Abstract
Background and purpose
Curcumin, an active constituent of rhizomes of Curcuma longa Linn, exhibits a variety of biological activities such as anti-inflammation and anti-oxidant. The present study aims to examine the effects of curcumin on oxidative stress, inflammation and apoptosis in L-arginine induced acute pancreatitis (AP) in mice.
Methods
Male ICR mice were randomly divided into 4 groups. Control group received intraperitoneal injection (i.p.) of 1% DMSO as a vehicle. AP group received two doses of i.p. L-arginine (L-Arg) 450 mg/100 g body weight (BW) at 1-hour interval. AP plus low-dose curcumin group received i.p. curcumin 50 mg/kg BW 1 hour before L-Arg injection and then once daily for 3 days. AP plus high-dose curcumin group received i.p. curcumin 200 mg/kg BW 1 hour before L-Arg injection and then once daily for 3 days. All mice were sacrificed at 72 hours. Pancreatic tissue was obtained for histological evaluation, immunohistochemical studies for nuclear factor-kappa beta (NF-kβ), apoptosis and myeloperoxidase (MPO), and Western blot analyses for 4-Hydroxynonenal (4-HNE). Blood samples were collected for amylase analysis.
Results
Mean body weight was significantly lower in AP group than in control group, while in curcumin group, body weight was maintained. The serum amylase, number of MPO positive cells, NF-kB positive cells, TUNEL positive cells, and 4-HNE expression significantly increased in AP group when compared with control group, but decreased in low and high-dose curcumin groups. Mice in AP group developed severe pancreatic inflammation, edema and fat necrosis. While mice in low and high-dose curcumin groups showed a significant improvement in histopathological scores. There was no significant difference between low and high doses of curcumin.
Conclusion
Curcumin could attenuate acute pancreatitis via anti-oxidant, anti-inflammation and anti-apoptosis property leading to the improvement in pancreatic damage.
Keywords: Biochemistry, Cell biology, Physiology, Systems biology, Curcumin, Pancreatitis, Apoptosis, 4-HNE, Myeloperoxidase
1. Introduction
Acute pancreatitis (AP) is one of the most common gastrointestinal causes for hospital admissions in the US with rising incidence globally (Shah et al., 2018; Yadav and Lowenfels, 2006). Severe acute pancreatitis (SAP) is associated with high morbidity, mortality (Whitcomb, 2006; Zerem, 2014) and significant financial burden (Andersson et al., 2013). Due to the lack of disease-specific therapies, the treatment of SAP is mainly supportive care. The pathophysiology of SAP involves a cascade of events from trypsinogen activation, acinar cell injury/death, systemic inflammatory responses and multi-organ dysfunction (Bhatia et al., 2005). The ongoing search for specific treatment of SAP by targeting one of these pathogenic steps has not yielded fruitful results.
Curcumin or diferuloylmethane is the principle bioactive component of rhizomes of Curcuma longa which is accounted for 77% of curcuminoids present in turmeric (Lestari and Indrayanto, 2014). It exerts several biological activities including anti-inflammatory, anti-oxidant, and anti-microbial effects (Amalraj et al., 2016; Maheshwari et al., 2006). With these properties, we hypothesized that curcumin would be beneficial in the treatment of AP by acting directly at the pathogenesis of AP. To prove this hypothesis, we used the mice model of L-arginine (L-Arg) induced AP. This experimental model has been consistently shown to produce acute necrotizing pancreatitis and a good model to study the effects of curcumin in several aspects of AP (Hegyi et al., 2004; Tashiro et al., 2001).
2. Materials and methods
2.1. Animal preparation
Four-week-old male ICR mice weighing 25–30 grams were purchased from the National Laboratory Animal Center, Salaya Campus, Mahidol University. All animal procedures were approved by Animal Care and Use Committee, Faculty of Medicine, Chulalongkorn University (IRB No 12/2560). Animals were housed in a temperature-controlled room at 25–30 °C with a 12-hour light and dark cycle and had free access to drinking water and standard rat chow. All animals were allowed to acclimate to the new environment for 1 week prior to the initiation of the experiment.
2.2. Preparation of L-arginine and curcumin
L-arginine solution was prepared by dissolving L-arginine powder (Sigma Aldrich Co Pvt. Ltd., USA) in 0.9% normal saline and adjusting the pH to 7 with 5 N HCl. Curcumin powder (Cayman Chemical Company, USA) was dissolved in 1% dimethyl sulfoxide (DMSO) to make a solution.
