Abstract Abstract
Three strains of the genus Diaporthe were isolated from different plant hosts in south-western China. Phylogenetic analyses of the combined ITS, β-tubulin, tef1 and calmoudulin dataset indicated that these strains represented three independent lineages in Diaporthe. Diaporthe millettiaesp. nov. clustered with D. hongkongensis and D. arecae, Diaporthe osmanthisp. nov. grouped with D. arengae, D. pseudomangiferae and D. perseae and Diaporthe strain GUCC9146, isolated from Camellia sinensis, was grouped in the D. eres species complex with a close relationship to D. longicicola. These species are reported with taxonomic descriptions and illustrations.
Keywords: Diaporthe , phylogeny, taxonomy, 2 new taxa
Introduction
Genus Diaporthe has been well-studied in recent years by Udayanga et al. (2011, 2012), incorporating morphological and molecular data and recommending appropriate genes to resolve species limitations in the genus. Since these revolutionary papers, 43 novel Diaporthe species have been described from China with morphological and phylogenetic evidence (Huang et al. 2013, 2015; Fan et al. 2016; Gao et al. 2014, 2015, 2016, 2017; Yang et al. 2017a,b, 2018; Yang et al. 2016; Du et al. 2016; Dissanayake et al. 2017a). Dissanayake et al. (2017b) provided an update of the genus with additional 15 species (7 new and 8 known species) from Italy based on molecular evidence. New records and species have also been reported by Hyde et al. (2016), Rossman et al. (2016), Chen and Kirschner (2017), Guarnaccia et al. (2018), Perera et al. (2018), Tibpromma et al. (2018) and Wanasinghe et al. (2018).
Three strains of Diaporthe were isolated from different medicinal plants collected in Guizhou and Guangxi during a survey of fungal diversity in south-western China. All the strains produced conidiomata containing alpha- and beta-conidia, typical of Diaporthe. This paper describes these three collections using molecular evidence, based on the analysis of combined ITS, β-tubulin, tef1 and calmoudulin datasets, as Diaporthe millettiae sp. nov. and D. osmanthi sp. nov. and D. longicicola with a new host record from Camellia sinensis.
Materials and methods
Isolation and morphological studies
The samples were collected from Guizhou and Guangxi provinces. The Diaporthe strains were isolated using the single-spore method (Chomnunti et al. 2014). Colonies, growing from single spores, were transferred to potato-dextrose agar (PDA) and incubated at room temperature (28 °C). Following 2–3 weeks of incubation, morphological characters were recorded as in Udayanga et al. (2011, 2015). Conidia and conidiophores were observed using a compound microscope (Olympus BX53). The holotype specimens are deposited in the Herbarium of Department of Plant Pathology, Agricultural College, Guizhou University (HGUP). Ex-type cultures are deposited in the Culture Collection at the Department of Plant Pathology, Agriculture College, Guizhou University, China (GUCC). Taxonomic information of the new taxa was submitted to MycoBank (http://www.mycobank.org) and Facesoffungi (http://www.facesoffungi.org).
DNA extraction and sequencing
Fungal cultures were grown on PDA medium until they nearly covered the whole Petri-dish (90 mm diam.) at 28 °C. Fresh fungal mycelia were scraped from the surface with sterilised scalpels. A BIOMIGA Fungus Genomic DNA Extraction Kit (GD2416) was used to extract fungal genome DNA. DNA amplification was performed in a 25 μl reaction volume system which contained 2.5 μl 10 × PCR buffer, 1 μl of each primer (10 μM), 1 μl template DNA and 0.25 μl Taq DNA polymerase (Promega, Madison, WI, USA). Primers ITS4 and ITS5 (White et al. 1990) were used to amplify the ITS region. Three protein-coding gene fragments (β-tubulin, tef1 and calmoudulin) were amplified by the primers Bt2a/Bt2b (Glass and Donaldson 1995), CAL228F/CAL737R and EF1-728F/EF1-986R (Carbone and Kohn 1999). Gene sequencing was performed with an ABI PRISM 3730 DNA autosequencer using either a dRhodamine terminator or Big Dye Terminator (Applied Biosystems Inc., Foster 19 City, California). The sequences of both strands of each fragment were determined for sequence confirmation. The DNA sequences were submitted to GenBank and their accession numbers were provided in Table 1.
Table 1.
GenBank accession numbers of isolates include in this study.
