Abstract
Objective
To study the putative effects of Advanced Oxidation Protein Products (AOPPs) and Advanced Glycation End Products (AGEs) in the development and progression of cardiovascular disease (CVD).
Methodology
AGEs, AOPPs, e-NOS, lipid profile, circulating stress and inflammatory biomarkers were evaluated among fifty cardiovascular patients and fifty controls. Independent student’s t-test was done for statistical analysis.
Results
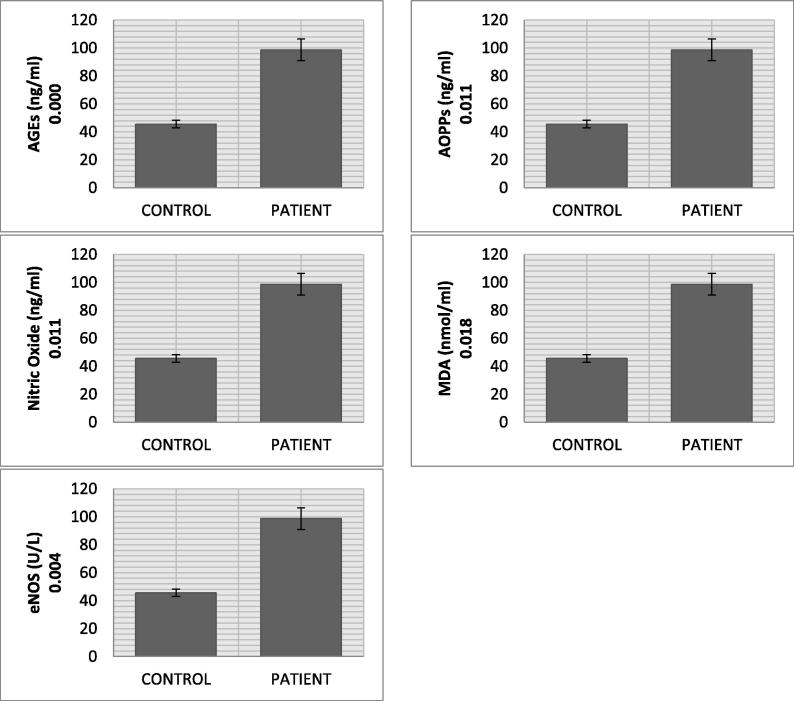
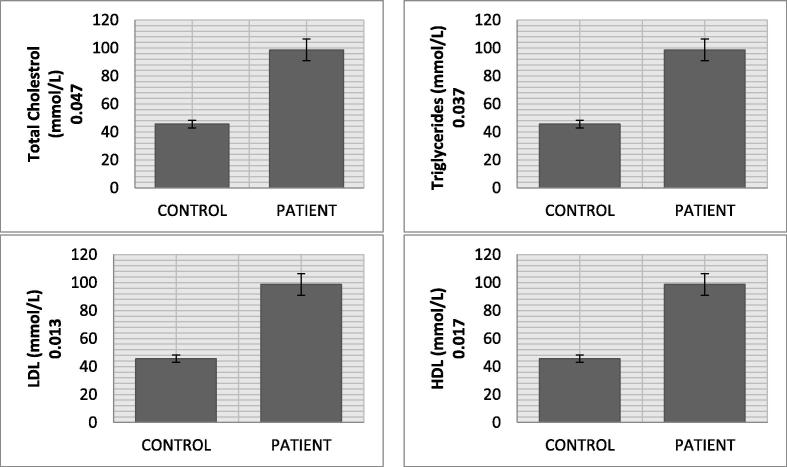
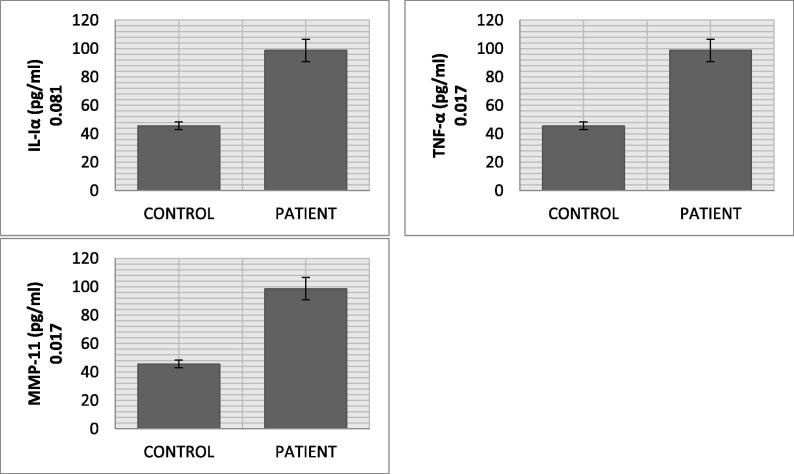
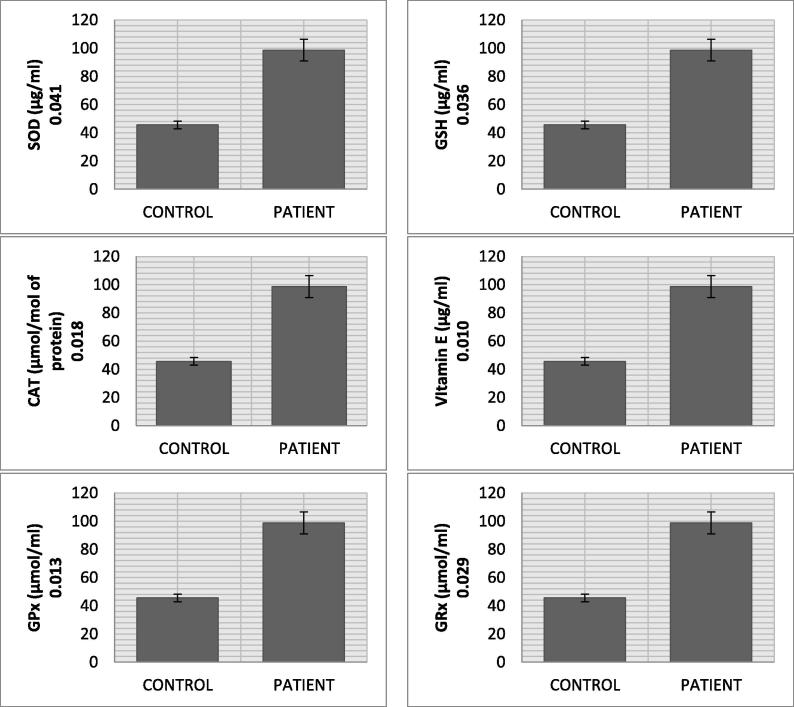
The malondialdehyde mean level in CVD patients (5.45 nmol/ml) was significantly higher than control (1.36 nmol/ml) (p value = 0.018). Nitric oxide in CVD patients (55.72 ng/ml) was remarkably increased as compared to normal subjects (19.19 ng/ml). A significant change in the mean serum level of AGEs in CVD patients (2.74 ng/ml) and normal individuals (0.85 ng/ml) was recorded (p value = 0.000). The AOPPs also showed significant increased levels in CVD group (132.07 ng/ml) in comparison with normal subjects (83.05 ng/ml) (p value = 0.011). The mean eNOS serum level in CVD group (15.50 U/L) was higher than control group (11.28 U/L) (p value = 0.004). Cardiovascular disease patients, in comparison with healthy controls, showed increased level of total cholesterol (5.48 mmol/L vs 4.45 mmol/L), triglycerides (2.59 mmol/L vs 1.24 mmol/L), and low density lipoprotein (2.47 mmol/L vs 2.31 mmol/L) along with decrease in high density lipoprotein (1.39 mmol/L vs 1.74 mmol/L). The mean MMP-11 serum levels in CVD group (98.69 ng/ml) was almost double of control group (45.60 ng/ml) (p value = 0.017). The mean serum level of TNF-α and IL1-α were 32.16 pg/ml and 6.64 pg/ml in CVD patient. The significant decreasing trend of SOD (p value = 0.041), CAT (p value = 0.018), GSH (p value = 0.036) and GRx (p value = 0.029) but increasing drift of GPx (0.023) level was observed in CVD patients.
Conclusion
This study provides strong evidence that CVD patients presented with elevated oxidative stress, enhanced inflammation and lipid profile in their serum. Therefore, the study strongly approves that AGEs, AOPPs, inflammatory and lipoxidative biomarkers hold predictive potential in causing and aggravating the disease, thus by controlling these factors CVD progression can be inhibited.