2.3. Experimental protocols
Male ICR mice were randomly divided into 4 groups (n = 6 in each group). Control group received intraperitoneal (i.p.) injection of 1% DMSO once daily for 3 days. AP group received two i.p. injections of L-Arg (total dose of 450 mg/100 g body weight) at an interval of 1 hour to induce AP. This model was modified from Dawra and colleagues (Dawra and Saluja, 2012). AP plus low-dose curcumin (AP + low dose cur) group received i.p. curcumin 50 mg/kg body weight 1 hour before L-Arg injection followed by i.p. curcumin 50 mg/kg body weight once daily for 3 days. AP plus high-dose curcumin (AP + high dose cur) group received i.p. curcumin 200 mg/kg body weight 1 hour before L-Arg injection followed by i.p. curcumin 200 mg/kg body weight once daily for 3 days. We chose curcumin 200 mg/kg body weight based on a study by Samuhasaneeto and colleagues with slight modification to fit the current model (Samuhasaneeto et al., 2009) and the purpose of using curcumin 50 mg/kg body weight was to evaluate whether a lower dose had similar effects to a higher dose (Shafik and Abou-Fard, 2016).
All mice were sacrificed at 72 hours after L-Arg administration by intraperitoneal injection of overdosed sodium thiopental (50 mg/kg body weight). Pancreas was rapidly removed and separated from the surrounding lymph nodes and fat. Pancreatic tissue samples were divided into 2 parts. The first part was fixed in 10% formalin solution for histological evaluation and immunohistochemical studies for nuclear factor-kappa beta (NF-kβ), myeloperoxidase (MPO), and apoptosis. The second part was placed in liquid nitrogen and stored at -80 °C until further measurement of 4-Hydroxynonenal (4-HNE) by Western blot analysis. Blood samples were obtained by cardiac puncture and allowed to clot for 30 minutes at 25 °C. Clotted blood was then centrifuged at 4 °C with the speed of 3,000 x g for 10 minutes. Serum samples were obtained and stored at -80 °C until amylase analysis. Serum amylase levels were measured using a Hitachi 7600-020 automatic biochemical analyzer (Hitachi Ltd., Tokyo, Japan).
2.4. Pathological examination of pancreas and assessment of apoptotic acinar cells
Histological slides were reviewed under light microscope by an experienced pathologist who was blinded to the experiment. A histopathologic scoring system suggested by H. E. V. DE COCK (De Cock et al., 2007) was modified to grade the severity of acute pancreatitis. In brief, the grading system was based on a point system for 3 lesions: neutrophilic inflammation, interstitial edema and mesenteric fat necrosis. The sum of the points for each lesion was calculated with a maximal score of 9. A score of 0 was considered normal. A score of 1–3 total points was considered mild AP; 4–6, moderate; and 7–9, severe AP. This scoring system was also used by another study from our group (Sriko et al., 2018).
Percentage of apoptotic acinar cells were determined by Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) method using ApopTag® Peroxidase In Situ Apoptosis Detection kit (Millipore, CA, USA). The procedure was performed according to the manufacturer's instruction. The number of TUNEL positive cells, defined by cells with dark brown nuclei, was counted by the Aperio ImageScope software (Leica Biosystems Imaging, Inc., MD, USA) and expressed as percentage of positive cells.
2.5. Immunohistochemistry for NF-kβ expression
After pancreatic tissue samples were fixed in 10% formalin for 24–48 hours, they were embedded in paraffin and sliced at a thickness of 4 μm. Tissue sections were then deparafinized with xylene and ethanol for 10 minutes and the antigen was retrieved with citrate buffer pH 6.0 in microwave for 13 minutes. Slides were incubated with 3% hydrogen peroxide to block endogenous peroxidase activity for 5 minutes and with 3% normal horse serum to block nonspecific binding for 20 minutes. Tissues were then washed with phosphate-buffered saline (PBS). Subsequently, sections were incubated overnight with a polyclonal antibody against the p65 subunit of NF-kβ (Abcam, MA, USA) at a dilution of 1:150 at 4 °C overnight and washed again with PBS. Slides were then incubated with secondary antibody (Abcam, MA, USA) for 30 minutes and appropriate horseradish-peroxidase–labeled polymers (BioCare, Concord, CA) were performed. When the development of color with diaminobenzidine (DAB) was detected, sections were counterstained with hematoxylin. Under light microscopy, positive cells were pancreatic acinar cells with dark brown-stained nuclei. The number of positive-stained cells was counted by the Aperio ImageScope software (Leica Biosystems Imaging, Inc., MD, USA) in 10 randomly selected fields at 40x magnification and expressed as percentage of positive cells.
