Abstract
As a complicated micro-ecosystem, gut microbes are closely related to metabolic disease, immune disease and tumor (such as constipation. Long-term constipation would cause intestinal mucosal injury, enteritis, ileus, etc., thus inducing intestine cancer). In this research, intestine cancer model group and Codonopsis foetens treatment group were successfully constructed, and the variation of intestinal microbes were analyzed by 16S rRNA sequence. Results showed that there were changes in bacteria abundance of Firmicutes, Bacteroidetes, Proteobacteria, Deferribacteres, Tenericutes, and Actinobacteria, etc. Codonopsis foetens could directly or indirectly affect the growth and metabolism of Deferribacteres by altering the nutritional ingredient and pH value of intestine “medium”, thus affecting the occurrence and development of intestinal microbes.
Keywords: Codonopsis foetens, Deferribacteres, Intestine tumor, 16S rRNA sequence
1. Introduction
According to data from disease center, intestine cancer is a disease with high morbidity and high mortality. Rectum is the predominant predilection site of intestine cancer, followed by sigmoid and others. The intestinal tract is a dynamic and complicated world of microbes (Deng, 2013, Mehvish and Barkat, 2018a, Mehvish and Barkat, 2018b). Closely related to the metabolism activities of the host, the numbers and varieties of intestinal microbes are normally relatively stable (Gosalbes et al., 2012, Rabbani et al., 2018). A status of dynamic balance is maintained by the interrelation and interaction of intestinal microbes. Once these balance were broken, it may do harm to the health of host by excreting toxins, invading mucosa, activating carcinogens and participating in inflammation, etc. (Deng, 2013, Munir et al., 2018), eventually leading to diseases (such as constipation, Long-term constipation would cause intestinal mucosal injury, enteritis, ileus, etc., thus inducing intestine cancer). Abundant evidence showed that various diseases like obesity, diabetics, colorectal cancer and irritable bowel syndrome accompanied by changes of intestinal microbes. Also, intestinal microbes disorder plays a significant role in the pathogenesis of these diseases (Liu, 2018, Liu et al., 2015, Aziz et al., 2013, Wang et al., 2017a, Wang et al., 2017b, Wang et al., 2014, Bäckhed et al., 2004, Koeth et al., 2013, Hooper and Gordon, 2001, Larsson et al., 2012, Qin et al., 2010, Qin et al., 2012, Ley et al., 2006, Tremaroli and Bäckhed, 2012, Mehvish and Barkat, 2018a, Mehvish and Barkat, 2018b).
Preliminary study showed that long-term constipation would induce the occurrence of intestine cancer (Luan et al., 2016). Long-term constipation would cause characteristic changes of intestinal microbes (such as numbers, variety, etc.), further inducing intestinal microbes’ disorder and participating in the occurrence and development of bowel diseases.
Deferribacteres is a kind of bacteria that could gain energy by obligatory or facultative anaerobic metabolism. The iron metabolism of Deferribacteres in intestinal flora is definitely related to bowel iron balance. Abnormal iron metabolism would increase the risks of diseases and promote tumor growth (Chen et al., 2017, Khattak et al., 2018).
The growth of microorganisms is affected by pH value, temperature, moisture, and oxygen (Monod, 1949), and bacteria account for about 50% of the fecal volume (Pashankar and Loening-Baucke, 2005, Li et al., 2018). Thus it should be seen that the rest of food residue is a good “medium” for the intestinal flora (Liu et al., 2015). In the treatment group of preliminary study, the effect of Codonopsis foetens as the intestinal microbiological culture medium was equivalent to the nutrient substance in the microbiological culture medium. Codonopsis foetens directly or indirectly changed the intestinal bacteria micro-ecology by altering the nutrient composition of the intestinal “medium”, pH, and the like, further affecting the occurrence and development of intestinal cancer. Luan et al. (2018) performed a research on the effects of Codonopsis foetens on human colon cancer HCT116 and SW480 cell lines, of which results showed that Codonopsis foetens inhibited cell autophagy and induced apoptosis of colon cancer cells via activating the NF-κB pathway and promoting nuclear transportation of P65.
