Abstract
The current study evaluated the hazards of Zinc oxide nanoparticles (ZnONPs) on Nile Tilapia liver and gill antioxidants enzymes activities and antioxidants genes expressions. The ameliorative action of vitamins E and C mixture was investigated. Two hundred males of Nile Tilapia were exposed to one and two mg L−1 of ZnONPs either with or without vitamin C and E mixture for 7 and 15 days. Glutathione reductase (GR), glutathione peroxidase (GPx) and glutathione-S-transferase (GST) activities and gene expression as well glutathione (GSH) and lipid peroxide (LPO) levels were investigated. The results revealed that the exposure to ZnONPs could induce alterations in the liver and gills antioxidants and LPO of Nile Tilapia. Moreover, the mixture of vitamin E and C highly effective in alleviation the toxic effect of ZnONPs.
Keywords: Zinc oxide nanoparticles toxicity, Oreochromis nilotica, Antioxidant enzymes, Gene expression
1. Introduction
In the last few years, Zinc oxide nanoparticles (ZnONPs) are widely used in industry (Pilar et al., 2017), waste-water treatment (Chen et al., 2004), and environmental remediation (Saddick et al., 2017). ZnONPs are spread to the aquatic environments through bathing, sewage effluent (Handy and Shaw, 2007) and other engineering applications (Nagaveni et al., 2004) producing a huge hazards for humans, ecosystem and aquatics (Nowack and Bucheli, 2007). ZnONP produces their hazards by many ways (Saddick et al., 2017). It may induce the generation of oxidative stresses by disruption of cellular metabolism (Long et al., 2006) or deplete the cellular enzymatic and non enzymatic antioxidants (Brown et al., 2004), damaging the cellular lipids, protein and nuclear DNA (Afifi et al., 2016). ZnONPs inhibited superoxide dismutase (SOD), catalase (CAT), GPx activities and increased lipid peroxides in juvenile carp (Linhua and Lei, 2012). The literatures on the toxicity of ZnONPs have focused on acute exposure or early developmental stages to aquatic organisms (Ji et al., 2011). It is well known that, Vitamins E and C are potent antioxidants and act by scavenging the free radicals and compensate the decrease in reduced glutathione (Van Gaal et al., 2006). Vitamin E is an important component in human diet and considered the most effective lipo-soluble antioxidant found in the biological system (Musalmah et al., 2002). The possible molecular mechanism of ZnONPs toxicity on Nile Tilapia did not adequately studded. This work aimed to assess the effect of different concentrations of ZnONPs on antioxidants enzymes activities and antioxidants gene expression as well as the possible protective effects of a combination of vitamins E and C on the liver and lung of Nile Tilapia.
2. Materials and methods
2.1. ZnONPs preparation and characterization
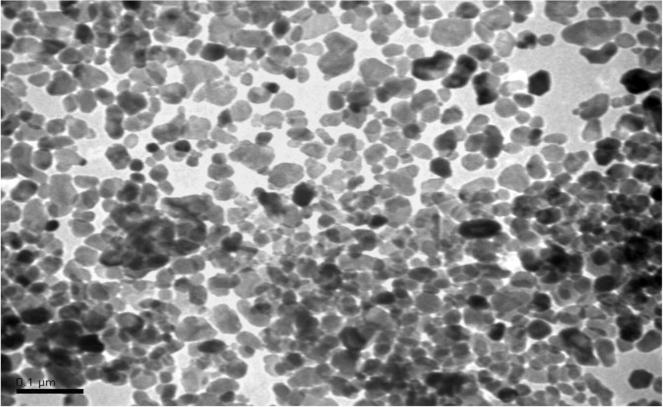
ZnONPs dispersion was supplied from Sigma-Aldrich, Steinheim, Germany (CAS Number 1314-13-2) of concentration 50 wt% in H2O, average particle size (APS) was <35 nm. The particle size distribution (hydrodynamic diameter) was <100 nm using dynamic light scattering (DLS) technique, pH 7 ± 0.1 (for aqueous systems) and density 1.7 ± 0.1 g mL−1 at 25 °C. Suspensions of ZnONPs in a concentration of one and two mg L−1 were daily prepared in distilled water using sonicator (JL-360, Shanghai, USA) for their dispersion. For characterize the ZnONPs shape and size, a small drop of aqueous ZnONPs solution was air dried by directly placing it onto a 300-mesh carbon-coated copper grid then examined under the transmission electron microscope (TEM) (JEM-1011, JEOL, Japan) (Fig. 1). Inductive coupled plasma mass spectrometry (ICP-MS) was applied for quantification of ZnONPs concentrations in the aquariums at zero, 12 and 24 h of exposure.