2.6. Immunohistochemistry for determination of myeloperoxidase (MPO) activity
The antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, California, USA). Formalin-fixed, paraffin-embedded samples were cut into 4-μm sections and each tissue section was deparaffinized and rehydrated with graded ethanol. Antigen retrieval was performed using EDTA antigen repairing buffer (pH 8.0) in microwave for 13 minutes. After natural cooling, tissues were placed in phosphate-buffered saline (PBS) at pH 7.4 twice for 5 minutes each. Tissues were then incubated with 3% hydrogen peroxide solution at room temperature for 25 minutes in the dark. Subsequently, slides were incubated with an antibody against MPO (Dako, Denmark; 1:500) overnight at 4 °C in a humid chamber. Then tissues were incubated in an anti-goat secondary antibody (Dako, Denmark; 1:1000) for 30 minutes at 25 °C. Finally, sections were counterstained with hematoxylin. Under a light microscope, the positive cells were defined as those with dark brown nuclei. The numbers of MPO positive cells were counted in 10 randomly selected fields at 40x magnification. The results were expressed as the average number of positive-stained cells per high-power field (HPF).
2.7. Western blotting for detection of 4-hydroxynonenal (4-HNE)
Western blot analysis was adopted from Levine et al. (1994). For the detection of protein bound HNE, 100 mL of pancreatic samples were homogenized in a solution containing 12% sodium dodecyl sulfate (SDS), 6% 2-mercaptoethanol, 50 mM Tris pH 7.8, and 30% glycerol. Protein concentrations in pancreatic homogenates were measured using bicinchoninic acid (BCA) assay kit (Pierce®, Thermo scientifric, lnc., IL, USA) according to the manufacturer's instruction. Samples were divided into aliquots of 10 μg, which were then heated at 95 °C for 5 minutes before being loaded to the gels and subsequently transferred to the PVDF membrane. The PDVF membrane was then probed with primary antibody against HNE-histidine (clone 1g4) (R&D Systems, Inc., USA) in a 1:500 dilution followed by peroxidase-conjugated antimouse-IgG in a 1:3000 dilution (R&D Systems, Inc., USA). Chemiluminescence was detected on polaroid-films using the ECL minicamera. Data were presented as relative ratio between HNE expression and glyceraldehyde 3-phosphate dehydrogenase (GADPH), which was used as loading control.
2.8. Statistical analysis
Data were expressed as mean ± standard deviation (SD). One-way ANOVA and post-hoc Tukey HSD were used for comparisons amongst groups. A p-value of less than 0.05 was considered statistically significant. All analyses were performed using the SPSS for windows version 17.0 (SPSS, Inc., Chicago, IL, USA).
3. Results
3.1. Changes in general appearance and body weight of mice
Comparing with the control group, mice in the AP group were less active with downier fur. Curcumin treatment in both low and high doses improved general appearance of mice. Body weight at the beginning of the experiment was similar amongst groups. Body weight changes before and after experimentation (ΔBW = BW at day 4 – BW at day 0) are summarized in Fig. 1. Significant weight loss was noted in AP group compared with weight gain in controls (ΔBW -0.69 ± 0.68 vs. 2.23 ± 1.52 g, respective, p < 0.01). Mice in low-dose curcumin group gained significant weight as compared with mice in AP group (ΔBW 1.57 ± 0.43 vs. -0.69 ± 0.68 g, respectively, p < 0.05). There were no significant differences in body weight changes between low and high-dose curcumin groups (ΔBW 1.57 ± 0.43 vs. 1.15 ± 1.51 g, respectively, p > 0.05).
Fig. 1.
The delta body weight between groups. (*P < 0.05 versus control group; #P < 0.05 versus AP group).
3.2. The effects of curcumin on serum amylase
Serum amylase levels in each group are shown in Fig. 2. Serum amylase levels were markedly increased in AP group when compared with control group (8438.50 ± 2371.55 vs. 5880 ± 1561.98 U/L, respectively, p < 0.05). Curcumin treatment, both in low and high doses, led to lower amylase levels as compared to those in AP group (4802 ± 486.85 U/L in low-dose group and 5069 ± 255.73 U/L in high-dose group, p < 0.01 for both analyses). There were no significant differences in amylase levels between low and high-dose curcumin groups.
Fig. 2.
Serum amylase level. Data are presented as mean ± SD (*P < 0.05 versus control group; #P < 0.05 versus AP group).
