Abstract
To describe the effects of sodium-glucose co-transporter 2 (SGLT2) inhibitors on serum uric acid (SUA) in patients with type 2 diabetes mellitus (T2DM). PubMed, EMBASE, and CENTRAL were searched for randomized controlled trials of SGLT2 inhibitors in patients with T2DM up to Aug 10, 2017, without language or date restrictions. Thirty-one studies totaling 13,650 patients were included. SGLT2 inhibitors significantly decreased SUA levels compared with placebo, canagliflozin WMD –37.02 μmol/L, 95% CI [–38.41, –35.63], dapagliflozin WMD –38.05 μmol/L, 95% CI [–44.47, –31.62], empagliflozin WMD –42.07 μmol/L, 95% CI [–46.27, –37.86]. The drug class effect of SUA reduction suggesting SGLT2 inhibitors might be beneficial for diabetic patients with hyperuricemia.
Abbreviations: SGLT2, sodium-glucose co-transporter 2; RCTs, randomized controlled trials; CKD, chronic kidney disease; N, number of patients; CANA, canagliflozin; DAPA, dapagliflozin; EMPA, empagliflozin; PLA, placebo
Keywords: Meta-analysis, Serum uric acid, SGLT2 inhibitor, Type 2 diabetes
1. Introduction
Chronic hyperuricemia is a well-known established risk factor for the development of gout, kidney stones, and nephropathy (Bobulescu and Moe, 2012, Grassi et al., 2013). Recent epidemiological study found that elevated serum uric acid (SUA) levels has been associated with hypertension, atherosclerosis, cardiovascular disease, and chronic kidney disease (Feig et al., 2002). The role of serum uric acid as an independent risk factor for cardiovascular disease was investigated using a systematic review and meta-analysis, and showed that hyperuricemia does increase the risk of cardiovascular disease and mortality (Kim et al., 2010). Many different studies showed that hyperuricemia was as well an independent risk factor for hypertension (Kuwabara et al., 2014). Sodium-glucose co-transporter 2 (SGLT2) inhibitor, a well-tolerated (Loeffler et al., 2012) newly approved oral hypoglycemic drug, induce urine glucose excretion by inhibiting SGLT2 at the S1 segment of the proximal tubule (Desai et al., 2017). SGLT2 inhibitors control blood glucose level and improve glycemic control in patients with T2D in an insulin independent method (Idris and Donnelly, 2009). Moreover, SGLT2 inhibitors are characterized by their added benefits to address unmet clinical needs, such as weight loss, blood pressure control, and possible lipid lowering effect (Kalra, 2014, Majewski and Bakris, 2015, Bolinder et al., 2014). A post hoc analysis assessed the effect of canagliflozin on serum uric acid level in type 2 diabetes mellitus patients (Abdulghani et al., 2016). Here, we aim to examine and to describe the effect of SGLT2 inhibitors on SUA level in patients with T2DM.
2. Materials and methods
2.1. Search strategy
The electronic databases of PubMed, Embase and the Cochrane Central Register of Controlled Trials (CENTRAL) were searched to identify eligible RCTs using relevant search terms described in (Table 1). We identified articles published up to Aug 10, 2017, without restrictions on language, year of publication or publication status. An additional manual search of the references of included trials, relevant meta-analyses.
Table 1.
Search strategy.