| Species | Culture no. | GenBank no. | |||
|---|---|---|---|---|---|
| ITS | tef1 | β-tubulin | calmoudulin | ||
| Diaporthe alleghaniensis | CBS 495.72 | KC343007 | KC343733 | KC343975 | KC343249 |
| D. ambigua | CBS 114015 | AF230767 | GQ250299 | KC343978 | KC343252 |
| D. anacardii | CBS 720.97* | KC343024 | KC343750 | KC343992 | KC343266 |
| D. arecae | CBS 161.64 | KC343032 | KC343758 | KC344000 | KC343274 |
| D. arengae | CBS 114979 | KC343034 | KC343760 | KC344002 | KC343276 |
| D. baccae | CBS 136972 | KJ160565 | KJ160597 | MF418509 | MG281695 |
| D. beilharziae | BRIP 54792 | JX862529 | JX862535 | KF170921 | – |
| D. betulae | CFCC 50470 | KT732951 | KT733017 | KT733021 | KT732998 |
| D. bicincta | CBS 121004 | KC343134 | KC343860 | KC344102 | KC343376 |
| D. biguttusis | CGMCC 3.17081 | KF576282 | KF576257 | KF576306 | – |
| D. celastrina | CBS 139.27 | KC343047 | KC343773 | KC344015 | KC343289 |
| D. celeris | CBS 143349 | MG281017 | MG281538 | MG281190 | MG281712 |
| D. charlesworthii | BRIP 54884m* | KJ197288 | KJ197250 | KJ197268 | – |
| D. cinerascens | CBS 719.96 | KC343050 | KC343776 | KC344018 | KC343292 |
| D. cotoneastri | CBS 439.82 | FJ889450 | GQ250341 | JX275437 | JX197429 |
| D. decedens | CBS 109772 | KC343059 | KC343785 | KC344027 | KC343301 |
| D. elaeagni | CBS 504.72 | KC343064 | KC343790 | KC344032 | KC343306 |
| D. ellipicola | CGMCC 3.17084 | KF576270 | KF576245 | KF576291 | – |
| D. eres | CBS 138594 | KJ210529 | KJ210550 | KJ420799 | KJ434999 |
| D. foeniculina | CBS 187.27 | KC343107 | KC343833 | KC344075 | KC343349 |
| D. goulteri | BRIP 55657a | KJ197289 | KJ197252 | KJ197270 | – |
| D. helianthi | CBS 592.81 | KC343115 | GQ250308 | KC343841 | JX197454 |
| D. hongkongensis | CBS 115448 | KC343119 | KC343845 | KC344087 | KC343361 |
| D. inconspicua | CBS 133813 | KC343123 | KC343849 | KC344091 | KC343365 |
| D. longicicola | GUCC9146 | MK398676 | MK480611 | MK502091 | MK502088 |
| D. longicicola | CGMCC 3.17091 | KF576267 | KF576242 | KF576291 | – |
| D. macinthoshii | BRIP 55064a* | KJ197290 | KJ197251 | KJ197269 | – |
| D. millettia | GUCC9167 | MK398674 | MK480609 | MK502089 | MK502086 |
| D. oncostoma | CBS 589.78 | KC343162 | KC343888 | KC344130 | KC343404 |
| D. osmanthusis | GUCC9165 | MK398675 | MK480610 | MK502090 | MK502087 |
| D. perseae | CBS 151.73 | KC343173 | KC343899 | KC344141 | KC343415 |
| D. phragmitis | CBS 138897 | KP004445 | – | KP004507 | – |
| D. pseudomangiferae | CBS 101339 | KC343181 | KC343907 | KC344149 | KC343423 |
| D. pseudophoenicicola | CBS 462.69 | KC343184 | KC343910 | KC344152 | KC343426 |
| D. rosicola | MFLU 17.0646 | NR157515 | MG829270 | MG843877 | MG829274 |
| D. saccarata | CBS 116311 | KC343190 | KC343916 | KC344158 | KC343432 |
| D. stitica | CBS 370.54 | KC343212 | KC343938 | KC344180 | KC343454 |
| D. vaccinii | CBS 160.32 | AF317578 | GQ250326 | KC344196 | KC343470 |
| Valsa ambiens | CFCC 89894 | KR045617 | KU710912 | KR045658 | – |
Ex-type isolates were labeled with bold.
Phylogenetic analyses
DNA sequences from our three strains and reference sequences downloaded from GenBank (Dissanayake et al. 2017a, b), Guarnaccia et al. (2018) and Wanasinghe et al. (2018) were analysed by maximum parsimony (MP) and maximum likelihood (ML). Sequences were optimised manually to allow maximum alignment and maximum sequence similarity, as detailed in Manamgoda et al. (2012). MP analyses were performed in PAUP v. 4.0b10 (Swofford 2003), using the heuristic search option with 1,000 random taxa additions and tree bisection and re-connection (TBR) as the branch swapping algorithm. Maxtrees = 5000 was set to build the phylogenetic tree. The characters of the alignment document were ordered according to ITS+tef1+β-tubulin+CAL for GUCC9165 and GUCC9167 and tef1+β-tubulin for GUCC9146 with equal weight and gaps were treated as missing data. The Tree Length (TL), Consistency Indices (CI), Retention Indices (RI), Rescaled Consistency Indices (RC) and Homoplasy Index (HI) were calculated for each tree generated. The resulting Phylip file was used to make ML and Bayesian trees by the CIPRES Science Gateway (https://www.phylo.org/portal2/login.action) and RAxML-XSEDE with 1000 bootstrap inferences.