Fig. 1.
TEM photomicrograph of ZnONPs, which shows that the APS is 30 ± 5 nm.
2.2. Fish preparation and management
Two hundred males of Nile Tilapia weight 90 ± 5 g, length 15 ± 3 cm were reared in twenty aquaria (n = 10 fish/aquarium), in one hundred litter water (pH 7.16 ± 0.3, temperature 28 ± 2 °C and 7.0 ± 0.5 mg L−1 of dissolved oxygen) that was changed daily. The water aeration was done using water aeration system (Eheim Liberty 150 Bio-Espumador cartridges). A Fish commercial diet formulated of 31% proteins, 6% lipids, 37% carbohydrates, 1.5% total phosphorus and 2.5% fibers was used for fish feeding. The fish were acclimatized for 15 days before the experiment began. Institutional and National Guidelines for the care and use of fisheries were followed.
2.3. Fish grouping and induction ZnONPs toxicity
Fishes were randomly divided into five groups, 40 fishes in each group (4 replicates). The first group was leaved as control; the 2nd and 3rd, groups were exposed to ZnONPs of one and two mg L−1 respectively. The 4th and 5th groups were exposed to ZnONPs of one and two mg L−1 and feed on diet containing 500 mg Kg−1 diet of vitamins C and E. After seven and 15 days of the exposure, twenty fishes of each group were anesthetized on ice and killed by transaction of the spinal cord. Liver and gills were quickly removed, weighed, rinsed with ice-cold saline, frozen in liquid nitrogen, and kept at −80 °C until be used. For biochemical analysis the individual fresh liver and gill samples were homogenated and centrifuged following Puerto et al. (2009) then the supernatant was separated and kept at −80 °C.
2.4. Glutathione, LPO concentrations and enzymatic assays
Liver and gills LPO products, protein and GSH contents were quantized by the methods of Esterbauer and Cheeseman, 1990, Bradford, 1976, Ellman, 1959. Liver and gills GST, GR and GPx activities were determined using the methods of Habig et al., 1974, Beutler, 1969, Lawrence and Burk, 1979 respectively.
2.5. Molecular assays and gene expressions
Liver and gills GST, GR and GPx genes expression was quantified using real time PCR. Total RNA was extracted by RNeasy Mini Kit (Qiagen) according to the instructions of manufacturer. Then, total RNA was used for production of cDNA. Five μL of cDNA was mixed with 2x SYBR® Green PCR mix with ROX from BioRad and 10 pmol/μL of each primer (Table 1). For each sample, the threshold cycle (Ct) values were used for determination of mRNA concentration. The data was expressed as a fold changes in mRNA expression relative to β-actin mRNA levels and calculated using the 2−DD CT method.
Table 1.
Oligonucleotide sequences of primers GPx, glutathione peroxidase; GR, glutathione reducatse; GST, glutathione-S-transferase and ß atin genes.
| Gene | Forward 5′->3′ | Reverse 5′->3′ |
|---|---|---|
| GPx | CCAAGAGAACTGCAAGAACGA | CAGGACACGTCATTCCTACAC |
| GR | CATTACCGAGACGCGGAGTT | CAGTTGGCTCAGGATCATTTGT |
| GST | TAATGGGAGAGGGAAGATGG | CTCTGCGATGTAATTCAGGA |
| ß atin | CAATGAGAGGTTCCGTTGC | AGGATTCCATACCAAGGAAGG |
2.6. Statistical analysis
Statistical package for social science (SPSS Inc., Chicago, IL, version 20, USA) was used for data analysis. One way ANOVA was used for groups comparison. The inter-grouping homogeneity was tested using Duncan's test.
3. Results
3.1. The actual exposure to ZnO NPs
During the experiment, the actual ZnONPs concentrations in the water tanks were determined (Table 2). Some loss of ZnONPs was observed that increased by time.
Table 2.