| Data source | Search strategy |
|---|---|
| PubMed | #1 (((Na+/glucose OR ‘sodium glucose’ OR ‘sodium dependent glucose’) AND (transporter 2 OR cotransporter OR ‘co transporter’)) OR ((SGLT2 OR SGLT 2))) AND (inhibitor OR inhibitor*) #2 SGLT2i OR ‘SGLT 2i’ OR SGLT2Is OR ‘SGLT 2is’ #3 atigliflozin #4 bexagliflozin OR egt0001442 OR egt0001474 OR thr 1442 #5 bi 44,847 #6 canagliflozin OR invokana OR jnj 28431754 OR ta 7284 #7 dapagliflozin OR farxiga OR bms 512,148 #8 egt0001474 #9 empagliflozin OR jardiance OR bi 10,773 OR bi 10,773 #10 ertugliflozin OR pf 04971729 OR mk-8835 #11 gsk-1614235 #12 ipragliflozin OR suglat OR asp1941 OR asp 1941 #13 isis 388,626 OR isis-sglt2rx ):TI, AB #14 luseogliflozin OR lusefi OR ts-071 OR ts 71 #15 remogliflozin OR OR bhv091009 OR gsk 189,075 #16 sergliflozin OR gw 869,682 #17 shr3824 #18 sotagliflozin OR lx421 OR lx 4211 OR lp 802,034 #19 ta-7284 #20 tofogliflozin OR apleway OR deberza OR csg452 or csg 452 or r 7201 #21 OR #1-#20 #22 ((clinical [tiab] AND trial [tiab]) OR clinical trials [mh] OR clinical trial [pt] OR random*[tiab] OR RCT OR RCTs OR random allocation [mh] OR therapeutic use [sh]) #23 ((animals [mh] NOT humans [mh]) OR (review [pt] OR meta-analysis [pt] OR conference abstract [pt])) #24 #21 AND #22 NOT #23 |
| Embase | #1 (‘sodium glucose transporter’ OR ‘sodium glucose cotransporter’ OR ‘sodium glucose co transporter’ OR sglt2 OR ‘sglt 2’ AND (inhibitor OR inhibitors)):TI, AB #2 (‘sglt 2i’ OR sglt2i OR sglt2is):TI, AB #3 (‘atigliflozin’):TI, AB #4 (‘bexagliflozin’ OR ‘egt0001442’ OR ‘egt0001474’ OR ‘thr 1442’):TI, AB #5 (‘bi 44847’):TI, AB #6 (‘canagliflozin’ OR ‘invokana’ OR ‘jnj 28431754’ OR ‘ta 7284’):TI, AB #7 (‘dapagliflozin’ OR ‘farxiga’ OR ‘bms 512148’):TI, AB #8 (‘egt0001474’):TI, AB #9 (‘empagliflozin’ OR ‘jardiance’ OR ‘bi 10773’ OR ‘bi 10773’):TI, AB #10 (‘ertugliflozin’ OR ‘pf 04971729’ OR ‘mk-8835’):TI, AB #11 (‘gsk-1614235’):TI, AB #12 (‘ipragliflozin’ OR ‘suglat’ OR ‘asp1941’ OR ‘asp 1941’):TI, AB #13 (‘isis 388626’ OR ‘isis-sglt2rx’):TI, AB #14 (‘luseogliflozin’ OR ‘lusefi’ OR ‘ts-071’ OR ‘ts 71’):TI, AB #15 (‘remogliflozin’ OR OR ‘bhv091009’ OR ‘gsk 189075’):TI, AB #16 (‘sergliflozin’ OR ‘gw 869682’):TI, AB #17 (‘shr3824’):TI, AB #18 (‘sotagliflozin’ OR ‘lx421’ OR ‘lx 4211’ OR ‘lp 802034’):TI, AB #19 (‘ta-7284’):TI, AB #20 (‘tofogliflozin’ OR ‘apleway’ OR ‘deberza’ OR ‘csg452’ or ‘csg 452’ or ‘r 7201’):TI, AB #21 OR 3#-20# #22 rct* OR random #23 NOT medline #24 (#1 OR #2 OR #21) AND #22 AND #23 |
| CENTRAL | #1Na glucose cotransporter 2 OR sodium Glucose cotransporter 2 OR sodium glucose co transporter 2 OR SGLT2 OR SGLT 2 OR atigliflozin OR bexagliflozin OR egt0001442 OR bi 44,847 OR bi 44,847 OR canagliflozin OR canagliflozin OR invokana OR jnj 28,431,754 OR dapagliflozin OR dapagliflozin OR farxiga OR bms 512,148 OR egt0001474 OR empagliflozin OR empagliflozin OR jardiance OR bi 10,773 OR ertugliflozin OR ertugliflozin OR pf04971729 OR mk-8835 OR gsk-1614235 OR ipragliflozin OR ipragliflozin OR suglat OR asp1941 OR isis 388,626 OR isis-sglt2rx OR luseogliflozin OR luseogliflozin OR lusefi OR ts-071 OR remogliflozin etabonate OR remogliflozin OR gsk 189,075 OR sergliflozin OR sergliflozin OR gw 869,682 OR shr3824 OR sotagliflozin OR sotagliflozin OR lx4211 OR ta-7284 OR tofogliflozin OR tofogliflozin OR apleway OR deberza: TI,AB,KY #2 [Trials] #3 #1 AND #2 |
2.2. Study selection
Studies meeting the following criteria were included: (1) population: patients with T2DM; (2) intervention: SGLT2 inhibitor monotherapy or as add-on to other hypoglycemic therapy; (3) comparison: placebo control or standard care; (4) outcome: serum uric acid changes from baseline; and (5) design: randomized controlled trials (RCTs); (6) follow-up duration at least 12 weeks. Conference abstracts were excluded because of the lack of detailed information assessing the trials’ characteristics, definition of outcomes and trial quality.