3.3. The effects of curcumin on pancreatic histopathology and apoptosis
Table 1 represents the detailed grading of histopathologic changes in each group, while Table 2 depicts the severity of pancreatitis with mean scores in each group. All mice in AP group developed severe pancreatitis based on the scoring system, whereas the majority of mice in control (n = 5), low-dose (n = 5) and high-dose curcumin groups (n = 5) had normal pancreatic histology with only 1 mouse in each group developed mild pancreatitis. Curcumin-treated mice also had similar pancreatitis scores to control mice. Fig. 3 are representative images of pancreatic pathology in each group.
Table 1.
Histopathologic scores of the pancreas in each group. The grading scores in each categories (inflammation, edema and necrosis) are presented in the table.
| Group | N | Inflammation |
Edema |
Necrosis |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | ||
| Control | 6 | 6 | - | - | - | 5 | 1 | - | - | 6 | - | - | - |
| AP | 6 | - | - | 1 | 5 | - | 1 | 2 | 3 | - | 1 | 1 | 4 |
| AP + Low cur | 6 | 6 | - | - | - | 5 | 1 | - | - | 6 | - | - | - |
| AP + High cur | 6 | 6 | - | - | - | 6 | - | - | - | 5 | 1 | - | - |
Table 2.
The sum of pancreatic pathological scores and the severity of pancreatitis in each group.
| Pathologic scores | ||||||
|---|---|---|---|---|---|---|
| Group | Number | Normal (0) | Mild (1–3) | Moderate (4–6) | Severe (7–9) | Mean ± SD |
| Control | 6 | 5 | 1 | 0 | 0 | 0.17 ± 0.41 |
| AP | 6 | 0 | 0 | 0 | 6 | 7.67 ± 0.82 |
| AP + Low cur | 6 | 5 | 1 | 0 | 0 | 0.17 ± 0.41 |
| AP + High cur | 6 | 5 | 1 | 0 | 0 | 0.17 ± 0.41 |
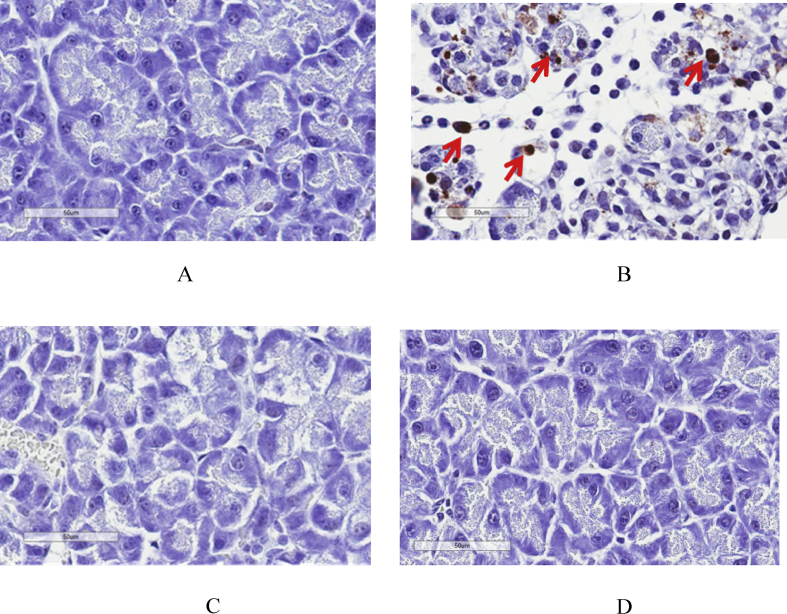
Fig. 3.
Representative images of pancreatic histopathology by H & E staining (40X). (A) Control group, (B) AP group, (C) Low-dose curcumin group and (D) High-dose curcumin group. Arrows indicate inflammation, edema and fat necrosis.
Fig. 4a are representative images of TUNEL staining in each group. Apoptotic acinar cells are those with dark brown stain. As shown in Fig. 4b, the percentage of apoptotic acinar cells in AP group was significantly higher than in control group (2.72 ± 2.72 vs. 0.50 ± 0.33%, respectively, p < 0.05). The percentages of apoptotic acinar cells in both low-dose (0.46 ± 0.13%) and high-dose curcumin groups (0.37 ± 0.08%) closely resembled that of controls but were much lower than in AP group (p < 0.05 for low-dose and p < 0.01 for high-dose curcumin).
Fig. 4.
a. Representative images of TUNEL staining for evaluation of apoptotic acinar cells (40X). (A) Control group, (B) AP group, (C) Low-dose curcumin and (D) High-dose curcumin. Arrows indicate apoptotic acinar cells. b. The percentage of TUNEL positive cells in each group. Data are presented as mean ± SD (*P < 0.05 versus control group; #P < 0.05 versus AP group).