Therefore, by using metagenomic sequencing technology combined with bioinformatics analysis techniques, this study aimed to qualitatively and quantitatively study the effects of various aspects including intestinal structural composition, microbial diversity, and abundance in intestine cancer group and Codonopsis foetens treatment group, further screening for microbial markers in both groups.
2. Materials and methods
2.1. The construction of long-term constipation model
Intestinal cancer model and Codonopsis foetens treatment model in mice 21 healthy Kunming mice were routinely fed in laboratory for 3 days and later designated into 7 groups, including blank group (B), 1,2-dimethylhydrazine induced intestinal cancer group (CaD), long-term constipation group (C), long-term constipation induced intestinal cancer group (CCa), 1,2-dimethylhydrazine induced intestinal cancer with treatment group (CaDT), long-term constipation with treatment group (CT), long-term constipation induced intestinal cancer with treatment group (CCaT). According to methods mentioned in references, 2.5 mg/(kg*d) of loperamide hydrochloride were administered orally by gavage to mice in all groups except for blank group, constructing constipation mice model, while mice in blank group were gavaged with equal amounts of saline. Gastrogavage was performed for 2 consecutive weeks, and successful construction of constipation model was confirmed with intestine propelling rates and defecation rates. 1,2-dimethylhydrazine were injected intraperitoneally to mice of CaD group and successful construction of intestinal cancer model was confirmed by pathomorphology 6 weeks later. To construct 3 Codonopsis foetens treatment groups
Namely the 1,2-dimethylhydrazine induced intestinal cancer with treatment group (CaDT), long-term constipation with treatment group (CT) and long-term constipation induced intestinal cancer with treatment group (CCaT), total extract of Codonopsis foetens were given by gavage to mice of 1,2-dimethylhydrazine induced intestinal cancer group (CaD), long-term constipation group (C) and long-term constipation induced intestinal cancer group (CCa).
2.2. Fecal sampling and testing
After 4 weeks of treatment, the contents of the rectum were collected under aseptic conditions from mice of blank group, intestinal cancer group and Codonopsis foetens treatment group. Samples were preserved in liquid nitrogen for further 16S rRNA sequence.
2.3. Data processing
SPSS 13.0 software was used for statistical analysis. One-way analysis of variance was used for statistical analysis. Comparison between groups was performed using LSD multiple comparisons. Non-parametric factorial Kruskal-Wallis (KW) sum-rank test and Linear Discriminant Analysis (LDA) software LEfSe were used for detecting significant abundance difference characteristics, screening groups with significant different abundance, and evaluating the effect of each component (species) on this discrepancy. Software FLASH, Trimmomatic; with platform being Usearch (version 7.0 http://drive5.com/uparse/) were used for data deconvolution and parameter settings. RDP classifier Bayesian algorithm was used for taxonomic analysis of OTU Sequence with 97% similar level, referred to the 16S bacteria and archaeal ribosomal database Silva (Release 128 http://www.arb-silva.de).
3. Results and analysis
3.1. Preliminary research showed that long-term constipation could induce intestine tumor
Preliminary research was based on long-term constipation group (C) induced by loperamide hydrochloride and blank group (B). Successful construction of intestinal cancer model was confirmed by pathomorphology (showed at Figs. 1, 2, 3 and Table 1).
Fig. 1.

Typical picture of constipation model (fecal was solid and aggregated in mass in intestinal tract).
Fig. 2.
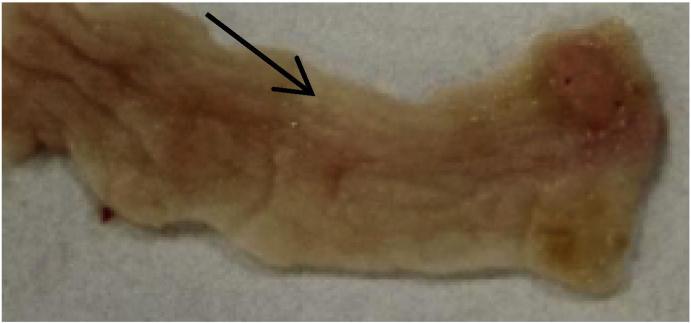
Typical picture of intestinal tumor morphology (arrow showed macroscopic sarcoma in intestinal tract).