2.3. Data extraction and quality assessment
The following information was extracted: first author, year of publication, study design, sample size, patient characteristics, intervention (type of SGLT2 inhibitor and dose regime) and comparison (placebo or standard care), follow-up duration, and outcome (change in SUA from baseline). For SUA level, a conversion factor of 1 mg/dL to 59.485 μmol/L was adopted. If multiple reports were retrieved on the same population, only the most complete and/or more recently reported data were used. If 2 arms (ie, PLA + A vs B + A) were evaluated in the trials, according to B vs PLA treatment. Two reviewers (Li YL and Guo Y) independently extracted the data and assessed the quality of each RCT. Any disagreements were resolved by consensus or referral to a third reviewer (Xin YK).
The Cochrane risk of bias tool was used to assess the quality of the RCTs based on five domains: random sequence generation (selection bias), allocation concealment (selection bias), blinding (performance bias and detection bias), incomplete outcome data (attrition bias) and selective reporting (reporting bias) (Table 2).
Table 2.
Risk of bias graph.
 |
2.4. Statistical analysis
Weighted mean differences (WMD) (95% CI) in SUA level were calculated using a random-effects model to evaluate each SGLT2 inhibitor separately and by dose. Heterogeneity was quantified using I2, with I2 values > 50% representing moderate heterogeneity. The significance level for all outcome and heterogeneity analyses was set at p ≤ 0.05. All outcomes were pooled using RevMan5.3 software.
3. Results
3.1. Characteristics of included trials
Of 2858 articles screened, 31 trials met the eligibility criteria (Fig. 1). The following information were extracted from each eligible RCT: first author and publication year, patient characteristics (background treatments, mean age, race, baseline HbA1c, and BMI), interventions (control), mean BUN, creatinine, and eGFR levels, variance measure and the number of participants in the treatment and control arms for all reported periods (Table 2). Baseline SUA levels were within normal reference ranges for all studies.
Fig. 1.

Flow chart.
3.2. Outcome analysis
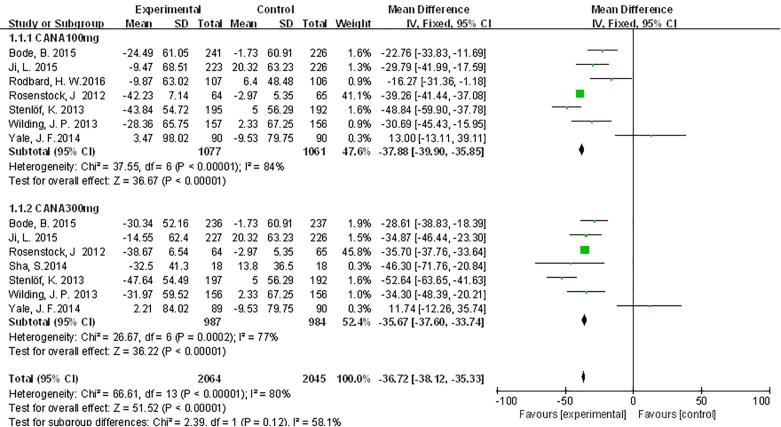
SGLT2 inhibitors significantly decreased SUA levels compared with placebo, canagliflozin WMD –37.02 μmol/L, 95% CI [–38.41, –35.63], dapagliflozin WMD –38.05 μmol/L, 95% CI [–44.47, –31.62], empagliflozin WMD –42.07 μmol/L, 95% CI [–46.27, –37.86]. For patients receiving canagliflozin 100 mg, SUA levels were significantly decreased compared with those receiving PLA WMD −37.88 μmol/L, 95% CI [−39.9, −35.85] (Fig. 2). Canagliflozin 300 mg had the same effects as canagliflozin 100 mg compared with PLA for SUA levels, which were significantly increased compared with those receiving PLA WMD −36.25 μmol/L, 95% CI [−38.17, −34.34]; (Fig. 2).