Results
Phylogenetic analyses
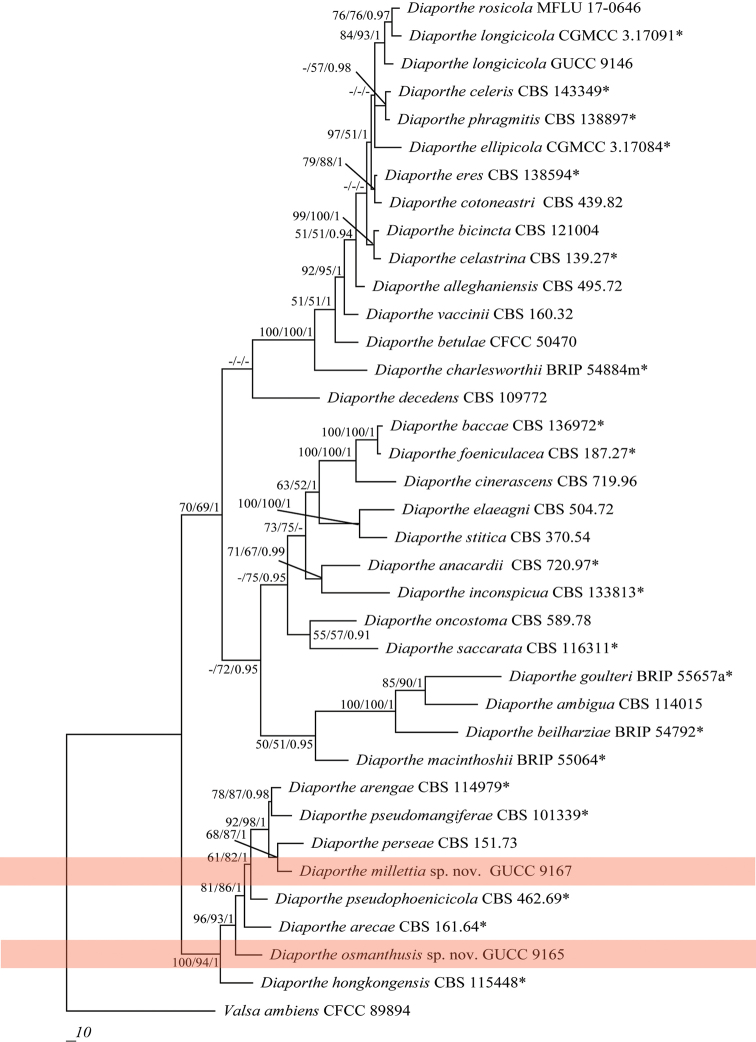
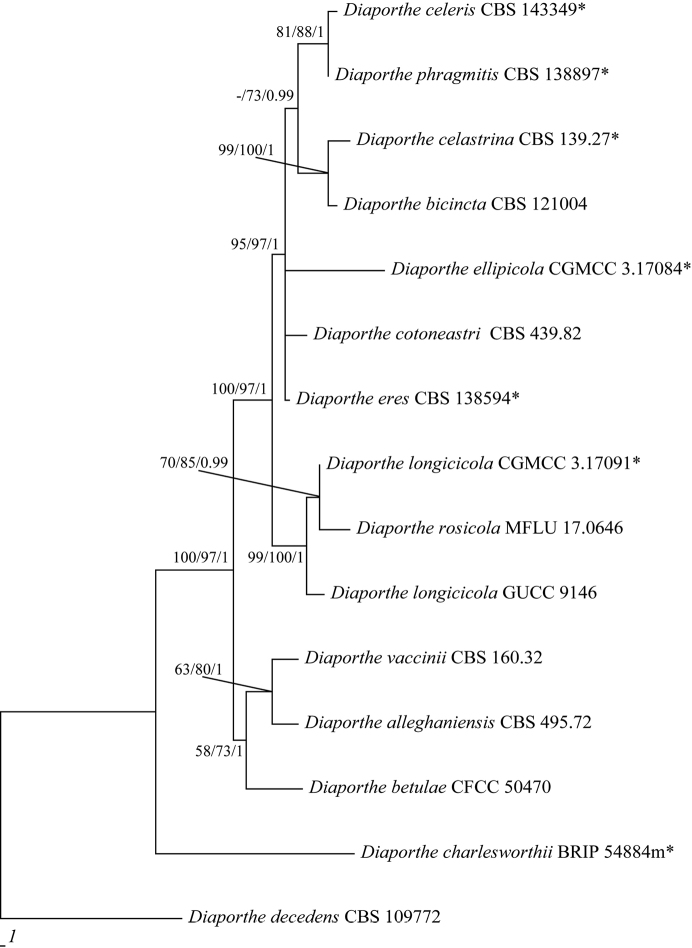
Three Diaporthe strains isolated from different plant hosts were sequenced. PCR products of 456–465 bp (ITS), 292–303 bp (tef1), 666–690 bp (β-tubulin) and 336–345 bp (CAL) were obtained. By alignment with the single gene region and then combination according to the order of ITS, tef1, β-tubulin and CAL with Valsa ambiens (CFCC 89894), only 1833 characters were obtained, viz. ITS: 1–492, tef1: 493–801, β-tubulin: 802–1469, CAL: 1470–1833, with 500 parsimony-informative characters. This procedure yielded eleven parsimonious trees (TL = 2169, CI = 0.58, RI = 0.71, RC = 0.41 and HI = 0.42), the first one being shown in Figure 1. All Diaporthe species clustered together, although without credible support for bootstrap and BPP values (Figure 1). Phylogenetic analysis of strains GUCC9165 and GUCC9167, using the four gene loci, confirmed them as well-resolved species (Figure 1). Strain GUCC 9165 formed an independent branch adjacent to D. arecae and D. hongkongensis (MP: 100%, ML: 94% and BPP: 1). Strain GUCC 9167 grouped with the branch which included D. arengae, D. perseae and D. pseudomangiferae (MP: 92%, ML: 98% and BPP: 1). Strain GUCC 9146 was aligned to the branch having D. longicicola and D. rosicola in the Diaporthe eres species-complex (Figure 2), with high statistical support (MP: 84%, ML: 93% and BPP: 1). This strain also showed a close relationship to D. eres and D. cotoneastri. In addition, we also compared the DNA base pair differences between our strains and related species in different gene regions (Suppl. material 1: Table S1). In Diaporthe strain GUCC9165, the four genes had 64 base pair differences with D. arecae and 119 with D. hongkongensis, the main differences being with β-tubulin and tef1. Strain GUCC9167 had 52 base pair differences with D. arengae, 61 with D. perseae and 64 with D. pseudomangiferae, wherein the base distinction was primarily in the β-tubulin and tef1 gene region. The β-tubulin sequences of D. eres and D. longicicola were apparently shorter than in strain GUCC 9146. The CAL sequences of D. rosicola were shorter than GUCC 9146. The DNA sequence of CAL for Diaporthe longicicola was not available (Gao et al. 2015). Integrating available DNA information, we discovered that 28 base pair differences were shown between GUCC 9146 and D. eres, 51 between GUCC 9146 and D. cotoneastri, 26 between GUCC 9146 and D. rosicola and 22 (only three genes) between GUCC 9146 and D. longicicola. Meanwhile, the phylogenetic analysis, based on only tef1 and β-tubulin for the D. eres species-complex (Figure 2), also indicated that GUCC 9146 clustered with D. longicicola and D. rosicola which obtained support values of MP: 99%, ML: 100% and BPP: 1 and maintained a closer relationship with D. longicicola.