Fig. 3.
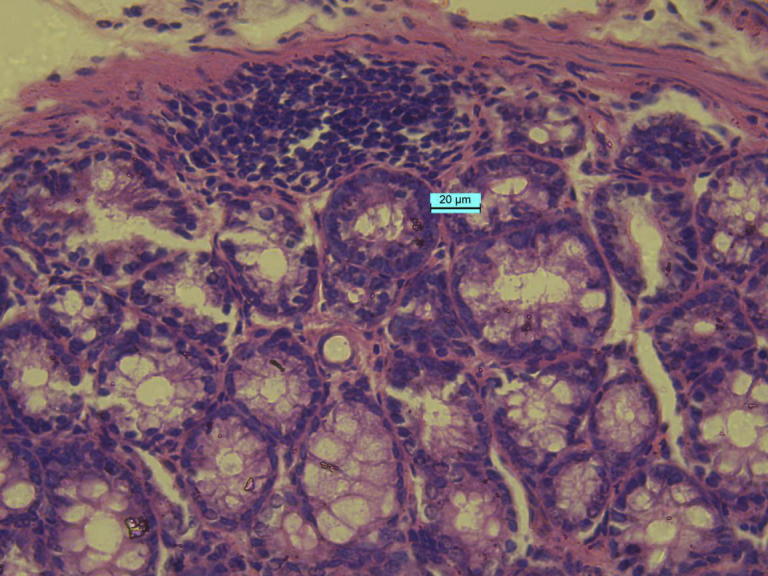
Intestinal tumor section under 40× magnification (pathological section of intestinal tumor cells, with varied cellular size and morphology, irregular nuclear, denser coloration, revealing different characteristics from maternal cells).
Table 1.
Intestinal propulsion rates of mice in constipation model group (C) and blank group (B).
| Total length of small intestine | The length of carbon particle propelling | Propulsion rate (%) | |
|---|---|---|---|
| C | 57 | 29 | 50.88 |
| B | 59 | 55 | 93.22 |
3.2. Intestinal microbial sequencing data filtration and Tags splicing in a mouse model of intestinal cancer
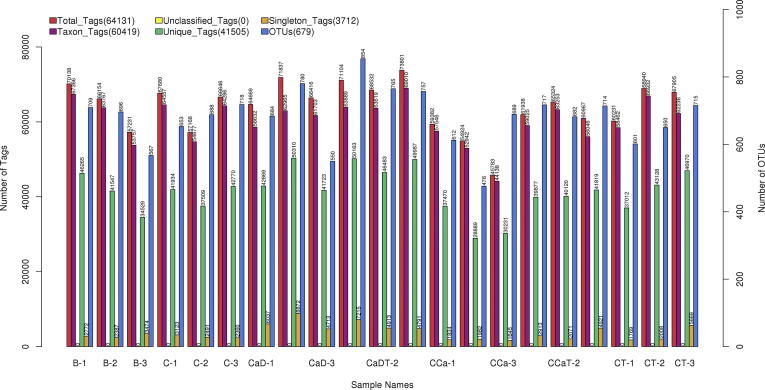
The results in Fig. 4 showed that after sequencing analysis of the 16s rRNA V3-V4 region, the original down-stream data was pre-processed. By filtering the original data, Tags spliced, Tags filtered and Tags region chimera, the final Effective Tags was obtained and OTU clustering analysis was performed.
Fig. 4.
OTUS and Tags diagram of different samples.
3.3. Comparative analysis of OTU in intestinal cancer model and Codonopsis foetens treatment group
The microbial communities of different habitats have a certain degree of similarity and specificity in species distribution. According to the OTU abundance information, we carried out the Venn diagram analysis to compare the OTU common or unique characteristics between the different intestinal cancer models and the Codonopsis foetens treatment group. As shown in Fig. 5, we can see that both intestinal cancer models and the Codonopsis foetens treatment group have special specific microbial community characteristics.
Fig. 5.
Intestinal cancer models and the Codonopsis foetens treatment group.