Fig. 2.
Meta-analysis of weighted mean difference and 95% confidence intervals for changes in serum uric acid level for canagliflozin.
Dapagliflozin had the same effects as canagliflozin compared with PLA, which were significantly decreased compared with those receiving PLA, dapagliflozin 5 mg WMD −37.81 μmol/L, 95% CI [−42.29, −33.33], dapagliflozin 10 mg WMD −38.93 μmol/L, 95% CI [−50.03, −27.83]; (Fig. 3).
Fig. 3.
Meta-analysis of weighted mean difference and 95% confidence intervals for changes in serum uric acid level for dapagliflozin.
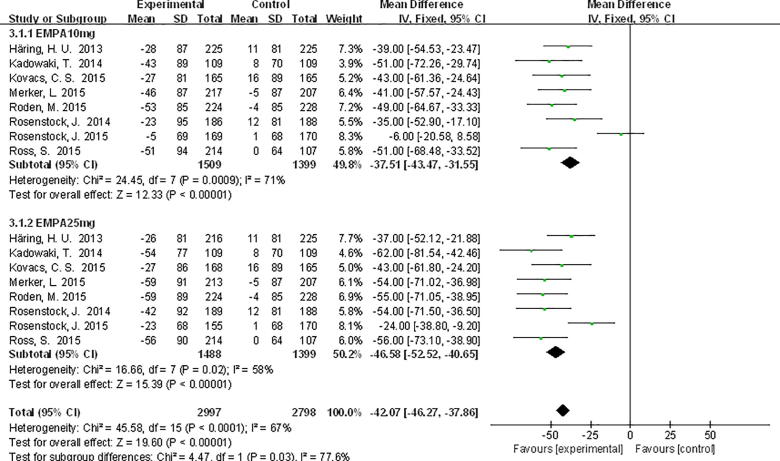
For patients receiving empagliflozin 10 mg, SUA levels were significantly decreased compared with PLA WMD −37.51 μmol/L, 95% CI [−43.47, −31.55], and WMD −46.58 μmol/L, 95% CI [−52.52, −40.65] for empagliflozin 25 mg (Fig. 4).
Fig. 4.
Meta-analysis of weighted mean difference and 95% confidence intervals for changes in serum uric acid level for empagliflozin.
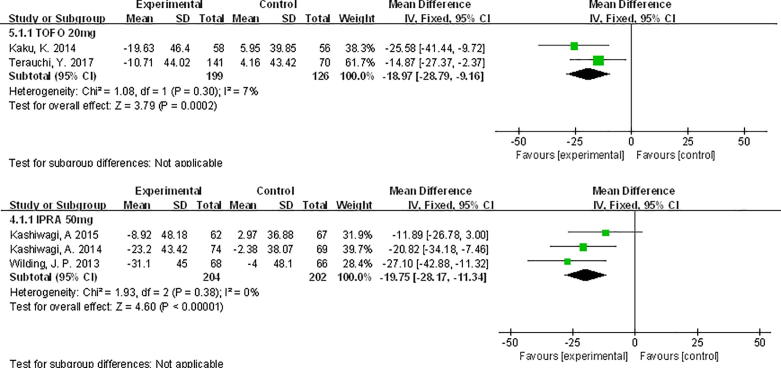
Compared with PLA, SUA levels were decreased, tofogliflozin 20 mg WMD −18.97 μmol/L, 95% CI [−28.79, −9.16], and ipragliflozin 50 mg WMD −19.75 μmol/L, 95% CI [−28.17, −11.34] (Fig. 5).
