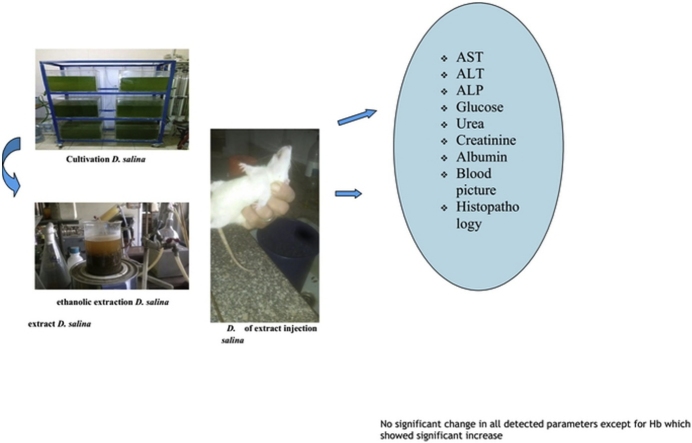
Graphical abstract
Keywords: Dunaliella salina, Liver function enzymes, Blood picture, Histopathological examination
Highlights
-
•
No significant effect of Dunaliella salina on hematological and biochemical studies.
-
•
Hb levels showed significant increase in both gender of mice and rats post oral administration of Dunaliella salina.
-
•
No pathological changes were observed in cardiac, hepatic and renal tissues.
-
•
Chronic study revealed the oral safety of Dunaliella salina.
Abstract
The chronic toxicity of the Dunaliella salina microalgae was examined to evaluate its toxicity by the exposure of laboratory animals to high doses of Dunaliella salina and to estimate the possibility of using it as a safe supplement. Different hematological and biochemical analysis including complete blood picture (CBC), liver function enzyme activities; aminotransferases (ALT and AST), alkaline phosphatase (ALP), total bilirubin, kidney function tests; urea, creatinine, and albumin, as well as blood glucose level, were measured. The histopathological investigation was also carried out on hepatic, renal and cardiac architectures to examine its safety. Treatment with the dose 100 mg /kg body weight of D. salina powder daily for three consecutive months did not show any signs of toxicity in both genders and in mice and rats (no mortality, no hair loss, no diarrhea, no patches of yellow color appearance, etc…..). Moreover, abnormalities on behavior, food and water intakes and health status among the treated animals were not observed. CBC profile revealed a significant increase in hemoglobin (Hb) level in treating male and female mice and rats compared to their related control levels. The biochemical analysis clearly showed an insignificant change in liver enzyme activities, blood glucose level at a dose of 100 mg/kg. Also, an insignificant reduction in total urea and creatinine levels in both genders of mice and rats were noticed. Histopathological investigation showed normal architectures of all organs. Hence we can conclude that Dunaliella salina has been proven a safe profile up to 100 mg/kg body weight, however, it succeeded to stimulate the Hb synthesis compared to control groups, showing its benifits to be used safely as food additives or protective and curative agent in different diseases in future.
1. Introduction
Dunaliella salina Chlorophyceae unicellular green algae are considered one of the most important species for commercial production due to the presence of β-carotene and other active compounds such as lutein zeaxanthin chlorophyll, and polyunsaturated fatty acids. Dunaliella salina because of β-carotene abundance, is seen as a potent antioxidant and vitamin A precursor so, it utilized as a health food supplement, food coloring agent, cosmetics additive [1]. There is a large body of evidence documented that Dunaliella salina may promote health-modulating effects and participate in diminishing the risk of developing different metabolic diseases [2].
It was reported that Dunaliella salina rich carotenoid as trans -β- carotene and 9- or 9′- cis -β-carotene, lutein, and zeaxanthin are considered the major identified carotenoids with the potent antioxidant efficiency [3].
No information is available in the literature up- till now for long-term toxicity study of Dunaliella salina (chronic toxicity). So, the present work is regarded as the first study concerning the chronic study of Dunaliella salina using the dose 100 mg/kg body weight daily supplemented orally for three consecutive months to both genders of mice and rats. In this study, hematological and biochemical parameters including liver enzymes activity, kidney function tests, and blood glucose level were evaluated. Besides, histopathological examination of cardiac, hepatic and renal tissues of both male and female mice and rats was investigated.
2. Materials and methods
2.1. Dunaliella salina cultivation in laboratory scale
Dunaliella salina species was isolated from water of River Nile using media named BG11 for isolation and purification of algae. Dunaliella salina was grown in 2 L of media for two weeks [4,5]. After the growth, the biomass of algae was harvested and grown for an additional two weeks. Then, the algal biomass was harvested again and grown in a plastic jar whose 17 L capacity and containing 15 L of culture media. The temperature of culture was 20 ± 3 °C with aeration. Stable fluorescent light was applied for the culture with intensity ≈2500 lx.
2.2. Dunaliella salina cultivation in the Photo-bioreactor
Post 10 days of growing the inoculums were put in a vertical photobioreactor with the capacity of 4000 L, automated and computerized.