3.4. Comparative analysis of alpha diversity of intestinal microflora in intestinal cancer model and Codonopsis foetens treatment group
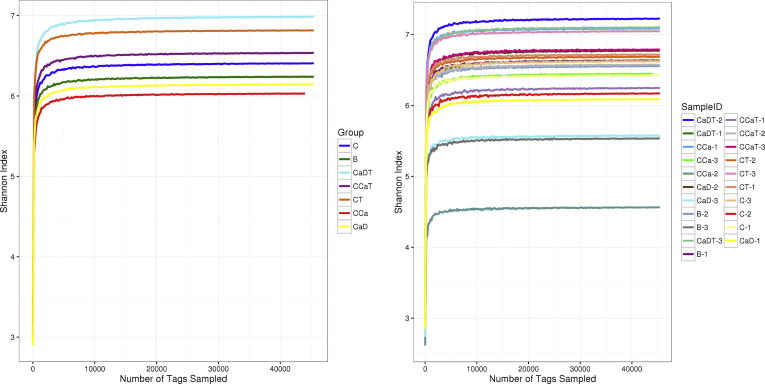
OTU clustering were performed on all samples collected from model group and Shannon Index were calculated with program to evaluate diversity of specific habitats or eco-system. Results showed that Shannon Index decreased in intestinal cancer model group while it was up-regulated in Codonopsis foetens treatment group compared with those of corresponding disease group (Fig. 6).
Fig. 6.
Change of Shannon Index in intestinal microflora of model group.
3.5. Comparative analysis of Beta diversity of intestinal microflora in intestinal cancer model and Codonopsis foetens treatment group
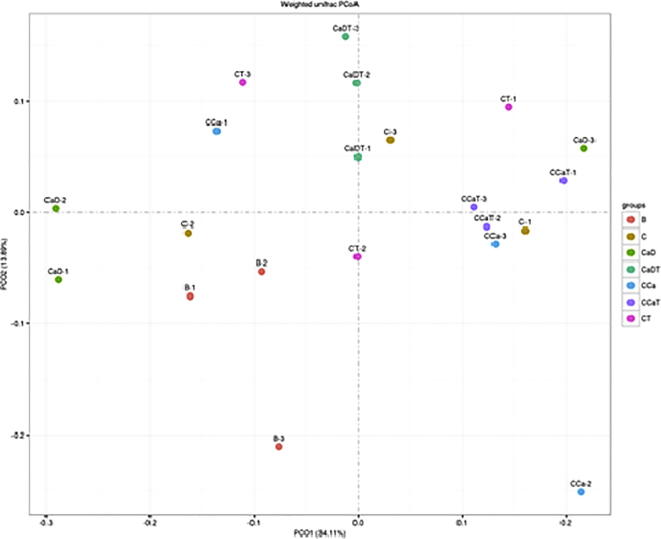
Beta diversity refers to diversity between different ecosystems. It is the rate of change of species composition along the environmental gradient or between communities, which represents biodiversity as reflection of environmental heterogeneity. Based on the species abundance information of the OTU list, Principal Component Analysis (PCA) and Principal Co-ordinate Analysis (PCoA, Principal Co-ordinate Analysis) methods are adopted. As shown in Fig. 7, results showed that the intestinal cancer model group and Codonopsis foetens treatment group were distinguishable, and the bacteria structure in CaDT treatment group and the CCaT treatment group had compact distribution and high similarity.
Fig. 7.
Beta diversity of intestinal microflora in model group.