3.4. Effects of curcumin on MPO activity and NF-kB expression
As shown in Fig. 5a and b, the number of MPO positive cells was significantly higher in AP mice as compared with control mice (29.57 ± 3.18 vs. 0.08 ± 0.27 cells/HPF, respectively, p < 0.001). Curcumin treatment, both in low and high doses, reduced MPO activities significantly when comparing with AP mice (1.25 ± 0.67 cells/HPF in low-dose group and 0.75 ± 0.4 cells/HPF in high-dose group, p < 0.001 for both comparisons).
Fig. 5.
a. Representative images of immunohistochemistry for MPO in mice pancreas (40X). (A) Control group, (B) AP group, (C) Low-dose curcumin and (D) High-dose curcumin. Arrows indicate MPO positive cells. b. Numbers of MPO positive cells per HPF in mice pancreas. Data are presented as mean ± SD (*P < 0.05 versus control group; #P < 0.05 versus AP group).
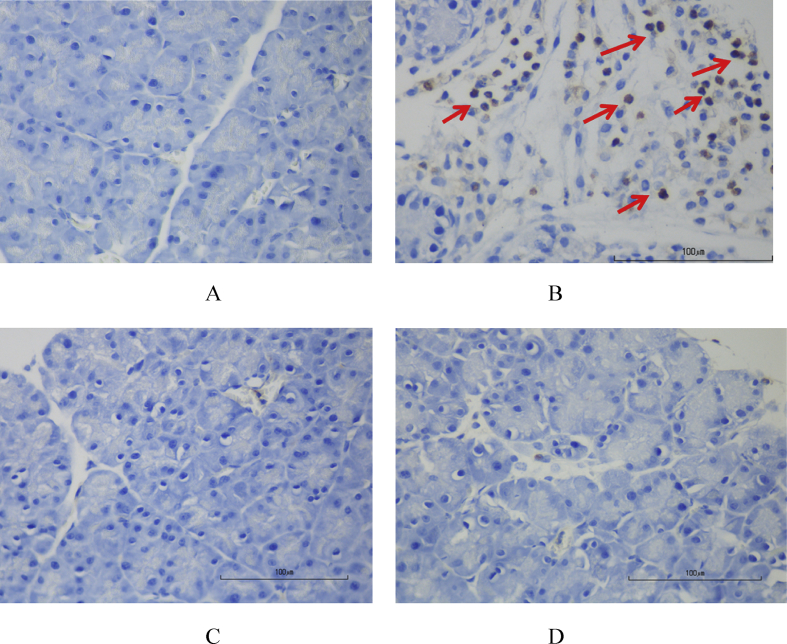
As shown in Fig. 6a and b, the percentage of NF-kβ-positive cells in AP group was significantly higher than in control group (47.95 ± 12.98 vs. 6.27 ± 2.31%, respectively, p < 0.001). With curcumin administration, the percentage of NF-kβ-positive cells significantly declined in both low-dose (3.63 ± 1.61%, p < 0.001) and high-dose group (11.68 ± 6.44%, p < 0.001) as compared with AP group.
Fig. 6.
a. Representative images of immunohistochemistry for NF-kB expression in mice pancreas (40X). (A) Control group, (B) AP group, (C) Low-dose curcumin and (D) High-dose curcumin. Arrows indicate NF-kβ positive cells. b. The percentage of NF-kβ positive cells in each groups. Data are presented as mean ± SD (*P < 0.05 versus control group; #P < 0.05 versus AP group).
3.5. Effects of curcumin on 4-HNE expression
The relative ratio of 4-HNE expression was significantly higher in AP group as compared with control group (2.59 ± 0.46 vs. 1.38 ± 0.70, respectively, p < 0.05), whereas the relative ratio of 4-HNE expression in the low-dose (1.01 ± 0.73, p < 0.01) and high-dose curcumin groups (1.46 ± 0.23, p < 0.05) were significantly lower than in AP group but closely resembled that of control group (Fig. 7).
Fig. 7.
Western blot analysis of 4-HNE expression in pancreas. (A) Western blot analysis of 4-HNE expression in pancreas. (B) Densitometric analysis of the 4-HNE relative to GAPDH. All data are presented as mean ± SD (*P < 0.05 versus control groups; #P < 0.05, ##P < 0.01 versus AP groups).
4. Discussion
We found that L-Arg could successfully induce AP as evidenced by serum amylase elevation and pancreatic histopathological changes. Mice in AP group lost weight and developed noticeable deterioration in general appearance compared with control group. Tani and colleagues also reported similar findings of which rats with L-Arg induced AP gained significantly less weight than control rats (Tani et al., 1990). In addition, the improvement in general appearance, curcumin treatment in both low and high doses reduced serum amylase to the similar levels of control group. The decline in serum amylase levels was in line with the normalization of pancreatic histopathology in curcumin-treated mice. Similar to our study, Yu and colleagues demonstrated the reduction in serum amylase levels and histopathological scoring in curcumin-treated AP mice as compared with caerulein-induced AP mice (Yu et al., 2011).