2.3. Animals
Male and female Swiss mice with an average weight of 20–30 g as well as the male and female Wistar albino rats weighing 120–130 g were obtained from Animal House Lab, National Research Centre (NRC), Dokki, Giza were used in this study. Animals were acclimatized for one week before the starting of the experiment (adaptation period). The animals were housed with a well-ventilation (22 ± 20 °C) with a cycle of twelve hours. Normal basal diets and water were supplied ad libitum. Animals were cared according to the Ethical Committee guidelines of NRC, Giza, Egypt for animal experiments, with ethical approval no: 13,115.
2.4. Experimental design
One hundred and twenty male and female mice and rats were used in this study; thirty mice and rats for each gender were supplemented orally with 100 mg /kg body weight of Dunaliella salina powdered and were divided into eight groups as follows:
Groups 1 and 2: Control male and female mice (15 mice each) were daily orally administered 0.9% normal saline solution for three consecutive months.
Groups 3 and 4: Male and female mice (15 mice each) were supplanted orally daily with 100 mg/ kg body weight of powdered of Dunaliella salina for three consecutive months.
Groups 5 and 6: Control male and female rats (15 rats each) as described aforementioned.
Groups 7 and 8: Male and female rats were administered orally daily with 100 mg/kg body weight of powdered of Dunaliella salina daily for three consecutive months (15 mice each). The animals were observed daily for behavioral, food and water intakes.
After the three months, all animals were sacrificed. Fasting blood samples were collected by puncture of the sublingual vein and left for clotting then, centrifuged at 3000 rpm for 15 min to separate serum for liver and kidney function tests [[6], [7], [8]]. Biochemical parameters were determined in serum using Biodiagnostic kits (Bio diagnostics Co., Egypt).
For hepatic, cardiac and renal tissues; the histopathological examination was carried out, tissue slices were fixed in 10% formaldehyde and embedded in paraffin wax blocks. Sections of 5 μm thick were stained with hematoxylin and eosin (H&E) then examined under a light microscope for determination of pathological changes.
2.5. Hematological parameters
Erythrocytes count, hemoglobin (Hb) levels and hematocrit (PCVt) were determined immediately in blood samples [9]. White blood cell (WBC) count was determined as described by Schaperclaus et al. [10].
2.6. Biochemical parameters