The actual ZnONPs concentrations (mg L−1) in the exposure water.
| Concentrations (mg L−1) | Time (hours) |
||
|---|---|---|---|
| Zero | 12 | 24 | |
| Control | nd | nd | nd |
| 1 | 1 ± 0.003 | 0.96 ± 0.003 | 0.92 ± 0.001 |
| 2 | 2 ± 0.005 | 1.98 ± 0.006 | 1.95 ± 0.004 |
| 1 + vitaminsa | 1 ± 0.003 | 0.93 ± 0.003 | 0.90 ± 0.001 |
| 2 + vitaminsa | 2 ± 0.004 | 1.96 ± 0.006 | 1.93 ± 0.004 |
nd = not detected.
Vitamins E + C in concentration of 500 mg Kg−1 diet (250 mg of each).
3.2. Effects of ZONPs on antioxidant enzymes activities and lipid peroxide levels in fish tissues
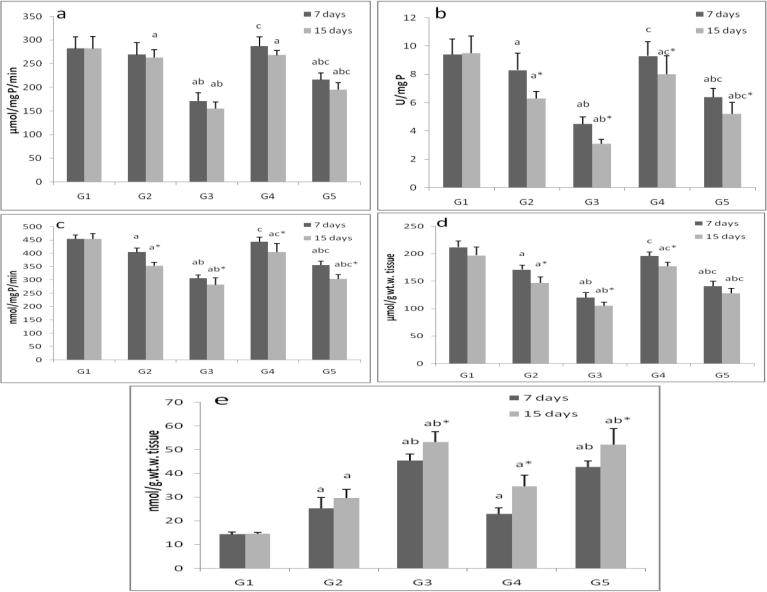
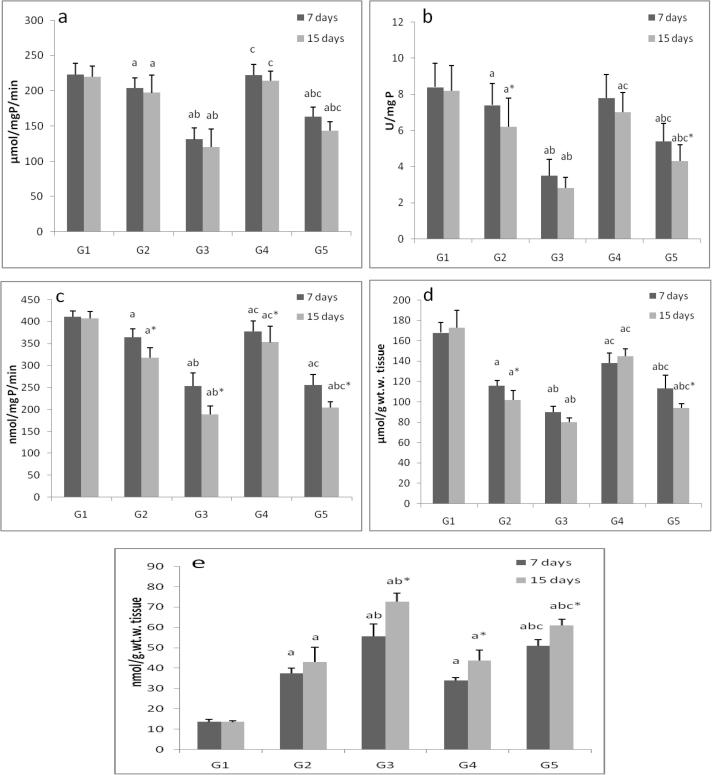
ZnONPs inhibited (p < 0.05) GPx, GR and GST activities, depleted GSH levels and increased lipid peroxide (LPO) in liver and gills tissues in a time- and dose-dependent manner. Vitamins mixture induced the studied enzymes activities and reduced LPO levels (Fig. 2, Fig. 3).
