Abstract
Background
Short sleep may be a risk factor for atrial fibrillation. However, previous investigations have been limited by lack of objective sleep measurement and small sample size. We sought to determine the association between objectively measured sleep duration and atrial fibrillation.
Methods
All 31,079 adult patients undergoing diagnostic polysomnography from 1999 to 2015 at multiple sites within a large hospital network were identified from electronic medical records. Prevalent atrial fibrillation was identified by continuous ECG during polysomnography. Incident atrial fibrillation was identified by diagnostic codes and 12-lead ECGs. Logistic regression and Cox proportional hazards modeling were used to examine the association of sleep duration and atrial fibrillation prevalence and incidence, respectively, adjusting for age, sex, BMI, hypertension, coronary artery disease, cerebrovascular disease, peripheral vascular disease, heart failure, and sleep apnea severity.
Results
We identified 404 cases of prevalent atrial fibrillation among 30,061 individuals (mean age ± SD, 51.0 ± 14.5 years; 51.6% women) undergoing polysomnography. After adjustment, each 1-h reduction in sleep duration was associated with a 1.17-fold (95% CI, 1.11-1.30) increased risk of prevalent atrial fibrillation. Among 27,589 patients without atrial fibrillation at baseline, we identified 1,820 cases of incident atrial fibrillation over 4.6 years median follow-up. After adjustment, each 1-h reduction in sleep duration was associated with a 1.09-fold (95% CI, 1.05-1.13) increased risk for incident atrial fibrillation.
Conclusions
Short sleep duration is independently associated with prevalent and incident atrial fibrillation. Further research is needed to determine whether interventions to extend sleep can lower atrial fibrillation risk.
Key Words: cardiology, epidemiology, sleep apnea, sleep medicine
Abbreviations: AF, atrial fibrillation; AHI, apnea-hypopnea index; MESA, Multi-Ethnic Study of Atherosclerosis; N1, stage 1 nonrapid eye movement; N2, stage 2 nonrapid eye movement; N3, stage 3 nonrapid eye movement; R, rapid eye movement sleep stage; TST, total sleep time
FOR EDITORIAL COMMENT, SEE PAGE 421
OSA has been associated with multiple cardiovascular conditions including atrial fibrillation (AF).1, 2 Short sleep duration has also been reported to be a risk factor for a range of adverse cardiometabolic outcomes including obesity, hypertension, and impaired glucose tolerance.3, 4, 5 Reduced sleep duration has substantial public health importance because the prevalence of chronically restricted sleep is high,6, 7 with national survey data indicating approximately 35% of US adults get ≤ 6 h of sleep per night.8
Known AF risk factors explain only about one-half of the population attributable risk, highlighting the need for identification of novel risk factors for this disease.9, 10, 11 Beyond sleep apnea, impaired sleep may be a risk factor for AF.12, 13 However, prior research has been limited by small sample size, lack of objective quantification of sleep, lack of prospective follow-up, or inadequate accounting of sleep apnea severity.
We designed this study to examine the relation between objectively measured sleep duration and prevalent and incident AF. We hypothesized that short sleep duration, independent of sleep apnea severity, would be associated with both prevalent and incident AF.
Materials and Methods
Participants and Study Design
All patients ≥18 years of age who underwent in-laboratory, full-night diagnostic polysomnography at one of six University of Pittsburgh Medical Center sleep laboratories between March 1999 and December 2015 were eligible for inclusion. We excluded 11,349 patients with split-night (half-diagnostic, half-therapeutic) studies. In patients who underwent more than one diagnostic polysomnogram during the study period, only the most recent study was used.
This investigation was conducted in accordance with the Declaration of Helsinki and approved by the University of Pittsburgh institutional review board (PRO17060661). Informed consent was waived because of the retrospective study design.
Polysomnography
All included sleep studies were in-laboratory, diagnostic-only studies (no split-night, positive pressure titration, or home studies). Studies included single-lead ECG, electroencephalography, electrooculography, submental and bilateral tibial electromyography, thoracic and abdominal effort sensors, airflow measurement via oronasal thermistor and nasal pressure transducer, snore sensor, and pulse oximetry. Sleep stage for each 30-s epoch was determined as stage 1 nonrapid eye movement (N1), stage 2 nonrapid eye movement (N2), or stage 3 nonrapid eye movement (N3) or rapid eye movement sleep stage (R) by usual criteria.14 Total time in N1, N2, N3, and R sleep was summed to calculate total sleep time (TST). Apneas and hypopneas were scored using standard criteria with hypopneas requiring a 30% decrease in nasal pressure flow signal accompanied by a 4% reduction in oxyhemoglobin saturation. At one sleep laboratory, hypopneas were scored based on alternative criteria (a 30% decrease in nasal pressure flow signal with either 3% oxyhemoglobin desaturation or arousal) from the start of electronic records keeping at that laboratory in May 2003 until September 2007 (comprising 9.7% of the total patients). To ensure that results were not biased by the inclusion of sleep studies scored using the alternate hypopnea criteria, we performed sensitivity analyses excluding such patients (e-Appendix 1). The apnea-hypopnea index (AHI) was computed as the sum total of apneas and hypopneas divided by TST.15 After identification of eligible polysomnogram reports in the electronic health record, processing algorithms were used to extract data from report text, including age, sex, sleep center, BMI, AHI, TST, and sleep stage duration.