Keywords: Cardiovascular diseases, Advanced glycation end products, Advanced oxidation protein products, Endothelial nitric oxide synthase, Matrix metalloproteinase-11
1. Introduction
Cardiovascular diseases are foremost fatality and disability all over the world rampant due to unhealthy food intake, obesity, and stressed environmental and genetic factors. On a global level, CVD is the most common underlying cause of death, accounts for approximately 31% i.e. 17 million CVD related deaths out of total 54 million deaths. In United State alone, it accounts for approximately 800,000 deaths i.e. one out of every three to four deaths (Benjamin et al., 2017). The percentage of premature deaths ranges from 4% to 42% in developed and under-developed countries (United Nations WHO, 2013). The developing countries like Pakistan, India, Bangladesh, Sri Lanka and Nepal consists of 20% of the world population and also considered in the regions with highest rate of cardiovascular diseases (Goyal and Yusuf, 2006). CVD have been majorly linked to four behavioral risk factors i.e. alcohol overuse, tobacco smoking, unhealthy diet and physical inactivity (Alwan, 2011). Long term exposure of behavioral risk factor contributes to high blood pressure, overweight, obesity, dyslipidaemia and hyperglycemia. These all myriads of causative factors lead to inflammation, oxidative and mechanical stress, endothelial impairment and eventually vascular remodeling. Therefore, for proper management of CVD patients, clinical assessment of these parameters, according to recent national institutes of health panel, can be included to improve our ability to identify the risk of CVD (Naghavi et al., 2003, Benjamin et al., 2017).
1.1. Intercellular adhesion molecule 1 (ICAM-1), and vascular cell adhesion molecule 1 (VCAM-1)
Inflammatory factors such as ICAM-1, VCAM-1, TNF-α, IL-6, IL-1α and CRP play significant roles in facilitating vascular inflammation and blocking RAAS (Pacurari et al., 2014). Non-enzymatic oxidative alteration of proteins resulting in increased advanced oxidation protein products (AOPPs), advanced glycation end-products (AGEs) levels and e-NOS, used as biomarkers are closely linked with biochemical changes in increased oxidative stress condition and play a vital role in prognosis of risk factors. AGEs accumulation with age contribute to changes in the structure and function of the cardiovascular system like vascular stiffening, reduced central compliance of arteries, myocardial abnormalities, atherosclerosis and endothelial dysfunction which is further enhanced by the presence of diabetes, high blood pressure and renal diseases (Peppa et al., 2004).
Systemic levels of AOPPs are augmented in diabetes mellitus, atherosclerosis, CVD and chronic renal disease. Nitric oxide has a range of intracellular effects including vasodilatation, endothelial regeneration, leukocytes chemotaxis inhibition and platelet adhesion. Atherosclerotic endothelial damage leads to decrease in endothelial nitric oxide synthase (e-NOS) bioactivity with consequent abnormal release of NO along with a local raised degradation of NO by promoting reactive oxygen species (ROS) production with consequent oxidation sensitive mechanism cascade in the arterial wall. Oxidative stress increases the production of reactive oxygen species (ROS) that endogenously leads to the activation of antioxidant defense mechanism. It is also involved in the modification of DNA, proteins, carbohydrates, lipids and other biological macromolecules (Dalle-Donne et al., 2005). Thus, oxidative stress markers should be identified having potential to integrate multiple processes that trigger cardiovascular pathogenicity (Ho et al., 2013). In addition, by understanding the underlying disease mechanism, we can get a vantage point of the potential predictive markers and novel treatment targets.
In the present research article, our focus is the insinuation of AOPPs and AGEs in the development of CVDs.
2. Methodology
We assessed AGEs AOPPs, inflammatory cytokines, lipid profile and oxidative stress markers for fifty CVD patients screened at Punjab Institute of Cardiology, Lahore, Pakistan. We included fifty healthy individuals as controls.
We took 5 ml of peripheral blood samples from each participants and immediately centrifuged for serum collection, and stored at −70 °C until assayed. We measured all the variable under investigation with previously described methods (GSH (Moron et al., 1979), CAT (Aebi, 1974), SOD (Kakkar et al., 1984), MDA (Ohkawa et al., 1979), GPx (David and Richard, 1983), GRx (David and Richard, 1983), Vit-E (Rosenberg and Culik, 1959), NO (Moshage et al., 1995), AOPPs and AGEs (Kalousova et al., 2002)) or by Commercial Kits like e-NOS by Cayman’s e-NOS ELIZA Kit, TNF-α by BioVendor Human TNF-α ELIZA Kit, IL-1 α by BioVendor Human IL-1α ELIZA Kit, MMP-11 by BioVendor Human MMP-11 ELIZA Kit. Lipid profiling of TCh, TG, LDL, and HDL were done by available commercial Human Diagnostics Kits.
2.1. Inclusion and exclusion criteria
The inclusion criteria were based on patients age ranging from 20 to 70 years, and suffering from ischemic heart diseases. The participants with history of addiction (alcohol, cigarette etc.) and on pre-diagnosis medications (aspirin, lipid lowering drugs etc.), were excluded from this study.