Fig. 5.
Meta-analysis of weighted mean difference and 95% confidence intervals for changes in serum uric acid level for tofogliflozin 20 mg and ipragliflozin 50 mg.
No significant association was revealed between the UA lowering effect of specific SGLT2 inhibitor with either dose.
4. Discussion
In this systematic review, we mainly evaluated the effects of SGLT2 inhibitors on SUA levels in patients with T2DM, a decrease in SUA levels was observed for SGLT2 inhibitors compared with PLA.
The mechanism by which SGLT2 inhibitors reduce serum uric acid has not been clearly understood. However, some studies suggested that it may possibly involve the renal SLC2A9 (GLUT9) transporter. The SLC2A9 gene encodes a facilitative glucose transporter and has two splice variants that are highly expressed in the apical membrane of the proximal tubule in nephron, a key site for urate handling in the kidney, found to transport both uric acid and d-glucose. A study was done to clarify the mechanism that may lead to the decreased of uric acid, where serum uric acid and urinary excretion rate of uric acid were analyzed after the oral administration of luseogliflozin to healthy subjects. This study confirmed that serum uric acid decreased as a result of the increase in the urinary excretion rate of uric acid, which correlated with the increase in urinary glucose excretion. SGLT2 inhibitor treatment leads to an increase in glucose excretion in the urine, which could eventually lead to an increased exchange of uric acid in the apical membrane of tubular cells leading to an increased release of uric acid from blood into the urine, thus decreasing serum uric acid levels (Heerspink et al., 2013, Mcgill, 2014, Wilding et al., 2009).
There was previous meta-analysis have been published (Zhao et al., 2017). Compared with that previous study, we also considered SUA-reduction as a major outcome, and analyzed the effect of existing SGLT2 inhibitors on uric acid compared with placebo. It provides more accurate evidence for the reaction of reducing uric acid by SGLT2 inhibitors.
Some limitations of our meta-analysis, which may reduce the strength of the evidence, such as, the length of the experiment is inconsistent, background treatment was inconsistent, basal uric acid levels are inconsistent etc. Secondly, we did not exclude trials enrolling CKD patients, whose SUA level might be elevated throughout disease progression. We could not observed about direct interaction between SGLT2 inhibitor and major uric acid transporters in vitro study. Articles included were hardly clinical homogeneity, so the presence of heterogeneity is inevitably substantial.
5. Conclusion
In conclusion, SGLT2 inhibitors were found in general to reduce serum uric acid levels in patients with T2DM. Given the associated clinical disorders with hyperuricemia, it is of course beneficial to lower serum uric acid in T2DM, and therefore, given the beneficial effects that SGLT2 inhibitors were found to have on serum uric acid reduction, this would give them an advantage over other oral antidiabetic agents.
6. Declarations
6.1. Ethics approval and consent to participate
Not applicable.
7. Consent to publish
All authors of the manuscript have read and agreed to its content and are accountable for all aspects of the accuracy and integrity of the manuscript in accordance with ICMJE criteria.
8. Availability of data and material
I can confirm I have included a statement regarding data and material availability in the declaration section of my manuscript.
Conflict of interest
I confirm that I have read BioMed Central’s guidance on competing interests and have included a statement in the manuscript indicating that none of the authors have any competing interests.
Acknowledgment
The work was financially supported by National Nature Science Foundation of China (Grant No.: U1404805 and 81141059).
Footnotes
Peer review under responsibility of King Saud University.