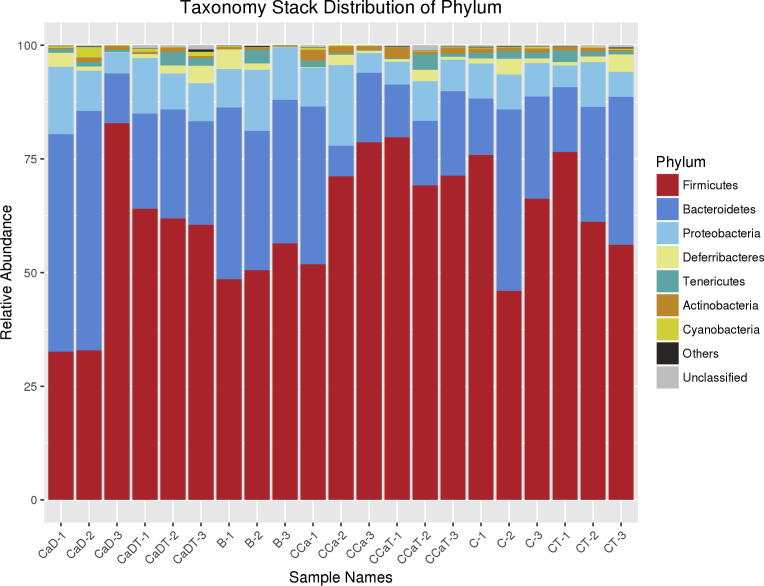
3.6. Differential analysis of species abundance
In order to investigate the effects of intestinal cancer model and Codonopsis foetens treatment group on the structure and composition of intestinal microflora, we performed clustering analysis on similar OTU at the level of phylum and class (as shown in Fig. 8). At phylum level, there were 6 major groups of intestinal microflora, including Firmicutes, Bacteroidetes, Proteobacteri, Deferribacteres, Tenericute and Actinobacteria. The abundance of Firmicutes and Bacteroidetes was higher in the intestinal cancer model and Codonopsis foetens treatment group, both being dominant bacteria groups. In control group, the abundance of Firmicutes was as high as 51.82%, while the abundance of Bacteroidetes was 33.3%, the abundance of Proteobacteria was 11.19% and the abundance of Deferribacteres is 1.9%.
Fig. 8.
Stack map of species distribution at phylum level.
The abundance of Firmicutes was higher in the intestinal cancer model group (CCa group) (67.2%) compared with those of the control group while the abundance of Bacteroidetes decreased (18.92%) compared with the control group. Moreover, the abundance of Proteobacteria and Deferribacteres were both lower than the control group, with the abundance being 10.1% and 0.9%, respectively.
In Codonopsis foetens treatment group (CCaT group), the abundance of Firmicutes was 73.4% while Bacteroidetes was 14.75%, Proteobacteria 6.8% and Deferribacteres 1.2%. This indicated that the abundance of intestinal microflora in both intestinal cancer model group and Codonopsis foetens treatment group was significantly different, especially Deferribacteres, Fimmicutes, Bacteroidetes, and Deformations, which are four major groups that contributing greatly on the intestinal micro-ecology.
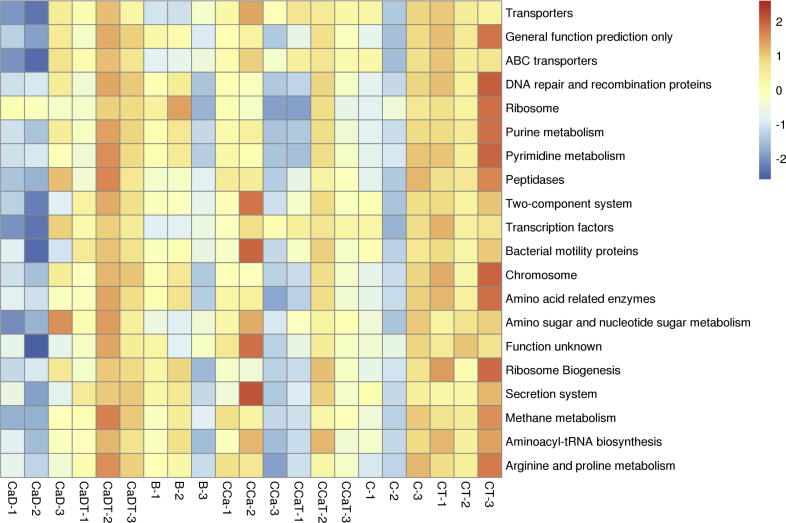
3.7. Kegg pathway analysis on intestinal cancer model and Codonopsis foetens treatment group
According to the OTU species annotation and abundance information, Picrust software was used for function prediction. The abundance of each pathway was obtained. As shown in Fig. 9, 20 enrich metabolic pathways were observed in abundance heat map, which are Arachidonic acid metabolic pathways, ABC transporters, Adipocytokine signaling pathway, Alanine, aspartate and glutamate metabolism, and the like.