2.2. Ethical approval
All the experimental procedures were approved by the ethical committee of the University of Lahore and informed written consent was taken prior to the start of study from all participants according to Helsinki's declaration.
3. Statistical analysis
All data was analyzed statistically by SPSS (ver 16) and expressed as mean ± S.D. Independent t-test was applied to compare the results among control and CVD patients. While Pearson correlation was used to correlate different parameters with each other. P value < 0.05 was statistically significant.
4. Results
Result of the current study presents highly significant pattern of data regarding oxidative stresses and biochemical markers between and within the groups (Table 1 and Fig. 1). The mean serum level of AGE in CVD patients (2.74 ± 0.25 ng/ml) was significantly higher (p value = 0.000) than normal individuals (0.85 ± 0.04 ng/ml). AOPP had also shown significantly increased levels (p value = 0.011) in CVD group (132.07 ± 13.79 ng/ml) than individuals (83.05 ± 7.31 ng/ml). We found significantly higher expression of e-NOS (p value = 0.004), MDA (p value = 0.018) and NO (p value = 0.011) as evident from their mean values in CVD (15.50 ± 1.64 U/L, 5.45 ± 1.09 nmol/ml and 55.72 ± 2.16 ng/ml) and control group (11.28 ± 1.34 U/L, 1.36 ± 0.031 nmol/ml and 19.19 ± 2.31 ng/ml). Lipid profile (TCh, Tg, LDL, and HDL) of CVD patients showed an increased level (TCh = 5.48 ± 0.33 mmol/L, Tg = 2.59 ± 0.535 mmol/L, LDL = 2.47 ± 0.19 mmol/L) as compared to healthy individuals (TCh = 4.45 ± 0.971 mmol/L, Tg = 1.24 ± 0.157 mmol/L and LDL = 2.31 ± 0.091 mmol/L), whereas HDL level was lower in CVD group (1.39 ± 0.031 mmol/L vs 1.74 ± 0.017 mmol/L). The mean IL1-α, TNF-α and MMP-11 serum levels in CVD patients (6.64 ± 0.78 pg/ml, 32.16 pg/ml and 98.69 ng/ml) were significantly higher than healthy individuals (5.68 ± 0.53 pg/ml, 29.57 pg/ml and 45.60 ng/ml). The significant decreasing trend of CAT, SOD, GSH and GRx was recorded in CVD patients as compared to normal individuals (CAT: 1.43 ± 0.011 µmol/mol of protein vs 4.27 ± 0.007 µmol/mol of protein; SOD: 0.24 ± 0.002 µg/ml vs 0.46 ± 0.003 µg/ml; GSH: 4.91 ± 0.077 µg/ml vs 9.77 ± 1.73 µg/ml; GRx: 6.03 ± 1.21 µmol/ml vs 7.31 ± 1.67 µmol/ml). The GPx levels of CVD patients (2.33 ± 0.021 µmol/ml) had shown increasing trend as compared to normal individuals (1.59 ± 0.007 µmol/ml) (p value = 0.023). Vitamin E level was also significantly low in CVD patients (2.19 ± 0.73 µg/ml) than healthy persons (2.95 ± 0.66 µg/ml) (see Fig. 2).
Table 1.
Measurements of expression of biomarkers in cardiovascular diseases and healthy controls.
| Variables | Control | Patients | P value (P < 0.05) |
|---|---|---|---|
| Stress variable biomarkers | |||
| MDA (nmol/ml) | 1.36 ± 0.031 | 5.44 ± 1.09 | 0.018 |
| Nitric oxide (ng/ml) | 19.19 ± 2.31 | 55.72 ± 2.16 | 0.011 |
| Ages (ng/ml) | 0.85 ± 0.04 | 2.74 ± 0.25 | 0.000 |
| AOPPS (ng/ml) | 83.05 ± 7.31 | 132.07 ± 13.79 | 0.011 |
| eNOS (U/L) | 11.28 ± 1.34 | 15.5 ± 1.64 | 0.004 |
| Lipid profile biomarkers | |||
| Total cholesterol (mmol/L) | 4.45 ± 0.971 | 5.48 ± 0.33 | 0.047 |
| Triglycerides (mmol/L) | 1.24 ± 0.15 | 2.59 ± 0.53 | 0.037 |
| LDL (mmol/L) | 2.31 ± 0.091 | 2.47 ± 0.19 | 0.013 |
| HDL (mmol/L) | 1.74 ± 0.017 | 1.39 ± 0.031 | 0.017 |
| Inflammatory biomarkers | |||
| IL-Iα (pg/ml) | 5.68 ± 0.53 | 6.64 ± 0.78 | 0.081 |
| TNF-α (pg/ml) | 29.57 ± 2.12 | 32.16 ± 3.79 | 0.017 |
| MMP-11 (ng/ml) | 45.6 ± 2.71 | 98.69 ± 7.77 | 0.017 |
| Anti-oxidative biomarkers | |||
| SOD (µg/ml) | 0.46 ± 0.003 | 0.24 ± 0.0021 | 0.041 |
| Glutathione (µg/ml) | 9.77 ± 1.73 | 4.91 ± 0.077 | 0.036 |
| Catalase (µmol/mol of protein) | 4.27 ± 0.007 | 1.43 ± 0.011 | 0.018 |
| Vitamin E (µg/ml) | 2.95 ± 0.66 | 2.19 ± 0.73 | 0.010 |
| GPx (µmol/ml) | 1.59 ± 0.007 | 2.33 ± 0.021 | 0.023 |
| GRx (µmol/ml) | 7.3 ± 1.67 | 6.03 ± 1.21 | 0.029 |
Fig. 1.