Ascertainment of AF
We designed cross-sectional and longitudinal analyses with prevalent and incident AF as the primary outcomes, respectively. For the cross-sectional analysis, prevalent AF was defined as AF or atrial flutter captured on polysomnography ECG. We classified rhythm as one of the following: sinus, AF, or indeterminate. Rhythms were deemed indeterminate if the interpreting physician reported an atrial rhythm other than sinus or AF or did not report an atrial rhythm. We performed two sensitivity analyses using alternative definitions for prevalent AF, detailed in e-Appendix 1, e-Table 1.
In the longitudinal analysis, we used both clinical encounters and 12-lead ECGs (inpatient and outpatient) to identify incident AF. For clinical encounters, International Classification of Diseases, Ninth Revision code 427.3x or International Classification of Diseases, 10th Revision code I48.xx identified AF. For ECGs, we developed parsing programs to classify rhythm based on interpreting cardiologist report. Patients with AF on baseline polysomnography, clinical encounter with AF diagnosis, or AF on ECG prior to and including the date of polysomnogram were excluded from longitudinal analysis. The date of incident AF was defined as the earliest of clinical encounter for AF or ECG showing AF. Patient time was censored at the earliest of (1) incident AF, (2) last available diagnostic or procedure code, or (3) December 31, 2016.
Covariates
Self-reported race was extracted from administrative records. BMI was calculated from height and weight as obtained according to each sleep laboratory’s protocol on the night of the test. Measures were extracted from sleep reports and therefore represent patient biometrics at the time of the index sleep study. Definitions for comorbidities were similar to those used in prior studies: heart failure (International Classification of Diseases, Ninth Revision codes 428.xx, 402.01, 402.11, 425, 429.3, or 514), hypertension (401.xx, 402.xx, 403.xx, 404.xx, or 405.xx), COPD (491.xx, 492.xx, 493.2, 494.xx, or 496), coronary artery disease (410.xx, 411.xx, 412, 414.xx, or 429.2, or procedure codes 36.0x, 36.1x, 36.2, or 00.66), peripheral arterial disease (440.0, 440.2x, 440.9, or 443.9) and cerebrovascular disease (430.xx, 431.xx, 433.xx, or 434.xx, or procedure codes 00.61-00.65).16, 17, 18, 19, 20, 21, 22
Validation
We validated data extraction algorithms by comparing results with manual examination of sleep study reports by a blinded physician (A. R. S.) in a random sample of 467 patients. Absolute agreement, defined as the percent of all cases evaluated where there was an exact match between the algorithm and the blinded reviewer’s assessment, was ≥ 99.5% for each of the following: TST, sleep stages, AHI, and cardiac rhythm. In an assessment of 300 12-lead ECGs, our ECG extraction algorithm was 100% accurate in AF classification against the criterion standard of original ECG interpretation by two blinded physicians (M. V. G. and A. R. S.).
Statistical Analysis
Logistic regression was used to relate TST and prevalent AF in multivariable-adjusted models. All models included age, age-squared, sex, center, BMI, AHI, heart failure, hypertension, COPD, coronary artery disease, peripheral artery disease, and cerebrovascular disease as covariates. Baseline characteristics were compared between patients with missing covariate data and those with complete data (e-Table 2). TST was modeled categorically in 1-h bins, then continuously because of linear appearance. A quadratic relation was formally tested by a TST-squared term. Multivariable logistic regression models were also used to assess the association between duration of each sleep stage and AF, adjusting for the same previously mentioned covariates along with all other sleep stages.
After excluding patients with prevalent AF by baseline polysomnography, ECG, or clinical encounter, AF-free survival was compared by TST using Kaplan-Meier survival curves and log-rank test. The proportional hazards assumption was assessed by plotting scaled Schoenfeld residuals, which revealed no clear pattern over time. The relationship between TST, modeled categorically, and incident AF was assessed using Cox proportional hazards models adjusting for the same set of covariates as in the cross-sectional analysis. These analyses demonstrated a linear relationship between TST and AF risk; therefore, TST was subsequently modeled continuously. We then evaluated time in each sleep stage as a predictor of incident AF.
In both cross-sectional and longitudinal analyses, we tested for interaction between TST and sleep apnea by inclusion of a TST × AHI term. Effect modification by age (< 70 vs ≥ 70 years), sex, obesity (BMI < 30 vs ≥ 30 kg/m2), and race (black vs white vs other because of relatively low numbers of nonblack and nonwhite patients) was also tested by additional models including multiplicative interaction terms in the longitudinal analysis. Tests of significance were two-sided with α = 0.05. Statistical analyses were conducted using R v3.4.0 (The R Project for Statistical Computing).
Results
Prevalent AF
A total of 31,206 patients had a diagnostic sleep study during the study period. After exclusions for age < 18 years, missing TST, or non-sinus/non-AF cardiac rhythm, 30,061 patients remained (e-Fig 1). Overall, the included patients had a mean age ± SD of 51.0 ± 14.5 years and 51.6% were women. TST averaged 4.7 ± 1.3 h. There was a greater prevalence of comorbidities and higher age with shorter sleep duration (Table 1).
Table 1.