Fig. 2.
The activities of liver enzymes, a; glutathione peroxidase, b; glutathione reductase, c; glutathione-s-transferase, d; reduced glutathione and e; lipid peroxide in control group (G1), zinc oxide nanoparticles groups (G2and G3) and zinc oxide nanoparticles with vitamin mixture groups (G4 and G5). Values are Expressed as mean ± SD (n 20). Significance levels (p < 0.05) observed are: a = in comparison to control group, b = when 2 mg ZONPs groups versus 1 mg ZONPs groups are compared, c = when ZONPs + vitamins groups versus their respective ZONPs groups are compared, * = when 15 days treated groups compared with their respective 7 days treated groups.
Fig. 3.
The activities of gills enzymes, a; catalase, b; superoxide dismutase, c; glutathione peroxidase, d; glutathione reductase, e; glutathione-s-transferase, f; lipid peroxide and g; reduced glutathione in control group (G1), zinc oxide nanoparticles groups (G2and G3) and zinc oxide nanoparticles with vitamin mixture groups (G4 and G5). Values are Expressed as mean ± SD (n 20). Significance levels (p < 0.05) observed are: a = in comparison to control group, b = when 2 mg ZONPs groups versus 1 mg ZONPs groups are compared, c = when ZONPs + vitamins groups versus their respective ZONPs groups are compared, * = when 15 days treated groups compared with their respective 7 days treated groups.
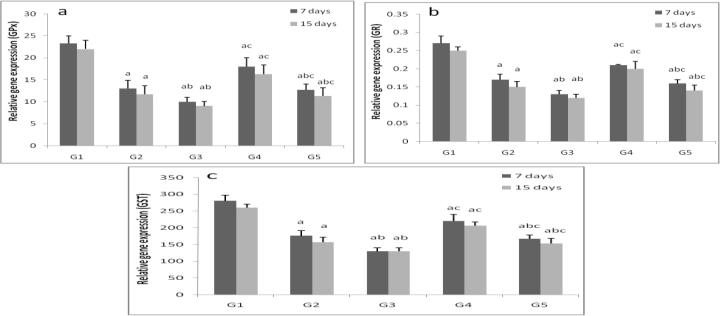
3.3. Effects of ZONPs on the relative gene expression of antioxidant enzymes in fish tissues
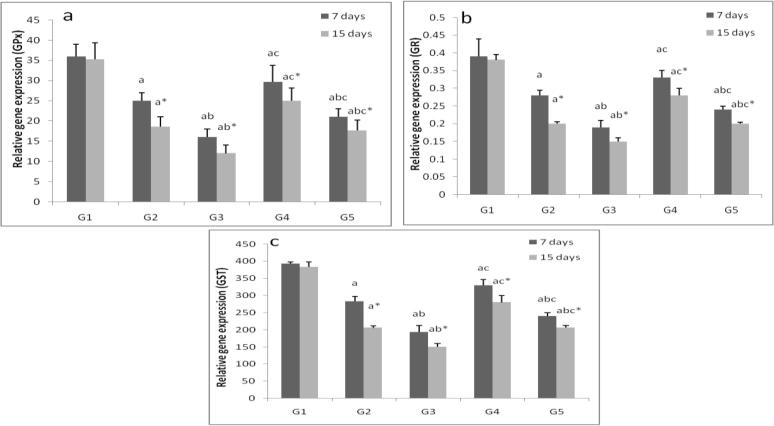
ZnONPs suppressed (p < 0.05) the GPx, GR and GST gene expression in liver and gills tissues in a time and dose dependent manner. Vitamins mixture induced the studied genes expression (Fig. 4, Fig. 5).
Fig. 4.