Attracted to the pancreatic tissues by chemokines, neutrophils are important players in the development of acute inflammation in AP (Vonlaufen et al., 2007). Neutrophils exert their action through the release of MPO, protease and reactive oxygen species (ROS). In this study, we determined neutrophil activities through the measurement of MPO positivity in pancreatic tissues. Our results demonstrated a significant rise in MPO activities in AP mice comparing with controls. With curcumin treatment, the number of MPO-positive cells declined markedly to the level seen in control group. In accordance with our study, Shafik and colleagues showed lower MPO activities for both pre- and post L-Arg treatment with curcumin as compared with AP group (Shafik and Abou-Fard, 2016).
NF-kβ activation is a key event in the development of AP. Pathologic calcium signaling and ROS generation have been shown to be important mediators in the activation of NF-kβ (Jakkampudi et al., 2016). NF-kβ leads to the production of several inflammatory cytokines, such as tumor necrosis factor (TNF)-α, interleukin (IL)-1b, IL-2, IL-6 and IL-18, and various chemokines such as IL-8. A study using transgenic mice demonstrated that the higher level of NF-kβ activity was associated with increased severity of AP and persistent elevation of NF-kβ levels could lead to changes seen in chronic pancreatitis (Huang et al., 2013). In this study, we found the increased NF-kβ expression in mice with AP which was attenuated by the treatment with both low and high-dose curcumin. The changes in NF-kβ expression correlated with the severity of pancreatitis on histopathology. Previous studies showed that curcumin inhibited NF-kβ activation through the blockage of I kappa B kinase (IKK) activation and I kappa B (IkB) degradation (Jobin et al., 1999; Pan et al., 2000). Similar to our findings, Gukovsky and colleagues studied the effects of curcumin in rat models of AP and found that curcumin inhibited both the increase in NF-κB binding activity and IκB degradation leading to the amelioration of pancreatitis (Gukovsky et al., 2003).
Oxidative stress is one of the major events that occurs after pancreatic acinar injury leading to the acceleration of inflammatory responses and multi-organ dysfunction (Robles et al., 2013; Shi et al., 2005). Lipid peroxidation of polyunsaturated fatty acids, a consequence of oxidative stress, produces 4-HNE which can be used as an oxidative stress marker. In this study, 4-HNE expression almost doubled in mice with AP as compared with control mice and the administration of curcumin in both doses could restore the changes in 4-HNE expression indicating the amelioration of oxidative stress. In animal models of alcohol-induced liver disease and CCl4-induced liver injury, curcumin was shown to increase glutathione production and reduce 4-HNE expression, thus improving liver histopathology and biochemical tests in both conditions (Lee et al., 2016; Varatharajalu et al., 2016). In a clinical study of tropical pancreatitis, 6-week treatment with curcumin led to the increased levels of glutathione and decreased levels of malonyldialdehyde, another marker of oxidative stress (Durgaprasad et al., 2005). The aforementioned evidence suggested that curcumin worked as an anti-oxidant and could be used for treatment of several inflammatory conditions including AP.
An in vitro study using rat pancreatic acinar AR4-2J cells demonstrated that L-Arg could induce acinar cell apoptosis through the change in pancreatitis-associated protein (PAP) gene expression (Motoo et al., 2000). Furthermore, products of lipid peroxidation, such as 4-HNE have been implicated in the development of apoptosis through the induction of Fas expression, and induction, phosphorylation, and nuclear translocation of p53 and subsequent activation of caspase3 (Awasthi et al., 2008). Our results confirmed the findings from previous observation that the degree of acinar cell apoptosis was significantly higher in L-Arg induced AP mice than that observed in control mice. With curcumin treatment in both low and high doses, the number of apoptotic acinar cells declined to the level seen in control mice. Despite not being studied directly in AP, curcumin has been shown to reduce apoptosis in other conditions. Wang and colleagues demonstrated that curcumin reduced the expression of apoptotic genes, such as CytC, Casp3 and Casp8 in non-alcoholic steatohepatitis rats that were treated with curcumin (Wang et al., 2015).
In conclusion, curcumin treatment in both low and high doses could reduce inflammation, oxidative stress, apoptosis and thus alleviating pancreatic histopathologic changes in L-Arg induced acute pancreatitis mice.