Baseline and Sleep Characteristics of the Cross-Sectional Study Cohort by Sleep Duration on Polysomnography
| Characteristic | Total Sleep Time |
||||
|---|---|---|---|---|---|
| ≥ 6 h (n = 3,989) | 5-6 h (n = 10,232) | 4-5 h (n = 8,624) | 3-4 h (n = 4,210) | < 3 h (n = 3,006) | |
| Age, y | 44.9 ± 13.9 | 47.8 ± 13.4 | 52.4 ± 13.8 | 55.8 ± 14.5 | 58.7 ± 14.8 |
| Female | 2,181/3,989 (54.7) | 5,429/10,231 (53.1) | 4,474/8,623 (51.9) | 2,044/4,210 (48.6) | 1,369/3,006 (45.5) |
| Race | |||||
| White | 3,230/3,989 (81.0) | 8,336/10,232 (81.5) | 7,141/8,624 (82.8) | 3,466/4,210 (82.3) | 2,454/3,006 (81.6) |
| Black | 535/3,989 (13.4) | 1,367/10,232 (13.4) | 1,106/8,624 (12.8) | 565/4,210 (13.4) | 416/3,006 (13.8) |
| Other | 224/3,989 (5.6) | 529/10,232 (5.2) | 377/8,624 (4.4) | 179/4,210 (4.3) | 136/3,006 (4.5) |
| BMI, kg/m2 | 32.8 ± 8.1 | 33.7 ± 8.4 | 34.4 ± 8.9 | 34.8 ± 9.2 | 35.5 ± 10.1 |
| Congestive heart failure | 191/3,989 (4.8) | 577/10,232 (5.6) | 744/8,624 (8.6) | 505/4,210 (12.0) | 495/3,006 (16.5) |
| COPD | 300/3,989 (7.5) | 845/10,232 (8.3) | 932/8,624 (10.8) | 627/4,210 (14.9) | 570/3,006 (19.0) |
| Hypertension | 1,233/3,989 (30.9) | 3,710/10,232 (36.3) | 3,787/8,624 (43.9) | 2,238/4,210 (53.2) | 1,670/3,006 (55.6) |
| Coronary artery disease | 323/3,989 (8.1) | 1,005/10,232 (9.8) | 1,227/8,624 (14.2) | 770/4,210 (18.3) | 688/3,006 (22.9) |
| Peripheral arterial disease | 109/3,989 (2.7) | 280/10,232 (2.7) | 337/8,624 (3.9) | 237/4,210 (5.6) | 221/3,006 (7.4) |
| Cerebrovascular disease | 120/3,989 (3.0) | 268/10,232 (2.6) | 322/8,624 (3.7) | 214/4,210 (5.1) | 173/3,006 (5.8) |
| Apnea-hypopnea index, h−1 | 13.9 ± 20.1 | 16.0 ± 20.3 | 19.9 ± 23.2 | 22.8 ± 26.1 | 27.0 ± 28.9 |
| N1 sleep, min | 15 ± 15 | 16 ± 18 | 18 ± 19 | 19 ± 21 | 18 ± 19 |
| N2 sleep, min | 275 ± 54 | 230 ± 44 | 196 ± 39 | 157 ± 35 | 94 ± 40 |
| N3 sleep, min | 35 ± 38 | 30 ± 34 | 22 ± 29 | 15 ± 23 | 8 ± 15 |
| R sleep, min | 65 ± 33 | 51 ± 27 | 36 ± 23 | 22 ± 19 | 10 ± 14 |
Values are reported as mean ± SD or No. of patients/total No. of patients (%). N1 = stage 1 nonrapid eye movement; N2 = stage 2 nonrapid eye movement; N3 = stage 3 nonrapid eye movement; R = rapid eye movement sleep stage.
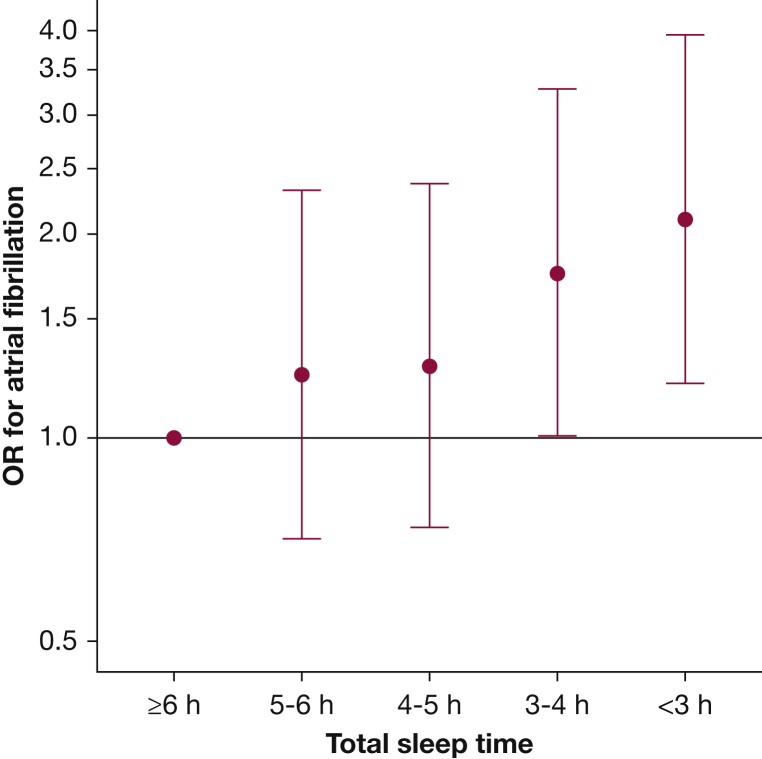
AF during sleep study was identified in 404 patients (1.3%). The crude prevalence of AF per TST category is shown in e-Table 1. Compared with those who slept ≥ 6 h, the odds of AF were 2.10 times greater (95% CI, 1.20-3.94) in those who slept < 3 h and 1.75 times greater (95% CI, 1.00-3.28) in those who slept 3 to 4 h after multivariable adjustment. The odds of AF increased linearly with each 1-h decrement in TST (Fig 1) (P = .004 for trend). Inclusion of a TST-squared term did not improve model fit (likelihood ratio test, P = .50). When modeled continuously, each 1-h reduction in TST was associated with 1.17-fold (95% CI, 1.08-1.27) higher odds of AF. The association between TST and AF risk was not modified by sleep apnea severity (P = .74). In sensitivity analyses, changing the criteria for AF did not meaningfully change the association with short sleep (e-Appendix 1).