Fig. 9.
The heat map of pathway distribution of intestinal cancer model and Codonopsis foetens treatment group.
4. Discussion
4.1. Intestinal cancer would affect the abundance of Deferribacteres
There are a large number of microbial communities in the intestine and a dynamic balance is maintained with the host organism. Also, different bacterial groups are kept at a certain proportional relationship. Based on 1,2-dimethylhydrazine-induced intestinal cancer model, we successfully constructed model of long-term constipation induced intestinal cancer. And treatment group was constructed with oral administration of Codonopsis foetens. The results showed that there were significant changes in intestinal microbes of intestinal cancer model and Codonopsis foetens treatment group.
The species abundance of Deferribacteres, Bacteroidetes, Firmicutes in the mice intestine had changed. The abundance of Deferribacteres was 1.9% in the control group, 0.9% in the cancer model group, and 1.2% in the Codonopsis foetens treatment group. Such tendency indicates that the iron metabolism of Deferribacteres is definitely related to the intestinal micro-ecology. In preliminary treatment group, the effect of Codonopsis foetens as the intestinal microbiological culture medium was equivalent to the nutrient substance in the microbiological culture medium. Codonopsis foetens directly or indirectly changed the intestinal bacteria micro-ecology by altering the nutrient composition of the intestinal “medium”, pH, and the like, further affecting the occurrence and development of intestinal cancer.
Deferribacteres is a kind of bacteria that could gain energy through obligate or facultative anaerobic metabolism. The iron metabolism of Deferribacteres in intestinal flora is definitely related to bowel iron balance. By altering the expression of iron-metabolizing proteins, increasing iron intake, reducing the amount of iron storage in tumor cells and iron loss, more iron could be mobilized and utilized by tumor cell metabolism. Abnormal iron metabolism increases the risk of cancer and promotes tumor growth (Chen et al., 2017, Nazihah et al., 2018, Khan et al., 2018).
In the intestinal cancer model group and the Codonopsis foetens treatment group, the abundances of Bacteroidetes and Firmicutes of mice intestinal tract were changed, which were irregular. Bacteroidetes is a kind of bacteria that promotes carbohydrate fermentation and participates in sugar, bile acid, and steroid metabolism. Firmicutes is a group of bacteria that can ferment cellulose to butyrate, and can be transformed into pathogenic pathogens by symbiosis. Subsequent studies are needed to determine the relationship between species abundance changes of Deferribacter, Bacteroidetes, and Firmicutes and the intestinal cancer, and to further elucidate the relationship between structural changes of intestinal microflora and intestinal cancer.
4.2. Codonopsis foetens could affect the metabolic pathways of Deferribacteres in constipation-type intestinal tumors
Changes of cellular metabolism is an important feature of tumors, and it interacts as both cause and effect of tumor occurrence and development. The occurrence and development of tumor will cause disorders in various metabolic pathways, such as the glycometabolism pathway, mitochondrial biosynthesis, amino acid metabolism, lipid metabolism and others (Zhou et al., 2016). In this study, PICRUSt software was used to perform functional annotations of KEGG Pathway. By applying significant enrichment analysis in KEGG metabolic pathways, 20 metabolic pathways including Ascorbate and aldarate metabolism (arachidonin acid pathway), ABC transporters, Adipocytokine signaling pathways, Alanine, aspartate and glutamate metabolism, indicating abnormal metabolic pathways such as glucose metabolism, amino acids, and lipid metabolism, accompanied by changes in metabolites. Subsequent studies should be focused on relevant metabolic pathways and important metabolites.
5. Conclusions
In this study, we carried out differential analysis of the effects of Codonopsis foetens on Deferribacteres in constipation-type intestinal tumor. Subsequent experiments are needed to further investigate the intestinal microflora (Deferribacteres, Firmicutes, Bacteroides). Combined with metabolomics studies, metabolites are analyzed and biomarkers that influence the development of intestinal tumors are identified.