Stress variable biomarkers profile of CVD patients.
Fig. 2.
Lipid profile biomarkers of CVD patients.
5. Discussion
AGE and AOPP, the novel oxidative biomarkers have emerged as chief inducers of cardiovascular events. AOPP are dityrosine containing biomarkers of oxidative damage, generated under the stressed conditions are responsible to trigger the inflammatory process (Piwowar, 2014). In the present study, significant increase of AOPPs was found in CVD patients which is concurrent with the study of Skvarilova et al., which demonstrated significantly elevated levels of AOPPs in acute coronary syndrome patients (Skvarilova et al., 2005). AOPPs have believed to have positive correlation with NO (r = +0.195*) while negative correlation with CAT (r = −0.306*) (see Fig. 3).
Fig. 3.
Inflammatory biomarkers profile of CVD patients.
AGEs are heterogeneous compounds generated through non enzymatic glycation of protein, lipids and nucleic acid, and has various biological effects mediated through intracellular and extracellular matrix proteins or through interacting with RAGE receptors on cell surface, and are linked to premature atherosclerosis. CVD patients showed significantly increased levels of AGEs which is concurrent with the study of Kilhovd et al., showing the high serum level of AGEs in coronary heart disease (Kilhovd et al., 2005). A strong positive significant correlation was found between AGE and NO (r = 0.447**) (see Fig. 4).
Fig. 4.
Antioxidant biomarkers profile of CVD patients.
Nitric oxide, an endothelium vasodilator, is produced by nitric oxide synthase enzyme. Superoxide anion (O2−) may react with excess NO and limit the reaction that leads to the formation of peroxynitrite (ONOO−), a trigger of lipid peroxidation (Byrne et al., 2003). Endothelial dysfunction and oxidative stress are caused by e-NOS uncoupling through different mechanisms. Firstly, the production of NO by e-NOS which allows the generation of free radicals that attacks other cellular targets. Secondly, enzyme contributes to oxidative stress by producing O2−. Partial uncoupling of e-NOS act as peroxynitrite generator, produces O2− and NO simultaneously leading to increased oxidative stress (Cai and Harrison, 2000). Our study showed significantly increased levels of NO and e-NOS in CVD patients than controls. This implies uncoupling of e-NOS and the excess formation of NO that will react with O2− to generate peroxynitrite. We found positive significant correlations between NO vs. GSH (r = 0.303*), AGEs vs NO (r = 0.447**), e-NOS vs. SOD (r = 0.292*).
In the present study, we observed increased level of Tg, TCh, LDL in cardiac patients as compared to healthy individuals which is concurrent with the study of Reiner et al., which showed increase values of Tg, TCh, LDL might cause CVD in the absence of other risk factors (Reiner et al., 2011). Statistically significant positive correlation was observed in between triglycerides and e-NOS (r = 0.393**). During premature CVD, elevated levels of TCh and LDL were observed along with prompt dyslipidaemia. Whereas, in case of atherogenic lipid triads increase in LDL, TCh, Tg with reduced HDL levels (Reiner et al., 2011).