Figure 1.
Risk of prevalent atrial fibrillation by sleep duration on polysomnography. ORs ± 95% CIs for prevalent atrial fibrillation, by total sleep time, compared with patients with total sleep time ≥ 6 h, adjusted for age, age-squared, sex, center, BMI, congestive heart failure, hypertension, COPD, coronary artery disease, peripheral arterial disease, cerebrovascular disease, and apnea-hypopnea index. P = .004 for test of linear trend.
Longitudinal Analysis for Incident AF
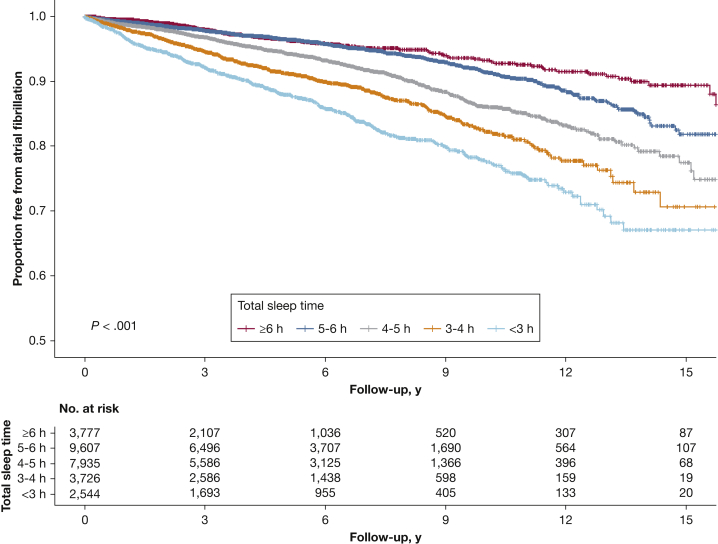
After excluding patients with prevalent AF on polysomnography, those with evidence of AF in the electronic record, and patients with no follow-up, 27,589 patients remained for longitudinal analysis (e-Fig 1). There were 1,820 cases of incident AF identified over a median follow-up of 4.6 years (range, 0-17 years), for a crude incidence rate of 12.7 (95% CI, 12.1-13.3) per 1,000 person-years. As shown in Figure 2, a dose-response relationship was found between TST and AF-free survival, such that patients with shorter sleep duration had significantly lower AF-free survival (log-rank test, P < .001).
Figure 2.
Atrial fibrillation-free survival by sleep duration on polysomnography. Proportion free from atrial fibrillation for patients by total sleep time on polysomnography. Incident atrial fibrillation identified by first diagnostic code for atrial fibrillation or ECG showing atrial fibrillation among patients free of atrial fibrillation at time of index polysomnogram. There was a significant difference in freedom from atrial fibrillation by total sleep time using the log-rank test, P < .001.
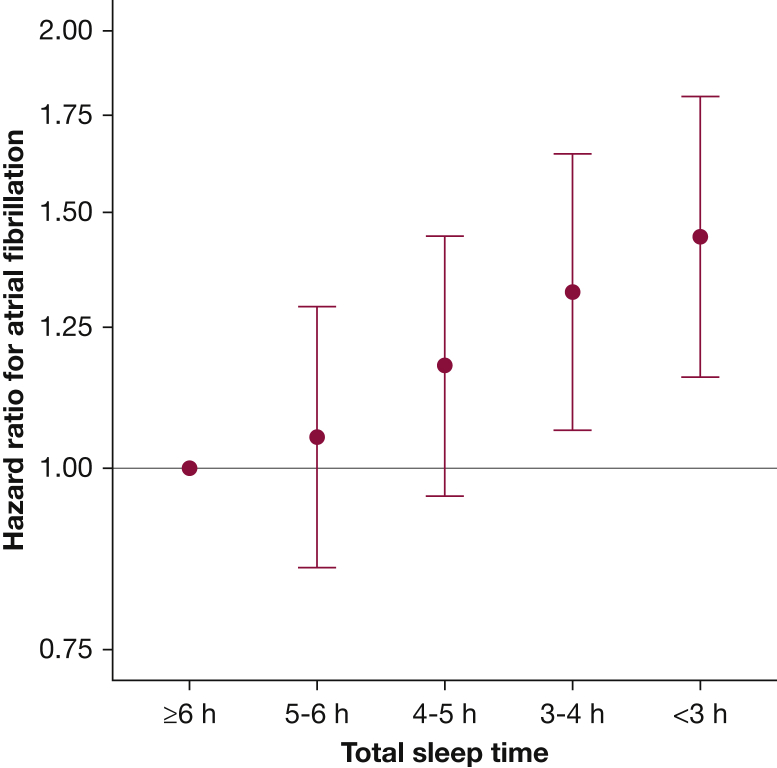
Compared with those who slept ≥ 6 h, the hazard of AF was 1.44 times greater (95% CI, 1.16-1.80) in those who slept < 3 h and 1.32 times greater (95% CI, 1.06-1.65) in those who slept 3 to 4 h after multivariable adjustment (Fig 3). When modeled continuously, each 1-h decrease in TST was associated with a 1.09-fold increase (95% CI, 1.05-1.13) in risk of incident AF after multivariable adjustment. There was no evidence for TST × AHI interaction (P = .72). There was no meaningful effect modification of the association between TST and AF incidence by age, sex, obesity, or race (e-Fig 2).
