT and Mrp4−/− mice. By contrast, NPSH values in Mrp4−/− mice are significantly greater at 12 and 24, but not at 48 h after APAP administration compared to their respective WT groups (Fig. 5C).
Fig. 5.
Analysis of hepatic glutathione content and expression of glutathione-related genes and proteins in WT and Mrp4−/− mice. A) mRNA levels of Gclc and Gclm; B) Western blot analysis of Gclc and Gclm; C) Hepatic non-protein sulfhydryl (NPSH) levels. Data are presented as mean ± S.E.M. (p ≤ 0.05). An asterisk “*” denotes significant difference between WT and Mrp4−/− mice.
4. Discussion
Drug transporters play a major role in detoxification processes, and changes in their expression and/or regulation can have profound effects in drug pharmacokinetics and pharmacodynamics [11,26,27,33]. A shift in hepatic APAP clearance due to altered expression of hepatic efflux transporters such as Mrp 2, 3 and 4 expression was theorized to be the cause of altered susceptibility to APAP-induced liver injury [34]. When this was proposed, studies in our laboratory were already showing that deficiency of Mrp3, a basolateral efflux transporter protein, protects mice from APAP toxicity [29]. Similarly, we also showed that Mrp2-deficient rats also exhibited resistance against APAP-induced hepatotoxicity due to the role of Mrp2 in mediating canalicular excretion of reduced glutathione, which is retained at significantly higher levels in the liver of these mutant rats [30]. Together, these studies highlight the importance of efflux drug transporters in altering not only the disposition of APAP and its metabolites, but also susceptibility towards APAP-induced liver injury. For Mrp4, we have previously shown that both in mice and humans, hepatic Mrp4 protein and gene expression is upregulated in livers after APAP intoxication and that suppression of Mrp4 upregulation increases the risk of liver injury due to APAP overdose [5,18]. Although the impact of genetic ablation of Mrp2 and 3 in APAP-induced liver injury has been fairly well-characterized, the role of Mrp4 as a genetic determinant of drug-induced hepatotoxicity was is still unknown. In this study, we evaluated whether Mrp4 has any role in altering APAP-induced liver injury or not.
The results presented here provide evidence that long-term post-APAP hepatotoxicity is not changed by the absence of Mrp4. Unexpectedly, Mrp4−/− mice had a lower incidence of liver injury only at 12 h post-APAP injection, as evidenced by decreased plasma ALT levels and histology scores. These changes in liver injury markers were not sustained at later time points. These early differences in liver injury can be attributed, in part, to decreased levels of Phase I enzymes expression in Mrp4−/− mice. Hepatic Cyp3a11 is involved in the bioactivation of APAP into its toxic metabolite NAPQI. Studies in Pregnane-X-Receptor (Pxr) knockout mice indicate that decreased hepatic Cyp3a11 expression may lead to decreased NAPQI formation, potentially explaining the reduced susceptibility of these mutant mice to liver injury by APAP [35]. As lower levels of Cyp3a11 gene expression in Mrp4−/− mouse liver increased at later time points post-APAP injection, the magnitude of APAP-induced hepatic toxicity also increased to values indistinguishable between null and wildtype mice. In addition to hepatic Cyp3a11, Cyp2e1 also plays an important role in APAP metabolism and it is well known that lack of Cyp2e1 protects against APAP-induced liver injury [36]. In Mrp4−/− mice, basal hepatic Cyp2e1 expression patterns are similar to Cyp3a11 expression. Together, these findings suggest that the differences in Phase I enzymes gene expression between genotypes indirectly contributed to the lower hepatotoxicity by APAP observed at the early time point examined, but are not sufficient to change the overall toxicity outcome.
Glutathione conjugation processes play an important role in the detoxification and mitigating oxidative stress produced by APAP intoxication [4]. Depletion and replenishment of hepatic glutathione alter susceptibility towards APAP intoxication and other xenobiotics that produce electrophilic and oxidative stress. Mrp4 is a transporter that is known to mediate the cellular efflux of glutathione and glutathione conjugates. Previous studies have shown that Mrp4 overexpression leads to increases in cellular export of glutathione [37]. The results of our studies show that basal glutathione levels are not altered in Mrp4−/− mice, but they were increased at 12 and 24 h after APAP treatment. There are alternative possibilities that might also account for elevated hepatic glutathione status in Mrp4−/− mice such as reduced hepatic toxicity and/or less GSH utilization or perhaps lower levels of basal Mrp2 in the Mrp4−/− mice. However, this latter possibility seems unlikely as Mrp2 levels are readily increased in the Mrp4−/− mice post-APAP treatment.