Liver function enzyme activities, alanine and aspartate aminotransferases (AST and ALT), as well as alkaline phosphatase (ALP), were estimated in mice and rat sera [[11] [12], respectively]. The levels of total urea and creatinine were determined according to the methods of Bartles et al. [13] and Fawcett and Scott [14]. The level of bilirubin was also determined [15]. Glucose level was measured using colorimetric kits according to the method of Trinder [16].
2.7. Statistical analysis
Statistical analysis for biochemical parts is carried out using the SPSS computer program (version 8) combined with a costate computer program, where unshared letters are significant at P≤0.05.
3. Results
Treatment of both male and female rats and mice with the dose 100 mg /kg body weight of powdered of Dunaliella salina daily for three consecutive months did not show any toxicity sign in both sex in mice and rats (no mortality, no hair loss, no diarrhea, no patches of yellow color appearance etc…..). In addition, abnormalities on behavior, food and water intakes, and health status among the treated animals did not observe.
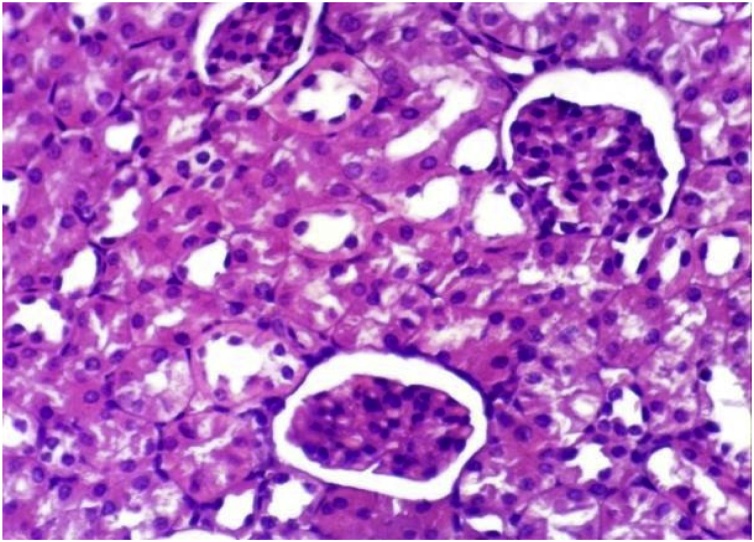
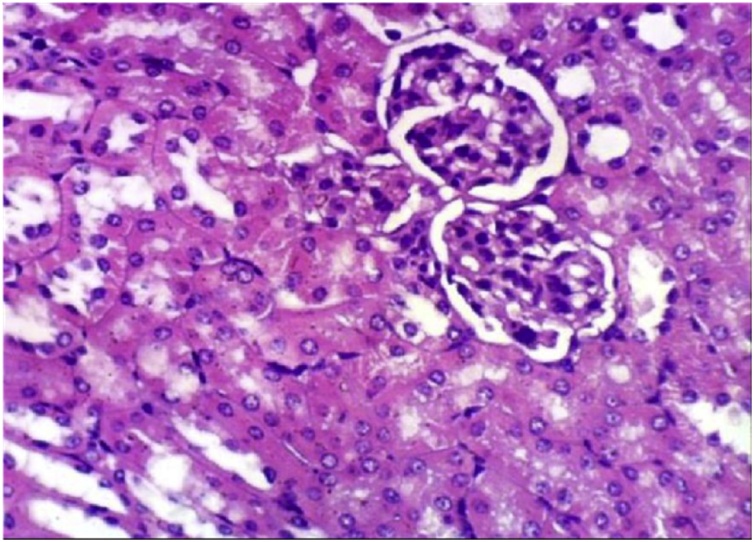
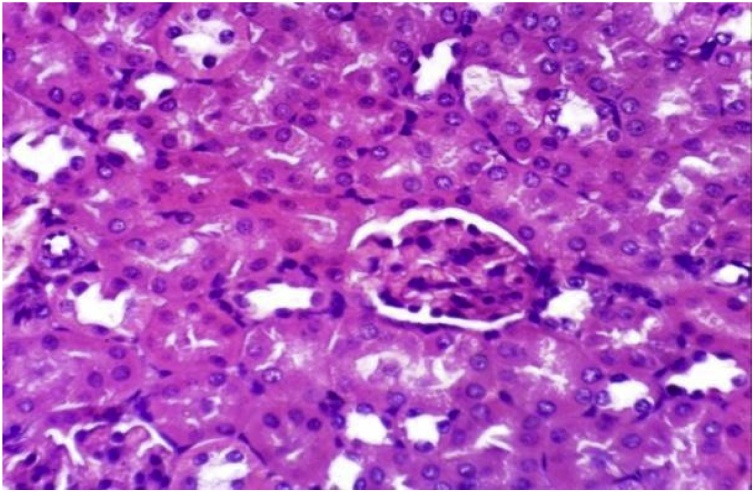
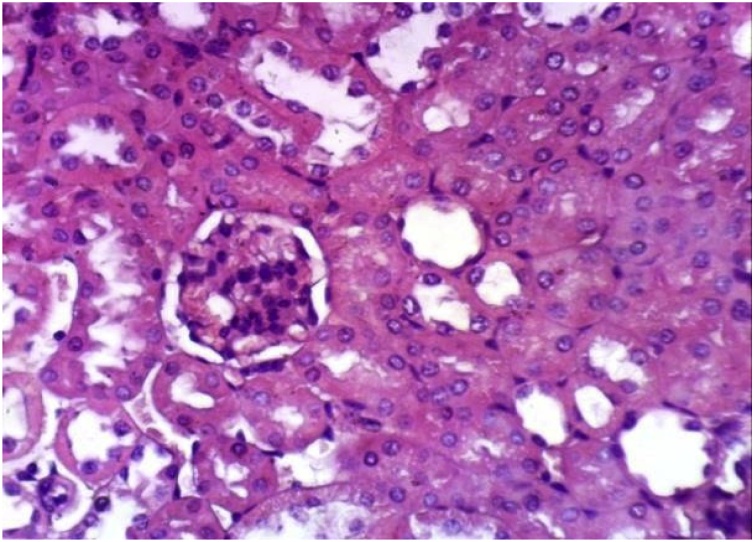
It has been clearly demonstrated that (Table 1, Table 4), no change in liver function enzyme activities and blood glucose level post chronic administration of Dunaliella salina to male and female mice and rats with 100 mg/Kg was observed. Additionally, the insignificant reduction was detected in total urea, creatinine and albumin levels post chronic administration of Dunaliella salina in both genders of mice and rats compared to their corresponding control levels (Table 2, Table 5). A significant increase in Hb level in treated male and female mice and rats while the insignificant change in the other parameters of CBC was detected as compared to their related control groups (Table 3, Table 6). Histopathological investigation revealed normal cardiac myocytes, normal histological structure of renal parenchyma as well as normal hepatic lobule in both sexes of mice and rats (Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5, Fig. 6, Fig. 7, Fig. 8, Fig. 9, Fig. 10, Fig. 11, Fig. 12, Fig. 13, Fig. 14, Fig. 15, Fig. 16, Fig. 17, Fig. 18, Fig. 19, Fig. 20, Fig. 21, Fig. 22, Fig. 23, Fig. 24, Fig. 25, Fig. 26, Fig. 27, Fig. 28, Fig. 29, Fig. 30, Fig. 31, Fig. 32, Fig. 33, Fig. 34, Fig. 35, Fig. 36, Fig. 37, Fig. 38, Fig. 39, Fig. 40, Fig. 41, Fig. 42, Fig. 43, Fig. 44, Fig. 45, Fig. 46, Fig. 47, Fig. 48, Fig. 49).