Figure 3.
Total sleep time as a predictor of incident atrial fibrillation. Hazard ratios for incident atrial fibrillation by total sleep time, with 95% CIs relative to patients sleeping ≥ 6 h. They were adjusted for age, age-squared, sex, center, BMI, congestive heart failure, hypertension, COPD, coronary artery disease, peripheral arterial disease, cerebrovascular disease, and apnea-hypopnea index. P < .001 for test of linear trend.
AF and Sleep Stages
Table 2 presents associations between sleep stage duration and AF. In cross-sectional analyses, each 1-h reduction in N2 sleep was associated with a 1.16 increase in the odds of prevalent AF (95% CI, 1.05-1.27), after multivariable adjustment including duration of other sleep stages (model 2). In longitudinal analyses, each 1-h reduction in N2 sleep was associated with a 1.07 times (95% CI, 1.03-1.12) higher risk of incident AF, whereas each 1-h reduction in R sleep was associated with a 1.17 times (95% CI, 1.04-1.30) higher risk. Overall, the magnitudes of the associations for N1 and N3 sleep were comparable with those estimated for N2 and R sleep, although not statistically significant.
Table 2.
Risk of Prevalent and Incident Atrial Fibrillation by Sleep Stage Duration on Polysomnography
| Sleep Component | OR for Prevalent Atrial Fibrillation (95% CI) | HR for Incident Atrial Fibrillation (95% CI) |
|---|---|---|
| N1 sleep, per hour shorter | ||
| Model 1a | 1.04 (0.77-1.44) | 1.05 (0.91-1.21) |
| Model 2b | 1.18 (0.86-1.65) | 1.13 (0.98-1.31) |
| N2 sleep, per hour shorter | ||
| Model 1 | 1.17 (1.06-1.28) | 1.07 (1.03-1.12) |
| Model 2 | 1.16 (1.05-1.27) | 1.07 (1.03-1.12) |
| N3 sleep, per hour shorter | ||
| Model 1 | 1.05 (0.82-1.38) | 1.08 (0.95-1.23) |
| Model 2 | 1.08 (0.83-1.42) | 1.12 (0.98-1.27) |
| R sleep, per hour shorter | ||
| Model 1 | 1.39 (1.08-1.82) | 1.21 (1.08-1.35) |
| Model 2 | 1.28 (0.98-1.68) | 1.17 (1.04-1.30) |
| Total sleep time, per hour shorter | ||
| Model 1 | 1.17 (1.08-1.27) | 1.09 (1.05-1.13) |
ORs for prevalent atrial fibrillation and HRs for incident atrial fibrillation are presented, per 1-h decrease in sleep stage duration, with 95% CIs. HR = hazard ratio. See Table 1 legend for expansion of other abbreviations.
Adjusting for age, age-squared, sex, center, BMI, congestive heart failure, hypertension, COPD, coronary artery disease, peripheral arterial disease, cerebrovascular disease, and apnea-hypopnea index.
Model 1, additionally adjusted for duration of all other sleep stages.
Discussion
In this large observational study of a clinical cohort referred for polysomnography, we found that objectively measured short sleep is an independent risk factor for prevalent and incident AF. Each 1-h reduction in sleep duration was associated with 17% greater risk of prevalent AF and 9% greater risk of incident AF. Notably, these results persisted after adjustment for sleep apnea severity, a known AF risk factor.2 In addition, we found no evidence that the association of sleep duration and AF risk varied by sleep apnea severity. Reductions in R and N2 sleep were associated with the largest effect on risk of AF, especially in the cross-sectional analysis. However, CIs were wide and all stages tended to have effect estimates in the same direction, toward higher risk of AF. Our findings demonstrate a potentially important association between short sleep duration and AF in a large cohort representative of patients seen in general practice.
Causal pathways between short sleep duration and AF are supported by both epidemiologic and physiologic evidence. Short sleep duration is recognized as an important risk factor for obesity, diabetes, and hypertension, all of which contribute to AF risk.23 Acute sleep deprivation has been shown to increase atrial electromechanical delay, a predictor of incident AF in unaffected individuals.24 Sleep deprivation also increases sympathetic nervous system activity and activates proinflammatory systems, additional pathways predisposing to AF.25, 26, 27
Our finding that short sleep duration is associated with a greater risk of AF supports and extends findings from prior studies. In both the Physician’s Health Study and the Kailuan Study, short self-reported sleep was associated with a 6% to 7% higher incident AF risk.28, 29 The attenuated risk compared with our results may be because of the use of self-report to assess sleep duration in these prior studies. In contrast with our results, polysomnographic sleep duration was not associated with incident AF risk in the Cardiovascular Health Study; however, the small number of incident events (n = 259) may have limited power.13 A cross-sectional analysis of the Multi-Ethnic Study of Atherosclerosis (MESA) cohort reported each hour reduction in sleep duration, as assessed by actigraphy, was associated with a 6% higher AF prevalence; however, this was not statistically significant.12 The MESA cohort was also limited in precision because of a total of only 100 prevalent cases of AF. Finally, a recent analysis of medical records in > 14 million California residents found a diagnosis of insomnia increased risk of incident AF by 36%, supporting the contention that poor sleep may be an AF risk factor independent of sleep apnea.13
Our analyses indicated a linear relationship between TST and AF. In contrast, analyses from both the Physician’s Health Study and the Kailuan Study reported a U-shaped relation, finding elevated risk for AF in those with self-reported sleep duration ≥ 8 h. The correlation between self-report and objectively assessed sleep duration is modest at best,30 and it appears self-reported long sleep duration may better reflect greater time in bed than time asleep per se.31, 32
Although we found evidence of a slightly larger association between R and N2 sleep and AF, the estimates for the effect of N1 and N3 sleep were in a similar direction, supporting the hypothesis that sufficient time in all sleep stages is protective for AF. Prior studies in this area have been mixed: N3 sleep was most strongly associated with AF in the MESA cohort analysis, whereas R sleep duration was most strongly associated in the Cardiovascular Health Study analysis.12, 13 Our results suggest adequate time in both stages may be important to minimize risk, with reductions in R sleep associated with particular vulnerability to AF. R sleep is generally accompanied by increases in heart rate, BP, and bursts of sympathetic activity interrupting the typical parasympathetic dominance seen during other sleep stages.33 However, nocturnal bradyarrhythmias are thought to occur exclusively during R sleep, perhaps because of abrupt withdrawal of sympathetic tone. Further, suppression of R sleep has been shown to increase cardiac arrhythmia susceptibility in an animal model.34 It is possible that variability in autonomic tone during R sleep serves a protective function against tachyarrhythmia not yet fully described.