Figure 1.
Parsimonious tree obtained from a combined analyses of an ITS, β-tubulin, calmoudulin and tef1 sequence dataset. MP, ML above 50% and BPP values above 0.90 were placed close to topological nodes and separated by “/”. The bootstrap values below 50% and BPP values below 0.90 were labelled with “-”. The tree is rooted with Valsa ambiens (CFCC89894). The branch of our new Diaporthe species is in pink.
Figure 2.
Parsimonious tree obtained from a combined analyses of a β-tubulin and tef1 sequence dataset (TL = 265, CI = 0.89, RI = 0.76, RC = 0.68 and HI = 0.11). MP, ML above 50% and BPP values above 0.90 were placed close to topological nodes and separated by “/”. The bootstrap values below 50% and BPP values below 0.90 were labelled with “-”. The tree is rooted with Diaporthe decedens (CBS 109772).
Taxonomy
Diaporthe millettiae
H. Long, K.D. Hyde & Yong Wang bis sp. nov.
18E3940B229E5A739D4216010B5CBA6D
MB829563
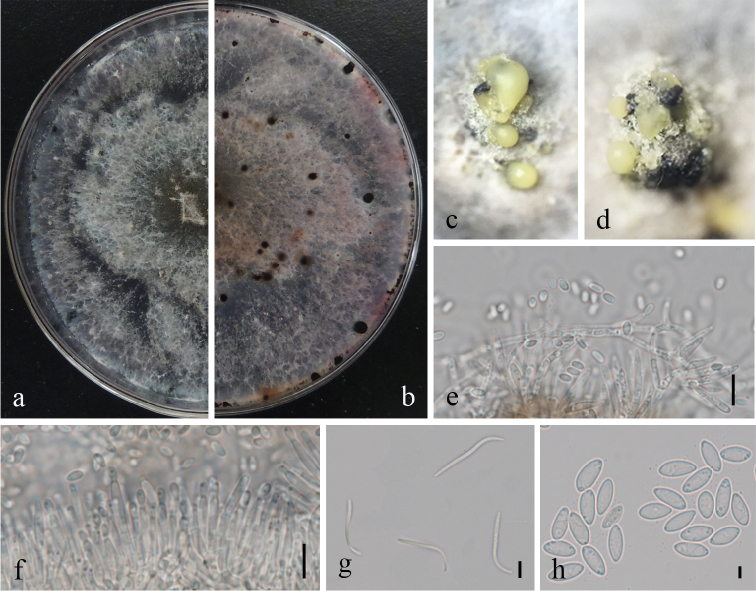
Figure 3.
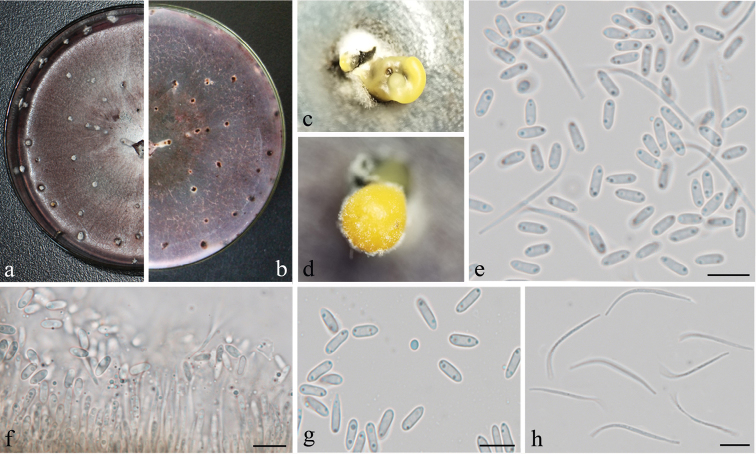
Diaporthe millettiae (GUCC9167). a–b upper (a) and lower (b) surface of colony on PDAc–d conidiomata e–f conidiophores, conidiogenous loci and conidia g β-conidia h α-conidia. Scale bars: 20 µm (e, f), 10 µm (g, h).
Diagnosis.
Characterised by larger J-shaped β-conidia.
Type.
China, Guangxi Province, Nanning City, from leaves of Millettia reticulata, 20 September 2016, Y. Wang, HGUP 9167, holotype, ex-type living culture GUCC 9167.
Description.
Colonies on PDA attaining 9 cm diam. after 10 days; coralloid with feathery branches at margin, adpressed, with apparent aerial mycelium, with numerous irregularly zonate dark stromata, isabelline becoming lighter towards the margin; reverse similar to surface, with zonations. Conidiomata pycnidial, multilocular, scattered, abundant on PDA after 3 wks, subglobose to irregular, 1.5–1.8 mm diam., ostiolate, with up to 1 mm necks when present. Conidiophores formed from the inner layer of the locular wall, sometimes reduced to conidiogenous cells, when present 1-septate, hyaline to pale yellowish-brown, cylindrical, 10–23 × 1–2.5 μm. Conidiogenous cells cylindrical to flexuous, tapered towards apex, hyaline, 8–18 × 1.5–3 μm. Alpha conidia abundant, fusiform, narrowed towards apex and base, mostly biguttulate, hyaline, 4.5–9 × 2–3.5 μm. Beta conidia scarce to abundant, flexuous to J-shaped, hyaline, 17.5–32 × 1–2 μm. Perithecia not seen.
Habitat and distribution.
Isolated from leaves of Millettia reticulata in China
Etymology.
Species epithet millettiae, referring to the host, Millettia reticulata from which the strain was isolated.
Notes.