Table 1.
Liver function enzyme activities in male and female mice post chronic administration of 100 mg /Kg Dunaliella salina.
| Groups | ALT (U/l) | AST (U/l) | Bilirubin(mg/dl) | ALP(U/l) | Glucose (mg/dl) |
|---|---|---|---|---|---|
| Control male mice | 30.11 ± 1.00a | 73.65 ± 3.10a | 0.79 ± 0.03a | 41.90 ± 3.10a | 100.50 ± 2.00a |
| Control female mice | 32.19 ± 2.64a | 71.80 ± 5.56a | 0.89 ± 0.01a | 42.00 ± 5.00 a | 90.50 ± 7.00 a |
|
Dunaliella salinamale mice (100 mg /Kg) |
31.00 ± 1.22a | 72.34 ± 6.10a | 0.84 ± 0.02a | 40.56 ± 4.10 a | 100.00 ± 4.90 a |
|
Dunaliellasalinafemale mice (100 mg /Kg) |
30.88 ± 3.45a | 81.00 ± 2.89a | 0.78 ± 0.03a | 43.20 ± 1.19a | 95.00 ± 6.30a |
Data are Means ± SD of 15 mice in treated group. Statistical analysis is carried out using Co-state and SPSS computer programs (version 7), where unshared letter is significant at P ≤ 0.05.
Table 4.
Liver function enzyme activities in male and female rats post chronic administration of 100 mg /Kg Dunaliella salina.
| Groups | ALT(U/l) | AST(U/l) | Bilirubin (mg/dl) | ALP(U/l) | Glucose (mg/dl) |
|---|---|---|---|---|---|
| Control male rats | 35.10 ± 1.44a | 76.70 ± 6.17a | 0.80 ± 0.01a | 44.00 ± 3.10a | 80.50 ± 5.00a |
| Control female rats | 33.22 ± 2.09a | 76.88 ± 4.20a | 0.82 ± 0.03a | 46.00 ± 3.00 a | 77.90 ± 3.00 a |
|
Dunaliella salina male rats (100 mg /Kg) |
34.00 ± 2.00a | 75.60 ± 3.76a | 0.84 ± 0.03a | 42.56 ± 2.67a | 82.00 ± 6.00 a |
|
Dunaliella salina female rats (100 mg /Kg) |
34.88 ± 3.00a | 71.80 ± 7. 00a | 0.78 ± 0.05a | 44.00 ± 1.19 a | 81.00 ± 2.30 a |
Data are Means ± SD of 15 mice in treated group. Statistical analysis is carried out using Co-state and SPSS computer programs (version 7), where unshared letter is significant at P ≤ 0.05.
Table 2.
Total urea, creatinine and albumin levels in serum of male and female mice post chronic administration of 100 mg /Kg Dunaliella salina.
| Groups | Urea (mg/dl) | Creatinine (mg/dl) | Albumin (mg/dl) |
|---|---|---|---|
| Control male mice | 31.00 ± 3.00a | 0.150 ± 0.01a | 4.80 ± 0.20a |
| Control female mice | 31.03 ± 2.92 a | 0. 144 ± 0.03a | 4.60 ± 0.3 a |
|
Dunaliella salina male mice (100 mg /Kg) |
26.00 ± 3.30 a | 0. 140 ± 0.01a | 4.9 ± 0.10 a |
|
Dunaliellasalina female mice (100 mg /Kg) |
26.30 ± 3.10 a | 0.145 ± 0.02a | 4.60 ± 0.12 a |
Data are Means ± SD of 15 mice in treated group. Statistical analysis is carried out using Co-state and SPSS computer programs (version 7), where unshared letter is significant at P ≤ 0.05.
Table 5.
Total urea, creatinine and albumin levels in serum of male and female rats post chronic administration of 100 mg /Kg Dunaliella salina.
| Groups | Urea (mg/dl) | Creatinine (mg/dl) | Albumin (mg/dl) |
|---|---|---|---|
| Control male rats | 30.00 ± 2.90a | 0. 110 ± 0.19a | 3.85 ± 0.60a |
| Control female rats | 31.11 ± 5.90 a | 0.089 ± 0.36a | 4.00 ± 0.90 a |
|
Dunaliella salina male rats (100 mg /Kg) |
27.10 ± 1.30 a | 0.088 ± 0.20a | 4.20 ± 0.80 a |
|
Dunaliella salina female rats (100 mg /Kg) |
28.30 ± 2.00 a | 0.085 ± 0.08a | 4.18 ± 0.12 a |
Data are Means ± SD of 15 mice in treated group. Statistical analysis is carried out using Co-state and SPSS computer programs (version 7), where unshared letter is significant at P ≤ 0.05.
Table 3.