Hepatic relative gene expression of glutathione peroxidase (a), glutathione reductase (b) and glutathione-s-transferase (c) in control group (G1), zinc oxide nanoparticles groups (G2and G3) and zinc oxide nanoparticles with vitamin mixture groups (G4 and G5). Values are Expressed as mean ± SD (n 5). Significance levels (p < 0.05) observed are: a = in comparison to control group, b = when 2 mg ZONPs groups versus 1 mg ZONPs groups are compared, c = when ZONPs + vitamins groups versus their respective ZONPs groups are compared, * = when 15 days treated groups compared with their respective 7 days treated groups.
Fig. 5.
Gills relative gene expression of glutathione peroxidase (a), glutathione reductase (b) and glutathione-s-transferase (c) in control group (G1), zinc oxide nanoparticles groups (G2and G3) and zinc oxide nanoparticles with vitamin mixture groups (G4 and G5). Values are Expressed as mean ± SD (n 5). Significance levels (p < 0.05) observed are: a = in comparison to control group, b = when 2 mg ZONPs groups versus 1 mg ZONPs groups are compared, c = when ZONPs + vitamins groups versus their respective ZONPs groups are compared, * = when 15 days treated groups compared with their respective 7 days treated groups.
4. Discussion
The decrease in ZnONPs that was observed in the tanks water during the experiment might be due to the aggregation of nanoparticle (Linhua and Lei, 2012). The continual aeration and changing of the water containing ZnONPs were used to decrease the aggregation of the particles and to overcome the nanoparticals loss. There is a relation between GSH and numerous effects that produced by ZONPs. GST, GR, and GPx activities were decreased in gills and liver of fish after seven, and 15 days of exposure to one mg L−1 or two mg L−1 ZONPs. GSH is the most important non protein thiol in all living cells; it has a vital role in protection of intracellular against toxins such as Cu and Zn through the action of GR, GST and GPx (Saddick et al., 2017). GSH concentration and GST activity evaluation has been reported in the context of their involvement in the metabolism of phase II detoxification. However, these molecules are also involved in the CAT activity and GR as cellular antioxidants. While the GST is a cytosolic enzyme, some membrane-bound forms have been described. The activities of SH group of glutathione with reactive molecules resulting from the activity of phase I enzymes are important when catalyzed by GST. Glutathione conjugation with these reactive molecules neutralizes their electrophilic site and makes them more soluble. The GPXs are the most important peroxidases for detoxification of hydroperoxides. The GST, catalyze the conjugation reactions of reduced glutathione GSH with electrophilic xenobiotics, resulting in an increase in solubility and facilitating their removal from the cell (Mofeed and Mosleh, 2013). It has been shown that many substrates of GST are resulted of oxidative stress such as peroxides. In addition, certain GST isoenzymes have glutathione peroxidase activities and are able to reduce lipid hydroperoxides to the corresponding alcohol (Bhattacharyya et al., 2014). While GPx, catalyzes the reduction of H2O2 to two molecules of water, in this reaction GSH is the source of hydrogen and converted to the oxidized form glutathione disulphide (GSSG) so GPx activity is strictly linked to GSH concentrations (Mofeed and Mosleh, 2013). The reduction in GPx activity when fish exposed to ZONPs only is an indicator for decreasing its capacity to break H2O2 and lipid peroxides, at the same time, this reduction may be resulted from over production of H2O2 or a direct action of heavy metals on the enzyme synthesis.
Analysis of ZnONPs modulated stress gene expression showed that the transcription levels of two oxidative stress genes (GPx and GR) in the liver, and gills were significantly repressed, particularly with two mg/L ZnONPs exposure for 15 days. The expression of these genes was up-regulated with vitamin C and E supplementation. They significantly increased in the supplemented groups in comparison with the ZnONPs exposure.
Vitamins which have antioxidant activities are protecting the cells against the damaging effects of the free radicals through preventing its production from the most powerful antioxidant vitamins; are A, C and E (Keleştemur, 2012). Treatment with vitamins E and C mixture significantly ameliorates the alterations induced by ZnONPs. Vitamin E ameliorated the oxidative stresses induced by ZnONPs in animals (Ognjanovic et al., 2003). But Cosic et al. (2007) reported the increase of DNA damage in presence of antioxidants as glutathione and vitamin C in in-vitro studies. Vitamin E is the most important fat-soluble antioxidants; it plays a key role in protection of lipoproteins, membrane’s phospholipids, and stored lipids from oxidation (Mekkawy et al., 2013); additionally, it plays an important role in the health of red blood corpuscle, capillaries, cardiac muscle and immunity (Halver, 2002). Moreover, the activities of examined enzymes were up-regulated in vitamins C and E supplemented groups if compared with their control groups. Such elevation could explain the antioxidant role of vitamin C and E to minimize the lipid peroxidation and production of ROS (Adikwu and Deo, 2013). Additionally, these findings were coincided with previous studies revealed that vitamin E played a major role in reducing inflammation as well as cleansing the body of free radicals. As vitamin E supplements lowered IL6 and mitochondrial and membrane damage dramatically in ZnONPs toxicity (Al-Attar, 2011).