Polysomnography is the criterion standard measurement of sleep duration; however, we note that the average TST across all included patients in this study was lower than might be expected. In several community-based epidemiologic studies, mean sleep duration as assessed by polysomnography tends to be approximately 6 h, which is significantly lower than what patients self-report, and equal to or lower than multiday actigraphy-measured duration.30, 35, 36 We would expect that in our study, single-night, in-laboratory sleep duration measured in a clinical population referred for symptomatic sleep disturbances would be even lower than the community-based average of 6 h.
Consideration of how well TST on single-night, in-laboratory polysomnography reflects habitual sleep duration is also warranted. It is well understood that patients suffer a first-night effect, wherein in-laboratory sleep duration is lower than at home because of the unfamiliarity of the setting. However, first-night effects appear to be universal, preserving the validity of between-subject comparisons.37, 38
Our study has notable strengths. We validated our algorithms for ascertainment of sleep variables and AF, demonstrating high accuracy. Our use of polysomnography to measure sleep, rather than self-report, reduces measurement error that have affected prior studies. Further, we were able to adjust for AHI, a quantitative measure of sleep apnea severity, which is an important risk factor for AF.1, 2 Although there are limitations in the use of diagnostic codes to identify AF,39 the addition of ECG data improves accuracy,40 and we validated our method. The large size of our cohort has allowed for more precise effect-size estimation, which has produced stronger evidence for the protective effect of sleep than has been seen in prior studies of AF.
Several potential limitations in this study should also be noted. As previously discussed, it is possible that our findings may be confounded by factors that influence the relative magnitude of the first-night effect on sleep duration in the laboratory. In addition, although we evaluated the relative contributions of each sleep stage to the overall relationship between TST and AF risk, the relative importance of arousals and sleep instability were not evaluated. Unfortunately, metrics of sleep fragmentation, such as arousals, stage shifting, and periodic limb movements, were not uniformly available for analysis and would be of interest in future analyses. An additional limitation is medical surveillance bias, wherein patients who are sicker (and perhaps shorter-sleeping) present to care more frequently and therefore have increased opportunities for AF diagnosis. We attempted to mitigate this by adjusting for cardiovascular comorbidities. Because of the retrospective, clinical nature of our study design and inclusion of multiple sleep laboratories over a long time frame, there was no way to standardize sleep laboratory hardware, data collection software, or procedures such as height and weight assessment. Although possibly enhancing external validity by mirroring general clinical practice, variation introduced by lack of standardization will likely lead to an increase in random error and therefore underestimation of the true effect size. Some patients may have had follow-up outside of our hospital system or may have asymptomatic AF which eludes detection. There is no reason to suspect, however, that either of these issues differentially affects patients with shorter or longer sleep times; therefore, inaccuracy of ascertainment of AF would also tend to bias findings toward the null hypothesis. Finally, although we adjusted for the strongest AF risk factors, we acknowledge the possibility of residual confounding.
Conclusions
In this large retrospective analysis, patients with short objectively measured sleep duration had increased risk for current and future AF independent of major risk factors. Poor sleep and AF are both growing global epidemics, increasing the public health and clinical importance of associations between these conditions. Further research is needed to identify the possible mediating mechanisms and to evaluate the potential of interventions to extend sleep duration as a low cost means to prevent AF.
Acknowledgments
Author contributions: M. V. G. is the guarantor and takes responsibility for the contents of the manuscript, the integrity of the data, and the accuracy of the data analysis. M. V. G. designed the study, collected the data, performed statistical analysis, and authored the initial and final drafts of the manuscript. J. W. M. and S. R. P. designed the study, contributed substantially to statistical analysis, and revised drafts of the manuscript. R. P. O. contributed substantially to statistical analysis and revised drafts of the manuscript. A. R. S., R. S. D., and M. I. S. contributed substantially to study design and data collection and integrity. All authors approved the final submitted version of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: S. R. P. has received grant funding through his institution from the American Sleep Medicine Foundation, the ResMed Foundation, Bayer Pharmaceuticals, and Philips Respironics. None declared (M. V. G., R. P. O., A. R. S., R. S. D., M. I. S., J. V. M.).
Role of sponsors: The sponsor played no role in the design, collection, and data analysis of the study, or the revision of the manuscript.