Blood profile picture of male and female mice post chronic administration of 100 mg /Kg Dunaliella salina.
| Biomarkers | Control male | Control Female | Treated male mice | Treated female mice |
|---|---|---|---|---|
| HB (g/L) | 12.20 ± 1.00a | 11.10 ± 1.20 a | 15.90 ± 1.29 b | 14. 80 ± 1.98b |
| RBCs(million cells/uL) | 6.30 ± 0.91a | 6.00 ± 0.90 a | 6.80 ± 0.98 a | 6.89 ± 0.98 a |
| PCV (%) | 41.80 ± 4.10a | 40.90 ± 3.50 a | 41.00 ± 2.97a | 41.65 ± 4.00a |
| WBCs(× 109/L) | 9.97 ± 112.50a | 10.00 ± 120.50 a | 10.500 ± 110.50 a | 10.067 ± 100.00 a |
| Neutrophils (×109/L) | 20.69 ± 1.00a | 19.65 ± 1.20 a | 18.00 ± 1.00a | 19.00 ± 1.00a |
|
Eosinophil (x100 cells /ul). |
1.69 ± 0.07a | 1.59 ± 0.05a | 1.65 ± 0.02a | 1.66 ± 0.04a |
| Lymphocyte(109/L) | 79.00 ± 8.20a | 78.00 ± 3.50 a | 77.00 ± 7.10a | 75.62 ± 7.10a |
| Monocyte(109/L) | 1.66 ± 0.05a | 1.63 ± 0.14 a | 1.60 ± 0.12 a | 1.59 ± 0.15a |
| Platelets (x 109/L) | 740.00 ± 50.00a | 770.00 ± 65.00 a | 800.00 ± 60.00 a | 780.00 ± 45.00 a |
Data are Means ± SD of 15 mice in treated group. Statistical analysis is carried out using Co-state and SPSS computer programs (version 7), where unshared letter is significant at P ≤ 0.05.
Table 6.
Blood profile picture of male and female rats post chronic administration of 100 mg /Kg Dunaliella salina.
| Biomarkers | Control male | Control Female | Treated male rats | Treated female rats |
|---|---|---|---|---|
| HB (g/L) | 11.20 ± 1.23a | 11.00 ± 1.980 a | 15.40 ± 1.20 b | 14. 99 ± 1.00b |
| RBCs (million cells/ul) | 6.00 ± 0.91a | 5.50 ± 0.60 a | 6.90 ± 0.68 a | 5.80 ± 0.56 a |
| PCV (%) | 33.50 ± 4.10a | 33.00 ± 3.50 a | 34.00 ± 3.90a | 33.00 ± 3.00a |
| WBCs(× 109/L) | 7. 600 ± 66.50a | 7.400 ± 70.50 a | 7.600 ± 65.00 a | 7.00 ± 54.00 a |
| Neutrophils (×109/L) | 21.00 ± 1.20a | 19.95 ± 1.70 a | 19.00 ± 1.10a | 19.45 ± 1.50a |
|
Eosinophil (x100 cells /uL). |
1.60 ± 0.07a | 1.60 ± 0.05a | 1.66 ± 0.05a | 1.69 ± 0.07a |
| Lymphocyte(109/L) | 72.00 ± 5.26a | 73.00 ± 5.80 a | 74.00 ± 4.90a | 74.00 ± 7.10a |
| Monocyte(109/L | 1.55 ± 0.06a | 1.53 ± 0.05 a | 1.50 ± 0.11a | 1.53 ± 0.10a |
| Platelets (x 109/L) | 740.00 ± 50.00a | 770.00 ± 65.00 a | 800.00 ± 60.00 a | 780.00 ± 45.00 a |
Data are Means ± SD of 15 mice in treated group. Statistical analysis is carried out using Co-state and SPSS computer programs (version 7), where unshared letter is significant at P ≤ 0.05.
Fig. 1.
Heart of mice from control female group showing normal cardiac myocytes (H & E X 400).
Fig. 2.
Heart of mice from control female group showing normal cardiac myocytes (H & E X 400).
Fig. 3.
Heart of mice from control male group showing normal cardiac myocytes (H & E X 400).
Fig. 4.
Heart of mice from control male group showing normal cardiac myocytes (H & E X 400).
Fig. 5.
Heart of mice from treated female group showing no histopathological alterations (H & E X 400).
Fig. 6.
Heart of mice from treated female group showing no histopathological alterations (H & E X 400).
Fig. 7.
Heart of mice from treated male group showing no histopathological alterations (H & E X 400).
Fig. 8.
Heart of mice from treated male group showing no histopathological alterations (H & E X 400).
Fig. 9.
Kidney of mice from control female group showing the normal histological structure of renal parenchyma (H & E X 400).
Fig. 10.
Kidney of mice from control female group showing the normal histological structure of renal parenchyma (H & E X 400).
Fig. 11.
Kidney of mice from control male group showing the normal histological structure of renal parenchyma (H & E X 400).
Fig. 12.
Kidney of mice from control male group showing the normal histological structure of renal parenchyma (H & E X 400).