Phylogenetic analysis combining four gene loci showed that Diaporthe millettiae (strain GUCC 9167) displayed a close relationship with D. arengae, D. pseudomangiferae and D. perseae with high bootstrap values (Figure 1). We compared the DNA base pair differences of the four gene regions, the main differences being in the β-tubulin and tef1 genes, especially tef1. Diaporthe millettiae produced two types of conidia (α, β), whereas D. pseudomangiferae only produced alpha conidia and D. perseae produced three types of conidia (α, β, γ). The β-conidia of D. arengae were smaller (20–25 × 1.5 μm) than those of Diaporthe millettiae (17.5–32 × 1–2 μm). The shape of β-conidia was also different. Conidiophores of D. arengae (10–60 μm) with more septa (0–6), were longer than those of D. millettiae (10–23 × 1–2.5 μm; 0-1-septate) (Gomes et al. 2013).
Diaporthe osmanthi
H. Long, K.D. Hyde & Yong Wang bis sp. nov.
AB53D9B53F1B5833915C42D14348045C
MB829564
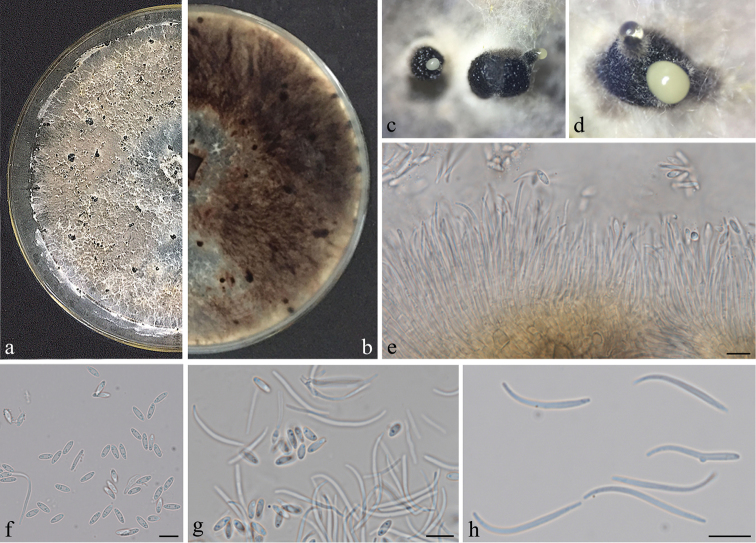
Figure 4.
Diaporthe osmanthi (GUCC9165). a–b upper (a) and lower (b) surface of colony on PDAc–d conidiomata e conidiophores, conidiogenous loci and conidia f α-conidia g two types of conidia h β-conidia. Scale bars: 10 µm (e, f, g, h).
Diagnosis.
Characterised by size of α-conidia and β-conidia.
Type.
China, Guangxi province, Nanning City, from leaves of Osmanthus fragrans, 20 September, 2016, Y. Wang, HGUP 9165, holotype, ex-type living culture GUCC 9165.
Description.
Colonies on PDA attaining 9 cm diam. after 10 days; coralloid with feathery branches at margin, adpressed, without aerial mycelium, with numerous irregularly zonated dark stromata, isabelline becoming lighter towards the margin; reverse similar to the surface with zonations more apparent. Conidiomata pycnidial and multilocular, scattered, abundant on PDA after 3 wks, globose, subglobose or irregular, up to 1–1.5 mm diam., ostiolate, necks absent or up to 1 mm. Conidiophores formed from the inner layer of the locular wall, reduced to conidiogenous cells or 1-septate, hyaline to pale yellowish-brown, cylindrical, 20.5–61 × 1–3 μm. Conidiogenous cells cylindrical to flexuous, tapered towards apex, hyaline, 10–15 × 1.5–3 μm. Alpha conidia abundant, fusiform, narrowed towards the apex and base, apparently biguttulate, hyaline, 5.5–8.5 × 2–3 μm. Beta conidia scarce to abundant, flexuous to J-shaped, hyaline, 20–31.5 × 1–2.5 μm. Perithecia not seen.
Habitat and distribution.
Isolated from leaves of Osmanthus fragrans in China.
Etymology.
Species epithet osmanthi, referring to the host, Osmanthus fragrans from which our strain was isolated.
Notes.
Diaporthe osmanthi (strain GUCC9165) formed an independent lineage, but was also related to D. arecae and D. hongkongensis (Figure 1). The sequences of β-tubulin and tef1 included about two-three differences between D. osmanthi (GUCC9165) and D. arecae (42) and D. hongkongensis (78) and thus they were different species according to the guidelines of Jeewon and Hyde (2016). Additionally, Diaporthe hongkongensis produced three types of conidia, but Diaporthe osmanthi did not produce γ-conidia. In addition, β-conidia of D. hongkongensis (18–22 μm) were shorter than those of Diaporthe osmanthi (Gomes et al. 2013). According to original description Srivastava et al. (1962), D. arecae also produced two types of conidia. The α-conidia (7.2–9.6 × 2.4 μm) were longer than in Diaporthe osmanthi, but its β-conidia (14.4–24 × 1.2 μm) were shorter and their shape also had some differences.
Diaporthe longicicola
Y.H. Gao & L. Cai, Fungal Biology 119(5): 303 (2015)
6147DDC1497558DB8A559F58761A6EC6
Figure 5.
Diaporthe longicicola (GUCC9146). a–b upper (a) and lower (b) surface of colony on PDAc–d conidiomata e two types of conidia f conidiophores, conidiogenous loci and conidia g α-conidia h β-conidia. Scale bars: 10 µm (e, f, g, h).
Description.