Additional information: The e-Appendix, e-Figures, and e-Tables can be found in the Supplemental Materials section of the online article.
Footnotes
Parts of this work have previously been presented at the following national meetings: AHA EPI/Lifestyle March 22, 2018, New Orleans, LA; and SLEEP June 6, 2018, Baltimore, MD.
FUNDING/SUPPORT: This work was supported by the National Institutes of Health [Grants HL083825 (to M. V. G.), HL082610 (to R. P. O.), HL127307 (to S. R. P.)]; and by the Doris Duke Charitable Foundation [Award 2015084 (to J. W. M.)].
Supplementary Data
References
- 1.Gami A.S., Pressman G., Caples S.M. Association of atrial fibrillation and obstructive sleep apnea. Circulation. 2004;110(4):364–367. doi: 10.1161/01.CIR.0000136587.68725.8E. [DOI] [PubMed] [Google Scholar]
- 2.Gami A.S., Hodge D.O., Herges R.M. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J Am Coll Cardiol. 2007;49(5):565–571. doi: 10.1016/j.jacc.2006.08.060. [DOI] [PubMed] [Google Scholar]
- 3.Patel S.R., Hu F.B. Short sleep duration and weight gain: a systematic review. Obesity. 2008;16(3):643–653. doi: 10.1038/oby.2007.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Faraut B., Touchette É., Gamble H. Short sleep duration and increased risk of hypertension. J Hypertens. 2012;30(7):1354–1363. doi: 10.1097/HJH.0b013e32835465e5. [DOI] [PubMed] [Google Scholar]
- 5.Rafalson L., Donahue R.P., Stranges S. Short sleep duration is associated with the development of impaired fasting glucose: the Western New York Health Study. Ann Epidemiol. 2010;20(12):883–889. doi: 10.1016/j.annepidem.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al Lawati N.M., Patel S.R., Ayas N.T. Epidemiology, risk factors, and consequences of obstructive sleep apnea and short sleep duration. Prog Cardiovasc Dis. 2009;51(4):285–293. doi: 10.1016/j.pcad.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Ayas N., White D., Manson J. A prospective study of sleep duration and coronary heart disease in women. Arch Int Med. 2003;163(2):205–209. doi: 10.1001/archinte.163.2.205. [DOI] [PubMed] [Google Scholar]
- 8.Liu Y., Wheaton A.G., Chapman D.P., Cunningham T.J., Lu H., Croft J.B. Prevalence of healthy sleep duration among adults — United States, 2014. MMWR Morb Mortal Wkly Rep. 2016;65(6):137–141. doi: 10.15585/mmwr.mm6506a1. [DOI] [PubMed] [Google Scholar]
- 9.Schnabel R.B. Can we predict the occurrence of atrial fibrillation? Clin Cardiol. 2012;35(suppl 1):5–9. doi: 10.1002/clc.20963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perez M.V., Wang P.J., Larson J.C. Risk factors for atrial fibrillation and their population burden in postmenopausal women: the Women’s Health Initiative Observational Study. Heart. 2013;99(16):1173–1178. doi: 10.1136/heartjnl-2013-303798. [DOI] [PubMed] [Google Scholar]
- 11.Benjamin E.J., Levy D., Vaziri S.M., D’Agostino R.B., Belanger A.J., Wolf P.A. Independent risk factors for atrial fibrillation in a population-based cohort: The Framingham Heart Study. J Am Heart Assoc. 1994;271(11):840–844. [PubMed] [Google Scholar]
- 12.Kwon Y., Gharib S.A., Biggs M.L. Association of sleep characteristics with atrial fibrillation: the Multi-Ethnic Study of Atherosclerosis. Thorax. 2015;70(9):873–879. doi: 10.1136/thoraxjnl-2014-206655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Christensen M.A., Dixit S., Dewland T.A. Sleep characteristics that predict atrial fibrillation. Hear Rhythm. 2018;15(9):1289–1295. doi: 10.1016/j.hrthm.2018.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kales A., Rechtschaffen A. U.S. National Institute of Neurological Diseases and Blindness, Neurological Information Network; Bethesda, MD: 1968. University of California Los Angeles Brain Information Service, NINDB Neurological Information Network (US). A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. [Google Scholar]
- 15.Berry R.B., Budhiraja R., Gottlieb D.J. Rules for scoring respiratory events in sleep: Update of the 2007 AASM manual for the scoring of sleep and associated events. J Clin Sleep Med. 2012;8(5):597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hicks L.S., Shaykevich S., Bates D.W., Ayanian J.Z. Determinants of racial/ethnic differences in blood pressure management among hypertensive patients. BMC Cardiovasc Disord. 2005;5(1):16. doi: 10.1186/1471-2261-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roger V.L., Weston S.A., Redfield M.M. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004;292(3):344–350. doi: 10.1001/jama.292.3.344. [DOI] [PubMed] [Google Scholar]