Fig. 13.
Kidney of mice from treated female group showing no histopathological alterations (H & E X 400).
Fig. 14.
Kidney of mice from control male group showing the normal histological structure of renal parenchyma (H & E X 400).
Fig. 15.
Kidney of mice from treated male group showing no histopathological alterations (H & E X 400).
Fig. 16.
no histopathological alterations of renal tissue (H & E X 400).
Fig. 17.
Kidney of mice from treated male group showing no histopathological alterations (H & E X 400).
Fig. 18.
Liver of mice from control female group showing the normal histological structure of hepatic lobule (H & E X 400).
Fig. 19.
Liver of mice from control male group showing slight hydropic degeneration of hepatocytes (H & E X 400). (mild +).
Fig. 20.
Liver of mice from control female group showing the normal histological structure of hepatic lobule (H & E X 400).
Fig. 21.
Liver of mice from treated female group showing slight hydropic degeneration of hepatocytes (H & E X 400). (mild +).
Fig. 22.
Liver of mice from control male group showing slight hydropic degeneration of hepatocytes (H & E X 400). (mild +).
Fig. 23.
Liver of mice from treated male group showing slight hydropic degeneration of hepatocytes (H & E X 400). (moderate ++).
Fig. 24.
Liver of mice from treated female group showing slight hydropic degeneration of hepatocytes (H & E X 400). (mild).
Fig. 25.
Liver of mice from treated malegroupshowingslight hydropic degeneration of hepatocytes (H & E X 400). (mild +).
Fig. 26.
Liver of rat from control female group showing the normal histological structure of hepatic lobule (H & E X 400).
Fig. 27.
Liver of rat from control female group showing the normal histological structure of hepatic lobule (H & E X 400).
Fig. 28.
Liver of rat from control male group showing the normal histological structure of hepatic lobule (H & E X 400).
Fig. 29.
histological structure of hepatic lobule (H & E X 400).
Fig. 30.
Liver of rat from treated female group showing no histopathological changes (H & E X 400).
Fig. 31.
Liver of rat from treated female group showing no histopathological changes (H & E X 400).
Fig. 32.
Liver of rat from treated male group showing no histopathological changes (H & E X 400).
Fig. 33.
Liver of rat from treated male group showing no histopathological changes (H & E X 400).
Fig. 34.
Kidney of rat from control female group showing the normal histological structure of renal parenchyma (H & E X 400).
Fig. 35.
Kidney of rat from control female group showing the normal histological structure of renal parenchyma (H & E X 400).
Fig. 36.
histological structure of renal parenchyma (H & E X 400).
Fig. 37.
Kidney of rat from control male group showing the normal histological structure of renal parenchyma (H & E X 400).
Fig. 38.
Kidney of rat from treated female group showing no histopathological alterations (H & E X 400).
Fig. 39.
Kidney of rat from treated female group showing no histopathological alterations (H & E X 400).
Fig. 40.
Kidney of rat from treated male group showing no histopathological alterations (H & E X 400).
Fig. 41.
Kidney of rat from treated male group showing no histopathological alterations (H & E X 400).
Fig. 42.
normal cardiac myocytes (H & E X 400).
Fig. 43.
Heart of rat from control female group showing normal cardiac myocytes (H & E X 400).
Fig. 44.
Heart of rat from control male group showing normal cardiac myocytes (H & E X 400).
Fig. 45.
Heart of rat from control male group showing normal cardiac myocytes (H & E X 400).
Fig. 46.
Heart of rat from treated female group showing no histopathological alterations (H & E X 400).
Fig. 47.
Heart of rat from treated female group showing no histopathological alterations (H & E X 400).
Fig. 48.
Heart of rat from treated male group showing no histopathological alterations (H & E X 400).
Fig. 49.
Heart of rat from treated male group showing no histopathological alterations (H & E X 400).
4. Discussion
Dunaliella salina microalgae algae have been classified as sources of food and do not yield toxins. In this aspect, Cyanotech [17] announced that the uptake of Dunaliella bardawil does not show adverse effects. In a good parallel result; Mokady et al. [18], illustrated that the powder of the Dunaliella bardawil algae (which is similar to the Dunaliella salina species), supplemented to rats and chicken for two -eight weeks, has no signs of toxicity up to 0.1% of beta-carotene in the diet and was unable to stimulate mutation of a gene or aberrations of chromosomes in lymphocytes of human. Further Mokady et al. [18], documented the safety of diets containing 0, 50 and 100 g/kg bw of Dunaliella bardawil ingested for one year and noticed, no significant differences in blood biochemistry and hematological parameters between the rats supplemented with algae and the control.