Colonies on PDA attaining 9 cm diam. in 10 days; coralloid with feathery branches at margin, adpressed, without aerial mycelium, without numerous irregularly zonated dark stromata, isabelline becoming lighter towards the margin; reverse similar to the surface with zonations more apparent. Conidiomata pycnidial and multilocular, scattered, abundant on PDA after 20 d, subglobose or irregular, 1.5–1.8 mm diam., ostiolate and up to 1 mm long. Conidiophores formed from the inner layer of the locular wall, densely aggregated, hyaline to pale yellowish-brown, cylindrical, tapering towards the apex, 15–25 × 1.5–2 μm. Alpha conidia abundant, ellipsoid to fusiform, apparently biguttulate, hyaline, 6–9 × 2–3 μm. Beta conidia scarce to abundant, flexuous to J-shaped, hyaline, 25.5–35.5 × 1–2.5 μm.
Habitat and distribution.
Isolated from leaves of Camellia sinensis in Duyun, Guizhou Province, China
Notes.
Phylogenetic analyses (Figures 1, 2) indicated that GUCC 9146 has a close relationship with D. longicicola, D. rosicola, D. eres and D. cotoneastri. Morphological comparison indicated that this strain was most similar to D. longicicola but not a related species by the width of alpha conidia and length of beta conidia (Udayanga et al. 2014; Gao et al. 2015).
Discussion
Phylogenetic analysis and morphology provide evidence for the introduction of Diaporthe millettiae and D. osmanthi as new species. In order to support the validity of these new species, we followed the guidelines of Jeewon and Hyde (2016) in comparing base pair differences (Suppl. material 1: Table S1). In accordance with Udayanga et al. (2014), we also believed that the ITS fragment was problematic for the D. eres species-complex. When not considering ITS, integration with morphological comparison was helpful and we concluded that GUCC 9146 is D. longicicola. Diaporthe longicicola was firstly reported on Lithocarpus glabra in Zhejiang Province, but our strain (GUCC 9146) was recovered from Camellia sinensis in Guizhou Province. Thus, this is the report of a new host and new location in China for D. longicicola.
Supplementary Material
Acknowledgements
This research is supported by the project funding of National Natural Science Foundation of China (No. 31560489), Genetically Modified Organisms Breeding Major Projects of China [2016ZX08010-003-009], Agriculture Animal and Plant Breeding Projects of Guizhou Province [QNYZZ2013-009], Key Laboratory of Superior Forage Germplasm in the Qinghai-Tibetan Plateau (2017-ZJ-Y12), Talent project of Guizhou science and technology cooperation platform ([2017]5788-5 and [2019]5641) and Guizhou science, technology department international cooperation base project ([2018]5806) and postgraduate education innovation programme of Guizhou Province (ZYRC[2014]004). Dr Kevin D. Hyde would like to thank “the future of specialist fungi in a changing climate: baseline data for generalist and specialist fungi associated with ants, Rhododendron species and Dracaena species (DBG6080013)” and “Impact of climate change on fungal diversity and biogeography in the Greater Mekong Subregion (RDG6130001)”.
Citation
Long H, Zhang Q, Hao Y-Y, Shao X-Q, Wei X-X, Hyde KD, Wang Y, Zhao D-G (2019) Diaporthe species in south-western China. MycoKeys 57: 113–127. https://doi.org/10.3897/mycokeys.57.35448
Funding Statement
The research is supported by the project of National Natural Science Foundation of China (No. 31560489), Genetically Modified Organisms Breeding Major Projects of China [2016ZX08010-003-009], Agriculture Animal and Plant Breeding Projects of Guizhou Province [QNYZZ2013-009], Key Laborary of Superior Forage Germplasm in the Qinghai-Tibetan Plateau (2017-ZJ-Y12), Talent project of Guizhou science and technology cooperation platform ([2017]5788-5) and Guizhou science, technology department international cooperation base project ([2018]5806) and postgraduate education innovation program of Guizhou Province (ZYRC[2014]004). Dr Kevin D. Hyde also would like to thank “the future of specialist fungi in a changing climate: baseline data for generalist and specialist fungi associated with ants, Rhododendron species and Dracaena species (DBG6080013)” and “Impact of climate change on fungal diversity and biogeography in the Greater Mekong Subregion (RDG6130001)”.
Supplementary materials
This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Hui Long, Qian Zhang, Yuan-Yuan Hao, Xian-Qiang Shao, Xiao-Xing Wei, Kevin D. Hyde, Yong Wang, De-Gang Zhao
The DNA bases difference between our strains and related taxa on four gene regions
Data type: molecular data
References
- Carbone I, Kohn LMA. (1999) A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 91(3): 553–556. 10.2307/3761358 [DOI] [Google Scholar]
- Chen KL, Kirschner R. (2017) Fungi from leaves of lotus (Nelumbo nucifera). Mycological Progress 17: 275–293. 10.1007/s11557-017-1324-y [DOI] [Google Scholar]
- Chomnunti P, Hongsanan S, Aguirre-Hudson B, Tian Q, Persoh D, Dhami MK, Alias AS, Xu JC, Liu XZ, Stadler M. (2014) The sooty moulds. Fungal Diversity 66: 1–36. 10.1007/s13225-014-0278-5 [DOI] [Google Scholar]
- Dissanayake AJ, Zhang W, Liu M, Hyde KD, Zhang W, Yan JY, Li XH. (2017a) Diaporthe species associated with peach tree dieback in Hubei, China. Mycosphere 8, 533–549. 10.5943/mycosphere/8/5/4 [DOI]
- Dissanayake AJ, Phillips AJL, Hyde KD, Yan JY, Li XH. (2017b) The current status of species in Diaporthe. Mycosphere 8: 1106–1156. 10.5943/mycosphere/8/5/5 [DOI] [Google Scholar]
- Du Z, Fan XL, Hyde KD, Yang Q, Liang YM, Tian CM. (2016) Phylogeny and morphology reveal two new species of Diaporthe from Betula spp. in China. Phytotaxa 269: 90–102. 10.11646/phytotaxa.269.2.2 [DOI] [Google Scholar]