5. Conclusion
ZnONPs sub-lethal doses significantly elevate the oxidative stress in Nile tilapia through increasing the LPO levels and decreasing GSH and inhibition of GR, GPx and GST activity and gene expression. The vitamin E and C mixture modulated the oxidative stress induced with ZnONPs.
Acknowledgments
Acknowledgments
This project was funded by the Deanship of Scientific Research (DSR), King Abdulaziz University, Jeddah, under grant no. (1-965-35-RG). The authors, therefore, acknowledge with thanks DSR technical and financial support.
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Peer review under responsibility of King Saud University.
References
- Adikwu E., Deo O. Hepatoprotective effect of vitamin C (ascorbic acid) Pharmacol. Pharm. 2013;4:84–92. [Google Scholar]
- Afifi M., Saddick S., Abu Zinada O. Toxicity of silver nanoparticles on the brain of Oreochromis niloticus and Tilapia zillii. Saudi J. Biol. Sci. 2016;23:754–760. doi: 10.1016/j.sjbs.2016.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Attar A.M. Antioxidant effect of vitamin E treatment on some heavy metals induced renal and testicular injuries in male mice. Saudi J. Biol. Sci. 2011;18(1):63–72. doi: 10.1016/j.sjbs.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler E. Effect of flavin compounds on the glutathione reductase activity: In vivo and in vitro studies. J. Clin. Invest. 1957;48:1966. doi: 10.1172/JCI106162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya A., Chattopadhyay R., Mitra S., Crowe S. Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 2014;94(2):329–354. doi: 10.1152/physrev.00040.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brown D., Donaldson K., Borm P., Schins R., Dehnhardt M., Gilmour P. Calcium and ROS-mediated activation of transcription factors and TNF-¦Á cytokine geneexpression in macrophages exposed to ultrafine particles. Am. J. Physiol. Lung Cell Mol. Physiol. 2004;286:344–353. doi: 10.1152/ajplung.00139.2003. [DOI] [PubMed] [Google Scholar]
- Chen J., Liu M., Zhang J., Ying X., Jin L. Photocatalytic degradation of organic wastes by electrochemically assisted TiO2 photocatalytic system. J. Environ. Manage. 2004;70:43–47. doi: 10.1016/j.jenvman.2003.09.019. [DOI] [PubMed] [Google Scholar]
- Cosic D.D., Bulat Z.P., Ninkovic M., Malicevic Z., Matovic V. Effect of subacute cadmium intoxication on iron and lipid peroxidation in mouse liver“. Toxicol. Lett. 2007;172:209. [Google Scholar]
- Ellman G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959;17:214–226. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Esterbauer H., Cheeseman K.H. Determination of aldehydic lipid peroxidation products: Malonaldehydeand4-hydroxynonetal. MethodsEnzymol. 1990;186:407–421. doi: 10.1016/0076-6879(90)86134-h. [DOI] [PubMed] [Google Scholar]
- Habig W., Pabst M., Jakoby W. J. Biol. Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- Halver J.E. The vitamins. In: Halver J.E., Hardy R.W., editors. Fish Nutrition. Academic Press; San Diego, CA: 2002. pp. 61–141. [Google Scholar]
- Handy R., Shaw B. Toxic effects of nanoparticles and nanomaterials: implications for public health, risk assessment and the public perception of nanotechnology. Health Risk Soc. 2007;9:125–144. [Google Scholar]
- Ji J., Long Z.F., Lin D.H. Toxicity of oxide nanoparticles to the green algae Chlorella sp. Chem. Eng. J. 2011;170:525–530. [Google Scholar]