In conclusion, our earlier studies showing that Mrp4 upregulation after APAP was indicative of a potential protective role of this transporter in APAP hepatotoxicity [5,17], and suggested that Mrp4 absence might promote liver damage provided the justification for the current studies investigating the susceptibility of Mpr4 knockout mice to APAP hepatotoxicity. Contrary to our predictions, the current findings indicate that lack of Mrp4 does not enhance susceptibility towards APAP-induced hepatotoxicity. Instead, these knockout mice had decreased liver injury at the earliest time point after APAP treatment. This seems to be related to compensatory changes in glutathione homeostasis and suggests a broader role for Mrp4 in hepatic glutathione homeostasis under APAP treatment, but possibly other hepatotoxic challenges. However, this lower susceptibility to toxicity was not sustained at later time points in these mice. This transient differential susceptibility to APAP hepatotoxicity may also be due to lower bioactivation of APAP in Mrp4-null mice, which exhibit lower levels of Phase I enzyme gene expression at early time points. As Phase I enzyme expression pattern changes toward expression values in wildtype mice following APAP, the magnitude of liver injury becomes nearly identical in both genotypes. Our laboratory continues to investigate the mechanistic role of increases in Mrp4 expression in liver repair and regeneration based on our previous observations indicating that upregulation of hepatic Mrp4 is observed nearly exclusively in proliferating hepatocytes in close proximity to the central vein [5]. Rather than a direct role in promoting compensatory cell proliferation, enhanced Mrp4 expression during drug liver injury may be a generalized compensatory response that increases the efflux of chemicals that if retained in the liver might promote damage to DNA and other cellular constituents during a critical period when active cell division and repair essential for full tissue recovery. In closing, our study has revealed a potential contribution of Mrp4 function in affecting glutathione to impact the kinetics of in hepatoprotection following chemical-induced liver injury by APAP. Given the existence of non-functional human MRP4 alleles [38] it is possible that population variation in these alleles contributes to variation in APAP hepatotoxicity. The studies also provide valuable information on basal differential gene expression of drug-metabolizing enzyme and transporters in mice deficient in Mrp4. The studies also provide the foundation for future studies investigating the role of Mrp4 in other models of liver tissue regeneration.
Declaration of Competing Interest
All authors declare no conflict of interest
Acknowledgments
We would like to acknowledge Oladimeji Aladelokun and Steven Toro for their help in performing animal experiments. This study was supported by P30 CA021765 Cancer Center Support grant, 5R01CA194206 and ALSAC (J.D.S).
References
- 1.Larson A.M., Polson J., Fontana R.J., Davern T.J., Lalani E., Hynan L.S., Reisch J.S., Schiodt F.V., Ostapowicz G., Shakil A.O., Lee W.M., Acute Liver Failure Study, G Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology. 2005;42:1364–1372. doi: 10.1002/hep.20948. [DOI] [PubMed] [Google Scholar]