Inflammation participates in the progression of CVD as TNF-α increases expression of vascular wall chemokines (VCAM-1) and augments multiplication of vascular smooth muscle cells (VSMCs) (Zhang et al., 2007). Elevated level of TNFα in CVD patients as compared to control, which was concurrent with the findings of Ridker et al. (2000), where also increased TNF-α levels were associated with recurrent coronary disease (Schonbeck et al., 1999). TNF-α showed a significant inverse correlation with SOD (r = −0.476**). IL-1α levels was also significantly high in CVD patients as compared to normal individuals, and present results were similar to the study of Chen et al. (2007), Elevated IL-1α levels causing tissue damage and disease aggravation in myocardial infarction (Walter et al., 2004). Substantial negative correlation was detected in between IL-1α and AOPPs (r = −0.276*) while positive correlated was observed in between TNFα and MMP-11 (stromelysin-3) (r = 0.166), it is one rare MMP involved in atherosclerosis (Jawalekar et al., 2010). The present study demonstrates significantly higher activity of MMP-11 in CVD group and positive correlation of MMP-11 with MDA (r = 0.405**), TCh (r = 0.297*), TG (r = 0.345*) and e-NOS (r = 0.358*) was observed, while inverse correlation was seen with SOD (r = −0.303*).
Present study reveals significant higher levels of MDA in the patients of cardiovascular disease as compared to healthy individuals. Therefore, atherosclerosis majorly contributes in the generation of free radicals that eventually leads to increase in lipid peroxidation. Current results are in accordance with Walter et al. (2004), who showed that serum elevated levels of TBARS in coronary artery disease and predict a key role in major cardiovascular events (Kumar et al., 2012). The present study also showed the significant positive correlation of MDA with TCh (r = 0.320*) and Tg (r = 0.394**).
In the present study, glutathione peroxidase (GPx) levels were increased in diseased groups as compared to normal individuals. GPx, which uses glutathione as a substrate, also converts hydrogen peroxide (H2O2) to water (H2O) and molecular oxygen (O2). The elevated level suggests that GPx activity enhances due to reduction in SOD and CAT to fight oxidative stress. SOD catalyzes dismutation of superoxide to H2O2. CAT is a tetrameric peroxidase enzyme which converts toxic substrate (H2O2) to neutral products (water and molecular oxygen) and plays a significant role in the antioxidant defense system and in adaptation to oxidant stress (Mates et al., 1999, Sindhu et al., 2005). It may be also due to increased concentration of lipo-peroxides which induce the production of antioxidant enzyme GPx (Robertson and Harmon, 2007). The current study showed significantly reduced levels of SOD and GRx in CVD patients as compared to normal individuals which are concurrent with Jawalekar et al. (2010), which demonstrated a significant reduced level of SOD and GRx in CVDs. Additionally a significant positive correlation of SOD was observed with GPx (r = 0.425), MMP-11 (r = −0.303*) and e-NOS (r = 0.292*).
The present study showed the reduced level of CAT in CVD victims as compared to controls, similar to the results of Kumar et al., which reported lower level of CAT in patients with cardiovascular diseases (Kumar et al., 2012). Vitamin E, a fat soluble antioxidant is involved in the prevention of CVDs through LDL oxidation which stimulates production of inflammatory markers involved in foam cell formation. It exerts cytotoxicity on endothelial cells, curbs the motility of tissue macrophages thus inhibiting NO induced vasodilation that lead to the formation of atherosclerotic plaque (Miwa et al., 1996). The current study demonstrated the decreased level of vitamin E in CVD patients as compared to healthy subjects.
6. Conclusion
The present study states strong and evident role of elevated oxidative stress in patients with cardiovascular disease. Patients with cardiovascular disease have shown enhanced inflammatory markers. Current study also approves that AGEs, AOPPs and lipoxidative biomarkers having potential role in the aggravation of disease. Hence, by controlling these factors can help in hampering disease progression.
Conflict of interest
There is no conflict of interest by any author.
Acknowledgements
This work was partially funded by Institute of Molecular Biology and Biotechnology, the University of Lahore, Lahore, Pakistan and (ii) Deanship of Research (HiCi grant No HiCi-1434-117-2) King Abdulaziz University, Jeddah, Saudi Arabia. We thank all lab-staff for their support. Authors also thank the research facilities provided by CEGMR and KFMRC at King Abdulaziz University, Jeddah, Saudi Arabia.
Footnotes
Peer review under responsibility of King Saud University.