References
- Abdulghani M., Prato S.D., Chilton R., Defronzo R.A. SGLT2 inhibitors and cardiovascular risk: lessons learned from the EMPA-REG OUTCOME study. J. Diab Care. 2016;39(5):717. doi: 10.2337/dc16-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobulescu I.A., Moe O.W. Renal transport of uric acid: evolving concepts and uncertainties. J. Adv. Chron. Kidney Disease. 2012;19(6):358–371. doi: 10.1053/j.ackd.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolinder J., Ljunggren Ö., Johansson L., Wilding J., Langkilde A.M., Sjöström C.D., Sugg J., Parikh S. Dapagliflozin maintains glycaemic control while reducing weight and body fat mass over 2 years in patients with type 2 diabetes mellitus inadequately controlled on metformin. J. Diab. Obes. Metabol. 2014;16(2):159–169. doi: 10.1111/dom.12189. [DOI] [PubMed] [Google Scholar]
- Desai M., Yavin Y., Balis D., Sun D., Xie J., Canovatchel W., Rosenthal N. Renal safety of canagliflozin, a sodium glucose co-transporter 2 inhibitor, in patients with type 2 diabetes mellitus. J. Diab. Obes. Metabol. 2017;19(6):897–900. doi: 10.1111/dom.12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feig D.I., Kang D.H., Johnson R.J. Uric acid and cardiovascular risk. J. Curr. Opin. Pharmacol. 2002;2(2):126–130. doi: 10.1016/s1471-4892(02)00143-1. [DOI] [PubMed] [Google Scholar]
- Grassi D. Chronic hyperuricemia, uric acid deposit and cardiovascular risk. J Curr. Pharmaceut. Des. 2013;19(13):-. doi: 10.2174/1381612811319130011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heerspink H.J.L., Zeeuw D.D., Wie L., Leslie B., List J. Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes. J. Diab. Obes. Metabol. 2013;15(9):853–862. doi: 10.1111/dom.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idris I., Donnelly R. Sodium-glucose co-transporter-2 inhibitors: an emerging new class of oral antidiabetic drug. J. Diab. Obes. Metabol. 2009;11(2):79. doi: 10.1111/j.1463-1326.2008.00982.x. [DOI] [PubMed] [Google Scholar]
- Kalra S. Sodium glucose co-transporter-2 (SGLT2) inhibitors: a review of their basic and clinical pharmacology. J. Diab. Ther. 2014;5(2):355–366. doi: 10.1007/s13300-014-0089-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.Y., Guevara J.P., Kim K.M., Choi H.K., Heitjan D.F., Albert D.A. Hyperuricemia and coronary heart disease: a systematic review and meta-analysis. J. Arthritis Care Res. 2010;62(2):170–180. doi: 10.1002/acr.20065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwabara M., Niwa K., Nishi Y., Mizuno A., Asano T., Masuda K., Komatsu I., Yamazoe M., Takahashi O., Hisatome I. Relationship between serum uric acid levels and hypertension among Japanese individuals not treated for hyperuricemia and hypertension. J. Hypertens. Res. Off. J. Japan. Soc. Hypertens. 2014;37(8):785–789. doi: 10.1038/hr.2014.75. [DOI] [PubMed] [Google Scholar]
- Loeffler Lauren F. Uric acid level and elevated blood pressure in U.S. Adolescents: National Health And Nutrition Examination Survey 1999–2006. J. Hypertens. 2012;59(4):811–817. doi: 10.1161/HYPERTENSIONAHA.111.183244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majewski C., Bakris G.L. Blood pressure reduction: an added benefit of sodium-glucose cotransporter 2 inhibitors in patients with type 2 diabetes. J. Diab. Care. 2015;38(3):429. doi: 10.2337/dc14-1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcgill J.B. The SGLT2 inhibitor empagliflozin for the treatment of type 2 diabetes mellitus: a bench to bedside review. J. Diab. Therapy. 2014;5(1):43–63. doi: 10.1007/s13300-014-0063-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilding J.P.H., Norwood P., T'Joen C., Bastien A., List J.F., Fiedorek F.T. A study of dapagliflozin in patients with type 2 diabetes receiving high doses of insulin plus insulin sensitizers: applicability of a novel insulin-independent treatment. J. Diab. Care. 2009;32(9):1656–1662. doi: 10.2337/dc09-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Xu L., Tian D., Xia P., Zheng H., Wang L., Chen L. Effects of sodium-glucose co-transporter 2 (SGLT2) inhibitors on serum uric acid level: a meta-analysis of randomized controlled trials. J. Diab. Obes. Metabol. 2017;20(2) doi: 10.1111/dom.13101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
I can confirm I have included a statement regarding data and material availability in the declaration section of my manuscript.