-
(1)
At phylum level, the abundance of both Deferribacteres and Bacteroidetes were lower in the intestinal cancer model while the abundance of Firmicutes was higher compared with control group. Moreover, the abundance of both Deferribacteres and Firmicutes were higher in the Codonopsis foetens treatment group while the abundance of Bacteroidetes was lower compared with intestinal cancer model group.
-
(2)
Codonopsis foetens directly or indirectly changed the intestinal bacteria micro-ecology by altering the nutrient composition of the intestinal “medium”, pH, and the like, further affecting the occurrence and development of intestinal cancer. Changes in the abundance of intestinal flora is closely related to the occurrence and development of intestinal cancer, further affecting intestinal micro-ecology.
Acknowledgements
This study was funded by National Natural Science Foundation of China (NSFC) (31860254/61363061/31660029) and Advanced and Characteristic Key Biological Disciplines of Yunnan Province (project serial code: 50097505). The thesis of “Scientific and Technological Innovation Team Construction Project for Protection and Utilization of Under-forest Biological Resources (project serial code: 51400605)” was completed through joint support.
Footnotes
Peer review under responsibility of King Saud University.
References
- Aziz Q., Dore J., Emmanuel A. Gut microbiota and gastrointestinal health: current concepts and future directions. Neurogastr. Motil. 2013;25(1):4–15. doi: 10.1111/nmo.12046. [DOI] [PubMed] [Google Scholar]
- Bäckhed F., Ding H., Wang T. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA. 2004;101(44):15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S.J., Wang X., Li W., Cui J.W. Ferritin provided a new prospective for tumor diagnosis and treatment. Electron. J. Metabol. Nutr. Cancer. 2017;4(2):242–246. [Google Scholar]
- Deng G.H. Southern Medical University; Guangzhou: 2013. Effect of Long-Term Treatment of Anti-Microbial Drugs on Mouse Gut Microbiota Determined with Illumina Sequence of 16S rRNA Tags. [Google Scholar]
- Gosalbes M.J., Abellan J.J., Durban A. Metagenomics of human microbiome: beyond 16s rRNA. Clin. Microbiol. Infect. 2012;18(4):47–49. doi: 10.1111/j.1469-0691.2012.03865.x. [DOI] [PubMed] [Google Scholar]
- Hooper L.V., Gordon J.I. Commensal host-bacterial relationships in the gut. Science. 2001;292(5519):1115–1118. doi: 10.1126/science.1058709. [DOI] [PubMed] [Google Scholar]
- Khan A., Fiaz M., Khan R.A., Khan J.B., Khan F.U., Wahab Z.U. Antimicrobial efficacy of Tamarix Dioca (L.) leaves and flowers. Sci. Herit. J. 2018;2(1):01–03. [Google Scholar]
- Khattak A.M., Ul-Haq Z., Barki Z., Ilyas M. Application of soft P-open set to binary soft structures. Acta Scient. Malaysia. 2018;2(2):23–26. [Google Scholar]
- Koeth R.A., Wang Z., Levison B.S. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013;19(5):576–585. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson E., Tremaroli V., Lee Y.S. Analysis of gut microbial regulation of host gene expression along the length of the gut and regulation of gut microbial ecology through MyD88. Gut. 2012;61(8):1124–1131. doi: 10.1136/gutjnl-2011-301104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley R.E., Peterson D.A., Gordon J.I. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124(4):837–848. doi: 10.1016/j.cell.2006.02.017. [DOI] [PubMed] [Google Scholar]
- Li Y., Yang P., Wang H. Collecting coal fired power environmental tax to promote wind power development and environmental improvement. Acta Scient. Malaysia. 2018;2(1):05–08. [Google Scholar]
- Liu C.X. Gut microbiota: the effects of health, illness and medicines. Chin. J. Antibiot. 2018;43(1):1–14. [Google Scholar]