References
- Aebi H. second ed. Elsevier; 1974. Catalase. Methods of Enzymatic Analysis; pp. 673–684. [Google Scholar]
- Alwan A. World Health Organization; 2011. Global Status Report on Noncommunicable Diseases 2010. [Google Scholar]
- Benjamin E.J., Blaha M.J., Chiuve S.E., Cushman M., Das S.R., Deo R., Floyd J., Fornage M., Gillespie C., Isasi C. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. 2017;135(10):e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne J.A., Grieve D.J., Cave A.C., Shah A.M. Oxidative stress and heart failure. Arch. Mal. Coeur Vaiss. 2003;96(3):214–221. [PubMed] [Google Scholar]
- Cai H., Harrison D.G. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ. Res. 2000;87(10):840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- Chen C.J., Kono H., Golenbock D., Reed G., Akira S., Rock K.L. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat. Med. 2007;13(7):851–856. doi: 10.1038/nm1603. [DOI] [PubMed] [Google Scholar]
- Dalle-Donne I., Scaloni A., Giustarini D., Cavarra E., Tell G., Lungarella G., Colombo R., Rossi R., Milzani A. Proteins as biomarkers of oxidative/nitrosative stress in diseases: the contribution of redox proteomics. Mass Spectrom. Rev. 2005;24(1):55–99. doi: 10.1002/mas.20006. [DOI] [PubMed] [Google Scholar]
- David, M., Richard, J., 1983. Glutathione reductase. In: Bermeyer, Hans, Ulrich, Jr. (Eds.), Methods of Enzymatic Analysis, pp. 258–265.
- Goyal A., Yusuf S. The burden of cardiovascular disease in the Indian subcontinent. Indian J. Med. Res. 2006;124(3):235–244. [PubMed] [Google Scholar]
- Ho E., Karimi Galougahi K., Liu C.C., Bhindi R., Figtree G.A. Biological markers of oxidative stress: applications to cardiovascular research and practice. Redox Biol. 2013;1:483–491. doi: 10.1016/j.redox.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jawalekar S.L., Kulkarni U., Surve V.T., Deshmukh Y.A. Role of oxidants and anti oxidants in patients with cardiovascular diseases. Asian J. Med. Sci. 2010;2(4):181–184. [Google Scholar]
- Kakkar, P., Das, B., Viswanathan, P., 1984. A Modified Spectrophotometric Assay of Superoxide Dismutase. [PubMed]
- Kalousova M., Skrha J., Zima T. Advanced glycation end-products and advanced oxidation protein products in patients with diabetes mellitus. Physiol. Res. 2002;51(6):597–604. [PubMed] [Google Scholar]
- Kilhovd B.K., Juutilainen A., Lehto S., Ronnemaa T., Torjesen P.A., Birkeland K.I., Berg T.J., Hanssen K.F., Laakso M. High serum levels of advanced glycation end products predict increased coronary heart disease mortality in nondiabetic women but not in nondiabetic men: a population-based 18-year follow-up study. Arterioscler. Thromb. Vasc. Biol. 2005;25(4):815–820. doi: 10.1161/01.ATV.0000158380.44231.fe. [DOI] [PubMed] [Google Scholar]
- Kumar E.P., Mukherjee R., Senthil R., Parasuraman S., Suresh B. Evaluation of oxidative stress and antioxidant status in patients with cardiovascular disease in rural populations of the nilgiris, South India. ISRN Pharmacol. 2012;2012:941068. doi: 10.5402/2012/941068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mates J.M., Perez-Gomez C., Nunez de Castro I. Antioxidant enzymes and human diseases. Clin. Biochem. 1999;32(8):595–603. doi: 10.1016/s0009-9120(99)00075-2. [DOI] [PubMed] [Google Scholar]
- Miwa K., Miyagi Y., Igawa A., Nakagawa K., Inoue H. Vitamin E deficiency in variant angina. Circulation. 1996;94(1):14–18. doi: 10.1161/01.cir.94.1.14. [DOI] [PubMed] [Google Scholar]
- Moron M.S., Depierre J.W., Mannervik B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim. Biophys. Acta. 1979;582(1):67–78. doi: 10.1016/0304-4165(79)90289-7. [DOI] [PubMed] [Google Scholar]
- Moshage H., Kok B., Huizenga J.R., Jansen P.L. Nitrite and nitrate determinations in plasma: a critical evaluation. Clin. Chem. 1995;41(6 Pt 1):892–896. [PubMed] [Google Scholar]