Declarations
Author contribution statement
Prasong Siriviriyakul: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data.
Thidarat Chingchit: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Naruemon Klaikeaw: Performed the experiments; Analyzed and interpreted the data.
Maneerat Chayanupatkul: Analyzed and interpreted the data; Wrote the paper.
Duangporn Werawatganon: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
This study was funded by the 90th Anniversary of Chulalongkorn University Fund (Ratchadaphiseksomphot Endowment Fund), Chulalongkorn University, Bangkok, Thailand.
References
- Amalraj A., Pius A., Gopi S. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives - a review. J. Tradit. Complementary Med. 2016;7(2):205–233. doi: 10.1016/j.jtcme.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson B., Appelgren B., Sjodin V. Acute pancreatitis--costs for healthcare and loss of production. Scand. J. Gastroenterol. 2013;48(12):1459–1465. doi: 10.3109/00365521.2013.843201. [DOI] [PubMed] [Google Scholar]
- Awasthi Y.C., Sharma R., Sharma A. Self-regulatory role of 4-hydroxynonenal in signaling for stress-induced programmed cell death. Free Radic. Biol. Med. 2008;45(2):111–118. doi: 10.1016/j.freeradbiomed.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia M., Wong F.L., Cao Y. Pathophysiology of acute pancreatitis. Pancreatology. 2005;5(2–3):132–144. doi: 10.1159/000085265. [DOI] [PubMed] [Google Scholar]
- Dawra R., Saluja A.K. L-arginine-induced experimental acute pancreatitis. Pancreapedia: Exocrine Pancreas Knowledge Base. 2012 [Google Scholar]
- De Cock H.E., Forman M.A., Farver T.B. Prevalence and histopathologic characteristics of pancreatitis in cats. Vet. Pathol. 2007;44(1):39–49. doi: 10.1354/vp.44-1-39. [DOI] [PubMed] [Google Scholar]
- Durgaprasad S., Pai C.G., Vasanthkumar A pilot study of the antioxidant effect of curcumin in tropical pancreatitis. Indian J. Med. Res. 2005;122(4):315–318. [PubMed] [Google Scholar]
- Gukovsky I., Reyes C.N., Vaquero E.C. Curcumin ameliorates ethanol and nonethanol experimental pancreatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2003;284(1):G85–95. doi: 10.1152/ajpgi.00138.2002. [DOI] [PubMed] [Google Scholar]
- Hegyi P., Rakonczay Z., Jr., Sári R. L-arginine-induced experimental pancreatitis. World J. Gastroenterol. 2004;10(14):2003–2009. doi: 10.3748/wjg.v10.i14.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H., Liu Y., Daniluk J. Activation of nuclear factor-kappaB in acinar cells increases the severity of pancreatitis in mice. Gastroenterology. 2013;144(1):202–210. doi: 10.1053/j.gastro.2012.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakkampudi A., Jangala R., Reddy B.R. NF-kappaB in acute pancreatitis: mechanisms and therapeutic potential. Pancreatology. 2016;16(4):477–488. doi: 10.1016/j.pan.2016.05.001. [DOI] [PubMed] [Google Scholar]
- Jobin C., Bradham C.A., Russo M.P. Curcumin blocks cytokine-mediated NF-kappa B activation and proinflammatory gene expression by inhibiting inhibitory factor I-kappa B kinase activity. J. Immunol. 1999;163(6):3474–3483. [PubMed] [Google Scholar]
- Lee H.-Y., Kim S.-W., Lee G.-H. Turmeric extract and its active compound, curcumin, protect against chronic CCl4-induced liver damage by enhancing antioxidation. BMC Complement Altern. Med. 2016;16(1):316. doi: 10.1186/s12906-016-1307-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lestari M.L., Indrayanto G. Curcumin. Profiles Drug Subst. Excipients Relat. Methodol. 2014;39:113–204. doi: 10.1016/B978-0-12-800173-8.00003-9. [DOI] [PubMed] [Google Scholar]
- Levine R.L., Williams J.A., Stadtman E.R. Carbonyl assays for determination of oxidatively modified proteins. Methods Enzymol. 1994;233:346–357. doi: 10.1016/s0076-6879(94)33040-9. [DOI] [PubMed] [Google Scholar]
- Maheshwari R.K., Singh A.K., Gaddipati J. Multiple biological activities of curcumin: a short review. Life Sci. 2006;78(18):2081–2087. doi: 10.1016/j.lfs.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Motoo Y., Taga K., Su S.B. Arginine induces apoptosis and gene expression of pancreatitis-associated protein (PAP) in rat pancreatic acinar AR4-2J cells. Pancreas. 2000;20(1):61–66. doi: 10.1097/00006676-200001000-00009. [DOI] [PubMed] [Google Scholar]