- Liu H.N., Chen Y.Z., Wu H., Liu T.T. Research of functional constipation and intestinal microbiota. Fudan Univ. J. Med. Sci. 2015;42(4):564–568. [Google Scholar]
- Luan Y.P., Li Y.M., Zhu L.N., Zheng S.Q., Mao D.C., Chen Z.X., Cao Y. Codonopis bulleynana Forest ex Diels inhibits autophagy and induces apoptosis of colon cancer cells by activating the NF-κB signaling pathway. Int. J. Mol. Med. 2018;41:1305–1314. doi: 10.3892/ijmm.2017.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan Y.P., Mao D.C., Li Y.M., Wu P.F., Ao C.Y. Effect of chronic constipation on dimethylhydrazine-induced intestinal cancer in Kunming Mice. J. Balkan Tribol. Assoc.-Biotribol. 2016;22(3A-Ⅱ):3383–3390. [Google Scholar]
- Mehvish S., Barkat M.Q. phytochemical and antioxidant screening of amomum subulatum, El ettaria Cardamomum, Emblica Officinalis, Rosa Damascene, Santalum Album And Valeriana officinalis and their effect on stomach, liver and heart. Matrix Sci. Pharma. 2018;2(2):21–26. [Google Scholar]
- Mehvish S., Barkat M.Q. Phytochemical and Antioxidant Screening Of Amomum Subulatum, Elettaria Cardamomum, Emblica Officinalis, Rosa Damascene, Santalum Album And Valeriana officinalis and their effect on stomach, liver and heart. Matrix Sci. Med. 2018;2(2):28–33. [Google Scholar]
- Monod J. The growth of bacterial cultures. Annu. Rev. Microbiol. 1949;3:371–394. [Google Scholar]
- Munir H., Shahid M., Subhani Z., Jabeen R. Activity-Guided isolation of a novel protein from foeniculum vulgare with antifungal and antibacterial activities. Matrix Sci. Pharma. 2018;2(2):01–03. [Google Scholar]
- Nazihah I., Zaini M.S., Shahari R., Che Amri C.N.A., Tajuddin N.M. Diversity and distribution of fern species in selected trail In Kuantan Pahang. Sci. Herit. J. 2018;2(1):04–09. [Google Scholar]
- Pashankar D.S., Loening-Baucke V. Increased prevalence of obesity in children with functional constipation evaluated in an academic medical center. Pediatrics. 2005;116(3):e377–e380. doi: 10.1542/peds.2005-0490. [DOI] [PubMed] [Google Scholar]
- Qin J., Li R., Raes J. A human gut microbial gene catalogue established by metagenomic sequence. Nature. 2010;464(7285):59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J., Li Y., Cai Z. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490(7418):55–60. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- Rabbani A.H., Hayat K., Gardezi F.H., Waheed A., Zahra A. A comparison of nalbuphine and pentazocine in controlling post-operative pain in dogs. Matrix Sci. Med. 2018;2(2):15–20. [Google Scholar]
- Tremaroli V., Bäckhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489(7415):242–249. doi: 10.1038/nature11552. [DOI] [PubMed] [Google Scholar]
- Wang B.H., Yao M.F., Lv L.X. The human microbiota in health and disease. Engineering. 2017;3:71–82. [Google Scholar]
- Wang W., Chen L., Zhou R. Increased proportions of Bifidobacterium and the Lactobacillus group and loss of butyrate-producing bacteria in inflammatory bowel disease. J. Clin. Microbiol. 2014;52(2):398–406. doi: 10.1128/JCM.01500-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.L., Wang B.H., Wu J.F. Modulation of gut microbiota in pathological states. Engineering. 2017;3(1):83–89. [Google Scholar]
- Zhou X., Wang Y.P., Xu Y.Y., Lei Q.Y. Metabolic dysfunction and cancer development. Chem. Life. 2016;36(6):739–744. [Google Scholar]
Further reading
- Luan Y.P., Cao Y., Mao D.C., Li Y.M., Yue X.Q., Zhao Y.J., Xiong F., Rong J., He C.Z. RNA sequencing reveals the differences between chemical and chronic constipation induction of intestinal tumor. Biomed. Res. 2017;28(22):10053–10061. [Google Scholar]
- Yang J.H., Zhao Z.H., Guo W.B., Guo J.L. Effects of Deoxynivalenol on intestinal microbiota of mice analyzed by Illumina-MiSeq high throughput sequencing technology. Chin. J. Anim. Nutr. 2017;29(1):158–167. [Google Scholar]