- Naghavi M., Libby P., Falk E., Casscells S.W., Litovsky S., Rumberger J., Badimon J.J., Stefanadis C., Moreno P., Pasterkamp G., Fayad Z., Stone P.H., Waxman S., Raggi P., Madjid M., Zarrabi A., Burke A., Yuan C., Fitzgerald P.J., Siscovick D.S., de Korte C.L., Aikawa M., Airaksinen K.E., Assmann G., Becker C.R., Chesebro J.H., Farb A., Galis Z.S., Jackson C., Jang I.K., Koenig W., Lodder R.A., March K., Demirovic J., Navab M., Priori S.G., Rekhter M.D., Bahr R., Grundy S.M., Mehran R., Colombo A., Boerwinkle E., Ballantyne C., Insull W., Jr., Schwartz R.S., Vogel R., Serruys P.W., Hansson G.K., Faxon D.P., Kaul S., Drexler H., Greenland P., Muller J.E., Virmani R., Ridker P.M., Zipes D.P., Shah P.K., Willerson J.T. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part II. Circulation. 2003;108(15):1772–1778. doi: 10.1161/01.CIR.0000087481.55887.C9. [DOI] [PubMed] [Google Scholar]
- Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Pacurari M., Kafoury R., Tchounwou P.B., Ndebele K. The renin-angiotensin-aldosterone system in vascular inflammation and remodeling. Int. J. Inflamm. 2014;2014 doi: 10.1155/2014/689360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppa M., Uribarri J., Vlassara H. The role of advanced glycation end products in the development of atherosclerosis. Curr. Diab. Rep. 2004;4(1):31–36. doi: 10.1007/s11892-004-0008-6. [DOI] [PubMed] [Google Scholar]
- Piwowar A. Biochemical and clinical aspects of advanced oxidation protein products in kidney diseases and metabolic disturbances. Postepy Hig. Med. Dosw. (Online) 2014;68:179–190. doi: 10.5604/17322693.1088754. [DOI] [PubMed] [Google Scholar]
- Reiner Z., Catapano A.L., De Backer G., Graham I., Taskinen M.R., Wiklund O., Agewall S., Alegria E., Chapman M.J., Durrington P., Erdine S., Halcox J., Hobbs R., Kjekshus J., Filardi P.P., Riccardi G., Storey R.F., Wood D. ESC/EAS Guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS) Eur. Heart J. 2011;32(14):1769–1818. doi: 10.1093/eurheartj/ehr158. [DOI] [PubMed] [Google Scholar]
- Ridker P.M., Rifai N., Pfeffer M., Sacks F., Lepage S., Braunwald E. Elevation of tumor necrosis factor-alpha and increased risk of recurrent coronary events after myocardial infarction. Circulation. 2000;101(18):2149–2153. doi: 10.1161/01.cir.101.18.2149. [DOI] [PubMed] [Google Scholar]
- Robertson R.P., Harmon J.S. Pancreatic islet β-cell and oxidative stress: the importance of glutathione peroxidase. FEBS Lett. 2007;581(19):3743–3748. doi: 10.1016/j.febslet.2007.03.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg H.R., Culik R. Effect of α-lipoic acid on vitamin C and vitamin E deficiencies. Arch. Biochem. 1959;80:86–93. [Google Scholar]
- Schonbeck U., Mach F., Sukhova G.K., Atkinson E., Levesque E., Herman M., Graber P., Basset P., Libby P. Expression of stromelysin-3 in atherosclerotic lesions: regulation via CD40-CD40 ligand signaling in vitro and in vivo. J. Exp. Med. 1999;189(5):843–853. doi: 10.1084/jem.189.5.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindhu R.K., Ehdaie A., Farmand F., Dhaliwal K.K., Nguyen T., Zhan C.D., Roberts C.K., Vaziri N.D. Expression of catalase and glutathione peroxidase in renal insufficiency. Biochim. Biophys. Acta. 2005;1743(1–2):86–92. doi: 10.1016/j.bbamcr.2004.08.013. [DOI] [PubMed] [Google Scholar]
- Skvarilova M., Bulava A., Stejskal D., Adamovska S., Bartek J. Increased level of advanced oxidation products (AOPP) as a marker of oxidative stress in patients with acute coronary syndrome. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2005;149(1):83–87. [PubMed] [Google Scholar]
- United Nations WHO, D. O. E. A. S. A., Population Division, 2013. (2013). World Population Prospects: The 2012 Revision, Highlights and Advance Tables. Working Paper No. ESA/P/WP.228.
- Walter M.F., Jacob R.F., Jeffers B., Ghadanfar M.M., Preston G.M., Buch J., Mason R.P. Serum levels of thiobarbituric acid reactive substances predict cardiovascular events in patients with stable coronary artery disease: a longitudinal analysis of the PREVENT study. J. Am. Coll. Cardiol. 2004;44(10):1996–2002. doi: 10.1016/j.jacc.2004.08.029. [DOI] [PubMed] [Google Scholar]
- Zhang L., Peppel K., Sivashanmugam P., Orman E.S., Brian L., Exum S.T., Freedman N.J. Expression of tumor necrosis factor receptor-1 in arterial wall cells promotes atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2007;27(5):1087–1094. doi: 10.1161/ATVBAHA.0000261548.49790.63. [DOI] [PMC free article] [PubMed] [Google Scholar]