- 2.Lee W.M. Acetaminophen (APAP) hepatotoxicity-Isn’t it time for APAP to go away? J. Hepatol. 2017;67:1324–1331. doi: 10.1016/j.jhep.2017.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banerjee S., Melnyk S.B., Krager K.J., Aykin-Burns N., McCullough S.S., James L.P., Hinson J.A. Trifluoperazine inhibits acetaminophen-induced hepatotoxicity and hepatic reactive nitrogen formation in mice and in freshly isolated hepatocytes. Toxicol. Rep. 2017;4:134–142. doi: 10.1016/j.toxrep.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGill M.R., Jaeschke H. Metabolism and disposition of acetaminophen: recent advances in relation to hepatotoxicity and diagnosis. Pharm. Res. 2013;30:2174–2187. doi: 10.1007/s11095-013-1007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aleksunes L.M., Campion S.N., Goedken M.J., Manautou J.E. Acquired resistance to acetaminophen hepatotoxicity is associated with induction of multidrug resistance-associated protein 4 (Mrp4) in proliferating hepatocytes. Toxicol. Sci.: Off. J. Soc. Toxicol. 2008;104:261–273. doi: 10.1093/toxsci/kfn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campion S.N., Johnson R., Aleksunes L.M., Goedken M.J., van Rooijen N., Scheffer G.L., Cherrington N.J., Manautou J.E. Hepatic Mrp4 induction following acetaminophen exposure is dependent on Kupffer cell function. Am. J. Physiol. Gastrointest. Liver Physiol. 2008;295:G294–304. doi: 10.1152/ajpgi.00541.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rudraiah S., Rohrer P.R., Gurevich I., Goedken M.J., Rasmussen T., Hines R.N., Manautou J.E. Tolerance to acetaminophen hepatotoxicity in the mouse model of autoprotection is associated with induction of flavin-containing monooxygenase-3 (FMO3) in hepatocytes. Toxicol. Sci.: Off. J. Soc. Toxicol. 2014;141:263–277. doi: 10.1093/toxsci/kfu124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Russel F.G., Koenderink J.B., Masereeuw R. Multidrug resistance protein 4 (MRP4/ABCC4): a versatile efflux transporter for drugs and signalling molecules. Trends Pharmacol. Sci. 2008;29:200–207. doi: 10.1016/j.tips.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 9.Leggas M., Adachi M., Scheffer G.L., Sun D., Wielinga P., Du G., Mercer K.E., Zhuang Y., Panetta J.C., Johnston B., Scheper R.J., Stewart C.F., Schuetz J.D. Mrp4 confers resistance to topotecan and protects the brain from chemotherapy. Mol. Cell. Biol. 2004;24:7612–7621. doi: 10.1128/MCB.24.17.7612-7621.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maher J.M., Slitt A.L., Cherrington N.J., Cheng X., Klaassen C.D. Tissue distribution and hepatic and renal ontogeny of the multidrug resistance-associated protein (Mrp) family in mice. Drug Metab. Dispos. 2005;33:947–955. doi: 10.1124/dmd.105.003780. [DOI] [PubMed] [Google Scholar]
- 11.Klaassen C.D., Aleksunes L.M. Xenobiotic, bile acid, and cholesterol transporters: function and regulation. Pharmacol. Rev. 2010;62:1–96. doi: 10.1124/pr.109.002014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Assem M., Schuetz E.G., Leggas M., Sun D., Yasuda K., Reid G., Zelcer N., Adachi M., Strom S., Evans R.M., Moore D.D., Borst P., Schuetz J.D. Interactions between hepatic Mrp4 and Sult2a as revealed by the constitutive androstane receptor and Mrp4 knockout mice. J. Biol. Chem. 2004;279:22250–22257. doi: 10.1074/jbc.M314111200. [DOI] [PubMed] [Google Scholar]
- 13.Zollner G., Wagner M., Fickert P., Silbert D., Gumhold J., Zatloukal K., Denk H., Trauner M. Expression of bile acid synthesis and detoxification enzymes and the alternative bile acid efflux pump MRP4 in patients with primary biliary cirrhosis. Liver Int.: Off. J. Int. Assoc. Study Liver. 2007;27:920–929. doi: 10.1111/j.1478-3231.2007.01506.x. [DOI] [PubMed] [Google Scholar]
- 14.Donepudi A.C., Cheng Q., Lu Z.J., Cherrington N.J., Slitt A.L. Hepatic transporter expression in metabolic syndrome: phenotype, serum metabolic hormones, and transcription factor expression. Drug Metab. Dispos. 2016;44:518–526. doi: 10.1124/dmd.115.066779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mennone A., Soroka C.J., Cai S.Y., Harry K., Adachi M., Hagey L., Schuetz J.D., Boyer J.L. Mrp4-/- mice have an impaired cytoprotective response in obstructive cholestasis. Hepatology. 2006;43:1013–1021. doi: 10.1002/hep.21158. [DOI] [PubMed] [Google Scholar]