The current data showed insignificant difference in total urea and creatinine levels in mice and rats supplemented with Dunaliella salina. Nevertheless, a significant increase in blood hemoglobin level of both gender of rats and mice post chronic administration of 100 mg /kg body weight of Dunaliella salina, for three consecutive months. In this aspect, El-Baz et al. [19], demonstrated that Dunaliella salina rich with a high antioxidant level of carotenoids such as β-carotene, astaxanthin, and fucoxanthin, causing an elevation in antioxidant enzyme activities, reduced inflammatory cytokines as a result of inhibition of oxidative stress (lipid peroxidation). So, Dunaliella salina powdered will preserve kidney function through normalization of urea and creatinine levels. This may be linked to the antioxidant properties of Dunaliella; since ROS may be implicated in the Glomerular Filtration Rate (GFR) impaired [[20], [21], [22]]. The increase in Hb level, which detected in the present study may be attributed to the B-carotene is considered as a precursor of vitamin A and a single oxygen quencher, so could affect the immune system through both pathways of antioxidant and retinoid [23]. Also, Dunaliella salina contains a high percentage of 9-cis b-carotene, which has been detected to be more potent than all-trans b-carotene in modulating cell-cell interaction through gap junctions [24]. Hence, Dunaliella salina rich with β-carotene has been shown to be a potent immune-stimulants, attenuating the erythropoietic tissue, resulting in the viability of the cells might be affected [25,26]. In opposite results, Nakano et al. and Rehulka [27,28], showed the interaction of carotenoids with hematological factors resulting in fluctuating in its concentrations compared to control. These controversial results may be attributed to, the route and dose of ingestion and physiological condition.
Histopathological examination revealed no differences in cardiac, renal and hepatic architectures between control and animals daily feed with 100 mg /kg bw of Dunaliella salina for three months. Similar results were obtained by Mokady et al. [18], who observed no difference in renal architecture post supplementation of rats for one year with 10 g /kg bw Dunaliella bardawil., suggesting the safety of consumption of Dunaliella bardawil for human [18].
5. Conclusion
Marked elevation in Hb level was observed in both sexes of mice and rats compared to control which may be related to the potent immune-stimulant activity from β- carotene.No significant differences were observed in all hematological and biochemical parameters as well as in histopathological examination of cardiac, renal and hepatic architectures of both gender of rats and mice post chronic administration of 100 mg/kg bw of Dunaliella salina for three consecutive months.
Declaration of Competing Interest
The authors declared no conflict of interest regarding this study.
Author contributions
All authors contributed to collecting and analyzing data. All authors participated in writing every part of this study. All authors read and approved the final version.
Acknowledgments
This work was supported by the alliance entitled "Integrated Pharmaceutical Alliance (IPA)". This alliance is funded by the Academy of Scientific Research and Technology under the "Egypt Research and Technology Alliances (EG-KTA) Program".
References
- 1.Tsai C.F., Lu F.J., Hsu Y.W. Protective effects of Dunaliella salina – a-carotenoids-rich alga –against ultraviolet B-induced corneal oxidative damage in mice. Mol. Vis. 2012;18:1540–1547. [PMC free article] [PubMed] [Google Scholar]
- 2.Havas F., Krispin S., Meléndez-Martínez A.J., von Oppen-Bezalel L. Preliminary data on the safety of phytoene- and phytofluene-rich products for human use including topical application. J. Toxicol. 2018 doi: 10.1155/2018/5475784. Volume Article ID 5475784, 8 [pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu C.C., Lin J.T., Lu F.J., Chou F.P., Yang D.J. Determination of carotenoids in Dunaliella salina cultivated in Taiwan and antioxidant capacity of the algal carotenoid extract. Food Chem. 2008;109:439–446. doi: 10.1016/j.foodchem.2007.12.043. [DOI] [PubMed] [Google Scholar]
- 4.Stein J. Culture Methods and Growth Measurements. Cambridge University Press; 1975. Handbook of phycological methods; p. 448. [Google Scholar]