- 18.Gerber L.M., Mann S.J., McDonald M.V., Chiu Y.-L., Sridharan S., Feldman P.H. Diuretic use in black patients with uncontrolled hypertension. Am J Hypertens. 2013;26(2):174–179. doi: 10.1093/ajh/hps029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cooke C.R., Joo M.J., Anderson S.M. The validity of using ICD-9 codes and pharmacy records to identify patients with chronic obstructive pulmonary disease. BMC Heal Serv Res. 2011;11(37):1–10. doi: 10.1186/1472-6963-11-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hlatky M.A., Ray R.M., Burwen D.R. Use of medicare data to identify coronary heart disease outcomes in the women’s health initiative. Circ Cardiovasc Qual Outcomes. 2014;7(1):157–162. doi: 10.1161/CIRCOUTCOMES.113.000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Polinski J.M., Shrank W.H., Glynn R.J., Huskamp H.A., Roebuck M.C., Schneeweiss S. Beneficiaries with cardiovascular disease and the part D coverage gap. Circ Cardiovasc Qual Outcomes. 2012;5(3):387–395. doi: 10.1161/CIRCOUTCOMES.111.964866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thacker E.L., Muntner P., Zhao H. Claims-based algorithms for identifying Medicare beneficiaries at high estimated risk for coronary heart disease events: a cross-sectional study. BMC Health Serv Res. 2014;14(1):195. doi: 10.1186/1472-6963-14-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.St-Onge M.P., Grandner M.A., Brown D. Sleep duration and quality: impact on lifestyle behaviors and cardiometabolic health: a scientific statement from the American Heart Association. Circulation. 2016;134(18):e367–e386. doi: 10.1161/CIR.0000000000000444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Esen Ö., Akçakoyun M., Açar G. Acute sleep deprivation is associated with increased atrial electromechanical delay in healthy young adults. Pacing Clin Electrophysiol. 2011;34(12):1645–1651. doi: 10.1111/j.1540-8159.2011.03186.x. [DOI] [PubMed] [Google Scholar]
- 25.Castro-Diehl C., Diez Roux A.V., Redline S. Sleep duration and quality in relation to autonomic nervous system measures: the Multi-Ethnic Study of Atherosclerosis (MESA) Sleep. 2016;39(11):1919–1926. doi: 10.5665/sleep.6218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanagasabai T., Ardern C.I. Contribution of inflammation, oxidative stress, and antioxidants to the relationship between sleep duration and cardiometabolic health. Sleep. 2015;38(12):1905–1912. doi: 10.5665/sleep.5238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo Y., Lip G.Y.H., Apostolakis S. Inflammation in atrial fibrillation. J Am Coll Cardiol. 2012;60(22):2263–2270. doi: 10.1016/j.jacc.2012.04.063. [DOI] [PubMed] [Google Scholar]
- 28.Khawaja O., Sarwar A., Albert C.M., Gaziano J.M., Djoussé L. Sleep duration and risk of atrial fibrillation (from the Physicians’ Health Study) Am J Cardiol. 2013;111(4):547–551. doi: 10.1016/j.amjcard.2012.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song Q., Liu X., Hu W. Long sleep duration is an independent risk factor for incident atrial fibrillation in a Chinese population: a prospective cohort study. Sci Rep. 2017;7(1):3679. doi: 10.1038/s41598-017-04034-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matthews K.A., Patel S.R., Pantesco E.J. Similarities and differences in estimates of sleep duration by polysomnography, actigraphy, diary, and self-reported habitual sleep in a community sample. Sleep Heal. 2018;4(1):96–103. doi: 10.1016/j.sleh.2017.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel S.R., Malhotra A., Gottlieb D.J., White D.P., Hu F.B. Correlates of long sleep duration. Sleep. 2006;29(7):881–889. doi: 10.1093/sleep/29.7.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patel S.R., Blackwell T., Ancoli-Israel S., Stone K.L. Sleep characteristics of self-reported long sleepers. Sleep. 2012;35(5):641–648. doi: 10.5665/sleep.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holty J.E.C., Guilleminault C. REM-related bradyarrhythmia syndrome. Sleep Med Rev. 2011;15(3):143–151. doi: 10.1016/j.smrv.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 34.Almeida F.R., Perry J.C., Futuro-Neto H.A. Cardiovascular function alterations induced by acute paradoxical sleep deprivation in rats. Clin Exp Hypertens. 2014;36(8):567–571. doi: 10.3109/10641963.2014.881843. [DOI] [PubMed] [Google Scholar]
- 35.Jackson C.L., Patel S.R., Ii W.B.J., Lutsey P.L., Redline S. Agreement between self-reported and objectively measured sleep duration among white, black, Hispanic, and Chinese adults in the United States: Multi-Ethnic Study of Atherosclerosis. Sleep. 2018;41(6):zsy057. doi: 10.1093/sleep/zsy057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Silva G.E., Goodwin J.L., Sherrill D.L. Relationship between reported and measured sleep times: the Sleep Heart Health Study (SHHS) J Clin Sleep Med. 2007;3(6):622–630. [PMC free article] [PubMed] [Google Scholar]
- 37.Toussaint M., Luthringer R., Schaltenbrand N. First-night effect in normal subjects and psychiatric inpatients. Sleep. 1995;18(6):463–469. doi: 10.1093/sleep/18.6.463. [DOI] [PubMed] [Google Scholar]
- 38.Hirscher V., Unbehaun T., Feige B., Nissen C., Riemann D., Spiegelhalder K. Patients with primary insomnia in the sleep laboratory: Do they present with typical nights of sleep? J Sleep Res. 2015;24(4):383–389. doi: 10.1111/jsr.12280. [DOI] [PubMed] [Google Scholar]
- 39.Thigpen J.L., Dillon C., Forster K.B. Validity of international classification of disease codes to identify ischemic stroke and intracranial hemorrhage among individuals with associated diagnosis of atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2015;8(1):8–14. doi: 10.1161/CIRCOUTCOMES.113.000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khurshid S., Keaney J., Ellinor P.T., Lubitz S.A. A simple and portable algorithm for identifying atrial fibrillation in the electronic medical record. Am J Cardiol. 2016;117(2):221–225. doi: 10.1016/j.amjcard.2015.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.