- 16.Li C., Krishnamurthy P.C., Penmatsa H., Marrs K.L., Wang X.Q., Zaccolo M., Jalink K., Li M., Nelson D.J., Schuetz J.D., Naren A.P. Spatiotemporal coupling of cAMP transporter to CFTR chloride channel function in the gut epithelia. Cell. 2007;131:940–951. doi: 10.1016/j.cell.2007.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aleksunes L.M., Slitt A.M., Cherrington N.J., Thibodeau M.S., Klaassen C.D., Manautou J.E. Differential expression of mouse hepatic transporter genes in response to acetaminophen and carbon tetrachloride. Toxicol. Sci.: Off. J. Soc. Toxicol. 2005;83:44–52. doi: 10.1093/toxsci/kfi013. [DOI] [PubMed] [Google Scholar]
- 18.Barnes S.N., Aleksunes L.M., Augustine L., Scheffer G.L., Goedken M.J., Jakowski A.B., Pruimboom-Brees I.M., Cherrington N.J., Manautou J.E. Induction of hepatobiliary efflux transporters in acetaminophen-induced acute liver failure cases. Drug Metab. Dispos. 2007;35:1963–1969. doi: 10.1124/dmd.107.016170. [DOI] [PubMed] [Google Scholar]
- 19.Cheepala S.B., Pitre A., Fukuda Y., Takenaka K., Zhang Y., Wang Y., Frase S., Pestina T., Gartner T.K., Jackson C., Schuetz J.D. The ABCC4 membrane transporter modulates platelet aggregation. Blood. 2015;126:2307–2319. doi: 10.1182/blood-2014-08-595942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghanem C.I., Rudraiah S., Bataille A.M., Vigo M.B., Goedken M.J., Manautou J.E. Role of nuclear factor-erythroid 2-related factor 2 (Nrf2) in the transcriptional regulation of brain ABC transporters during acute acetaminophen (APAP) intoxication in mice. Biochem. Pharmacol. 2015;94:203–211. doi: 10.1016/j.bcp.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 21.Manautou J.E., Hoivik D.J., Tveit A., Hart S.G., Khairallah E.A., Cohen S.D. Clofibrate pretreatment diminishes acetaminophen’s selective covalent binding and hepatotoxicity. Toxicol. Appl. Pharmacol. 1994;129:252–263. doi: 10.1006/taap.1994.1250. [DOI] [PubMed] [Google Scholar]
- 22.Donepudi A.C., Aleksunes L.M., Driscoll M.V., Seeram N.P., Slitt A.L. The traditional ayurvedic medicine, Eugenia jambolana (Jamun fruit), decreases liver inflammation, injury and fibrosis during cholestasis. Liver Int.: Off. J. Int. Assoc. Study Liver. 2012;32:560–573. doi: 10.1111/j.1478-3231.2011.02724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ellman G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 24.Kim W.R., Flamm S.L., Di Bisceglie A.M., Bodenheimer H.C., Public Policy Committee of the American Association for the Study of Liver, D Serum activity of alanine aminotransferase (ALT) as an indicator of health and disease. Hepatology. 2008;47:1363–1370. doi: 10.1002/hep.22109. [DOI] [PubMed] [Google Scholar]
- 25.Morgan J.A., Cheepala S.B., Wang Y., Neale G., Adachi M., Nachagari D., Leggas M., Zhao W., Boyd K., Venkataramanan R., Schuetz J.D. Deregulated hepatic metabolism exacerbates impaired testosterone production in Mrp4-deficient mice. J. Biol. Chem. 2012;287:14456–14466. doi: 10.1074/jbc.M111.319681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Slitt A.L., Cherrington N.J., Maher J.M., Klaassen C.D. Induction of multidrug resistance protein 3 in rat liver is associated with altered vectorial excretion of acetaminophen metabolites. Drug Metab. Dispos. 2003;31:1176–1186. doi: 10.1124/dmd.31.9.1176. [DOI] [PubMed] [Google Scholar]