- 5.Pikula K.S., Zakharenko A.M., Chaika V.V., Stratidakis A.K., Kokkinakis M., Waissi G.V., Rakitskii N., Sarigiannis D.A., Hayes A.W., Coleman M.D., Tsatsakisa A., Golokhvas K.S. Toxicity bioassay of waste cooking oil-based biodiesel on marine microalgae. Toxicol. Rep. 2019;6 doi: 10.1016/j.toxrep.2018.12.007. 111–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kale O.E., Awodele O., Akindele A.J. Subacute and subchronic oral toxicity assessments of Acridocarpus meathmannii(DC.) Guill. & Perr. Root in Wistar rats. Toxicol. Rep. 2019;6:161–175. doi: 10.1016/j.toxrep.2019.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanaka W., Yokoyamaa D., Matsuura Y., Nozaki M., Hirozawa N., Kunitake H., Sakono M. Sakakibara, Subchronic toxicity evaluation of leaves from rabbit eye blueberry (Vaccinium virgatum)in rats. Toxicol. Rep. 2019;6:161–175. doi: 10.1016/j.toxrep.2019.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tiwari R.K., Singh S., Pandey R.S. Assessment of acute toxicity and biochemical responses to chlorpyrifos, cypermethrin and their combination exposed earthworm, Eudrilus eugenie. Toxicol. Rep. 2019;6 doi: 10.1016/j.toxrep.2019.03.007. 288-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thrall M.A. Lippincott Williams & Wilkins; USA: 2004. Veterinary Hematology, and Clinical Chemistry; pp. 277–288. 241. [Google Scholar]
- 10.Schaperclaus W., Kulow H., Schreckenbach K. Hematological, and serological technique. In: Kothekar V.S., editor. 2nd ed. Vol. 1. New Delhi, Gulab primlani, Oxonian press Pvt. Ltd; 1991. pp. 71–108. (Fish Disease). [Google Scholar]
- 11.Reitman A., Frankel S. A colorimetric method for the determination of serum glutamate - oxaloacetate and glutamate pyruvate transaminase. Am. J. Clin.Pathol. 1957;28:56–61. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 12.Belfield A., Goldberg D.M. Determination of alkaline phosphatase activity (ALP) Enzyme. 1971;12 doi: 10.1159/000459586. 561-266. [DOI] [PubMed] [Google Scholar]
- 13.Bartles H., Bohrnes M., Heirlis C. Determination of creatinine methods. Clinical Chemistry Acta. 1972;37:193–196. [Google Scholar]
- 14.Fawcett J.K., Scott J.E. Determination of urea concentration methods. J. Clin. Pathol. 1960;13:156–159. doi: 10.1136/jcp.13.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walter M., Gerade H. A colorimetric method for determination bilirubin in serum and plasma. Micro. Chem. J. 1970;15:231–236. [Google Scholar]
- 16.Trinder P. Determination of blood glucose using 4-aminophenazone. J. Clin. Pathol. 1969;22:246–250. doi: 10.1136/jcp.22.2.246-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corporation C. Woodinville; Washington, USA: 1988. Ten-day Konatene (TM) Feeding Study: Effects on Serum Beta-carotene Levels. Unpublished Summary Report Submitted to WHO by Cyanotech Corporation. [Google Scholar]
- 18.Mokady S., Abramovici A., Cogan U. The safety evaluation of Dunaliella bardawil as a potential food supplement. Food Chem. Toxicol. 1990;27(4):221–226. doi: 10.1016/0278-6915(89)90159-2. [DOI] [PubMed] [Google Scholar]
- 19.El-Baz F.K., Aly H.F., Abdo S.M., Mahmoud R., Saad S.A. Dunaliella salina alleviates renal dysfunction and suppresses inflammatory cytokines in STZ-induced diabetic rats. Int. J. Pharma Sci.Rev. Res. 2016;38:210–215. [Google Scholar]
- 20.Hazarikaa I., Geethaa K.M., Sundarib P.S., Madhu D. Acute oral toxicity evaluation of extracts of Hydrocotyle sibthorpioides in Wister albino rats as per OECD 425 TG. Toxicol. Rep. 2019;6:321–328. doi: 10.1016/j.toxrep.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Youssef M.I., Mutar T.F., Kamel M.A. Hepato-renal toxicity of oral sub-chronic exposure to aluminum oxide and/or zinc oxide nanoparticles in rats. Toxicol. Rep. 2019;6:336–346. doi: 10.1016/j.toxrep.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fang J., Xu X., Jiang L., Qiao J., Zhou H., Li K. Preliminary results of toxicity studies in rats following low-dose and short-term exposure to methyl mercaptan. Toxicol. Rep. 2019;6:431–438. doi: 10.1016/j.toxrep.2019.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alishahi M., Karamifar M., Mesbah M., Zarei M. Hemato-immunological responses of Heros severus fed diets supplemented with different levels of Dunaliella salina. Fish Physiol. Biochem. 2014;40:57–65. doi: 10.1007/s10695-013-9823-5. [DOI] [PubMed] [Google Scholar]
- 24.Zhang L.X., Cooney R.V., Bertram J.S. Carotenoids up-regulate Connexin 43 gene expression independent of their provitamin A or antioxidant properties. Cancer Res. 1992;52:5707–5712. [PubMed] [Google Scholar]
- 25.Supamattaya K., Kiriratnikom S., Boonyaratpalin M. Effect of a Dunaliella extract on growth performance, health condition, immune response and disease resistance in black tiger shrimp (Penaeus monodon) Aquaculture. 2005;248:207–216. [Google Scholar]
- 26.Tavares D.M., Martins M.L., Nascimento K.S. Evaluation of the hematological parameters in Piaractus mesopotamicus with Argulus sp. Infestation and treatment with organophosphate. Revista Brasileria de Zoologia. 1999;16:553–555. [Google Scholar]
- 27.Nakano T., Tosa M., Takeuchi M. Improvement of biochemical features in fish health by red yeast and synthetic astaxanthin. Agric.Food Chem. 1995;43:1570–1573. [Google Scholar]
- 28.Rehulka J. Influence of astaxanthin on the growth rate, condition, and some blood indices of rainbow trout (Oncorhynchus mykiss) Aquaculture. 2000;190:27–47. [Google Scholar]