- Fan XL, Hyde KD, Udayanga D, Wu XY, Tian CM. (2016) Diaporthe rostrata, a novel ascomycete from Juglans mandshurica associated with walnut dieback. Mycological Progress 14: 82. 10.1007/s11557-015-1104-5 [DOI]
- Gao YH, Sun W, Su YY, Cai L. (2014) Three new species of Phomopsis in Gutianshan Nature Reserve in China. Mycological Progress 13: 111–121. 10.1007/s11557-013-0898-2 [DOI] [Google Scholar]
- Gao YH, Su YY, Sun W. (2015) Diaporthe species occurring on Lithocarpus glabra in China, with descriptions of five new species. Fungal Biology 119: 295–309. 10.1016/j.funbio.2014.06.006 [DOI] [PubMed] [Google Scholar]
- Gao YH, Liu F, Cai L. (2016) Unravelling Diaporthe species associated with Camellia. Systematics and Biodiversity 14: 102–117. 10.1080/14772000.2015.1101027 [DOI] [Google Scholar]
- Gao YH, Liu F, Duan W, Crous PW, Cai L. (2017) Diaporthe is paraphyletic. IMA fungus 8: 153–187. 10.5598/imafungus.2017.08.01.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass NL, Donaldson GC. (1995) Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Applied and Environmental Microbiology 61: 1323–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes RR, Glienke C, Videira SIR, Lombard L, Groenewald JZ, Crous PW. (2013) Diaporthe: a genus of endophytic, saprobic and plant pathogenic fungi. Persoonia- Molecular Phylogeny and Evolution of Fungi 31: 1–41. 10.3767/003158513X666844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarnaccia V, Groenewald JZ, Woodhall J, Armengol J, Cinelli T, Eichmeier A, Ezra D, Fontaine F, Gramaje D, Gutierrez-Aguirregabiria A, Kaliterna J, Kiss L, Larigno P. (2018) Diaporthe diversity and pathogenicity revealed from a broad survey of grapevine diseases in Europe. Persoonia - Molecular Phylogeny and Evolution of Fungi 40: 135–153. 10.3767/persoonia.2018.40.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang F, Hou X, Dewdney MM, Fu YS, Chen GQ, Hyde KD, Li HY. (2013) Diaporthe species occurring on citrus in China. Fungal Diversity 61: 237–250. 10.1007/s13225-013-0245-6 [DOI] [Google Scholar]
- Huang F, Udayanga D, Wang XH, Hou X, Mei XF, Fu YS, Hyde KD, Li HY. (2015) Endophytic Diaporthe, associated with citrus: a phylogenetic reassessment with seven new species from China. Fungal Biology, 119: 331–347. 10.1016/j.funbio.2015.02.006 [DOI] [PubMed] [Google Scholar]
- Hyde KD, Hongsanan S, Jeewon R, Bhat DJ, McKenzie EHC, Jones EBG, Phookamsak R, Ariyawansa HA, Boonmee S, Zhao Q, Abdel-Aziz FA, Abdel-Wahab MA, Banmai S, Chomnunti P, Cui BK, Daranagama DA, Das K, Dayarathne MC, de Silva NI, Dissanayake AJ, Doilom M, Ekanayaka AH, Gibertoni TB, Goes-Neto A, Huang SK, Jayasiri SC, Jayawardena RS, Konta S, Lee HB, Li WJ, Lin CG, Liu JK, Lu YZ, Luo ZL, Manawasinghe IS, Manimohan P, Mapook A, Niskanen T, Norphanphoun C, Papizadeh M, Perera RH, Phukhamsakda C, Richter C, Santiago ALCM de A, Ricardo DS, Senanayake IC, Tanaka K, Tennakoon TMDS, Thambugala KM, Tian Q, Tibpromma S, Thongbai B, Vizzini A, Wanasinghe D, Wijayawardene NN, Wu HX, Yang J, Zeng XY, Zhang H, Zhang JF, Bulgakov T, Camporesi E, Bahkali AH, Amoozegar M, Araujo-Neta LS, Ammirati JF, Baghela A, Bhatt RP, Bojantchev D, Buyck B, da Silva GA, Ferreira de Lima, Catarina Leticia, Vilela de Oliveira RJ, Fragoso de Souza CA, Dai YC, Dima B, Duong TT, Ercole E, Mafalda-Freire F, Ghosh A, Hashimoto A, Kamolhan S, Kang JC, Karunarathna Samantha C, Kirk PM, Kytovuori I, Lantieri A, Liimatainen K, Liu ZY, Liu XZ, Lucking R, Medardi G, Mortimer PE, Thi TTN, Promputtha I, Raj KNA, Reck MA, Lumyong S, Shahzadeh-Fazeli SA, Stadler M, Soudi MR, Su HY, Takahashi T, Tangthirasunun N, Uniyal P, Wang Y, Wen TC, Xu JC, Zhang ZK, Zhao YC, Zhou JL, Zhu L. (2016) Fungal diversity notes 367–490: taxonomic and phylogenetic contributions to fungal taxa. Fungal Diversity 80: 1–270. 10.1007/s13225-016-0366-9 [DOI] [Google Scholar]
- Jeewon R, Hyde KD. (2016) Establishing species boundaries and new taxa among fungi: recommendations to resolve taxonomic ambiguities. Mycosphere 7: 1669–1677. 10.5943/mycosphere/7/11/4 [DOI] [Google Scholar]
- Manamgoda DS, Cai L, Bahkali AH, Chukeatirote E, Hyde KD. (2011) Cochliobolus: an overview and current status of species. Fungal Diversity 51: 3–42. 10.1007/s13225-011-0139-4 [DOI] [Google Scholar]
- Manamgoda DS, Cai L, McKenzie EHC, Crous PW, Madrid H, Chukeatirote E, Shivas RG, Tan YP, Hyde KD. (2012) A phylogenetic and taxonomic re-evaluation of the Bipolaris-Cochliobolus-Curvularia complex. Fungal Diversity 56: 131–144. 10.1007/s13225-012-0189-2 [DOI] [Google Scholar]
- Nilsson RH, Hyde KD, Pawlowska J, Ryberg M. et al. (2014) Improving ITS sequence data for identification of plant pathogenic fungi. Fungal Diversity 67: 11–19. 10.1007/s13225-014-0291-8 [DOI] [Google Scholar]
- Perera RH, Hyde KD, Dissanayake AJ, Jones EBG, Liu JK, Wei D, Liu ZY. (2018) Diaporthe collariana sp. nov., with prominent collarettes associated with Magnolia champaca fruits in Thailand. Studies in Fungi 3: 141–151. 10.5943/sif/3/1/16 [DOI] [Google Scholar]
- Rossman AY, Allen WC, Braun U, Castlebury LA, Chaverri P, Crous PW, Hawksworth DL, Hyde KD, Johnston P, Lombard L, Romberg M, Samson RA, Seifert KA, Stone JK, Udayanga D, White JF. (2016) Overlooked competing asexual and sexually typified generic names of Ascomycota with recommendations for their use or protection. IMA Fungus 7: 289–308. 10.5598/imafungus.2016.07.02.09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava HC, Zakia B, Govindarajan VS. (1962) Fruit rot of areca nut caused by a new fungus. Mycologia 54: 5–11. 10.1080/00275514.1962.12024974 [DOI] [Google Scholar]
- Swofford D. (2003) PAUP* – Phylogenetic analysis using parsimony (*and other methods). Version 4. Sinauer Associates, Sunderland.