- 27.Canet M.J., Merrell M.D., Hardwick R.N., Bataille A.M., Campion S.N., Ferreira D.W., Xanthakos S.A., Manautou J.E., HH A.K., Erickson R.P., Cherrington N.J. Altered regulation of hepatic efflux transporters disrupts acetaminophen disposition in pediatric nonalcoholic steatohepatitis. Drug Metab. Dispos. 2015;43:829–835. doi: 10.1124/dmd.114.062703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen C., Hennig G.E., Manautou J.E. Hepatobiliary excretion of acetaminophen glutathione conjugate and its derivatives in transport-deficient (TR-) hyperbilirubinemic rats. Drug Metab. Dispos. 2003;31:798–804. doi: 10.1124/dmd.31.6.798. [DOI] [PubMed] [Google Scholar]
- 29.Manautou J.E., de Waart D.R., Kunne C., Zelcer N., Goedken M., Borst P., Elferink R.O. Altered disposition of acetaminophen in mice with a disruption of the Mrp3 gene. Hepatology. 2005;42:1091–1098. doi: 10.1002/hep.20898. [DOI] [PubMed] [Google Scholar]
- 30.Silva V.M., Thibodeau M.S., Chen C., Manautou J.E. Transport deficient (TR-) hyperbilirubinemic rats are resistant to acetaminophen hepatotoxicity. Biochem. Pharmacol. 2005;70:1832–1839. doi: 10.1016/j.bcp.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 31.Botta D., Shi S., White C.C., Dabrowski M.J., Keener C.L., Srinouanprachanh S.L., Farin F.M., Ware C.B., Ladiges W.C., Pierce R.H., Fausto N., Kavanagh T.J. Acetaminophen-induced liver injury is attenuated in male glutamate-cysteine ligase transgenic mice. J. Biol. Chem. 2006;281:28865–28875. doi: 10.1074/jbc.M605143200. [DOI] [PubMed] [Google Scholar]
- 32.McConnachie L.A., Mohar I., Hudson F.N., Ware C.B., Ladiges W.C., Fernandez C., Chatterton-Kirchmeier S., White C.C., Pierce R.H., Kavanagh T.J. Glutamate cysteine ligase modifier subunit deficiency and gender as determinants of acetaminophen-induced hepatotoxicity in mice. Toxicol. Sci.: Off. J. Soc. Toxicol. 2007;99:628–636. doi: 10.1093/toxsci/kfm165. [DOI] [PubMed] [Google Scholar]
- 33.Cherkas Y., McMillian M.K., Amaratunga D., Raghavan N., Sasaki J.C. ABC gene-ranking for prediction of drug-induced cholestasis in rats. Toxicol. Rep. 2016;3:252–261. doi: 10.1016/j.toxrep.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ghanem C.I., Ruiz M.L., Villanueva S.S., Luquita M.G., Catania V.A., Jones B., Bengochea L.A., Vore M., Mottino A.D. Shift from biliary to urinary elimination of acetaminophen-glucuronide in acetaminophen-pretreated rats. J. Pharmacol. Exp. Ther. 2005;315:987–995. doi: 10.1124/jpet.105.090613. [DOI] [PubMed] [Google Scholar]
- 35.Guo G.L., Moffit J.S., Nicol C.J., Ward J.M., Aleksunes L.A., Slitt A.L., Kliewer S.A., Manautou J.E., Gonzalez F.J. Enhanced acetaminophen toxicity by activation of the pregnane X receptor. Toxicol. Sci.: Off. J. Soc. Toxicol. 2004;82:374–380. doi: 10.1093/toxsci/kfh286. [DOI] [PubMed] [Google Scholar]
- 36.Lee S.S., Buters J.T., Pineau T., Fernandez-Salguero P., Gonzalez F.J. Role of CYP2E1 in the hepatotoxicity of acetaminophen. J. Biol. Chem. 1996;271:12063–12067. doi: 10.1074/jbc.271.20.12063. [DOI] [PubMed] [Google Scholar]
- 37.Lai L., Tan T.M. Role of glutathione in the multidrug resistance protein 4 (MRP4/ABCC4)-mediated efflux of cAMP and resistance to purine analogues. Biochem. J. 2002;361:497–503. doi: 10.1042/0264-6021:3610497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krishnamurthy P., Schwab M., Takenaka K., Nachagari D., Morgan J., Leslie M., Du W., Boyd K., Cheok M., Nakauchi H., Marzolini C., Kim R.B., Poonkuzhali B., Schuetz E., Evans W., Relling M., Schuetz J.D. Transporter-mediated protection against thiopurine-induced hematopoietic toxicity. Cancer Res. 2008;68:4983–4989. doi: 10.1158/0008-5472.CAN-07-6790. [DOI] [PMC free article] [PubMed] [Google Scholar]