- Pan M.H., Lin-Shiau S.Y., Lin J.K. Comparative studies on the suppression of nitric oxide synthase by curcumin and its hydrogenated metabolites through down-regulation of IkappaB kinase and NFkappaB activation in macrophages. Biochem. Pharmacol. 2000;60(11):1665–1676. doi: 10.1016/s0006-2952(00)00489-5. [DOI] [PubMed] [Google Scholar]
- Robles L., Vaziri N.D., Ichii H. Role of oxidative stress in the pathogenesis of pancreatitis: effect of antioxidant therapy. Pancreat. Disord. Ther. 2013;3(1) doi: 10.4172/2165-7092.1000112. 112-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuhasaneeto S., Thong-Ngam D., Kulaputana O. Curcumin decreased oxidative stress, inhibited NF-kappaB activation, and improved liver pathology in ethanol-induced liver injury in rats. J. Biomed. Biotechnol. 2009;2009:981963. doi: 10.1155/2009/981963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafik N.M., Abou-Fard G.M. Ameliorative effects of curcumin on Fibrinogen-like protein-2 gene expression, some oxido-inflammatory and apoptotic markers in a rat model of l-arginine-induced acute pancreatitis. J. Biochem. Mol. Toxicol. 2016;30(6):302–308. doi: 10.1002/jbt.21794. [DOI] [PubMed] [Google Scholar]
- Shah A.P., Mourad M.M., Bramhall S.R. Acute pancreatitis: current perspectives on diagnosis and management. J. Inflamm. Res. 2018;11:77–85. doi: 10.2147/JIR.S135751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C., Andersson R., Zhao X. Potential role of reactive oxygen species in pancreatitis-associated multiple organ dysfunction. Pancreatology. 2005;5(4–5):492–500. doi: 10.1159/000087063. [DOI] [PubMed] [Google Scholar]
- Sriko J., Werawatganon D, Klaikaew N., Siriviriyakul P. Genistein attenuated severity of L-arginine-induced acute pancreatitis. J. Physiol. Biomed. Sci. 2018;31(1):12–17. [Google Scholar]
- Tani S., Itoh H., Okabayashi Y. New model of acute necrotizing pancreatitis induced by excessive doses of arginine in rats. Dig. Dis. Sci. 1990;35(3):367–374. doi: 10.1007/BF01537416. [DOI] [PubMed] [Google Scholar]
- Tashiro M., Schafer C., Yao H. Arginine induced acute pancreatitis alters the actin cytoskeleton and increases heat shock protein expression in rat pancreatic acinar cells. Gut. 2001;49(2):241–250. doi: 10.1136/gut.49.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varatharajalu R., Garige M., Leckey L.C. Protective role of dietary curcumin in the prevention of the oxidative stress induced by chronic alcohol with respect to hepatic injury and antiatherogenic markers. Oxidative Med. Cell. Longev. 2016;2016:5017460. doi: 10.1155/2016/5017460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonlaufen A., Apte M.V., Imhof B.A. The role of inflammatory and parenchymal cells in acute pancreatitis. J. Pathol. 2007;213(3):239–248. doi: 10.1002/path.2231. [DOI] [PubMed] [Google Scholar]
- Wang L., Lv Y., Yao H. Curcumin prevents the non-alcoholic fatty hepatitis via mitochondria protection and apoptosis reduction. Int. J. Clin. Exp. Pathol. 2015;8(9):11503–11509. [PMC free article] [PubMed] [Google Scholar]
- Whitcomb D.C. Clinical practice. Acute pancreatitis. N. Engl. J. Med. 2006;354(20):2142–2150. doi: 10.1056/NEJMcp054958. [DOI] [PubMed] [Google Scholar]
- Yadav D., Lowenfels A.B. Trends in the epidemiology of the first attack of acute pancreatitis: a systematic review. Pancreas. 2006;33(4):323–330. doi: 10.1097/01.mpa.0000236733.31617.52. [DOI] [PubMed] [Google Scholar]
- Yu W.-G., Xu G., Ren G.-J. Preventive action of curcumin in experimental acute pancreatitis in mouse. Indian J. Med. Res. 2011;134(5):717–724. doi: 10.4103/0971-5916.91009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerem E. Treatment of severe acute pancreatitis and its complications. World J. Gastroenterol. 2014;20(38):13879–13892. doi: 10.3748/wjg.v20.i38.13879. [DOI] [PMC free article] [PubMed] [Google Scholar]