- Keleştemur G.T. The antioxidant vitamin (A, C, E) and the lipid peroxidation levels in some tissues of juvenile rainbow trout (Oncorhynchusmykiss, W. 1792) at Different oxygen levels. Iranian J. Fisheries Sci. 2012;11(2):315–324. [Google Scholar]
- Lawrence R.A., Burk R.F. Glutathione peroxidase activity in seleniumdeficient rat liver. Biochem. Biophys. Res. Commun. 1979;71:952–958. doi: 10.1016/0006-291x(76)90747-6. [DOI] [PubMed] [Google Scholar]
- Linhua H., Lei C. Oxidative stress responses in different organs of carp (Cyprinus carpio) with exposure to ZnO nanoparticles. Ecotoxicol. Environ. Saf. 2012;80:103–110. doi: 10.1016/j.ecoenv.2012.02.017. [DOI] [PubMed] [Google Scholar]
- Long T.C., Saleh N., Tilton R.D., Lowry G.V., Veronesi B. Titanium dioxide (P 25) producesreactive oxygen species in immortalized brain microglia (BV 2): implications fornanoparticle neurotoxicity. Environ. Sci. Technol. 2006;40:4346–4352. doi: 10.1021/es060589n. [DOI] [PubMed] [Google Scholar]
- Mekkawy I.A.A., Mahmoud U.M., Mohammed R.H. Protective effects of tomato paste and vitamin e on atrazine-induced hematological and biochemical characteristics of clariasgariepinus (burchell, 1822. Glo. Adv. Res. J. Environ. Sci. Toxicol. 2013;2(1):11–21. [Google Scholar]
- Mofeed J., Mosleh Y. Toxic responses and antioxidative enzymes activity of Scenedesmusobliquus exposed to fenhexamidand atrazine, alone and in mixture. Ecotoxicol. Environ. Saf. 2013;95:234–240. doi: 10.1016/j.ecoenv.2013.05.023. [DOI] [PubMed] [Google Scholar]
- Musalmah M., Fairuz A.H., Gapor M.T., Ngah W.Z.W. Effect of vitamin E on plasma malondialdehyde, antioxidant enzyme levels and the rates of wound closures during wound healing in normal and diabetic rats. Asia Pac. J. Clin. Nutr. 2002;11:S448–S451. doi: 10.1046/j.1440-6047.11.s.7.6.x. [DOI] [PubMed] [Google Scholar]
- Nagaveni K., Sivalingam G., Hegde M.S., Madras G. Photocatalytic degradation of organic compounds over comebustion-synthesized nano-TiO 2. Environ Sci Technol. 2004;38:1600–1604. doi: 10.1021/es034696i. [DOI] [PubMed] [Google Scholar]
- Nowack B., Bucheli T. Occurrence, behavior and effects of nanoparticles in theenvironment. Environ Pollut. 2007;150:5–22. doi: 10.1016/j.envpol.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Ognjanovic B.I., Pavlovic S.Z., Maletic S.D., Zikic R.V., Stajn A.S., Radojicic R.M. Protective influence of vitamin E on antioxidant defense system in the blood of rats treated with cadmium. Physiol. Res. 2003;52:563–570. [PubMed] [Google Scholar]
- Pilar M., Llanas H., Marics L., Mitjans M. In vitro comparative skin irritation induced by nano and non-nano zinc oxide. Nanomaterials. 2017;7:56–64. doi: 10.3390/nano7030056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puerto M., Prieto A.I., Pichardo S., Moreno I., Jos A., Moyano R., Camean A.M. Effects of dietaryN-acetylcysteine (NAC) on the oxidative stress induced in tilapia (Oreochromis niloticus) exposed to a microcystin-producing cyanobacterial water bloom. Environ. Toxicol. Chem. 2009;28:1679–1686. doi: 10.1897/08-520.1. [DOI] [PubMed] [Google Scholar]
- Saddick S., Afifi M., Abu Zinada O. Effect of zinc nanoparticles on oxidative stress-related genes and antioxidant enzymes activity in the brain of Oreochromis niloticus and Tilapia zillii. Saudi J. Biol. Sci. 2017;24:1672–1678. doi: 10.1016/j.sjbs.2015.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gaal L., Mertens I., De Block C. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444(7121):875–880. doi: 10.1038/nature05487. [DOI] [PubMed] [Google Scholar]