- Tibpromma S, Hyde KD, Bhat JD, Mortimer PE, Xu JC, Promputtha I, Doilom M, Yang JB, Tang AMC, Karunarathna SC. (2018) Identification of endophytic fungi from leaves of Pandanaceae based on their morphotypes and DNA sequence data from southern Thailand. Mycokeys 33: 25–67. 10.3897/mycokeys.32.23670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udayanga D, Liu XZ, McKenzie EHC, Chukeatirote E, Bahkali AH, Hyde KD. (2011) The genus Phomopsis: biology, applications, species concepts and names of common phytopathogens. Fungal Diversity 50: 189–225. 10.1007/s13225-011-0126-9 [DOI] [Google Scholar]
- Udayanga D, Liu XZ, Crous PW, McKenzie EHC, Chukeatirote E, Hyde KD. (2012) A multi-locus phylogenetic evaluation of Diaporthe (Phomopsis). Fungal Diversity 56: 157–171. 10.1007/s13225-012-0190-9 [DOI] [Google Scholar]
- Udayanga D, Castlebury LA, Rossman AY, Chukeatirote E, Hyde KD. (2014) Insights into the genus Diaporthe: phylogenetic species delimitation in the D. eres species complex. Fungal Diversity 67: 203–229. 10.1007/s13225-014-0297-2 [DOI] [Google Scholar]
- Udayanga D, Castlebury LA, Rossman AY, Hyde KD. (2015) Species limits in Diaporthe: molecular re-assessment of D. citri, D. cytosporella, D. foeniculina and D. rudis. Persoonia 32: 83–101. 10.3767/003158514X679984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanasinghe DN, Phukhamsakda C, Hyde KD, Jeewon R, Lee HB, Jones EBG, Tibpromma S, Tennakoon DS, Dissanayake AJ, Jayasiri SC, Gafforov Y, Camporesi E, Bulgakov TS, Ekanayake AH, Perera RH, Samarakoon MC, Goonasekara ID, Mapook A, Li WJ, Senanayake IC, Li JF, Norphanphoun C, Doilom M, Bahkali AH, Xu JC, Mortimer PE, Tibell L, Tibell S, Karunarathna SC. (2018) Fungal diversity notes 709–839: taxonomic and phylogenetic contributions to fungal taxa with an emphasis on fungi on Rosaceae. Fungal Diversity 89: 1–236. 10.1007/s13225-018-0395-7 [DOI] [Google Scholar]
- White TJ, Bruns TD, Lee S, Taylor J. (1990) Amplification and direct sequencing of fungal ribosomal genes for phylogenetics. In: Gelfand M, Sninsky JI, White TJ (Eds) PCR protocols: a guide to methods and applications, Academic Press, USA, 315–322. 10.1016/B978-0-12-372180-8.50042-1 [DOI]
- Yang Q, Fan X, Du Z, Liang Y, Tian C. (2017a) Diaporthe camptothecicola sp. nov. on Camptotheca acuminata in China. Mycotaxon 132: 591–601. 10.5248/132.591 [DOI]
- Yang Q, Fan XL, Du Z, Tian CM. (2017b) Diaporthe juglandicola sp. nov. (Diaporthales, Ascomycetes) evidenced by morphological characters and phylogenetic analysis. Mycosphere 8: 817–826. 10.5943/mycosphere/8/5/3 [DOI] [Google Scholar]
- Yang Q, Du Z, Tian CM. (2018) Phylogeny and morphology reveal two new species of Diaporthe from Traditional Chinese Medicine in Northeast China. Phytotaxa 336: 159–170. 10.11646/phytotaxa.336.2.3 [DOI] [Google Scholar]
- Yang Y, Guo YX, Zhang YK, WuH Zhang M. (2016) Diaporthe henanensis sp. nov. an endophytic fungus in Ziziphus jujuba from China. Mycotaxon 131: 645–652. 10.5248/131.645 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Hui Long, Qian Zhang, Yuan-Yuan Hao, Xian-Qiang Shao, Xiao-Xing Wei, Kevin D. Hyde, Yong Wang, De-Gang Zhao
The DNA bases difference between our strains and related taxa on four gene regions
Data type: molecular data